Abstract
Objectives
To determine the association between neutrophil gelatinase-associated lipocalin (NGAL) levels and cardiovascular and all-cause mortality in community-dwelling older adults.
Background
NGAL is a novel marker best known for its role in rapidly identifying acute kidney injury. Although expressed in atherosclerosis, its association with cardiovascular disease (CVD) in the community has not been reported.
Methods
We measured plasma NGAL levels in 1393 Rancho Bernardo Study participants without CVD, mean age 70. Participants were followed for a mean of 11 years.
Results
During follow-up, 436 participants died (169 from CVD). In models adjusted for traditional CVD risk factors and creatinine clearance, NGAL was a significant predictor of CVD mortality (HR per SD log increase: 1.33, 95% CI[1.12–1.57]), all-cause mortality (HR 1.19[1.07–1.32]), and a combined cardiovascular endpoint(HR 1.26[1.10–1.45]). After further adjusting for NT-proBNP and CRP, NGAL remained an independent predictor of each outcome. NGAL improved the c-statistic (0.835 to 0.842) for prediction of CVD death (p=0.001). Net reclassification improvement (NRI>0) with the addition of NGAL was 18% (p=0.02); the integrated discrimination index was also significant (p=0.01). Participants with NGAL and NT-proBNP above the median had increased risk of CVD death vs. those with only NT-proBNP elevated (HR 1.43[1.12–1.82]).
Conclusions
Plasma NGAL is a significant predictor of mortality and CVD in community-dwelling older adults, independent of traditional risk factors and kidney function, and adds incremental value to NT-proBNP and CRP. The potential impact of these results includes providing insight into new mechanisms of CVD and the possibility of improving screening, intervention and prevention.
Keywords: Cardiovascular disease, biomarkers, elderly, natriuretic peptides, risk factors
Introduction
Neutrophil gelatinase-associated lipocalin (NGAL) is a protein belonging to the lipocalin family, and is best known clinically as a novel and early marker of acute kidney injury (1). NGAL is expressed by activated neutrophils and various epithelial cells (2), and is also produced by renal tubular cells in response to injury (1). NGAL plays a role in tumor development (3), and may have a protective role against bacterial infection (4), apoptosis (5), and oxidative stress (6).
Recent studies have demonstrated that NGAL is expressed at high levels in the heart and in atherosclerotic plaques (7), where it has been shown to inhibit the degradation of matrix metalloproteinase-9 (8). Matrix metalloproteinase-9 is known to play a critical role in acute coronary syndromes, serving to weaken vessels and destabilize plaques, leading to an increased risk of plaque rupture (9). Despite this putative role of NGAL in atherosclerosis and acute coronary syndromes, human studies of the relationship between NGAL and cardiovascular disease (CVD) are limited. A few small cross-sectional studies have demonstrated increased levels of urine, serum, or plasma NGAL among patients with heart failure (10,11), coronary artery disease (12,13), and stroke (14,15); and three small observational studies in heart failure patients (n=46, n=236, and n=186 respectively) (16–18), plus one in stroke patients (n=144) (19), suggested that higher NGAL levels were associated with increased mortality. The association between NGAL and CVD or mortality in the general community has never been reported. We tested the hypothesis that plasma NGAL is associated with CVD and mortality in a population of community-dwelling older adults.
Methods
Study Population
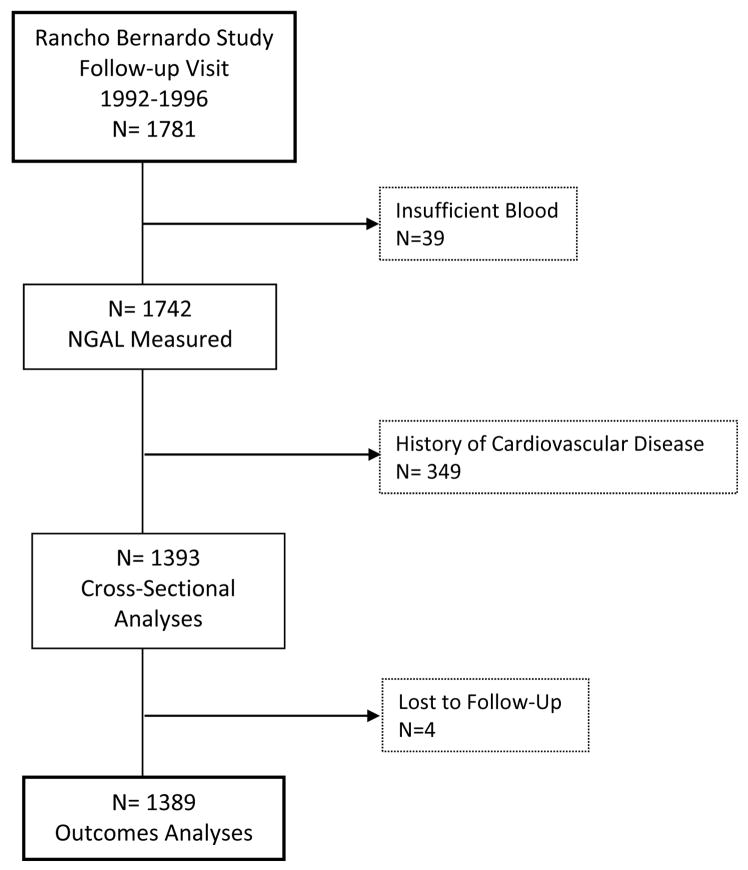
The Rancho Bernardo Study is an ongoing, prospective, population-based study of the epidemiology of cardiovascular and other chronic diseases. Between 1972 and 1974, all adults between 30 and 80 years old who resided in Rancho Bernardo, a community in Southern California, were invited to participate in a study of heart disease risk factors; 5052 (82%) enrolled. In 1992–1996, 1781 of the surviving participants attended a follow-up study visit, which served as the baseline visit for the present analyses. Sufficient blood was available for measurement of NGAL in 1742 (97.8%) of the participants; the 1393 (80.0%) who had no history of CVD at the time of this follow-up study visit are the focus of these analyses. Prevalent CVD at baseline was defined as a history of physician-diagnosed myocardial infarction, coronary revascularization, stroke, transient ischemic attack, or peripheral arterial disease. Four participants lost to follow-up immediately after their study visit were not included in outcomes analyses (Figure 1). All participants provided written informed consent; the study protocol was approved by the human research protection program at the University of California at San Diego.
