SUMMARY
Much has been learned by identifying the molecules that can be recognized by IgE from patients with allergies. Increasingly, by correlating patterns of sensitization with clinical features, it has become possible to distinguish molecules responsible for primary sensitization (complete allergens) from those that are more likely cross-reactive targets. In the case of animal allergens, evolutionary distance seems to be an important factor in determining allergenicity. However, until more is understood regarding the mechanistic details of primary sensitization, including the participation of molecules that stimulate innate immune responses and the repertoire of T-cell antigens, molecules that may or may not themselves be important B-cell antigens, we will not be able to explain fundamental questions, such as why peanut allergy is more severe than soy allergy or why tick exposure is associated with clinically relevant sensitization to a carbohydrate epitope.
Keywords: Food allergy, Allergen, Plant food allergens, Animal food allergens, Peanut, Soy, Cross-reactive carbohydrate determinants, Alpha-gal
As of September 2011, there were 12,273 recognized protein families in the Pfam database, yet only 255 (2.1%) of those families are represented among allergens. Among food allergens, only 71 (0.6%) families are represented (from ~400 described allergens); and among the top 20 families, a mere 0.16% of protein families, account for 80% of all described food allergens (Table 1).1 At the same time, it is not the case that simple elements (ie, primary or secondary structure) are constrained among allergens. For instance, examples of all common polypeptide folds are found without apparent strong overrepresentation of some.2,3 These observations suggest that there are determinants of food allergy, that is, structural or functional properties of certain proteins that play a significant role in determining what makes them allergens. In this review, the authors briefly review the categorization of food allergens and then draw on 3 examples of food allergies with disparate clinical presentations to discuss the potential relationships between allergen structure and function and the immune responses induced in humans.
Table 1. Common food allergy protein families ranked by number of identified allergens in each family.
| Family | Source | Rank by Number of Identified Family Members Associated with Food Allergy |
Rank by Number of Identified Family Members Associated with Aero/Contact Allergy |
Additional Notes |
|---|---|---|---|---|
| Prolamin superfamily | Plant | 1 | 4 | Includes cereal storage (gliadins), 2S albumin (eg, Arah 2/6), LTPs |
| Tropomyosin | Animal | 2 | 5 | Dominant crustacean allergen |
| Cupin superfamily | Plant | 3 | Rare; 1 described inhalant, 1 described contact |
Dominant family of legume and nut allergens |
| Profilin | Plant | 4 | 1 | Highly cross-reactive |
| EF-hand domain | Plant, animal | 5 | 2 | For ingestion, exclusively associated with fish, shellfish |
| PR-10 | Plant | 6 | 18 | Bet v 1 related |
| Alpha/beta-caseins | Animal, mammal | 7 | Not described | Milk allergens |
| Heveinlike domain | Plant | 8 | Latex allergy only | Includes some chitinases; latex, banana, avocado |
| Class I chitinases | Plant | 9 | Latex allergy only | Latex, banana, avocado (also chestnut, grape, corn) |
| Oleosins | Plant | 10 | Not described | ?Major sesame, minor peanut, hazelnut allergens |
| Lipocalin | Animal: arthropod and mammalian |
11 | 6 | Beta-lactoglobulin from milk in this family |
| Beta-1,3-glucanase | Plant | 12 | Latex (Hev b 2) olive tree pollen |
Described in tomato, potato, banana, bell pepper |
| Papainlike cysteine protease |
Plant | 13 | Der p 1, Der f 1 | Kiwi, pineapple, papaya (fairly rare) |
| Thaumatinlike protein |
Plant | 14 | Cedar/cypress pollen | Apple, cherry, grape, kiwi, pepper |
| Expansin, C-term | Plant: all grasses | Not described | 3 | Major allergens of grass pollen |
| Trypsinlike serine proteases |
Animal: arthropod and mammalian |
Not described | 9 | Bee and bumblebee sting, mite and roach inhalant |
| Enolase | Fungi and plants | Not described | 10 | — |
| Expansin, N-term | Plant: all grasses except 1 | Very low; 1 food allergen described from kiwi |
8 | — |
| Subtillsinlike serine protease |
Fungi (1 identified from bacteria) |
Very low; 1 food allergen described from musk melon |
7 | — |
Data from Radauer C, Bublin M, Wagner S, et al. Allergens are distributed into few protein families and possess a restricted number of biochemical functions. J Allergy Clin Immunol 2008;121(4):847–52.
WHAT IS A FOOD ALLERGEN?
The simplest definition of an allergen is a substance that causes an allergic reaction, broadly speaking, a hypersensitivity immune response, but usually refers to a type I– or immunoglobulin E (IgE)–mediated hypersensitivity response. This definition is vague, allowing for both principal and proximate causes. In practical terms, allergens are defined by being recognized by IgE from patients. Some allergens included by this definition are not very potent inducers of primary allergic immune responses, in other words, they are weak allergenic immunogens; but if IgE capable of binding them is present (perhaps because of cross-reactivity with a strong immunogen), they can trigger effector responses. Other structures (eg, some glycans discussed later) are inducers of IgE and are recognized by IgE in binding assays but may be impotent triggers of allergic responses. A molecule with the capacity to induce sensitization and trigger reactions has been termed a complete allergen3; Ara h 2 from peanut seems to meet this definition. Mal d 1, by contrast, is an incomplete allergen because it can trigger reactions by being cross-reactive with the dominant aeroallergen from birch pollen, Bet v 1, but is not an important immunogen. It is important to recognize that defining allergens purely by the IgE response may overlook the importance of molecules that predominantly stimulate T cells or other immune cells and the potential importance of that for the pathogenesis of both IgE- and perhaps non–IgE-mediated food allergy.
Food allergens, for this review, include molecules, mostly proteins, that are recognized by IgE and found in the diet, whether or not they are complete allergens. We recognize that defining what is a food allergen is greatly influenced by culture and circumstance. Many proteins that may not be important at all as food allergens in a particular culture may be significant in another population, and proteins that are rarely or never described as food allergens in patients may be capable of being an allergen under contrived conditions or experimental models, particularly when combined with strong T helper (Th)-2 adjuvants. That said, by recognizing that there is significant homology across many plant and animal taxa that are sources of food, one can be impressed by the observation that some protein families are significantly overrepresented (eg, prolamins) and that some species of plant or animal are also overrepresented as strongly polarizing allergen sources in many cultures (eg, crustaceans, peanut).
STRUCTURAL FEATURES
It seems intuitive that structural features, including stability during food processing and digestion, are important factors in determining allergenic potency of ingested molecules. The food-pollen syndromes, best represented by PR-10 sensitization, exemplify this concept as the structural instability of those proteins correlates with the observation that cooking destroys allergenicity and that ingestion of any form is rarely if ever associated with systemic reactions. Digestibility, on the other hand, as a predictor of food allergenicity has been tested empirically with mixed results.4-7 There are multiple potential explanations for the weak correlation between digestibility and food allergenicity, including limitations of in vitro systems used to mimic digestion, food matrix effects that are lost when assessing purified proteins, alteration of protein structure during protein preparation, relative abundance of proteins in whole food, and others. However, whatever the explanation, we know that IgE-mediated activation of effector cells requires cross-linking and, therefore, interaction with multivalent ligands that must, therefore, possess a complex structure. Food allergens, thus, must either survive or bypass digestion in sufficient amounts to provoke immune responses.
Protein glycosylation has been shown to contribute to protein stability,8,9 although it is also likely to enhance immunogenicity in other ways. That glycosylation can enhance the allergic immunogenicity of proteins has been demonstrated by creating neo-conjugates of specific glycans to non-glycosylated carrier proteins.10 The immunogenicity of nonmammalian glycans had already been suggested by the demonstration of ubiquitous glycan-specific antibodies in humans from a variety of clinical contexts as well as in murine models.11,12 These structures are antigenic structures that are distinct from endogenous glycans. Much like the glycans that underlie ABO blood group incompatibility, responses to them are simply not edited out during immune development and can therefore induce immune responses. Some glycans are recognized by specific innate immune pattern receptors within the C-type lectin receptor (CLR) family, and this seems to enhance or modulate their immunogenicity, as discussed later.
Lipid binding is a common feature of both food and respiratory allergens.13 Many prolamins (the largest food allergen group, including nonspecific lipid transfer proteins, 2S albumins, prolamin storage proteins, and α-amylase/trypsin inhibitors), lipocalins, and some cupins interact with lipids; and this may protect them from degradation and enhance their absorption from the gastrointestinal (GI) tract.14 The lipocalin milk allergen, β-lactoglobulin, for example, is more stable when lipid bound.15
Macromolecular aspects of protein structure are also known to be relevant in the context of food allergen immune responses and mucosal immunity more broadly. Particulate antigens are predominantly processed and presented in Peyer patches via specialized microfold cell uptake, and this can be a route of strong humoral immune induction. In contrast, soluble antigens can be efficiently absorbed across the intestinal epithelium and tend to be less immunogenic. Mammalian milk is a complex colloidal fluid in which caseins (α, β, κ) are largely contained in large micelles that are in suspension and are predominantly presented to the immune system via Peyer patches, whereas whey proteins (eg, β-lactoglobulin and α-lactalbumin) are highly soluble and rapidly transported across the intestinal epithelium. One functional consequence of this in an animal model of milk allergy is that caseins are more potent inducers of antibodies, including IgE; but for animals sensitized to both casein and whey proteins, it is the whey proteins that induce stronger systemic allergic reactions on exposure, possibly because they are so rapidly absorbed.16 Many food allergens have a tendency to aggregate because of the presence of repetitive sequences, rheomorphic structures, or the ability to oligomerize.17 This tendency may favor their capacity to induce sensitization in the gut by being preferentially presented in Peyer patches.
FUNCTIONAL FEATURES
In addition to passive aspects of structure, part of what may make an allergen potent may be what immunostimulatory capacity it has intrinsically or by intimate association with other molecules. Some of the same features that enhance immunogenicity by increasing stability or aggregation (eg, glycosylation, lipid binding) might also stimulate innate immune responses.
