Abstract
Nell-1 is a growth factor required for normal skeletal development and expression of extracellular matrix proteins required for bone and cartilage cell differentiation. We identified the transcription factor nuclear factor of activated T cells (Nfatc2) as a primary response gene of Nell-1 through a microarray screen, with validation using real-time polymerase chain reaction (PCR). We investigated the effects of recombinant Nell-1 protein on the chondrogenic cell line ATDC5 and primary mouse chondrocytes. The osteochondral transcription factor Runx2 was investigated as a possible intermediary between Nell-1 and Nfatc2 using adenoviral overexpression of wild-type and dominant-negative Runx2. Nell-1 transiently induced both transcription and translation of Nfatc2, an effect inhibited by transduction of dominant-negative Runx2, suggesting that Runx2 was necessary for Nfatc2 induction. Differentiation assays revealed inhibitory effects of Nell-1 on ATDC5 cells. Although proliferation was unaffected, expression of chondrocyte-specific genes was decreased, and cartilage nodule formation and proteoglycan accumulation were suppressed. siRNA knockdown of Nfatc2 significantly reversed these inhibitory effects. To elucidate the relationship between Nell-1, Runx2, and Nfatc2 in vivo, their presence and distribution were visualized in femurs of wild-type and Nell1-deficient mice at both neonatal and various developmental stages using immunohistochemistry. All three proteins colocalized in the perichondrium of wild-type femurs but stained weakly or were completely absent in Nell1-deficient femurs at neonatal stages. Thus Nfatc2 likely plays an important role in Nell-1-mediated osteochondral differentiation in vitro and in vivo. To our knowledge, this is the first demonstration that Nfatc2 is a primary response gene of Nell-1.
Keywords: NELL-1, NFATC2, RUNX2, OSTEOCHONDRAL DEVELOPMENT, PERICHONDRIUM
Introduction
Chondrogenesis, a dynamic cellular process that leads to establishment of various types of cartilage, is important during both embryogenesis and adult life. The complex process of chondrogenesis involves several stages, including condensation of mesenchymal cells, proliferation of chondroprogenitor cells, differentiation of chondrocytes, and formation of cartilage.(1) Progress through these stages is mediated by a complex differentiation pathway that involves coordinate expression of various extracellular matrix and transcription factors as well as regulation by growth factors and cytokines.
Nel-like molecule 1 (Nell-1) was first isolated from human calvarial bones and characterized by overexpression in patho-logicsuture specimens of human craniosynostosis patients.(2) Nell-1 is a secreted protein containing a signal peptide sequence and six epidermal growth factor (EGF)–like domains.(3–5) It accelerates osteogenic differentiation and bone formation(2,6,7) and also contributes to proper terminal chondrocyte differentiation during fetal bone development.(8) Thus far, most published reports have focused on the function and mechanism of Nell-1 with respect to osteogenic differentiation and bone formation.(9–12) However, we have reported recently that Nell-1 promotes proliferation of rabbit chondrocytes in a 3D alginate hydrogel microenvironment with deposition of cartilaginous extracellular matrix.(13) In addition, Nell-1 overexpression results in abnormal cartilage formation,(14) whereas loss of Nell-1 function results in reduced gene expression of extracellular matrix proteins critical for osteogenesis and chondrogenesis.(15)
In this study we revealed roles for Nell-1 in the molecular regulation of chondrogenesis using ATDC5 cells, a murine embryonal carcinoma-derived chondroprogenitor cell line that undergoes a multistep chondrogenic differentiation process beginning with mesenchymal condensation and culminating in cartilage formation in vitro.(16,17) The ATDC5 cell line is a commonly used model for dissecting molecular mechanisms underlying the regulation of chondrocyte differentiation during endochondral bone formation.(18,19) Using a microarray screen, and confirming with real-time polymerase chain reaction (PCR), we observed Nell-1-induced upregulation of Nfatc2, a gene encoding a member of the nuclear factor of activated T cells (NFAT) family of transcription factors. We found that Nell-1 could transiently induce Nfatc2 expression without the requirement of new protein synthesis. Importantly, to confirm our results from the ATDC5 line in nonimmortalized, normally chondrogenic cells, we also validated our results with primary chondrocytes harvested from neonatal mouse costal cartilage. We found that Runx2 is required for Nell-1 protein–induced Nfatc2 mRNA expression in vitro and that Nfatc2 and Runx2 may be involved in Nell-1-mediated perichondrium differentiation in vivo. Finally, we explored the relationship between Nell-1, Runx2, and Nfatc2 in vivo in the developing femurs of age-matched wild-type and Nell1-deficient neonatal mice and embryos at various developmental stages. Our results identify new roles for Nell-1 in the regulation of chondrogenic differentiation, by which the homeostasis and programmed osteochondral differentiation of perichondrial cell populations likely are maintained.
Materials and Methods
Cell culture
ATDC5 cells were obtained from the RIKEN Cell Bank (Tsukuba, Japan) and cultured in ATDC maintenance medium composed of a 1:1 mixture of Dulbecco’s modified Eagle’s medium (DMEM) and Ham’s F-12 medium (Invitrogen, Carlsbad, CA, USA) containing 5% fetal bovine serum (FBS; Invitrogen), 10 mg/mL of human transferrin (Sigma-Aldrich, St Louis, MO, USA), and 30 nM sodium selenite (Sigma-Aldrich) at 37°C in a humidified atmosphere consisting of 5% CO2 and 95% air. Primary mouse costal chondrocytes were isolated from P0 to P5 C57BL/6 neonates by blunt dissection of ribcages followed by collagenase digestion, as described previously,(20) and maintained in DMEM supplemented with 10% FBS, 50 μg/mL of l-ascorbic acid, and 10 mM glycerol-2-phosphate.
For primary response gene studies, subconfluent ATDC5 cells or primary chondrocytes were subjected to serum starvation (0.1% FBS) for 18 hours and either left unstimulated or stimulated with recombinant human Nell-1 protein (rhNell-1) for the indicated times. When necessary, cycloheximide (CHX, 10 μg/mL; Sigma-Aldrich) or actinomycin D (ActD, 10 μg/mL; Sigma-Aldrich) was added 30 minutes prior to rhNell-1 induction.
For gene expression studies of chondrogenesis and alcian blue staining, ATDC5 cells were seeded at a density of 6 ×104 cells/well in 6-well plates (Corning, Lowell, MA, USA) and cultured in ATDC5 differentiation medium (ATDC5 maintenance medium supplemented with 10 μg/mL of bovine insulin; Sigma-Aldrich). When necessary, 800 or 2000 ng/mL of rhNell-1 was added to the differentiation medium. The medium was replaced every 3 days.
RNA isolation and quantitative real-time PCR
Total RNA from ATDC5 cells for microarray and real-time PCR evaluation was extracted using TRIzol reagent (Invitrogen). Following ethanol precipitation, total RNA was applied to an RNeasy column (Qiagen, San Diego, CA, USA) for further purification and treated with DNase per the manufacturer’s protocol. cDNA was synthesized from 1 μg of total RNA using the SuperScript III Reverse-Transcriptase Kit (Invitrogen) in a final volume of 20 μL. Real-time PCR reactions were performed routinely in triplicate in 96-well plates using the 7300 Real-Time PCR System instrument (Applied Biosystems, Foster City, CA, USA). For gene expression studies of chondrogenesis, TaqMan Gene Expression Master Mix primer and probe sets were purchased from Applied Biosystems: Col2α1 (Mm00491889_m1), Sox9 (Mm00448840_m1), Acan (Mm00545807_m1), Runx2 (Mm00501580_ml), Col10α1 (Mm00487041_m1), and Gapdh (Mm99999915_g1). For primary response gene studies, Power SYBR Green PCR Master Mix (Applied Biosystems) was used; the sequence and product length for each primer pair were as follows: Gapdh (GenBank Accession Number AK002273.1), forward primer 5′-ATT CAA CGG CAC AGT CAA GG-3′, reverse primer 5′-GAT GTT AGT GGG GTC TCG CTC-3′, product length 91 bp; Nfatc2 (GenBank Accession Number AK161174.1), forward primer 5′-CTT TCA GAT GGG AAT AAA CGT C-3′, reverse primer 5′-TCC TAC TCA CAT AGC AAC AGC A-3′, product length 108 bp.
