Abstract
Weight reduction induces bone loss by several factors, and the effect of higher protein (HP) intake during caloric restriction on bone mineral density (BMD) is not known. Previous study designs examining the longer-term effects of HP diets have not controlled for total calcium intake between groups and have not examined the relationship between bone and endocrine changes. In this randomized, controlled study, we examined how BMD (areal and volumetric), turnover markers, and hormones [insulin-like growth factor 1 (IGF-1), IGF-binding protein 3 (IGFBP-3), 25-hydroxyvitamin D, parathyroid hormone (PTH), and estradiol] respond to caloric restriction during a 1-year trial using two levels of protein intake. Forty-seven postmenopausal women (58.0 ± 4.4 years; body mass index of 32.1 ± 4.6 kg/ m2) completed the 1-year weight-loss trial and were on a higher (HP, 24%, n =26) or normal protein (NP, 18%, n =21) and fat intake (28%) with controlled calcium intake of 1.2 g/d. After 1 year, subjects lost 7.0% ± 4.5% of body weight, and protein intake was 86 and 60 g/d in the HP and NP groups, respectively. HP compared with NP diet attenuated loss of BMD at the ultradistal radius, lumbar spine, and total hip and trabecular volumetric BMD and bone mineral content of the tibia. This is consistent with the higher final values of IGF-1 and IGFBP-3 and lower bone-resorption marker (deoxypyridinoline) in the HP group than in the NP group ( p <.05). These data show that a higher dietary protein during weight reduction increases serum IGF-1 and attenuates total and trabecular bone loss at certain sites in postmenopausal women.
Keywords: TRABECULAR BONE, DIET, PROTEIN, HORMONES, INSULIN-LIKE GROWTH FACTOR 1, OBESITY, WEIGHT LOSS, CORTICAL BONE
Introduction
Dietary protein plays an important role in the maintenance of bone health.(1) A regular supply of amino acids is required to offset losses during proteolysis and in maintenance of bone structure. Large epidemiologic studies that have evaluated the relationship between dietary protein intake and bone have shown a beneficial impact of protein on bone health(2,3) similar to clinical intervention studies in patients with osteoporosis.(4) On the other hand, there is some concern about greater acid load and increased urinary calcium excretion with higher intake of protein causing low bone mineral density (BMD) and greater fracture risk.(5,6) Since the negative effects of high-protein (HP) diets on BMD and fracture risk have been found to be influenced by low intakes of calcium,(7,8) controlled trials with adequate calcium intake are needed to determine the independent effect of protein on BMD.
Caloric restriction (CR) is often associated with bone mobilization and loss, and this is observed largely in older women and men.(9) This increase in bone mobilization may occur for a variety of reasons, including a decrease in intake of calcium and/or other nutrients,(10) a decrease in calcium absorption,(11) reduced weight bearing,(12) and/or hormonal changes.(9) There is also a decrease in serum insulin-like growth factor 1 (IGF-1) levels with CR,(13) and this too may negatively affect bone. A higher protein intake increases IGF-1 and calcium absorption, which may indirectly preserve bone mass.(1,4,14)
Previous studies that have examined the role of dietary protein on BMD after 3 to 6 months of CR have focused on either HP and dairy intakes compared with lower protein intakes with insufficient calcium or high calcium intakes in both groups.(15–17) These trials have shown either attenuated bone loss in HP group or a negative influence of HP diets on bone. Thus the role of protein in the maintenance of bone mass during a longer period of CR is unclear, and use of a control group that is not deficient in calcium intake and with the recommended intake of calcium has not been undertaken previously. The goal in this study was to examine the role of HP intake compared with normal protein (NP) intake on BMD during CR for 1 year in postmenopausal women with controlled and recommended calcium and vitamin D intakes between groups.
Subjects and Methods
Subjects
Postmenopausal women who reported no menstruation for at least 2 years prior to the study were recruited. All participants were between the ages of 50 and 70 years and free from any disease states or medications known to influence bone metabolism. Before initiation of any study procedures, subjects signed an informed consent approved by the institutional review board at Rutgers University and an external advisory board. This trial was registered at clinicaltrials.gov (NCT00473031).
Screening for eligibility
All participants had to pass a three-step screening process that included a telephone, laboratory, and physical screening. The telephone screening included questions about current diseases and medications and participation in prior weight-loss programs in the past few months. Participants had a comprehensive metabolic panel test and physical screening prior the study. They were screened for high fasting blood glucose, abnormal creatinine clearance, blood urea nitrogen (BUN), uric acid, calcium, and phosphorus. For patients who were taking thyroid medications, a stable dose for the past 2 or more years was required for inclusion, and none reported any change in medication when questioned monthly during the intervention. Subjects with a body mass index (BMI) of between 25 and 40 kg/ m2 were included. Participants also were screened at baseline for osteoporosis and excluded when their T-score was less than −2.5 at the hip or spine. Subjects who passed all the parts of screening were considered eligible for the study.
Study design
Subjects were recruited in four cohorts in the spring months between the years of 2005 and 2008. They were enrolled in the lifestyle behavior-modification weight-loss program for 1 year and randomized to a HP (30% of total calories) or NP diet (18% of total calories) using a randomization program (SAS, Version 9.2; SAS Institute, Cary, NC, USA). To determine caloric requirements, resting energy expenditure (REE) was measured at baseline using indirect calorimetry (VMax, Sensor Medics, Yorba Linda, CA, USA). This was done after an overnight fast and about 30 minutes of rest prior to the taking a steady-state measurement. Participants then received an individually tailored diet plan (about 500 to 600 kcal deficit per day) that included appropriate food selections and portion sizes using the diabetic exchange lists. There were 36 weight-loss counseling sessions by a registered dietitian throughout the 1-year intervention. Participants also received individual counseling sessions for specific diet concerns as needed. Physical activity of the participants was monitored using a pedometer, and subjects reported pedometer steps at least 3 days a month during the intervention. The average daily physical activity level was quantitated using their respective metabolic equivalents and was estimated from all active, incidental active, and passive activities that were recorded periodically. These postmenopausal women were instructed to maintain the same level of physical activity throughout the intervention.
Weight and height
Weight and height were measured with a balance-beam scale and stadiometer, respectively (Detecto, Webb City, MO, USA). At each morning visit, weight was recorded with minimal clothing.
Food records
All participants were instructed to maintain food records for at least 1 week per month and were asked to enter details of food quantity, servings, and method of preparation throughout the intervention. In addition, participants also completed a food frequency questionnaire (FFQ) and 24-hour recall with the dietitian once every 3 months to ensure adherence to diet. Dietary intakes were analyzed using FoodWorks software (Version 10, FoodWorks, Long Valley, NJ, USA).