Examples of pathways that are directly stimulated by allergens are growing in literature. For example, glycans in house dust mite extract induce Th2 differentiation via binding to the C-type lectin Dectin-2 on dendritic cells (DC) and the subsequent production of cysteinyl leukotrienes.18 The mannose receptor (MR), another CLR, binds to and mediates the internalization of a variety of allergens, such as Der p1 and p2 (house dust mite) and Ara h1 (peanut). MR also mediated Der p1–induced Th2 skewing by human monocyte derived DCs (MoDC) through the upregulation of indoleamine 2,3-dioxygenase (IDO) activity.19
IDO has also been associated with immune tolerance, particularly, although not exclusively, in models of Th1 inflammation. In fact, many pathways or mediators (eg, CLRs, thymic stromal lymphopoietin (TSLP), OX40 ligand (OX40L), retinoic acid) that under some conditions have been associated with Th2 induction have also been implicated in tolerance. In both the induction of Treg and Th2, the suppression of interleukin (IL)-12 and other features of alternative DC activation may be shared. As an example, the activation of the CLR SIGNR1 (the murine paralogue of DC-SIGN) on lamina propria DC (LPDC) had a tolerogenic rather than a Th2-skewing effect: bovine serum albumin (BSA) coupled with mannoside (Man-BSA) selectively targeted SIGNR1-expressing LPDC and induced the production of IL-10, which promoted the generation of CD4+ type 1 regulatory T cells. In mice sensitized with BSA, treatment with Man-BSA substantially reduced the BSA-induced anaphylactic response.20
Additional examples of functional allergen properties include the cystein protease allergens, including both respiratory and food allergens from plants (see below), signaling via protease activated receptors (PARs),21 and the functional mimicry of MD-2 by the dust mite allergen, Der p 2, possibly modulating TLR4 sensitivity to natural ligands.22
PLANT FOOD ALLERGENS
About 65% of the plant food allergens belongs to one of the following classes of structurally related protein superfamilies: (1) the prolamin superfamily; (2) the cupin super-family; and (3) the pathogenesis-related proteins (PR-10) family, of which Bet v 1 is the best known.23,24
The prolamin superfamily includes seed storage proteins of cereals, lipid transfer proteins (LTPs), alpha-amylase/protein inhibitors and 2S albumins. Their 3-dimensional (3D) structure consists of a compact tertiary structure with 4 alpha-helices stabilized by disulfide bridges and a central cavity for lipid binding. There are 8 conserved cysteine residues with cys-cys and cys-X-cys motifs. Despite their homologous structures, the sequence similarity is low between members of the prolamin superfamily. The seed storage proteins/prolamins of wheat are known to cause baker’s asthma and celiac diseases. The alpha-amylase/trypsin inhibitors from several cereals, such as wheat, barley, and rice, are shown to be involved in allergy. Some of the clinically relevant allergenic proteins that belong to this protein superfamily are the following: Ara h 2, Ara h 6, Sin a 1, Ber e 1, Ses i 2, Jug v 1, and so forth. The nonspecific LTPs are known to be major food allergens in fruits from the Rosacea family. The presence of specific IgE to LTP is considered to be a significant risk factor for allergy and may even serve as a diagnostic marker.25
The prominent allergens of the cupin superfamily of proteins are the 7S (vicilins) and 11S globulins. The 7S globulins include Ara h 1, Jug r 2, and Ses i 3. Some examples of 11S globulins are Ara h 3, soybean glycinins, Ber e 2, Cor a 9, and Fag e 1.14 The 3D structure of proteins that belong to the cupin family consists of a series of antiparallel β-sheets associated with α-helix and forms a cavity (cuplike structure, hence the name cupin; cupa in Latin means “barrel”).26 This structure is also found in several proteins of the lipocalin family involved in the transport of hydrophobic ligands, including β-lactoglobulin of milk. Profilins are highly conserved, small, actin-binding proteins that are ubiquitously expressed in all eukaryotic cells.27
Plant profilins share about 70% of the amino acid sequence homology among themselves. Profilins consist of a compact globular structure with central 7 stranded antiparallel beta sheets enclosed by alpha helices.28 Because of its conserved amino acid sequences, about 20% to 30% of all patients with tree pollen allergies react to profilin.29,30 Moreover, sensitization to profilin is considered a risk factor for developing multiple pollen-associated food allergies.25
Bet v 1–related proteins are ubiquitously distributed and comprised of 8 superfamilies that include PR-10, major latex proteins, and proteins involved in alkaloid biosynthesis. The 3D conformation of the Bet v 1–related proteins provides a large, Y-shaped hydrophobic pocket that binds to plant steroids.31
ANIMAL FOOD ALLERGENS
Although in theory all proteins have the potential to become allergens, this is not usually the case. In fact, before an epitope can result in an allergenic response, it requires that the human immune system has first discriminated self from nonself. This requirement is likely to be particularly true when related to animal food allergens. For instance, any antibody response in humans to vertebrate tropomyosins might be expected to cause autoimmune disease.24 Others have alluded to the notion that allergy to mammalian proteins likely approaches the limit of the human immune system to discriminate self from foreign antigens.32 Thus, unlike most plant allergens, almost all the animal food allergen families have an equivalent or counterpart in the human protein repertoire that may influence the human immune system response. Recent work has classified animal food allergens into 3 main families: tropomyosins, EF-hand proteins, and caseins.24 In each of these 3 main animal food allergen families, the ability to act as an allergen seems to be related to the relative identity to human homologs such that proteins closely related to human homologs are significantly less likely to be allergenic. In the 3 families of allergens analyzed, proteins with a sequence identity of roughly 54% to human homologs were all allergenic, whereas those with a sequence identity greater than 63% to human homologs were rarely allergenic.24 In keeping with this finding, others have suggested that a low degree of similarity to a host’s proteome is required for immunogenicity.33
Of the tropomyosin family, the animal food allergenic varieties are reportedly confined to invertebrate groups, namely mollusks and arthropods.24 Although there are 4 types of mammalian muscle tropomyosins, none of these have been reported to be sources of an IgE response in humans.34 Likewise, no human IgE response has been identified to tropomyosins from birds or fish. The absence of such a response might be expected because these sequences are greater than or equal to 90% identical to at least 1 human tropomyosin.24
EF-hand proteins compose the second largest animal food allergen family, and this is largely made up of parvalbumins.24 Parvalbumins themselves are divided into α- and β-parvalbumins. The α-parvalbumins, generally considered to be nonallergenic, are found primarily in the muscle of fish and amphibians.24 On the contrary, β-parvalbumins are found in a variety of fish species; retain allergenic potential; and, interestingly, are absent from human muscle.35
Of the caseins that have been shown to elicit an IgE response in humans, the general principle is that, again, the closer the sequence in homology to the human equivalent the less likely an IgE response will occur. One exception worth mentioning in the setting of animal food allergy is that of BSA. Sensitization to BSA is the main predictive marker of the cross-reactivity to cow’s milk that is present in 73% to 93% of patients with beef allergies, despite the fact that BSA shares 76% identity to its human homolog.24,36 Cross-reactivity to BSA is frequently outgrown in children, and this may be caused by the high homology to human serum albumin, whereby BSA may elicit a less-effective immune response than other cow’s milk proteins (ie, caseins).37,38
In sum, it may be that both the source of the food allergen and the route of sensitization are important in determining which protein families predominate, with different families represented in plant versus animal food allergen families.
WHAT CAN WE LEARN FROM 3 DISPARATE EXAMPLES OF FOOD ALLERGY?
Peanut Allergy
In the United States, approximately 1% of the population is peanut allergic, and this prevalence seems to have risen.39 In several studies, peanut allergen is also disproportionately implicated as a trigger of severe reactions among those who are affected. The humoral immune response to peanut allergens in susceptible individuals is more strongly IgE biased, in comparison, for example, with the immune response to milk allergens, against which atopic individuals tend to make both IgE and IgG. Although atopy is clearly a risk factor for peanut allergy, isolated peanut allergy is common40 in part because peanut allergy is more persistent than other common food allergies. Contrast that with soy allergy, discussed later, which is rarely an isolated allergy.
There are 12 peanut allergens recognized in Allergome, although several of these include isomers and some distinctly named proteins (eg, Ara h 2 and Ara h 6), are paralogs related as tandem gene duplicates. The major plant allergen families are all represented. For example, Ara h 1, 2, and 6 are prolamins, whereas Ara h 3 is a cupin, Ara h 8 is a PR-10 protein, and Ara h 9 is a profilin. Additional identified allergens include the lectin, peanut agglutinin, and an 18kD oleosin. The most immunodominant allergens, at least in populations with primary peanut allergy, are Ara h 2, Ara h 6 and Ara h 1. Geographic variation in sensitization to specific peanut allergens has been recognized, but it is reactivity to Ara h 2 and Ara h 6 that has increasingly emerged as the major risk factor for significant clinical reactivity.41
The major peanut allergen and glycoprotein Ara h 1 induces activation of human DC and enhances the induction of Th2 differentiation by these DC in naïve T cells. Ara h 1 was further shown to bind to the CLR, DC-SIGN. De-glycosylated Ara h 1 lacked this Th2-skewing effect, indicating that allergen-bound carbohydrate structures may act as a Th2-skewing adjuvant.42 Correspondingly, other studies have reported that glycation of proteins enhances their uptake by DC and their T-cell immunogenicity,43 as well as their ability to induce Th2 differentiation.44,45 Incubation of DC with Lewis-x trisaccharides suppressed the production of IL-12,45 which suggests one mechanism for the enhanced Th2 skewing induced by these glycans.