Microarray data analysis
To screen for primary response genes that are not regulated by newly synthesized proteins, ATDC5 cells were subjected to serum starvation for 18 hours, followed by treatment with the protein synthesis inhibitor CHX (10 μg/mL) for 30 minutes and then PBS (control) or 100 ng/mL of rhNell-1 for another 30 minutes. Total RNA samples were sent to the UCLA DNA Microarray Center, where target preparation and hybridization to the Affymetrix Murine 430 2.0 GeneChip (Affymetrix, Santa Clara, CA, USA) were performed per the manufacturer’s protocol. This GeneChip contains over 39,000 full-length mouse genes and expressed sequence tag clusters from the UniGene database. Data obtained from the hybridization were preprocessed using Affymetrix GeneChip Command Console Software (AGCC) and Expression Console Software (Affymetrix) to generate probe-set intensity data. Expression values were further filtered by retaining only probe sets with a fold change of at least 1.5 in rhNell-1-treated samples compared with controls. Results were submitted to the NCBI Gene Expression Omnibus (GEO) with Accession Number GSE23570.
Total protein extraction and Western blot analysis
ATDC5 cells were seeded at a density of 2 ×106 in a 10-cm cell culture dish; treated with rhNell-1 for 0, 1, 3, 6, 8, or 10 hours; and washed twice with ice-cold PBS solution. For total-protein extracts, cells were resuspended for 15 minutes in 300 μL of radioimmunoprecipitation assay buffer (ThermoFisher Scientific, Rockford, IL, USA) with 1× protease inhibitor (Sigma-Aldrich) and 1×phosphatase inhibitor (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) added. Protein lysates were spun at 15,000g for 15 minutes at 4°C, and supernatants were used for Western blotting.
Then 30 μg of total protein combined with 5 ×loading buffer (ThermoFisher Scientific) was boiled for 10 minutes, separated by SDS-PAGE (4% stacking and 12% resolving gel), and electro-transferred to a nitrocellulose membrane (GE Healthcare, Piscataway, NJ, USA) at 100 V for 1 hour at 4°C. The membrane was blocked for 1 hour with 5% nonfat milk in Tris-buffered saline plus 0.05% Tween 20 incubated with anti-Nfatc2 primary antibody (Cat. No. ab2722, Abcam, Cambridge, MA, USA) at 1:800 dilution in 5% nonfat milk/TBST overnight at 4°C, washed with TBST, and incubated with anti-goat IgG-mouse peroxidase–conjugated secondary antibody (ThermoFisher Scientific) at 1:10,000 dilution in 5% nonfat milk/TBST for 1 hour. Following incubation, the membrane was washed with TBST, and proteins were visualized using the Immun-Star WesternC Chemiluminescent Kit (Bio-Rad, Hercules, CA, USA) per the manufacturer’s instructions. The protein loading control was performed using anti-β-actin primary antibody (Santa Cruz Biotechnology) and its corresponding secondary antibody (ThermoFisher Scientific). Quantitation of Western blot intensity was performed using Quantity One software (Bio-Rad).
ATDC5 proliferation assay
Cell proliferation was determined using a DNA assay as described previously.(21) ATDC5 cells were seeded at 5000 cells/well in 24-well plates containing RPMI 1640 medium supplemented with 0.5% FBS, 10 mg/mL of human transferrin, 30 nM sodium selenite, 10 μg/mL of bovine insulin, and 1% penicillin/streptomycin for 0, 3, 7, 14, and 21 days. At each time point, samples were digested in proteinase K (Invitrogen) solution for 30 minutes at 55°C, and total DNA was quantified subsequently using the Quant-iT PicoGreen dsDNA Kit (Invitrogen). The concentration of DNA in solution was converted to cell numbers using a standard curve of cell number against unit fluorescence for ATDC5 cells.
Alcian blue staining
ATDC5 cells were washed twice with PBS, fixed with methanol at −20° for 2 minutes, stained with 0.1% alcian blue 8GX (Sigma-Aldrich) in 0.1 N HCl overnight, and rinsed repeatedly with distilled water. For quantitative analysis, each culture plate stained with alcian blue was extracted with 150 μL of 6 M guanidine-HCl for 2 hours at room temperature. The extracted dye was transferred to 96-well plates, and optical density was measured at 620 nm.
Small interfering RNA experiments
RNA knockdown experiments were performed using chemically synthesized and annealed small interfering RNA (siRNA) specific to Nfatc2 (Cat No. Mm_Nfatc2_2_HP, Qiagen). When ATDC5 cells reached 30% confluence, cells were transfected with 50 nM Nfatc2 siRNA or nontarget negative control siRNA (Qiagen) using Lipofectamine RNAiMax (Invitrogen) per the manufacturer’s protocol.
Gene transduction of ATDC5 cells
Adenoviral vectors encoding full-length Runx2 cDNA (Ad-Runx2) and LacZ (Ad-LacZ) were generated as described previously.(22,23) Adenoviral vector encoding dominant-negative Runx2 (Ad-DN-Runx2) was kindly provided by Dr Riko Nishimura (Department of Biochemistry, Osaka University Graduate School/Faculty of Dentistry, Osaka, Japan).(24) ATDC5 cells were plated at a density of 5 ×105 cells/well in 6-well plates and then cultured in maintenance medium for 24 hours to reach 80% confluence; then they were transduced at a multiplicity of infection of 100 pfu/cell of Ad-LacZ, Ad-Runx2, or Ad-DN-Runx2. After another 24 hours, cells were treated with PBS or 800 ng/mL of rhNell-1 for 3 hours. RNA was isolated for real-time PCR to quantitate Nfatc2 and Runx2 gene expression.
Immunocytochemical analysis
Serum-starved ATDC5 cells (1 ×105) cultured on glass coverslips in 24-well plates were treated with PBS or 800 or 2000 ng/mL of rhNell-1 for 3 hours, followed by two rinses with ice-cold PBS and fixation with ice-cold methanol for 15 minutes. The fixed cells were permeabilized with PBS containing 0.25% Triton X-100 (PBST) for 10 minutes and blocked with 1% (w/v) bovine serum albumin (BSA) in PBST for 30 minutes at room temperature. After blocking, cells were incubated with anti-Nfatc2 antibody in blocking buffer at room temperature for 1 hour and then overnight at 4°C in a humidified chamber. Following three washes with PBST, cells were incubated with a biotinylated anti-mouse IgG secondary antibody (1:200; Vector Laboratories, Burlingame, CA, USA) in PBS for 1 hour at room temperature. After three more washes with PBS, cells were incubated with Alexa Fluor 488–conjugated streptavidin (Invitrogen) in PBS at room temperature for 30 minutes. After another three washes with PBS, cells were counterstained with Hoechst 33342 (Sigma-Aldrich) for 1 minute, rinsed with PBS, and then mounted onto glass slides. Fluorescence was observed using an Olympus (Center Valley, PA, USA) BX51 microscope.
Immunohistochemistry
Nell1-deficient neonatal mice(15) and wild-type littermates were euthanized and fixed in 10% formalin, and femurs were isolated and embedded in paraffin. Mouse embryos were collected from pregnant C57BL/6 mice, where age was defined as E0.5 on the day the vaginal plug was observed. Then 5-μm-thick paraffin sections were dewaxed in xylenes and rehydrated in ethanol baths. Endogenous peroxidases were blocked by incubating sections in 3% hydrogen peroxide for 20 minutes at room temperature. Sections were incubated with anti-Nell-1, anti-
Runx2, or anti-Nfatc2 primary antibodies at room temperature for 1 hour and overnight at 4°C and then incubated with a biotinylated secondary antibody for 1 hour at room temperature. Positive immunoreactivity was detected using Vectastain ABC kit (Vector Laboratories) and AEC chromogenic substrate (Dako, Carpinteria, CA, USA) per the manufacturer’s instructions. A negative control was performed by replacing primary antibody solutions with PBS. Sections were counterstained with hematoxylin for 30 seconds, followed by rinsing 5 minutes in running water. Photomicrographs were acquired using an Olympus BX51 microscope and MicroFire digital microscope camera with PictureFrame software (Optronics, Goleta, CA, USA).
Statistical analysis
Statistical significance was determined using Student’s t test at a confidence level of 95% ( p <.05).