Supplements
Subjects in the HP group were specifically counseled to increase protein intake from the diet with lean meat, fish, legumes, and dairy. In addition, subjects also were offered a whey protein supplement (Beneprotein, Nestle HealthCare Nutrition, successor-in-interest to Novartis Medical Nutrition, Minneapolis, MN, USA) to increase total protein intake and were asked to consume at least 1 scoop of the powder per day (1 scoop =6 g protein). Participants also completed a protein FFQ with the clinical coordinator to estimate protein intake once every month. Adherence to protein intake was monitored by absolute increase in protein intake from baseline, percent protein intake, as well as BUN. All subjects completed a calcium FFQ at baseline to estimate calcium supplementation. Subjects were stabilized to a calcium intake of 1.2 g/d beginning 1 month prior to the intervention and throughout the study period. If calcium intake from food and multivitamin (Nature Made Multi 50 +, Mission Hills, CA, USA) was below 1.2 g/d, subjects were given a calcium supplement without added vitamin D (Citracal, Bayer, NJ, USA) to meet 1.2 g/d. All participants were given a multivitamin that contained 400 IU of vitamin D3. Once every month, subjects completed a calcium FFQ to ensure adherence to the recommended daily calcium intake from diet and supplements.
Bone and body composition
Bone and body composition was measured using dual-energy X-ray absorptiometry (DXA; Lunar Prodigy Advanced, GE Lunar, Madison, WI, USA; coefficient of variation <1% for all sites, except 2% in radius) using enCORE 2004 software (Version 8.10.027, GE Lunar). BMD and bone mineral content (BMC) were measured at the ultradistal (UD) radius, 1/3 radius, femoral neck (FN), lumbar spine (L2–L4), total hip, and total body. In addition, fat mass and lean mass also were measured. All measurements were performed at baseline and at 6 and 12 months. Lumbar spine X-rays were examined for vertebral exclusion criteria specified by the International Society of Clinical Densitometry (ISCD) official positions 2007.(18) A vertebra was excluded if it showed a local structural change, artifact, or evidence of anatomic abnormality with a T-score difference more than 1.0 between the vertebra in question and adjacent vertebrae.
Peripheral quantitative computed tomography (pQCT)
Volumetric BMD, BMC, and geometric and bone strength properties of the tibia were measured using pQCT (Stratec XCT 3000; Orthometrix, White Plains, NY, USA). Sectional images were standardized at specific sites (4% and 38%) using distal tibia as the anatomic marker. The scans were acquired at 0.5-mm voxel and a slice thickness of 2.4 mm. A scout view was used to determine the positioning of the cross-sectional measurements along the tibia and was set by the integrated software (STRATEC XCT-3000, Version 5.4). Trabecular bone parameters are reported at the 4% site and cortical bone at the 38% site, as described previously.(19) The coefficient of variation (CV) was less than 1.7% for tibial and cortical volumetric BMD, BMC, area, and geometry.
Blood and urine analysis
Fasting blood and urine samples were collected at baseline and at weeks 4, 12, 24, 38, and 52. Serum 25-hydroxyvitamin D [25(OH)D] andestradiol (E2) were measured by radioimmunoassay (RIA; DiaSorin, Stillwater, MI, USA; CV <12.5% for vitamin D; DSL, Webster, TX, USA; CV <8.9% for E2). Our laboratory also participates in the Vitamin D External Quality Assessment Scheme, which monitors the performance of our 25(OH)D assay. Intact parathyroid hormone (iPTH) was determined by immunoradioassay (Scantibodies, Santee, CA, USA, CV <6.8%). Urea nitrogen in serum was measured using the liquid urea nitrogen reagent set (Pointe Scientific, Canton, MI, USA, CV <4.6%). Pyridinoline (PYD, CV <8%) and deoxypyridinoline (DPD, CV <10%) were measured in the urine by reverse-phase HPLC and fluorescence detection and normalized for creatinine excretion. Bone-formation markers osteocalcin (OC; BTI, Stoughton, MA, USA, CV <9%) and propeptide of type 1 collagen (P1NP; UNIQ P1NP RIA, Orion Diagnostica, Espoo, Finland, CV <10.2%) were measured by RIA. Serum N-telopeptide of type 1 collagen (NTX) was measured by ELISA (Osteomark, Princeton, NJ, USA, CV <4.6%). Insulin =like growth factor 1 (IGF-1) and IGF-binding protein 3 (IGFBP-3) were measured using immunoradiometric assay (DSL, Webster, TX, USA, CV <7.4% and <3.9%, respectively).
Statistical analysis
The influence of diet (HP versus NP) and time (0, 6, or 12 months) on BMD, BMC, fat and lean tissue, hormones, bone turnover markers, and nutrient intake was measured using two-factor repeated-measures ANOVA. Tukey’s post-hoc analysis was performed when the model F ratio was significant. One-way ANOVA was used to examine differences between the percent changes in BMD from baseline between the two groups. Paired t tests for comparison of means were used to determine changes in outcome variables within a group compared with baseline. Values are expressed as mean ±SD. p Values ≤.05 were considered significant. A power analysis was performed with α set at 0.05, with the value of β set at 0.90, using BMD from subgroup analysis from a previous study(2) to evaluate the effect of protein intake. This analysis indicated that 15 participants per group would be necessary to avoid a type II error. Our goal was to include at least 5 additional participants per group to account for two potential baseline covariates.
Results
Participants
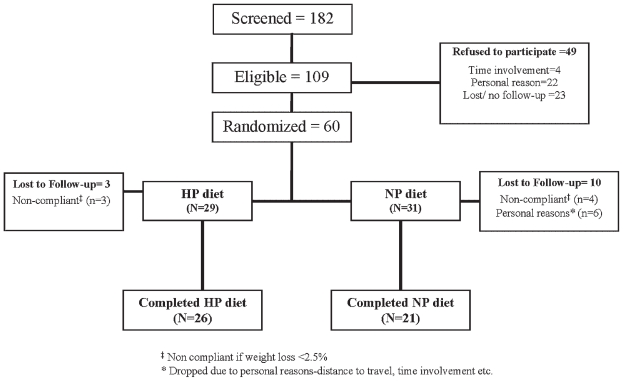
We screened a total of 182 women over a period of 4 years, of whom 60 subjects were enrolled for the study (Fig. 1). Women were randomized to either the HP group (n =29) or the NP group (n =31). Three subjects from the HP group and 4 from the NP group were dropped from the study owing to noncompliance with weight loss, that is, lost less than 2.5% of their initial body weight over the first 4 months. Six subjects who were randomized to the NP group were dropped from the study owing to a lack of time commitment and an inability to the attend biweekly sessions. Subjects were primarily white (n =53) and African American (n =6), and there was 1 Asian. Forty-seven subjects including 5 African Americans and 1 Asian completed the 1-year trial, with 26 subjects completing the HP diet and 21 the NP diet. In the HP group, there were 3 African Americans, 22 whites, and 1 Asian, and in the NP group, there were 2 African Americans and 19 whites.
Fig. 1.
Flowchart of study participants.
