Abstract
Schizophrenia is a widespread psychiatric disorder, affecting 1% of people. Despite this high prevalence, schizophrenia is not well treated because of its enigmatic developmental origin. We explore here the developmental etiology of endophenotypes associated with schizophrenia using a regulated transgenic approach in mice. Recently, a polymorphism that increases mRNA levels of the G-protein subunit Gαs was genetically linked to schizophrenia. Here we show that regulated overexpression of Gαs mRNA in forebrain neurons of mice is sufficient to cause a number of schizophrenia-related phenotypes, as measured in adult mice, including sensorimotor gating deficits (prepulse inhibition of acoustic startle, PPI) that are reversed by haloperidol or the phosphodiesterase inhibitor rolipram, psychomotor agitation (hyperlocomotion), hippocampus-dependent learning and memory retrieval impairments (hidden water maze, contextual fear conditioning), and enlarged ventricles. Interestingly, overexpression of Gαs during development plays a significant role in some (PPI, spatial learning and memory and neuroanatomical deficits) but not all of these adulthood phenotypes. Pharmacological and biochemical studies suggest the Gαs-induced behavioral deficits correlate with compensatory decreases in hippocampal and cortical cyclic AMP (cAMP) levels. These decreases in cAMP may lead to reduced activation of the guanine exchange factor Epac (also known as RapGEF 3/4) as stimulation of Epac with the select agonist 8-pCPT-2′-O-Me-cAMP increases PPI and improves memory in C57BL/6J mice. Thus, we suggest that the developmental impact of a given biochemical insult, such as increased Gαs expression, is phenotype specific and that Epac may prove to be a novel therapeutic target for the treatment of both developmentally regulated and non-developmentally regulated symptoms associated with schizophrenia.
Keywords: GNAS, cAMP, Rap-GEF 4, learning and memory, cognition, schizophrenia
Introduction
Schizophrenia is a devastating psychiatric disorder of multifactorial origin. It is characterized by positive (for example, hallucinations, psychosis), negative (for example, social withdrawal, flattened affect) and cognitive symptoms (for example, attention and memory deficits).1 Positive symptoms usually emerge after adolescence and are fairly well treated in ~70% of patients by currently available antipsychotics. In contrast, negative and cognitive symptoms—the severity of which tends to predict functional outcome— can emerge in preadolescence and are often unchanged by current therapies.2,3 It is generally thought that schizophrenia-related symptoms observed in adults are due to developmental abnormalities; however, direct evidence to this effect is minimal.4 Fortunately, genetic tools in mice now exist that enable reversible expression of disease-relevant molecules across the lifespan, and we use such an approach here to test the developmental hypothesis of schizophrenia.
The G-protein subunit Gαs (also known as GNAS1) may be one molecule contributing to the pathophysiology of schizophrenia. Increased efficacy of Gαs signaling is observed in striatum and mononuclear leukocytes from patients with schizophrenia5,6 and is reversed with antipsychotic treatment.7 Further, the T393C polymorphism in Gαs has been genetically linked to deficit schizophrenia in an Italian population sample, such that the 393TT genotype was observed significantly more in these patients (37.1%) relative to controls (22.8%).8 Interestingly, the 393TT genotype appears to increase Gαs mRNA expression, as measured in bladder tumors and adipose tissue of patients suffering from transitional cell carcinoma (brain was not examined).9 Following acute activation—either by a ligand-bound receptor10 or by non-canonical mechanisms independent of a receptor10–14—Gαs stimulates adenylyl cyclase (AC), thereby increasing cyclic AMP (cAMP) levels. Chronic stimulation of Gαs, however, triggers a protein kinase A (PKA)-dependent compensatory degradation of cAMP by phosphodiesterases (PDEs) in select brain regions (hippocampus and cortex, but not striatum;15,16). PDEs are categorized into 11 families and are the only know enzymes to break down cyclic nucleotides.17,18 Interestingly, abnormalities in the PDE-4 family, a family of cAMP-specific, PKA-regulated isoforms,17–20 has been implicated in schizophrenia directly and through an interaction with the major mental illness susceptibility gene Disrupted in schizophrenia 1.21–23
The cAMP cascade modulates an array of processes within the central nervous system, including behavioral domains relevant to schizophrenia such as sensorimotor gating15,24 and learning and memory. 16,25–28 As such, the cAMP cascade has been of great interest in the search for central nervous system therapeutics.29,30 Targeting the cAMP cascade may be particularly fruitful for the treatment of schizophrenia as rolipram, a drug that increases cAMP levels by inhibiting PDE-4,31 has shown antipsychotic-like, memory-enhancing and antidepressant-like effects in preclinical models.15,24,32–37
cAMP stimulates cyclic nucleotide-gated channels (CNGC), PKA and the more recently characterized guanine exchange protein activated by cAMP (Epac).38 Although much is known of PKA, fairly little is understood of the role Epac has in CNS function, although this is quickly changing with the advent of an Epac-selective agonist (8-pCPT-2′-O-Me- cAMP).39 What we do know of Epac suggests this downstream target of cAMP may be particularly relevant to schizophrenia. First, Epac appears to be involved in glutamate release at peripheral and central synapses,40–42 and a loss of glutamatergic signaling (along with heightened dopaminergic signaling) is thought to be important in the manifestation of schizophrenia.43 Second, Epac stimulates integrin function,44 and a loss of integrin signaling has been implicated in the pathophysiology of schizophrenia (for example,45,46). Finally, Epac appears to regulate neuronal excitability in cerebellar neurons47 as well as hippocampal synaptic plasticity,48 and both of these brain regions have been implicated in the neuropathophysiology of schizophrenia.49–51
To determine if elevated expression of Gαs is sufficient to trigger developmentally regulated endophenotypes (also known as intermediate or subphenotypes) associated with schizophrenia, we developed transgenic mice that overexpress Gαs under the control of the reversible tetracycline system (Figure 1). Behaviorally, we focus on endophenotypes that are reliably modeled in mice52 and closely resemble behaviors tested in humans.49,53,54 These measures of interest include sensorimotor gating (prepulse inhibition of acoustic startle, PPI), psychomotor agitation (locomotor activity) and hippocampus- dependent learning and memory (spatial water maze and contextual fear conditioning). Neuroanatomy and biochemical compensation within the cAMP cascade are also examined. We show that overexpressing Gαs is sufficient to cause schizophrenia-related phenotypes in adults and that maximal expression of some—but not all—phenotypes in Gαs adults requires overexpression during development. Phenotypes triggered by Gαs overexpression appear due to compensatory decreases in cAMP, and we identify Epac as a novel therapeutic target for the treatment of schizophrenia.
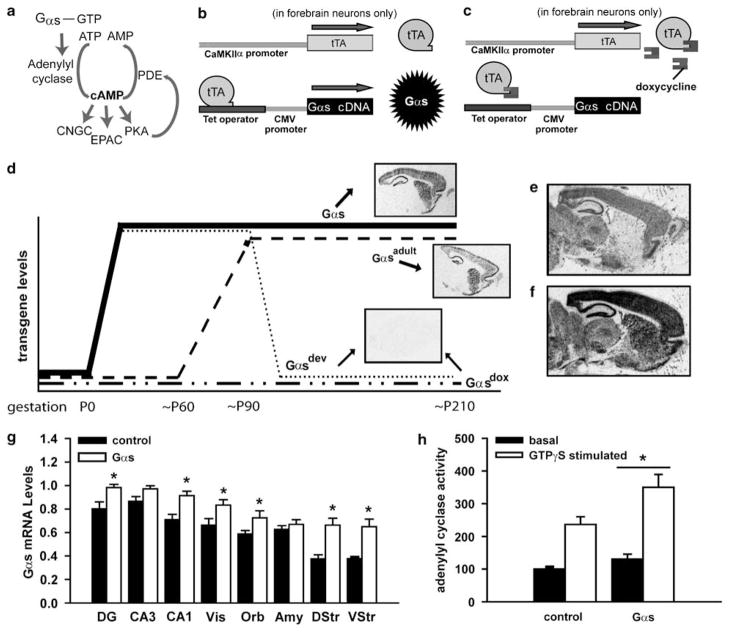
Figure 1.
Gαs mice show temporally and spatially controlled transgene expression in hippocampus, cortex and striatum. (a) Once activated, either through traditional receptor stimulation of a heterotrimer or noncanonical mechanisms independent of a heterotrimer, Gαs stimulates cAMP (cAMP) synthesis by adenylyl cyclase. cAMP, in turn, stimulates cyclic nucleotide-gated channels (CNGCs), exchange protein activated by cAMP (Epac), and protein kinase A (PKA). Chronic Gαs signaling triggers negative feedback through phosphodiesterases (PDEs),15,16 the only known enzymes to degrade cAMP,18 ultimately leading to compensatory decreases in cAMP levels in select brain regions.15 (b) Expression of the Gαs transgene is spatially restricted to forebrain neurons because expression of the tTA transcription factor is driven by the CaMKIIα promoter. (c) Gαs overexpression is temporally controlled by doxycycline (dox; by food supply) administration, which prevents tTA from binding the tet operator. (d) Here, we test mice that express Gαs throughout both development and adulthood (i.e., never receive dox; Gαs), throughout development but not at time of testing (i.e., receive dox≥2.5 weeks in adulthood; Gαsdev), during adulthood only (i.e., raised on dox until 2 months of age; Gαsadult), or never (i.e., raised and maintained on dox throughout life; Gαsdox). To verify expression of the transgene effectively increases total Gαs mRNA levels, we conducted autoradiographic in situ hybridization in (e) control (CT) and (f) Gαs mice. (g) Quantification indicates that expression of the transgene increases total levels of Gαs mRNA (endogenous + transgenic) in select regions (n = 7–8; F(7,91) = 4.15, P < 0.001), which leads to (h) increased adenylyl cyclase activity (as measured in parahippocampal cortex; n = 6–8; F(1,12) = 5.99, P = 0.031). Data graphed mean±s.e.m. DG, dentate gyrus; Vis, visual cortex; Orb, orbital cortex; Amy, amygdala; DStr, dorsal striatum; VStr, ventral striatum, vs CT, *P < 0.05–0.001.
Materials and methods
Subjects
Expression of the Gαs transgene, which contains a hemagglutinin epitope,55 is regulated by the tetracycline operator (tetO). Thus, for the Gαs cDNA to be transcribed, co-expression of a tetracycline-responsive transcription factor (tTA) is required (Figures 1b and e). Use of such a bigenic system allows for spatial and temporal control over transgene expression because expression of tTA was spatially restricted to forebrain neurons using the CaMKIIα promoter and transgene expression can be suppressed with doxycycline (dox), which blocks the ability of tTA to bind the tetO promoter (Figures 1c and f).56 We chose to restrict expression of the transgene to forebrain neurons (using the CaMKIIα promoter56) to prevent peripheral effects of the transgene that could confound the interpretation of behavioral data (that is, effects on brain stem, musculature or organs that could influence the general health and well-being of an animal, thus, preventing them from performing a task independent of effects of cognition). Comparison of endogenous vs transgenic expression of Gαs can be seen in Figure 1. The tetO-Gαs transgene was microinjected into pronuclei from superovulated B6SJLF1/J mice by the Transgenic and Chimeric Mouse Facility at the University of Pennsylvania. The line reported here (line 1593, chosen for its high levels of transgene expression throughout forebrain structures) was backcrossed onto C57BL/6J for 3–5 generations before being mated to CaMKIIα-tTA mice (N20+ on C57BL/6J) for initial characterization of control and transgenic offspring. The tetO-Gαs line was then backcrossed to N10–12 before again being mated with the CaMKIIα-tTA mice for further characterization of control and transgenic offspring. There was no loss or gain of phenotypes with additional backcrossing; thus, data were collapsed across generations.
