Abstract
Proton pump inhibitors (PPIs) are the most potent gastric acid suppressing drugs available, and their use is widespread. An emerging concern about chronic PPI therapy is whether these drugs impair intestinal calcium absorption, resulting in a negative calcium balance and thereby potentially causing bone loss. The objective of this study was to evaluate the acute effect of the PPI esomeprazole or placebo on intestinal calcium absorption in healthy adults. Twelve young adults participated in a placebo-controlled, double-blind, crossover study. There were two 3-week interventions that included a 14-day adjustment period (designed to stabilize calcium homeostasis) followed by 6 days of a diet containing 800 mg of calcium and 2.1 g/kg of protein (intervention). During the last 3 days of the adjustment period and throughout the intervention period, subjects consumed esomeprazole or placebo. Half the subjects underwent 24-hour continuous gastric acid pH monitoring. Intestinal calcium absorption was measured using dual-stable calcium isotopes at the end of each intervention. Treatment with esomprazole significantly increased gastric pH (mean pH on PPI 5.38 ± 0.13, mean pH on placebo 2.70 ± 0.44, p =.005). Neither calcium absorption (PPI 34.2% ± 2.4%, placebo 31.5% ± 2.1%, p =.24) nor urinary calcium (PPI 321 ± 38 mg/34 hours, placebo 355 ± 37 mg/34 hours, p =.07) differed between the PPI and placebo groups. It is concluded that short-term gastric acid suppression by PPIs does not attenuate intestinal calcium absorption in healthy young adults.
Keywords: PROTON PUMP INHIBITORS, GASTRIC pH, INTESTINAL CALCIUM ABSORPTION, URINE CALCIUM
Introduction
Proton pump inhibitors (PPIs) are the most potent acid-suppressing drugs available, and millions of individuals worldwide use these medications to treat common disorders such as gastroesophageal reflux disease and peptic ulcers.(1,2) PPIs specifically target the H+,K+-ATPase pump of the gastric parietal cell, thereby inhibiting the final step in gastric acid production. An emerging concern about chronic PPI therapy is that it may inhibit intestinal calcium absorption. It has been suggested that stomach acid is required to free ingested calcium from the food matrix, thereby making it more bioavailable for absorption in the intestine. If true, then the millions of individuals using PPIs may be at increased risk for calcium malabsorption, negative calcium balance, bone loss, and increased fractures. Concerns about the effects of PPIs on calcium homeostasis have been heightened by two retrospective case-controlled studies that reported an increased risk of fracture in patients with long term PPI use.(1,3) A recent prospective study in 1211 postmenopausal women found that omeprazole use was associated with an increased risk of vertebral fractures.(4) Another study, however, failed to find such an association.(5)
Studies exploring the role of gastric acid in facilitating intestinal calcium absorption are inconclusive. Recker and colleagues reported more than 20 years ago that absorption of calcium (from calcium carbonate) was impaired in fasting achlorhydric patients compared with fasting healthy adults.(6) However, when the calcium carbonate was administered to achlorhydric patients in the fed state, calcium absorption was normal.(6) O’Connell and colleagues demonstrated reduced fractional absorption of radioactive labeled calcium carbonate (45Ca) in fasting subjects taking PPIs.(7) In contrast, two studies in which calcium absorption was measured using the intestinal lavage technique found no effect of PPIs on absorption.(8,9)
The purpose of our study was to determine if inhibiting gastric acid production with a commonly prescribed PPI drug (esomeprazole) would affect calcium absorption. The study employed a crossover design in which each study subject served as his or her own control, thereby eliminating the confounding effect of interindividual variation on calcium absorption. We chose to use a high-protein diet for all interventions in our study because available data indicate that this would augment gastric acid production and intestinal calcium absorption,(10–13) allowing us the best opportunity to quantify the contribution of gastric acid to calcium absorption.
