Abstract
Parathyroid hormone (PTH) increases both the number of osteoclast in bone and the number of early hematopoietic stem cells (HSCs) in bone marrow. We previously characterized the phenotype of multiple populations of bone marrow cells with in vitro osteoclastogenic potential in mice. Here we examined whether intermittent administration of PTH influences these osteoclast progenitor (OCP) populations. C57BL/6 mice were treated with daily injections of bPTH(1–34) (80 μg/kg/day) for 7 or 14 days. We found that PTH caused a significant increase in the percentage of TN/CD115+CD117high and TN/CD115+CD117int cells ( p <.05) in bone marrow on day 7. In contrast, PTH decreased the absolute number of TN/CD115+CD117low cells by 39% on day 7 ( p <.05). On day 14, there was no effect of PTH on osteoclast progenitor distribution in vivo. However, PTH treatment for 7 and 14 days did increase receptor activator of NF-κB ligand (RANKL)– and macrophage colony-stimulating factor (M-CSF)–stimulated in vitro osteoclastogenesis and bone resorption in TN/CD115+ cells. In the periphery, 14 days of treatment increased the percentage and absolute numbers of HSCs (Lin−CD117+Sca-1+) in the spleen ( p <.05). These data correlated with an increase in the percent and absolute numbers of HSCs in bone marrow on day 14 ( p <.05). Interestingly, the effects on hematopoietic progenitors do not depend on osteoclast resorption activity. These results suggest that in vivo PTH treatment increased in vitro osteoclastogenesis and resorption without altering the number of osteoclast precursors. This implies that in vivo PTH induces sustained changes, possibly through an epigenetic mechanism, in the in vitro responsiveness of the cells to M-CSF and RANKL.
Keywords: PARATHYROID HORMONE, ANABOLIC RESPONSE, OSTEOCLAST PROGENITORS, OSTEOPOROSIS, BONE REMODELING
Introduction
Osteoclasts are unique giant multinucleated cells of hematopoietic origin and constitute the main class of cells that are able to resorb bone.(1) As such, they are critical elements in bone remodeling, the process that regulates the balance between bone formation (mediated by osteoblasts) and bone resorption (mediated by osteoclasts).(2,3) Osteoclasts are generated from myeloid progenitors through a progression that involves the fusion of mononuclear precursor cells. Pivotal for the development of functional osteoclasts is the inducing cytokine, receptor activator of NF-κB ligand (RANKL). This molecule is a tumor necrosis factor family member expressed predominantly by osteoblast lineage cells with the ability to bind to its transmembrane receptor, RANK, expressed on osteoclast precursors.(4,5) The identification of RANK/RANKL interactions as the main signal regulating osteoclastogenesis originates from in vitro studies of osteoclast differentiation in which hematopoietic cells and bone marrow stromal cells were cocultured in the presence of parathyroid hormone (PTH) or 1α,25-dihydroxyvitamin D3 [1α,25(OH)2D3](4,5) These studies also indicated that osteoclastogenesis depended on cell-to-cell interactions between myeloid progenitors and osteoblasts or other cells of mesenchymal origin.
PTH is the major systemic regulator of calcium homeostasis and bone metabolism. PTH is known to maintain calcium homeostasis in bone and kidney and indirectly in the gastrointestinal tract. PTH also regulates phosphorus metabolism. The PTH/parathyroid hormone–related protein (PTHrP) receptor is present on osteoblasts and other mesenchymal-derived cells in the bone marrow microenvironment. In contrast, most of the literature agrees that osteoclasts and their precursor cells lack high-affinity PTH receptors.(3,6–8)
Therefore, it has been proposed that the effects of PTH on osteoclasts are indirect.(9) The PTH receptor on target cells is activated by the binding of PTH or PTHrP.(10) In addition, PTH upregulates RANKL gene expression and protein production on osteoblasts and stromal cells and therefore can activate osteoclasts precursors to develop into mature resorbing osteoclasts.(11) Interestingly, the in vivo effect of PTH depends on the dose and frequency of administration. Continuous administration of PTH induces catabolic effects on bone, whereas intermittent PTH administration exerts anabolic effects.(12–19) Currently, once-daily injection of human PTH(1–34) is approved by the Food and Drug Administration as treatment for osteoporosis in the United States. In addition, it could have potential for the treatment of other conditions that require new bone formation. Most studies have been focused on the effects of PTH on osteoblasts, and recent published literature has shown that PTH also could modulate hematopoietic parameters.(20) However, direct studies of the effects of PTH on osteoclast progenitor populations and osteoclast development have not been done in a comprehensive fashion. This is important because a complete understanding on how PTH modulates different cell types in the bone microenvironment is crucial for developing new strategies to treat osteoporosis and bone fractures, as well as designing stem cell–based therapies (ie, hematopoietic stem cell transplantation and mobilization and harvesting of hematopoietic progenitors).(20–23)
We have identified a discrete population of cells containing most of the in vitro osteoclast precursor (OCP) activity in murine bone marrow.(24) In this study we examined whether these populations of OCPs were affected by in vivo administration of PTH to mice. In addition, we examined the effects of PTH on the distribution of early hematopoietic progenitors in the murine bone marrow and spleen because PTH is known to modulate early hematopoiesis in vivo.(25)
Materials and Methods
Mice
Eight-week-old C57BL/6 male mice were purchased from Charles River Laboratories (Wilmington, MA, USA). All the animals were housed in sterile microisolators or in Thoren microisolators and given water and rodent chow ad libitum. Animals were housed in the Center for Laboratory Animal Care at the University of Connecticut Health Center. All animals were used according to protocols approved by the Animal Care Committee of the University of Connecticut Health Center.
In vivo treatment
Mice were injected subcutaneously with bovine PTH(1–34) (Bachem America, Torrance, CA, USA) at a dose of 80 μg/kg per day for 7 or 14 days. As controls, parallel groups of mice received subcutaneous injections of PBS.