All transgenic mice were bred and housed at the University of Pennsylvania on a 12 h light–dark cycle with ad libitum access to food and water. Approximately equal numbers of male and female Gαs mice ranging from 2 to 7 months of age were compared to control littermates. The control group (CT) was composed of wild-type mice (having neither transgene), monogenic tetO-Gαs mice and monogenic CaMKIIα-tTA mice for comparison relative to bigenic tetO-Gαs/CaMKIIα-tTA (referred to hereafter as Gαs mice). For experiments employing dox, 200 mg/kg was administered with the chow (Bio-serve, Frenchtown, NJ, USA). To parse out the effects of transgene expression during development vs adulthood, adult Gαs and control littermates (> 2 months old) were tested under one of four dox conditions: no dox (Gαs), dox at some age after 2 months old (tested ≥2.5 weeks on dox; Gαsdev), dox from conception until 2–2.5 months of age (tested ≥2 months following dox removal; Gαsadult) or lifelong dox (from conception to kill; Gαsdox). The lifelong dox condition was only used in experiments where dox given to adults (Gαsdev group) failed to reverse a phenotype. Generally speaking, every data set represents both behaviorally naive in addition to non-naive mice or naive mice only. With regards to Gαs mice and their control littermates, a single cohort of mice was initially tested on all behaviors described herein with a minimum of 1-week separating tests (open field, startle/PPI, contextual (CFC) and cue-dependent fear conditioning and then water maze). Subsequent naive cohorts were then tested in PPI, CFC and water maze to ensure that phenotypes observed in the first cohort were not due to testing history. Gαs mice exhibited the behavioral deficits described herein whether behaviorally naive or non-naive, so data were collapsed. Similar approaches were taken with Gαsdev and Gαsadult mice. See the Supplementary Discussion regarding justification of sample sizes.
Male C57BL/6J mice were also housed at the University of Pennsylvania on a 12 h light–dark cycle with ad libitum access to food and water. Mice were allowed to acclimate to the facility for a minimum of 1 week before cannulation (for Epac agonist studies). All experiments were conducted in accordance with National Institutes of Health Guidelines for Animal Care and Use, and all experiments were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
Autoradiographic in situ hybridization
In situ hybridization was conducted as previously described.15–16 Two (α-35S)-dATP labeled probes were used, one specific for transgenic Gαs (Figures 1d–f; 5′-CTCACCATCGCTGTTGGACGCGTAATCCGGCACG TCCTCTTCGCCGCC-3′) and one that labeled both endogenous and transgenic Gαs (Supplementary Figure S1; 5′-TTGATCTCCTGCACTTTAGTGGCCTTCTCACCATCGCTGTTG-3′). Measures for total Gαs were taken in sections approximately 2.32mm lateral from bregma.
Biochemistry
Biochemical experiments were conducted as previously described.15 Briefly, mice were killed by cervical dislocation, brains were harvested fresh and regions of interest from a single hemisphere were immediately dissected out and placed in tubes sitting on dry ice. AC activity was measured in membrane preparations from parahippocampal cortex (loosely defined as cortical regions overlying the hippocampus) of control and Gαs mice killed from the home cage or following a vehicle (saline) treatment using a modification of the method presented by Salomon et al.57 cAMP levels were measured using a radioimmune competition assay according to manufacturer’s directions (PerkinElmer, Wellesley, MA, USA).
PPI
Startle and PPI were measured as previously described (SR-Lab, San Diego, CA, USA)15 To measure startle, pulses of 90–120 dB were presented in random order (background set to 68 dB). To calculate PPI (%PPI), the 40 ms startling pulse of 120 dB was preceded (by 100 ms) by a 20-ms nonstartling prepulse (72, 76 or 84 dB). PPI for a given prepulse intensity was calculated using the following formula: (100−(average startle response for prepulse trials/average startle response for pulse-only trials)×100).
Open field
Locomotor activity was measured in a 41×41cm open field (San Diego Instruments, San Diego, CA, USA) equipped with 16×16 motion detector beams (beam every 2.54 cm). Activity was recorded for 30 min. Horizontal (breaks of two contiguous beams in the horizontal place) and vertical activities (breaks of a beam 5 cm above the floor) were measured and the percentage of horizontal activity that occurred in the periphery (outer two beams on all sides) was calculated.
Water maze
Spatial learning and memory was assayed using the Morris water maze.58 Animals were first trained to sit for 20 s on a submerged platform in a bucket (three trials in 1 day). After bucket training, animals were trained to locate an 11cm diameter platform located within a 117cm diameter pool of white tinted 21 °C water. Training involved two trials per day with a 5-min intertrial interval. For the visible-platform maze, animals were trained for 5 days to locate a submerged platform that was located just beneath the surface of the cloudy water but was marked by a visible cue. For the hidden maze, subjects were trained for 9 days to find an unmarked, submerged platform. If mice did not reach the platform within 60 s, they were placed on the platform. Mice remained on the platform for 20 s at the end of each trial before being returned to their home cage. Mice were brought back to the maze 1 or 21 days after training for a ‘probe’ trial, in which the platform was removed. Performance (latency to platform, time in quadrant, crossings over an area twice the diameter of the platform, swim speed, thigmotaxis as defined as the outer 10% of the pool) was tracked using an automated system (HVS Image, San Diego, CA, USA).
Fear conditioning
Mice were trained to associate a 1.5 mA. footshock (unconditioned stimulus, US) with a training context and an auditory cue (white noise), as previously described.16 In experiments using transgenic mice, subjects were handled for 2 min per day for 3 days before training. In the experiment testing the Epac agonist, subjects were not handled. During training, mice were placed in the box and 2 min into the session the cue (conditioned stimulus, CS) was played for 30 s, during the last 2 s of which the footshock was administered. For testing cued fear memory, mice were placed into a different experimental chamber for 5 min either 1 h (short-term memory) or 24 h following training (long-term memory), and the cue played the last 3 min of the session. For testing contextual fear memory, mice were returned to the training chamber for 3 min, either 1 h, 24 h or 2.5 weeks following training (the last to allow time for removing or adding dox between training and retrieval).
Neuroanatomy
Nissl staining was conducted as previously described using thionin16 and the size (mm2) of various regions of interest were quantified using ImageJ 1.23. (NIH, Washington, DC, USA). An average measurement was calculated for each region across three adjacent sections approximately 0.84–1.32mm lateral from bregma. Magnetic resonance imaging (MRI) was performed in vivo on a 4.7T horizontal bore magnet (Varian Inc., Palo Alto, CA, USA) in the Small Animal Imaging Core at the Hospital of the University of Pennsylvania using a circularly polarized birdcage coil custom built for mouse brain imaging. Mice were anesthetized with 1% isoflurane in air. Body temperature was maintained at 37 °C and heart rate was monitored using an MRI-compatible system (SA Instruments, Stony Brook, NY, USA). T2-weighted images of the entire mouse brain were acquired using a three-dimensional fast spin echo pulse sequence (TR = 3 s, effective TE = 40 ms, ETL = 16, NEX= 2, acquisition matrix = 256×128×128, dimensions = 3.2×1.6×1.6mm, 125 μm isotropic resolution, scan time = 100 min). For each three-dimensional data set, the brain and ventricles were segmented in a semiautomated fashion using thresholding (3D Slicer software).59
Drugs
Haloperidol (Ben Venue Laboratories Inc. Bedford, OH, USA) was dissolved in saline with lactic acid, pH 3.0–3.8 (0.1 mg/ml) and administered at a dose of 1.0 mg/kg, i.p., 30 min before experiment. In the haloperidol PPI and cAMP experiments, a between-subjects design was used. Rolipram (Sigma-Aldrich, St Louis, MO, USA) was dissolved in 2% dimethyl sulfoxide in saline (0.066 mg/ml) and administered at a dose of 0.66 mg/kg, i.p., 15 min before experiment. In the PPI experiment examining rolipram, a within-subjects design was used where half of subjects were tested under vehicle and half were tested under rolipram and then subjects were tested again (a minimum of 1 week later) under the opposite treatment. 5 nmol of 8-pCPT-2′-O-Me-cAMP (Biolog, Germany), a selective Epac agonist, was dissolved in 3 μl of 1× phosphate-buffered saline and administered i.c.v. immediately before the experiment, using a between-subjects design. Doses for these drugs were based on our previous reports using these or similar drugs.15,24
Surgeries
Implantation of a 22-gauge guide cannula (Plastics One Inc., Roanoke, VA, USA) into the lateral ventricle (anteroposterior from the bregma −0.4mm, lateral 1.1mm and 2.3mm in depth) was conducted on 8- to 10-week-old C57BL/6J mice as previously described.15 To verify cannulae placement, animals were killed after testing and their brains were coronally sectioned (20 μm sections) with a cryostat. Cannula tracks were plainly visible on Nissl staining, and placement within the ventricle was confirmed by an investigator blinded to treatment condition.
Data analysis
Statistical analyses were conducted using SigmaStat (version 2.03; Systat, Point Richmond, CA, USA) and Statistica (version 6.1; Statsoft, Tulsa, OK, USA). Data were analyzed by t-test, analysis of variance (ANOVA) or repeated-measures ANOVA as appropriate. When significant effects (between more than two groups) or interactions were found by ANOVAs, post hoc comparisons were made using Student–Newman–Keuls range statistics. Statistical outliers greater than two standard deviations from the mean were dropped from analyses (Figure 2d, 6 of 120 data points; Figure 4B1, 6 of 261 data points; Figure 4C1, 11 of 296 data points; Figure 4C2 1 of 160 data points; Figure 8a, 1 of 33 data points). In biochemical experiments, balanced groups of animals were raised at different times and killed on different days. Therefore, to control for variability due to day of killing, data for each group were normalized and expressed as a percentage of the control (or control vehicle) mean as previously described.15 Normalized data were then combined for statistical analyses. Significance was determined at P < 0.05. All data are expressed as mean±s.e.m.
Figure 2.
Overexpression of Gαs is required throughout both development and adulthood to cause deficits in prepulse inhibition in adult Gαs mice. (a) There is no difference between control (CT) and Gαs mice in the magnitude of the startle response to a range of decibel (dB) intensities (n = 27 per genotype). (b) In contrast, Gαs mice show a significant reduction in the ability to gate the startle response (to 120 dB), as indicated by decreased prepulse inhibition (%PPI; F(1,104) = 11.19, P = 0.002). Interestingly, neither (c) developmental (n = 18–19 per genotype) nor (d) adulthood overexpression of Gαs (n=20 per genotype) alone is sufficient to reduce prepulse inhibition of acoustic startle (PPI). (e) Despite the developmental nature of the PPI deficit, haloperidol (1 mg/kg, i.p.) fully rescues the PPI deficit exhibited by Gαs mice (n = 6–9 per group; F(1,54) = 4.60, P = 0.01). Data graphed mean±s.e.m. main effect of genotype, @P = 0.002; post hoc, vs all other groups, *P < 0.05.
