Abstract
Objective
To investigate the distribution of mast cells and dendritic cell (DC) subsets in paired muscle and skin (lesional/nonlesional) from untreated children with juvenile dermatomyositis (DM).
Methods
Muscle and skin biopsy samples (4 skin biopsy samples with active rash) from 7 patients with probable/definite juvenile DM were compared with muscle and skin samples from 10 healthy pediatric controls. Mast cell distribution and number were assessed by toluidine blue staining and analyzed by Student’s t-test. Immunohistochemical analysis was performed to identify mature DCs, myeloid DCs (MDCs), and plasmacytoid DCs (PDCs) by using antibodies against DC-LAMP, blood dendritic cell antigen 1 (BDCA-1), and BDCA-2, respectively. Myxovirus resistance protein A (MxA) staining indicated active type I interferon (IFN) signaling; positive staining was scored semiquantitatively and analyzed using the Mann-Whitney U test.
Results
Both inflamed and nonlesional skin from patients with juvenile DM contained more mast cells than did skin from pediatric controls (P = 0.029), and comparable numbers of mast cells were present in lesional and nonlesional skin. Interestingly, mast cell numbers were greater in skin than in paired muscle tissue from patients with juvenile DM (P = 0.014) and were not increased in muscle from patients with juvenile DM compared with control muscle. Both muscle and skin from patients with juvenile DM showed more mature PDCs and MxA staining than did their corresponding control tissues (P < 0.05). In both muscle and skin from patients with juvenile DM and in pediatric control muscle, there were fewer MDCs than PDCs, and the distributions of MDCs and PDCs were similar in pediatric control skin samples.
Conclusion
The identification of mast cells in skin (irrespective of rash) from patients with juvenile DM, but not in paired muscle tissue, suggests that they have a specific role in juvenile DM skin pathophysiology. In skin from patients with juvenile DM, increased numbers of PDCs and increased expression of type I IFN–induced protein suggest a selective influence on T cell differentiation and subsequent effector function.
Juvenile dermatomyositis (DM) is a rare systemic autoimmune vasculopathy, comprising ~85% of the pediatric idiopathic inflammatory myopathies, and is characterized clinically by symmetric proximal muscle weakness and pathognomonic skin rashes (1,2). Environmental triggers, such as season of the year (3), together with genetic predisposition (4–6) have been implicated as precipitating factors in the development of juvenile DM symptoms. Antecedent illnesses, primarily respiratory and/or gastrointestinal infections (for which antibiotics were administered in 63% of cases), preceded the onset of definite symptoms of juvenile DM (7) and were confirmed in a separate patient population (8).
Both humoral and cell-mediated components participate in vascular and muscle damage in juvenile DM (9). Skin manifestation of juvenile DM was associated with decreased nailfold end row loop capillaries rather than with muscle symptoms, implying a stronger association of microvascular damage with cutaneous findings (10,11). Study of gene expression profiles of skeletal muscle in untreated juvenile DM showed that most of the differentially expressed and up-regulated genes were inducible by type I interferons α and β (IFNα/β) (9). Further, O’Connor et al demonstrated that messenger RNA levels of the type I IFN–inducible gene myxovirus resistance protein A (MxA) in peripheral blood mononuclear cells were significantly higher in juvenile DM and were positively associated with muscle weakness, but not with the extent or severity of inflammatory skin involvement, again suggesting an organ-specific pathophysiology (12).
Type I IFNs, cytokines with potent antiviral, antimicrobial, antiproliferative, and immunomodulatory properties (13), have been implicated in the pathogenesis of several rheumatic autoimmune diseases, including cutaneous lupus erythematosus (14), systemic lupus erythematosus (SLE) (15), and adult DM (16). Higher levels of IFNα activity were also observed in serum samples from children with juvenile DM who had a shorter duration of untreated disease at diagnosis. This suggests that IFNα played a critical role in the initiation, but perhaps not in the perpetuation, of inflammatory disease (17).
One of the major sources of IFNα in children with viral infection and inflammation are plasmacytoid dendritic cells (PDCs) (18). Mature PDCs have been identified in the inflamed muscle of patients with juvenile DM, localized mainly in perivascular areas, thus suggesting an active participation in muscle inflammation (18,19). In contrast, there do not seem to be any reports identifying PDCs in skin of patients with juvenile DM. One goal of this study was to investigate different subsets of DCs in paired muscle and skin (lesional and nonlesional) of patients with juvenile DM, as well as to ascertain whether type I IFN signaling was present in skin of patients with juvenile DM as previously demonstrated in muscle.
Skin inflammation is one of the hallmarks of juvenile DM and often persists longer than evidence of muscle involvement (1). The rash is erythematous, present over areas of trauma (knees, elbows, finger joints), often itchy, and may be accompanied by urticaria. Vascular telangiectasia (manifested as dilated reddish brown ovals) is a common component and is often seen in the capillaries of the eyelids, similar to findings in mastocytosis (20). In juvenile DM with severe skin involvement, the serum IgE may be elevated, cutaneous scarring may be so severe as to induce alopecia of the scalp (21), and anasarca has been reported as well (1). Corticosteroids, the mainstay of current therapy for juvenile DM (1), also impair mast cell function and specifically inhibit mast cell degranulation (22).
In other types of dermatitis (23) and inflammatory connective tissue diseases such as systemic sclerosis and SLE (24), mast cells have been identified as playing a key role in establishing inflammation. As regulatory cells in the immune system, they are induced via various surface markers and release both pro- and antiinflammatory cues that can influence T cells, natural killer (NK) cells, and DCs (25,26). Therefore, we searched for mast cells in both muscle and skin from children with juvenile DM to ascertain whether they participated in juvenile DM pathophysiology.
PATIENTS AND METHODS
Population of patients with juvenile DM and pediatric controls
Seven children (1 male and 6 females) newly diagnosed as having untreated probable/definite juvenile DM were enrolled in the study after age-appropriate Institutional Review Board (IRB)–approved informed consent was given (no. 2002-11762). The diagnosis was made by the attending physician (LMP) based on the clinical criteria of Bohan and Peter (2). Magnetic resonance imaging (MRI)–directed muscle biopsy samples were obtained. A skin biopsy sample was also obtained from the edge of the incision for the muscle biopsy sample. The sites of the biopsies and degree of skin involvement at the biopsy sites are presented in Table 1, with demographic, clinical, and genetic data. The Disease Activity Score (DAS) for the skin lesions in children with juvenile DM is a 9-point scale composed of 4 domains: 1) intensity of skin involvement (0–4); 2) distribution of skin involvement (0–3); 3) presence/absence of vasculitis (0–1); 4) presence/absence of Gottron’s papules (0–1) (10). The second domain, distribution of skin involvement, was defined using 4 categories that recorded increasing area of skin inflammation: 0 = none; 1 = focal including areas of joint-related skin; 2 = diffuse including extensor surfaces of limbs and shawl areas; 3 = generalized including trunk involvement. These combined scores measured the disease activity of skin (27). The mean ± SD age of the children with juvenile DM at the time of the biopsies was 4.91 ± 2.58 years.