Peanut extract has also been shown to activate complement in murine and human serum, resulting in the formation of C3a. In susceptible mice, peanut extract induced anaphylactic shock, which was dependent on C3a as well as on platelet-activating factor and histamine. No involvement of DC was shown in this response46; however, complement split products can activate DC and regulate adaptive immunity.47,48Both C3- and C3a-deficient mice are resistant to asthma in an Aspergillus-induced model.49
Soy Allergy
Soy allergy usually manifests in childhood. The prevalence of soy allergy in adults is still poorly described40 but estimated to be around 0.3% to 0.4%.50,51 Soy allergy is spontaneously resolved in about 50% of children with soy allergies by 7 years of age.52 Allergic symptoms to soy may include atopic dermatitis, enterocolitis, and other IgE-mediated multisystem reactions.53,54 Even though soy is recognized as one of the big 8 food allergens, it is rather uncommon to see allergic reactions exclusive to soy.55 Soy allergy is often present in individuals who are allergic to multiple foods and birch pollen.56 About 6% of atopic children57 and 10% to 14% of infants with a cow milk allergy also suffer from concomitant soy allergy.58-60 Moreover, anaphylaxis caused by soy is very rare.57,61 Isolated soy allergy seems to be rare; for example, 88% of children with soy allergies also have peanut allergies.52
Soybean Allergens and Their Cross-Reactivity with Other Allergenic Proteins
Soybeans contain allergens that belong to all the categories of the protein superfamilies previously discussed. So far, about 28 potential allergens have been identified based on IgE binding from patients with a soy allergy.62,63 Among them, 16 proteins are characterized as allergens by the Allergome database. However, the reactivity of these allergens is highly variable among individual patients with no correlation with severity of symptoms. Only a few of them are recognized as major allergens by the International Union of Immunologic Societies of Allergen Nomenclature Subcommittee: Gly m 1, Gly m 2, Gly m 3, and Gly m 4. Other dominant allergens in soy are the soybean Kunitz trypsin inhibitor, the thiol-protease Gly m Bd 30k, the alpha subunit of β-conglycinin (BC, Gly m 5), the acidic chain of the major storage protein glycinin (G, Gly m 6) G1 subunit, the basic chain of the G2 subunit,64 and 2S albumin. For a full list of allergenic proteins in soy, refer to article by L’Hocine and colleagues.65
Gly m1 and Gly m2 are soybean hull proteins and have been shown to be aeroallergens. These proteins, in addition to the Kunitz trypsin inhibitor, have been implicated in the development of allergic asthma.66 Gly m 1 (also known as Gly m Bd30K/P34 or P34) is a soybean vacuolar protein.67,68 Gly m Bd30K shares a high sequence similarity to thiol proteinases of the papain family. Another notable member of this group is Der p 1, which is the major allergen in the dust mite. Birch pollen–related allergens, Gly m 3 and Gly m 4, belong to the profilin family of proteins. Gly m 3 is highly homologous with Bet v 2, with an amino acid sequence identity of 73%. The IgE-binding epitopes of Gly m 3 are highly conformational because fragments of Gly m 3 fail to bind IgE.69 Gly m 4, also called as PR-10 protein, and starvation-associated message 22, is a stress-related protein with a 50% homology with Bet v 1. Gly m 4 is a major cross-reactive allergen in patients with a birch pollen allergy.70 Serum IgE from 85% of patients with birch pollen allergies reacts to Gly m 4.71 About 10% of Central European patients sensitized to birch pollen are reported to have concomitant soy allergy, mainly caused by cross-reactivity to Gly m 4.56
Major seed storage proteins Gly m 5 (β-conglycinin, 7 S) and Gly m 6 (glycinin, 11 S) were found to be major allergens in a cohort of 30 patients with Double-blind, placebocontrolled food challenge (DBPCFC)-confirmed soy allergy, which suggests that these molecules are good diagnostic markers of component-resolved in vitro testing.64 However, another study by Vissers72 found high IgE binding to Gly m 4 but not Gly m 5 and Gly m 6. Patients who are allergic to peanuts but clinically non–soy allergic tested positive for Gly m 5 and 6.
Glycinin (11S) and β-conglycinin (7S) belong to storage proteins that belong to the cupin family.73 Burks and colleagues,74,75 Ogawa and colleagues,76 and Beardslee and colleagues77 all have identified IgE binding to different subunits of β-conglycinin. Changes in the ionic strength and pH alter the conformation structure of glycinin and have significant effect on the immunogenicity in terms of IgG1 binding.78
Because soy proteins are considered to be less immunogenic than cow milk protein,79 soy-based formula is usually used as an alternative in infants with a cow milk allergy. However, it is common to see coexisting soy allergy in patients with a cow milk allergy. Osterballe and colleagues80 have shown that about 17% of patients with a cow milk allergy are also sensitized to soy. In a study with 10 patients with a cow milk allergy but never exposed to soy, their serum reacted to components in soy extract by competitive inhibition enzyme-linked immunosorbent assay. The authors have identified the cross-reactive protein to be a 30 KD fraction of 11S globulin in soy.81
Similarity of Soybean and Peanut Allergens: Why Soy is Less Allergenic than Peanut
Several soy allergens share high sequence homology to their counterparts in peanut (Table 2). Soybean β-conglycinin Gly m 5 is the closest homolog of Ara h 1, with 51% amino acid sequence identity and a similar 3D structure. In addition, Ara h 3 and soybean glycinin Gly m 6 share similar 3D structure to that of Ara h 1 despite less sequence homology.82 However, the incidence of soy allergy is much less than that of peanut.83 Although fatal events after consumption of soy have been reported,84,85 those patients also had severe peanut allergies. In a double-blind, placebo-controlled food challenge study in 30 European patients, 67% of patients with a soy allergy reported concomitant peanut allergy.86 Because of the high amino acid homology between peanut and soy, soy protein has been tried as an immunotherapeutic agent for peanut allergy in mouse models.87 In this study, peanut-allergic mice were able to get desensitized with crude soy extract. Intraperitoneal injection of peanut-sensitized mice with soy extract resulted in suppressed immune response to peanut and markedly reduced clinical symptoms on peanut challenge.87
Table 2. Amino acid homology between peanut and soy allergens.
| % Similarity % Identity |
Soy | |||||
|---|---|---|---|---|---|---|
| Gly m Conglycinin | Gly m Glycinin G1 | Gly m Glycinin G2 | ||||
| 7S Globulin | 11S Globulin | 11S Globulin | 2S Albumin 1 | 2S Albumin 3 | ||
| Peanut | ||||||
|
| ||||||
| Ara h 1 | 7S globulin | 52.2 | 23.0 | 24.0 | 7.1 | 6.4 |
| 40.0 | 14.2 | 13.9 | 3.8 | 3.0 | ||
|
| ||||||
| Ara h 2 | 2S albumin | 6.6 | 7.1 | 7.4 | 44.2 | 41.5 |
| 3.4 | 5.1 | 4.7 | 35.6 | 34.8 | ||
|
| ||||||
| Ara h 3 | 11S globulin | 24.9 | 65.3 | 65.6 | 7.4 | 6.7 |
| 15.5 | 57.3 | 57.2 | 4.3 | 4.1 | ||
Homology between peanut and soy allergens as determined by Vector-NTI software.
Numbers in bold represent comparisons of proteins that fall in similar categories.
The percentage of amino acids that are similar or identical are shown as straight and italicized text, respectively.
Soy protein is potentially allergenic. However, the reaction threshold (ie, safe for 90% of allergic patients) to soy is as high as 400 mg compared with 0.1 mg for peanut.88,89 In a retrospective study of DBPCFC, Sicherer and colleagues90 reported that about 28% of children with a soy allergy reacted to an initial dose of 500 mg of soy (200 mg soy protein). Ballmer-Weber and colleagues86 reported a range of 454 mg to 50 g of soy required to achieve objective allergic symptoms, is at least one order of magnitude higher than observed in peanut allergy. It is not clear why soy protein showed a low reaction threshold and diminished immunoreactivity.
Soy has been a part of staple food in Asia for centuries. Anecdotal evidences suggest that high consumption of traditional soy food, particularly fermented soy food in Far Eastern countries, could account for the lower incidence of several chronic health conditions. Comparatively, much of the soy-food in the Western world is in the form of processed protein isolates and subtle food additives. Investigation of why the incidence and severity of soy allergy is low compared with peanut and other foods would be informative in designing a therapy for other food allergies. It is possible that the intrinsic allergenicity of soy could be different in terms of glycosylation91,92 and IgE binding.93 Kroghsbo and colleagues93 have compared immune response with 7S globulins of peanut, hazelnut, soy, and pea in Brown Norway rats. Peanut 7S globulins induced IgE of higher avidity than other related proteins. Soy induced IgE of least avidity. Susceptibility to heat74,94 and hydrolysis95,96 have been shown to result in the reduced allergenicity of soy.
Emerging evidence suggests that the presence of anti-inflammatory phytochemicals in soy could be one of the reasons for their reduced allergenicity. Several phytochemicals are present in soybean: isoflavones, saponins, phytates, sterols, lignans, and so forth. Most of them present as glycosides in soybean. The microbiota in the gut converts glycosides to active aglycones, which have antiinflammatory and immunomodulatory properties. The levels of bioactive phytochemicals are increased in fermented soy food as a result of the hydrolysis of isoflavone glycosides. These immunomodulatory phytochemicals are well known to have beneficial properties in various disorders, including food allergy.97-99
MAMMALIAN MEAT ALLERGY
Carbohydrates Induce and Bind IgE
Although the most frequent allergens involved in anaphylactic reactions are proteins found in peanuts, tree nuts, fish, shellfish, bee and wasp venoms, and haptens in drugs and latex,100-103 carbohydrate moieties are also present in many foods and can induce antiglycan IgE responses.11 Because carbohydrate moieties can share significant structural homologies beyond that of proteins, they are prone to extensive cross-reactivity. These cross-reactive epitopes are called cross-reactive carbohydrate determinants (CCDs); however, the clinical significance of IgE antibodies directed against them is unclear.104-106
The study of carbohydrates as food antigens began in the 1970s when a Japanese group reported the structure of a protease from pineapple stems.107 It was subsequently shown that this protease, bromelain, contained an oligosaccharide with 2 structural features that had not been found in mammalian glycoproteins: core α1,3-fucose and xylose (Fig. 1).108 In fact, xylose and core-3-linked fucose may be the most common carbohydrate epitopes recognized by human IgE antibodies.109
Fig. 1.