Subjects and Methods
Study overview
We conducted a randomized, placebo-controlled, double-blind crossover study in 12 healthy young adults (6 women and 6 men). The protocol consisted of two cycles that included a 2-week adjustment period (designated as day –13 to day 0) followed by 6 days of a high-protein diet (the experimental period, designated days 1 through 6), during which study subjects took either 20 mg of esomeprazole (Astra Zeneca, Wilmington, DE, USA) or a placebo twice daily. During the adjustment period, the subjects modified their diets to contain moderate amounts of protein, sodium, calcium, and caffeine. The 2-week adjustment period and 6-day experimental period then were repeated once more so that all subjects received both esomeprazole and placebo in random order. During the last 6 days of the adjustment period and the entire experimental period, all food was prepared for subjects by the metabolic kitchen at Yale and was formulated to contain identical amounts of protein, calcium, phosphorus, and sodium. Fasting blood samples and timed urine collections were taken at the start and end of the experimental period. During the last 1.5 days of the experimental period, all subjects underwent calcium absorption measurements using dual-stable isotopes. Six of the 12 subjects underwent continuous gastric pH monitoring from the morning of day 5 to the morning of day 6 (to coincide with the measurement of intestinal calcium absorption).
Subjects
All subjects were between 18 and 45 years of age. The mean age of the men was 29.2 ± 7.9 years, and their average weight and body mass index (BMI) were 75.8 ± 7.9 kg and 24.9 ± 1.7 kg/m2, respectively. The mean age of the women was 32.5 ± 8.7 years, and their average weight and BMI were 59.5 ± 5.4 kg and 22.3 ± 1.2 kg/m2, respectively. Exclusion criteria included medications known to affect calcium metabolism (including bisphosphonates, estrogen, oral contraceptives, calcitonin, parathyroid hormone, anabolic steroids, testosterone, growth hormone, and diuretics), amenorrhea, pregnancy, smoking, eating disorders, diabetes, renal or gastrointestinal disease, bone disease, nephrolithiasis, or intensive daily physical exercise. Subjects stopped consuming all vitamin and mineral supplements during the entire study. The racial background of the subjects was either white or Asian. Subjects continued their usual activities at home, school, and work during the study. The study was approved by the Human Investigation Committee at Yale University. Informed consent was obtained from each participant.
Adjustment and experimental periods
During the first 8 days of the adjustment period, subjects were instructed by the research dietitian to consume 1.2 g of protein per kilogram, 800 mg (20 mmol) of calcium, 2300 mg (100 mmol) of sodium, and adequate energy for weight maintenance. During the last 6 days of the adjustment period (days −5 to 0), the diet was provided by the metabolic kitchen to more completely control the intake of calcium, sodium, and protein prior to absorption testing. Between days −5 to +6, subjects reported to the metabolic kitchen to receive their meals and record their body weight. Subjects began taking esomeprazole or placebo 3 days prior to the experimental diet (day −2), and were instructed to take 20 mg 1 hour before breakfast and 20 mg 1 hour before dinner throughout the remainder of the experimental period (days −2 through +6).
All experimental diets (days 1 through 6) were individually calculated to contain 2.1 g of protein per kilogram, 800 mg (20 mmol) of calcium, 2300 mg (100 mmol) of sodium, and 1240 mg (40 mmol) of phosphorus. Energy intake was adjusted with simple sugars and fats to maintain body weight. Subjects were instructed to consume all the food and beverages provided, and no other food was allowed. Distilled water was given ad libitum. Caffeine-containing beverages were limited to one serving per day, and alcohol was not permitted throughout the entire study. The macronutrient and mineral composition of the experimental diets was calculated from the U.S. Department of Agriculture Handbook No. 8 and manufacturers’ information. The primary sources of calcium in the experimental diets were dairy foods. However, if a subject preferred not to consume dairy, we provided him or her with a chewable calcium carbonate supplement (eg, Tums, GlaxoSmithKline, Pittsburgh, PA, USA). The increase in protein intake from the adjustment to the experimental diet was accomplished by adding both animal and vegetable sources of protein to the diet. The adjustment and experimental diets (including all foods and Tums supplements) remained identical within a subject between the two interventions. Pill counts were done at the end of each intervention to assess adherence to the study protocol.
Sample collection and analyses
Fasting blood samples were obtained on days −2 and +5 during each cycle of the study for measures of parathyroid hormone (PTH), total and ionized calcium, 1,25-dihydroxyvitamin D, and creatinine. A timed 24-hour urine collection was done on day −3 for measurements of calcium, sodium, and creatinine. Stomach pH was recorded for 24 hours using a nasogastric pH probe (GERD Check pH Monitoring System, Sandhill Scientific, Highlands Ranch, CO, USA) on day 5 of each cycle in half the subjects (3 women and 3 men). Subjects were chosen for the gastric pH monitoring based on their willingness to participate in this phase of the study; however, there is no reason to suspect that the findings in these individuals would differ from those in the entire group. The position of the pH probe was confirmed radiographically each time it was placed.