Table 1.
Demographic, clinical, serologic, and genetic data of the patients with juvenile dermatomyositis*
| Patient/sex | Race | Age at muscle biopsy, years | Duration of untreated disease, months | DAS for muscle strength and function† | DAS for skin involvement‡ | TNFα −308 genotype | Serum IFNα activity level§ | Muscle biopsy location | Skin involvement (rash at biopsy site)¶ |
|---|---|---|---|---|---|---|---|---|---|
| 1/M | Caucasian | 2.58 | 1.12 | 4.00 | 5.00 | GA | 12.31 | Right VL | Mild, diffuse (+) |
| 2/F | Hispanic | 9.00 | 10.55 | 0.50 | 5.00 | GG | 3.28 | Right VL | Mild, focal (−) |
| 3/F | Caucasian | 3.12 | 14.23 | 6.00 | 9.00 | GA | 0.51 | Right VL | Generalized (+) |
| 4/F | Caucasian | 2.92 | 5.65 | 3.00 | 6.00 | GG | 7.58 | Left VL | Moderate, focal (−) |
| 5/F | Caucasian | 7.44 | 2.04 | 8.00 | 7.00 | GG | 26.19 | Left VL | Severe, diffuse (+) |
| 6/F | Caucasian | 6.09 | 2.40 | 2.50 | 5.00 | GA | 25.59 | Right VL | Mild, focal (−) |
| 7/F | Caucasian/Indian | 3.22 | 1.68 | 4.00 | 6.00 | GG | 0.31 | Right VL | Mild, diffuse (+) |
The mean ± SD age at muscle biopsy was 4.91 ± 2.58 years, the mean ± SD duration of untreated disease was 5.38 ± 5.11 months, the mean ± SD Disease Activity Score (DAS) for muscle strength and function was 4.00 ± 2.43, and the mean ± SD DAS for skin involvement was 6.14 ± 1.46. TNFα = tumor necrosis factor α; IFNα = interferon-α; VL = vastus lateralis.
From 0 (normal) to 11 (maximal impairment).
From 0 (normal) to 9 (severe).
Sum of the number of standard deviations above the mean for healthy pediatric controls.
+ = clinically evident rash present; − = clinically evident rash absent (see Patients and Methods).
A sliver of skin was obtained at the incision site during operations for orthopedic reconstructive surgery in 4 male patients and 2 female patients who had no evidence of dermatologic or immunologic disease, and 4 additional pediatric control muscle samples were obtained. All samples were obtained after age-appropriate, IRB-approved informed consent was given (no. 2001-11715). The mean ± SD age of the 10 pediatric controls was 11.07 ± 5.70 years, which was slightly older than the test population.
Clinical definitions
The severity of the juvenile DM symptoms was assessed using a validated scoring system, the total DAS, by one physician (LMP) at the time of the clinical visit prior to the diagnostic muscle biopsy as previously described (27). The duration of untreated disease was defined as the interval of time between the first recognized symptom of rash and/or weakness (disease onset) and the obtainment of the muscle biopsy sample prior to treatment (19).
Determination of tumor necrosis factor α (TNFα) −308 polymorphism
A single basepair substitution of an A allele for the more common G allele at the TNFα −308 promoter site was confirmed using polymerase chain reaction as previously described. Digestion of the amplified product with the Nco I restriction enzyme confirmed the genotype as GG, GA, or AA (6).
Processing of muscle and skin biopsy samples
A diagnostic muscle biopsy sample was obtained from the vastus lateralis in the area of inflammation as defined by an MRI (T2-weighted, fat-suppressed image). A skin biopsy sample was also obtained from the edge of the incision site of the muscle biopsy. Muscle and skin samples were immediately divided into 2 sections, one (snap-frozen and stored in liquid nitrogen at −180°C) to be used for molecular studies and the other to be used for immunohistochemistry. Serial transverse 8 μm fresh frozen slides were prepared from the biopsy materials to be used for immunohistochemical analysis.
Mast cell staining
Frozen muscle and skin biopsy tissue sections were fixed in Carnoy’s fixative (60% ethanol, 30% chloroform, 10% glacial acetic acid) for 1 hour, followed by staining with toluidine blue (0.5% toluidine blue in 0.5N HCl) for 4 hours. The sections were then dehydrated and mounted in xylene-based medium for viewing.
Immunohistochemistry
Cold acetone–fixed frozen muscle and skin tissue sections were blocked and incubated with the following primary antibodies overnight at 4°C: anti–DC-LAMP (1:10 dilution; Beckman Coulter), anti–blood dendritic cell antigen 1 (anti–BDCA-1) (1:25 dilution; Miltenyi Biotec), and anti–BDCA-2 (1:50 dilution), and anti-MxA (1:100 dilution) (Santa Cruz Biotechnology). The sections were incubated with biotinylated secondary antibody (Jackson ImmunoResearch) at room temperature for 1 hour. Avidin–biotin complex and diaminobenzidine reagent kits (Vector) were used according to the instructions of the manufacturer to detect the secondary antibody. Counterstaining with hematoxylin and Scott’s bluing solution (Ricca Chemical) was performed. Substitutions of the primary antibodies with mouse IgG isotype control for anti–DC-LAMP and anti–BDCA-1 (catalog no. 08-6599; Zymed) and with goat IgG isotype control for anti–BDCA-2 and anti-MxA (catalog no. ab37388; Abcam) served as negative controls. Human tonsil sections were used as positive tissue controls.
Image acquisition
Images of stained tissue sections were acquired using Openlab computer software 4.04 (Improvision) and a DMR-HC microscope (Leica Microsystems) coupled to a Photometrics CoolSNAP CCD camera (RS Photometrics). Images were then edited using Photoshop CS2 software (Adobe Systems).