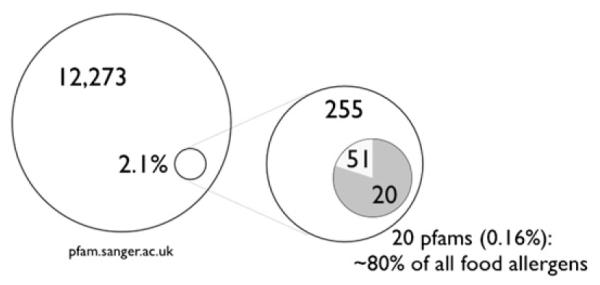
Protein families present among allergens (255) and food allergens (71) relative to the total number of described family members in the Pfam database. (Data from Radauer C, Bublin M, Wagner S, et al. Allergens are distributed into few protein families and possess a restricted number of biochemical functions. J Allergy Clin Immunol 2008;121(4):847–52.)
CCDS in Allergy: A Review of the Controversy
The link between plant-based glycans and allergy was made by Aalberse and colleagues104 in 1981. They showed that IgE from patients’ sera cross-reacted with extracts from several foods and insect venoms. This work laid the foundation for later studies, which indicated that asparagine-linked (N-glycans) were involved in IgE binding to honeybee venom phospholipase A2 (PLA2; Api m 1).110 Ultimately, the structural basis for the cross-reactivity of IgE with insect and plant glycoproteins was shown to be core α1,3-fucose.111 The binding to Api m 1 from sera of patients with a bee allergy was inhibited by glycopeptides from pineapple stem bromelain.111
Current estimates are that 15% to 30% of allergic patients generate specific antiglycan IgE.105,106,112,113 This frequent occurrence of sensitization to CCDs contrasts with their apparent inability to produce clinical symptoms. Evidence against a significant clinical impact of carbohydrate-directed IgE comes from several aforementioned studies and was recently reviewed.106,113,114 A clinically benign role for CCDs is now being questioned because several studies have shown the ability of anti-CCD IgE to trigger mediator release from basophils.115-118 Although preliminary studies in the authors’ laboratory using basophils isolated from patients with IgE to alpha-gal showed evidence of activation in response to a carbohydrate antigen, the ability of this IgE antibody to trigger a mediator release in vivo remains an area of active investigation. In the aforementioned study by Mari113 that examined bromelain sensitization, skin testing to bromelain revealed few positives. This finding may be expected, though, given that degranulation requires cross-linking on the surface of the mast cell and that bromelain contains only one oligosaccharide chain.119 It has also been suggested that antibodies to uncharged carbohydrate epitopes would have low affinity,120 rendering skin testing less reliable. However, the evidence that IgE antibodies to CCDs are of low affinity is poor, and recent work has indicated that these antibodies have affinities comparable with IgG antibodies.121
Adding to this growing literature, recent work from the authors’ group has shown that IgE antibodies specific for the carbohydrate alpha-gal are capable of eliciting serious, even fatal, reactions.122 The authors subsequently extended that observation by confirming that IgE antibodies to alpha-gal are associated with an unusual form of delayed anaphylaxis, which follows 3 to 6 hours after eating meat that carries alpha-gal (eg, beef, pork, or lamb).123 In contrast to previously described CCD motifs of xylose and core-3-linked fucose, which populate plants and insects, the alpha-gal epitope is abundantly expressed on cells and tissues of nonprimate mammals.124,125 This expression pattern makes alpha-gal potentially clinically relevant either as a food allergen (eg, beef, pork, lamb) or as an inhaled allergen (eg, cat, dog).123,126
Galactose-α-1,3-Galactose (Alpha-Gal): Epitope Characteristics and Clinical Relevance
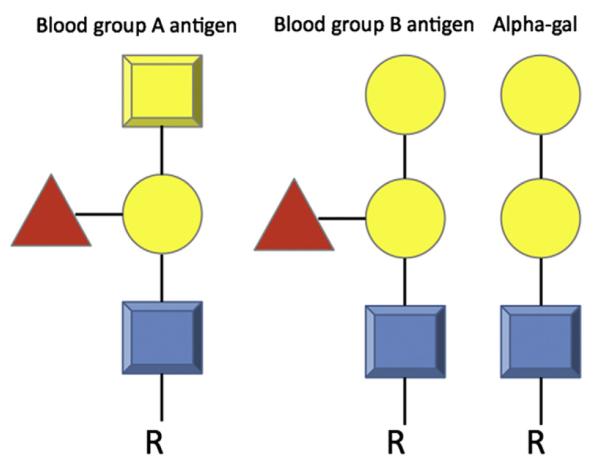
Although alpha-gal has not had a long history within the allergy literature, the alpha-gal epitope and its naturally occurring IgG antibody have been the subject of much important and thoughtful work from other scientific perspectives. Karl Landsteiner speculated about the existence of a blood-group-B–like substance on mammalian cells in the 1930s, and this was likely to be alpha-gal. One can reason this because blood group B antigen and the alpha-gal epitope differ only in that blood group B antigen has a fucose-linked α-1,2 to the penultimate galactose (Fig. 2). Likewise, medical and surgical transplant communities are familiar with alpha-gal: the naturally occurring antibody to alpha-gal in humans, apes, and Old World monkeys is responsible for the hyperacute rejection of pig xenografts by binding alpha-gal epitopes on the porcine cells.
Fig. 2.
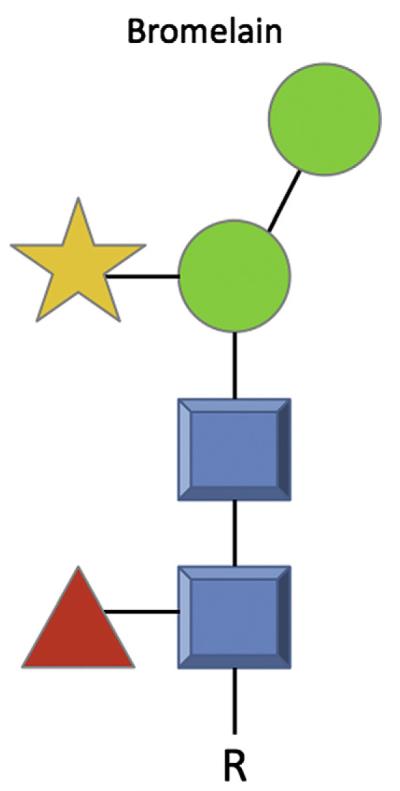
Bromelain showing the core α1,3-fucose (red triangle) and xylose (orange star) as referenced in the text. NB The oligosaccharide structures are shown in the symbols suggested by the Consortium of Functional Glycomics, such that the blue squares represent N-acetylglucosamine, green circles represent mannose, yellow symbolizes galactose, whereas orange star and red triangle are xylose and fucose, respectively. R, an organic molecule.
The alpha-gal epitope induces the production of a highly specific antigal IgG antibody through continuous antigenic stimulation by carbohydrate antigens on GI bacteria of the normal flora,127 much akin to the production of antibodies to blood group A or B antigens (Fig. 3).128 Inactivation of the α1,3GT gene is thought to have occurred roughly 28 million years ago,129 such that humans, apes, and Old World monkeys possess a pseudogene found on chromosome 9. The exon that codes for the main catalytic domain contains 2 point mutations (deletions) that produce a frame-shift and a premature stop codon.130 The result is that although all lower mammals make alpha-gal as a glycosylation product, humans do not and instead produce antigal antibodies.
Fig. 3.
Comparison of blood group A antigen, blood group B antigen, and galactose-α-1,3-galactose (alpha-gal). Note that the lack of a core fucose residue separates the structure of blood group B antigen from alpha-gal.
Recently Identified IgE to Alpha-Gal
While investigating IgE antibodies in the serum of patients who experienced a hypersensitivity reaction to the chimeric monoclonal antibody cetuximab, Chung and colleagues122 identified control patients without cancer who also had serum IgE antibodies that bound to cetuximab. These IgE antibodies were shown to be specific for an alpha-gal moiety found on the asparagine at position 88 in the murine heavy chain portion of cetuximab. The authors have recently broadened this work and described a cohort of patients with IgE antibodies to alpha-gal who experience delayed symptoms of anaphylaxis, angioedema, or urticaria after eating mammalian meat.123 This report was the first to identify food allergy related to IgE antibodies to the alpha-gal carbohydrate epitope. Whether IgE to alpha-gal is specific for this moiety only or, as with CCDs, has the ability to bind other epitopes is currently unknown (ie, whether this is actually IgE directed against alpha-gal or IgE that binds alpha-gal and other closely related epitopes). The authors’ experience is that patients with IgE to alphagal describe no oral allergy syndrome-type symptoms; report generalized urticaria or frank anaphylaxis starting 3 to 6 hours after eating beef, pork, or lamb; and have a consistent pattern of both skin testing and serum IgE antibody results.114,123
The elucidation of IgE to alpha-gal has reversed the conclusion of prior work showing that binding of cat IgA and IgM was caused by an IgE-independent cross-linking with the patients’ anti-glycan IgM.131 In fact, this antiglycan IgM has now been shown to be IgE to alpha-gal.132 Recent work by Wong and colleagues133 hinted at the existence of another CCD in mammalian tissue. The authors’ description of IgE to alpha-gal in a cohort of patients who report delayed symptoms after eating mammalian meat fits the notion of Wong and colleagues, and alpha-gal may well be a clinically relevant CCD that is specifically found in mammalian tissue.
Evidence for the Role of Tick Bites
Since establishing the assay for IgE antibodies to alpha-gal, large numbers of sera have been screened. The results showed that these IgE antibodies were regionally distributed and that they were also associated with a novel form of anaphylaxis.122,123 As mentioned, these patients reported delayed symptoms after eating mammalian meat, but they had had no trouble with chicken, turkey, or fish.114,123 Thus, their symptoms matched the specificity of IgE antibodies present in their serum, which accurately reflected the known distribution of alpha-gal in mammals.120,123 In most cases, these patients were adults who had consumed red meat for many years before they developed the delayed reactions. The implication was that some new exposure had triggered the production of IgE antibodies to alpha-gal.