On day 5 of the experimental diet, oral 44Ca (0.25 mg/kg total) was administered in three divided doses and delivered with each meal in proportion to the calcium content of the meal, thus correcting for potential differences in calcium bioavailability between meals. Each oral calcium isotope was equilibrated in milk for 8 to 24 hours prior to administration. Immediately after breakfast, the subjects received an intravenous infusion of 0.022 mg/kg of 42Ca over a 5-minute interval. The intravenous line then was flushed with saline to ensure that the entire isotope dose was delivered. All urine passed for the next 34 hours was collected in acid-washed containers in pools of 8, 12, and 14 hours. The three urine collections were combined, and we report data for these 34-hour urine collections at the end of each experimental period.
Calcium isotope ratios were measured using a Thermoquest magnetic sector thermal ionization mass spectrometer (Triton TI, Bremen, Germany). A ratio was created between each administered calcium isotope (42Ca and 44Ca) and another naturally occurring calcium isotope (48Ca). All isotopes were corrected for isotopic fractionation by normalizing the data to the 43:48Ca ratio. Fractional calcium absorption was determined as the ratio of the cumulative oral tracer recovery to the cumulative intravenous tracer recovery in the 34-hour urine collection obtained after dosing. Relative standard deviations for the isotopes used typically are under 0.3%.
Statistical analysis
The baseline data are presented as mean ± SD, and the day 5 data are presented as mean ± SEM. A Student’s t test was used initially to evaluate sex differences. Since no significant differences in calcium homeostasis were observed, the two groups were pooled for all subsequent analyses. Paired t tests were used to evaluate differences between the two treatments at baseline, at the end of the intervention, and the change within the intervention. All statistical analyses were performed using SPSS Version 12.0 (SPSS Science, Chicago, IL, USA). A probability of p <.05 was considered significant.
Sample size was estimated in two ways. O’Connell(7) observed a mean difference of 6.6% ± 5.5% in intestinal calcium absorption when study subjects were treated with omeprazole 20 mg/day for 7 days. Based on that effect size, a sample size of 12 would provide more than 90% power with α =0.05. We also estimated sample size based on the assumption that the observed 7.7% ± 3.5% difference in mean calcium absorption in normal subjects ingesting a diet containing 1.0 g/kg of protein versus 2.1 g/kg is due to an attendant increase in gastric acid production.(13) Using an effect size of 7.7% ± 3.5% (mean ± SD), a sample size of 12 provides power of 90% at α =0.05. We therefore chose a sample size of 12.
Results
All subjects tolerated the study without difficulty. Body weight remained constant throughout for all subjects. The nutrient contents of the experimental diets during the placebo and the esomeprazole interventions were identical, with average values as follows:140.0 ± 18.4 g of protein, 800 ± 4 mg (20.0 ± 0.1 mmol) of calcium, 1190 ± 25 mg (38.4 ± 0.8 mmol) of phosphorus, 2208 ± 299 mg (96.0 ± 13.0 mmol) of sodium, 272 ± 36 mg (11.2 ± 1.5 mmol) of magnesium, 2629 ± 460 mg (67.4 ± 11.8 mmol) of potassium, and 14.3 ± 4.7 g of fiber. Pill counts indicated more than 90% overall compliance. There was no difference in compliance rates between the placebo and PPI interventions.
As expected, male subjects had higher glomerular filtration rates (GFRs) than did female subjects. Baseline GFRs before esomeprazole treatment were 142.2 ± 12.6 and 95.5 ± 30.4 mL/ min in men and women, respectively ( p <.01). Baseline GFRs before placebo treatment were 136.5 ± 23.1 and 98.2 ± 13.1 mL/ min in men and women, respectively ( p <.01). Baseline measures of serum total and ionized calcium, midmolecule PTH, 1,25-dihydroxyvitamin D, 24-hour urine sodium, calcium, GFR, calcium per kilogram of body weight, and calcium-creatinine ratio were not different at baseline, and all values were within normal range (Table 1). Table 2 and Fig. 2 present the day 5 data. Similar to the findings at baseline, there were no differences on day 5 between the PPI treatment group and the placebo treatment group in serum total and ionized calcium, midmolecule PTH, or 1,25-dihydroxyvitamin D. There also were no significant differences in the change from baseline to day 5 between PPI and placebo treatments in serum total and ionized calcium, midmolecule PTH, and 1,25-dihydroxyvitamin D (data not shown).