Antibodies and flow cytometry
All the antibodies used for the fractionation of hematopoietic progenitors from bone marrow and for phenotypic analyses are available commercially. These include anti-B-cell-lineage cell antibody (mAb): anti-CD45R/B220 (RA3-6B2); anti-T-cell-lineage mAbs: anti-CD3 (145-2C11), anti-CD4 (GK1.5), and anti-CD8 (53.6.7); anti-NK cell mAb: NK1.1 (PK136); anti-monocyte/macrophage mAb: anti-CD11b/Mac-1 (M1/70); anti-granulocyte mAb: anti-Ly-6G/Gr-1 (RB6-8C5); and anti-erythroid progenitor monoclonal mAb (Ter119) and anti-progenitor cell mAbs: anti-Ly-6A/E Sca-1 (E13.161.7), anti-ckit/CD117 (2B8), anti-c-fms/CD115 (AFS98), anti-IL-7Ra/CD127 (A7R34), and anti-flt3/CD135 (A2F10). All these antibodies were obtained directly conjugated to fluorochromes or biotinylated from e-Biosciences (San Diego, CA, USA) or BioLegend (San Diego, CA, USA).
Labeling of cells for flow cytometric analysis or cell sorting was performed by standard staining procedures, wherein directly conjugated or biotinylated mAb plus second-step reagents were added sequentially to the cell preparation of interest. All antibodies were optimized in term of their concentration and titration. All staining was done on ice. Dead cells were identified and excluded from our analyses by their ability to incorporate propidium iodide (PI). Flow cytometric analysis and sorting were performed in a BD-FACS Aria (BD Biosciences, San Jose, CA, USA) equipped with five lasers and 18 fluorescence detectors. All the data were analyzed using FlowJo software from Tree Star, Inc. (Ashland, OR, USA).
Isolation of hematopoietic precursors
For the isolation of osteoclast progenitors, bone marrow cells were harvested for single-cell suspensions by flushing femurs and tibias with staining medium [1 × Hank’s balanced salt solution (HBSS), 10 mM HEPES, and 2% newborn calf serum] using a 21-gauge needle. Cells were spun down and lysed by hypotonic shock with 2 mL of red blood cell lysing buffer (Sigma, St Louis, MO, USA), washed with staining medium, and spun down again. The pellet was resuspended in staining medium and filtered through Nytex mesh. Cells were counted in a hemocytometer by trypan blue exclusion. Cells were stained with a mixture of antibodies containing anti-CD3, anti-B220/CD45R coupled with allophycocyanin (APC) Alexa750, anti-CD11b coupled with Pacific blue, anti-CD117 coupled with APC, and biotinylated anti-CD115. Incubation on ice proceeded for 45 minutes, followed by washing in staining medium. As a second step, cells were stained with streptavidin coupled with phycoerythrin (PE). Finally, cells were resuspended in staining medium containing 1 μg/mL of PI.
For analyses of early hematopoietic progenitors, cells suspensions were stained with a cocktail of biotinylated antibodies against mature hematopoietic markers. These included lymphoid markers CD3, CD4, CD8, CD45R/B220, and NK1.1; myeloid markers CD11b and Ly-6G (Gr-1), and the erythroid marker Ter119. After incubation and washing, cells were stained subsequently with streptavidin coupled with PE–Texas red, anti-CD117 coupled with APC, anti-Sca1 coupled with FITC, anti-CD135 coupled with PE, and anti-CD127 coupled with Pacific blue. Cells then were resuspended in staining medium containing PI.
Osteoclast progenitor cultures and quantification
Sorted OCP cells were spun down and resuspended in α-minimum essential medium (α-MEM) (Gibco BRL, Carlsbad, CA, USA) with 10% fetal bovine serum (FBS), penicillin, streptomycin, recombinant mouse macrophage colony-stimulating factor (M-CSF; R&D Systems, Minneapolis, MN, USA) and RANKL, both at 30 ng/mL. The cells were plated in five replicates per mouse in 96-well plates for both groups, vehicle- and PTH-treated mice, and incubated at 37 °C with 5% CO2. Fresh medium was changed every 48 hours. To quantify tartrate-resistant acid phosphatase–positive (TRACP+) multinuclear and mononuclear cells in vitro, cells were fixed with 2.5% glutaraldehyde in PBS for 30 minutes and stained for TRACP using the Leukocyte Acid Phosphatase Kit (Sigma) according to the manufacturer’s instructions. Osteoclasts were identified as TRACP+ cells with more than three nuclei. TRACP+ mononuclear cells also were counted.
Bone resorption
Sorted osteoclast progenitor populations from either vehicle- or PTH-treated mice were tested for their ability to resorb bone. Osteoclast progenitors at a density of 1000 cells were seeded in triplicates on bovine cortical bone slices in 96-well plates containing α-MEM (Gibco BRL) with 10% FBS, penicillin, streptomycin, recombinant mouse M-CSF (R&D Systems), and RANKL (both at 30 ng/mL) for 12 days. The bone slices were then fixed with 2.5% glutaraldehyde in PBS, stained for TRACP (as described previously), and visualized by microscopy to ensure that osteoclasts have formed on the bone slices. To visualize resorption pits, bones were sonicated to remove osteoclasts, and resorption pits then were stained with 1% toluidine blue in 1% sodium borate. To evaluate the ability of osteoclasts to resorb bone, we measured the area of individual pits of each group using the Via-160 video image measurement system (Boeckeler Instruments, Tucson, AZ, USA). Data were analyzed to compare the PTH-treated group with the vehicle-treated group.
Osteoclasts area measurements
The size of osteoclasts grown on tissue culture dishes was measured as the area of multinuclear TRACP+ osteoclasts from vehicle- and PTH-treated groups and evaluated using the Via-160 video image measurement system (Boeckeler Instruments).
