Abstract
The development of carbohydrate based anti-cancer vaccines is of high current interests. Herein, the latest development in this exciting field is reviewed. After a general introduction about tumor associated carbohydrate antigens and immune responses, the review is focused on the various strategies that have been developed to enhance the immunogenecity of these antigens. The results from animal studies and clinical trials are presented.
1. Introduction
Cancer is one of the most harmful diseases. According to a report from the American Cancer Society,[1] cancer is the second leading cause of death in economically developed countries, and the third leading cause of death in developing countries. In 2008 alone, there were nearly 12.7 million new cancer cases and close to 7.6 million people lost their battle to cancer worldwide. It is predicted that by 2050, there can be as many as 27 million new cases and 17.5 million deaths caused by this disease.[2]
Traditional methods for anti-cancer therapy include chemotherapy, ionizing radiation and surgery. While tremendous progress has been made in these areas during the past decades, cancer still remains extremely difficult to cure and prevent. Therefore, it is imperative that novel methods are continuously developed to combat cancer. In view of the great success in vaccines against many infectious diseases, immunotherapies and anti-cancer vaccines are attractive alternative approaches for cancer prevention and treatment.
Anti-cancer vaccine is based on the idea of harnessing the awesome power of the body’s immune system to fight cancer. As early as 1893, Coley observed that an immune response to a fulminant bacterial infection could eliminate established sarcomas.[3] However, little was known at that time about the immune system. In 1950s, Burnet and Thomas proposed the concept ‘immunosurveillance’, where immunological cells can sense and eliminate continuously arising transformed cells.[4] After much debate, the immunosurveillance theory is now widely accepted.
Several immunotherapeutic methods against cancer have been used successfully in the clinic. The first therapeutic monoclonal antibody (mAb), rituximab was approved by the US FDA for the treatment of B-cell non-Hodgkin’s lymphoma by targeting CD20 present on B cell surfaces.[5] By 2010, ten mAbs have been approved by the US FDA for cancer treatment.[6] Besides mAbs, a dendritic cell based therapeutic vaccine, Provenge, was approved in 2010 to fight advanced prostate cancer.[6] These developments highlight the potential for immunotherapy against cancer. However, many of these treatments can only prolong patients’ lives by several months. Therefore, continual research is needed to develop highly effective anti-cancer vaccines and immunotherapies.
2. Tumor Associated Carbohydrate Antigens
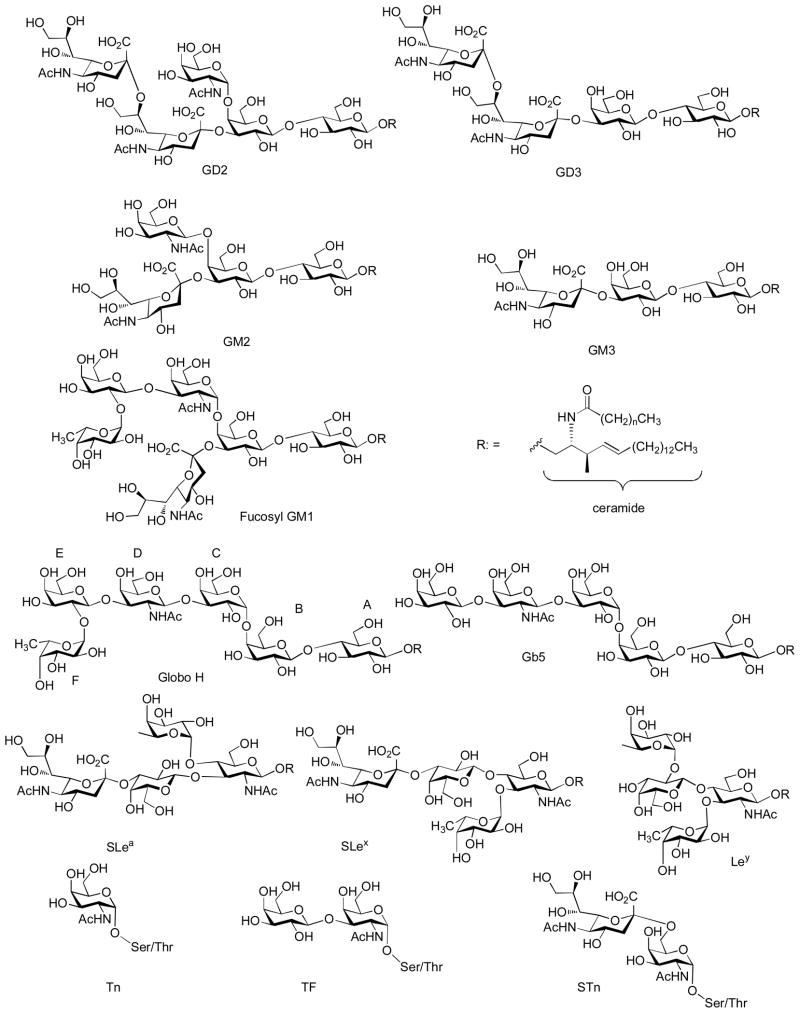
On cancer cell surface, there are a variety of antigenic carbohydrate structures, with some representative examples shown in Figure 1.[7–9] These tumor associated carbohydrate antigens (TACAs) can be divided into two classes: (1) glycolipid antigens, where carbohydrates are linked to ceramide lipids and anchored to the lipid bilayer on the cell surface through hydrophobic hydrophobic interactions. The glycolipids can be further divided to several families. These include the gangliosides such as GD2, GD3, GM2, GM3, and fucosyl GM1; globo-series Globo-H, Gb3, Gb4, and Gb5; lacto-series (type 1) SLea, Leb, and Lea-Leb and neolacto-series (type 2) SLex, Ley, and Ley-Lex; (2) glycoprotein antigens including Tn, TF and STn linked to the hydroxyl group of serine or threonine in glycoproteins.