Results
Induction of NFAT mRNA expression by Nell-1 in ATDC5 cells
We hypothesize that the growth factor Nell-1 affects chondrogenic differentiation by stimulating a signal-transduction pathway involving primary response genes, which subsequently activate multiple molecular mediators. To identify primary response gene candidates induced by Nell-1, we first used microarray analysis to uncover differences between the gene expression profiles of control ATDC5 cells treated with PBS and ATDC5 cells treated with 100 ng/mL of rhNell-1. We found that the expression of three members of the NFAT family of transcription factors, Nfatc1, Nfatc2, and Nfat5, was increased between 1.52- to 2.27-fold compared with PBS at 30 minutes after Nell-1 treatment (Table 1). Expression levels were further confirmed by real-time PCR (Table 1). Subsequent multiple-time-point studies demonstrated that among these NFAT family members, only Nfatc2 demonstrated a consistent response. Notably, Nell-1 did not immediately upregulate Runx2 expression in the context of a screen for Nell-1 primary response genes (Table 1).
Table 1.
Microarray Results With Verification by Real-Time PCR
| Fold change detected by | ||
|---|---|---|
| Gene | Microarray | Real-time PCR |
| Nfatc1 | 1.55 ×0.05* | 1.63 ×0.08* |
| Nfatc2 | 2.27 ×0.09** | 2.13 ×0.10* |
| Nfat5 | 1.52 ×0.04* | 2.04 ×0.11** |
| Runx2 | 1.05 ×0.11 | 1.20 ×0.17 |
p <.05;
p <.01 compared with untreated controls.
Induction of Nfatc2 mRNA expression by Nell-1 is time- and dose-dependent and does not require new protein synthesis
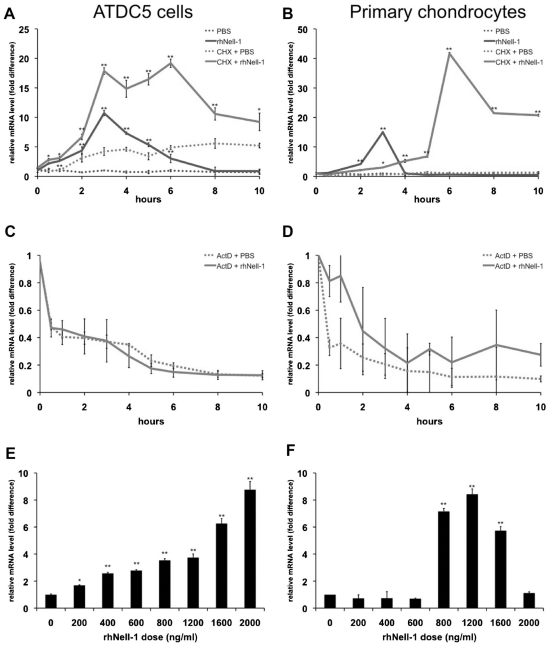
To analyze the time dependence of Nell-1-induced Nfatc2 expression, ATDC5 cells were treated for 0 to 10 hours with a fixed dose of 800 ng/mL of rhNell-1, a relatively high dose, in order to ensure a marked response effect. Real-time PCR analysis showed that Nell-1 rapidly and transiently induced Nfatc2 mRNA levels, which peaked at 3 hours and then returned to almost baseline levels after 8 hours (Fig. 1A). To confirm these results in bona fide chondrogenic cells, we harvested primary chondrocytes from neonatal mice and subjected them to the same time course of treatment with 800 ng/mL of rhNell-1. Nfatc2 was maximally induced at 3 hours and returned to baseline levels soon thereafter (Fig. 1B).
Fig. 1.
Nfatc2 is a primary response target induced by rhNell-1. Nfatc2 transcription level was quantitated by real-time PCR analysis. ATDC5 cells (A) or primary mouse chondrocytes (B) were treated with PBS or 800 ng/mL of rhNell-1 for 0 to 10 hours. Results for cells pretreated with CHX to inhibit protein synthesis are as indicated. ATDC5 cells (C) or primary mouse chondrocytes (D) were treated with PBS or 800 ng/mL of rhNell-1 for 0 to 10 hours in the presence of actinomycin D to inhibit transcription. ATDC5 cells (E) or primary mouse chondrocytes ( F) were treated with rhNell-1 in doses ranging from 0 to 2000 ng/mL. Data represent the mean ±SD from tests of samples in triplicate. *p <.05; **p <.01 versus corresponding PBS-treated controls.
To determine whether Nfatc2 is a primary response target of Nell-1 induction, and to determine whether Nell-1 is required for Nfatc2 gene transcription, ATDC5 cells and primary chondrocytes were treated with either the protein synthesis inhibitor cycloheximide (CHX) or the transcription inhibitor actinomycin D (ActD) prior to rhNell-1 treatment. In both cell types, pretreatment with CHX did not inhibit but rather enhanced Nell-1-induced Nfatc2 mRNA levels for the duration of the experiment (Fig. 1A, B). However, pretreatment with ActD completely inhibited this induction (Fig. 1C, D).
To determine the dose dependence of Nell-1-induced Nfatc2 expression, ATDC5 cells were treated with 0 to 2000 ng/mL of rhNell-1 for a fixed time of 3 hours. Nfatc2 mRNA levels showed an increasing trend with increasing doses of Nell-1 (Fig. 1E). When primary chondrocytes were treated with a dose range of rhNell-1, Nfatc2 expression was not induced at doses lower than 800 ng/mL, and Nfatc2 induction was not observed when 2000 ng/mL of Nell-1 was added (Fig. 1F).
Nell-1 induces Nfatc2 protein synthesis in ATDC5 cells
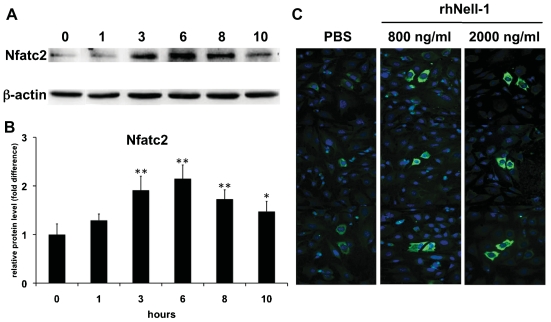
To address the effect of Nell-1 on Nfatc2 protein translation, we performed Western blot analysis of extracts from ATDC5 cells treated with 800 ng/mL of rhNell-1 for 0 to 10 hours using an Nfatc2 protein-specific antibody. As expected, Nfatc2 protein levels were increased significantly after rhNell-1 treatment, starting at 3 hours, peaking at 6 hours, and declining thereafter (Fig. 2A, B). The time at which the Nfatc2 protein level reached its peak is about 3 hours later than the peak of its mRNA level, which may be accounted for by the time required for protein translation. In addition, immunocytochemical staining confirmed that Nell-1 treatment increased Nfatc2 protein level after 3 hours (Fig. 2C).
Fig. 2.
rhNell-1 induces Nfatc2 protein synthesis in ATDC5 cells. (A) Western blot of total lysates of ATDC5 cells treated with 800 ng/mL of rhNell-1 for 0 to 10 hours. β-actin was included as a protein loading control. (B) Quantitative plot of Nfatc2 protein level normalized to β-actin using Quantity One software. Data represent the mean ± SD from tests of samples in triplicate. *p <.05; **p <.01 versus control (0 hour). (C) Subcellular localization of Nfatc2 protein in ATDC5 cells with or without 800 ng/mL of rhNell-1 treatment for 3 hours. Nfatc2 protein was stained with anti-Nfatc2 antibody (green), and nuclei were counterstained with Hoechst 33342 (blue). Representative image from one of three independent experiments. Original magnification ×200.
Effects of Nell-1 on chondrogenesis in ATDC5 cells
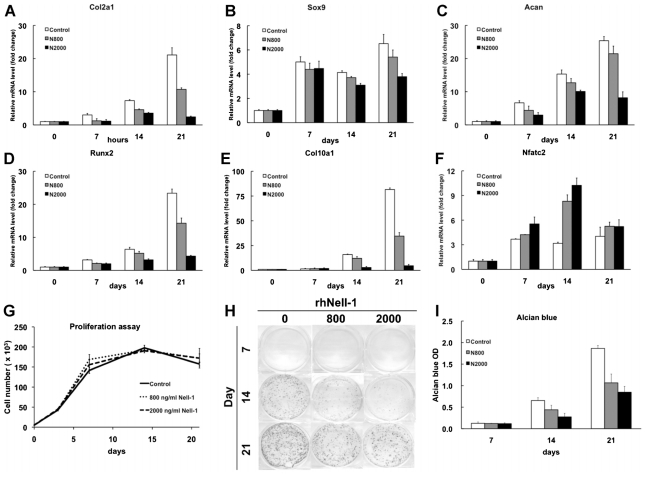
To investigate the ability of Nell-1 to influence chondrogenesis, ATDC5 cells were cultured in differentiation medium with or without rhNell-1 for 21 days. Insulin was added to the medium because it has been reported to accelerate chondrogenic differentiation of ATDC5 cells.(25) Sustained exposure to rhNell-1 led to a marked decrease in mRNA expression of the proliferating chondrocyte markers Col2a1, Sox9, and Acan and the hypertrophic chondrocyte markers Runx2 and Col10a1 in cells cultured for 14 and 21 days (Fig. 3A–E). Importantly, Nfatc2 gene expression was increased significantly on days 7 and 14 of Nell-1 stimulation (Fig. 3F). Cell proliferation analysis revealed that cell number did not differ between cells cultured in the presence or absence of Nell-1, suggesting that Nell-1 had no significant effect on cell proliferation (Fig. 3G).