Weight, bone, and body composition changes
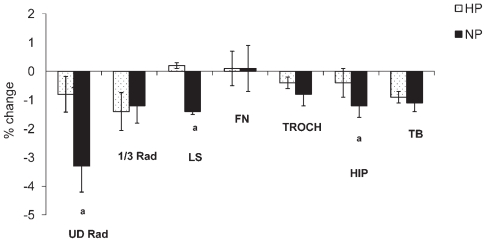
The mean age (58 ± 4 years) and REE (1365 ± 177 kcal/d) at baseline were not significantly different between the groups. Subjects in the HP group lost 6.6% ± 4.0% and subjects in the NP group lost 7.4% ± 5.2% of their initial body weight with no difference between groups at baseline or during the intervention (Table 1). Subjects lost 11.7% ± 10.1% of fat mass and 2.7% ± 4.0% of lean mass during the intervention with no difference between groups. In addition, there was a decrease in BMD at the ultradistal radius, 1/3 radius, hip, and total body and BMC at the lumbar spine and femoral neck ( p <.05) at 1 year compared with baseline. There was an interaction between diet and time observed for BMD at the ultradistal radius, lumbar spine, and total hip ( p <.05; Table 1). Subjects in the NP group compared with the HP group lost significantly more BMD at the ultradistal radius (−3.3% ± 4.2% versus −0.9% ± 3.2%), lumbar spine (−1.4% ± 3.6% versus 0.2% ± 3.4%), and total hip (−1.2% ± 1.8% versus −0.4% ± 1.3%) (Fig. 2). For the entire group of overweight/obese women at baseline, osteopenia at the lumbar spine and femoral neck was 23% and 43%, respectively, and 2% to 4% had osteoporosis. At the end of the study, the total cases of osteopenia remained the same, and osteoporosis increased to 6% at the lumbar spine and femoral neck.
Table 1.
| HP (n = 26) | NP (n =21) | p Value | |||||
|---|---|---|---|---|---|---|---|
| Baseline | 12 months | Baseline | 12 months | Diet | Time | Diet × Time | |
| Age (years) | 58.5 ± 4.1 | 57.4 ± 4.7 | |||||
| Weight (kg) | 88.5 ± 15.1 | 82.8 ± 15.4 | 82.7 ± 12.2 | 76.6 ± 11.7 | .151 | <.001 | .755 |
| Fat mass (kg) | 39.6 ± 9.5 | 35.4 ± 10.6 | 36.9 ± 8.4 | 32.4 ± 8.3 | .321 | <.001 | .834 |
| Lean mass (kg) | 45.2 ± 6.4 | 44.0 ± 5.8 | 41.7 ± 5.5 | 40.3 ± 4.6 | .034 | <.001 | .504 |
| BMD (g/cm2) | |||||||
| UD radius | 0.37 ± 0.05 | 0.37 ± 0.05 | 0.37 ± 0.07 | 0.36 ± 0.06 | .837 | .002 | .032 |
| 1/3 radius | 0.69 ± 0.06 | 0.68 ± 0.06 | 0.68 ± 0.08 | 0.67 ± 0.08 | .419 | .012 | .490 |
| Lumbar spinea | 1.24 ± 0.17 | 1.25 ± 0.18 | 1.13 ± 0.17 | 1.12 ± 0.17 | .063 | .193 | .028 |
| Trochanter | 0.82 ± 0.11 | 0.82 ± 0.12 | 0.75 ± 0.09 | 0.74 ± 0.10 | .032 | .287 | .512 |
| Femoral neck | 0.94 ± 0.10 | 0.94 ± 0.11 | 0.89 ± 0.10 | 0.89 ± 0.10 | .085 | .887 | .884 |
| Total hip | 1.02 ± 0.12 | 1.01 ± 0.12 | 0.94 ± 0.01 | 0.93 ± 0.10 | .020 | .004 | .050 |
| Total body | 1.20 ± 0.09 | 1.19 ± 0.09 | 1.14 ± 0.10 | 1.12 ± 0.10 | .023 | .007 | .951 |
| BMC (g) | |||||||
| UD radius | 1.31 ± 0.19 | 1.28 ± 0.19 | 1.28 ± 0.21 | 1.28 ± 0.20 | .846 | .246 | .119 |
| 1/3 radius | 1.67 ± 0.20 | 1.69 ± 0.23 | 1.61 ± 0.26 | 1.61 ± 0.26 | .289 | .553 | .591 |
| Lumbar spine | 54.0 ± 11.3 | 54.3 ± 12.1 | 46.5 ± 9.7 | 46.1 ± 9.6 | .047 | .009 | .469 |
| Trochanter | 10.1 ± 2.1 | 10.3 ± 2.1 | 9.0 ± 1.6 | 9.3 ± 1.9 | .047 | .354 | .739 |
| Femoral neck | 5.0 ± 0.7 | 4.9 ± 0.7 | 4.4 ± 0.6 | 4.4 ± 0.5 | .003 | .042 | .706 |
| Total hip | 32.1 ± 3.9 | 32.0 ± 4.0 | 29.1 ± 3.3 | 29.0 ± 3.5 | .007 | .661 | .930 |
| Total body | 2523 ± 299 | 2520 ± 338 | 2352 ± 408 | 2340 ± 357 | .058 | .258 | .177 |
Values are mean ± SD (all variables); UD =ultra distal radius.
16 subjects had their lumbar spine BMD scans reanalyzed according to ISCD exclusion criteria.
A two-factor repeated-measures ANOVA was performed with time (0, 6, and 12 months) and diet (HP or NP) as independent variables.
Fig. 2.
The percent change in bone mineral density (BMD) compared with baseline at all sites in the HP and NP groups at 1 year. Comparison of percent change between two groups was done by one-way ANOVA. ap <.05.
Changes in trabecular and cortical bone
The effect of protein intake on trabecular and cortical bone and geometry is presented in Table 2. There was a decrease in tibial total volumetric BMD accompanied by an increase in total area over the 1-year period ( p <.05). There was a greater decrease in total volumetric BMD and trabecular volumetric BMD and BMC in the NP group compared with the HP group over time ( p <.05). In addition, there also was a trend toward increase cortical volumetric BMD and decrease in cortical area over time ( p <.09), without differences between the HP and NP diets. Tibia muscle area did not decrease significantly over time or differ between groups. Fat area around the tibia decreased over time in both groups ( p <.001), and this decrease was greater in the NP group than in the HP group ( p <.05).
Table 2.