Measurement of serum IFNα activity and serum IgE levels
The untreated serum collected from each patient during the diagnostic clinic visit prior to biopsy was available. The IFNα activity was assessed in deidentified patient serum samples using reporter cells (WISH cells, no. CCL125; American Type Culture Collection) at the University of Chicago according to the methods described by Niewold et al (15). Total IgE assay in deidentified serum samples was performed by the Clinical Immunology Laboratory at Children’s Memorial Hospital (Chicago, IL) using ImmunoCAP (Phadia) according to the instructions of the manufacturer.
Statistical analysis
Mast cells in muscle and skin tissue sections were identified by the characteristic metachromatic staining of secretory granules with toluidine blue, appearing as red-purple against the blue background when viewed under the microscope. The number of mast cells was counted on entire tissue sections by an observer (SS) who was blinded to patient diagnosis (juvenile DM versus pediatric control), under high-power field (63× magnification) on 2 separate occasions, and the average of the 2 independent counts was calculated. The area of the tissue section (mm2) was measured, and the number of mast cells per mm2 was calculated. Student’s t-test was performed for the comparison of the mean number of mast cells between the various tissue sources. P values less than 0.05 were considered significant.
The presence of DCs—either myeloid DCs (MDCs) or PDCs—and their maturation state in muscle and skin sections were examined using antibodies to the following markers: BDCA-1 (MDCs), BDCA-2 (PDCs), and DC-LAMP (mature DCs). A positive stain for MxA was used as an indicator of active type I IFN signaling. The results were evaluated by 2 independent observers (one of whom was SS) who were blinded to the nature of the staining and the patient diagnosis (juvenile DM versus pediatric control). Positive staining was defined as areas of brown staining against the blue background of counterstain, comparable with the positive control slides. The expression of these markers was scored semiquantitatively (0 = none; 1 = weak; 2 = moderate; 3 = strong). Since the scoring was semiquantitative and not normally distributed, the nonparametric Mann-Whitney U test was used for statistical analysis. P values less than 0.05 were considered significant, and P values less than 0.01 were considered highly significant.
Spearman’s rank correlation was used to see whether the staining pattern of DCs (as well as their maturity) and the staining pattern of the type I IFN signaling marker MxA were correlated between muscle and skin from patients with juvenile DM. The Wilcoxon signed rank test was further performed to compare scores for DC-LAMP, BDCA-1, BDCA-2, and MxA staining in muscle and skin from patients with juvenile DM. The correlation of serum IFNα activity levels with scores for BDCA-2 and MxA staining in muscle and skin from patients with juvenile DM, the Disease Activity Scores, and the duration of untreated disease were also analyzed using Spearman’s rank correlation.
RESULTS
Mast cell infiltration in skin from patients with juvenile DM is elevated compared with that in skin from pediatric controls
When stained with toluidine blue, mast cells appeared spindle-shaped with purplish-red metachromatic granules present in the cytoplasm against the blue background. In control skin, rare mast cells were diffusely scattered without forming clusters, predominantly localized to the upper dermis. They were mostly found in the perivascular areas, whereas they were completely absent in epidermal layers. Skin from patients with juvenile DM showed increased density of mast cell infiltration, forming loose clusters in the perivascular area throughout dermis. Both in muscle from patients with juvenile DM and in pediatric control muscle, the numbers of mast cells were reduced compared with the numbers in the skin and were located primarily around the blood vessels with very few in perifascicular areas (Figure 1). Both in muscle from patients with juvenile DM and in pediatric control muscle, the mast cells showed no or minimal degranulation, whereas a range of degranulation was seen in control skin and, apparently to a far greater extent, in skin from patients with juvenile DM.
Figure 1.
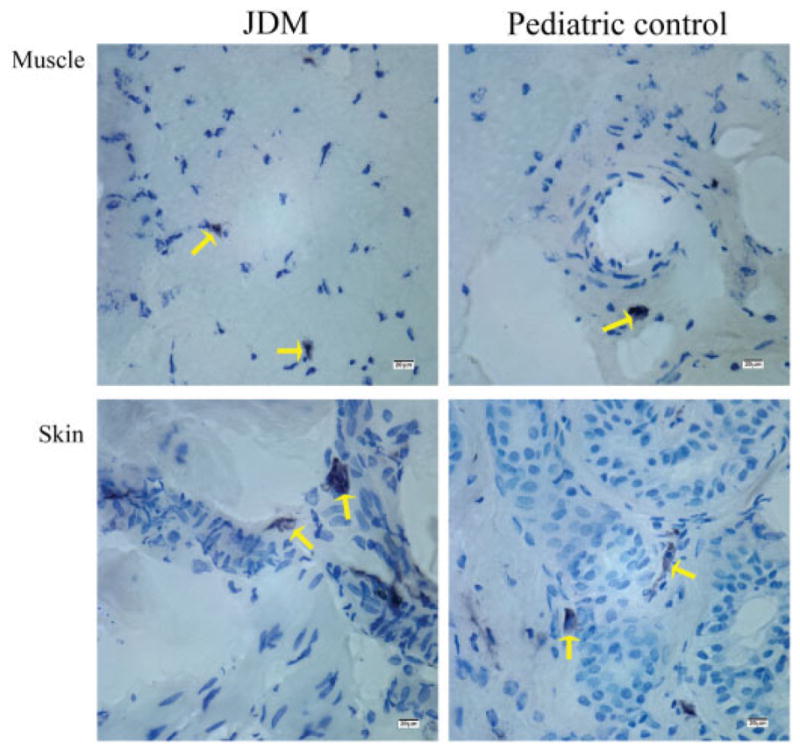
Mast cells stained with toluidine blue in paired muscle and skin samples from patients with juvenile dermatomyositis (JDM) and from pediatric controls. Arrows indicate spindle-shaped mast cells with metachromatic staining of granules (purple) present in the cytoplasm against a blue background (original magnification × 63).