Fig. 3.
Chondrogenic effects of rhNell-1 on ATDC5 cells. ATDC5 cells were cultured in differentiation medium with or without 800 or 2000 ng/mL of rhNell-1 for 21 days. Relative mRNA levels of Col2α1 (A), Sox9 (B), Acan (C), Runx2 (D), Col10α1 (E), and Nfatc2 ( F) compared with Gapdh were quantitated by real-time PCR. (G) The effect of Nell-1 on ATDC5 cell proliferation over 21 days was quantitated by DNA content. (H) Cell surface proteoglycans were detected by alcian blue staining on days 7, 14, and 21. (I) Quantitative assessment of alcian blue staining. Data represent the mean ± SD from tests of samples in triplicate. *p <.05; **p <.01 versus control.
When cultured in the presence of insulin, ATDC5 cells aggregate and form cartilaginous nodules, an important phenotype characteristic of chondrogenic differentiation.(25) On days 14 and 21, many nodules strongly staining for alcian blue were observed, indicating differentiation of ATDC5 cells into chondrocytes and concomitant production of significant levels of proteoglycan. However, addition of 800 or 2000 ng/mL of rhNell-1 to ATDC5 cells cultured with insulin significantly inhibited nodule formation and proteoglycan accumulation (Fig. 3H, I).
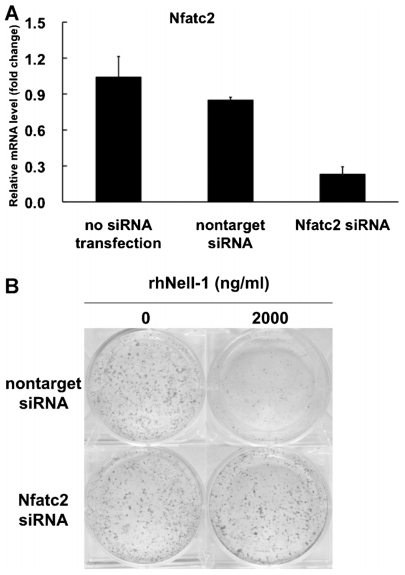
Effect of Nfatc2 siRNA on Nell-1-mediated inhibition of ATDC5 chondrogenic differentiation
To investigate whether Nfatc2 expression mediates the effects of rhNell-1 on chondrogenic differentiation of ATDC5 cells, we used siRNA to attenuate Nfatc2 expression. siRNA efficiency was tested on day 3 after transfection by real-time PCR, which showed 70% inhibition of Nfatc2 mRNA levels (Fig. 4A). After incubation in differentiation medium with or without rhNell-1 for 14 days, proteoglycan accumulation was detected by alcian blue staining (Fig. 4B). Without Nell-1 stimulation, there was no significant difference between cells cultured in differentiation medium and cells transfected with nontarget control versus Nfatc2 siRNA. With Nell-1 stimulation, proteoglycan accumulation in cells transfected with nontarget control siRNA was inhibited significantly, comparable with results from untransfected cells (Fig. 3H). However, this inhibition was abolished when cells were transfected with Nfatc2 siRNA. These results suggest that rhNell-1 inhibition of chondrogenic differentiation depends on Nfatc2 expression.
Fig. 4.
The effect of Nfatc2 siRNA on Nell-1-mediated inhibition of ATDC5 cell chondrogenic differentiation. (A) Nfatc2 siRNA efficiency test. Cells were cultured in maintenance medium for 3 days after transfection, and Nfatc2 gene expression was detected by real-time PCR. Data represent the mean ± SD from tests of samples in triplicate. **p <.01 compared with nontarget control siRNA. (B) ATDC5 cells were transfected with nontarget control siRNA or Nfatc2 siRNA on day 1 and cultured in differentiation medium with or without 2000 ng/mL of rhNell-1. Proteoglycan production was assessed by alcian blue staining on day 14.
Nell-1 induction of Nfatc2 mRNA expression requires Runx2 in ATDC5 cells
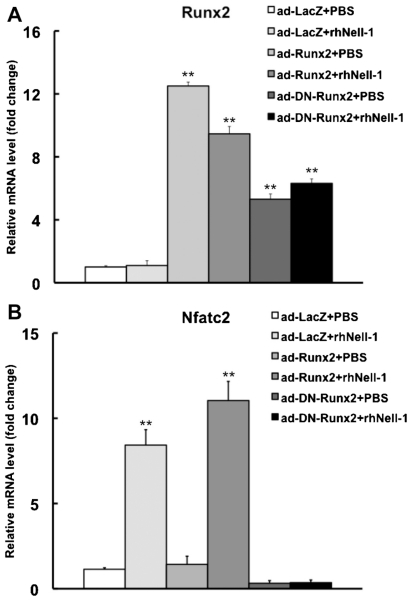
Previous work reported that Runx2 regulates Nfatc2 transcription,(26) whereas Nell-1 induces Runx2 phosphorylation.(12) To further explore whether Runx2 is a possible mediator of Nell-1-induced Nfatc2 expression, ATDC5 cells were transduced with adenovirus containing either LacZ (Ad-LacZ), Runx2 (Ad-Runx2), or a dominant-negative mutant of Runx2 (Ad-DN-Runx2) and then treated with PBS or 800 ng/mL of rhNell-1 for 3 hours on the following day. We confirmed that Ad-Runx2 and Ad-DN-Runx2 increased Runx2 expression levels (Fig. 5A). Gene expression analysis revealed that Nell-1 could induce Nfatc2 expression in Ad-LacZ-transduced cells, as expected, and that this induction was mildly enhanced in Ad-Runx2-transduced cells. On the other hand, in cells transduced with Ad-DN-Runx2, Nfatc2 expression level was decreased about threefold compared with Ad-LacZ-transduced cells, and Ad-DN-Runx2-transduced cells lost the ability to induce Nfatc2 expression in response to rhNell-1 stimulation (Fig. 5B).
Fig. 5.
Nell-1 induction of Nfatc2 mRNA expression requires Runx2. ATDC5 cells were transduced with Ad-LacZ, Ad-Runx2, or Ad-DN-Runx2 for 24 hours and then treated with PBS or 800 ng/mL of rhNell-1 for 3 hours. Real-time PCR was used to detect Nfatc2 mRNA expression level. Data represent the mean ± SD from tests of samples in triplicate. **p <.01 versus corresponding PBS-treated controls.