Figure 1. Flowchart of Rancho Bernardo Study Participants.
Flowchart showing which Rancho Bernardo Study participants were included in the present analyses.
Data Collection
Data collected at the 1992–1996 research clinic visit served as the baseline for these analyses. Medical histories and information about physical activity (exercise 3+ times per week, yes/no), alcohol consumption (1+drinks per day versus less or none), and current smoking (yes/no) were obtained using standard questionnaires developed by the Rancho Bernardo Research Group. Blood pressure, height and weight were measured, and body mass index (BMI; kg/m2) was calculated. Diabetes mellitus was defined as a fasting morning plasma glucose level ≥126mg/dl, reported physician diagnosis, or use of diabetes-specific medication. Hypertension was defined as resting blood pressure ≥140mmHg systolic or ≥90mmHg diastolic, reported physician diagnosis, or use of anti-hypertensive medication. Estimated creatinine clearance (CrCl) was calculated using the Cockroft-Gault formula; results were not materially different after substituting the estimated glomerular filtration rate by the MDRD formula instead of the CrCl, and only the latter data are shown. Participants were followed with periodic clinic visits and annual mailed questionnaires through July 30, 2009, an average of 11.0±3.7 years.
Definition of Endpoints
The primary outcomes were CVD death, all-cause mortality, and the combined cardiovascular endpoint which was defined as the first incidence of coronary revascularization (percutaneous coronary intervention or coronary artery bypass graft surgery), non-fatal myocardial infarction, or CVD death. Death certificates were obtained for decedents and coded by a certified nosologist using the International Classification of Disease–9th Revision criteria. CVD death included deaths assigned codes 390–459.
Laboratory Methods
Morning blood samples were obtained by venipuncture after a 12 hour fast. Serum and plasma were separated, and lipids, lipoproteins, and glucose levels were measured on fresh samples; the remaining samples were stored frozen at −70°C. N-terminal pro B-type natriuretic peptide (NT-proBNP), NGAL, and C-reactive protein (CRP) were measured in 2009–2010 in EDTA plasma that had been collected at the 1992–1996 study visit and had been stored frozen at −70°C. NT-proBNP was measured using the Elecsys® proBNP sandwich immunoassay (measurable range 5–35,000 pg/ml; Roche Diagnostics, Indianapolis, Indiana); three of the 1391 participants did not have sufficient plasma for this measurement. Plasma NGAL was measured using a screening NGAL test developed on the Luminex platform as a competitive immunoassay (measureable range 5–2000 ng/mL, limit of quantification 5 ng/L, Alere Inc., Waltham, MA). Intra-assay coefficient of variation (CV) was 8%, and inter-assay CV was 11% at an NGAL level of 67 ng/mL. CRP was also measured on the Luminex platform with a competitive immunoassay (measureable range 0.004–10.000 mg/dL; intra-assay CV 7%, inter-assay CV 10%).
Statistical Analysis
Continuous variables are presented as means±standard deviation; most laboratory values were not normally distributed, and are presented as medians (quartile 1–quartile 3). Dichotomous variables are presented as percentages. NGAL, NT-proBNP, and CRP were log10 transformed for incorporation in subsequent analyses.
Single-predictor associations between the clinical variables listed in Table 1 and logNGAL levels were determined by linear regression analysis. Backward multivariable regression analysis including variables with significant individual associations was used to determine which covariates were independently associated with logNGAL levels; repeating the analysis with forward regression analysis yielded identical results.
Table 1.
Baseline characteristics of the study population
| Age | 70 ± 11 |
| % Male | 35.8 |
| Vital Signs | |
| Heart rate, bpm | 65 ± 11 |
| Systolic BP, mm Hg | 135 ± 22 |
| Diastolic BP, mm Hg | 76 ± 9 |
| Cardiovascular Risk Factors | |
| Hypertension, % | 49.0 |
| Current smoking, % | 7.7 |
| Ever smoked, % | 55.1 |
| Diabetes, % | 12.4 |
| Medication Use | |
| Aspirin, % | 26.1 |
| Lipid-Lowering, % | 9.6 |
| Nutrition and Activity | |
| Body mass index, kg/m2 | 25.5 ± 4.0 |
| Waist-hip ratio, cm/cm | 0.83 ± 0.09 |
| Exercise ≥3x/wk, % | 71.0 |
| Alcohol ≥3x/wk, % | 45.2 |
| Laboratory Values | |
| NT-proBNP*, pg/mL | 112 (56–211) |
| CRP*, mg/dL | 0.78 (0.37–1.71) |
| Fasting glucose*, mg/dL | 94 (88–100) |
| CrCl*, ml/min | 62 (48–79) |
| Total cholesterol*, mg/dL | 209 (187–234) |
| Triglycerides*, mg/dL | 103 (74–146) |
| HDL*, mg/dL | 57 (46–70) |
| LDL*, mg/dL | 127 (106–148) |
Median (Quartile 1 - Quartile 3)
BP=blood pressure; BPM=beats per minute; CrCl=creatinine clearance; CRP = C-reactive protein; CVD=cardiovascular disease; HDL=high-density lipoprotein; LDL=low-density lipoprotein; mm Hg = millimeters of mercury; NT-proBNP=N-terminal pro B-type natriuretic peptide.