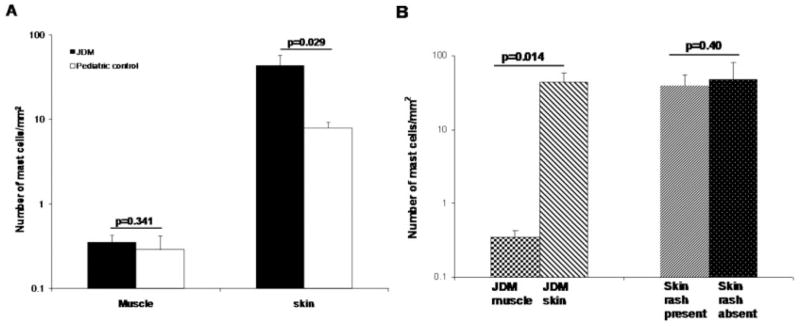
The number of mast cells infiltrating skin from patients with juvenile DM was significantly higher than the number in control skin (mean ± SD 43.16 ± 39.90/mm2 versus 7.90 ± 3.63/mm2; P = 0.029) (Figure 2A). However, there was no significant difference between the number of mast cells infiltrating muscle from patients with juvenile DM and the number infiltrating control muscle (mean ± SD 0.35 ± 0.21/mm2 versus 0.28 ± 0.26/mm2; P = 0.341) (Figure 2A). Within the patients with juvenile DM, the number of mast cells in skin was significantly higher than in the child’s corresponding muscles (P = 0.014) (Figure 2B). Furthermore, only 4 of 7 patients with juvenile DM had generalized or diffuse skin involvement with presence of rash at the site of biopsy. Of note, the number of mast cells was very similar in both lesional and nonlesional skin from patients with juvenile DM, and comparable numbers of mast cells were present both in rash at the biopsy site and in apparently normal skin at the biopsy site (P = 0.40) (Figure 2B), indicating that histologic evidence of inflammation in patients with juvenile DM was present despite lack of clinical manifestations.
Figure 2.
Mast cell distribution in muscle and skin samples from patients with juvenile dermatomyositis (JDM) and from pediatric controls. Mast cells, which were identified by toluidine blue staining, were counted in the entire muscle and skin sections, and the number of mast cells/mm2 in the respective tissue sections was calculated (n = 7 samples for muscle and skin from patients with juvenile DM, n = 4 samples for pediatric control muscle, and n = 6 samples for pediatric control skin). Values are the mean and SD. The y-axis is in logarithmic scale. P values less than 0.05 were considered significant. A, Comparison between mast cell numbers in muscle and skin samples from patients with juvenile DM and from pediatric controls. B, Comparison between mast cell numbers in muscle and skin samples from patients with juvenile DM and between mast cell numbers in skin samples with and without rash from patients with juvenile DM.
Infiltration of mature PDCs in muscle from patients with juvenile DM compared with pediatric control muscle
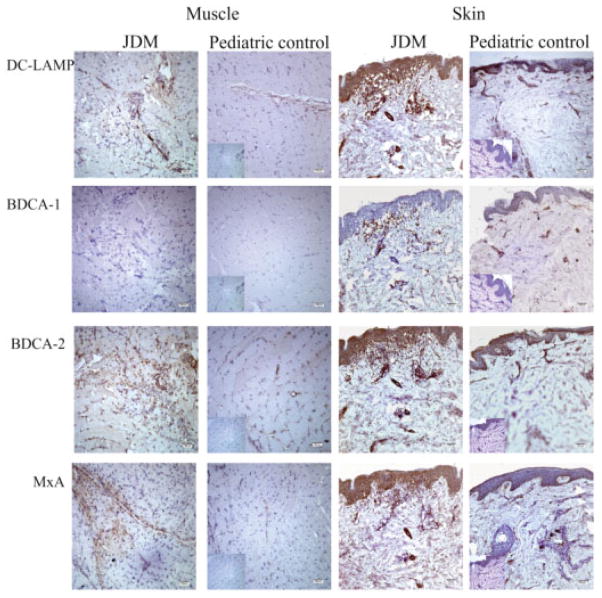
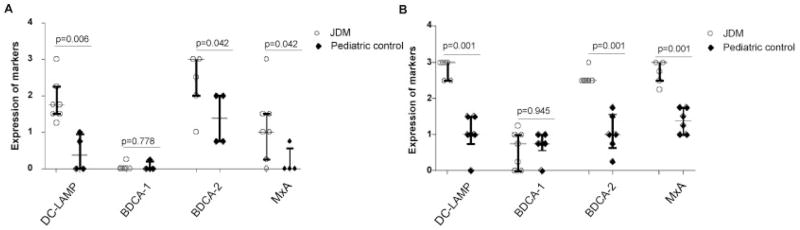
Immunohistochemical analysis was performed to identify mature DCs, MDCs, and PDCs by using antibodies against DC-LAMP, BDCA-1, and BDCA-2, respectively (Figure 3). Increased numbers of mature DCs, positive for DC-LAMP, were observed in muscle from patients with juvenile DM, primarily in the perivascular and perifascicular areas with some infiltration of the endomysium. Only 2 of 4 control muscle samples showed the presence of mature DCs, limited to the perivascular area, and there were significantly fewer mature DCs in control muscle than in muscle from patients with juvenile DM (P = 0.006) (Figure 4A). Both control muscle and muscle from patients with juvenile DM were not significantly positive for MDCs, but 1 patient sample and 1 control sample had a negligible amount of positive staining. PDCs, identified by BDCA-2, were localized to the same regions as the positive DC-LAMP stains both in control muscle and in muscle from patients with juvenile DM. Of note, BDCA-2–positive cells slightly exceeded DC-LAMP–positive cells in number, indicating that DCs in muscle from patients with juvenile DM were plasmacytoid in origin and were mostly mature. The number of PDCs in muscle from patients with juvenile DM was significantly higher than that in control muscle (P = 0.042). In contrast, the PDCs in control muscle were primarily immature cells.
Figure 3.
Immunohistochemical staining of dendritic cell (DC) markers and the type I interferon (IFN)–induced gene product myxovirus resistance protein A (MxA) on muscle and skin samples from patients with juvenile dermatomyositis (JDM) and from pediatric controls (original magnification × 10). DC-LAMP was used to detect mature DCs, blood dendritic cell antigen 1 (BDCA-1) was used to detect myeloid DCs, and BDCA-2 was used to detect plasmacytoid DCs. Type I IFN signaling was measured by staining for MxA protein, one of the type I IFN–induced gene products. Positive staining is indicated by brown color. Insets represent isotype controls for the respective markers and tissues.
Figure 4.