Figure 4.
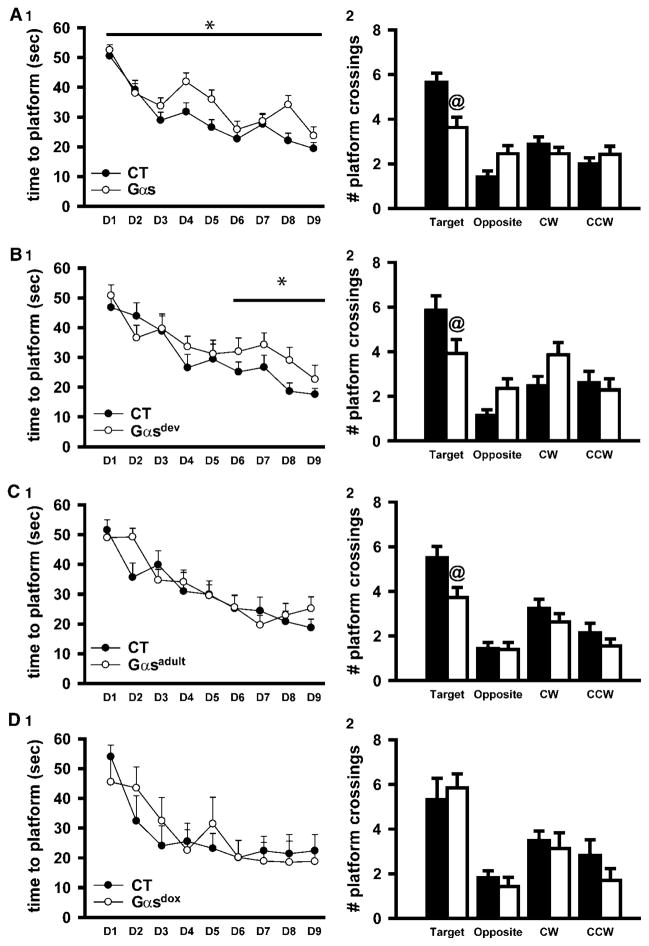
Overexpression of Gαs during development is necessary and sufficient to induce spatial learning impairments, whereas overexpression during development or adulthood is sufficient to induce deficits in spatial memory in adult Gαs mice. Mice were trained to find a platform hidden in a Morris water maze. (A1) Relative to control littermates, Gαs mice (n = 32–35 per genotype) show deficient spatial learning (longer latencies to find a hidden platform; F(1,520) = 6.82, P = 0.011) and (A2) impaired spatial memory (fewer crossings over the platform location; F(3,195) = 8.10, P < 0.001). (B1) Developmental overexpression of Gαs (n = 14–15 per genotype) is sufficient to cause these deficits in spatial learning (F(1,78) = 5.16, P = 0.031) and (B2) memory (F(3,75)= 4.286, P = 0.008). (C1) In contrast, adulthood overexpression of Gαs (n = 20 per genotype) is not sufficient to trigger the deficits in spatial learning, but (C1) is sufficient to impair spatial memory (F(1,112) = 8.52, P = 0.006). (D1) Importantly, Gαsdox mice with lifelong transgene suppression (n = 6–7 per genotype) show normal spatial learning and (D2) memory. Data graphed mean±s.e.m. T, target; O, opposite; CW, clockwise; CCW, counterclockwise, vs CT, *P < 0.01–0.001.
Figure 8.
Acute stimulation of the cAMP (cAMP) target Epac during adulthood increases prepulse inhibition of acoustic startle (PPI) and improves hippocampus-dependent associative memory in C57BL/6J mice. To determine if Epac signaling might impact behaviors noted to be altered in Gαs mice, we tested the effect of the Epac agonist 8-pCPT-2′-O-Me-cAMP (5 nmol, i.c.v.) on PPI and contextual fear memory retrieval. (a) C57BL/6J mice treated with 8-pCPT-2′-O-Me-cAMP (5 nmol, i.c.v.; n = 6) exhibit significantly increased levels of PPI across prepulse intensities, relative to mice treated with vehicle (n=5; F(1,17) = 8.18, P = 0.019). (b) At 1 h following contextual fear training, C57BL/6J mice trained with the footshock (US) received either vehicle (n = 12) or the Epac agonist (n = 8) and all mice trained without the US received the Epac agonist (n = 4) to test for nonspecific effects of the drug on mobility. Immediately following drug treatment, mice were tested for short-term contextual memory. Animals were retested 24 h following training without any additional drug treatment. Relative to vehicle- and agonist-treated mice trained without the US, 8-pCPT-2′-O-Me-cAMP-treated mice trained with the US show significantly higher levels of freezing across the short-term and long-term memory tests (F(2,18) = 4.48, P = 0.024). This suggests that stimulation of Epac facilitates retrieval (as indicated by improved performance during the short-term memory test) and consolidation of contextual fear memory (as indicated by carryover of the effect to the drug-free long-term memory test). Data graphed mean±s.e.m. Post hoc, vs vehicle and non-shocked 8-pCPT-2′-O-Me-cAMP, *P < 0.05; vs vehicle and shocked 8-pCPT-2′-O-Me-cAMP, #P < 0.05.
Results
Gαs mice show reversible overexpression of Gαs in cortex, striatum and hippocampus
Gαs mice show high levels of transgene expression restricted to the cortex, hippocampus, dorsal striatum and ventral striatum (Figure 1), and low levels in the amygdala and in the inferior colliculus. Expression of the transgene effectively increases total levels of Gαs mRNA in select regions (Figures 1e, f). As a result, Gαs mice show increased basal and GTPγs-stimulated AC activity relative to control littermates (Figure 1h).
Gαs overexpression throughout development and adulthood is required to produce PPI deficits in adult Gαs mice that are reversible with antipsychotic treatment
One of the most widely studied endophenotypes associated with schizophrenia is impaired sensorimotor gating, as measured by PPI. Although Gαs mice show normal startle responses to a range of decibel intensities (Figure 2a), they show a decreased ability to gate the startle response as indicated by significantly reduced PPI (Figure 2b). Interestingly, both Gαsdev (Figure 2c) and Gαsadult mice (Figure 2d) show normal PPI relative to control littermates, suggesting both developmental and adulthood overexpression of Gαs are necessary for manifestation of the PPI deficit in adult Gαs mice. Importantly, administration of the typical antipsychotic haloperidol (1mg/kg, i.p.) reverses the PPI deficits observed in Gαs mice (Figure 2e).
Adulthood overexpression of Gαs is necessary and sufficient to cause hyperactivity in adult Gαs mice
Another hallmark endophenotype associated with schizophrenia is psychomotor agitation, which can be quantified in humans 54 or mice as an increase in locomotor activity.52 In an open field, Gαs mice show a significant increase in horizontal locomotor activity (Figure 3a) and a trend toward an increase in vertical movements relative to control littermates. Gαsadult mice, but not Gαsdev mice, show significantly increased horizontal movements relative to control littermates (Figure 3c). There is no effect of the transgene on preference for the periphery of the open field—nor for the open arms of an elevated zero maze (data not shown)—suggesting Gαs mice show no difference in anxiety-related behaviors.
Figure 3.
Overexpression of Gαs during adulthood is necessary and sufficient to trigger hyperactivity in adult Gαs mice. (a) Gαs mice exhibit significantly higher levels of horizontal activity relative to controls (n = 8–9 per genotype; t(15) = 2.66, P = 0.018). (b) Although developmental overexpression is not sufficient to cause this hyperactivity (Gαsdev; n = 9–10 per genotype), (c) adulthood overexpression of Gαs is (n = 6 per genotype; t(10) = 4.02, P = 0.002), vs CT, *P < 0.02–0.002.
Developmental overexpression of Gαs is necessary and sufficient to cause spatial learning deficits in adult Gαs mice
In addition to gating disturbances and psychomotor agitation, patients with schizophrenia suffer from memory deficits that have been linked to hippocampal pathophysiology.50 As such, we tested hippocampus- dependent spatial learning and memory in the hidden-platform Morris water maze. It is important to first note that Gαs mice perform normally in a visible-platform water maze task (data not shown), indicating they are physically capable of the behaviors required to solve the hidden-platform maze. Both Gαs mice (Figure 4A1) and Gαsdev mice (Figure 4B1) show significantly greater escape latencies during training relative to control littermates, suggesting a deficit in spatial learning. In contrast, Gαsadult mice show normal performance during acquisition (Figure 4C1), as do Gαsdox mice (Figure 4D1).
During a platform-free probe trial 1 day following the final training session, Gαs mice show significantly impaired memory for the platform location relative to control littermates as indicated by less time spent in the target quadrant (effect of genotype×quadrant: F(3,195) = 4.77, P = 0.003; post hoc Gαs vs CT within target, P = 0.001), a greater average proximity from the platform location (t(65) = 3.12, P = 0.003) and fewer crossings over the target quadrant platform location (Figure 4A2). These memory deficits are long lasting as Gαs mice show the same profile of impaired probe performance 21 days following training (data not shown). Gαs mice also swim significantly faster (t(65) = 3.42, P = 0.001) and exhibit more thigmotaxis (t(65) = 2.18, P = 0.038). Similarly, Gαsdev mice spend significantly less time in the target quadrant, (effect of genotype×quadrant: F(3,81) = 3.86, P = 0.012; post hoc Gαs vs CT within target, P = 0.012), demonstrate a greater average proximity from the platform location (t(27) = 2.34, P = 0.027) and make fewer crossings over the target quadrant platform location (Figure 4B2). Gαsdev mice do not, however, show altered swim speed nor thigmotaxis. In contrast, Gαsadult mice show an attenuated impairment in probe performance (relative to Gαs mice) by spending an equivalent amount of time in the target quadrant as a whole relative to control littermates, but making significantly fewer crossings over the specific target platform location (Figure 4C2). Gαsadult mice also exhibit increased thigmotaxis relative to control littermates (t(36) = 2.52, P = 0.016). Importantly, Gαsdox mice show normal performance on all measures (Figure 4D2), ensuring effects noted above in Gαs mice are specifically due to expression of the transgene. Together, these data show that Gαs mice exhibit spatial learning and memory deficits that are separable from effects on performance measures (for example, swim speed).
Adulthood overexpression of Gαs is necessary and sufficient to impair retrieval of hippocampus-dependent associative memory in adult Gαs mice
Given that Gαsadult mice show normal spatial learning across days on the hidden water maze but impaired spatial memory suggests that Gαs overexpression during adulthood affects memory retrieval specifically. To determine explicitly if Gαs overexpression affects acquisition, consolidation and/or retrieval, we turned to a one-trial Pavlovian fear conditioning task in which mice learn to associate a training context and cue (an auditory CS) with a 1.5mA footshock. Gαs mice show normal levels of freezing during the training session relative to control littermates (Figure 5a). Gαs mice also show normal short-term and long-term memory for cued fear (Figures 5b and c), confirming the mice have an intact amygdala, are able to process fearful stimuli and can freeze at high levels. In contrast, Gαs mice show significantly impaired short-term (Figure 5b) and long-term memory for hippocampus-dependent contextual fear (Figure 5c). Gαsdev mice do not show an impairment in long-term memory for contextual fear (Figure 5d); however, Gαsadult mice do show lower levels of contextual fear relative to control littermates 24 h following training (Figure 5e).