Cox proportional hazard regression models were used to determine the association of log10 NGAL with each endpoint. Missing datapoints (<0.01% of data) were mean substituted. Model 1 adjusted for age and sex. Model 2 additionally adjusted for traditional cardiovascular risk factors, including categorically defined diabetes, hypertension, and current smoking, plus continuously defined systolic blood pressure, total cholesterol, high density lipoprotein (HDL), kidney function, and BMI. Model 3 additionally adjusted for logNT-proNBP and logCRP. Analyses were also performed with NGAL as a binary variable (above or below the median), and Kaplan Meier cumulative survival plots were constructed with log-rank tests used to compare groups. To help understand to what extent NGAL levels reflect kidney function as opposed to other, unmeasured factors, an additional exploratory analysis was performed; this model evaluated the other covariates from Model 3 but without logNGAL. We tested for interactions between logNGAL levels and both sex and diabetes using Cox proportional hazard models with interaction terms, and by performing sex- and diabetes-stratified analyses.
In fully adjusted analyses, both logNGAL and logNT-proBNP were independent predictors of each outcome, but logCRP was not. Therefore, to further understand the incremental benefit of NGAL when combined with NT-proBNP levels, we divided participants into 4 groups based upon whether their levels were above or below the median for each of these two biomarkers. Kaplan Meier cumulative survival plots were again constructed, and groups compared using the log-rank test. We tested for an interaction between NGAL group and NT-proBNP group using Cox proportional hazard models. Cox models were also used to compare groups after adjusting for traditional CVD risk factors, CrCl, and BMI.
Receiver operating characteristic (ROC) curves were constructed and areas under the ROC curves (C-statistic) were calculated using a method adapted for survival models (20), to evaluate the incremental improvement in discrimination with logNGAL, for each outcome. LogNGAL was added to two separate models: first, to the baseline model including the covariates from Model 2 (traditional CVD risk factors plus CrCl and BMI), and second to the Model 3 covariates (which also included logNT-proBNP and logCRP). Cox model increment tests were used to assess whether global model fit improved with the addition of logNGAL. For comparison, the C-statistics were also evaluated for the addition of NT-proBNP and of CRP to Model 2.
Model calibration was assessed using a Hosmer-Lemeshow test modified for use with Cox proportional hazards models (21). Integrated discrimination improvement (IDI) and net reclassification improvement (NRI) for the addition of logNGAL to Model 2 were calculated according to the methods of Pencina et al (22). Because NRI calculations are highly sensitive to chosen cut-points, and because there are no pre-specified cut-points for long-term follow-up in elderly individuals with these specific outcomes, the category-free NRI (NRI>0) was utilized. The NRI>0 also facilitates comparison with other studies. Both the “event NRI” and the “non-event NRI” were also calculated (23). The event NRI is the amount of correct reclassification that occurred among participants who had events, while the non-event NRI is the amount of correct reclassification among participants who did not have events. For reclassification analyses, we estimated risk at 12 years.
A two-tailed p<0.05 was considered statistically significant. Data were analyzed using SPSS 19.0 (Chicago, IL).
Results
Baseline Characteristics
The mean age of the overall study population (n=1742) at baseline was 71±11 years. The median NGAL level was 200 ng/mL (150–287 ng/mL) and was slightly higher among the 349 participants with prevalent CVD (median 246 ng/mL [172–348 ng/mL]). Subsequent analyses were performed only on the subset comprised of the 1393 participants who were free of known CVD at baseline. Baseline characteristics of the study population are shown in Table 1. In this group, the mean age at baseline was 70±11 years and 36% were men. The median NGAL level was 192 ng/mL (146–270 ng/mL) and was similar in both sexes (199 ng/mL in men vs. 189 ng/mL in women, p=0.14). The 95% range of NGAL concentrations was from 71 to 610 ng/mL.
Correlates of NGAL Levels
Correlations between various demographic, clinical and laboratory variables and NGAL levels are shown in Table 2. The strongest individual correlations were with higher CRP, older age, lower CrCl, and higher NT-proBNP levels. Weaker associations were seen with increased systolic blood pressure, lower total and HDL cholesterol, hypertension, diabetes, and relative abstinence from alcohol. Higher heart rate, fasting glucose level, and lower triglyceride levels were also weakly associated with higher NGAL levels. NGAL levels were not significantly associated with BMI, smoking status, frequent exercise, or use of lipid-lowering medications.
Table 2.
Individual and Multivariable Covariates of logNGAL Levels
| Variable | Individual | Multivariable* | ||
|---|---|---|---|---|
| r | p | β | p | |
| Demographics | ||||
| Age | 0.22 | <0.001 | ||
| Male Sex | 0.04 | N/S | ||
| Vital Signs | ||||
| Heart Rate | 0.05 | 0.04 | ||
| SBP | 0.12 | <0.001 | ||
| DBP | 0.02 | N/S | ||
| Cardiovascular Risk Factors | ||||
| Hypertension | 0.08 | <0.01 | ||
| Current Smoking | 0.004 | N/S | ||
| Diabetes | 0.05 | 0.04 | ||
| Nutrition and Activity | ||||
| Waist-Hip Ratio | 0.04 | N/S | ||
| Body Mass Index | 0.01 | N/S | ||
| Exercise 3x/wk | 0.00 | N/S | ||
| Alcohol 3+ drinks/wk | −0.06 | 0.03 | ||
| Medications | ||||
| Aspirin | 0.003 | N/S | ||
| Lipid-Lowering | 0.001 | N/S | ||
| Laboratory Values | ||||
| log NT-proBNP | 0.19 | <0.001 | ||
| log CRP | 0.35 | <0.001 | 0.34 | <0.001 |
| log Fasting Glucose | 0.05 | 0.04 | ||
| log CrCl | −0.22 | <0.001 | −0.24 | <0.001 |
| log Triglycerides | −0.05 | N/S | ||
| log Total Cholesterol | −0.07 | <0.01 | ||
| log HDL | −0.07 | 0.01 | −0.11 | <0.001 |
| log LDL | −0.01 | N/S | ||
Abbreviations as in Table 1; N/S = not significant.