Grading of numbers of cells positive for immunohistochemical markers of DCs (DC-LAMP for mature DCs, BDCA-1 for myeloid DCs, and BDCA-2 for plasmacytoid DCs) and for the type I IFN–induced gene product, MxA, in muscle and skin tissue sections. Samples from patients with juvenile dermatomyositis were compared with those from pediatric controls. The y-axis shows the semiquantitative scores of DC markers for DC-LAMP, BDCA-1, and BDCA-2 and for type I IFN signaling with MxA protein for muscle (A) and skin (B). Horizontal lines represent the median; bars represent the interquartile range. P values were calculated using the Mann-Whitney U test. See Figure 3 for definitions.
Type I IFN signaling in muscle from patients with juvenile DM, as indicated by MxA protein expression
The type I IFN–inducible protein MxA was assessed to verify functional type I IFN signaling. The MxA protein was identified in 6 of 7 muscle samples from patients with juvenile DM, and was preferentially expressed by myofibers located primarily in the perifascicular area. The MxA staining was either diffuse throughout muscle fibers and/or present along the edges of specific fibers. Some fibers were atrophic, but a few normal-appearing muscle fibers expressed MxA as well. A few scattered inflammatory cells around blood vessels also stained positive for MxA. Almost all control muscle samples were negative for MxA, except for 1 sample in which there was weak expression along the perifascicular area (Figure 3). Expression of MxA in muscle from patients with juvenile DM was significantly higher than that in control muscle (P = 0.042) (Figure 4A).
Enhanced expression of mature PDCs and MxA protein indicates presence of type I IFN signaling in skin from patients with juvenile DM
To investigate one aspect of the inflammatory response in skin from patients with juvenile DM, we performed immunohisto-chemical analysis with markers for DCs and a type I IFN–inducible protein, MxA (Figure 3). Overall, mature DCs, indicated by DC-LAMP staining, were present in all parts of the epidermis and throughout the dermal layers, primarily at the periphery of blood vessels. All skin from patients with juvenile DM had strong expression of DC-LAMP, which was not associated with the presence of clinically evident rash. Control skin biopsy samples had weak expression of DC-LAMP, which localized along the epidermal basal membrane and blood vessels in the dermis and was much less intense than in skin from patients with juvenile DM (P = 0.001) (Figure 4B).
A few BDCA-1–positive cells of myeloid origin were scattered sparsely in the dermal layers, but were completely absent from the epidermis and blood vessels both in control skin and in skin from patients with juvenile DM. Strong expression of both BDCA-2 and MxA protein was observed, similar to that of DC-LAMP in skin from patients with juvenile DM, indicating the presence of both mature PDCs and IFNα signaling. Control skin biopsy samples demonstrated more cells positive for BDCA-2 and MxA than for DC-LAMP, illustrating that not all of the PDCs were mature. In addition, the expression of BDCA-2 and MxA protein was higher in skin from patients with juvenile DM than in control skin (both P = 0.001) (Figure 4B).
In summary, the expression of all DC markers and type I IFN–induced MxA was increased in skin compared with muscle in patients with juvenile DM, and this increase was significant for DC-LAMP (P = 0.007) and MxA (P = 0.011). However, the staining pattern of DC-LAMP, BDCA-1, BDCA-2, and MxA was not correlated between muscle and skin of patients with juvenile DM.
Serum IFNα activity and total IgE levels
High levels of serum IFNα activity were measured in sera from untreated children with juvenile DM (Table 1), as reported previously (17), but this activity did not correlate with any of the variables tested. In the present study, the total IgE levels measured in serum samples from each patient fell within the age-based normal range values used in the Clinical Immunology Laboratories in Children’s Memorial Hospital (data not shown).
DISCUSSION
We believe that this is the first study investigating mast cells in paired muscle and skin biopsy samples from patients with juvenile DM. Our results show that mast cells are more prevalent in skin than in muscle in patients with juvenile DM, and that the presence of active rash is not associated with the number of mast cells present. As seen in a study of scleroderma, in which unaffected skin had an increased number of total and degranulated mast cells and the involved skin became fibrotic within 1.5 years of biopsy (28), we speculate that nonlesional skin with an increased number of mast cells in juvenile DM may develop more severe damage if not properly and effectively treated. In this investigation, all the untreated children with juvenile DM had DAS skin scores of ≥4, which indicated that they had at least a moderate level of skin involvement.
Mast cells have pro- and antiinflammatory functions in both the innate and the adaptive immune systems, making them a critical element in the patho-physiology of a range of skin diseases (23). One of the pathways that activates the cutaneous mast cells is exposure to sunlight, since both ultraviolet A (UVA) and UVB radiation can reach to the reticular dermal layers where mast cells are located. UVB exposure can trigger mast cells to produce interleukin-8 (IL-8), which assists recruitment of neutrophils to the site of inflammation (29), and neutrophils are a component of the inflammatory response in skin in DM (30) and are also found in association with a diffuse distribution of mast cells in all layers of the skin in a Sheltie model of DM (31). Skin lesions created in SLE patients by exposure to UVB showed up-regulation of IFNα, MxA, and endothelial cell adhesion molecules (32). In addition, UVA can suppress the established secondary immune memory and response by acting on cis-urocanic acid and calcitonin gene-related peptide, which target mast cells, or by acting on products of mast cells such as histamine, platelet-activating factor, and IL-10 (33). These studies show that mast cells are important in establishing inflammation in skin and suggest that skin flares in juvenile DM may be precipitated by circumstances that activate mast cells.
In contrast, the role of mast cells in skeletal muscles has not been well characterized. For example, an increased congregation of mast cells, both intact cells as well as those undergoing degranulation, was reported in muscle from children with Duchenne’s muscular dystrophy (34). Blocking mast cell degranulation by administration of cromolyn reduced dystrophic muscle necrosis (35), which suggested that one function of mast cells may be to induce necrosis of myofibers. However, mast cells were often more apparent in regenerating myofibers than in those undergoing fibrosis (36) and appear to contribute to cardiac pathology as well (37). The scarcity of mast cells in muscle in juvenile DM implies that cellular immune components other than mast cells might be central to persistence of myositis, as opposed to the chronic skin inflammation.