Table 1.
Average Baseline Values of the Calcium-Related Metabolitesa
| Placebo | PPI | Differenceb | p Valuec | |
|---|---|---|---|---|
| Serum minerals (mmol/L) | ||||
| Calcium | 2.41 ± 0.06 | 2.38 ± 0.07 | −0.03 ± 0.07 | .19 |
| Ionized calcium | 1.27 ± 0.03 | 1.25 ± 0.03 | −0.02 ± 0.05 | .20 |
| Serum hormones (pmol/L) | ||||
| Midmolecule PTH | 35.1 ± 8.5 | 35.6 ± 10.6 | 0.6 ± 7.0 | .79 |
| 1,25(OH)2D3 | 142.0 ± 34.8 | 136.6 ± 28.0 | −5.4 ± 19.3 | .35 |
| 24-hour urine (mmol) | ||||
| Sodium | 83.3 ± 36.8 | 79.3 ± 29.8 | −4.4 ± 47.8 | .76 |
| Calcium | 3.88 ± 1.88 | 4.16 ± 2.34 | 0.28 ± 1.46 | .53 |
| GFR (mL/min) | 117.3 ± 26.8 | 118.8 ± 33.0 | 1.5 ± 23.2 | .83 |
| Ca/kgd | 2.15 ± 1.20 | 2.26 ± 1.45 | 0.11 ± 1.13 | .75 |
| Calcium/creatininee | 0.17 ± 0.26 | 0.11 ± 0.04 | −0.07 ± 0.26 | .39 |
All variables are presented as mean ± SD.
Mean change between placebo and PPI as mean ± SD.
Two-tailed paired t test between placebo and PPI baseline values.
Ca/kg =urinary calcium (mg/day)/body weight (kg).
Calcium:creatinine =urinary calcium (mg/day)/urinary creatinine (mg/day).
Table 2.
Effect of PPI and Placebo Treatment on Day 5 of the Experimental Perioda
| Placebo | PPI | Differenceb | p Valuec | |
|---|---|---|---|---|
| Serum minerals (mmol/L) | ||||
| Calcium | 2.32 ± 0.03 | 2.31 ± 0.02 | −0.01 ± 0.02 | .69 |
| Ionized calcium | 1.22 ± 0.01 | 1.22 ± 0.01 | 0.01 ± 0.01 | .32 |
| Serum hormones (pmol/L) | ||||
| Midmolecule PTH | 39.3 ± 2.6 | 38.2 ± 2.6 | −1.1 ± 1.9 | .57 |
| 1,25(OH)2D3 | 147.2 ± 10.5 | 159.2 ± 12.5 | 12.0 ± 8.2 | .17 |
| 34-hour urine calcium (mmol) | 8.86 ± 0.92 | 8.00 ± 0.94 | −0.86 ± 0.43 | .07 |
All variables are presented as mean ± SEM.
Mean change between placebo and PPI as mean ± SEM.
Two-tailed paired t test between placebo and PPI treatments.
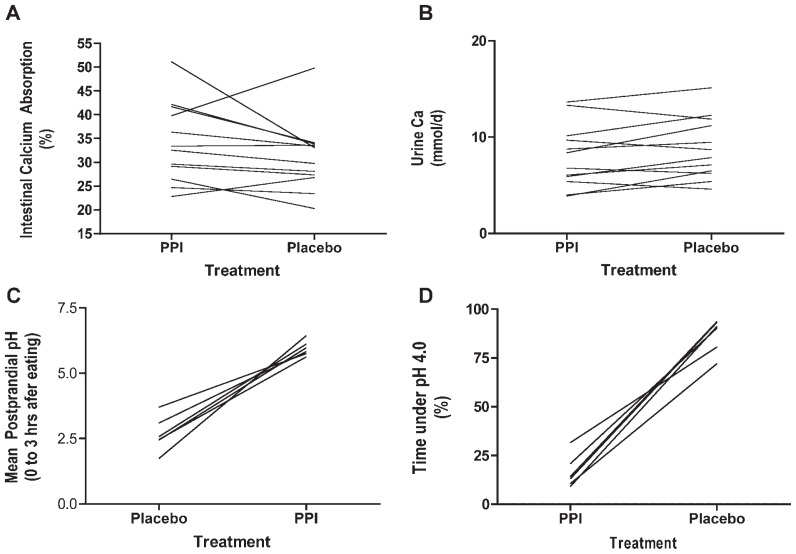
Fig. 2.