Trabecular and Cortical Volumetric BMD, BMC, and Geometry Over 12 Months in the Two Treatment Groupsa,b
| HP (n =26) | NP (n =21) | p Valueb | |||||
|---|---|---|---|---|---|---|---|
| Baseline | 12 months | Baseline | 12 months | Diet | Time | Diet × Time | |
| Total BMC (mg) | 301.3 ± 40.5 | 304.8 ± 46.4 | 280.1 ± 36.1 | 276.2 ± 32.5 | .036 | .718 | .061 |
| Total vBMD (mg/cm3) | 298.8 ± 33.3 | 296.9 ± 34.3 | 306.8 ± 45.7 | 295.8 ± 40.2 | .679 | .031 | .049 |
| Trabecular BMC (mg) | 104.0 ± 18.8 | 110.9 ± 26.6 | 97.1 ± 20.3 | 95.3 ± 16.8 | .083 | .581 | .043 |
| Trabecular BMD (mg/cm3) | 228.6 ± 31.7 | 232.9 ± 33.8 | 237.6 ± 58.7 | 225.2 ± 32.4 | .831 | .207 | .041 |
| Total area (mm2) | 1012.3 ± 117.0 | 1036.3 ± 161.9 | 926.4 ± 134.2 | 945.2 ± 128.6 | .021 | .026 | .700 |
| Cortical BMC (mg) | 325.5 ± 31.6 | 325.6 ± 31.2 | 294.7 ± 41.2 | 291.9 ± 40.8 | .005 | .423 | .173 |
| Cortical volumetric BMD (mg/cm3) | 1147.1 ± 27.3 | 1148.6 ± 24.0 | 1126.7 ± 42.6 | 1131.8 ± 43.8 | .014 | .093 | .697 |
| Cortical area (mm2) | 284.0 ± 27.0 | 282.7 ± 27.5 | 261.1 ± 31.3 | 257.3 ± 29.5 | .008 | .076 | .237 |
| Cortical thickness (mm) | 5.2 ± 0.5 | 5.1 ± 0.5 | 4.7 ± 0.6 | 4.7 ± 0.6 | .011 | .169 | .475 |
| Peripheral circulation (mm) | 71.5 ± 4.0 | 71.6 ± 3.6 | 70.3 ± 4.1 | 70.1 ± 3.9 | .228 | .663 | .419 |
| Polar moment of inertia (mm4) | 24,442 ± 5039 | 24,212 ± 4910 | 22,231 ± 4544 | 21,925 ± 3992 | .110 | .127 | .929 |
| Stress-strain index (mm3) | 1590 ± 525 | 1600 ± 239 | 1423 ± 253 | 1425 ± 239 | .025 | .774 | .412 |
| Muscle area (mm2) | 6894 ± 1093 | 6707 ± 1025 | 6523 ± 1125 | 6438 ± 1010 | .112 | .098 | .300 |
| Fat area (mm2) | 5218 ± 1439 | 4856 ± 1606 | 5663 ± 1750 | 4892 ± 1454 | .544 | <.001 | .034 |
Mean ± SD.
A two-factor repeated-measures ANOVA was performed with time (0, 6, and 12 months) and diet (HP or NP) as independent variables.
Nutrient intake during the intervention
Nutrient intake from food and supplements is presented in Table 3. Intake of total calories, carbohydrates, and fat was significantly lower ( p <.01) during the intervention. As expected, subjects in the HP group consumed significantly higher protein (86 versus 60 g/d) than those in the NP group ( p <.001). The percent of protein calories was 24% versus 18% in the HP and NP groups, respectively. Women on the HP diet had a greater intake of dairy and meat ( p <.01) and tended to have higher intake of eggs ( p <.08) than those on the NP diet. The whey protein supplement contributed to less than 4% of total daily protein intake. Differences in protein intake between groups also were supported by the finding that BUN was higher in the HP group than in the NP group ( p <.01). Subjects consumed 1128 ± 202 mg of calcium per day from diet, multivitamin, and calcium supplements with no differences between the groups during the intervention.
Table 3.
| HP (n =26) | NP (n =21) | p Valueb | |||||
|---|---|---|---|---|---|---|---|
| Baseline | Months 1–12 of intakea | Baseline | Months 1–12 of intake | Diet | Time | Diet × Time | |
| % Protein | 18.4 ± 4.2 | 23.6 ± 4.6 | 16.7 ± 4.5 | 17.8 ± 2.8 | <.001 | <.001 | .021 |
| Protein (g) | 79.6 ± 20.2 | 86.3 ± 11.3 | 70.0 ± 24.3 | 60.1 ± 10.2 | <.001 | .955 | .045 |
| Carbohydrate (g) | 199.4 ± 62.7 | 153.1 ± 48.3 | 199.4 ± 65.6 | 157.1 ± 33.8 | .983 | .002 | .336 |
| Fat (g) | 74.3 ± 34.2 | 59.5 ± 13.7 | 68.9 ± 36.9 | 56.9 ± 21.7 | .755 | .013 | .937 |
| Energy (kcal) | 1733 ± 505 | 1480 ± 270 | 1672 ± 577 | 1375 ± 283 | .765 | <.001 | .474 |
| Calcium (mg)d | 1226 ± 286 | 1127 ± 240 | 1204 ± 174 | 1129 ± 164 | .538 | .281 | .980 |
| Vitamin D (μg) | 10.4 ± 0.7 | 10.6 ± 0.7 | 10.5 ± 0.9 | 10.4 ± 0.5 | .868 | .517 | .877 |
| Mg (mg) | 361± 131 | 352 ± 67 | 343 ± 112 | 348± 73 | .322 | <.001 | .640 |
| Phosphorus (mg) | 1270 ± 432 | 1238 ± 238 | 1182 ± 313 | 1063 ± 218 | .009 | .766 | .225 |
| Na (mg) | 3190 ± 1129 | 2475 ± 437 | 2938 ± 859 | 2166 ± 298 | .026 | <.001 | .574 |
| Vitamin K (μg) | 126 ± 128 | 137 ± 91 | 88 ± 86 | 115 ± 50 | .139 | .142 | .374 |
Mean ± SD; daily nutrient intake is an average over 12 months (3-day food diaries from each month). Intake includes multivitamins (200 mg of calcium, 10 μg of vitamin D, 48 μg of phosphorous, 100 mg of magnesium, 10 μg of vitamin K), individualized calcium supplement and whey protein powder.
A two-factor repeated-measures ANOVA was performed with time (0, 6, and 12 months) and diet (HP or NP) as independent variables.
Nutrient intake did not differ between the groups at baseline.
Prestablilization intake of calcium was 802 ± 351 and 730 ± 272 mg/d in HP and NP groups, respectively.