Information about the dynamics of mast cell interaction with other immune cells has narrowed the gap in our understanding of autoimmune diseases and has also increased our understanding of the role of mast cells as central organizers. Mast cells may modulate T cell response by presenting antigen directly to CD8+ T cells via class I major histocompatibility complex molecules, thus leading to antigen-specific T cell activation and proliferation. Mast cells can influence T cell maturation along either the Th1 or Th2 pathway, either directly by release of specific mediators or indirectly by their action on DCs (38). It has been shown that PDCs and type I IFN can selectively induce Th1-biased inflammatory immune response in patients with cutaneous lupus erythematosus (14). In vitro experiments show that mast cells also affect the ability of murine Treg cells to suppress effector T cells, which ultimately establishes the Th17-mediated inflammatory response (39), recently found to be highly up-regulated in the muscle of children with juvenile DM (40). By producing CXCL8, mast cells also recruit NK cells to respond to early viral stimuli (26). Similarly, ratio-dependent direct cross-talk between NK cells and DCs can influence DC functions (41).
It has been documented that PDCs are the predominant type of DCs in muscle in DM and are found in the endomysium, perimysium, and perivascular regions (42). Similarly, our results demonstrated that inflamed, untreated muscle in juvenile DM was infiltrated with mature PDCs, primarily localized in the perivascular area, as reported previously (18,19). In the present study, we did not identify MDCs in muscle in juvenile DM, but they have been characterized in other inflammatory myopathies, such as polymyositis (PM) and inclusion body myositis (43), as well as in juvenile PM, in which levels of IFNα were in the normal range (44). These results suggest that the specific DC subsets can differentially influence disease pathophysiology and disease outcomes. Recent investigation showed that the dissection of the cellular infiltrate components in muscle biopsy samples in juvenile DM can help to design individualized therapeutic interventions (45).
PDCs are the major producers of type I IFN. The high levels of serum IFNα activity measured in patients with juvenile DM in a previous cohort (17) as well as in the present study may be a reflection of activated PDCs. Furthermore, IFNα itself can also modulate both DC subsets—MDCs and PDCs—and alter their maturity and migratory and stimulatory functions. High levels of IFNα activity can also act in a negative feedback loop to prevent further activation of PDCs during the ongoing immune response (13). Effective type I IFN signaling can be confirmed by evaluating IFN-inducible genes and proteins. In muscle in adult DM, microarray studies have shown substantially high up-regulation of IFNα/β-inducible gene transcripts including the promoter sequences. Type I IFN signaling was further evident by the strong expression of MxA by myofibers in the perifascicular and perivascular regions (42). Similarly, muscle in juvenile DM in the present study showed increased expression of MxA protein in myofibers in the perifascicular region and in some inflammatory cells around blood vessels. Type I IFN signaling is also present in skin lesions in DM, as shown by MxA expression in the epidermis and in the inflammatory infiltrates as well as by expression of IFNγ-inducible 10-kd protein, which recruits lymphocytes to the site of inflammation (30). PDCs were the major infiltrating DCs in lesional skin in adult DM and were localized only in epidermis, whereas in LE they were localized only in dermis (46). Unlike skin in DM and LE, skin in juvenile DM contained PDC infiltrates in both the epidermal and dermal layers as well as around blood vessels. This implies that PDCs could have greater impact on skin inflammation in juvenile DM than in other rheumatic diseases with cutaneous involvement. The presence of MxA protein in skin in juvenile DM supports the hypothesis that microbial factors could be associated with the initiation of the inflammatory process in juvenile DM, as was seen in an early study of dermatoses in which MxA was expressed only in skin with acute viral lesions but not in skin with nonviral lesions (47). Studies of cyclosporin A (CSA) suggest that the drug inhibits histamine release from mast cells (48), and CSA is often an effective drug in the therapy of juvenile DM (49).
Other agents to be considered for their specific effect on mast cells are bispecific antibodies that target both activating (Fcε receptor type I [FcεRI]) and inhibitory (FcγRIIB) receptors and suppress mast cell activity (50). It is axiomatic that a further understanding of the mechanisms of cellular interactions in skin in juvenile DM will teach us a great deal more about the often drug-resistant cutaneous inflammation and disease pathophysiology, perhaps leading to more effective interventions.
Acknowledgments
Dr. Niewold’s work was supported by the NIH (grant K08-AI-083790), the Arthritis National Research Foundation, Eng Tan Scholar Award, and the Lupus Research Institute. Dr. Pachman’s work was supported by the NIH (grant R01-AR-48289 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases), the Cure JM Foundation, and the Macy’s Miracle Foundation.
Footnotes
Dr. Wershil has received consulting fees, speaking fees, and/or honoraria from Prometheus Laboratories (less than $10,000).
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Pachman had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Shrestha, Wershil, Pachman.
Acquisition of data. Shrestha, Wershil, Sarwark, Niewold, Philipp, Pachman.
Analysis and interpretation of data. Shrestha, Niewold, Pachman.
References
- 1.Feldman BM, Rider LG, Reed AM, Pachman LM. Juvenile dermatomyositis and other idiopathic inflammatory myopathies of childhood. Lancet. 2008;371:2201–12. doi: 10.1016/S0140-6736(08)60955-1. [DOI] [PubMed] [Google Scholar]