In vivo distribution of Nell-1, Nfatc2, and Runx2 in wild-type and Nell1-deficient mice
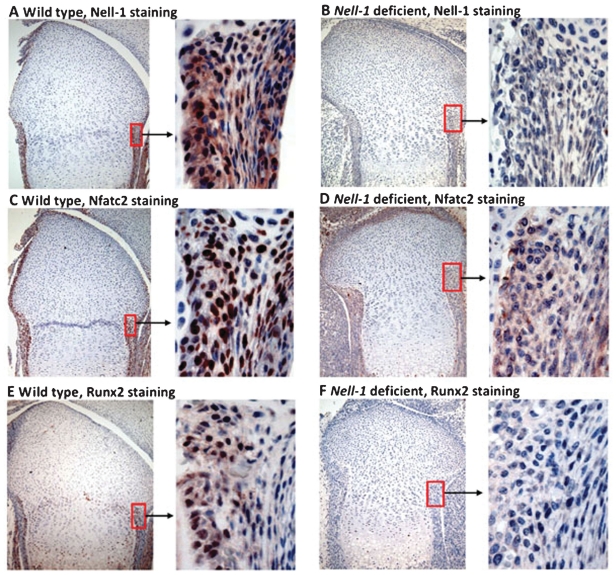
To explore the relationship between Nell-1, Nfatc2, and Runx2 during osteochondrogenesis in vivo, we analyzed their expression patterns in the distal femurs of neonatal mice with either wild-type or deficient Nell1(15) expression. Immunohistochemistry revealed similar distribution of Nell-1 and Nfatc2 in wild-type mice, with colocalization predominantly in the nuclei of perichondrial cells (Fig. 6A, C). In contrast, both Nell-1 and Nfatc2 were absent or weakly present in the perichondrium of Nell1-deficient mouse femurs (Fig. 6B, D). In addition, Runx2 exhibited a different expression pattern from Nell-1 and Nfatc2; it was present only in the inner chondrogenic layer but not the outer fibrous layer of the perichondrium of wild-type femurs (Fig. 6E), whereas Runx2 was completely absent in Nell1-deficient femurs (Fig. 6F). Similarly, the intracellular distribution of Nell-1, Nfatc2, and Runx2 was localized predominantly to the nuclei of positive cells in distal femurs of samples from various embryonic stages of mouse development (Supplemental Fig. S1). However, the expression patterns of positive cells in embryonic samples differed from those in neonatal samples. In particular, most chondrocytes in the distal end of E15.5 and E16.5 femurs stained strongly for Nell-1 and Nfatc2 but not Runx2 without obvious positivity of perichondrial cells (Supplemental Fig. S1A–F). Interestingly, chondrocytes with relatively rich cartilaginous matrix in E18.5 femurs rarely expressed Nell-1, Nfatc2, or Runx2 (Supplemental Fig. S1G–I), which was similar to the neonatal pattern. Runx2 was profoundly expressed by cells in and around the primary ossification center, along with some prehypertrophic chondrocytes in the femurs at all three stages studied (data not shown).
Fig. 6.
Immunohistochemical detection of Nell-1, Nfatc2, and Runx2 in distal femurs of wild-type and Nell1-deficient neonatal mice. Staining for Nell-1 in wild-type (A) and Nell1-deficient (B) mice. Staining for Nfatc2 in wild-type (C) and Nell1-deficient mice (D). Staining for Runx2 in wild-type (E) and Nell1-deficient mice (F). Left column: Low magnification (original at ×100) of distal femur; right column: High magnification (original at ×400) emphasizing perichondrium area.
Discussion
Cartilage, a complex connective tissue of diverse embryonic origin, contains a unique set of extracellular matrix (ECM) molecules and serves multiple prenatal and postnatal functions. Chondrocytes are the major cell type in cartilage and are responsible for the precise production of ECM at different stages of differentiation in several different types of cartilage tissue.(27) The differentiation of chondrocytes from mesenchymal cells occurs along a multistep differentiation pathway involving coordinate expression of transcription factors and regulation by growth factors. For instance, the transcription factor Sox9 is required for both mesenchymal condensation and physiologic inhibition of hypertrophic conversion of proliferating chondrocytes. In addition, Sox9 null cells do not express chondrogenic marker genes such as Col2α1, Col9α2, Col11α2, and Acan.(28) Parathyroid hormone–related peptide (PTHrP) is known as an important local factor for chondrogenesis, promoting chondrocyte proliferation and inhibiting chondrocyte differentiation into the hypertrophic phenotype.(29) The transforming growth factor β (TGF-β) superfamily, including the TGF-β subfamily and bone morphogenetic protein (BMP) subfamily, is essential for proper chondrocyte differentiation and production of cartilage matrix. During development of the growth plate, they play distinct roles. TGF-β signaling inhibits the hypertrophic differentiation to preserve an adequate number of chondrocytes, whereas BMP signaling promotes the maturation of chondrocytes to facilitate ossification.(30–32)
In this study, we searched for primary response genes of the growth factor Nell-1 in order to uncover possible roles for Nell-1 in chondrogenesis. Nell-1 is a novel growth factor believed to specifically target cells committed to the osteochondral lineage.(2,6) Nell1-deficient mice exhibit reduced expression of numerous extracellular matrix proteins required for differentiation of bone and cartilage precursor cells.(15) As a secreted molecule, Nell-1 is expected to bind to cell surface receptors and activate signal-transduction cascades. We have reported that Nell-1 transiently activates the mitogen-activated protein kinase (MAPK) signaling cascade, induces the phosphorylation of Runx2, and promotes the rapid intracellular accumulation of Tyr-phosphorylated proteins,(12) but the exact molecular and cellular mechanisms underlying the regulation of chondrogenesis and osteogenesis by Nell-1 remain unknown. For our in vitro studies, we elected to use ATDC5, which is a well-studied embryonal carcinoma-derived chondrogenic cell line.(16,25,33) Importantly, to determine whether our observed results in ATDC5 cells were sufficiently representative of bona fide chondrogenic cells, we validated our real-time PCR experiments in primary chondrocytes and confirmed similar responses to Nell-1. In both cases, blockade of translation by CHX with or without Nell-1 stimulation resulted in sustained elevated expression of Nfatc2 instead of a transient elevation, suggesting that the regulation of Nell-1-induced Nfatc2 expression may require synthesis of a protein repressor that allows tight temporal control of Nfatc2 expression; similar mechanisms have been described in other systems.(34) Although we have not identified the repressor(s), selectively altering its expression may shed light on the role of Nfatc2 during development.
Significant differences between ATDC5 and primary chondrocytes still may exist; indeed, although a similar trend of induction of Nfatc2 expression was observed in both ATDC5 cells and primary chondrocytes (Fig. 1A, B), the levels and onset of maximal induction differed between the two cell types. This could be attributed to inherent fundamental differences between the two cell types. ATDC5 cells are not chondrocytes per se; they are chondrogenic, immortalized, relatively undifferentiated cells whose differentiation to a chondrocyte-like phenotype is insulin-dependent.(16,18,25,33) In contrast, primary chondrocytes are already committed and do not require insulin to maintain the chondrocyte phenotype in our culture system.(20) In addition, given the large number of cells required for this experiment, interindividual variability in the response to Nell-1 resulting from harvesting primary chondrocytes from multiple litters of pups could play a role. As a result, we elected to use ATDC5 cells for our subsequent experiments to minimize this variability and improve the consistency of our results.