Figure 5.
Overexpression of Gαs during adulthood is necessary and sufficient to cause deficits in the retrieval of a hippocampus-dependent associative memory in adult Gαs mice. Mice were trained to associate a context and a conditioned stimulus (CS, an auditory cue) with an unconditioned stimulus (US, a 1.5mA footshock). (a) Relative to control littermates, Gαs mice exhibit equivalent levels of freezing during the training session (n = 64–65 per genotype). (b) When tested for short-term memory 1 h following training, Gαs mice show normal levels of cued freezing (n = 11 per genotype) but decreased levels of contextual freezing (n = 15–16 per genotype; t(29) = 2.39, P = 0.024). (c) Similarly, when tested for long-term memory 24 h following training, Gαs mice show normal levels of cued freezing (n = 25 per genotype) but lower levels of contextual freezing (n = 31–32 per genotype; t(61) = 5.14, P < 0.001), indicating a specific impairment in hippocampus-dependent contextual fear memory. (d) Developmental overexpression of Gαs (n = 4 per genotype; see Supplement for discussion of sample sizes) does not trigger this deficit in long-term contextual fear memory; however, (e) adulthood overexpression of Gαs is sufficient to impair (n = 19 per genotype; t(35) = 2.93, P = 0.006). (f) Interestingly, Gαstraining mice (i.e., express the transgene at training but not retrieval) show normal performance relative to control littermates. In contrast, Gαsretrieval mice (i.e., express the transgene at retrieval but not training) show impaired performance relative to control littermates (n = 8 per group; t(14) = 2.32, P = 0.036). Data graphed mean±s.e.m. CT, *P < 0.05–0.001.
To determine if the Gαs-induced deficits in contextual fear memory are due to an effect on acquisition, consolidation or retrieval, we compared control littermates to Gαs mice that expressed the transgene during training but not retrieval (that is, administered dox starting 24 h after training through the day of the retrieval test; Gαstraining) or expressed the transgene during retrieval but not training (that is, administered dox 2.5 weeks before training until 24 h following training; Gαsretrieval). Levels of freezing to the training context were assessed 2.5 weeks following training to allow sufficient time for transgene suppression/reexpression. Gαstraining mice show normal levels of freezing relative to control littermates (Figure 5f). In contrast, Gαsretrieval mice show significantly lower levels of freezing relative to control littermates (Figure 5f).
Developmental or adulthood overexpression of Gαs is sufficient to produce enlarged lateral ventricles and a reduced striatum in adult Gαs mice
To determine if Gαs overexpression leads to schizophrenia- like alterations in neuroanatomy, we measured the sizes of various transgene-expressing brain regions by histology and total brain volume by MRI. Inspection of histological sections reveals that Gαs expression triggers a dramatic enlargement of the lateral ventricles in Gαs mice relative to control littermates (Figures 6a and b), which appears to occur independent of an increase in total brain volume. MRI analyses confirm that the enlarged ventricles noted in Gαs mice are not simply due to an increase in overall brain volume. In fact, Gαs mice demonstrate a strong trend toward a reduction in total brain volume (Figures 6c–f; Gαs, n = 5, 381.89±8.4mm3; CT, n=4, 415.57±15.0mm3; t(7) = 2.06, P = 0.078). Not surprisingly, even 6 weeks of adulthood transgene suppression fails to reverse the Gαs-induced ventricular enlargement. Relative to control littermates, Gαsdev mice show a striking enlargement of the lateral ventricles that corresponds with a significant diminution of the dorsal and ventral striatum (Figure 6i). A reduction is also observed in frontal cortex and dorsal hippocampus, although not to the level of statistical significance (Figure 6i). Measures from ventral hippocampus taken from a separate cohort of Gαs mice showed a similar small, nonsignificant reduction (data not shown). Gαsadult mice also show significantly enlarged lateral ventricles as well as a smaller dorsal and ventral striatum relative to control littermates; however, the extent of the change in the lateral ventricles and ventral striatum is somewhat less than that observed in Gαsdev mice (Figure 6j). Importantly, Gαsdox mice show normal neuroanatomical measures relative to control littermates (Figure 6k).
Figure 6.
Developmental or adulthood overexpression of Gαs is sufficient to cause enlarged lateral ventricles and a smaller dorsal and ventral striatum in adult Gαs mice. Enlargement of the lateral ventricles within Gαs mice is visibly noticeable upon inspection of Nissl-stained sagital sections, coronal MRIs and three-dimensional surface renderings from adult control (a, c, e and g) and Gαs mice (b, d, f and h). (i) Not surprisingly, these Gαs-induced anatomical alterations persist even when the transgene is suppressed for more than 6 weeks, as evidenced by significantly enlarged ventricles in Gαsdev mice (as per quantification of Nissl-stained sagital sections, expressed %CT; n = 9–10 per genotype; t(18) = 3.01, P = 0.007). The enlargement in ventricles corresponds with a loss in both dorsal (t(18) = 3.84, P < 0.001) and ventral striatum (t(18) = 3.41, P = 0.003), as opposed to an increase in total brain size. (j) Interestingly, adulthood overexpression of Gαs is sufficient to elicit the same pattern of neuroanatomical changes (n = 29 for each genotype; LV, t(54) = 2.87, P = 0.006; DStr, t(54) = 2.79, P = 0.007; VStr, t(53) = 2.35, P = 0.023), although the magnitude of change is less than that observed in Gαsdev mice (Gαsdev vs Gαsadult: LV—t(36) = 2.13, P = 0.04; VStr—t(45) = 2.87, P = 0.006). (k) Importantly, Gαsdox mice that never express the transgene show normal anatomical measures (n = 8 for each genotype). Data graphed mean±s.e.m. LV, lateral ventricles; Hipp, hippocampus; FCx, frontal cortex; DStr, dorsal striatum; VStr, ventral striatum within each region, vs CT, *P < 0.025–0.001; vs Gαsdev, #P < 0.01.
Behavioral deficits caused by Gαs overexpression appear due to compensatory decreases in cAMP and are reversible by pharmacologically increasing cAMP signaling
To identify the downstream consequence of Gαs overexpression/increased AC activity that causes the above-noted behavioral phenotypes, and to determine in which brain region Gαs overexpression acts to alter given behaviors, we next quantified cAMP levels across brain regions in which total Gαs mRNA levels were significantly increased in the transgenic mice. On the basis of previous reports examining the effects of a constitutively active isoform of Gαs (Gαs*) on cAMP levels,15 we anticipated Gαs overexpression would cause compensatory decreases in cAMP levels, in select brain regions. As seen previously in Gαs* mice, Gαs mice exhibit significantly decreased cAMP levels in parahippocampal cortex, frontal cortex and hippocampus (Figure 7a), in contrast to significantly increased cAMP levels in striatum. There is no significant effect of genotype on cAMP levels of the inferior colliculus and cerebellum, the latter a control region that lacks transgene expression.
Figure 7.
Behavioral deficits in Gαs mice correlate with compensatory decreases in cAMP (cAMP) and can be rescued by drugs that increase cAMP. (a) Gαs mice (per genotype, n = 8–9 per region) show increased cAMP levels (pmol cAMP/mg protein expressed as % CT) in striatum (t(14) = 2.17, P = 0.048) but compensatorily decreased cAMP levels in hippocampus and cortex (Hipp, t(13) = 2.26, P = 0.042; PCx, t(13) = 2.25, P = 0.042; FCx, t(13) = 2.29, P = 0.039). (b) Developmental overexpression of Gαs is not sufficient to cause permanent alterations in cAMP levels as evidenced in Gαsdev mice (per genotype, n = 4–11 per region). (c) In contrast, adulthood overexpression of Gαs is sufficient to alter cAMP levels, but only in the hippocampus (for each genotype, n = 11–17 per region; t(19) = 2.13P = 0.046), suggesting that this compensatory decrease in hippocampal cAMP levels underlies the hyperactivity, spatial memory, and contextual fear deficits caused by Gαs overexpression. (d) In addition, the compensatory decrease in parahippocampal cAMP levels appears to mediate the Gαsinduced prepulse inhibition of acoustic startle (PPI) deficit because the dose of haloperidol (1 mg/kg, i.p.) that rescues the PPI deficit in Gαs mice selectively increases parahippocampal cAMP levels (expressed as %CT-veh) in Gαs mice (n = 7–16 per group per region; F(1,61) = 5.17, P = 0.026). Consistent with this correlation between increasing cortical cAMP levels and rescuing PPI deficits, (e) rolipram (0.66 mg/kg, i.p.), a drug that increases cAMP levels, also rescues the Gαs-induced PPI deficits (n = 16 per genotype; F(1,60) = 6.62, P = 0.015). Data graphed mean±s.e.m. Hipp, hippocampus; PCx, parahippocampal cortex; FCx, frontal cortex; Str, striatum; IC, inferior colliculus; Cb, cerebellum vs control, *P < 0.05; main effect of genotype, @P < 0.05–0.001; post hoc, vs all other groups, #P < 0.05–0.001.
To identify which of the regional cAMP changes noted above were particularly relevant to the behavioral deficits exhibited by Gαs mice, we compared and contrasted cAMP changes in Gαsdev and Gαsadult mice. Gαsdev mice show normal cAMP levels across all regions, suggesting developmental overexpression is not sufficient to cause permanent alterations in cAMP signaling (Figure 7b). Gαsadult mice, however, do show significantly altered cAMP levels, but only in hippocampus (Figure 7c). Together, these results suggest that the compensatory decrease in hippocampal cAMP levels likely mediates the hyperactivity, contextual memory deficit and, to some extent, the spatial memory impairment as these co-occur in Gαsadult mice. That said, studies here cannot rule out the possibility that the behavioral profile observed in Gαsadult mice may be due to alterations in long-term plasticity mechanisms (for example, gene expression changes, epigenetic modifications and so on.) triggered by transient increases in striatal and/or cortical cAMP signaling during development.
To determine whether the decreases in cortical cAMP or the increases in striatal cAMP levels correlate with the PPI deficits observed in Gαs mice, we next measured cAMP levels following administration of haloperidol at the dose that rescues the PPI deficits of Gαs mice. In parahippocampal cortex, haloperidol (1 mg/kg, i.p.) selectively increases cAMP levels in Gαs mice (Figure 7d). In contrast, haloperidol increases striatal cAMP levels selectively in control mice (F(1,47) = 5.84, P = 0.02). Given that haloperidol increases both PPI and parahippocampal cAMP levels in Gαs mice, we hypothesized that the PDE-4 inhibitor rolipram, which increases cAMP levels, would also increase PPI in Gαs mice. Across prepulse intensities, rolipram (0.66 mg/kg, i.p.) selectively increases PPI in Gαs mice (Figure 7e).