- 2.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts) N Engl J Med. 1975;292:344–7. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 3.Vegosen LJ, Weinberg CR, O’Hanlon TP, Targoff IN, Miller FW, Rider LG. Seasonal birth patterns in myositis subgroups suggest an etiologic role of early environmental exposures. Arthritis Rheum. 2007;56:2719–28. doi: 10.1002/art.22751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reed AM, Pachman L, Ober C. Molecular genetic studies of major histocompatibility complex genes in children with juvenile dermatomyositis: increased risk associated with HLA-DQA1 *0501. Hum Immunol. 1991;32:235–40. doi: 10.1016/0198-8859(91)90085-n. [DOI] [PubMed] [Google Scholar]
- 5.Mamyrova G, O’Hanlon TP, Monroe JB, Carrick DM, Malley JD, Adams S, et al. Immunogenetic risk and protective factors for juvenile dermatomyositis in Caucasians. Arthritis Rheum. 2006;54:3979–87. doi: 10.1002/art.22216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pachman LM, Liotta-Davis MR, Hong DK, Kinsella TR, Mendez EP, Kinder JM, et al. TNFα-308A allele in juvenile dermatomyositis: association with increased production of tumor necrosis factor α, disease duration, and pathologic calcifications. Arthritis Rheum. 2000;43:2368–77. doi: 10.1002/1529-0131(200010)43:10<2368::AID-ANR26>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 7.Pachman LM, Lipton R, Ramsey-Goldman R, Shamiyeh E, Abbott K, Mendez EP, et al. History of infection before the onset of juvenile dermatomyositis: results from the National Institute of Arthritis and Musculoskeletal and Skin Diseases Research Registry. Arthritis Rheum. 2005;53:166–72. doi: 10.1002/art.21068. [DOI] [PubMed] [Google Scholar]
- 8.Manlhiot C, Liang L, Tran D, Bitnun A, Tyrrell PN, Feldman BM. Assessment of an infectious disease history preceding juvenile dermatomyositis symptom onset. Rheumatology (Oxford) 2008;47:526–9. doi: 10.1093/rheumatology/ken038. [DOI] [PubMed] [Google Scholar]
- 9.Tezak Z, Hoffman EP, Lutz JL, Fedczyna TO, Stephan D, Bremer EG, et al. Gene expression profiling in DQA1*0501+ children with untreated dermatomyositis: a novel model of pathogenesis. J Immunol. 2002;168:4154–63. doi: 10.4049/jimmunol.168.8.4154. [DOI] [PubMed] [Google Scholar]
- 10.Smith RL, Sundberg J, Shamiyah E, Dyer A, Pachman LM. Skin involvement in juvenile dermatomyositis is associated with loss of end row nailfold capillary loops. J Rheumatol. 2004;31:1644–9. [PubMed] [Google Scholar]
- 11.Christen-Zaech S, Seshadri R, Sundberg J, Paller AS, Pachman LM. Persistent association of nailfold capillaroscopy changes and skin involvement over thirty-six months with duration of untreated disease in patients with juvenile dermatomyositis. Arthritis Rheum. 2008;58:571–6. doi: 10.1002/art.23299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Connor KA, Abbott KA, Sabin B, Kuroda M, Pachman LM. MxA gene expression in juvenile dermatomyositis peripheral blood mononuclear cells: association with muscle involvement. Clin Immunol. 2006;120:319–25. doi: 10.1016/j.clim.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cicinnati VR, Kang J, Hou J, Lindemann M, Koop K, Tuting T, et al. Interferon-α differentially affects homeostasis of human plasmacytoid and myeloid dendritic cells. J Interferon Cytokine Res. 2009;29:145–59. doi: 10.1089/jir.2008.0011. [DOI] [PubMed] [Google Scholar]
- 14.Wenzel J, Worenkamper E, Freutel S, Henze S, Haller O, Bieber T, et al. Enhanced type I interferon signalling promotes Th1-biased inflammation in cutaneous lupus erythematosus. J Pathol. 2005;205:435–42. doi: 10.1002/path.1721. [DOI] [PubMed] [Google Scholar]
- 15.Niewold TB, Hua J, Lehman TJ, Harley JB, Crow MK. High serum IFN-α activity is a heritable risk factor for systemic lupus erythematosus. Genes Immun. 2007;8:492–502. doi: 10.1038/sj.gene.6364408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walsh RJ, Kong SW, Yao Y, Jallal B, Kiener PA, Pinkus JL, et al. Type I interferon–inducible gene expression in blood is present and reflects disease activity in dermatomyositis and polymyositis. Arthritis Rheum. 2007;56:3784–92. doi: 10.1002/art.22928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niewold TB, Kariuki SN, Morgan GA, Shrestha S, Pachman LM. Elevated serum interferon-α activity in juvenile dermatomyositis: associations with disease activity at diagnosis and after thirty-six months of therapy. Arthritis Rheum. 2009;60:1815–24. doi: 10.1002/art.24555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopez de Padilla CM, Vallejo AN, McNallan KT, Vehe R, Smith SA, Dietz AB, et al. Plasmacytoid dendritic cells in inflamed muscle of patients with juvenile dermatomyositis. Arthritis Rheum. 2007;56:1658–68. doi: 10.1002/art.22558. [DOI] [PubMed] [Google Scholar]
- 19.Chen YW, Shi R, Geraci N, Shrestha S, Gordish-Dressman H, Pachman LM. Duration of chronic inflammation alters gene expression in muscle from untreated girls with juvenile dermatomyositis. BMC Immunol. 2008;9:43. doi: 10.1186/1471-2172-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horny HP. Mastocytosis: an unusual clonal disorder of bone marrow-derived hematopoietic progenitor cells. Am J Clin Pathol. 2009;132:438–47. doi: 10.1309/AJCPPXHMN5CJOXHZ. [DOI] [PubMed] [Google Scholar]
- 21.Pachman LM. Juvenile dermatomyositis and other inflammatory myopathies in children. Philadelphia: Butterworth Heinemann; 2003. [Google Scholar]
- 22.Zhou J, Liu DF, Liu C, Kang ZM, Shen XH, Chen YZ, et al. Glucocorticoids inhibit degranulation of mast cells in allergic asthma via nongenomic mechanism. Allergy. 2008;63:1177–85. doi: 10.1111/j.1398-9995.2008.01725.x. [DOI] [PubMed] [Google Scholar]
- 23.Navi D, Saegusa J, Liu FT. Mast cells and immunological skin diseases. Clin Rev Allergy Immunol. 2007;33:144–55. doi: 10.1007/s12016-007-0029-4. [DOI] [PubMed] [Google Scholar]
- 24.Rimkevicius A, Mackiewicz Z. Mast cells in the inflammatory connective tissue diseases. Acta Medica Lituanica. 2006;13:77–82. [Google Scholar]
- 25.Mazzoni A, Siraganian RP, Leifer CA, Segal DM. Dendritic cell modulation by mast cells controls the Th1/Th2 balance in responding T cells. J Immunol. 2006;177:3577–81. doi: 10.4049/jimmunol.177.6.3577. [DOI] [PubMed] [Google Scholar]