Bisphosphonate treatment
Mice were divided into three groups: (1) vehicle, (2) PTH, and (3) PTH +alendronate. (1) Vehicle-treated mice were pretreated with one injection containing PBS and 0.1% BSA (alendronate vehicle); seven days later vehicle-treated mice were given daily injections containing PBS, 0.1% BSA, and 1 mM HCl (PTH vehicle) for 14 days. (2) For this PTH group, mice were treated with daily injections of bovine PTH(1–34) (80 μg/kg) for 14 days. (3) Mice in this group were pretreated with one injection of alendronate (100 μg/kg), followed by two more injections, once weekly, 7 and 14 days after pretreatment. At the end of the pretreatment with alendronate, mice were treated with bovine PTH(1–34) (80 μg/kg) for 14 days. Bone marrow and spleens from all three groups were harvested the following day after the last PTH injection, processed as indicated earlier, and stained with antibodies for hematopoietic progenitors. Samples were analyzed by flow cytometry as described earlier. The levels of cross-linked C-telopeptide (CTX) were measured using the RatLaps EIA (AC-06F1) from Immunodiagnostic Systems (IDS, Scottsdale, AZ, USA).
Statistical analysis
All data were analyzed using GraphPad (La Jolla, CA, USA) Prism 5 software using an unpaired Student’s t test.
Results
The effect of PTH(1–34) on osteoclast progenitor populations in the bone marrow
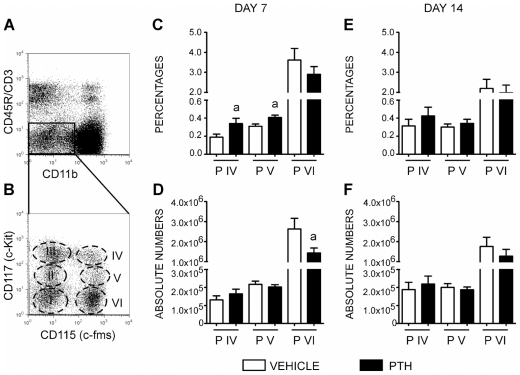
We have previously identified three OCP populations in murine bone marrow with high osteoclastogenic potential.(24) As depicted in Fig. 1A, B, these populations share the phenotype CD3−CD45R−CD11blowCD115+ and are further separated by their reactivity to CD117 as CD117high (population IV), CD117int (population V), and CD117low (population VI).
Fig. 1.
The effect of PTH on osteoclast progenitors. (A) FACS plots of the TN fraction (CD45R−/CD3−/CD11blow). (B) Distribution of the TN fraction into six populations (I to VI) in the context of CD115 and CD117 cell surface markers. Analysis of percentage (C) and absolute number (D) of osteoclast progenitors (populations IV to VI) after 7 daily injections with PTH. Analysis of percentage (E) and absolute number ( F) of osteoclast progenitors (populations IV to VI) after 14 daily injections with PTH. The data represent the mean ± SEM values of four replicates per mouse of two independent experiments with a total of 11 mice for the vehicle-treated group (white bars) and 10 mice for PTH-treated group (black bars). Statistically significant difference between vehicle and PTH groups (a) p ≤.05.
In this study, we evaluated the effect of intermittent PTH administration on osteoclast progenitor (OCP) populations, measuring the distribution of populations IV to VI on days 7 and 14 after treatment. As shown in Fig. 1C, D, on day 7 of PTH treatment, the osteoclast progenitor populations in the bone marrow showed a significant increase in the percentages of population IV (vehicle =0.1890 ± 0.0342 versus PTH =0.3400 ± 0.0583, p =.042) and population V on day 7 (vehicle =0.3090 ± 0.0258 versus PTH =0.4064 ± 0.0269, p =.018). No changes were observed in the percentages of population VI. In contrast, PTH decreased the absolute numbers of population VI on day 7 (vehicle =2.7 × 106 ± 5.1 × 105 versus PTH =1.5 × 106 ± 2.4 × 105, p =.047). Interestingly, there was no effect of PTH on the percentage or absolute number of osteoclast progenitors in mice on day 14 (Fig. 1E, F). Hence intermittent PTH administration induced a transient modulation of osteoclast progenitor populations.
Administration of PTH increases the in vitro osteoclastogenic potential of bone marrow OCP populations in C57BL/6 mice
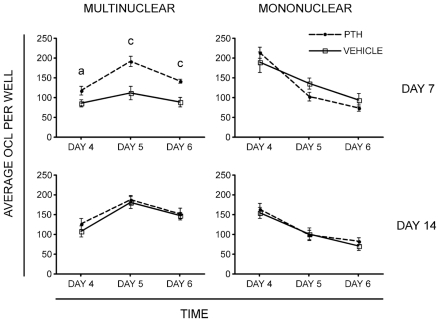
We next examined the in vitro osteoclastogenic potential of osteoclast progenitors from C57BL/6 mice treated with daily injections of bPTH(1–34) for 7 or 14 days. As an osteoclast progenitor population, we isolated cells with the phenotype of CD3−CD45R−CD11blowCD115+ (TN/CD115+) from bone marrow. This population contains the majority of the in vitro osteoclastogenic activity in bone marrow. Populations containing over 99.1% purified cells were cultured with M-CSF and RANKL in 96-well plates at a density of 1000 cells/well. The formation of osteoclast-like cells (OCLs) was evaluated on days 4, 5, and 6 of culture. Cells were fixed, stained for TRACP, and multinuclear versus mononuclear TRACP+ cells were counted. We observed that 7 days of bPTH treatment increased in vitro osteoclastogenesis by approximately 1.4- to 2.0-fold compared with cells from vehicle-treated mice depending on how long the cells were cultured in vitro (Fig. 2). In these time-course experiments, a significant increase was observed in multinuclear TRACP+ osteoclasts in the PTH-treated group on day 4 (vehicle = 86 ± 9 versus PTH =118 ± 11, p =.042), on day 5 (vehicle = 112 ± 17 versus PTH =192 ± 13, p =.002) and on day 6 of culture (vehicle =89 ± 12 versus PTH =142 ± 5, p =.001). In addition, there was a trend toward a decrease in the number of mononuclear TRACP+ osteoclasts in the PTH-treated group on day 5 (vehicle =136 ± 14 versus PTH =103 ± 11, p =.086). The decreased in mononuclear osteoclasts may be related to the higher rate of multinuclear osteoclast formation in the PTH group. As observed when analyzing the distribution of OCPs, we found no effect of 14 days of PTH treatment on the number of osteoclasts that formed in cultures from bone marrow OCP cells that were treated with M-CSF and RANKL. Therefore, the increase in osteoclastogenic potential from these progenitors was transient and could be related to the transient increase in certain progenitor populations.