R2 = 0.18, β = standardized regression coefficient.
Three variables were independently associated with higher NGAL levels in multivariable analysis: CRP, lower CrCl, and lower HDL cholesterol levels. The adjusted R2 value of this model was 0.18 with identical results whether forward or backward selection was used.
NGAL Levels and Outcomes
Participants were followed for a mean of 11.0 ± 3.7 years (maximum 16.2 years), during which time there were 436 deaths (31%), including 169 (39%) which were due to cardiovascular causes. There were 101 participants who suffered a fatal or non-fatal myocardial infarction during follow-up, and 75 who had coronary revascularization (25 of whom also had a myocardial infarction). As these events were not mutually exclusive, 254 participants experienced a first CVD event when defined as the composite of MI, revascularization, or CVD death.
Multivariable Cox proportional hazards models were used to evaluate the adjusted risk of death per each 1 standard deviation (SD) increase in log10 NGAL level (Table 3). After adjusting for age and sex (Model 1), the hazard ratio (HR) for CVD death per 1 SD increase in NGAL was 1.34 (95% confidence interval [CI] 1.15–1.58, p<0.001). The risk estimate was only minimally attenuated after further adjusting for traditional CVD risk factors, kidney function, and BMI (Model 2). In unadjusted and adjusted models, log10 NGAL levels were also associated with all-cause mortality (Model 2 HR 1.19 [1.07–1.32], p=0.001) and of the combined cardiovascular outcome of coronary revascularization, myocardial infarction or CVD death (Model 2 HR 1.26 [1.10–1.45], p=0.001).
Table 3.
Multivariable Cox Proportional Hazard Models for Risk of Death or CVD per 1 Standard Deviation Increase in log10NGAL and Other Biomarkers
| Cardiovascular Mortality | All-Cause Mortality | Combined Cardiovascular Endpoint† | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| # of Deaths/Events | 169 | 436 | 254 | |||
| Unadjusted | 1.72 (1.47–2.01)** | <0.001 | 1.48 (1.34–1.64)** | <0.001 | 1.48 (1.30–1.69)** | <0.001 |
| Model 1 | 1.34 (1.15–1.58)** | <0.001 | 1.22 (1.10–1.35)** | <0.001 | 1.29 (1.13–1.47)** | <0.001 |
| Model 2 | 1.33 (1.12–1.57)* | 0.001 | 1.19 (1.07–1.32)* | 0.001 | 1.26 (1.10–1.45)* | 0.001 |
| Model 3 | ||||||
| NGAL | 1.25 (1.05–1.48) | 0.01 | 1.14 (1.03–1.28) | 0.02 | 1.21 (1.05–1.39) | 0.01 |
| NT-proBNP | 1.80 (1.48–2.18)** | <0.001 | 1.42 (1.26–1.61)** | <0.001 | 1.52 (1.29–1.79)** | <0.001 |
| CRP | 1.09 (0.90–1.31) | 0.38 | 1.06 (0.95–1.19) | 0.29 | 1.08 (0.93–1.26) | 0.31 |
Model 1 - Adjusted for age and sex.
Model 2 - Adjusted for Model 1 + diabetes, hypertension, and current smoking (dichotomous variables) and systolic blood pressure, total cholesterol, HDL, creatinine clearance, and body mass index.
Model 3 - Adjusted for Model 2 + log10NT-proBNP and log10CRP.
Bold values are significant at p<0.05.
values are significant at p<0.01.
values are significant at p<0.001.
Combined Cardiovascular Endpoint = coronary revascularization, myocardial infarction, or CVD death.
CI = confidence interval; HR = hazard ratio.
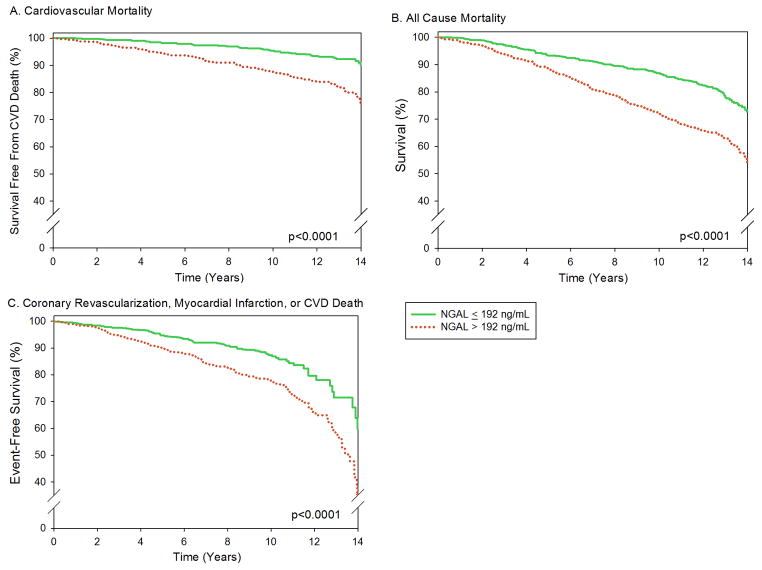
As a sensitivity analysis, we also evaluated NGAL as a binary variable with participants grouped based on an NGAL level above or below the median (192 ng/mL) (Figure 2). Adjusted Cox proportional hazard models for each endpoint were again performed, and results did not materially differ (Supplemental Table 1).
Figure 2. NGAL Level and Risk of Death or CVD.