Changes in hormones and binding proteins during intervention
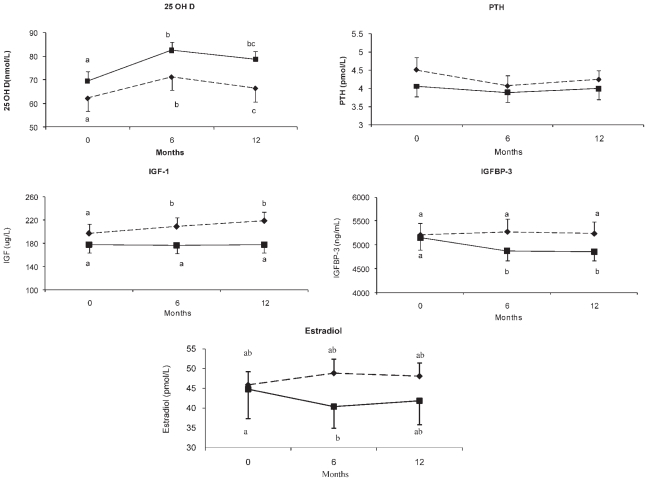
There was a significant effect of weight loss independent of diet on serum levels of PTH, 25(OH)D, and IGF-1 during the intervention ( p <.05; Fig. 3). PTH decreased during the 1-year period ( p <.05), whereas there was a rise in 25(OH)D ( p <.001). Those in the NP group showed a trend for higher values for 25(OH)D ( p <.08) compared with the HP group. In addition, there was a tendency for estradiol to increase over time in the HP group compared with the NP group ( p <.06). PTH and 25(OH)D levels did not differ significantly between groups during the intervention. Serum levels of IGF-1 increased significantly over time in the HP group ( p <.05) compared with the NP group. Serum IGF-1 increased by 20% ± 37% during intervention compared with baseline (p <.01) in HP group compared with an insignificant rise of 3% ± 18% ( p =.92) in the NP group. Serum IGFBP-3 remained unchanged in the HP group but decreased significantly in the NP group ( p <.05).
Fig. 3.
Changes in 25-hydroxyvitamin D [25(OH)D], parathyroid hormone (PTH), estradiol, insulin-like growth factor 1 (IGF-1), and insulin-like growth factor–binding protein 3 (IGFBP-3) during the intervention in the two groups. The influence of diet over time on these hormones was analyzed by two-factor repeated-measures ANOVA (diet × time =D × T). Values with different superscript letters are significantly different. Diamond and dotted line represents HP (n =26); square and solid lines represent NP (n =21).
Changes in bone turnover during intervention
The changes in bone turnover markers during the intervention period are shown in Table 3. PYD increased during the 1 year of CR ( p <.05), and this increase was independent of diet. Those in the NP group showed a trend for higher values P1NP (p <.06) compared with the HP group. Resorption marker DPD increased significantly in the NP group compared with the HP group over time ( p <.05), and a similar tendency ( p <.08) also was observed for PYD. There were no significant differences in the bone-formation markers OC and P1NP between the groups during intervention.
Discussion
HP intake is a popular means of weight reduction because in the short term it has shown to result in greater weight loss, fat loss, and preservation of lean mass compared with high-carbohydrate, low-fat diets.(20) However, longer-term studies show that a greater weight loss and/or fat loss on a higher-protein diet is not sustained and is similar to that seen with a standard high-carbohydrate diet after 1 to 2 years,(21,22) as observed in this study as well. This is the first study that delineates the influence of protein intake during weight reduction on bone that controls for dietary intake of calcium and other micronutrients and, more important, compares the effect of a HP diet on a control group with sufficient and recommended calcium intake. We show that CR for 1 year with a protein intake of 24% of total calories attenuates loss of bone at the radius, hip, and spine and increases IGF-1 compared with a diet with 18% protein intake.
During weight-stable conditions, an increase in dietary protein intake has a positive influence on the bone. An HP diet has been shown to increase serum levels of IGF-1,(4,23) which promote osteoblast proliferation and matrix formation(24) and may, in turn, increase bone mass and reduce fracture risk.(4) A higher protein intake (2.1 g/kg) compared with moderate intake (1.0 g/kg) also has been shown to increase intestinal calcium absorption, which leads to a parallel increase in urinary calcium and decrease in bone turnover markers.(25) In this 10-day study(25) and another short-term (8-week isotopic tracer) study examining protein intake.(26) there were trends toward better calcium retention during HP intakes. It is interesting that with higher calcium intake (~1500 mg), there was no protein-related increase in calcium absorption.(27) Because a higher protein intake is associated with an increase in muscle mass and promotes collagen synthesis, this too may contribute to acquisition of bone mass.(1)
Similar to previous findings from our laboratory and others,(10,28,29) we observed a loss of bone mass at a few sites with CR. In rodents, protein-calorie malnutrition has been shown to decrease trabecular and cortical BMD(30); the effect of HP and CR on these compartments has not been examined in humans. We show that higher protein intake attenuated the loss of BMD at the lumbar spine, ultradistal radius, total hip, and trabecular bone at the tibia compared with the NP diet. Multiple clinical intervention trials have examined the role of higher dietary protein on bone mass and turnover during CR, although none of these trials controls for calcium intake between groups, and findings are not consistent.(15–17,31,32) The importance of dietary calcium on bone and during weight loss is well established.(10,29,33) These previous studies that address the role of higher dietary protein intake during dieting have accomplished this by increasing both dairy and calcium intake and were compared with calcium-insufficient (~600 mg/d), high-carbohydrate diets. Some of these high calcium and protein trials(15,16) have shown a positive impact of the diet on maintenance of bone mass during CR, similar to calcium supplementation studies without higher protein intake.(10,29,33) In contrast, a recent CR study with very high calcium intake in all women resulted in a greater total-body BMD loss in the HP group, but the study is limited by its short duration (12 weeks) and an absence of measuring specific bone sites.(17) This study is the first to examine the effect of 1 year of CR on bone and to demonstrate that dietary protein alone, with calcium and vitamin D at recommended intakes, will attenuate bone loss during CR.
The increase in IGF-1 levels during the intervention in the HP group compared with the NP group may have mediated a greater preservation of bone in the HP group. There is a well-known anabolic effect of IGF-1 on bone(24,34); protein and calorie deprivation lowers IGF-1(13) and is associated with lower BMD.(34) We observed a 20% increase in IGF-1 in the HP group, whereas it remained unchanged in the NP group. Many studies have evaluated the effect of total protein intake using animal (meat, milk) or plant foods rich in protein such as soy on the serum levels of IGF-1.(4,35–38) These studies suggest that both animal and plant protein intakes contribute to the increase in IGF-1. In this study, the women consumed a mixture of both animal and plant proteins. The increase in IGF-1 levels in response to protein intake in some(4,35) but not all studies(36) also shows a positive effect of IGF-1 on bone turnover and density. In one such study, supplementation with three servings of milk per day increased IGF-1 by 10% and decreased bone resorption in adult men and women.(35) Similarly, meat supplementation to up to 24% of total calories increased IGF-1 by 25% and also decreased bone turnover.(37) In another trial, elderly malnourished patients who received a whey protein supplementation (20 g/d) for 6 months following hip fracture (4) had a 35 μg/L (62%) increase in IGF-1 and attenuated bone loss compared with baseline. The greater increase in IGF-1 following protein supplementation in the Schruch study compared with this study is likely due to their older and malnourished population that started out with lower baseline IGF-1 levels. In addition, we observed a decrease in IGFBP-3 over time with weight loss in the NP group that is consistent with the decrease found with fasting,(39) but there was no decrease in the HP group. The relationship between IGFBP-3 and BMD is a weakly positive one, and serum levels are low in osteoporosis.(40) Overall, the absence of a change in IGFBP-3 and rise in IGF-1 support the notion that IGF-1 contributes to BMD preservation in the HP group. However we did not find an increase in bone turnover markers in the HP group. We suggest that a modest 20% increase in serum IGF-1 in response to the HP diet is too small to have a measurable effect on bone-formation markers. This is in contrast to the dramatic increases with exogenous treatment of IGF-1 that increase both formation and resorption markers.(41) In addition, these findings are consistent with findings in other dietary protein studies showing no rise in bone formation and a decrease in bone resorption owing to HP intake.(4,37) It is possible that the small decline in bone-resorption markers can explain the attenuated bone loss. A trend for a higher estradiol, as in this study, may be another mechanism besides IGF-1 that is attenuating bone loss on an HP diet. In addition, greater calcium absorption with higher protein intake(25) may explain the decline in bone-resorption markers.