Fig. 2.
In vitro osteoclastogenesis. Osteoclast progenitors were FACS sorted and plated in 96-well plates at a density of 1000 cells/well, and TRACP assays were performed at different time points as indicated. (Upper left panel) The TRACP+multinuclear OCLs on day 7 after PTH treatment. (Upper right panel) The TRACP+ mononuclear cells on day 7 after PTH treatment. (Lower left panel) The TRACP+ multinuclear OCLs on day 14 of PTH treatment. (Lower right panel) The TRACP+ mononuclear cells on day 14 of PTH treatment. The data represent the mean ± SEM values of four replicates per mouse of two independent experiments with a total of 8 mice for the vehicle-treated group and 10 mice for the PTH-treated group. Statistically significant difference between vehicle and PTH groups (a) p ≤.05, (c) p ≤.001.
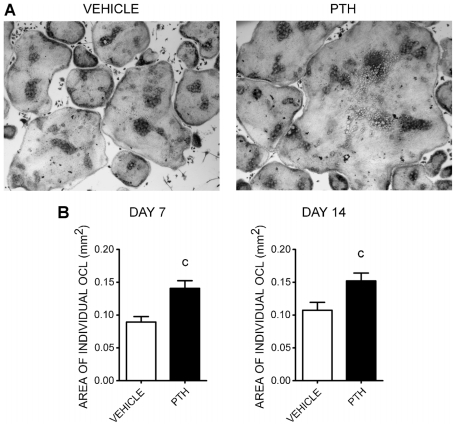
In vitro TRACP+ osteoclasts from PTH-treated mice are larger in size than those from vehicle-treated mice
Initial evaluation of OCLs generated in vitro from PTH-treated animals suggested that they generated larger multinucleated cells. Therefore, we measured the size of multinucleated OCLs. Fluorescence-activated cell sorted (FACS) osteoclast progenitors (TN/CD115+) from 7- or 14-day vehicle- or PTH-treated mice were cultured with M-CSF and RANKL. On day 5 of culture, we performed TRACP assays and measured the area of individual osteoclasts from both groups. As shown in Fig. 3, we found that osteoclasts from the PTH-treated group were larger than those from the vehicle-treated group. Seven days of in vivo PTH treatment increased the size of osteoclasts significantly compared with the vehicle-treated group (vehicle =0.0895 ± 0.0082 mm2 versus PTH =0.1408 ± 0.0115 mm2, p =.0003). Interestingly, cultures of cells treated with PTH for 14 days also had a significant increase in the size of osteoclasts compared with the vehicle-treated group (vehicle =0.1074 ± 0.012 mm2 versus PTH =0.1519 ± 0.012 mm2, p =.0089) despite the lack of an effect at this time point of PTH treatment on osteoclast progenitors or the number of osteoclasts that formed in the cultures.
Fig. 3.
Measurement of the sizes of multinuclear TRACP+ OCLs in vitro. (A) Microphotographs of TRACP+ multinuclear OCLs from FACS-sorted osteoclast progenitors (TN/CD115+) on day 5 of culture with M-CSF and RANKL derived from vehicle- or PTH-treated mice. (B) Areas of individual OCLs measured from 7 days (left) or 14 days (right) of treatment with PTH. The data represent the mean values of individual OCLs from the vehicle-treated group (white bar, n =255) and the PTH-treated group (black bar, n =258) on day 7 and the vehicle-treated group (white bar, n =244) and PTH-treated group (black bar, n =248) on day 14. The data represent the mean ± SEM values of four replicates per mouse of two independent experiments with a total of 8 mice for the vehicle-treated group and 10 mice for the PTH-treated group. Statistically significant difference between vehicle and PTH groups (c) p ≤.001.
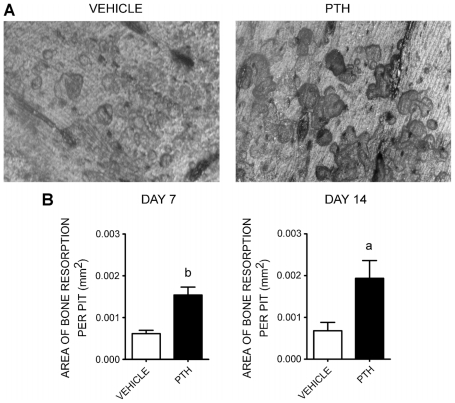
Osteoclasts from PTH-treated mice formed larger resorption pits than those from vehicle-treated mice
To evaluate whether PTH treatment affected resorption activity, we plated purified osteoclast progenitors (TN/CD115+) from vehicle- or PTH-treated mice onto bovine cortical bone slices in the presence of M-CSF and RANKL. Twelve days after seeding, bone slices were stained for TRACP to identify osteoclasts. Bone slices were sonicated and then stained with toluidine blue to identify resorption pits. We measured the area of individual bone resorption pits from each group, as shown in Fig. 4. We observed that in vitro osteoclasts derived from 7- or 14-day PTH-treated mice formed larger resorption pits than those from vehicle-treated mice (7 days: vehicle =0.000618 ± 0.000079 mm2 versus PTH =0.001542 ± 0.00019 mm2, p =.01; 14 days: vehicle = 0.000681 ± 0.00020 mm2 versus PTH =0.001934 ± 0.00043 mm2, p =.03).