Using a microarray screen followed by real-time PCR validation, we found that the Nfatc2 mRNA and protein were induced by Nell-1 in a time- and dose-dependent manner in ATDC5 cells. Pretreatment with CHX resulted in chronically high Nfatc2 expression, indicating that protein synthesis was not necessary for Nfatc2 production and strongly suggesting that Nfatc2 is a primary response gene of Nell-1. The NFAT family of proteins, originally identified in T cells,(35) consists of transcription factors that induce cytokine gene expression and are central during the immunologic response.(36–39) Their activation is controlled by dephosphorylation by calcineurin, a Ca2+-dependent phosphatase,(40) followed by translocation from the cytoplasm to the nucleus.(41) Further studies showed that NFATs regulate different genes in various cell types(42) and that NFAT signaling is also involved in bone resorption and bone formation.(43–45) However, NFATs play variable roles in chondrogenesis.(46,47) For example, NFAT4 is known to promote chondrogenesis.(47) In contrast, Nfatc2-deficient adult mice have shown enhancement of cartilage phenotype in load-bearing joints caused by hyperproliferation of articular chondrocytes and ectopic chrondrogenesis in the periarticular soft tissues.(46) Nfatc2-deficient chondrocytes possess an intrinsic increase in proliferation and express higher levels of chondrocyte marker genes (Col2α1 and Col10α1) in vitro than wild-type cells. Additionally, Nfatc2 is specifically regulated during chondrogenic differentiation of mesenchymal cells in vitro. Therefore, Nfatc2 is believed to be a repressor/inhibitor of chondrogenesis.(46) That the calcineurin inhibitor FK506 (tacrolimus) induces chondrogenic differentiation of ATDC5 cells(16) supports this conclusion. Collectively, these results suggest that chondrocyte differentiation is inhibited via the calcineurin-NFAT signaling pathway and that agents targeting this pathway may promote cartilage formation.(48) Recently, histopathologic changes in the skeletal system of Nfatc2-deficient mice have been described, showing that expression of cartilage matrix genes such as Acan, Col2α1, Col9α1, and Col11α1 is decreased, but expression of Col10α1 and genes coding for proteinases and proinflammatory cytokines is increased in mouse joints before 3 months. It was suggested that the phenotype of Nfatc2-deficient mice is more consistent with the repair process in human osteoarthritis than chondrocyte proliferation.(49) In addition, some recent studies have indicated that Nfatc2 also controls the balance between anabolic and catabolic pathways of cartilage formation.(49–51)
In this study, we did not observe any significant nuclear translocation of Nfatc2 on Nell-1 stimulation. Thus, although Nell-1 increases Nfatc2 protein levels, it alone may not be sufficient to increase Nfatc2 activity. Rather, Nell-1 may need to interact with other signaling cascades or molecular factors that activate the calcineurin pathway in order to synergistically enhance Nfatc2 activity. In fact, NFATs bind only weakly to DNA and require a partner protein (eg, AP1 or GATA4, but this varies among cell types) to initiate transcriptional activation.(52) NFAT transactivation involves not only its nuclear translocation but also the intrinsic function of its transactivation domain.(53) Increasing evidence supports the notion that enhancement of the N-terminal transactivating domain of Nfatc2 plays a role in its activation.(54,55)
Addition of insulin to ATDC5 induces chondrogenic differentiation,(25) but we found that adding Nell-1 suppressed both chondrocyte gene expression and alcian blue staining phenotype. Interestingly, we found no significant effect on cell proliferation by Nell-1 stimulation. Taken together, these data suggest that Nell-1 could decrease the rate of differentiation of ATDC5 cells from prechondrocytes to mature chondrocytes without detectable inhibition of cell proliferation. siRNA knockdown of Nfatc2 expression and adenoviral transduction of dominant-negative Runx2 suggest that Nell-1-mediated inhibition of chondrogenic differentiation is Nfatc2- and Runx2-dependent, respectively. Thus, our analysis of cellular and molecular phenotype suggests Nell-1-mediated inhibition of chondrogenic differentiation in ATDC5 cells and participation of Nfatc2 in this process. In contrast, we reported that Nell-1 promoted proliferation and differentiation of rabbit chondrocytes seeded within a 3D scaffold(13) and reduced proliferation via Sox9 regulation and accelerated chondrocyte hypertrophy and endochondral bone formation in a distracted rat inter-maxillary suture organ culture model.(56) These paradoxical effects may be related to different cell types, differentiation stages, culture conditions, methods, timing, and dose of Nell-1 protein. In addition, our study was conducted on isolated mouse cells of a single type in monolayer in vitro culture, which differs from the experimental conditions of either of our previous studies. These paradoxical results suggest that Nell-1 may not elicit a universal response in chondrocytes; rather, we expect that the effect will depend on cell and species type, differentiation status, specific cell culture conditions, influence of other cells in the culture system, and anatomic context.
Runt homology domain transcription factor 2 (Runx2) is a multifunctional transcription factor that controls skeletal development by regulating the expression of many extracellular matrix protein genes during chondrocyte and osteoblast differentiation.(57) Runx2 is required for chondrocyte maturation and regulates Col10α1 expression in hypertrophic chondrocytes as well as expression of Spp1, Ibsp, and Mmp13 in terminal hypertrophic chondrocytes.(58) In Runx2-deficient mice, chondrocyte maturation is severely but not completely disrupted.(59)
A previous report showed that Runx2 gene expression is upregulated in prehypertrophic and hypertrophic ATDC5 -cells.(60) Another study demonstrated that Runx2 regulates Nfatc2 by binding to its promoter in C3H10T1/2 mesenchymal cells,(26) while Nell-1 protein is involved in Runx2 phosphorylation.(12) In addition, this study demonstrated that Nell-1-induced Nfatc2 expression does not depend on de novo protein synthesis but is mediated by preexisting proteins. Thus we predicted that Runx2 might be a mediator of Nell-1-regulated induction of Nfatc2 during chondrogenesis. Our adenoviral transduction experiments showed that Nell-1-induced Nfatc2 expression in cells transduced with Ad-LacZ, but overexpressing Runx2 itself did not affect Nfatc2 expression. This phenomenon was less obvious than it was in previous studies in C3H10T1/2 cells.(26) In contrast, a dominant-negative mutant of Runx2 suppressed endogenous Nfatc2 expression as well as Nell-1-induced Nfatc2 expression. Therefore, upregulation of Nfatc2 expression by Nell-1 likely requires adequate Runx2 expression and/or access to OSE2 sites in the Nfatc2 promoter in ATDC5 cells. Our findings suggest that Nell-1 induction of Nfatc2 expression requires Runx2.
Finally, our immunohistochemistry results suggest that Nell-1 is required for proper Nfatc2 expression in vivo because Nell1-deficient mice fail to express Nell-1, Runx2, or Nfatc2. The fetal growth plate is an ideal location to examine chondrogenesis in vivo because it contains distinct cellular zones corresponding to the differentiation status of the chondrocytes within. Nell-1, Runx2, and Nfatc2 were colocalized in the perichondrium and osteoblasts in trabecular bone (data not shown) of wild-type mice, and all were absent or stained only weakly in Nell1-deficient mice, suggesting that Nell-1, Runx2, and Nfatc2 have a close relationship in vivo at neonatal stages and that Nell-1 plays a significant role in Nfatc2 and Runx2 expression. Notably, the predominant intranuclear localization of endogenous Nell-1 was unexpected but not completely surprising because we (manuscript in preparation) and others(61) have reported that Nell-1 can bind nuclear proteins. In addition, we have evidence that Nell-1 can exist as an alternative isoform (data not shown) that may preferentially localize or transfer to the nucleus when activated.
In fetal bone development, some perichondrial cells become osteoblasts that populate both future cortical and trabecular bone, whereas others become chondrocytes.(62) Perichondrial cells have two broad functions: First, they signal to underlying chondrocytes and receive signals from these cells in turn, and second, they provide cells that become osteoblasts and chondrocytes in a carefully orchestrated fashion.(62) We suggest that Nfatc2 may be involved in Nell-1-mediated differentiation of perichondrial cells into osteoblasts or chondrocytes and that Nell-1 may inhibit chondrocyte proliferation and hypertrophy in the perichondrium through Runx2 because Runx2 is known to inhibit chondrocyte proliferation and hypertrophy in the perichondrium.(53) Interestingly, analysis of E15.5 and E16.5 embryos revealed patterns of Nell-1, Nfatc2, and Runx2 expression in the developing femoral cartilage distinct from those at the later E18.5 and neonatal stages. This preliminary observation may suggest that coordinate regulation of chondrocyte maturation in appendicular bone by Nell-1 and Nfatc2 is more important at earlier developmental stages, whereas Runx2 is more important during the transition of prehypertrophic to hypertrophic chondrocytes, as well as during ossification of the bone collar. The effects Nell-1 and Nfatc2 may have on cartilaginous tissues and chondrocytes in skeletally mature mice remain undefined, and their determination is an important goal for a comprehensive understanding of our current findings.
In conclusion, our results demonstrate for the first time that Nfatc2 is a primary response gene of Nell-1. Future studies will be aimed at elucidating a precise mechanism for Nfatc2 function in chondrogenesis and identifying specific interactions between Nfatc2 and potential binding partners. An improved understanding of the mechanism by which Nell-1 drives chondrogenic differentiation will be essential not only for delineating the respective roles of Nell-1 and Nfatc2 during cartilage development but also determining their roles in cartilage repair and regeneration in adult life and engineering of cartilage tissue to replace or repair injuries or age-related damage in human patients.
Supplementary Data
Acknowledgments
W Chen, X Zhang, and RK Siu contributed equally to the work.
We would like to thank Dr Renny Franceschi at the University of Michigan for providing Runx2 adenovirus, Dr Riko Nishimura at Osaka University for providing DN-Runx2 adenovirus, and Kristine Estrada (Karen Lyons lab) at UCLA for technical assistance with primary mouse chondrocytes. This work was supported by the NIH/NIDCR Grants R21-DE0177711, R01-DE01607, T32-DE007296, UC Discovery Grant 07–10677, US Army Medical Research Acquisition Activity (Log No. 07128099), Musculoskeletal Transplant Foundation Grant 20082668, and the Thomas R. Bales Endowed Chair.