Not only did the known distribution of the immediate reactions to cetuximab match to the area of the highest prevalence of Rocky Mountain spotted fever, the authors also heard histories from patients that their reactions to red meat started after receiving multiple tick bites.134 The authors have reported the evidence that tick bites in the United States can induce IgE antibodies to alpha-gal, and the evidence included, following the response prospectively in 3 cases, a strong correlation with histories of tick bites, epidemiologic evidence that these antibodies are not found in regions where tick bites are rare, and the correlation with IgE antibodies specific for tick proteins.134 In addition, there is epidemiologic evidence that these IgE antibodies are found in areas where tick bites are common, that the responses correlate with prolonged pruritic reactions to tick bites, and that IgE antibodies to alpha-gal correlate with the presence of IgE antibodies to tick proteins.134 Furthermore, the currently known distribution of delayed anaphylactic reactions to red meat fits the known distribution of the lone star tick, Amblyomma americanum.134 As the deer population expands, it will be interesting to follow whether there is an increase in the incidence of diagnosed mammalian meat allergy.
Summary of Allergy to Glycans
The discovery of IgE antibodies to the oligosaccharide galactose alpha -1, 3-galactose has made it possible to investigate several novel aspects of allergic disease. The obvious thing is that the glycosylation of therapeutic recombinant molecules, particularly monoclonal antibodies, can create a risk for severe hypersensitivity reactions. In addition, because these IgE antibodies also bind to a wide range of mammalian proteins, the authors recognized the syndrome of delayed anaphylaxis to mammalian meat.123 However, the most interesting feature of their reactions may be that first symptoms (ie, itching or urticaria) occur 3 to 6 hours after eating meat and would normally be regarded as spontaneous or idiopathic anaphylaxis. Understanding the factors that control the delay may provide real insight into the factors that control anaphylaxis. Moreover, understanding how ticks induce this form of response will be important as we explore the control of IgE antibody responses in general.
ACKNOWLEDGMENTS
The authors would like to thank Youngshin Han, PhD and Galina Grishina, MS for providing the sequence analysis data.
Funding sources: The Jaffe Food Allergy Institute (M.M.), NIH (S.C., W.S.).
Footnotes
Conflict of interest: The authors have no conflict of interest.
REFERENCES
- 1.Radauer C, Bublin M, Wagner S, et al. Allergens are distributed into few protein families and possess a restricted number of biochemical functions. J Allergy Clin Immunol. 2008;121(4):847–52. e7. doi: 10.1016/j.jaci.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 2.Aalberse RC, Stadler BM. In silico predictability of allergenicity: from amino acid sequence via 3-D structure to allergenicity. Mol Nutr Food Res. 2006;50(7):625–7. doi: 10.1002/mnfr.200500270. [DOI] [PubMed] [Google Scholar]
- 3.Aalberse RC. Structural biology of allergens. J Allergy Clin Immunol. 2000;106(2):228–38. doi: 10.1067/mai.2000.108434. [DOI] [PubMed] [Google Scholar]
- 4.Astwood JD, Leach JN, Fuchs RL. Stability of food allergens to digestion in vitro. Nat Biotechnol. 1996;14(10):1269–73. doi: 10.1038/nbt1096-1269. [DOI] [PubMed] [Google Scholar]
- 5.Bannon GA. What makes a food protein an allergen? Curr Allergy Asthma Rep. 2004;4(1):43–6. doi: 10.1007/s11882-004-0042-0. [DOI] [PubMed] [Google Scholar]
- 6.Fu T, Abbott UR, Hatzos C. Digestibility of food allergens and nonallergenic proteins in simulated gastric fluid and simulated intestinal fluid-a comparative study. J Agric Food Chem. 2002;50(24):7154–60. doi: 10.1021/jf020599h. [DOI] [PubMed] [Google Scholar]
- 7.Herman RA, Woolhiser MM, Ladics GS, et al. Stability of a set of allergens and non-allergens in simulated gastric fluid. Int J Food Sci Nutr. 2007;58(2):125–41. doi: 10.1080/09637480601149640. [DOI] [PubMed] [Google Scholar]
- 8.Pedrosa C, Felice FG, Trisciuzzi C, et al. Selective neoglycosylation increases the structural stability of vicilin, the 7S storage globulin from pea seeds. Arch Biochem Biophys. 2000;382(2):203–10. doi: 10.1006/abbi.2000.2024. [DOI] [PubMed] [Google Scholar]
- 9.Wormald MR, Dwek RA. Glycoproteins: glycan presentation and protein-fold stability. Structure. 1999;7(7):R155–60. doi: 10.1016/s0969-2126(99)80095-1. [DOI] [PubMed] [Google Scholar]
- 10.Bencúrová M, Hemmer W, Focke-Tejkl M, et al. Specificity of IgG and IgE antibodies against plant and insect glycoprotein glycans determined with artificial glycoforms of human transferrin. Glycobiology. 2004;14(5):457–66. doi: 10.1093/glycob/cwh058. [DOI] [PubMed] [Google Scholar]
- 11.Altmann F. The role of protein glycosylation in allergy. Int Arch Allergy Immunol. 2007;142(2):99–115. doi: 10.1159/000096114. [DOI] [PubMed] [Google Scholar]
- 12.Faveeuw C, Mallevaey T, Paschinger K, et al. Schistosome N-glycans containing core α3-fucose and core β2-xylose epitopes are strong inducers of Th2 responses in mice. Eur J Immunol. 2003;33(5):1271–81. doi: 10.1002/eji.200323717. [DOI] [PubMed] [Google Scholar]
- 13.Wilson IB, Zeleny R, Kolarich D, et al. Analysis of Asn-linked glycans from vegetable foodstuffs: widespread occurrence of Lewis a, core alpha1,3-linked fucose and xylose substitutions. Glycobiology. 2001;11(4):261–74. doi: 10.1093/glycob/11.4.261. [DOI] [PubMed] [Google Scholar]
- 14.Mills EN, Jenkins JA, Alcocer MJC, et al. Structural, biological, and evolutionary relationships of plant food allergens sensitizing via the gastrointestinal tract. Crit Rev Food Sci Nutr. 2004;44(5):379–407. doi: 10.1080/10408690490489224. [DOI] [PubMed] [Google Scholar]
- 15.Considine T, Patel HA, Singh H, et al. Influence of binding of sodium dodecyl sulfate, all-trans-retinol, palmitate, and 8-anilino-1-naphthalenesulfonate on the heat-induced unfolding and aggregation of beta-lactoglobulin B. J Agric Food Chem. 2005;53(8):3197–205. doi: 10.1021/jf0481756. [DOI] [PubMed] [Google Scholar]
- 16.Roth-Walter F, Berin MC, Arnaboldi P, et al. Pasteurization of milk proteins promotes allergic sensitization by enhancing uptake through Peyer’s patches. Allergy. 2008;63(7):882–90. doi: 10.1111/j.1398-9995.2008.01673.x. [DOI] [PubMed] [Google Scholar]
- 17.Breiteneder H, Mills EN. Molecular properties of food allergens. J Allergy Clin Immunol. 2005;115(1):14–23. doi: 10.1016/j.jaci.2004.10.022. quiz: 24. [DOI] [PubMed] [Google Scholar]
- 18.Barrett NA, Rahman OM, Fernandez JM, et al. Dectin-2 mediates Th2 immunity through the generation of cysteinyl leukotrienes. J Exp Med. 2011;208(3):593–604. doi: 10.1084/jem.20100793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Royer P, Emara M, Yang C, et al. The mannose receptor mediates the uptake of diverse native allergens by dendritic cells and determines allergen-induced T cell polarization through modulation of IDO activity. J Immunol. 2010;185(3):1522–31. doi: 10.4049/jimmunol.1000774. [DOI] [PubMed] [Google Scholar]
- 20.Zhou Y, Kawasaki H, Hsu S, et al. Oral tolerance to food-induced systemic anaphylaxis mediated by the C-type lectin SIGNR1. Nat Med. 2010;16(10):1128–33. doi: 10.1038/nm.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reed CE, Kita H. The role of protease activation of inflammation in allergic respiratory diseases. J Allergy Clin Immunol. 2004;114(5):997–1008. doi: 10.1016/j.jaci.2004.07.060. quiz: 1009. [DOI] [PubMed] [Google Scholar]
- 22.Trompette A, Divanovic S, Visintin A, et al. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature. 2009;457(7229):585–8. doi: 10.1038/nature07548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mills E, Madsen C, Shewry P. Food allergens of plant origin–their molecular and evolutionary relationships. Trends in Food Science and Technology. 2003;(14):145–56. [Google Scholar]
- 24.Jenkins JA, Breiteneder H, Mills EN. Evolutionary distance from human homologs reflects allergenicity of animal food proteins. J Allergy Clin Immunol. 2007;120(6):1399–405. doi: 10.1016/j.jaci.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 25.Hoffmann-Sommergruber K, Mills EN. Food allergen protein families and their structural characteristics and application in component-resolved diagnosis: new data from the EuroPrevall project. Anal Bioanal Chem. 2009;395(1):25–35. doi: 10.1007/s00216-009-2953-z. [DOI] [PubMed] [Google Scholar]