A compound that stimulates Epac, a downstream target of cAMP, exhibits antipsychotic-like effects in C57BL/6J
Given the correlation between decreased cortical cAMP and PPI deficits noted above and elsewhere15 and the correlation between decreased hippocampal cAMP and the locomotor, spatial memory, and contextual memory deficits noted herein (Table 1), we next investigated what downstream target of cAMP might mediate the effects of Gαs overexpression on these behavioral measures. Our previous work suggests that a loss of PKA signaling is not sufficient to reduce PPI and may, in fact, rescue deficits caused by constitutive activation of Gαs signaling.15,16 Therefore, we turned to another downstream target of cAMP, Epac. As there is no selective Epac antagonist available, we tested the effects of the selective Epac agonist 8-pCPT-2′-O-Me-cAMP in PPI and retrieval for contextual fear (Figure 8). Owing to unavailability of transgenic mice at the time of testing, we turned to C57BL/6J mice as this is the background strain of our transgenic mice and this strain has low basal PPI that appears to have similar pharmacological sensitivities to those shown here in the Gαs mice.24 Indeed, a number of typical and atypical antipsychotics are reported to increase C57BL/6J PPI, making this strain a viable model for predicting antipsychotic-like effects.60,61 Across prepulse intensities, 8-pCPT-2′-O-Me- cAMP (5 nmol, i.c.v.) significantly increases PPI relative to vehicle in C57BL/6J mice (Figure 8a). There is no significant effect of the drug on the startle response itself (veh, 51.44±12.0; Gαs, 46.73±16.2), suggesting the increase in PPI caused by the Epac agonist reflects an increase in sensorimotor gating.
Table 1.
Summary of phenotypes observed in adult Gαs mice (i.e., > 2 months old) when the transgene is overexpressed during development and/or adulthood
| Phenotype | Period of transgene expression
|
||
|---|---|---|---|
| Development and adulthood | Developmenta | Adulthoodb | |
| ↑ striatal cAMP | + + | − | − |
| ↓ Cortical cAMP | + + | − | − |
| ↓ PPI | + + | − | − |
| ↓ Spatial learning | + + | + + | − |
| ↑ Ventricles | + + | + + | + |
| ↓ Ventral striatum size | + + | + + | + |
| ↓ Spatial memory | + + | + + | + |
| ↓ Dorsal striatum size | + + | + + | + + |
| ↓ Hippocampal cAMP | + + | − | + + |
| ↑ Locomotion | + + | − | + + |
| ↓ Contextual memory retrieval | + + | − | + + |
| ↑ Paired-pulse facilitationc | + + | − | NM |
| ↑ Long-term potentiationc | + + | − | NM |
(++), phenotype strongly present; (+), phenotype moderately present; (−), phenotype absent; cAMP, cyclic AMP; NM, not measured.
Transgene expressed throughout development and early adulthood but suppressed at time of testing due to a minimum of 2.5 weeks of dox administration.
Transgene expressed only after 2 months of age.
In fear conditioning, 8-pCPT-2′-O-Me-cAMP (5 nmol, i.c.v.) improves memory for contextual fear (Figure 8b). Post hoc analyses show that when animals are trained with a shock and receive 8-pCPT-2′-O-Me-cAMP immediately before a short-term memory test, they exhibit significantly higher levels of freezing during both the short-term memory test as well as a subsequent drug-free long-term memory test, relative to vehicle-treated animals as well as animals that receive 8-pCPT-2′-O-Me-cAMP but have no shock paired with the context.
Discussion
We show here that increased expression of the G-protein subunit Gαs within forebrain neurons of mice is sufficient to trigger biochemical, behavioral and anatomical phenotypes commonly associated with schizophrenia. Interestingly, we show that some (PPI deficits, spatial learning and memory impairments, and ventricular enlargement), but not all, phenotypes require Gαs overexpression during development for full symptomatic expression in adulthood. These results support the suggestion that neurodegenerative influences overlie neurodevelopmental insults to produce the full spectrum of schizophrenia-related phenotypes.62,63 Importantly, we show it is possible to pharmacologically reverse in the adult phenotypes that are, in part, due to developmental insult (as shown in PPI). Our results in mice argue that the increases in Gαs expression and activity that have been previously measured in patients are likely to have functional significance. Further, our results suggest that specific intracellular signaling molecules— in particular PDEs and Epac—may represent novel classes of therapeutic targets for the treatment of schizophrenia.
Deficits observed in Gαs mice are strikingly reminiscent of phenotypes associated with schizophrenia
It has been suggested that an ‘ideal’ mouse model for studying schizophrenia would emulate three behavioral endophenotypes (also referred to as intermediate or subphenotypes) associated with the disease.64 We show here that Gαs mice exhibit deficits across three disease-relevant behavioral domains: psychomotor agitation,65 sensorimotor gating53 and hippocampus-dependent learning and memory retrieval.49,50 Importantly, Gαs mice also show responsiveness to antipsychotics—a class of drugs used to treat patients with schizophrenia. In addition to exhibiting these behavioral impairments, Gαs mice also recapitulate biochemical and neuroanatomical features associated with schizophrenia.
Biochemically, Gαs mice mimic the increase in mRNA levels associated with a polymorphism genetically linked to schizophrenia8,9 as well as the increased efficacy of Gαs signaling that has been noted in tissue from schizophrenia patients.5–7 Several studies have also noted increased expression and/or efficacy of Gαs signaling in patients with bipolar disorder (for example,66), a disease with overlapping symptomatology. Given the region-specific effects observed in Gαs mice, we further hypothesize that select cases of schizophrenia may be due to sporadic tissue-specific mutations in Gαs that increase function (for example, specifically within neocortex). It is well known that Gαs, in particular, appears vulnerable to such sporadic tissue-specific mutations (for example,67,68). Although cAMP levels have not been directly measured in the brains of patients with schizophrenia, a number of studies have shown decreased cAMP production in platelets of patients with schizophrenia,69 which may be emulated in the compensatory decreases in cAMP levels measured in hippocampus and cortex of Gαs mice.
Neuroanatomically, Gαs mice show strikingly enlarged lateral ventricles at the expense of a reduction in striatal volume, two of the most commonly documented anatomical alterations associated with schizophrenia.70 Gαs mice also show small, nonsignificant decreases in the size of frontal cortex and hippocampus, which may reflect more subtle neuroanatomical lesions (for example, reduced dendritic arborization) of interest to future studies. The anatomical alterations observed in Gαs mice appear due to both impaired development and enhanced degeneration, as animals that overexpress Gαs only after 2 months of age do manifest these deficits, but to a slightly lesser degree than those observed in animals expressing Gαs throughout development. Enhanced degeneration (for example, of the neuropil) is consistent with observations in patients.63
A longstanding question in the field of neuropsychiatric research is whether gross anatomical changes noted in the brains of patients cause symptoms or simply occur in parallel. Such a question has significant implications for pharmacological treatments as it has been suggested that therapeutics would not likely reverse such significant anatomical lesions.71 In Gαs mice, the substantial neuroanatomical changes appear to have relatively little effect on behavior. For example, Gαsdev mice show normal PPI, locomotion and contextual fear memory despite having enlarged ventricles/reduced striata. The only behavioral phenotype for which a relationship to the neuroanatomical changes cannot be ruled out is the spatial learning and memory deficit. It is possible that damage to the ventral striatum contributes to the spatial learning and memory deficit caused by Gαs overexpression given evidence demonstrating a role for this region in spatial memory consolidation.72 The apparent minimal behavioral effect of the Gαs-induced anatomical changes is reminiscent of findings in the area of Parkinson’s disease where more than 80% of cells in the substantia nigra must die off before physical symptoms emerge.73 If findings here in mice translate to humans, our results suggest that the effectiveness of pharmacological therapeutics should not be limited by the neuroanatomical changes that are observed in patients with schizophrenia.
Effects of in vivo Gαs overexpression support a non-canonical view of G-protein signaling
The traditional view of heterotrimeric G-protein signaling describes a process whereby a GDP-bound α-subunit forms a heterotrimer with a β-γ-subunit, which is then only activated (GTP displacement of the bound GDP) when the heterotrimer is recruited to a ligand-bound G-protein-coupled receptor.10 With such a definition it is easily understandable why the loss of one G-protein subunit negatively impacts biology, as has previously been shown;74,75 however, this scenario makes less clear how overexpression of one subunit alone impacts physiology as shown here and elsewhere.76
A growing body of literature suggests that the traditional view of G-protein signaling must be revised to include several non-canonical mechanisms that activate α-subunits.11–14 For example, in vitro evidence suggests there is a low level of spontaneous activation of Gαs when not bound to a β-γ subunit.11 Further, microtubules are able to activate free Gαs subunits (that is, Gαs-GDP) by transferring a phosphate from the microtubule cap to the bound GDP, resulting in Gαs-GTP.12,77 If these mechanisms are active in vivo, it is reasonable to expect that increasing the pool of available Gαs-GDP would eventually lead to increased levels of Gαs-GTP, without a need for a complimentary increase in β-γ levels.
Alternatively, overexpressing Gαs could elicit effects by acting as a sink for free β-γ subunits, which would otherwise inhibit AC type I (ACI).78 We believe it unlikely, however, that phenotypes observed in Gαs mice are related to increased activity of ACI because overexpression of ACI within the forebrain elicits phenotypes opposite to those observed here.79
Overexpression of Gαs may cause behavioral deficits via a loss of cAMP stimulation of Epac signaling
The various behavioral alterations noted in Gαs mice appear to correlate with distinct regional decreases in hippocampal and cortical cAMP levels, and rescue of behavioral deficits appears possible when these cAMP deficits are reversed, as shown in PPI with haloperidol and the PDE-4 inhibitor rolipram. Although the Gαs-induced decreases in cAMP appear largely the consequence of compensatory increases in PDE activity,15,16 hyperdopaminergic (by D2, 3, 4 receptor-mediated inhibition of AC)80 and/or hypoglutamatergic signaling (by reduced calcium/calmodulin stimulation of AC)81 may also have a role, both of which are commonly hypothesized to contribute to endophenotypes of schizophrenia (for example, Costa et al.46). Indeed, Gαs mice show enhanced sensitivity to haloperidol, suggesting increased activity at the D2 receptor. Glutamatergic signaling has not yet been empirically tested in our model, however, it is interesting to note that chronic administration of the glutamatergic antagonist phencyclidine appears to reduce cAMP signaling in hippocampus and cortex, as measured by decreased phosphorylation of a number of members of the cAMP cascade.82
Decreasing hippocampal cAMP levels appears sufficient to trigger hyperlocomotion, impairments in contextual memory retrieval and—to some extent—deficits in spatial memory, as these phenotypes co-occur when Gαs is overexpressed only during adulthood (Table 1). Although studies here do not distinguish the contribution of ventral vs dorsal hippocampus, our results are consistent with lesion studies showing that neonatal ventral hippocampal lesions trigger hyperlocomotion in adult rats83,84 as do adulthood lesions to the dorsal or entire hippocampus. 85,86 It will be of interest to future studies to determine if Gαs mice share similar neurochemical, electrophysiological and neuroanatomical changes in striatum and/or prefrontal cortex as have been described in mice with neonatal ventral hippocampal lesions (for example,87,88). The hippocampal dysfunction measured in Gαs mice is also consistent with patient literature describing substantial hippocampal pathophysiology in schizophrenia.49,50
In contrast, the sensorimotor gating deficits and antipsychotic responsiveness observed in Gαs mice appears particularly tied to cAMP levels in the cortical regions surrounding the hippocampus (Figure 7d). Although it is challenging to map strictly homologous cortical subregions from rodent to human, we believe the regional specificity of the sensorimotor gating deficits and antipsychotic responsiveness observed in Gαs mice correlates well with findings in patient studies. Studies in patients with schizophrenia have repeatedly associated abnor- mal activity within the temporal cortex to altered attentional processing,89 various aspects of auditory hallucinations90–93 and dysfunctional emotional processing.94–96 Further, studies have suggested that D2/D3 receptor binding in temporal cortex is particularly important for the therapeutic action of antipsychotics (for example, references97–99). The functional alterations reported in temporal cortex of patients with schizophrenia may be explained by the anatomical100–104 and molecular abnormalities that have been measured within this brain region.105–108 Thus, the fact that behavioral deficits in Gαs mice appear due to decreased cAMP signaling specifically within the hippocampus and cortical regions surrounding the hippocampus is consistent with findings in patients describing dysfunction within these brain regions.