Fig. 4.
Bone resorption assay. Osteoclast progenitors were FACS sorted and plated onto bovine cortical bone slices in 96-well plates at a density of 1000 cells/well. TRACP assays were performed on day 12 after culturing with M-CSF and RANKL to identify osteoclasts on bone, and then resorption pits were stained with toluidine blue. (A) Representative microphotograph of bone resorption pits from vehicle-treated mice (left) and PTH-treated mice (right). (B) Measurement of individual resorption pit area (mm2) on day 7 (left) and day 14 (right) of treatment. The data represent the mean ± SEM values of triplicates of bovine cortical bone slices per group from two independent experiments with a total of 6 mice for the vehicle-treated group and 6 mice for the PTH-treated group. Statistically significant difference between vehicle and PTH groups (a) p ≤.05, (b) p ≤.01.
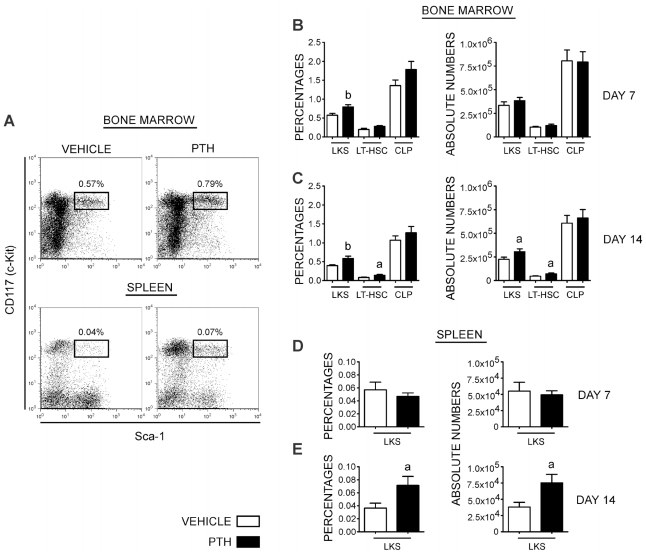
PTH affects the dynamics of early hematopoietic progenitors in the bone marrow and periphery
Several recent reports have shown that exogenous PTH treatment could significantly modify early hematopoietic parameters. These include increases in the number of early hematopoietic progenitors in the bone marrow(25) and a potentiating effect on cytokines that induce their egress into the periphery. This has led to the proposal that PTH treatment could be used as a more effective way to mobilize progenitors, facilitating their harvest from blood for hematopoietic stem cell–based therapies.(20) To test whether these effects also were evident in our experiments, we examined the effects of PTH treatment in vivo on days 7 and 14 on the percentages and absolute numbers of early hematopoietic precursors, as assessed by the phenotype Lin−CD117+Sca1+ (LKS). As depicted in Fig. 5, we found a statistically significant increase in the percentages of LKS in the bone marrow at 7 days (control =0.57 ± 0.051 versus PTH =0.7924 ± 0.062, p =.01) and at 14 days (control =0.397 ± 0.023 versus PTH =0.588 ± 0.059, p =.004) of treatment. However, when the absolute number of these cells was analyzed, we found a significant increase on day 14 (control =2.26 × 105 ± 2.2 × 104 versus PTH =3.10 × 105 ± 3.1 × 104, p =.04) but not on day 7 of treatment (control =3.34 × 105 ± 3.8 × 104 versus PTH =3.83 × 105 ± 3.5 × 104, p =.35).
Fig. 5.
PTH regulates hematopoietic stem cells in bone marrow and spleen. (A) Representative dot plots of LKS cells (Lin−CD117+Sca-1+) in bone marrow (upper panel) and spleen (lower panel) from vehicle-treated (left) and PTH-treated (right) mice. All plots were gated on negative populations for lineage markers. (B) Representative bar graphs of the percentage (left) and absolute numbers (right) of LKS cells, LT-HSCs, and CLPs in the bone marrow on day 7 (upper graphs) and (C) day 14 (lower graphs) with PTH treatment. (D) Representative bar graphs of percentage (left) and absolute numbers of LKS cells in spleen on day 7 and (E) day 14 with PTH treatment. The data represent the mean ± SEM values from four independent experiments with a total of 20 mice for the vehicle-treated group and 20 mice for the PTH-treated group. Statistically significant difference between vehicle and PTH groups (a) p ≤.05, (b) p ≤.01.
The LKS population is heterogeneous and includes early self-renewing hematopoietic stem cells with the ability to reconstitute the hematopoietic system of lethally irradiated recipients in a long-term fashion. To test whether this population (LT-HSC) was affected by PTH treatment, we evaluated the LKS fraction that was negative for the CD135 molecule (Lin−CD117+Sca-1+CD135−).(26–28) The distribution of this population followed the same changes observed for the LKS population with an almost significant increase in its percentage on day 7 (control =0.201 ± 0.031 versus PTH =0.280 ± 0.024, p =.051) and no significant changes in absolute number. In contrast, on day 14 of PTH treatment, there was an increase in both the percentage (control =0.084 ± 0.008 versus PTH =0.144 ± 0.022, p =.02) and absolute number (control =4.7 × 105 ± 4.91 × 103 versus PTH =7.2 × 104 ± 1.01 × 104, p =.03) of LT-HSC.
We also evaluated the effect of PTH treatment on the distribution of common lymphoid progenitors (CLPs) with the phenotype Lin−CD117+CD127+(29) and found no differences between vehicle- and PTH-treated mice on either day 7 or day 14.
Because of the reported ability of PTH to mobilize early hematopoietic progenitors, we evaluated the presence of LKS cells in the periphery. Seven days after treatment, there were no changes in the distribution of LKS cells in the spleens of PTH-treated animals compared with vehicle-treated controls. In contrast, on day 14, PTH treated animals showed a significant increase in the percentage (control =0.037 ± 0.008 versus PTH =0.072 ± 0.014, p =.03) and absolute number (control =3.81 × 104 × 7.3 × 103 versus PTH =7.53 × 104 ± 1.31 × 104; p =.02) of LKS cells in the spleen.