Women in the HP group tended to increase serum estradiol over time more compared than those in the NP group. The slight decrease in estradiol in the NP group owing to CR is consistent with previous findings during weight loss, as observed in our laboratory and by others.(11,42) It is interesting to note that in a rodent model, a lower compared with a higher protein diet is associated not only with lower IGF-1 levels but also with estrogen deficiency and may contribute to the loss of bone mineral and strength.(34) To our knowledge, there has been no study to specifically examine the effect of an HP diet on serum levels of estradiol, and the absence of a decrease in estradiol supports the attenuated bone loss and resorption in the HP group. Other trials that have evaluated small physiologic differences in estradiol in older individuals have showed that differences as low as 3.7 pg/ mL (13.6 pmol/L)(43,44) are associated with higher BMD values. Thus it is possible that while the approximately 7 pmol higher estradiol in the HP group compared with the NP group is a small difference, it may have acted in concert with other factors, such as higher IGF-1, to attenuate bone loss in the HP group.
Caloric restriction is associated with changes in several other hormones, such as an increase in cortisol(29) and PTH in the absence of calcium supplementation, all of which may mediate the bone loss during this process. A rise in PTH owing to CR may be due to the decrease in calcium absorption(11) and/or a reduced intake of calcium. Similar to previous findings in our laboratory and others.(29,45) we observed a rise in 25(OH)D and a decrease in PTH over the 1-year period of CR. It is possible that a loss of fat mass is associated with release of vitamin D from the adipose tissue,(46) resulting in an increase in serum levels of 25(OH)D and thereby suppressing PTH levels.
Some limitations of this study include the following: There is a concern for artifacts associated with projection-based axial DXA measurements owing to excess fat tissue surrounding the bone in obese individuals and changes in the surrounding soft tissue owing to weight reduction.(47) However, the cross-sectional measurements of peripheral sites (ie, tibia and radius) with less fat thickness than at central sites show similar bone changes. Also, we use a control group with similar weight loss to help address this issue. In addition, subjects in the HP group increased their protein intake to only 24% of calories, although the goal was higher. However, we did achieve a 26 g/d difference in protein intake between groups, which was a more attainable intake for older individuals, so it may have more practical implications. Also, we did not examine other sex steroids that also may have been influenced by weight loss and affect bone. There are also several strengths to this study. Careful monitoring of calcium and protein intake during the intervention included biweekly checks of compliance and periodic assessment of biomarkers. This is also the first 1-year randomized weight-loss trial that evaluates protein intake on bone and controls for calcium intake between groups. In contrast, previous studies consisted of low calcium intake in the NP groups and 3 to 6 months of weight loss,(15,16,31,32) and some were followed longer by a weight-stable period.(15,31,32) In addition, ISCD vertebral exclusion criteria were applied, which can be even more important in an obese population owing to their higher incidence of osteoarthritis. Furthermore, this study examines the bone response in a variety of approaches by including measurements of both areal and volumetric bone and geometry and examining potential endocrine regulators.
In summary, a higher-protein diet that increases serum IGF-1 attenuates bone loss during CR at certain sites in postmenopausal women over a 1-year period. With a significant percentage of the older population being on weight-loss diets, bone loss has become a major concern. A higher-protein diet at 24% of total calories with recommended calcium and vitamin D intake preserves BMD and can be recommended to postmenopausal women during caloric restriction.
Table 4.
| HP (n =26) | NP (n =21) | p Valueb | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Turnover markers | Baseline | 6 months | 12 months | Baseline | 6 months | 12 months | Diet | Time | Diet × Time |
| Osteocalcin (ng/mL) | 9.2 ± 1.7 | 9.5 ± 2.5 | 9.5 ± 2.5 | 10.0 ± 2.4 | 10.0 ± 2.4 | 10.0 ± 2.5 | .342 | .925 | .405 |
| P1NP (μg/L) | 48.2 ± 16.3 | 44.7 ± 16.7 | 46.1 ± 16.4 | 56.6 ± 15.4 | 53.4 ± 16.1 | 56.6 ± 17.6 | .058 | .215 | .910 |
| NTX (BCE) | 12.5 ± 3.7 | 12.3 ± 3.7 | 12.1 ± 3.5 | 13.0 ± 6.4 | 14.6 ± 7.8 | 13.1 ± 4.8 | .268 | .571 | .319 |
| PYD/C (nmol/mmol) | 22.6 ± 8.9a | 24.8 ± 8.6a | 4.9 ± 9.5a | 24.9 ± 16.2a | 24.8 ± 11.0 | 28.3 ± 18.7b | .332 | .018 | .084 |
| DPD/C (nmol/mmol) | 10.5 ± 6.3a | 9.8 ± 4.4a | 8.9 ± 3.9b | 9.3 ± 5.5a | 9.6 ± 3.9a | 10.8 ± 6.2a | .906 | .399 | .049 |
Mean ± SD; P1NP =propeptide of type 1 collagen; NTX =N-telopeptide of type 1 collagen; PYD/C =pyridinoline/creatinine; DPD/C =deoxypyridinoline/ creatinine.
A two-factor repeated-measures ANOVA showing effect of diet (NP or HP), time (0, 6, and 12 months), and diet × time interaction. Values with different superscript letters are significantly different.
Acknowledgments
We thank the laboratory and clinical staff for their invaluable technical and clinical assistance. We also would like to thank L Taich, MD, for her examination and interpretation of radiographic spine images. We appreciate the commitment of the volunteers in this study. This work was supported by grants from the National Institutes of Health (RO1-AG12161) and a Busch Biomedical Award to SAS.
Footnotes
Disclosures
SAS contributed to study design, data analysis and interpretation, and manuscript preparation. DS contributed to coordination of the study, recordkeeping, data collection, management and interpretation, laboratory and statistical analysis, and manuscript preparation. HAS contributed to laboratory analysis and quality control. YS contributed to study design and statistical analysis. RZ contributed to coordination of the volunteers and data collection and management. TS contributed to bone density scan analysis and critical review of the manuscript. CG contributed to pQCT scan analysis and critical review of the manuscript.