- 26.Dunwell JM, Purvis A, Khuri S. Cupins: the most functionally diverse protein superfamily? Phytochemistry. 2004;65(1):7–17. doi: 10.1016/j.phytochem.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 27.Witke W. The role of profilin complexes in cell motility and other cellular processes. Trends Cell Biol. 2004;14(8):461–9. doi: 10.1016/j.tcb.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Radauer C, Willerroider M, Fuchs H, et al. Cross-reactive and species-specific immunoglobulin E epitopes of plant profilins: an experimental and structurebased analysis. Clin Exp Allergy. 2006;36(7):920–9. doi: 10.1111/j.1365-2222.2006.02521.x. [DOI] [PubMed] [Google Scholar]
- 29.Ballmer-Weber BK, Wangorsch A, Bohle B, et al. Component-resolved in vitro diagnosis in carrot allergy: does the use of recombinant carrot allergens improve the reliability of the diagnostic procedure? Clin Exp Allergy. 2005;35(7):970–8. doi: 10.1111/j.1365-2222.2005.02294.x. [DOI] [PubMed] [Google Scholar]
- 30.Lüttkopf D, Ballmer-Weber BK, Wüthrich B, et al. Celery allergens in patients with positive double-blind placebo-controlled food challenge. J Allergy Clin Immunol. 2000;106(2):390–9. doi: 10.1067/mai.2000.108711. [DOI] [PubMed] [Google Scholar]
- 31.Markovic-Housley Z, Degano M, Lamba D, et al. Crystal structure of a hypoallergenic isoform of the major birch pollen allergen Bet v. 1 and its likely biological function as a plant steroid carrier. J Mol Biol. 2003;325(1):123–33. doi: 10.1016/s0022-2836(02)01197-x. [DOI] [PubMed] [Google Scholar]
- 32.Spitzauer S. Allergy to mammalian proteins: at the borderline between foreign and self? Int Arch Allergy Immunol. 1999;120(4):259–69. doi: 10.1159/000024278. [DOI] [PubMed] [Google Scholar]
- 33.Kanduc D, Lucchese A, Mittelman A. Individuation of monoclonal anti-HPV16 E7 antibody linear peptide epitope by computational biology. Peptides. 2001;22(12):1981–5. doi: 10.1016/s0196-9781(01)00539-3. [DOI] [PubMed] [Google Scholar]
- 34.Ayuso R, Lehrer SB, Tanaka L, et al. IgE antibody response to vertebrate meat proteins including tropomyosin. Ann Allergy Asthma Immunol. 1999;83(5):399–405. doi: 10.1016/S1081-1206(10)62837-2. [DOI] [PubMed] [Google Scholar]
- 35.Wild LG, Lehrer SB. Fish and shellfish allergy. Curr Allergy Asthma Rep. 2005;5(1):74–9. doi: 10.1007/s11882-005-0059-z. [DOI] [PubMed] [Google Scholar]
- 36.Restani P, Beretta B, Fiocchi A, et al. Cross-reactivity between mammalian proteins. Ann Allergy Asthma Immunol. 2002;89(6 Suppl 1):11–5. doi: 10.1016/s1081-1206(10)62116-3. [DOI] [PubMed] [Google Scholar]
- 37.Fiocchi A, Restani P, Riva E, et al. Meat allergy: I–Specific IgE to BSA and OSA in atopic, beef sensitive children. J Am Coll Nutr. 1995;14(3):239–44. doi: 10.1080/07315724.1995.10718502. [DOI] [PubMed] [Google Scholar]
- 38.Werfel SJ, Cooke SK, Sampson HA. Clinical reactivity to beef in children allergic to cow’s milk. J Allergy Clin Immunol. 1997;99(3):293–300. doi: 10.1016/s0091-6749(97)70045-9. [DOI] [PubMed] [Google Scholar]
- 39.Liu AH, Jaramillo R, Sicherer SH, et al. National prevalence and risk factors for food allergy and relationship to asthma: results from the National Health and Nutrition Examination Survey 2005-2006. J Allergy Clin Immunol. 2010;126(4):798–806. e13. doi: 10.1016/j.jaci.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sicherer SH. Epidemiology of food allergy. J Allergy Clin Immunol. 2011;127(3):594–602. doi: 10.1016/j.jaci.2010.11.044. [DOI] [PubMed] [Google Scholar]
- 41.Nicolaou N, Poorafshar M, Murray C, et al. Allergy or tolerance in children sensitized to peanut: prevalence and differentiation using component-resolved diagnostics. J Allergy Clin Immunol. 2010;125(1):191–7. e1–13. doi: 10.1016/j.jaci.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 42.Shreffler WG, Castro RR, Kucuk ZY, et al. The major glycoprotein allergen from Arachis hypogaea, Ara h 1, is a ligand of dendritic cell-specific ICAM-grabbing nonintegrin and acts as a Th2 adjuvant in vitro. J Immunol. 2006;177(6):3677–85. doi: 10.4049/jimmunol.177.6.3677. [DOI] [PubMed] [Google Scholar]
- 43.Ilchmann A, Burgdorf S, Scheurer S, et al. Glycation of a food allergen by the Maillard reaction enhances its T-cell immunogenicity: role of macrophage scavenger receptor class A type I and II. J Allergy Clin Immunol. 2010;125(1):175–83. e1–11. doi: 10.1016/j.jaci.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 44.Hilmenyuk T, Bellinghausen I, Heydenreich B, et al. Effects of glycation of the model food allergen ovalbumin on antigen uptake and presentation by human dendritic cells. Immunology. 2010;129(3):437–45. doi: 10.1111/j.1365-2567.2009.03199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hsu S, Tsai T, Kawasaki H, et al. Antigen coupled with Lewis-x trisaccharides elicits potent immune responses in mice. J Allergy Clin Immunol. 2007;119(6):1522–8. doi: 10.1016/j.jaci.2007.01.034. [DOI] [PubMed] [Google Scholar]
- 46.Khodoun M, Strait R, Orekov T, et al. Peanuts can contribute to anaphylactic shock by activating complement. J Allergy Clin Immunol. 2009;123(2):342–51. doi: 10.1016/j.jaci.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carroll MC. The complement system in regulation of adaptive immunity. Nat Immunol. 2004;5(10):981–6. doi: 10.1038/ni1113. [DOI] [PubMed] [Google Scholar]
- 48.Hawlisch H, Belkaid Y, Baelder R, et al. C5a negatively regulates toll-like receptor 4-induced immune responses. Immunity. 2005;22(4):415–26. doi: 10.1016/j.immuni.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 49.Drouin SM, Corry DB, Hollman TJ, et al. Absence of the complement anaphylatoxin C3a receptor suppresses Th2 effector functions in a murine model of pulmonary allergy. J Immunol. 2002;169(10):5926–33. doi: 10.4049/jimmunol.169.10.5926. [DOI] [PubMed] [Google Scholar]
- 50.Becker W, Brasseur D. Opinion of the Scientific Panel on Dietetic Products, Nutrition and Allergies on a request from the Commission relating to the evaluation of allergenic foods for labeling purposes. The EFSA Journal. 2004;32:1–197. [Google Scholar]
- 51.Zuidmeer L, Goldhahn K, Rona RJ, et al. The prevalence of plant food allergies: a systematic review. J Allergy Clin Immunol. 2008;121(5):1210–8. e4. doi: 10.1016/j.jaci.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 52.Savage JH, Kaeding AJ, Matsui EC, et al. The natural history of soy allergy. J Allergy Clin Immunol. 2010;125(3):683–6. doi: 10.1016/j.jaci.2009.12.994. [DOI] [PubMed] [Google Scholar]
- 53.Kattan JD, Cocco RR, Järvinen KM. Milk and soy allergy. Pediatr Clin North Am. 2011;58(2):407–26. x. doi: 10.1016/j.pcl.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sicherer SH, Sampson HA. Food allergy: recent advances in pathophysiology and treatment. Annu Rev Med. 2009;60:261–77. doi: 10.1146/annurev.med.60.042407.205711. [DOI] [PubMed] [Google Scholar]
- 55.Bruno G, Giampietro PG, Guercio MJD, et al. Soy allergy is not common in atopic children: a multicenter study. Pediatr Allergy Immunol. 1997;8(4):190–3. doi: 10.1111/j.1399-3038.1997.tb00159.x. [DOI] [PubMed] [Google Scholar]
- 56.Mittag D, Vieths S, Vogel L, et al. Soybean allergy in patients allergic to birch pollen: clinical investigation and molecular characterization of allergens. J Allergy Clin Immunol. 2004;113(1):148–54. doi: 10.1016/j.jaci.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 57.Magnolfi CF, Zani G, Lacava L, et al. Soy allergy in atopic children. Ann Allergy Asthma Immunol. 1996;77(3):197–201. doi: 10.1016/S1081-1206(10)63255-3. [DOI] [PubMed] [Google Scholar]
- 58.Bhatia J, Greer F. American Academy of Pediatrics Committee on Nutrition. Use of soy protein-based formulas in infant feeding. Pediatrics. 2008;121(5):1062–8. doi: 10.1542/peds.2008-0564. [DOI] [PubMed] [Google Scholar]
- 59.Klemola T, Vanto T, Juntunen-Backman K, et al. Allergy to soy formula and to extensively hydrolyzed whey formula in infants with cow’s milk allergy: a prospective, randomized study with a follow-up to the age of 2 years. J Pediatr. 2002;140(2):219–24. doi: 10.1067/mpd.2002.121935. [DOI] [PubMed] [Google Scholar]
- 60.Zeiger RS, Sampson HA, Bock SA, et al. Soy allergy in infants and children with IgE-associated cow’s milk allergy. J Pediatr. 1999;134(5):614–22. doi: 10.1016/s0022-3476(99)70249-0. [DOI] [PubMed] [Google Scholar]
- 61.David TJ. Anaphylactic shock during elimination diets for severe atopic eczema. Arch Dis Child. 1984;59(10):983–6. doi: 10.1136/adc.59.10.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Awazuhara H, Kawai H, Maruchi N. Major allergens in soybean and clinical significance of IgG4 antibodies investigated by IgE- and IgG4-immunoblotting with sera from soybean-sensitive patients. Clin Exp Allergy. 1997;27(3):325–32. [PubMed] [Google Scholar]