Footnotes
Disclosures
Bone Biologics, Inc., licensed Nell-1-related patents from UCLA. CS, KT, and XZ are founders of Bone Biologics, Inc., and inventors of the related patents. CTC is the founder of NellOne, a company developing cardiovascular applications for the Nell-1 protein. All the other authors state that they have no conflicts of interest.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Bilezikian JP, Raisz L, Rodan G. Principles of Bone Biology. 2d ed. San Diego, CA: Academic Press; 2002. [Google Scholar]
- 2.Ting K, Vastardis H, Mulliken JB, et al. Human NELL-1 expressed in unilateral coronal synostosis. J Bone Miner Res. 1999;14:80–89. doi: 10.1359/jbmr.1999.14.1.80. [DOI] [PubMed] [Google Scholar]
- 3.Bornstein P, McKinney CE, LaMarca ME, et al. Metaxin, a gene contiguous to both thrombospondin 3 and glucocerebrosidase, is required for embryonic development in the mouse: implications for Gaucher disease. Proc Natl Acad Sci USA. 1995;92:4547–4551. doi: 10.1073/pnas.92.10.4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuroda S, Oyasu M, Kawakami M, et al. Biochemical characterization and expression analysis of neural thrombospondin-1-like proteins NELL1 and NELL2. Biochem Biophys Res Commun. 1999;265:79–86. doi: 10.1006/bbrc.1999.1638. [DOI] [PubMed] [Google Scholar]
- 5.Kuroda S, Tanizawa K. Involvement of epidermal growth factor-like domain of NELL proteins in the novel protein-protein interaction with protein kinase C. Biochem Biophys Res Commun. 1999;265:752–757. doi: 10.1006/bbrc.1999.1753. [DOI] [PubMed] [Google Scholar]
- 6.Zhang X, Kuroda S, Carpenter D, et al. Craniosynostosis in transgenic mice overexpressing Nell-1. J Clin Invest. 2002;110:861–870. doi: 10.1172/JCI15375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang X, Carpenter D, Bokui N, et al. Overexpression of Nell-1, a craniosynostosis-associated gene, induces apoptosis in osteoblasts during craniofacial development. J Bone Miner Res. 2003;18:2126–2134. doi: 10.1359/jbmr.2003.18.12.2126. [DOI] [PubMed] [Google Scholar]
- 8.Siu RK, Zhang X, Ko T, et al. Nell-1 deficient mice exhibit abnormal strucutre in spinal and long bones. 31st Annual Meeting of the American Society for Bone and Mineral Research; Denver, CO, USA. 2009. [Google Scholar]
- 9.Cowan C, Aghaloo T, Chou Y, et al. Nell-1 induces osteogenic differentiation and bone formation within calvarial defects. J Am Coll Surg. 2005;201:S61. [Google Scholar]
- 10.Aghaloo T, Cowan CM, Chou YF, et al. Nell-1-induced bone regeneration in calvarial defects. Am J Pathol. 2006;169:903–915. doi: 10.2353/ajpath.2006.051210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cowan CM, Jiang X, Hsu T, et al. Synergistic effects of Nell-1 and BMP-2 on the osteogenic differentiation of myoblasts. J Bone Miner Res. 2007;22:918–930. doi: 10.1359/jbmr.070312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bokui N, Otani T, Igarashi K, et al. Involvement of MAPK signaling molecules and Runx2 in the NELL1-induced osteoblastic differentiation. FEBS Lett. 2008;582:365–371. doi: 10.1016/j.febslet.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee M, Siu RK, Ting K, Wu BM. Effect of Nell-1 delivery on chondrocyte proliferation and cartilaginous extracellular matrix deposition. Tissue Eng Part A. 2010;16:1791–1800. doi: 10.1089/ten.TEA.2009.0384. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X, Cowan CM, Jiang X, et al. Nell-1 induces acrania-like cranioskeletal deformities during mouse embryonic development. Lab Invest. 2006;86:633–644. doi: 10.1038/labinvest.3700430. [DOI] [PubMed] [Google Scholar]
- 15.Desai J, Shannon ME, Johnson MD, et al. Nell1-deficient mice have reduced expression of extracellular matrix proteins causing cranial and vertebral defects. Hum Mol Genet. 2006;15:1329–1341. doi: 10.1093/hmg/ddl053. [DOI] [PubMed] [Google Scholar]
- 16.Nishigaki F, Sakuma S, Ogawa T, Miyata S, Ohkubo T, Goto T. FK506 induces chondrogenic differentiation of clonal mouse embryonic carcinoma cells, ATDC5. Eur J Pharmacol. 2002;437:123–128. doi: 10.1016/s0014-2999(02)01269-4. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura Y, Takarada T, Kodama A, Hinoi E, Yoneda Y. Predominant promotion by tacrolimus of chondrogenic differentiation to proliferating chondrocytes. J Pharmacol Sci. 2009;109:413–423. doi: 10.1254/jphs.08315fp. [DOI] [PubMed] [Google Scholar]
- 18.Atsumi T, Miwa Y, Kimata K, Ikawa Y. A chondrogenic cell line derived from a differentiating culture of AT805 teratocarcinoma cells. Cell Differ Dev. 1990;30:109–116. doi: 10.1016/0922-3371(90)90079-c. [DOI] [PubMed] [Google Scholar]
- 19.Shukunami C, Ishizeki K, Atsumi T, Ohta Y, Suzuki F, Hiraki Y. Cellular hypertrophy and calcification of embryonal carcinoma-derived chondrogenic cell line ATDC5 in vitro. J Bone Miner Res. 1997;12:1174–1188. doi: 10.1359/jbmr.1997.12.8.1174. [DOI] [PubMed] [Google Scholar]
- 20.Gosset M, Berenbaum F, Thirion S, Jacques C. Primary culture and phenotyping of murine chondrocytes. Nat Protoc. 2008;3:1253–1260. doi: 10.1038/nprot.2008.95. [DOI] [PubMed] [Google Scholar]
- 21.Shea LD, Wang D, Franceschi RT, Mooney DJ. Engineered bone development from a pre-osteoblast cell line on three-dimensional scaffolds. Tissue Eng. 2000;6:605–617. doi: 10.1089/10763270050199550. [DOI] [PubMed] [Google Scholar]
- 22.Ohgushi H, Okumura M. Osteogenic capacity of rat and human marrow cells in porous ceramics. Experiments in athymic (nude) mice. Acta Orthop Scand. 1990;61:431–434. doi: 10.3109/17453679008993556. [DOI] [PubMed] [Google Scholar]
- 23.Nakagawa T, Sugiyama T, Shimizu K, et al. Characterization of the development of ectopic chondroid/bone matrix and chondrogenic/osteogenic cells during osteoinduction by rhBMP-2: a histochemical and ultrastructural study. Oral Dis. 2003;9:255–263. doi: 10.1034/j.1601-0825.2003.02912.x. [DOI] [PubMed] [Google Scholar]
- 24.Nishimura R, Hata K, Harris SE, Ikeda F, Yoneda T. Core-binding factor alpha 1 (Cbfa1) induces osteoblastic differentiation of C2C12cells without interactions with Smad1 and Smad5. Bone. 2002;31:303–312. doi: 10.1016/s8756-3282(02)00826-8. [DOI] [PubMed] [Google Scholar]
- 25.Shukunami C, Shigeno C, Atsumi T, Ishizeki K, Suzuki F, Hiraki Y. Chondrogenic differentiation of clonal mouse embryonic cell line ATDC5 in vitro: differentiation-dependent gene expression of parathyroid hormone (PTH)/PTH-related peptide receptor. J Cell Biol. 1996;133:457–468. doi: 10.1083/jcb.133.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thirunavukkarasu K, Pei Y, Moore TL, Wei T, Wang H, Chandrasekhar S. Regulation of NFATc2 gene expression by the transcription factor Runx2. Mol Biol Rep. 2007;34:1–10. doi: 10.1007/s11033-006-9001-2. [DOI] [PubMed] [Google Scholar]
- 27.Shum L, Nuckolls G. The life cycle of chondrocytes in the developing skeleton. Arthritis Res. 2002;4:94–106. doi: 10.1186/ar396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akiyama H. Control of chondrogenesis by the transcription factor Sox9. Mod Rheumatol. 2008;18:213–219. doi: 10.1007/s10165-008-0048-x. [DOI] [PubMed] [Google Scholar]
- 29.Li M, Amizuka N. [Histological function of PTHrP in cartilage] Clin Calcium. 2006;16:1221–1227. [PubMed] [Google Scholar]