Osteoclast activity is not responsible for the effect of PTH treatment on early hematopoietic progenitors
Alendronate is a second-generation nitrogen-containing bisphosphonate used for the treatment of osteoporosis. As an antiresorptive (antianabolic) agent, it is known to inhibit the enzyme farnesyl pyrophosphate synthase (FPPS), inducing apoptosis in osteoclasts and consequently suppressing osteoclast-mediated bone resorption.(34–37) Because we observed a significant increase in the percentage and number of LKS cells in the bone marrow and spleen (Fig. 5) and an increase in the activity of osteoclasts in vitro (Figs. 3 and 4) after 14 days of PTH treatment, we asked whether osteoclasts activity in the bone marrow affects hematopoietic progenitors or whether this effect was attributed to PTH effects independent of osteoclast activity. Therefore, we treated mice with either vehicle alone, PTH alone, or PTH in combination with alendronate for a 14-day period. As shown previously, in the bone marrow, the PTH treatment induced a modest increase in the number of early hematopoietic progenitors (LKS cells) from 3.65 × 105 ± 2.15 × 104 for vehicle to 4.61 × 105 ± 3.07 × 104 for PTH treatment ( p =.04). The same trend, although nonsignificant, was observed when vehicle (3.65 × 105 ± 2.15 × 104) was compared with mice treated with PTH plus alendronate (4.24 × 105 ± 8.26 × 104). In the spleen, as expected, there was evidence of a significant egress of LKS cells when vehicle-treated mice were compared with PTH-treated groups (1.88 × 104 ± 3.31 × 103 for vehicle versus 7.19 × 104 ± 7.53 × 103 for PTH treatment, p =.003). This effect was maintained on cotreatment of PTH with alendronate (1.88 × 104 ± 3.31 × 103 for vehicle versus 5.95 × 104 ± 5.58 × 103 for PTH with alendronate, p =.002).
To test the effectiveness of alendronate for limiting osteoclast resorption, we measured the levels of serum CTX in the different groups of mice and found increased levels in PTH-treated mice when compared with vehicle-treated mice (53.2 ± 6.8 ng/mL versus 34.7 ± 2.2 ng/mL, p <.05). This increased activity was not observed on cotreatment of mice with PTH plus alendronate (33.0 ± 3.2 ng/mL). This result indicates that alendronate treatment limited osteoclastogenic activity without altering the egress of early hematopoiesis progenitors to the periphery.
Discussion
PTH exerts different effects on bone in vivo depending on the method of treatment. Continuous administration results in a net increase in bone resorption. In contrast, when given intermittently (ie, daily injections), PTH induces a net increase in bone formation. However, the mechanisms underlying the paradoxical effects of PTH remain unknown. Most studies of the molecular and cellular mechanisms of the effects of PTH on bone have focused on osteoblast lineage cells. It remains unclear whether the main effects are due to its ability to activate osteoblast precursor cells or quiescent osteoblastic cells. It also may inhibit apoptosis in mature osteoblasts, which might prolong the anabolic effects of PTH.(12–19,30) However, to fully appreciate the responses of bone to PTH, it is also necessary to understand how PTH administration affects other type of cells in the bone microenvironment that are implicated in bone turnover. Osteoclasts are another potential target of PTH action because the physiologic activities of osteoblasts and osteoclasts are tightly coupled.
To date, no studies have reported the effects of PTH treatment on specific osteoclast progenitor populations. Here we demonstrate that daily administration of PTH to mice significantly increased the percentages of specific populations of osteoclast progenitors compared with vehicle-treated mice. It appears that these effects are a transient response to PTH because the number of osteoclasts progenitors was increased at 7 days of treatment but not at 14 days. Moreover, we think that this effect might be more specific for less mature populations of osteoclast precursors because we observed an increase only in the percentages of populations IV and V. From previous studies it is known that PTH induces a transient increase in the expression of RANKL on the surfaces of osteoblasts when administered on a daily basis.(22,23) These effects on osteoblasts may help to explain the increase that we observed in osteoclast precursor cells after 7 days of treatment (the acute response) and the return to normal levels on day 14. In addition, our in vitro osteoclastogenesis results found that the number of multinuclear TRACP+ osteoclasts in cultures of cells from PTH-treated mice was increased significantly after 7 days of treatment but not after 14 days. These in vitro results correlate with our studies in vivo demonstrating that daily PTH administration increased the percentages of less mature OCP populations on day 7 but not on day 14. Our previous work demonstrated that these less mature cells are more efficient at forming osteoclasts in vitro.(24)
Interestingly, we also observed that PTH treatment for both 7 and 14 days increased the size of osteoclasts that formed in cultures from OCP cells (TN/CD115+). In addition, these cells were more active at resorbing bone because they formed larger resorption pits than the osteoclasts from vehicle-treated mice. The ability of PTH treatment in vivo to enhance subsequent RANKL- and M-CSF–stimulated osteoclast formation and bone resorption in vitro does not appear to depend on changes in the percentages and/or absolute number of osteoclast precursor populations IV, V, and VI in bone marrow because they occurred in OCP cells from mice treated with PTH for 14 days, whereas PTH had no effect on the distribution of these populations. Rather, a more likely explanation is that intermittent in vivo PTH primed the cells and induced sustained changes in the OCP cells, possibly by an epigenetic mechanism. This, in turn, caused them to be more susceptible to M-CSF and RANKL stimulation. We are currently investigating the mechanisms by which these sustained changes may be occurring and attempting to identify the cells that respond and contribute to the overall in vivo effects of PTH on OCP progenitor populations.