Reduced cAMP levels would be expected to decrease signaling to all of the targets downstream of cAMP: PKA, Epac and CNGCs.38 Given that loss of PKA activity is not sufficient to impair PPI and appears even to rescue deficits caused by chronic Gαs signaling,15,16 we propose that the Gαs-induced deficits are at least partially due to reduced cAMP stimulation of Epac, although a role for CNGCs cannot be ruled out. As discussed above, a loss of Epac activity could impact a number of neurobiological processes hypothesized to be involved in schizophrenia, including glutamatergic signaling, integrin function and hippocampal plasticity.40–46,48–51,109 Consistent with the hypothesis that decreased Epac activation may contribute to the behavioral deficits observed in Gαs mice, we show here that increasing Epac activity with a selective agonist (8-pCPT-2′-O-Me- cAMP) increases PPI and improves contextual memory in C57BL/6J mice. It will be of great interest to future studies to more thoroughly explore the extent to which behavioral deficits in Gαs mice can be rescued by Epac agonists as well as other positive modulators of cAMP signaling, such as PDE-4 inhibitors beyond rolipram and PDE-10 inhibitors.
In conclusion, results presented here not only advance our understanding of fundamental cell biology, the molecular underpinnings of cognition and the progression of schizophrenia (that is, developmental vs nondevelopmental components), we also offer hope by identifying Epac as a novel therapeutic target able to improve both developmentally and nondevelopmentally regulated phenotypes.
Supplementary Material
Acknowledgments
We thank Dr Raquel Gur for helpful discussions, Dr Jean Richa for efforts in generating our transgenic mice and Jan Tokarczyk for technical assistance. We also acknowledge the following sources of funding: Tourettes Syndrome Association (MPK), NIMH T32 MH019112 (to MPK, R Gur, PI), Systems and Integrative Biology Training grant GM07517 (to CGV; M Nusbaum, PI), NIH T32 HL07953 (to CGV; AI Pack, PI), NIMH K08 MH067091 (SJK), NIAA AA09000 (GW), Merck, Whitehall and Packard Foundations as well as NIMH R01 MH60244, NIA R01 AG18199 and P50 MH 6404501 (Project 3 to TA; R Gur, Conte Center, PI). MPK and SJK are currently employed at Wyeth and AstraZeneca, respectively.
Footnotes
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
References
- 1.Gogos JA, Gerber DJ. Schizophrenia susceptibility genes: emergence of positional candidates and future directions. Trends Pharmacol Sci. 2006;27:226–233. doi: 10.1016/j.tips.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Miyamoto S, Duncan GE, Marx CE, Lieberman JA. Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry. 2005;10:79–104. doi: 10.1038/sj.mp.4001556. [DOI] [PubMed] [Google Scholar]
- 3.Murphy BP, Chung YC, Park TW, McGorry PD. Pharmacological treatment of primary negative symptoms in schizophrenia: a systematic review. Schizophr Res. 2006;88:5–25. doi: 10.1016/j.schres.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Rapoport JL, Addington AM, Frangou S, Psych MR. The neurodevelopmental model of schizophrenia: update 2005. Mol Psychiatry. 2005;10:434–449. doi: 10.1038/sj.mp.4001642. [DOI] [PubMed] [Google Scholar]
- 5.Memo M, Kleinman JE, Hanbauer I. Coupling of dopamine D1 recognition sites with adenylate cyclase in nuclei accumbens and caudatus of schizophrenics. Science. 1983;221:1304–1307. doi: 10.1126/science.6310753. [DOI] [PubMed] [Google Scholar]
- 6.Avissar S, Barki-Harrington L, Nechamkin Y, Roitman G, Schreiber G. Elevated dopamine receptor-coupled G(s) protein measures in mononuclear leukocytes of patients with schizophrenia. Schizophr Res. 2001;47:37–47. doi: 10.1016/s0920-9964(00)00038-4. [DOI] [PubMed] [Google Scholar]
- 7.Avissar S, Roitman G, Schreiber G. Differential effects of the antipsychotics haloperidol and clozapine on G protein measures in mononuclear leukocytes of patients with schizophrenia. Cell Mol Neurobiol. 2001;21:799–811. doi: 10.1023/a:1015164423918. [DOI] [PubMed] [Google Scholar]
- 8.Minoretti P, Politi P, Coen E, Di Vito C, Bertona M, Bianchi M, et al. The T393C polymorphism of the GNAS1 gene is associated with deficit schizophrenia in an Italian population sample. Neurosci Lett. 2006;397:159–163. doi: 10.1016/j.neulet.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 9.Frey UH, Eisenhardt A, Lummen G, Rubben H, Jockel KH, Schmid KW, et al. The T393C polymorphism of the G alphas gene (GNAS1) is a novel prognostic marker in bladder cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:871–877. doi: 10.1158/1055-9965.EPI-04-0720. [DOI] [PubMed] [Google Scholar]
- 10.McCudden CR, Hains MD, Kimple RJ, Siderovski DP, Willard FS. G-protein signaling: back to the future. Cell Mol Life Sci. 2005;62:551–577. doi: 10.1007/s00018-004-4462-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brandt DR, Ross EM. GTPase activity of the stimulatory GTP-binding regulatory protein of adenylate cyclase, Gs. Accumulation and turnover of enzyme-nucleotide intermediates. J Biol Chem. 1985;260:266–272. [PubMed] [Google Scholar]
- 12.Rasenick MM, Wang N. Exchange of guanine nucleotides between tubulin and GTP-binding proteins that regulate adenylate cyclase: cytoskeletal modification of neuronal signal transduction. J Neurochem. 1988;51:300–311. doi: 10.1111/j.1471-4159.1988.tb04870.x. [DOI] [PubMed] [Google Scholar]
- 13.Schaefer M, Petronczki M, Dorner D, Forte M, Knoblich JA. Heterotrimeric G proteins direct two modes of asymmetric cell division in the Drosophila nervous system. Cell. 2001;107:183–194. doi: 10.1016/s0092-8674(01)00521-9. [DOI] [PubMed] [Google Scholar]
- 14.Ugur O, Oner SS, Molinari P, Ambrosio C, Sayar K, Onaran HO. Guanine nucleotide exchange-independent activation of Gs protein by beta2-adrenoceptor. Mol Pharmacol. 2005;68:720–728. doi: 10.1124/mol.104.010306. [DOI] [PubMed] [Google Scholar]
- 15.Kelly MP, Isiegas C, Cheung YF, Yokarczyk J, Yang X, Esposito MF, et al. Constitutive activation of Galphas within forebrain neurons causes deficits in sensorimotor gating because of PKA-dependent decreases in cAMP. Neuropsychopharmacology. 2007;32:577–588. doi: 10.1038/sj.npp.1301099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly MP, Cheung YF, Favilla C, Kanes SJ, Houslay MD, Abel T. Inhibition of PKA rescues associative memory deficits caused by constitutive activation of the G-protein subunit Gαs within forebrain neurons. Learn Mem. 2008;15:75–83. doi: 10.1101/lm.723708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conti M, Beavo J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Ann Rev Biochem. 2007;76:481–511. doi: 10.1146/annurev.biochem.76.060305.150444. [DOI] [PubMed] [Google Scholar]
- 18.Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev. 2006;58:488–520. doi: 10.1124/pr.58.3.5. [DOI] [PubMed] [Google Scholar]
- 19.MacKenzie SJ, Baillie GS, McPhee I, MacKenzie C, Seamons R, McSorley T, et al. Long PDE4 cAMP specific phosphodiesterases are activated by protein kinase A-mediated phosphorylation of a single serine residue in upstream conserved region 1 (UCR1) Br J Pharmacol. 2002;136:421–433. doi: 10.1038/sj.bjp.0704743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vicini E, Conti M. Characterization of an intronic promoter of a cyclic adenosine 3′,5′-monophosphate (cAMP)-specific phosphodiesterase gene that confers hormone and cAMP inducibility. Mol Endocr. 1997;11:839–850. doi: 10.1210/mend.11.7.9941. [DOI] [PubMed] [Google Scholar]
- 21.Millar JK, Pickard BS, Mackie S, James R, Christie S, Buchanan SR, et al. DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science. 2005;310:1187–1191. doi: 10.1126/science.1112915. [DOI] [PubMed] [Google Scholar]
- 22.Millar JK, Mackie S, Clapcote SJ, Murdoch H, Pickard BS, Christie S, et al. Disrupted in schizophrenia 1 and phosphodiesterase 4B: towards an understanding of psychiatric illness. J Physiol. 2007;584:401–405. doi: 10.1113/jphysiol.2007.140210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murdoch H, Mackie S, Collins DM, Hill EV, Bolger GB, Klussmann E, et al. Isoform-selective susceptibility of DISC1/phosphodiesterase- 4 complexes to dissociation by elevated intracellular cAMP levels. J Neurosci. 2007;27:9513–9524. doi: 10.1523/JNEUROSCI.1493-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanes SJ, Tokarczyk J, Siegel SJ, Bilker W, Abel T, Kelly MP. Rolipram: a specific phosphodiesterase 4 inhibitor with potential antipsychotic activity. Neuroscience. 2007;144:239–246. doi: 10.1016/j.neuroscience.2006.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Livingstone MS, Sziber PP, Quinn WG. Loss of calcium/calmodulin responsiveness in adenylate cyclase of rutabaga, a Drosophila learning mutant. Cell. 1984;37:205–215. doi: 10.1016/0092-8674(84)90316-7. [DOI] [PubMed] [Google Scholar]
- 26.Connolly JB, Roberts IJ, Armstrong JD, Kaiser K, Forte M, Tully T, et al. Associative learning disrupted by impaired Gs signaling in Drosophila mushroom bodies. Science. 1996;274:2104–2107. doi: 10.1126/science.274.5295.2104. [DOI] [PubMed] [Google Scholar]
- 27.Bourtchouladze R, Abel T, Berman N, Gordon R, Lapidus K, Kandel ER. Different training procedures recruit either one or two critical periods for contextual memory consolidation, each of which requires protein synthesis and PKA. Learn Mem. 1998;5:365–374. [PMC free article] [PubMed] [Google Scholar]
- 28.Bourtchouladze R, Patterson SL, Kelly MP, Kreibich A, Kandel ER, Abel T. Chronically increased Gsalpha signaling disrupts associative and spatial learning. Learn Mem. 2006;13:745–752. doi: 10.1101/lm.354106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barco A, Pittenger C, Kandel ER. CREB, memory enhancement and the treatment of memory disorders: promises, pitfalls and prospects. Expert Opin Ther Targets. 2003;7:101–114. doi: 10.1517/14728222.7.1.101. [DOI] [PubMed] [Google Scholar]