Kaplan Meier cumulative survival curves based on NGAL levels above or below the median (192 ng/mL). Among participants with NGAL levels below the median there were 161 deaths (55 cardiovascular) and 94 instances of the combined endpoints; among those with NGAL levels above the median there were 275 deaths (114 cardiovascular) and 160 instances of the combined endpoint. A.) Cardiovascular disease (CVD) death. B.) All-cause mortality. C.) Combined cardiovascular endpoint (coronary revascularization, myocardial infarction, or CVD death).
To explore the pathophysiology behind the robust associations of NGAL with long-term CVD and mortality, we next sought to determine whether NGAL was acting as a surrogate (but superior) marker of either kidney function or inflammation. An exploratory analysis was performed with NGAL excluded from Model 3, to determine whether CrCl or CRP would manifest significant associations in the absence of NGAL. Neither CrCl nor CRP was independently predictive for any of the three endpoints.
We also tested for interactions between logNGAL levels and sex or diabetes in prediction of any of the three outcomes; none was significant (p interaction all >0.17).
Combinations of Markers
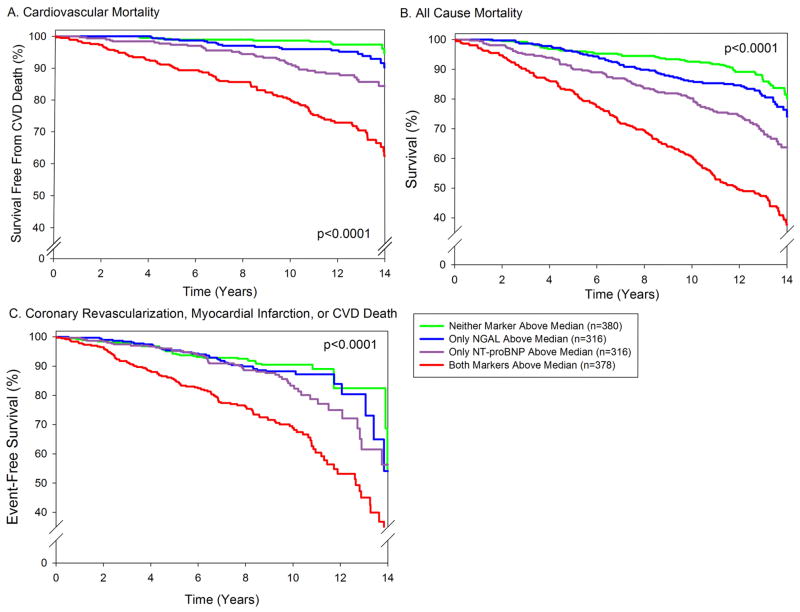
To investigate whether NGAL adds information that is complementary to NT-proBNP and CRP, two markers frequently measured for assessing prognosis in this and other settings, we further adjusted the Cox proportional hazard models for these two biomarkers (Model 3, Table 3 and Supplemental Table 1). After adjusting, both logNGAL and logNT-proBNP, but not logCRP, were independently associated with CVD death, all-cause mortality, and the combined cardiovascular endpoint. To further investigate the interplay between NGAL and NT-proBNP, we stratified participants into 4 groups based upon whether their level of each marker was above or below the median (192 ng/mL for NGAL, and 111 pg/mL for NT-proBNP). Participants with levels above the median for both NGAL and NT-proBNP had a significantly higher incidence of all 3 endpoints, compared with each of the other 3 groups (Figure 3). There was a borderline significant interaction between NGAL and NT-proBNP for prediction of all-cause mortality (p for interaction = 0.11) and the composite cardiac endpoint (p for interaction = 0.10), with NGAL levels showing a stronger association with outcomes among individuals whose NT-proBNP level was also elevated above the median.
Figure 3. Combination of NGAL and NT-proBNP and Risk of Death or CVD.
Kaplan Meier curves based on NGAL and NT-proBNP levels above or below the median (192 ng/mL for NGAL, and 111 pg/mL for NT-proBNP). Four groups are compared: those with both NGAL and NT-proBNP below the median (n=380; 57 deaths, 12 cardiovascular; 38 combined endpoint occurrences), those with only NGAL above the median (n=316; 67 deaths, 20 cardiovascular; 40 combined endpoint occurrences), those with only NT-proBNP above the median (n=316; 104 deaths, 43 cardiovascular; 56 combined endpoint occurrences), and those with both markers above the median (n=378; 207 deaths, 93 cardiovascular; 119 combined endpoint occurrences). Three participants did not have NT-proBNP measured. Log rank p-value <0.001 for each. A.) CVD death. B.) All-cause mortality. C.) Combined cardiovascular endpoint (coronary revascularization, myocardial infarction, or CVD death).
In adjusted analyses, individuals with both NGAL and NT-proBNP levels above the median had an increased risk of CVD death compared to those with only elevated NGAL (adjusted HR 1.59, 95% CI 1.18–2.16, p<0.01), and compared to those with only elevated NT-proBNP (adjusted HR 1.43, 95% CI 1.12–1.82, p<0.01). They also had an increased risk of all-cause mortality (adjusted HR 1.49, 95% CI 1.20–1.84, p<0.001) and of the combined cardiovascular endpoint (adjusted HR 1.65, 95% CI 1.24–2.20) compared to those with only one marker elevated.