- 30.Yang G, Yang X. Roles of TGF-b superfamily in the genesis, development and maintenance of cartilage. Yi Chuan. 2008;30:953–959. doi: 10.3724/sp.j.1005.2008.00953. [DOI] [PubMed] [Google Scholar]
- 31.Pogue R, Lyons K. BMP signaling in the cartilage growth plate. Curr Top Dev Biol. 2006;76:1–48. doi: 10.1016/S0070-2153(06)76001-X. [DOI] [PubMed] [Google Scholar]
- 32.Yang X, Chen L, Xu X, Li C, Huang C, Deng CX. TGF-beta/Smad3 signals repress chondrocyte hypertrophic differentiation and are required for maintaining articular cartilage. J Cell Biol. 2001;153:35–46. doi: 10.1083/jcb.153.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen L, Fink T, Zhang XY, Ebbesen P, Zachar V. Quantitative transcriptional profiling of ATDC5 mouse progenitor cells during chon-drogenesis. Differentiation. 2005;73:350–363. doi: 10.1111/j.1432-0436.2005.00038.x. [DOI] [PubMed] [Google Scholar]
- 34.Efrat S, Kaempfer R. Control of biologically active interleukin 2 messenger RNA formation in induced human lymphocytes. Proc Natl Acad Sci USA. 1984;81:2601–2605. doi: 10.1073/pnas.81.9.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shaw JP, Utz PJ, Durand DB, Toole JJ, Emmel EA, Crabtree GR. Identification of a putative regulator of early T cell activation genes. Science. 1988;241:202–205. [PubMed] [Google Scholar]
- 36.Crabtree GR, Clipstone NA. Signal transmission between the plasma membrane and nucleus of T lymphocytes. Annu Rev Biochem. 1994;63:1045–1083. doi: 10.1146/annurev.bi.63.070194.005145. [DOI] [PubMed] [Google Scholar]
- 37.Rao A. NF-ATp: a transcription factor required for the co-ordinate induction of several cytokine genes. Immunol Today. 1994;15:274–281. doi: 10.1016/0167-5699(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 38.Jain J, Loh C, Rao A. Transcriptional regulation of the IL-2 gene. Curr Opin Immunol. 1995;7:333–342. doi: 10.1016/0952-7915(95)80107-3. [DOI] [PubMed] [Google Scholar]
- 39.Serfling E, Avots A, Neumann M. The architecture of the interleukin-2 promoter: a reflection of T lymphocyte activation. Biochim Biophys Acta. 1995;1263:181–200. doi: 10.1016/0167-4781(95)00112-t. [DOI] [PubMed] [Google Scholar]
- 40.Loh C, Shaw KT, Carew J, et al. Calcineurin binds the transcription factor NFAT1 and reversibly regulates its activity. J Biol Chem. 1996;271:10884–10891. doi: 10.1074/jbc.271.18.10884. [DOI] [PubMed] [Google Scholar]
- 41.Masuda ES, Imamura R, Amasaki Y, Arai K, Arai N. Signalling into the T-cell nucleus: NFAT regulation. Cell Signal. 1998;10:599–611. doi: 10.1016/s0898-6568(98)00019-9. [DOI] [PubMed] [Google Scholar]
- 42.Horsley V, Pavlath GK. NFAT: ubiquitous regulator of cell differentiation and adaptation. J Cell Biol. 2002;156:771–774. doi: 10.1083/jcb.200111073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takayanagi H. The role of NFAT in osteoclast formation. Ann N Y Acad Sci. 2007;1116:227–237. doi: 10.1196/annals.1402.071. [DOI] [PubMed] [Google Scholar]
- 44.Winslow MM, Pan M, Starbuck M, et al. Calcineurin/NFAT signaling in osteoblasts regulates bone mass. Dev Cell. 2006;10:771–782. doi: 10.1016/j.devcel.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 45.Koga T, Matsui Y, Asagiri M, et al. NFAT and Osterix cooperatively regulate bone formation. Nat Med. 2005;11:880–885. doi: 10.1038/nm1270. [DOI] [PubMed] [Google Scholar]
- 46.Ranger AM, Gerstenfeld LC, Wang J, et al. The nuclear factor of activated T cells (NFAT) transcription factor NFATp (NFATc2) is a repressor of chondrogenesis. J Exp Med. 2000;191:9–22. doi: 10.1084/jem.191.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tomita M, Reinhold MI, Molkentin JD, Naski MC. Calcineurin and NFAT4 induce chondrogenesis. J Biol Chem. 2002;277:42214–42218. doi: 10.1074/jbc.C200504200. [DOI] [PubMed] [Google Scholar]
- 48.Sitara D, Aliprantis AO. Transcriptional regulation of bone and joint remodeling by NFAT. Immunol Rev. 2010;233:286–300. doi: 10.1111/j.0105-2896.2009.00849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang J, Gardner BM, Lu Q, et al. Transcription factor Nfat1 deficiency causes osteoarthritis through dysfunction of adult articular chondrocytes. J Pathol. 2009;219:163–172. doi: 10.1002/path.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thirunavukkarasu K, Pei Y, Moore TL, et al. Regulation of the human ADAMTS-4 promoter by transcription factors and cytokines. Biochem Biophys Res Commun. 2006;345:197–204. doi: 10.1016/j.bbrc.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 51.Yaykasli KO, Oohashi T, Hirohata S, et al. ADAMTS9 activation by interleukin 1 beta via NFATc1 in OUMS-27 chondrosarcoma cells and in human chondrocytes. Mol Cell Biochem. 2009;323:69–79. doi: 10.1007/s11010-008-9965-4. [DOI] [PubMed] [Google Scholar]
- 52.Al-Daraji WI, Grant KR, Ryan K, Saxton A, Reynolds NJ. Localization of calcineurin/NFAT in human skin and psoriasis and inhibition of calcineurin/NFAT activation in human keratinocytes by cyclosporin A. J Invest Dermatol. 2002;118:779–788. doi: 10.1046/j.1523-1747.2002.01709.x. [DOI] [PubMed] [Google Scholar]
- 53.Avots A, Buttmann M, Chuvpilo S, et al. CBP/p300 integrates Raf/Rac-signaling pathways in the transcriptional induction of NF-ATc during T cell activation. Immunity. 1999;10:515–524. doi: 10.1016/s1074-7613(00)80051-5. [DOI] [PubMed] [Google Scholar]
- 54.de Gregorio R, Iniguez MA, Fresno M, Alemany S. Cot kinase induces cyclooxygenase-2 expression in T cells through activation of the nuclear factor of activated T cells. J Biol Chem. 2001;276:27003–27009. doi: 10.1074/jbc.M100885200. [DOI] [PubMed] [Google Scholar]
- 55.San-Antonio B, Iniguez MA, Fresno M. Protein kinase Czeta phosphorylates nuclear factor of activated T cells and regulates its transactivating activity. J Biol Chem. 2002;277:27073–27080. doi: 10.1074/jbc.M106983200. [DOI] [PubMed] [Google Scholar]
- 56.Cowan CM, Cheng S, Ting K, et al. Nell-1 induced bone formation within the distracted intermaxillary suture. Bone. 2006;38:48–58. doi: 10.1016/j.bone.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 57.Komori T. Requisite roles of Runx2 and Cbfb in skeletal development. J Bone Miner Metab. 2003;21:193–197. doi: 10.1007/s00774-002-0408-0. [DOI] [PubMed] [Google Scholar]
- 58.Komori T. Regulation of bone development and extracellular matrix protein genes by RUNX2. Cell Tissue Res. 2009;339:189–195. doi: 10.1007/s00441-009-0832-8. [DOI] [PubMed] [Google Scholar]
- 59.Inada M, Yasui T, Nomura S, et al. Maturational disturbance of chondrocytes in Cbfa1-deficient mice. Dev Dyn. 1999;214:279–290. doi: 10.1002/(SICI)1097-0177(199904)214:4<279::AID-AJA1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 60.Enomoto H, Enomoto-Iwamoto M, Iwamoto M, et al. Cbfa1 is a positive regulatory factor in chondrocyte maturation. J Biol Chem. 2000;275:8695–8702. doi: 10.1074/jbc.275.12.8695. [DOI] [PubMed] [Google Scholar]
- 61.Lim J, Hao T, Shaw C, et al. A protein-protein interaction network for human inherited ataxias and disorders of Purkinje cell degeneration. Cell. 2006;125:801–814. doi: 10.1016/j.cell.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 62.Kronenberg HM. The role of the perichondrium in fetal bone development. Ann N Y Acad Sci. 2007;1116:59–64. doi: 10.1196/annals.1402.059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.