In addition to the findings we just described, we also studied the effects of daily administration of PTH for 7 and 14 days on early hematopoietic stem cells (LKS cells), long-term self-renewing HSCs (LT-HSCs), and common lymphoid progenitors (CLPs). The effects of PTH on hematopoietic progenitors from bone marrow and peripheral blood have been described previously.(20,31) However, to our knowledge, this study is the first to report the effect of PTH on cells in the spleen. We observed a significant increase in the percentage but not the absolute numbers of LKS cells and LT-HSCs on day 7 of PTH treatment. We found no changes in the percentage or absolute number of CLPs in the bone marrow and LKS cells in the spleen. Interestingly, on day 14, the percentages and absolute number of LKS cells and LT-HSCs in bone marrow and LKS cells in the spleen increased significantly. These results suggest that PTH affects the distribution of these early precursors, possibly by altering their niche.(20,25,32,33) These alterations could be due to a direct effect of PTH on the cells supporting the stability of hematopoietic niches or, alternatively, as a product of increased osteoclastogenesis. This latter possibility has been documented in recent years in studies showing that osteoclast activity may play a role in the dynamics of the hematopoietic niche.(38)
Because we observed that hematopoietic progenitors from bone marrow and spleen at 14 days of PTH treatment increased in percentage and number, and because this correlates with an increase in the activity of osteoclasts (bone-resorption assay), we tested the possibility that osteoclast activity in bone marrow might affect the distribution of hematopoietic progenitors in bone marrow and spleen. Even when alendronate efficiently blocked the increase in bone resorption induced by PTH treatment, we observed no significant differences in percentage or number of LKS cells from mice cotreated with PTH and alendronate compared with PTH treatment alone. These results suggest that the activity of osteoclasts in the bone marrow microenvironment does not influence the dynamics of hematopoietic progenitors and is not responsible for the egress of hematopoietic progenitors to the periphery. Thus the increase we observed of hematopoietic progenitors in the bone marrow and spleen should be caused by a more direct effect of PTH on cells responsible for maintaining the stability of hematopoietic niches.
In conclusion, we propose that PTH, by acting on cells of either the osteoblastic lineage or other populations, indirectly affects specific populations of OCPs by (1) transiently increasing their numbers in vivo, (2) priming them to become more responsive to M-CSF and RANKL, and (3) affecting their proliferation and ability to fuse and form larger osteoclasts. In turn, these changes caused by PTH have the potential to produce osteoclasts that are more active at resorbing bone in vitro. PTH also increased the number of hematopoietic progenitors in bone marrow and spleen, an effect that is independent of the activity of osteoclasts. Insights into the cellular and molecular mechanisms of the anabolic effects of PTH on osteoclastogenesis and bone might help to develop and/or optimize therapeutic strategies to modulate bone remodeling and improve bone quality. In addition, the identification of bona fide osteoclast precursors in peripheral tissues is crucial to understand the distribution and mobilization of these populations within the body after PTH treatment.
Acknowledgments
This work is supported by funds from the National Institutes of Health (AR048714) to JAL and HLA. We thank Judith Kalinowski and Sandra Jastrzebski for technical support.
Footnotes
Disclosures
All the authors state that they have no conflicts of interest.
References
- 1.Walker DG. Osteopetrosis cured by temporary parabiosis. Science. 1973;180:875. doi: 10.1126/science.180.4088.875. [DOI] [PubMed] [Google Scholar]
- 2.Raisz LG. Physiology and pathophysiology of bone remodeling. Clin Chem. 1999;45:1353–1358. [PubMed] [Google Scholar]
- 3.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 4.Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev. 1999;20:345–357. doi: 10.1210/edrv.20.3.0367. [DOI] [PubMed] [Google Scholar]
- 5.Lorenzo J, Horowitz M, Choi Y. Osteoimmunology: interactions of the bone and immune system. Endocr Rev. 2008;29:403–440. doi: 10.1210/er.2007-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuller K, Owens JM, Chambers TJ. Induction of osteoclast formation by parathyroid hormone depends on an action on stromal cells. J Endocrinol. 1998;158:341–350. doi: 10.1677/joe.0.1580341. [DOI] [PubMed] [Google Scholar]
- 7.Zaidi M. Skeletal remodeling in health and disease. Nat Med. 2007;13:791–801. doi: 10.1038/nm1593. [DOI] [PubMed] [Google Scholar]
- 8.Lupp A, Klenk C, Rocken C, Evert M, Mawrin C, Schulz S. Immunohistochemical identification of the PTHR1 parathyroid hormone receptor in normal and neoplastic human tissues. Eur J Endocrinol. 2010;162:979–986. doi: 10.1530/EJE-09-0821. [DOI] [PubMed] [Google Scholar]
- 9.Zhao W, Byrne MH, Boyce BF, Krane SM. Bone resorption induced by PTH is strinkgly diminished in collagenase-resistant mutant mice. J Clin Invest. 1999;103:517–524. doi: 10.1172/JCI5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Juppner H, Abou-Samra A, Freeman M, et al. A G Protein-linked receptor for parathyroid hormone and parathyroid hormone-related peptide. Science. 1991;254:1024–1026. doi: 10.1126/science.1658941. [DOI] [PubMed] [Google Scholar]
- 11.Lee SK, Lorenzo JA. Parathyroid hormone stimulates TRANCE and inhibits osteoprotegerin messenger ribonucleic acid expression in murine bone marrow cultures: correlation with osteoclasts-like cell formation. Endocrinology. 1999;140:3552–3561. doi: 10.1210/endo.140.8.6887. [DOI] [PubMed] [Google Scholar]