Discrimination and Reclassification
The area under the ROC curve (c-statistic) for prediction of CVD death improved from 0.835 to 0.842, with a highly significant increment test in the Cox model (p=0.001), with the addition of NGAL to a model adjusted for traditional CVD risk factors, kidney function, and body mass index (Model 2) (Supplemental Table 2). For comparison, the c-statistics are also shown for the addition of NT-proBNP and for CRP. Adding NGAL to the model that included NT-proBNP and CRP in addition to the risk factors (Model 3) also improved the c-statistic, from 0.851 to 0.855 (p=0.01). Improvements in the c-statistic for prediction of all-cause mortality were more modest but still significant. For all-cause mortality, the addition of NGAL to Model 2 resulted in a small increase in the c-statistic from 0.801 to 0.803 (p=0.001). It increased further from 0.806 to 0.808 (p=0.02) with the addition of NGAL to Model 3 (including NT-proBNP and CRP). The largest improvements in the c-statistic came with the use of NGAL for prediction of the combined cardiovascular endpoint, above and beyond both the risk factor model (Model 2), and the model with the other two biomarkers (Model 3).
Assessment with the Hosmer-Lemeshow test indicated good calibration for the addition of NGAL to the adjusted Model 2, for each of the 3 outcomes (p>0.22 for each). The IDI was also significant for the addition of NGAL to Model 2, for each of the 3 outcomes (IDI for CVD death=0.008, p=0.01; for all-cause mortality=0.005, p=0.002; and for the combined cardiovascular endpoint=0.009, p=0.009).
We assessed reclassification using the category-free NRI (NRI>0). The addition of NGAL to Model 2 resulted in a significant improvement in the NRI(>0) of 18.0% (p=0.02) for the CVD death endpoint. This was the result of both correct upward reclassification of those with events (net gain in reclassification of 11.2%), and from correct downward reclassification of non-events (net gain in reclassification of 6.9%). For all-cause mortality the NRI(>0) was 12.8% (p=0.02) (event NRI=4.4%, non-event NRI=8.4%), and for the combined coronary endpoint the NRI(>0) was 25.3% (p<0.001) (event NRI=14.3%, non-event NRI=11.1%). For comparison, the IDI and NRI(>0) for the addition of NT-proBNP or CRP to Model 2 are shown in Supplemental Table 3.
Discussion
Our study demonstrates that higher levels of plasma NGAL are independently associated with an increased risk of CVD death, all-cause mortality and the combined endpoint of coronary revascularization, non-fatal myocardial infarction, or CVD death, in a cohort of older community-dwelling adults with no antecedent clinical CVD. We also provide novel evidence that NGAL adds significantly to the predictive value of NT-proBNP and CRP in this setting. Finally, to our knowledge, this is the first study of community-dwelling individuals to report the clinical factors associated with NGAL levels, and the first longitudinal community-based study to report the prognostic value of NGAL levels.
Our findings suggest that plasma NGAL adds complementary information to established risk factors and biomarkers, including NT-proBNP and CRP. Although NGAL is known by many as a marker of acute renal injury, our results suggest that plasma NGAL provides important prognostic information and is not merely serving as a surrogate measure of renal function: in fact, correlation between NGAL and CrCl was quite modest (r = −0.22). In addition, CrCl was not predictive of any of the three outcomes, even after NGAL was removed from the models. These findings demonstrate clearly that plasma NGAL is providing prognostic information that is independent of glomerular filtration rate.
NGAL was weakly associated with several traditional CVD risk factors, including age, systolic blood pressure, hypertension, and diabetes. This is consistent with findings in a previous small study of 156 subjects with asymptomatic carotid plaque, in whom plasma NGAL levels were associated with age, blood pressure, and a history of hypertension (24). However, in the present study, none of these factors remained significantly associated with NGAL levels after taking into account other variables including CRP levels, kidney function, and cholesterol levels. Furthermore, the association between NGAL and outcomes was independent of these risk factors. CRP levels showed the strongest association with NGAL levels (r = 0.35); however, NGAL remained associated with mortality and CVD even after adjusting for CRP levels. In fact, CRP was not a significant predictor of any of the three outcomes studied, after adjusting for traditional CVD risk factors, NGAL, and NT-proBNP. To assess the possibility that NGAL and CRP were providing overlapping information, we removed NGAL from the models. Even with NGAL removed from the models, CRP was not a significant predictor of outcomes in adjusted models. Thus, despite the modest correlation between NGAL and CRP, our findings suggest that NGAL is reflecting a pathophysiology distinct from CRP.
We also found a weak but significant correlation between NGAL and NT-proBNP levels (r = 0.19). This is remarkably similar to the correlation reported by Yndestad et al. in the much smaller study of serum NGAL in a subgroup of 236 acute heart failure patients from the OPTIMAAL trial (r = 0.15) (17). In the present study, both NGAL and NT-proBNP levels were each associated with CVD death, all-cause mortality, and the combined cardiovascular outcome independent of one another. The two markers added complementary information, with by far the poorest outcomes seen in individuals with elevated levels of both biomarkers. This finding is in accordance with our current understanding of the distinct biologic pathways leading to expression of natriuretic peptides versus plasma NGAL. While natriuretic peptides are expressed primarily in the setting of myocardial strain (25), NGAL expression, though not fully delineated, is believed to be upregulated in settings of vascular damage and remodeling, and atherosclerosis, among others (26).
Animal models have demonstrated that NGAL is hyper-expressed in the myocardium following myocardial infarction (7,17). Its co-localization with MMP-9 (7) and its ability to enhance the proteolytic activity of MMP-9 (8) suggest that NGAL may be an active mediator of acute coronary syndromes and their negative sequelae, rather than a passive bystander. This potential role in the pathogenesis of CVD is one characteristic that may ultimately distinguish plasma NGAL from NGAL expressed in the urine, as well as from the host of other candidate biomarkers vying for recognition in the burgeoning field of CVD risk stratification. Irrespective of biology, NGAL may be a good candidate for risk assessment because it improves both the c-statistic and the NRI.
Several other features of NGAL also help to distinguish it from other novel CVD biomarkers. Unlike natriuretic peptides and other markers, NGAL levels are not greatly affected by BMI, age, or sex. The predictive ability of NGAL appears to be independent of traditional CVD risk factors, kidney function, and other markers, and NGAL was similarly predictive in both sexes. It improved both reclassification and discrimination, and a single baseline value was predictive of risk even a decade later. These favorable characteristics of NGAL improve the likelihood that it will ultimately prove to be a clinically useful tool for CVD risk stratification.