(A) Intestinal calcium absorption on day 5 of PPI and placebo treatments for each study subject (mean values are PPI 34.2% ± 2.4%, placebo 31.5% ± 2.1%, p =.24, paired t test). (B) The 34-hour urine calcium excretion on day 5 of PPI or placebo treatment for each study subject (mean values are PPI 8.00 ± 0.94, placebo 8.86 ± 0.92, p =.07, paired t test). (C) Postprandial gastric pH during and for the 3 hours following each meal for each study subject (mean values are placebo 2.67 ± 0.27, PPI 5.95 ± 0.12, p <.001, paired t test). (D) The percentage of time gastric pH was 4.0 or less during 24-hour nasogastric pH monitoring for each study subject taking PPI or placebo (mean values are PPI 16.7% ± 3.4%, placebo 86.8% ± 3.5%, p <.001, paired t test).
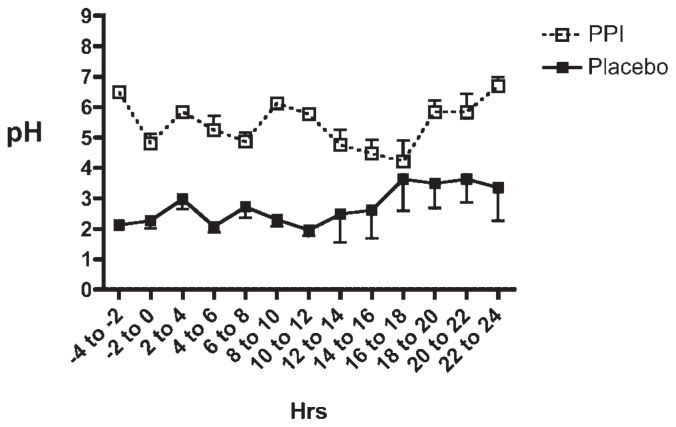
Thirty-four-hour urinary calcium values did not differ between the two experimental conditions (Table 2 and Fig. 2B), nor were there any consistent changes in intestinal calcium absorption between PPI and placebo treatments (Fig. 2A). In particular, the mean intestinal calcium absorption during treatment with esomeprazole was 34.2% ± 2.4%, and with placebo it was 31.5% ± 2.1% ( p =.24). PPI treatment produced the anticipated change in gastric acidity, with an average pH during PPI treatment of 5.38 ± 0.13 compared with an average pH during placebo treatment of 2.70 ± 0.44 ( p =.005). Mean pH data for every 2-hour increment throughout the 24 hours that gastric pH was measured during each intervention are shown in Fig. 1. As can be seen, there is no overlap in mean pH values at any point during the day, and many pH values during esomeprazole therapy were in the 5.5 to 7.0 range. The percent of time that gastric pH was less than 4.0 was significantly greater during the placebo treatment (86.8% ± 3.5%) compared with PPI treatment (16.7% ± 3.4%, p <.001; Fig. 2D). Furthermore, as shown in Fig. 2C, the mean 3-hour postprandial pH was significantly higher during PPI treatment than during placebo treatment (5.95 ± 0.12 versus 2.67 ± 0.2, p <.001). The 3-hour post-prandial pH data are particularly relevant because this is the time interval during which calcium would be absorbed from a meal.
Fig. 1.
Mean pH data for every 2-hour increment throughout the 24 hours that gastric pH was measured in each study subject while the subject was taking PPI or placebo. In most study subjects, pH measurements also were taken for the 4 hours before the 24-hour time period, and those data are included in the two time intervals −4 to −2 hours and −2 to 0 hours.