All the authors state that they have no conflicts of interest.
References
- 1.Conigrave AD, Brown EM, Rizzoli R. Dietary protein and bone health: roles of amino acid–sensing receptors in the control of calcium metabolism and bone homeostasis. Annu Rev Nutr. 2008;28:131–155. doi: 10.1146/annurev.nutr.28.061807.155328. [DOI] [PubMed] [Google Scholar]
- 2.Hannan MT, Tucker KL, Dawson-Hughes B, Cupples LA, Felson DT, Kiel DP. Effect of dietary protein on bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res. 2000;15:2504–2512. doi: 10.1359/jbmr.2000.15.12.2504. [DOI] [PubMed] [Google Scholar]
- 3.Munger RG, Cerhan JR, Chiu BC. Prospective study of dietary protein intake and risk of hip fracture in postmenopausal women. Am J Clin Nutr. 1999;69:147–152. doi: 10.1093/ajcn/69.1.147. [DOI] [PubMed] [Google Scholar]
- 4.Schurch MA, Rizzoli R, Slosman D, Vadas L, Vergnaud P, Bonjour JP. Protein supplements increase serum insulin-like growth factor-I levels and attenuate proximal femur bone loss in patients with recent hip fracture: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1998;128:801–809. doi: 10.7326/0003-4819-128-10-199805150-00002. [DOI] [PubMed] [Google Scholar]
- 5.Abelow BJ, Holford TR, Insogna KL. Cross-cultural association between dietary animal protein and hip fracture: a hypothesis. Calcif Tissue Int. 1992;50:14–18. doi: 10.1007/BF00297291. [DOI] [PubMed] [Google Scholar]
- 6.Feskanich D, Willett WC, Stampfer MJ, Colditz GA. Protein consumption and bone fractures in women. Am J Epidemiol. 1996;143:472–479. doi: 10.1093/oxfordjournals.aje.a008767. [DOI] [PubMed] [Google Scholar]
- 7.Sahni S, Cupples LA, McLean R, et al. Protective effect of high protein and calcium intake on the risk of hip fracture in the Framingham Offspring Cohort. J Bone Miner Res. 2010;25:2770–2776. doi: 10.1002/jbmr.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dawson-Hughes B, Harris SS. Calcium intake influences the association of protein intake with rates of bone loss in elderly men and women. Am J Clin Nutr. 2002;75:773–779. doi: 10.1093/ajcn/75.4.773. [DOI] [PubMed] [Google Scholar]
- 9.Shapses SA, Riedt CS. Bone, body weight, and weight reduction: what are the concerns? J Nutr. 2006;136:1453–1456. doi: 10.1093/jn/136.6.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jensen LB, Kollerup G, Quaade F, Sorensen OH. Bone minerals changes in obese women during a moderate weight loss with and without calcium supplementation. J Bone Miner Res. 2001;16:141–147. doi: 10.1359/jbmr.2001.16.1.141. [DOI] [PubMed] [Google Scholar]
- 11.Cifuentes M, Riedt CS, Brolin RE, Field MP, Sherrell RM, Shapses SA. Weight loss and calcium intake influence calcium absorption in overweight postmenopausal women. Am J Clin Nutr. 2004;80:123–130. doi: 10.1093/ajcn/80.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villareal DT, Fontana L, Weiss EP, et al. Bone mineral density response to caloric restriction-induced weight loss or exercise-induced weight loss: a randomized controlled trial. Arch Intern Med. 2006;166:2502–2510. doi: 10.1001/archinte.166.22.2502. [DOI] [PubMed] [Google Scholar]
- 13.Oster MH, Fielder PJ, Levin N, Cronin MJ. Adaptation of the growth hormone and insulin-like growth factor-I axis to chronic and severe calorie or protein malnutrition. J Clin Invest. 1995;95:2258–2265. doi: 10.1172/JCI117916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shapses SA, Sukumar D. Protein intake during weight loss: Effects on bone. In: Burckhardt P, Dawson-Hughes B, Weaver C, editors. Nutritional Influences on Bone Health. London: Springer; 2010. pp. 27–33. [Google Scholar]
- 15.Thorpe MP, Jacobson EH, Layman DK, He X, Kris-Etherton PM, Evans EM. A diet high in protein, dairy, and calcium attenuates bone loss over twelve months of weight loss and maintenance relative to a conventional high-carbohydrate diet in adults. J Nutr. 2008;138:1096–1100. doi: 10.1093/jn/138.6.1096. [DOI] [PubMed] [Google Scholar]
- 16.Skov AR, Haulrik N, Toubro S, Molgaard C, Astrup A. Effect of protein intake on bone mineralization during weight loss: a 6-month trial. Obes Res. 2002;10:432–438. doi: 10.1038/oby.2002.60. [DOI] [PubMed] [Google Scholar]
- 17.Campbell WW, Tang M. Protein intake, weight loss, and bone mineral density in postmenopausal women. J Gerontol A Biol Sci Med Sci. 2010;65:1115–1122. doi: 10.1093/gerona/glq083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewiecki EM, Gordon CM, Baim S, et al. International Society for Clinical Densitometry 2007 Adult and Pediatric Official Positions. Bone. 2008;43:1115–1121. doi: 10.1016/j.bone.2008.08.106. [DOI] [PubMed] [Google Scholar]
- 19.Sukumar D, Schlussel Y, Riedt CS, Gordon C, Stahl T, Shapses SA. Obesity alters cortical and trabecular bone density and geometry in women. Osteoporos Int. 2011;22:635–645. doi: 10.1007/s00198-010-1305-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Westerterp-Plantenga MS, Nieuwenhuizen A, Tome D, Soenen S, Westerterp KR. Dietary protein, weight loss, and weight maintenance. Annu Rev Nutr. 2009;29:21–41. doi: 10.1146/annurev-nutr-080508-141056. [DOI] [PubMed] [Google Scholar]
- 21.Sacks FM, Bray GA, Carey VJ, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360:859–873. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clifton PM, Keogh JB, Noakes M. Long-term effects of a high-protein weight-loss diet. Am J Clin Nutr. 2008;87:23–29. doi: 10.1093/ajcn/87.1.23. [DOI] [PubMed] [Google Scholar]
- 23.Holmes MD, Pollak MN, Willett WC, Hankinson SE. Dietary correlates of plasma insulin-like growth factor I and insulin-like growth factor binding protein 3 concentrations. Cancer Epidemiol Biomarkers Prev. 2002;11:852–861. [PubMed] [Google Scholar]
- 24.Price JS, Oyajobi BO, Oreffo RO, Russell RG. Cells cultured from the growing tip of red deer antler express alkaline phosphatase and proliferate in response to insulin-like growth factor-I. J Endocrinol. 1994;143:R9–16. doi: 10.1677/joe.0.143r009. [DOI] [PubMed] [Google Scholar]