Morrow and deLemos have proposed three criteria for evaluating novel biomarkers, including whether the marker is easy to measure and accurate, whether the marker adds new information, and whether the marker will improve clinical management (27). Our findings in the present study support, in part, an affirmative answer to the first two criteria. Although whether or not NGAL will improve clinical management must await further studies, the biology underlying the expression of this novel marker provides cause for optimism in this regard. Because of its possible causal and distinct role in acute coronary syndromes, NGAL may prove to be a suitable target for pharmacologic intervention, and with further study, measurement of NGAL levels may help to better match individuals with appropriate therapies.
Study Strengths and Limitations
Strengths of this study include the well-characterized, population-based sample of older adults, and the long-term follow-up. A significant limitation is the relative homogeneity of the cohort. Because the Rancho Bernardo Study population is largely white and middle to upper-middle class, these results may not be generalizable to other populations. However, in one regard, the homogeneity can be viewed as a strength to the extent that it limits confounding by socioeconomic status and access to health care.
Though significant, the absolute improvement in the c-statistic with NGAL was small, and the clinical utility of NGAL, if any, remains to be seen. Since no measures of albuminuria were included in the analysis, one cannot assume the observed relationships were completely independent of kidney disease, although they were independent of renal function as assessed by estimated CrCl.
Another limitation is the long-term storage of blood samples, which could raise questions about the stability of the analyte. Prior to measurement of NGAL levels, blood samples were stored, frozen at −70°C, for 14–18 years. Although the long-term stability of plasma NGAL is difficult to directly assess, the fact that NGAL levels showed strong associations with clinical outcomes argues that there is sufficient stability to preserve a clinical signal. It is unlikely that antigen would decay differentially based on different participant long-term outcomes, thereby creating a clinical signal where there was not one originally. However, the question of stability could still raise concerns of whether the particular median values identified would be the same in fresher samples. If some decay had occurred, this would lower the specific values identified in our study. NGAL was measured at one point in time. Future studies should evaluate whether serial measurement of NGAL might provide more specific information on risk of CVD events above and beyond the level at one point in time at baseline. Finally, the screening NGAL assay utilized for this study was not calibrated to the commercially available Alere Triage® NGAL Test and is known to give different values (28).
Conclusion
NGAL levels are independently associated with increased risk of CVD mortality, all-cause mortality, and cardiovascular disease during long-term follow-up of community-dwelling older adults free from prior CVD. Plasma NGAL provides information that is complementary to traditional CVD risk factors, NT-proBNP, and CRP. The potential impact of these results, if confirmed and expanded upon in future studies, includes providing insight into new mechanisms of CVD and the possibility of improving screening, intervention and prevention.
Supplementary Material
Acknowledgments
Funding Sources:
The Rancho Bernardo Study was funded by research grants AG07181 and AG028507 from the National Institute on Aging, and grant DK31801 from the National Institute of Diabetes and Digestive and Kidney Diseases. This work was also supported by grants from the American Heart Association (LBD & GAL) and the Sandra Daugherty Foundation (GAL). Alere Inc. performed the measurement of NGAL levels for this study, but the authors alone were responsible for data acquisition, data analysis, manuscript preparation, and all other aspects of the study.
Abbreviations
- BMI
body mass index
- CI
confidence interval
- CrCl
creatinine clearance
- CRP
C-reactive protein
- CV
coefficient of variation
- CVD
cardiovascular disease
- HDL
high density lipoprotein
- HR
hazard ratio
- IDI
integrated discrimination improvement
- NGAL
neutrophil gelatinase-associated lipocalin
- NRI
net reclassification improvement
- NT-proBNP
N-terminal pro B-type natriuretic peptide
- ROC
receiver operating characteristic
Footnotes
Relationships with Industry:
ASM has received research grants from Abbott Diagnostics and Roche Diagnostics, and is a consultant for Alere Inc.; LBD has received research grants from Alere Inc. and Roche Diagnostics; there are no other conflicts to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Devarajan P. NGAL in acute kidney injury: from serendipity to utility. Am J Kidney Dis. 2008;52:395–9. doi: 10.1053/j.ajkd.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Friedl A, Stoesz SP, Buckley P, Gould MN. Neutrophil gelatinase-associated lipocalin in normal and neoplastic human tissues. Cell type-specific pattern of expression. Histochem J. 1999;31:433–41. doi: 10.1023/a:1003708808934. [DOI] [PubMed] [Google Scholar]
- 3.Bolignano D, Donato V, Lacquaniti A, et al. Neutrophil gelatinase-associated lipocalin (NGAL) in human neoplasias: a new protein enters the scene. Cancer Lett. 2010;288:10–6. doi: 10.1016/j.canlet.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 4.Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell. 2002;10:1033–43. doi: 10.1016/s1097-2765(02)00708-6. [DOI] [PubMed] [Google Scholar]