Discussion
The purpose of this study was to evaluate the role of gastric pH in calcium absorption. We were motivated to conduct this study in an attempt to explain the results of two large retrospective, case-controlled studies reporting increased risk of hip fracture with long-term PPI use.(1,3) One plausible mechanism for the increased relative risk of hip fracture during long-term PPI use would be by reducing intestinal calcium absorption resulting in negative calcium balance and/or compensatory secondary hyperparathyroidism leading to increased skeletal turnover, bone loss, and increased fracture risk. However, the data from this study indicate that, at least in the short term, in healthy individuals, PPI therapy does not affect calcium absorption. The most important finding of our study is that blocking gastric acid production had no impact on intestinal calcium absorption during a diet in which acid production is high (eg, a diet rich in protein). We reasoned that if acid generation was important for intestinal calcium, a high-protein diet that stimulated both gastric acid production and calcium absorption would be an appropriate dietary setting in which to test this hypothesis. If we inhibited gastric acid production under these dietary conditions, we should maximize our ability to detect the contribution of gastric acid to intestinal calcium absorption. This required that we successfully block gastric acid production, which we were able to do, as demonstrated in Figs. 1 and 2. Further, after 5 days of the 2.1 g/kg of protein diet, urinary calcium did not differ between the PPI and placebo treatment groups, which is consistent with the absorption data and supports the conclusion that esomeprazole had no effect on absorption efficiency. The tight correlation between dietary and urinary sodium, the pill-count data, and the fact that gastric pH rose significantly and consistently in subjects while they were taking esomeprazole all support the conclusion that subject compliance was excellent.
Short-term clinical studies examining the effects of PPIs on intestinal calcium absorption have reached divergent conclusions.(6–9,14) Whether absorption is measured in the context of a meal or in the fasted state after ingestion of just a calcium salt influences the results. Two studies that evaluated calcium absorption during fasting concluded that PPIs (or achlorhydria) decreased calcium absorption.(6,7) O’Connell and colleagues(7) reported the effects of omeprazole on fractional calcium absorption using a 45Ca tracer in a randomized crossover study in 18 women over the age of 65 years. Fractional calcium absorption was determined directly from 1 g of 45Ca-labeled calcium carbonate given without food. They reported a 41% reduction in calcium absorption.(7) At least five studies measured intestinal calcium absorption from test meals or diets in subjects who were either achlorhydric or using gastric acid suppressors (PPI or histamine H2 receptor antagonists [H2RA]).(6,8,9,14,15) None of these studies used calcium isotopic methods to measure calcium absorption directly, but in the aggregate, they concluded that absorption was unaffected by acid inhibition (or achlorhydria) when calcium was provided with food. In our study, subjects received the oral calcium isotope as part of their three meals during PPI or placebo treatment. Therefore, our finding that calcium absorption was unaffected in the fed state by a PPI are consistent with the human literature. However in rats, inhibition of gastric acid production either with PPIs or with irradiation inhibited apparent calcium absorption (as calculated by dietary calcium – fecal calcium).(16–19)
It is unlikely that PPI-induced changes in the rate of bone resorption confounded our findings. Clinical studies and in vitro experiments have suggested either no effect or an antiresorptive action of PPIs.(20–22) It is uncertain how relevant the in vitro studies are to the effects of PPIs in vivo because plasma concentrations of PPIs are in the micromolar range, and omeprazole has been shown to inhibit PTH-induced bone resorption only at a concentration of 10−4 M.(22–24)
Calcium generally is thought to be absorbed in the ionized form throughout the small intestine but primarily in the proximal small bowel. Ionization of this mineral depends on an acidic medium to release calcium from a salt or food complex. Typical fasting gastric pH values in healthy individuals are 1 to 3, and calcium is highly soluble in this environment. Because dietary protein is known to increase gastric acid secretion,(10–12) we decided to study the effect of a PPI in the setting of a high-protein diet, thereby maximizing gastric acid output. When acidic gastric chyme enters the duodenum, it stimulates secretin production, causing pancreatic and intestinal epithelial secretion of bicarbonate. This results in a rise in duodenal pH(25,26) such that the majority of active calcium transport occurs in the slightly acidic milieu of the duodenum (average pH 5 to 6). Thus it may be that regardless of the pH of the gastric contents, the pH of the chyme in the duodenum remains relatively constant.
Our study had significant strengths. It used a paired study design and a high dose of a widely used PPI. We used state-of-the-art methods to measure intestinal calcium absorption and a controlled dietary intervention that should maximize gastric acid production. Finally, we documented that our intervention blocked gastric acid production. Three limitations of this study are its short duration, the fact that only healthy young individuals were included, and that our experimental diet was not typical of that ingested by most Americans. The long-term effects of PPI use on calcium homeostasis have not been studied extensively. This is an important unaddressed issue because individuals who are prescribed PPIs are often older and consume them chronically, not uncommonly for years or even decades. As noted earlier, our data are relevant only to calcium ingested in the context of a meal.