- 30.Houslay MD, Schafer P, Zhang KY. Keynote review: phosphodiesterase- 4 as a therapeutic target. Drug Discov Today. 2005;10:1503–1519. doi: 10.1016/S1359-6446(05)03622-6. [DOI] [PubMed] [Google Scholar]
- 31.Scuvee-Moreau J, Giesbers I, Dresse A. Effect of rolipram, a phosphodiesterase inhibitor and potential antidepressant, on the firing rate of central monoaminergic neurons in the rat. Arch Int Pharmacodyn Ther. 1987;288:43–49. [PubMed] [Google Scholar]
- 32.Barad M, Bourtchouladze R, Winder DG, Golan H, Kandel E. Rolipram, a type IV-specific phosphodiesterase inhibitor, facilitates the establishment of long-lasting long-term potentiation and improves memory. Proc Natl Acad Sci USA. 1998;95:15020–15025. doi: 10.1073/pnas.95.25.15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monti B, Berteotti C, Contestabile A. Subchronic rolipram delivery activates hippocampal CREB and Arc, enhances retention and slows down extinction of conditioned fear. Neuropsychopharmacology. 2006;31:278–286. doi: 10.1038/sj.npp.1300813. [DOI] [PubMed] [Google Scholar]
- 34.Bourtchouladze R, Lidge R, Catapano R, Stanley J, Gossweiler S, Romashko D, et al. A mouse model of Rubinstein–Taybi syndrome: defective long-term memory is ameliorated by inhibitors of phosphodiesterase 4. Proc Natl Acad Sci USA. 2003;100:10518–10522. doi: 10.1073/pnas.1834280100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang H, Huang Y, Jin SLC, Frith SA, Suvarna N, Conti M, et al. Antidepressant-like profile and reduced sensitivity to rolipram in mice deficient in the PDE4D phosphodiesterase enzyme. Neuropsychopharmacology. 2002;27:587. doi: 10.1016/S0893-133X(02)00344-5. [DOI] [PubMed] [Google Scholar]
- 36.Bonbon D, Breulet M, Gerard-Vandenhove M, Guiot-Goffioul F, Plomteux G, Sastre-y-Hernandez M, et al. Is phosphodiesterase inhibition a new mechanism of antidepressant action? A double blind double-dummy study between rolipram and desipramine in hospitalized major and/or endogenous depressives. Eur Arch Psychiatry Neurol Sci. 1988;238:2–6. doi: 10.1007/BF00381071. [DOI] [PubMed] [Google Scholar]
- 37.O’Donnell JM, Zhang HT. Antidepressant effects of inhibitors of cAMP phosphodiesterase (PDE4) Trends Pharmacol Sci. 2004;25:158–163. doi: 10.1016/j.tips.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 38.Kopperud R, Krakstad C, Selheim F, Doskeland SO. cAMP effector mechanisms. Novel twists for an ‘old’ signaling system. FEBS Lett. 2003;546:121–126. doi: 10.1016/s0014-5793(03)00563-5. [DOI] [PubMed] [Google Scholar]
- 39.Enserink JM, Christensen AE, de Rooij J, van Triest M, Schwede F, Genieser HG, et al. A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat Cell Biol. 2002;4:901–906. doi: 10.1038/ncb874. [DOI] [PubMed] [Google Scholar]
- 40.Kaneko M, Takahashi T. Presynaptic mechanism underlying cAMP-dependent synaptic potentiation. J Neurosci. 2004;24:5202–5208. doi: 10.1523/JNEUROSCI.0999-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhong N, Zucker RS. cAMP acts on exchange protein activated by cAMP/cAMP-regulated guanine nucleotide exchange protein to regulate transmitter release at the crayfish neuromuscular junction. J Neurosci. 2005;25:208–214. doi: 10.1523/JNEUROSCI.3703-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gekel I, Neher E. Application of an Epac activator enhances neurotransmitter release at excitatory central synapses. J Neurosci. 2008;28:7991–8002. doi: 10.1523/JNEUROSCI.0268-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sawa A, Snyder SH. Schizophrenia: diverse approaches to a complex disease. Science. 2002;296:692–695. doi: 10.1126/science.1070532. [DOI] [PubMed] [Google Scholar]
- 44.Rangarajan S, Enserink JM, Kuiperij HB, de Rooij J, Price LS, Schwede F, et al. Cyclic AMP induces integrin-mediated cell adhesion through Epac and Rap1 upon stimulation of the beta 2-adrenergic receptor. J Cell Biol. 2003;160:487–493. doi: 10.1083/jcb.200209105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bouvard D, Brakebusch C, Gustafsson E, Aszodi A, Bengtsson T, Berna A, et al. Functional consequences of integrin gene mutations in mice. Circ Res. 2001;89:211–223. doi: 10.1161/hh1501.094874. [DOI] [PubMed] [Google Scholar]
- 46.Costa E, Chen Y, Davis J, Dong E, Noh JS, Tremolizzo L, et al. REELIN and schizophrenia: a disease at the interface of the genome and the epigenome. Mol Interv. 2002;2:47–57. doi: 10.1124/mi.2.1.47. [DOI] [PubMed] [Google Scholar]
- 47.Ster J, De Bock F, Guerineau NC, Janossy A, Barrere-Lemaire S, Bos JL, et al. Exchange protein activated by cAMP (Epac) mediates cAMP activation of p38 MAPK and modulation of Ca2+dependent K+ channels in cerebellar neurons. Proc Natl Acad Sci USA. 2007;104:2519–2524. doi: 10.1073/pnas.0611031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gelinas JN, Banko JL, Peters MM, Klann E, Weeber EJ, Nguyen PV. Activation of exchange protein activated by cyclic-AMP enhances long-lasting synaptic potentiation in the hippocampus. Learn Mem. 2008;15:403–411. doi: 10.1101/lm.830008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hanlon FM, Weisend MP, Hamilton DA, Jones AP, Thoma RJ, Huang M, et al. Impairment on the hippocampal-dependent virtual Morris water task in schizophrenia. Schizophr Res. 2006;87:67–80. doi: 10.1016/j.schres.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 50.Boyer P, Phillips JS, Rousseau FL, Ilivitsky S. Hippocampal abnormalities and memory deficits: new evidence of a strong pathophysiological link in schizophrenia. Brain Res Rev. 2007;54:92–112. doi: 10.1016/j.brainresrev.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 51.Andreasen NC, Pierson R. The role of the cerebellum in schizophrenia. Biol Psychiatry. 2008;64:81–88. doi: 10.1016/j.biopsych.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arguello PA, Gogos JA. Modeling madness in mice: one piece at a time. Neuron. 2006;52:179–196. doi: 10.1016/j.neuron.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 53.Braff D, Stone C, Callaway E, Geyer M, Glick I, Bali L. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978;15:339–343. doi: 10.1111/j.1469-8986.1978.tb01390.x. [DOI] [PubMed] [Google Scholar]
- 54.Young JW, Minassian A, Paulus MP, Geyer MA, Perry W. A reverse translational approach to bipolar disorder. Rodent and human studies in the behavioral pattern monitor. Neurosci Biobehav Rev. 2007;31:882–886. doi: 10.1016/j.neubiorev.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Levis MJ, Bourne HR. Activation of the alpha subunit of Gs in intact cells alters its abundance, rate of degradation, and membrane avidity. J Cell Biol. 1992;119:1297–1307. doi: 10.1083/jcb.119.5.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mayford M, Kandel ER. Genetic approaches to memory storage. Trends Genet. 1999;15:463–470. doi: 10.1016/s0168-9525(99)01846-6. [DOI] [PubMed] [Google Scholar]
- 57.Salomon Y, Londos C, Rodbell M. A highly sensitive adenylate cyclase assay. Analyt Biochem. 1974;58:541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- 58.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Meth. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 59.Gering DT, Nabavi A, Kikinis R, Hata N, O’Donnell LJ, Grimson WE, et al. An integrated visualization system for surgical planning and guidance using image fusion and an open MR. J Magn Reson Imag. 2001;13:967–975. doi: 10.1002/jmri.1139. [DOI] [PubMed] [Google Scholar]
- 60.McCaughran J, Jr, Mahjubi E, Decena E, Hitzemann R. Genetics, haloperidol-induced catalepsy and haloperidol-induced changes in acoustic startle and prepulse inhibition. Psychopharmacologia. 1997;134:131–139. doi: 10.1007/s002130050434. [DOI] [PubMed] [Google Scholar]
- 61.Ouagazzal AM, Jenck F, Moreau JL. Drug-induced potentiation of prepulse inhibition of acoustic startle reflex in mice: a model for detecting antipsychotic activity? Psychopharmacologia. 2001;156:273–283. doi: 10.1007/s002130100763. [DOI] [PubMed] [Google Scholar]
- 62.Liebmann C. G protein-coupled receptors and their signaling pathways: classical therapeutical targets susceptible to novel therapeutic concepts. Curr Pharm Des. 2004;10:1937–1958. doi: 10.2174/1381612043384367. [DOI] [PubMed] [Google Scholar]
- 63.Berger GE, Wood S, McGorry PD. Incipient neurovulnerability and neuroprotection in early psychosis. Psychopharmacol Bull. 2003;37:79–101. [PubMed] [Google Scholar]
- 64.Powell CM, Miyakawa T. Schizophrenia-relevant behavioral testing in rodent models: a uniquely human disorder? Biol Psychiatry. 2006;59:1198–1207. doi: 10.1016/j.biopsych.2006.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gobbi G, Gaudreau PO, Leblanc N. Efficacy of topiramate, valproate, and their combination on aggression/agitation behavior in patients with psychosis. J Clin Psychopharmacol. 2006;26:467–473. doi: 10.1097/01.jcp.0000237945.35022.45. [DOI] [PubMed] [Google Scholar]
- 66.Schreiber G, Avissar S, Danon A, Belmaker RH. Hyperfunctional G proteins in mononuclear leukocytes of patients with mania. Biol Psychiatry. 1991;29:273–280. doi: 10.1016/0006-3223(91)91289-4. [DOI] [PubMed] [Google Scholar]
- 67.Levine MA. Clinical implications of genetic defects in G proteins: oncogenic mutations in G alpha s as the molecular basis for the McCune-Albright syndrome. Arch Med Res. 1999;30:522–531. doi: 10.1016/s0188-4409(99)00075-2. [DOI] [PubMed] [Google Scholar]
- 68.Weinstein LS, Yu S, Warner DR, Liu J. Endocrine manifestations of stimulatory G protein alpha-subunit mutations and the role of genomic imprinting. Endocr Rev. 2001;22:675–705. doi: 10.1210/edrv.22.5.0439. [DOI] [PubMed] [Google Scholar]
- 69.Kaiya H. Second messenger imbalance hypothesis of schizophrenia. Prostaglandins Leukot Essent Fatty Acids. 1992;46:33–38. doi: 10.1016/0952-3278(92)90056-o. [DOI] [PubMed] [Google Scholar]