- 26.Burke SM, Issekutz TB, Mohan K, Lee PW, Shmulevitz M, Marshall JS. Human mast cell activation with virus-associated stimuli leads to the selective chemotaxis of natural killer cells by a CXCL8-dependent mechanism. Blood. 2008;111:5467–76. doi: 10.1182/blood-2007-10-118547. [DOI] [PubMed] [Google Scholar]
- 27.Bode RK, Klein-Gitelman MS, Miller ML, Lechman TS, Pachman LM. Disease activity score for children with juvenile dermatomyositis: reliability and validity evidence. Arthritis Rheum. 2003;49:7–15. doi: 10.1002/art.10924. [DOI] [PubMed] [Google Scholar]
- 28.Claman HN. Mast cells and fibrosis. The relevance to scleroderma. Rheum Dis Clin North Am. 1990;16:141–51. [PubMed] [Google Scholar]
- 29.Endoh I, Di Girolamo N, Hampartzoumian T, Cameron B, Geczy CL, Tedla N. Ultraviolet B irradiation selectively increases the production of interleukin-8 in human cord blood-derived mast cells. Clin Exp Immunol. 2007;148:161–7. doi: 10.1111/j.1365-2249.2007.03332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wenzel J, Schmidt R, Proelss J, Zahn S, Bieber T, Tuting T. Type I interferon-associated skin recruitment of CXCR3+ lymphocytes in dermatomyositis. Clin Exp Dermatol. 2006;31:576–82. doi: 10.1111/j.1365-2230.2006.02150.x. [DOI] [PubMed] [Google Scholar]
- 31.Haupt KH, Prieur DJ, Hargis AM, Cowell RL, McDonald TL, Werner LL, et al. Familial canine dermatomyositis: clinicopathologic, immunologic, and serologic studies. Am J Vet Res. 1985;46:1870–5. [PubMed] [Google Scholar]
- 32.Reefman E, Kuiper H, Limburg PC, Kallenberg CG, Bijl M. Type I interferons are involved in the development of ultraviolet B-induced inflammatory skin lesions in systemic lupus erythaematosus patients. Ann Rheum Dis. 2008;67:11–8. doi: 10.1136/ard.2007.070359. [DOI] [PubMed] [Google Scholar]
- 33.Ullrich SE, Nghiem DX, Khaskina P. Suppression of an established immune response by UVA—a critical role for mast cells. Photochem Photobiol. 2007;83:1095–100. doi: 10.1111/j.1751-1097.2007.00184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gorospe JR, Tharp M, Demitsu T, Hoffman EP. Dystrophin-deficient myofibers are vulnerable to mast cell granule-induced necrosis. Neuromuscul Disord. 1994;4:325–33. doi: 10.1016/0960-8966(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 35.Radley HG, Grounds MD. Cromolyn administration (to block mast cell degranulation) reduces necrosis of dystrophic muscle in mdx mice. Neurobiol Dis. 2006;23:387–97. doi: 10.1016/j.nbd.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 36.Lefaucheur JP, Gjata B, Sebille A. Factors inducing mast cell accumulation in skeletal muscle. Neuropathol Appl Neurobiol. 1996;22:248–55. [PubMed] [Google Scholar]
- 37.Gavrisheva NA, Tkachenko SB. Mast cells in normal and diseased heart. Kardiologiia. 2003;43:59–65. In Russian. [PubMed] [Google Scholar]
- 38.Stelekati E, Bahri R, D’Orlando O, Orinska Z, Mittrucker HW, Langenhaun R, et al. Mast cell-mediated antigen presentation regulates CD8+ T cell effector functions. Immunity. 2009;31:665–76. doi: 10.1016/j.immuni.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 39.Piconese S, Gri G, Tripodo C, Musio S, Gorzanelli A, Frossi B, et al. Mast cells counteract regulatory T-cell suppression through interleukin-6 and OX40/OX40L axis toward Th17-cell differentiation. Blood. 2009;114:2639–48. doi: 10.1182/blood-2009-05-220004. [DOI] [PubMed] [Google Scholar]
- 40.Reed AM, Ernste F. The inflammatory milieu in idiopathic inflammatory myositis. Curr Rheumatol Rep. 2009;11:295–301. doi: 10.1007/s11926-009-0041-1. [DOI] [PubMed] [Google Scholar]
- 41.Piccioli D, Sbrana S, Melandri E, Valiante NM. Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J Exp Med. 2002;195:335–41. doi: 10.1084/jem.20010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greenberg SA, Pinkus JL, Pinkus GS, Burleson T, Sanoudou D, Tawil R, et al. Interferon-α/β-mediated innate immune mechanisms in dermatomyositis. Ann Neurol. 2005;57:664–78. doi: 10.1002/ana.20464. [DOI] [PubMed] [Google Scholar]
- 43.Greenberg SA, Pinkus GS, Amato AA, Pinkus JL. Myeloid dendritic cells in inclusion-body myositis and polymyositis. Muscle Nerve. 2007;35:17–23. doi: 10.1002/mus.20649. [DOI] [PubMed] [Google Scholar]
- 44.Pachman LM, Neiwold TB, Kariuki SN, Morgan GA, Geraci N, Chen YW. Decreased type-1 interferon α in sera from untreated children with juvenile polymyositis (JPM) compared with juvenile dermatomyositis (JDM), matched for short disease duration [abstract] Arthritis Rheum. 2008;58 (Suppl):S225. [Google Scholar]
- 45.Lopez de Padilla CM, Vallejo AN, Lacomis D, McNallan K, Reed AM. Extranodal lymphoid microstructures in inflamed muscle and disease severity of new-onset juvenile dermatomyositis. Arthritis Rheum. 2009;60:1160–72. doi: 10.1002/art.24411. [DOI] [PubMed] [Google Scholar]
- 46.McNiff JM, Kaplan DH. Plasmacytoid dendritic cells are present in cutaneous dermatomyositis lesions in a pattern distinct from lupus erythematosus. J Cutan Pathol. 2008;35:452–6. doi: 10.1111/j.1600-0560.2007.00848.x. [DOI] [PubMed] [Google Scholar]
- 47.Fah J, Pavlovic J, Burg G. Expression of MxA protein in inflammatory dermatoses. J Histochem Cytochem. 1995;43:47–52. doi: 10.1177/43.1.7822763. [DOI] [PubMed] [Google Scholar]
- 48.Stellato C, de Paulis A, Ciccarelli A, Cirillo R, Patella V, Casolaro V, et al. Anti-inflammatory effect of cyclosporin A on human skin mast cells. J Invest Dermatol. 1992;98:800–4. doi: 10.1111/1523-1747.ep12499960. [DOI] [PubMed] [Google Scholar]
- 49.Reiff A, Rawlings DJ, Shaham B, Franke E, Richardson L, Szer IS, et al. Preliminary evidence for cyclosporin A as an alternative in the treatment of recalcitrant juvenile rheumatoid arthritis and juvenile dermatomyositis. J Rheumatol. 1997;24:2436–43. [PubMed] [Google Scholar]
- 50.Karra L, Berent-Maoz B, Ben-Zimra M, Levi-Schaffer F. Are we ready to downregulate mast cells? Curr Opin Immunol. 2009;21:708–14. doi: 10.1016/j.coi.2009.09.010. [DOI] [PubMed] [Google Scholar]