The fact that our study did not show an effect on intestinal calcium absorption raises the question of a false-negative result (type 2 statistical error). As noted, our sample size, calculated using two different approaches, provided very robust power to observe an effect, and the p value on the primary outcome variable, intestinal calcium absorption, did not suggest even a trend toward a difference (Fig. 2A).
In summary, using dual-stable calcium isotopes, we found that short-term, profound inhibition of gastric acid secretion using a common PPI drug did not affect intestinal calcium absorption in healthy young adults when calcium was ingested as part of a meal. As noted earlier, our data are not applicable to calcium supplements taken at times separate from meals. Longer-term studies in older subjects ingesting lower levels of dietary protein are needed to determine whether our findings also apply under those conditions.
Acknowledgments
This study was supported by grants from the Yale Bone Center and the U.S. Department of Agriculture (USDA Agreements 97-35200-4420 and 00-35200-9579). This article also was made possible in part by CTSA Grant UL1 RR024139 from the National Center for Research Resources (NCRR), a component of the NIH, and the NIH Roadmap for Medical Research.
Footnotes
Disclosures
KLI and JEK contributed equally to this study. The contents of this study are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. All the authors state that they have no conflicts of interest.
References
- 1.Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296:2947–2953. doi: 10.1001/jama.296.24.2947. [DOI] [PubMed] [Google Scholar]
- 2.Sachs G, Shin JM, Briving C, Wallmark B, Hersey S. The pharmacology of the gastric acid pump: the H+,K+-ATPase. Annu Rev Pharmacol Toxicol. 1995;35:277–305. doi: 10.1146/annurev.pa.35.040195.001425. [DOI] [PubMed] [Google Scholar]
- 3.Vestergaard P, Rejnmark L, Mosekilde L. Proton pump inhibitors, histamine H2 receptor antagonists, and other antacid medications and the risk of fracture. Calcif Tissue Int. 2006;79:76–83. doi: 10.1007/s00223-006-0021-7. [DOI] [PubMed] [Google Scholar]
- 4.Roux C, Briot K, Gossec L, et al. Increase in vertebral fracture risk in postmenopausal women using omeprazole. Calcif Tissue Int. 2009;84:13–19. doi: 10.1007/s00223-008-9188-4. [DOI] [PubMed] [Google Scholar]
- 5.Kaye JA, Jick H. Proton pump inhibitor use and risk of hip fractures in patients without major risk factors. Pharmacotherapy. 2008;28:951–959. doi: 10.1592/phco.28.8.951. [DOI] [PubMed] [Google Scholar]
- 6.Recker RR. Calcium absorption and achlorhydria. N Engl J Med. 1985;313:70–73. doi: 10.1056/NEJM198507113130202. [DOI] [PubMed] [Google Scholar]
- 7.O’Connell MB, Madden DM, Murray AM, Heaney RP, Kerzner LJ. Effects of proton pump inhibitors on calcium carbonate absorption in women: a randomized crossover trial. Am J Med. 2005;118:778–781. doi: 10.1016/j.amjmed.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Bo-Linn GW, Davis GR, Buddrus DJ, Morawski SG, Santa Ana C, Fordtran JS. An evaluation of the importance of gastric acid secretion in the absorption of dietary calcium. J Clin Invest. 1984;73:640–647. doi: 10.1172/JCI111254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serfaty-Lacrosniere C, Wood RJ, Voytko D, et al. Hypochlorhydria from short-term omeprazole treatment does not inhibit intestinal absorption of calcium, phosphorus, magnesium or zinc from food in humans. J Am Coll Nutr. 1995;14:364–368. doi: 10.1080/07315724.1995.10718522. [DOI] [PubMed] [Google Scholar]
- 10.Busque SM, Kerstetter JE, Geibel JP, Insogna K. L-type amino acids stimulate gastric acid secretion by activation of the calcium-sensing receptor in parietal cells. Am J Physiol Gastrointest Liver Physiol. 2005;289:G664–G669. doi: 10.1152/ajpgi.00096.2005. [DOI] [PubMed] [Google Scholar]