- 12.Schiller PC, D’Ippolito G, Roos BA, Howard GA. Anabolic or catabolic responses of MC3T3-E1 osteoblastic cells to parathyroid hormone depend on time and duration of treatment. J Bone Miner Res. 1999;14:1504–1512. doi: 10.1359/jbmr.1999.14.9.1504. [DOI] [PubMed] [Google Scholar]
- 13.Mohan S, Kutilek S, Zhang C, et al. Comparison of bone formation response to parathyroid hormone (1–34), (1–31), and (2–34) in mice. Bone. 2000;27:471–478. doi: 10.1016/s8756-3282(00)00355-0. [DOI] [PubMed] [Google Scholar]
- 14.Frolic CA, Black EC, Cain RL, et al. Anabolic and catabolic bone effects of human parathyroid hormone (1–34) are predicted by duration of hormone exposure. Bone. 2003;33:372–379. doi: 10.1016/s8756-3282(03)00202-3. [DOI] [PubMed] [Google Scholar]
- 15.Koh AJ, Demiralp B, Neiva KG, et al. Cells of the osteoclast lineage as mediators of the anabolic actions of parathyroid hormone in bone. Endocrinology. 2005;146:4584–4596. doi: 10.1210/en.2005-0333. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Liu Y, Buhl K, Rowe DW. Comparison of the action of transient and continuous PTH on primary osteoblast cultures expressing differentiation stage-specific GFP. J Bone Miner Res. 2005;20:5–14. doi: 10.1359/JBMR.041016. [DOI] [PubMed] [Google Scholar]
- 17.Li X, Liu H, Qin L, et al. Determination of dual effects of parathyroid hormone on skeletal gene expression in vivo by microarray and network analysis. J Biol Chem. 2007;282:33086–33097. doi: 10.1074/jbc.M705194200. [DOI] [PubMed] [Google Scholar]
- 18.Pettway GJ, Meganck JA, Koh AJ, Keller ET, Goldstein SA, McCauley LK. Parathyroid hormone mediates bone growth through the regulation of osteoblast proliferation and differentiation. Bone. 2008;42:806–818. doi: 10.1016/j.bone.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Freitas PH, Li M, Ninomiya T, et al. Intermittent PTH administration stimulates pre-osteoblastic proliferation without leading to enhanced bone formation in osteoclast-less c-fos−/− mice. J Bone Miner Res. 2009;24:1586–1597. doi: 10.1359/jbmr.090413. [DOI] [PubMed] [Google Scholar]
- 20.Adams GB, Martin RP, Alley IR, et al. Therapeutic targeting of a stem cell niche. Nat Biotechnol. 2007;25:238–243. doi: 10.1038/nbt1281. [DOI] [PubMed] [Google Scholar]
- 21.Rodan GA, Martin TJ. Therapeutic approaches to bone disease. Science. 2000;289:1508–1514. doi: 10.1126/science.289.5484.1508. [DOI] [PubMed] [Google Scholar]
- 22.Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 23.Cheng Z, Yao W, Zimmermann EA, Busse C, Ritchie RO, Lane NE. Prolonged treatments with antiresorptive agents and PTH have different effects on bone strength and the degree of mineralization in old estrogen-deficient osteoporotic rats. J Bone Miner Res. 2009;24:209–220. doi: 10.1359/jbmr.81005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacquin C, Gran DE, Lee S, Lorenzo JA, Aguila HL. Identification of multiple osteoclast precursor populations in murine bone marrow. J Bone Miner Res. 2006;21:67–77. doi: 10.1359/JBMR.051007. [DOI] [PubMed] [Google Scholar]
- 25.Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the hematopoietic stem cell niche. Science. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 26.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 27.Uchida N, Weissman IL. Searching for hematopoietic stem cells: evidence that Thy-1. 1lo Lin- Sca-1+ cells are the only stem cells in C57BL/Ka-Thy-1.1 bone marrow. J Exp Med. 1992;175:175–184. doi: 10.1084/jem.175.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christensen JL, Weissman IL. Flk-2 is a marker in hematopoietic stem cell differentiation: A simple method to isolate long-term stem cells. Proc Natl Acad Sci USA. 2001;98:14541–14546. doi: 10.1073/pnas.261562798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 30.Jilka RL, O’Brien CA, Ali AA, Roberson PK, Weinstein RS, Manolagas SC. Intermittent PTH stimulates periosteal bone formation by actions on post-mitotic preosteoblasts. Bone. 2009;44:275–286. doi: 10.1016/j.bone.2008.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brunner S, Zaruba M, Huber B, et al. Parathyroid hormone effectively induces mobilization of progenitor cells without depletion of bone marrow. Exp Hematol. 2008;36:1157–1166. doi: 10.1016/j.exphem.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 32.Zhang J, Niu C, Ye L, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 33.Lo Celso C, Fleming HE, Wu JW, et al. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature. 2009;457:92–96. doi: 10.1038/nature07434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Beek ER, Lowik CWGM, Papapoulos SE. Effect of alendronate treatment on the osteoclastogenic potential of bone marrow cells in mice. Bone. 1997;20:335–340. doi: 10.1016/s8756-3282(97)00006-9. [DOI] [PubMed] [Google Scholar]
- 35.Luckman SP, Hughes DE, Coxon FP, Russell GG, Rogers MJ. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res. 1998;13:581–589. doi: 10.1359/jbmr.1998.13.4.581. [DOI] [PubMed] [Google Scholar]
- 36.Graham R, Russel G. Bisphosphonates: Mode of Action and Pharmacology. Pediatrics. 2007;119:S150–S162. doi: 10.1542/peds.2006-2023H. [DOI] [PubMed] [Google Scholar]
- 37.Amelio PD, Grimaldi A, Cristofaro MA, et al. Alendronate reduces osteoclast precursors in osteoporosis. Osteoporos Int. 2010;21:1741–1750. doi: 10.1007/s00198-009-1129-1. [DOI] [PubMed] [Google Scholar]
- 38.Kollet O, Dar A, Lapidot T. The multiple roles of osteoclasts in host defense: bone remodeling and hematopoietic stem cell mobilization. Annu Rev Immunol. 2007;25:51–69. doi: 10.1146/annurev.immunol.25.022106.141631. [DOI] [PubMed] [Google Scholar]