- 5.Tong Z, Wu X, Ovcharenko D, Zhu J, Chen CS, Kehrer JP. Neutrophil gelatinase-associated lipocalin as a survival factor. Biochem J. 2005;391:441–8. doi: 10.1042/BJ20051020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roudkenar MH, Halabian R, Ghasemipour Z, et al. Neutrophil gelatinase-associated lipocalin acts as a protective factor against H(2)O(2) toxicity. Arch Med Res. 2008;39:560–6. doi: 10.1016/j.arcmed.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Hemdahl AL, Gabrielsen A, Zhu C, et al. Expression of neutrophil gelatinase-associated lipocalin in atherosclerosis and myocardial infarction. Arterioscler Thromb Vasc Biol. 2006;26:136–42. doi: 10.1161/01.ATV.0000193567.88685.f4. [DOI] [PubMed] [Google Scholar]
- 8.Yan L, Borregaard N, Kjeldsen L, Moses MA. The high molecular weight urinary matrix metalloproteinase (MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil gelatinase-associated lipocalin (NGAL). Modulation of MMP-9 activity by NGAL. J Biol Chem. 2001;276:37258–65. doi: 10.1074/jbc.M106089200. [DOI] [PubMed] [Google Scholar]
- 9.Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res. 2002;90:251–62. [PubMed] [Google Scholar]
- 10.Damman K, van Veldhuisen DJ, Navis G, Voors AA, Hillege HL. Urinary neutrophil gelatinase associated lipocalin (NGAL), a marker of tubular damage, is increased in patients with chronic heart failure. Eur J Heart Fail. 2008;10:997–1000. doi: 10.1016/j.ejheart.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Poniatowski B, Malyszko J, Bachorzewska-Gajewska H, Malyszko JS, Dobrzycki S. Serum neutrophil gelatinase-associated lipocalin as a marker of renal function in patients with chronic heart failure and coronary artery disease. Kidney Blood Press Res. 2009;32:77–80. doi: 10.1159/000208989. [DOI] [PubMed] [Google Scholar]
- 12.Choi KM, Lee JS, Kim EJ, et al. Implication of lipocalin-2 and visfatin levels in patients with coronary heart disease. Eur J Endocrinol. 2008;158:203–7. doi: 10.1530/EJE-07-0633. [DOI] [PubMed] [Google Scholar]
- 13.Zografos T, Haliassos A, Korovesis S, Giazitzoglou E, Voridis E, Katritsis D. Association of neutrophil gelatinase-associated lipocalin with the severity of coronary artery disease. Am J Cardiol. 2009;104:917–20. doi: 10.1016/j.amjcard.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 14.Elneihoum AM, Falke P, Axelsson L, Lundberg E, Lindgarde F, Ohlsson K. Leukocyte activation detected by increased plasma levels of inflammatory mediators in patients with ischemic cerebrovascular diseases. Stroke. 1996;27:1734–8. doi: 10.1161/01.str.27.10.1734. [DOI] [PubMed] [Google Scholar]
- 15.Anwaar I, Gottsater A, Ohlsson K, Mattiasson I, Lindgarde F. Increasing levels of leukocyte-derived inflammatory mediators in plasma and cAMP in platelets during follow-up after acute cerebral ischemia. Cerebrovasc Dis. 1998;8:310–7. doi: 10.1159/000015873. [DOI] [PubMed] [Google Scholar]
- 16.Bolignano D, Basile G, Parisi P, Coppolino G, Nicocia G, Buemi M. Increased plasma neutrophil gelatinase-associated lipocalin levels predict mortality in elderly patients with chronic heart failure. Rejuvenation Res. 2009;12:7–14. doi: 10.1089/rej.2008.0803. [DOI] [PubMed] [Google Scholar]
- 17.Yndestad A, Landro L, Ueland T, et al. Increased systemic and myocardial expression of neutrophil gelatinase-associated lipocalin in clinical and experimental heart failure. Eur Heart J. 2009;30:1229–36. doi: 10.1093/eurheartj/ehp088. [DOI] [PubMed] [Google Scholar]
- 18.Maisel AS, Mueller C, Fitzgerald R, et al. Prognostic utility of plasma neutrophil gelatinase-associated lipocalin in patients with acute heart failure: The NGAL EvaLuation Along with B-type NaTriuretic Peptide in acutely decompensated heart failure (GALLANT) trial. Eur J Heart Fail. 2011;13:846–51. doi: 10.1093/eurjhf/hfr087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Falke P, Elneihoum AM, Ohlsson K. Leukocyte activation: relation to cardiovascular mortality after cerebrovascular ischemia. Cerebrovasc Dis. 2000;10:97–101. doi: 10.1159/000016037. [DOI] [PubMed] [Google Scholar]
- 20.Kremers WK. Technical Report Series #80. Rochester, MN: Mayo Clinic; 2007. Concordance for survival time data: fixed and time-dependent covariates and possible ties in predictor and time; pp. 1–17. [Google Scholar]
- 21.May S, Hosmer DW. A simplified method of calculating an overall goodness-of-fit test for the Cox proportional hazards model. Lifetime Data Anal. 1998;4:109–20. doi: 10.1023/a:1009612305785. [DOI] [PubMed] [Google Scholar]
- 22.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–72. doi: 10.1002/sim.2929. discussion 207–12. [DOI] [PubMed] [Google Scholar]
- 23.Pencina MJ, D’Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elneihoum AM, Falke P, Hedblad B, Lindgarde F, Ohlsson K. Leukocyte activation in atherosclerosis: correlation with risk factors. Atherosclerosis. 1997;131:79–84. doi: 10.1016/s0021-9150(96)06077-7. [DOI] [PubMed] [Google Scholar]
- 25.Daniels LB, Maisel AS. Natriuretic peptides. J Am Coll Cardiol. 2007;50:2357–68. doi: 10.1016/j.jacc.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 26.Bolignano D, Coppolino G, Lacquaniti A, Buemi M. From kidney to cardiovascular diseases: NGAL as a biomarker beyond the confines of nephrology. Eur J Clin Invest. 2010;40:273–6. doi: 10.1111/j.1365-2362.2010.02258.x. [DOI] [PubMed] [Google Scholar]
- 27.Morrow DA, de Lemos JA. Benchmarks for the assessment of novel cardiovascular biomarkers. Circulation. 2007;115:949–52. doi: 10.1161/CIRCULATIONAHA.106.683110. [DOI] [PubMed] [Google Scholar]
- 28.Inverness Medical. Triage NGAL package insert. San Diego, CA: 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.