- 25.Kerstetter JE, O’Brien KO, Caseria DM, Wall DE, Insogna KL. The impact of dietary protein on calcium absorption and kinetic measures of bone turnover in women. J Clin Endocrinol Metab. 2005;90:26–31. doi: 10.1210/jc.2004-0179. [DOI] [PubMed] [Google Scholar]
- 26.Roughead ZK, Hunt JR, Johnson LK, Badger TM, Lykken GI. Controlled substitution of soy protein for meat protein: effects on calcium retention, bone, and cardiovascular health indices in postmenopausal women. J Clin Endocrinol Metab. 2005;90:181–189. doi: 10.1210/jc.2004-0393. [DOI] [PubMed] [Google Scholar]
- 27.Hunt JR, Johnson LK, Fariba Roughead ZK. Dietary protein and calcium interact to influence calcium retention: a controlled feeding study. Am J Clin Nutr. 2009;89:1357–1365. doi: 10.3945/ajcn.2008.27238. [DOI] [PubMed] [Google Scholar]
- 28.Ricci TA, Heymsfield SB, Pierson RN, Jr, Stahl T, Chowdhury HA, Shapses SA. Moderate energy restriction increases bone resorption in obese postmenopausal women. Am J Clin Nutr. 2001;73:347–352. doi: 10.1093/ajcn/73.2.347. [DOI] [PubMed] [Google Scholar]
- 29.Riedt CS, Cifuentes M, Stahl T, Chowdhury HA, Schlussel Y, Shapses SA. Overweight postmenopausal women lose bone with moderate weight reduction and 1g/day calcium intake. J Bone Miner Res. 2005;20:455–463. doi: 10.1359/JBMR.041132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ammann P, Bourrin S, Bonjour JP, Meyer JM, Rizzoli R. Protein undernutrition-induced bone loss is associated with decreased IGF-I levels and estrogen deficiency. J Bone Miner Res. 2000;15:683–690. doi: 10.1359/jbmr.2000.15.4.683. [DOI] [PubMed] [Google Scholar]
- 31.Noakes M, Keogh JB, Foster PR, Clifton PM. Effect of an energy-restricted, high-protein, low-fat diet relative to a conventional high-carbohydrate, low-fat diet on weight loss, body composition, nutritional status, and markers of cardiovascular health in obese women. Am J Clin Nutr. 2005;81:1298–1306. doi: 10.1093/ajcn/81.6.1298. [DOI] [PubMed] [Google Scholar]
- 32.Bowen J, Noakes M, Clifton PM. A high dairy protein, high-calcium diet minimizes bone turnover in overweight adults during weight loss. J Nutr. 2004;134:568–573. doi: 10.1093/jn/134.3.568. [DOI] [PubMed] [Google Scholar]
- 33.Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med. 1997;337:670–676. doi: 10.1056/NEJM199709043371003. [DOI] [PubMed] [Google Scholar]
- 34.Yakar S, Rosen CJ, Beamer WG, et al. Circulating levels of IGF-1 directly regulate bone growth and density. J Clin Invest. 2002;110:771–781. doi: 10.1172/JCI15463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heaney RP, McCarron DA, Dawson-Hughes B, et al. Dietary changes favorably affect bone remodeling in older adults. J Am Diet Assoc. 1999;99:1228–1233. doi: 10.1016/S0002-8223(99)00302-8. [DOI] [PubMed] [Google Scholar]
- 36.Khalil DA, Lucas EA, Juma S, Smith BJ, Payton ME, Arjmandi BH. Soy protein supplementation increases serum insulin-like growth factor-I in young and old men but does not affect markers of bone metabolism. J Nutr. 2002;132:2605–2608. doi: 10.1093/jn/132.9.2605. [DOI] [PubMed] [Google Scholar]
- 37.Dawson-Hughes B, Harris SS, Rasmussen H, Song L, Dallal GE. Effect of dietary protein supplements on calcium excretion in healthy older men and women. J Clin Endocrinol Metab. 2004;89:1169–1173. doi: 10.1210/jc.2003-031466. [DOI] [PubMed] [Google Scholar]
- 38.Roughead ZK, Johnson LK, Lykken GI, Hunt JR. Controlled high meat diets do not affect calcium retention or indices of bone status in healthy postmenopausal women. J Nutr. 2003;133:1020–1026. doi: 10.1093/jn/133.4.1020. [DOI] [PubMed] [Google Scholar]
- 39.Grinspoon SK, Baum HB, Peterson S, Klibanski A. Effects of rhIGF-I administration on bone turnover during short-term fasting. J Clin Invest. 1995;96:900–906. doi: 10.1172/JCI118137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sugimoto T, Nishiyama K, Kuribayashi F, Chihara K. Serum levels of insulin-like growth factor (IGF) I, IGF-binding protein (IGFBP)-2, and IGFBP-3 in osteoporotic patients with and without spinal fractures. J Bone Miner Res. 1997;12:1272–1279. doi: 10.1359/jbmr.1997.12.8.1272. [DOI] [PubMed] [Google Scholar]
- 41.Ghiron LJ, Thompson JL, Holloway L, et al. Effects of recombinant insulin-like growth factor-I and growth hormone on bone turnover in elderly women. J Bone Miner Res. 1995;10:1844–1852. doi: 10.1002/jbmr.5650101203. [DOI] [PubMed] [Google Scholar]
- 42.O’Dea JP, Wieland RG, Hallberg MC, Llerena LA, Zorn EM, Genuth SM. Effect of dietery weight loss on sex steroid binding sex steroids, and gonadotropins in obese postmenopausal women. J Lab Clin Med. 1979;93:1004–1008. [PubMed] [Google Scholar]
- 43.Ettinger B, Ensrud KE, Wallace R, et al. Effects of ultralow-dose transdermal estradiol on bone mineral density: a randomized clinical trial. Obstet Gynecol. 2004;104:443–451. doi: 10.1097/01.AOG.0000137833.43248.79. [DOI] [PubMed] [Google Scholar]
- 44.Prestwood KM, Kenny AM, Kleppinger A, Kulldorff M. Ultralow-dose micronized 17beta-estradiol and bone density and bone metabolism in older women: a randomized controlled trial. JAMA. 2003;290:1042–1048. doi: 10.1001/jama.290.8.1042. [DOI] [PubMed] [Google Scholar]
- 45.Tzotzas T, Papadopoulou FG, Tziomalos K, et al. Rising serum 25-hydroxy-vitamin D levels after weight loss in obese women correlate with improvement in insulin resistance. J Clin Endocrinol Metab. 2010;95:4251–4257. doi: 10.1210/jc.2010-0757. [DOI] [PubMed] [Google Scholar]
- 46.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 47.Bolotin HH. A new perspective on the causal influence of soft tissue composition on DXA-measured in vivo bone mineral density. J Bone Miner Res. 1998;13:1739–1746. doi: 10.1359/jbmr.1998.13.11.1739. [DOI] [PubMed] [Google Scholar]