- 63.Shibasaki M, Suzuki S, Tajima S, et al. Allergenicity of major component proteins of soybean. Int Arch Allergy Appl Immunol. 1980;61(4):441–8. doi: 10.1159/000232472. [DOI] [PubMed] [Google Scholar]
- 64.Holzhauser T, Wackermann O, Ballmer-Weber BK, et al. Soybean (glycine max) allergy in Europe: Gly m 5 (beta-conglycinin) and Gly m 6 (glycinin) are potential diagnostic markers for severe allergic reactions to soy. J Allergy Clin Immunol. 2009;123(2):452–8. doi: 10.1016/j.jaci.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 65.L’Hocine L, Boye JI. Allergenicity of soybean: new developments in identification of allergenic proteins, cross-reactivities and hypoallergenization technologies. Crit Rev Food Sci Nutr. 2007;47(2):127–43. doi: 10.1080/10408390600626487. [DOI] [PubMed] [Google Scholar]
- 66.González R, Polo F, Zapatero L, et al. Purification and characterization of major inhalant allergens from soybean hulls. Clin Exp Allergy. 1992;22(8):748–55. doi: 10.1111/j.1365-2222.1992.tb02814.x. [DOI] [PubMed] [Google Scholar]
- 67.Kalinski A, Weisemann JM, Matthews BF, et al. Molecular cloning of a protein associated with soybean seed oil bodies that is similar to thiol proteases of the papain family. J Biol Chem. 1990;265(23):13843–8. [PubMed] [Google Scholar]
- 68.Kalinski A, Melroy DL, Dwivedi RS, et al. A soybean vacuolar protein (P34) related to thiol proteases is synthesized as a glycoprotein precursor during seed maturation. J Biol Chem. 1992;267(17):12068–76. [PubMed] [Google Scholar]
- 69.Rihs HP, Chen Z, Ruëff F, et al. IgE binding of the recombinant allergen soybean profilin (rGly m 3) is mediated by conformational epitopes. J Allergy Clin Immunol. 1999;104(6):1293–301. doi: 10.1016/s0091-6749(99)70027-8. [DOI] [PubMed] [Google Scholar]
- 70.van Zuuren EJ, Terreehorst I, Tupker RA, et al. Anaphylaxis after consuming soy products in patients with birch pollinosis. Allergy. 2010;65(10):1348–9. doi: 10.1111/j.1398-9995.2010.02357.x. [DOI] [PubMed] [Google Scholar]
- 71.Kleine-Tebbe J, Vogel L, Crowell DN, et al. Severe oral allergy syndrome and anaphylactic reactions caused by a Bet v. 1- related PR-10 protein in soybean, SAM22. J Allergy Clin Immunol. 2002;110(5):797–804. doi: 10.1067/mai.2002.128946. [DOI] [PubMed] [Google Scholar]
- 72.Vissers YM, Jansen AP, Ruinemans-Koerts J, et al. IgE component-resolved allergen profile and clinical symptoms in soy and peanut allergic patients. Allergy. 2011;66(8):1125–7. doi: 10.1111/j.1398-9995.2011.02575.x. [DOI] [PubMed] [Google Scholar]
- 73.Breiteneder H, Mills EN. Plant food allergens-structural and functional aspects of allergenicity. Biotechnol Adv. 2005;23(6):395–9. doi: 10.1016/j.biotechadv.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 74.Burks AW, Williams LW, Helm RM, et al. Identification of soy protein allergens in patients with atopic dermatitis and positive soy challenges; determination of change in allergenicity after heating or enzyme digestion. Adv Exp Med Biol. 1991;289:295–307. doi: 10.1007/978-1-4899-2626-5_22. [DOI] [PubMed] [Google Scholar]
- 75.Burks AW, Cockrell G, Connaughton C, et al. Identification of peanut agglutinin and soybean trypsin inhibitor as minor legume allergens. Int Arch Allergy Immunol. 1994;105(2):143–9. doi: 10.1159/000236816. [DOI] [PubMed] [Google Scholar]
- 76.Ogawa T, Bando N, Tsuji H, et al. Alpha-subunit of beta-conglycinin, an allergenic protein recognized by IgE antibodies of soybean-sensitive patients with atopic dermatitis. Biosci Biotechnol Biochem. 1995;59(5):831–3. doi: 10.1271/bbb.59.831. [DOI] [PubMed] [Google Scholar]
- 77.Beardslee TA, Zeece MG, Sarath G, et al. Soybean glycinin G1 acidic chain shares IgE epitopes with peanut allergen Ara h 3. Int Arch Allergy Immunol. 2000;123(4):299–307. doi: 10.1159/000053642. [DOI] [PubMed] [Google Scholar]
- 78.L’Hocine L, Boye JI, Jouve S. Ionic strength and pH-induced changes in the immunoreactivity of purified soybean glycinin and its relation to protein molec-ular structure. J Agric Food Chem. 2007;55(14):5819–26. doi: 10.1021/jf070281v. [DOI] [PubMed] [Google Scholar]
- 79.Piacentini GL, Benedetti M, Spezia E, et al. Anaphylactic sensitizing power of selected infant formulas. Ann Allergy. 1991;67(4):400–2. [PubMed] [Google Scholar]
- 80.Osterballe M, Mortz CG, Hansen TK, et al. The prevalence of food hypersensitivity in young adults. Pediatr Allergy Immunol. 2009;20(7):686–92. doi: 10.1111/j.1399-3038.2008.00842.x. [DOI] [PubMed] [Google Scholar]
- 81.Rozenfeld P, Docena GH, Añón MC, et al. Detection and identification of a soy protein component that cross-reacts with caseins from cow’s milk. Clin Exp Immunol. 2002;130(1):49–58. doi: 10.1046/j.1365-2249.2002.t01-1-01935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chruszcz M, Maleki SJ, Majorek KA, et al. Structural and immunologic characterization of Ara h 1, a major peanut allergen. J Biol Chem. 2011;286(45):39318–27. doi: 10.1074/jbc.M111.270132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sicherer SH, Sampson HA, Burks AW. Peanut and soy allergy: a clinical and therapeutic dilemma. Allergy. 2000;55(6):515–21. doi: 10.1034/j.1398-9995.2000.00119.x. [DOI] [PubMed] [Google Scholar]
- 84.Foucard T, Yman IM. A study on severe food reactions in Sweden–is soy protein an underestimated cause of food anaphylaxis? Allergy. 1999;54(3):261–5. doi: 10.1034/j.1398-9995.1999.00924.x. [DOI] [PubMed] [Google Scholar]
- 85.Yunginger JW, Nelson DR, Squillace DL, et al. Laboratory investigation of deaths due to anaphylaxis. J Forensic Sci. 1991;36(3):857–65. [PubMed] [Google Scholar]
- 86.Ballmer-Weber BK, Holzhauser T, Scibilia J, et al. Clinical characteristics of soybean allergy in Europe: a double-blind, placebo-controlled food challenge study. J Allergy Clin Immunol. 2007;119(6):1489–96. doi: 10.1016/j.jaci.2007.01.049. [DOI] [PubMed] [Google Scholar]
- 87.Pons L, Ponnappan U, Hall RA, et al. Soy immunotherapy for peanut-allergic mice: modulation of the peanut-allergic response. J Allergy Clin Immunol. 2004;114(4):915–21. doi: 10.1016/j.jaci.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 88.Bindslev-Jensen C, Briggs D, Osterballe M. Can we determine a threshold level for allergenic foods by statistical analysis of published data in the literature? Allergy. 2002;57(8):741–6. doi: 10.1034/j.1398-9995.2002.23797.x. [DOI] [PubMed] [Google Scholar]
- 89.Cordle CT. Soy protein allergy: incidence and relative severity. J Nutr. 2004;134(5):1213S–9S. doi: 10.1093/jn/134.5.1213S. [DOI] [PubMed] [Google Scholar]
- 90.Sicherer SH, Morrow EH, Sampson HA. Dose-response in double-blind, placebo-controlled oral food challenges in children with atopic dermatitis. J Allergy Clin Immunol. 2000;105(3):582–6. doi: 10.1067/mai.2000.104941. [DOI] [PubMed] [Google Scholar]
- 91.Amigo-Benavent M, Athanasopoulos V. Carbohydrate moieties on the in vitro immunoreactivity of soy [beta]-conglycinin. Food Research International. 2009;42(7):819–25. [Google Scholar]
- 92.Bando N, Tsuji H, Yamanishi R, et al. Identification of the glycosylation site of a major soybean allergen, Gly m Bd 30K. Biosci Biotechnol Biochem. 1996;60(2):347–8. doi: 10.1271/bbb.60.347. [DOI] [PubMed] [Google Scholar]
- 93.Kroghsbo S, Bøgh KL, Rigby NM, et al. Sensitization with 7S globulins from peanut, hazelnut, soy or pea induces IgE with different biological activities which are modified by soy tolerance. Int Arch Allergy Immunol. 2011;155(3):212–24. doi: 10.1159/000321200. [DOI] [PubMed] [Google Scholar]
- 94.Müller U, Weber W, Hoffmann A, et al. Commercial soybean lecithins: a source of hidden allergens? Zschr für Lebensmittel-Untersuchung und –Forschung A. 1998;207:341–51. [Google Scholar]
- 95.Tsumura K, Kugimiya W, Bando N. Preparation of hypoallergenic soybean protein with processing functionality by selective enzymatic hydrolysis. Food Sci Tech Res. 1999;5(2):171–5. [Google Scholar]
- 96.Tsuji H, Okada N, Yamanishi R. Fate of a major soybean allergen, Gly m Bd 30K, in rice-, barley-and soybean-koji miso (fermented soybean paste) during fermentation. Food Sci Tech Res. 1997;3(2):145–9. [Google Scholar]
- 97.Kang J, Badger TM, Ronis MJ, et al. Non-isoflavone phytochemicals in soy and their health effects. J Agric Food Chem. 2010;58(14):8119–33. doi: 10.1021/jf100901b. [DOI] [PubMed] [Google Scholar]
- 98.Masilamani M, Wei J, Bhatt S, et al. Soybean isoflavones regulate dendritic cell function and suppress allergic sensitization to peanut. J Allergy Clin Immunol. 2011;128(6):1242.e1–50.e1. doi: 10.1016/j.jaci.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 99.Messina M. A brief historical overview of the past two decades of soy and isoflavone research. J Nutr. 2010;140(7):1350S–4S. doi: 10.3945/jn.109.118315. [DOI] [PubMed] [Google Scholar]