- 70.Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Green MF, Nuechterlein KH, Gold JM, Barch DM, Cohen J, Essock S, et al. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biol Psychiatry. 2004;56:301–307. doi: 10.1016/j.biopsych.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 72.De Leonibus E, Oliverio A, Mele A. A study on the role of the dorsal striatum and the nucleus accumbens in allocentric and egocentric spatial memory consolidation. Learn Mem. 2005;12:491–503. doi: 10.1101/lm.94805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 74.Pineda VV, Athos J, Wang H, Celver J, Ippolito D, Boulay G, et al. Removal of G(ialpha1) constraints on adenylyl cyclase in the hippocampus enhances LTP and impairs memory formation. Neuron. 2004;41:153–163. doi: 10.1016/s0896-6273(03)00813-4. [DOI] [PubMed] [Google Scholar]
- 75.Krumins AM, Gilman AG. Targeted knockdown of G protein subunits selectively prevents receptor-mediated modulation of effectors and reveals complex changes in non-targeted signaling proteins. J Biol Chem. 2006;281:10250–10262. doi: 10.1074/jbc.M511551200. [DOI] [PubMed] [Google Scholar]
- 76.Yang X, Lee FY, Sr, Wand GS. Increased expression of Gs(alpha) enhances activation of the adenylyl cyclase signal transduction cascade. Mol Endocrinol. 1997;11:1053–1061. doi: 10.1210/mend.11.8.9957. [DOI] [PubMed] [Google Scholar]
- 77.Rasenick MM, Donati RJ, Popova JS, Yu JZ. Tubulin as a regulator of G-protein signaling. Meth Enzymol. 2004;390:389–403. doi: 10.1016/S0076-6879(04)90024-9. [DOI] [PubMed] [Google Scholar]
- 78.Tang WJ, Gilman AG, Tang WJ, Gilman AG. Type-specific regulation of adenylyl cyclase by G protein beta gamma subunits. Science. 1991;254:1500–1503. doi: 10.1126/science.1962211. [DOI] [PubMed] [Google Scholar]
- 79.Wang H, Ferguson GD, Pineda VV, Cundiff PE, Storm DR. Overexpression of type-1 adenylyl cyclase in mouse forebrain enhances recognition memory and LTP. Nat Neurosci. 2004;7:635–642. doi: 10.1038/nn1248. [DOI] [PubMed] [Google Scholar]
- 80.Neves SR, Ram PT, Iyengar R. G protein pathways. Science. 2002;296:1636–1639. doi: 10.1126/science.1071550. [DOI] [PubMed] [Google Scholar]
- 81.Chetkovich DM, Sweatt JD. nMDA receptor activation increases cyclic AMP in area CA1 of the hippocampus via calcium/calmodulin stimulation of adenylyl cyclase. J Neurochem. 1993;61:1933–1942. doi: 10.1111/j.1471-4159.1993.tb09836.x. [DOI] [PubMed] [Google Scholar]
- 82.Molteni R, Pasini M, Moraschi S, Gennarelli M, Drago F, Racagni G, et al. Reduced activation of intracellular signaling pathways in rat prefrontal cortex after chronic phencyclidine administration. Pharmacol Res. 2008;57:296–302. doi: 10.1016/j.phrs.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 83.Sams-Dodd F, Lipska BK, Weinberger DR. Neonatal lesions of the rat ventral hippocampus result in hyperlocomotion and deficits in social behaviour in adulthood. Psychopharmacology. 1997;132:303–310. doi: 10.1007/s002130050349. [DOI] [PubMed] [Google Scholar]
- 84.Flores G, Silva-Gomez AB, Ibanez O, Quirion R, Srivastava LK. Comparative behavioral changes in postpubertal rats after neonatal excitotoxic lesions of the ventral hippocampus and the prefrontal cortex. Synapse. 2005;56:147–153. doi: 10.1002/syn.20140. [DOI] [PubMed] [Google Scholar]
- 85.Bardgett ME, Baum KT, O’Connell SM, Lee NM, Hon JC. Effects of risperidone on locomotor activity and spatial memory in rats with hippocampal damage. Neuropharmacology. 2006;51:1156–1162. doi: 10.1016/j.neuropharm.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 86.Sloan HL, Good M, Dunnett SB. Double dissociation between hippocampal and prefrontal lesions on an operant delayed matching task and a water maze reference memory task. Behav Brain Res. 2006;171:116–126. doi: 10.1016/j.bbr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 87.Tseng KY, Lewis BL, Lipska BK, O’Donnell P. Post-pubertal disruption of medial prefrontal cortical dopamine–glutamate interactions in a developmental animal model of schizophrenia. Biol Psychiatry. 2007;62:730–738. doi: 10.1016/j.biopsych.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Flores G, Alquicer G, Silva-Gomez AB, Zaldivar G, Stewart J, Quirion R, et al. Alterations in dendritic morphology of prefrontal cortical and nucleus accumbens neurons in post-pubertal rats after neonatal excitotoxic lesions of the ventral hippocampus. Neuroscience. 2005;133:463–470. doi: 10.1016/j.neuroscience.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 89.Schneider F, Habel U, Reske M, Kellermann T, Stocker T, Shah NJ, et al. Neural correlates of working memory dysfunction in first-episode schizophrenia patients: an fMRI multi-center study. Schizophr Res. 2007;89:198–210. doi: 10.1016/j.schres.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 90.Whalley HC, Gountouna VE, Hall J, McIntosh A, Whyte MC, Simonotto E, et al. Correlations between fMRI activation and individual psychotic symptoms in un-medicated subjects at high genetic risk of schizophrenia. BMC Psychiatry. 2007;7:61. doi: 10.1186/1471-244X-7-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Plaze M, Bartres-Faz D, Martinot JL, Januel D, Bellivier F, de Beaurepaire R, et al. Left superior temporal gyrus activation during sentence perception negatively correlates with auditory hallucination severity in schizophrenia patients. Schizophr Res. 2006;87:109–115. doi: 10.1016/j.schres.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 92.Reulbach U, Bleich S, Maihofner C, Kornhuber J, Sperling W. Specific and unspecific auditory hallucinations in patients with schizophrenia: a magnetoencephalographic study. Neuropsychobiology. 2007;55:89–95. doi: 10.1159/000103907. [DOI] [PubMed] [Google Scholar]
- 93.Allen P, Amaro E, Fu CH, Williams SC, Brammer MJ, Johns LC, et al. Neural correlates of the misattribution of speech in schizophrenia. Br J Psychiatry. 2007;190:162–169. doi: 10.1192/bjp.bp.106.025700. [DOI] [PubMed] [Google Scholar]
- 94.Bediou B, Henaff MA, Bertrand O, Brunelin J, d’Amato T, Saoud M, et al. Impaired fronto-temporal processing of emotion in schizophrenia. Neurophysiol Clin. 2007;37:77–87. doi: 10.1016/j.neucli.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 95.Schneider F, Habel U, Reske M, Toni I, Falkai P, Shah NJ. Neural substrates of olfactory processing in schizophrenia patients and their healthy relatives. Psychiatry Res. 2007;155:103–112. doi: 10.1016/j.pscychresns.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 96.Sanjuan J, Lull JJ, Aguilar EJ, Marti-Bonmati L, Moratal D, Gonzalez JC, et al. Emotional words induce enhanced brain activity in schizophrenic patients with auditory hallucinations. Psychiatry Res. 2007;154:21–29. doi: 10.1016/j.pscychresns.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 97.Xiberas X, Martinot JL, Mallet L, Artiges E, Loc’H C, Maziere B, et al. Extrastriatal and striatal D(2) dopamine receptor blockade with haloperidol or new antipsychotic drugs in patients with schizophrenia. Br J Psychiatry. 2001;179:503–508. doi: 10.1192/bjp.179.6.503. [DOI] [PubMed] [Google Scholar]
- 98.Grunder G, Landvogt C, Vernaleken I, Buchholz HG, Ondracek J, Siessmeier T, et al. The striatal and extrastriatal D2/D3 receptorbinding profile of clozapine in patients with schizophrenia. Neuropsychopharmacology. 2006;31:1027–1035. doi: 10.1038/sj.npp.1300931. [DOI] [PubMed] [Google Scholar]
- 99.Stone JM, Davis JM, Leucht S, Pilowsky LS. Cortical dopamine D2/D3 receptors are a common site of action for antipsychotic drugs—an original patient data meta-analysis of the SPECT and PET in vivo receptor imaging literature. Schizophr Bull. 2008 doi: 10.1093/schbul/sbn009. (e-pub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Williams LM. Voxel-based morphometry in schizophrenia: implications for neurodevelopmental connectivity models, cognition and affect. Expert Rev Neurother. 2008;8:1049–1065. doi: 10.1586/14737175.8.7.1049. [DOI] [PubMed] [Google Scholar]
- 101.Wolf RC, Hose A, Frasch K, Walter H, Vasic N. Volumentric abnormalities associated with cognitive deficits in patients with schizophrenia. Eur Psychiatry. 2008 doi: 10.1016/j.eurpsy.2008.02.002. (e-pub ahead of print) [DOI] [PubMed] [Google Scholar]
- 102.Sim K, DeWitt I, Ditman T, Zalesak M, Greenhouse I, Goff D, et al. Hippocampal and parahippocampal volumes in schizophrenia: a structural MRI study. Schizophr Bull. 2006;32:332–340. doi: 10.1093/schbul/sbj030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Barta PE, Powers RE, Aylward EH, Chase GA, Harris GJ, Rabins PV, et al. Quantitative MRI volume changes in late onset schizophrenia and Alzheimer’s disease compared to normal controls. Psychiatry Res. 1997;68:65–75. doi: 10.1016/s0925-4927(96)02751-5. [DOI] [PubMed] [Google Scholar]
- 104.Casanova MF, Carosella N, Kleinman JE. Neuropathological findings in a suspected case of childhood schizophrenia. J Neuropsychiatry Clin Neurosci. 1990;2:313–319. doi: 10.1176/jnp.2.3.313. [DOI] [PubMed] [Google Scholar]
- 105.Beveridge NJ, Tooney PA, Carroll AP, Gardiner E, Bowden N, Scott RJ, et al. Dysregulation of miRNA 181b in the temporal cortex in schizophrenia. Hum Mol Genet. 2008;17:1156–1168. doi: 10.1093/hmg/ddn005. [DOI] [PubMed] [Google Scholar]
- 106.Akbarian S, Huang HS. Molecular and cellular mechanisms of altered GAD1/GAD67 expression in schizophrenia and related disorders. Brain Res Rev. 2006;52:293–304. doi: 10.1016/j.brainresrev.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 107.Katsel P, Davis KL, Gorman JM, Haroutunian V. Variations in differential gene expression patterns across multiple brain regions in schizophrenia. Schizophr Res. 2005;77:241–252. doi: 10.1016/j.schres.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 108.Ftouh S, Akbar MT, Hirsch SR, de Belleroche JS. Down-regulation of Dickkopf 3, a regulator of the Wnt signalling pathway, in elderly schizophrenic subjects. J Neurochem. 2005;94:520–530. doi: 10.1111/j.1471-4159.2005.03239.x. [DOI] [PubMed] [Google Scholar]
- 109.Bos JL. Epac proteins: multi-purpose cAMP targets. Trends Biochem Sci. 2006;31:680–686. doi: 10.1016/j.tibs.2006.10.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.