- 11.Konturek SJ, Tasler J, Cieszkowski M, Jaworek J. Comparison of intravenous amino acids in the stimulation of gastric secretion. Gastroenterology. 1978;75:817–824. [PubMed] [Google Scholar]
- 12.Richardson CT, Walsh JH, Hicks MI, Fordtran JS. Studies on the mechanisms of food-stimulated gastric acid secretion in normal human subjects. J Clin Invest. 1976;58:623–631. doi: 10.1172/JCI108509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kerstetter JE, O’Brien KO, Caseria DM, Wall DE, Insogna KL. The impact of dietary protein on calcium absorption and kinetic measures of bone turnover in women. J Clin Endocrinol Metab. 2005;90:26–31. doi: 10.1210/jc.2004-0179. [DOI] [PubMed] [Google Scholar]
- 14.Knox TA, Kassarjian Z, Dawson-Hughes B, et al. Calcium absorption in elderly subjects on high- and low-fiber diets: effect of gastric acidity. Am J Clin Nutr. 1991;53:1480–1486. doi: 10.1093/ajcn/53.6.1480. [DOI] [PubMed] [Google Scholar]
- 15.Heaney RP, Smith KT, Recker RR, Hinders SM. Meal effects on calcium absorption. Am J Clin Nutr. 1989;49:372–376. doi: 10.1093/ajcn/49.2.372. [DOI] [PubMed] [Google Scholar]
- 16.Chonan O, Takahashi R, Yasui H, Watanuki M. Effect of L-lactic acid on the absorption of calcium in gastrectomized rats. J Nutr Sci Vitaminol (Tokyo) 1998;44:869–875. doi: 10.3177/jnsv.44.869. [DOI] [PubMed] [Google Scholar]
- 17.Hara H, Suzuki T, Kasai T, Aoyama Y, Ohta A. Ingestion of guar-gum hydrolysate partially restores calcium absorption in the large intestine lowered by suppression of gastric acid secretion in rats. Br J Nutr. 1999;81:315–321. [PubMed] [Google Scholar]
- 18.Mahoney AW, Hendricks DG. Role of gastric acid in the utilization of dietary calcium by the rat. Nutr Metab. 1974;16:375–382. doi: 10.1159/000175510. [DOI] [PubMed] [Google Scholar]
- 19.Mahoney AW, Holbrook RS, Hendricks DG. Effects of calcium solubility on adsorption by rats with induced achlorhydria. Nutr Metab. 1975;18:310–317. doi: 10.1159/000175609. [DOI] [PubMed] [Google Scholar]
- 20.Mizunashi K, Furukawa Y, Katano K, Abe K. Effect of omeprazole, an inhibitor of H+,K(+)-ATPase, on bone resorption in humans. Calcif Tissue Int. 1993;53:21–25. doi: 10.1007/BF01352010. [DOI] [PubMed] [Google Scholar]
- 21.Kocsis I, Arato A, Bodanszky H, et al. Short-term omeprazole treatment does not influence biochemical parameters of bone turnover in children. Calcif Tissue Int. 2002;71:129–132. doi: 10.1007/s00223-001-2068-9. [DOI] [PubMed] [Google Scholar]
- 22.Tuukkanen J, Vaananen HK. Omeprazole, a specific inhibitor of H+-K+-ATPase, inhibits bone resorption in vitro. Calcif Tissue Int. 1986;38:123–125. doi: 10.1007/BF02556841. [DOI] [PubMed] [Google Scholar]
- 23.Sachs G, Shin JM, Howden CW. Review article: the clinical pharmacology of proton pump inhibitors. Aliment Pharmacol Ther. 2006;23(Suppl 2):2–8. doi: 10.1111/j.1365-2036.2006.02943.x. [DOI] [PubMed] [Google Scholar]
- 24.Hunt RH, Armstrong D, James C, et al. Effect on intragastric pH of a PPI with a prolonged plasma half-life: comparison between tenatoprazole and esomeprazole on the duration of acid suppression in healthy male volunteers. Am J Gastroenterol. 2005;100:1949–1956. doi: 10.1111/j.1572-0241.2005.41956.x. [DOI] [PubMed] [Google Scholar]
- 25.Evenepoel P. Alteration in digestion and absorption of nutrients during profound acid suppression. Best Pract Res Clin Gastroenterol. 2001;15:539–551. doi: 10.1053/bega.2000.0197. [DOI] [PubMed] [Google Scholar]
- 26.Kaunitz JD, Akiba Y. Acid-sensing protective mechanisms of duodenum. J Physiol Pharmacol. 2003;54(Suppl 4):19–26. [PubMed] [Google Scholar]