- 100.Kay AB. Allergy and allergic diseases. Second of two parts. N Engl J Med. 2001;344(2):109–13. doi: 10.1056/NEJM200101113440206. [DOI] [PubMed] [Google Scholar]
- 101.Kay AB. Allergy and allergic diseases. First of two parts. N Engl J Med. 2001;344(1):30–7. doi: 10.1056/NEJM200101043440106. [DOI] [PubMed] [Google Scholar]
- 102.Sampson HA. Food allergy. Part 2: diagnosis and management. J Allergy Clin Immunol. 1999;103(6):981–9. doi: 10.1016/s0091-6749(99)70167-3. [DOI] [PubMed] [Google Scholar]
- 103.Sampson HA. Food allergy. Part 1: immunopathogenesis and clinical disorders. J Allergy Clin Immunol. 1999;103(5 Pt 1):717–28. doi: 10.1016/s0091-6749(99)70411-2. [DOI] [PubMed] [Google Scholar]
- 104.Aalberse RC, Koshte V, Clemens JG. Immunoglobulin E antibodies that cross-react with vegetable foods, pollen, and Hymenoptera venom. J Allergy Clin Immunol. 1981;68(5):356–64. doi: 10.1016/0091-6749(81)90133-0. [DOI] [PubMed] [Google Scholar]
- 105.Kochuyt A, Hoeyveld EM, Stevens EA. Prevalence and clinical relevance of specific immunoglobulin E to pollen caused by sting- induced specific immuno-globulin E to cross-reacting carbohydrate determinants in Hymenoptera venoms. Clin Exp Allergy. 2005;35(4):441–7. doi: 10.1111/j.1365-2222.2005.02217.x. [DOI] [PubMed] [Google Scholar]
- 106.van der Veen MJ, van Ree R, Aalberse RC, et al. Poor biologic activity of cross-reactive IgE directed to carbohydrate determinants of glycoproteins. J Allergy Clin Immunol. 1997;100(3):327–34. doi: 10.1016/s0091-6749(97)70245-8. [DOI] [PubMed] [Google Scholar]
- 107.Ishihara H, Takahashi N, Oguri S, et al. Complete structure of the carbohydrate moiety of stem bromelain. An application of the almond glycopeptidase for structural studies of glycopeptides. J Biol Chem. 1979;254(21):10715–9. [PubMed] [Google Scholar]
- 108.Bouwstra JB, Spoelstra EC, Waard PD, et al. Conformational studies on the N-linked carbohydrate chain of bromelain. Eur J Biochem. 1990;190(1):113–22. doi: 10.1111/j.1432-1033.1990.tb15553.x. [DOI] [PubMed] [Google Scholar]
- 109.Wilson IB, Altmann F. Structural analysis of N-glycans from allergenic grass, ragweed and tree pollens: core alpha1,3-linked fucose and xylose present in all pollens examined. Glycoconj J. 1998;15(11):1055–70. doi: 10.1023/a:1006960401562. [DOI] [PubMed] [Google Scholar]
- 110.Weber A, Schröder H, Thalberg K, et al. Specific interaction of IgE antibodies with a carbohydrate epitope of honey bee venom phospholipase A2. Allergy. 1987;42(6):464–70. doi: 10.1111/j.1398-9995.1987.tb00364.x. [DOI] [PubMed] [Google Scholar]
- 111.Tretter V, Altmann F, Kubelka V, et al. Fucose alpha 1,3-linked to the core region of glycoprotein N-glycans creates an important epitope for IgE from honeybee venom allergic individuals. Int Arch Allergy Immunol. 1993;102(3):259–66. doi: 10.1159/000236534. [DOI] [PubMed] [Google Scholar]
- 112.Aalberse RC, van Ree R. Cross-reactive carbohydrate determinants. Clin Rev Allergy Immunol. 1997;15(4):375–87. doi: 10.1007/BF02737733. [DOI] [PubMed] [Google Scholar]
- 113.Mari A. IgE to cross-reactive carbohydrate determinants: analysis of the distribution and appraisal of the in vivo and in vitro reactivity. Int Arch Allergy Immunol. 2002;129(4):286–95. doi: 10.1159/000067591. [DOI] [PubMed] [Google Scholar]
- 114.Commins SP, Platts-Mills TA. Anaphylaxis syndromes related to a new mammalian cross-reactive carbohydrate determinant. J Allergy Clin Immunol. 2009;124(4):652–7. doi: 10.1016/j.jaci.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Foetisch K, Westphal S, Lauer I, et al. Biological activity of IgE specific for cross-reactive carbohydrate determinants. J Allergy Clin Immunol. 2003;111(4):889–96. doi: 10.1067/mai.2003.173. [DOI] [PubMed] [Google Scholar]
- 116.Fötisch K, Altmann F, Haustein D, et al. Involvement of carbohydrate epitopes in the IgE response of celery-allergic patients. Int Arch Allergy Immunol. 1999;120(1):30–42. doi: 10.1159/000024217. [DOI] [PubMed] [Google Scholar]
- 117.Iacovacci P, Afferni C, Butteroni C, et al. Comparison between the native glycosylated and the recombinant Cup a1 allergen: role of carbohydrates in the histamine release from basophils. Clin Exp Allergy. 2002;32(11):1620–7. doi: 10.1046/j.1365-2222.2002.01516.x. [DOI] [PubMed] [Google Scholar]
- 118.van Ree R, Aalberse RC. Specific IgE without clinical allergy. J Allergy Clin Immunol. 1999;103(6):1000–1. doi: 10.1016/s0091-6749(99)70169-7. [DOI] [PubMed] [Google Scholar]
- 119.Lommerse JP, Kroon-Batenburg LM, Kamerling JP, et al. Conformational analysis of the xylose-containing N-glycan of pineapple stem bromelain as part of the intact glycoprotein. Biochemistry. 1995;34(25):8196–206. doi: 10.1021/bi00025a027. [DOI] [PubMed] [Google Scholar]
- 120.Galili U. The alpha-gal epitope and the anti-Gal antibody in xenotransplantation and in cancer immunotherapy. Immunol Cell Biol. 2005;83(6):674–86. doi: 10.1111/j.1440-1711.2005.01366.x. [DOI] [PubMed] [Google Scholar]
- 121.Jin C, Hantusch B, Hemmer W, et al. Affinity of IgE and IgG against cross-reactive carbohydrate determinants on plant and insect glycoproteins. J Allergy Clin Immunol. 2008;121(1):185–90. e2. doi: 10.1016/j.jaci.2007.07.047. [DOI] [PubMed] [Google Scholar]
- 122.Chung CH, Mirakhur B, Chan E, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med. 2008;358(11):1109–17. doi: 10.1056/NEJMoa074943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Commins SP, Satinover SM, Hosen J, et al. Delayed anaphylaxis, angioedema, or urticaria after consumption of red meat in patients with IgE antibodies specific for galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2009;123(2):426–33. doi: 10.1016/j.jaci.2008.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Macher BA, Galili U. The Galalpha1,3Galbeta1,4GlcNAc-R (alpha-Gal) epitope: a carbohydrate of unique evolution and clinical relevance. Biochim Biophys Acta. 2008;1780(2):75–88. doi: 10.1016/j.bbagen.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Spiro RG, Bhoyroo VD. Occurrence of alpha-D-galactosyl residues in the thyroglobulins from several species. Localization in the saccharide chains of the complex carbohydrate units. J Biol Chem. 1984;259(15):9858–66. [PubMed] [Google Scholar]
- 126.Paschinger K, Fabini G, Schuster D, et al. Definition of immunogenic carbohydrate epitopes. Acta Biochim Pol. 2005;52(3):629–32. [PubMed] [Google Scholar]
- 127.Galili U, Mandrell RE, Hamadeh RM, et al. Interaction between human natural anti-alpha-galactosyl immunoglobulin G and bacteria of the human flora. Infect Immun. 1988;56(7):1730–7. doi: 10.1128/iai.56.7.1730-1737.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Springer GF, Horton RE. Blood group isoantibody stimulation in man by feeding blood group-active bacteria. J Clin Invest. 1969;48(7):1280–91. doi: 10.1172/JCI106094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Koike C, Fung JJ, Geller DA, et al. Molecular basis of evolutionary loss of the alpha 1,3-galactosyltransferase gene in higher primates. J Biol Chem. 2002;277(12):10114–20. doi: 10.1074/jbc.M110527200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Larsen RD, Rivera-Marrero CA, Ernst LK, et al. Frameshift and nonsense mutations in a human genomic sequence homologous to a murine UDP-Gal:beta-D-Gal(1,4)-D-GlcNAc alpha(1,3)-galactosyltransferase cDNA. J Biol Chem. 1990;265(12):7055–61. [PubMed] [Google Scholar]
- 131.Adédoyin J, Johansson SG, Grönlund H, et al. Interference in immunoassays by human IgM with specificity for the carbohydrate moiety of animal proteins. J Immunol Methods. 2006;310(1-2):117–25. doi: 10.1016/j.jim.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 132.Grönlund H, Adédoyin J, Commins SP, et al. The carbohydrate galactose-alpha-1,3-galactose is a major IgE-binding epitope on cat IgA. J Allergy Clin Immunol. 2009;123(5):1189–91. doi: 10.1016/j.jaci.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wong KN, Wang X, Lee W, et al. Deglycosylation of meat extracts reduced the binding of cross-reactive antibodies to meat; XXV Congress of the European Academy of Allergology and Clinical Immunology; Vienna (Austria). June 10-14, 2006. [Google Scholar]
- 134.Commins SP, James HR, Kelly LA, et al. The relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide galactose-α-1,3-galactose. J Allergy Clin Immunol. 2011;127(5):1286–93. e6. doi: 10.1016/j.jaci.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]