Figure 1.
Representative structures of TACAs.
TACAs are expressed at much higher levels on malignant cells and the high density of these antigens distinguish tumor from their normal cell counterparts.[7–9] For example, the mAb M2590, which specifically reacts with melanoma (not with normal melanocytes), recognizes GM3 gangliosides on the cell surface only when it is above a threshold density level.[10] TACAs are among the most frequently found antigens on cancer cell surface.[11] They are present in tumors more frequently than oncogene products such as rask and HER2/neu, and their correlation with cancer progression is much stronger than inactivation of tumor suppressing genes such as p53.[12] Furthermore, TACAs are shared by many cancer cell types; an example is gangliosides GM2 and GD2 expressed in large quantities on melanoma, sarcomas or neuroblastomas cells (Table 1).[13,14] Ley and Globo H antigens are expressed on a variety of epithelial cancers including breast, prostate and ovarian cancers.[7,15,16] Thus vaccines against TACAs open up the possibility of targeting multiple types of cancer using a single construct.
Table 1.
Major TACAs Identified in Cancer Tissues
| Cancer Type | Tumor Antigens |
|---|---|
| Melanoma | GM2, GM3, GD2 |
| Neuroblastoma | GM2, GD2, polysialic acid |
| Sarcoma | GM2, GD2, GD3 |
| B cell lymphoma | GM2, GD2 |
| Small-cell lung | GM2, fucosyl-GM1, polysialic acid, Globo H |
| Breast | GM2, Globo H, TF(c), Tn(c), Ley |
| Prostate | GM2, Globo H, Tn(c), TF(c), STn(c), Ley |
| Lung | GM2, Globo H, Ley |
| Colon | GM2, Tn, STn(c), TF(c), Ley |
| Ovary | GM2, Globo H, STn(c), TF(c), Ley |
| Stomach | GM2, Ley, Lea, SLea |
Cancer stem cells are a subpopulation of cancer cells with stem-cell like characteristics of self-renewal, which are capable of regenerating and differentiating into multiple cell types in a particular cancer. For an effective anti-cancer therapy, cancer stem cells should be targeted as well to prevent the re-emergence of cancer.[17] Recently, there are several reports suggesting that TACAs are significantly expressed in cancer stem cells.[18,19] For example, the Wong group disclosed that Globo H was expressed in 8/40 (20%) breast cancer stem cells and Gb5, the pentasaccharide precursor or Globo H, was expressed in 25/40 (62.5%) of breast cancer stem cells.[19] Therefore, these attributes render TACAs attractive targets for anti-cancer immunotherapy development.
3. Challenges associated with the development of anti-cancer vaccines targeting TACAs
The immune system adapts to the challenge of cancer through two possible mechanisms, the humoral immune response and the cellular response. Cell-mediated immunity involves the activation of a variety of immune cells, including macrophages, DCs, antigen-specific CD4+ helper T (Th) cells, and CD8+ cytotoxic T (Tc) cells. An antigen is typically ingested by antigen presenting cells (APCs) such as DCs, which in turn process the antigen and present a small portion, known as T cell epitopes to activate Tc and Th cells. Th cells release various cytokines to help potentiate the immune system, while upon stimulation by the epitopes and the cytokines the Tc cells recognize and kill tumor cells expressing the antigens.
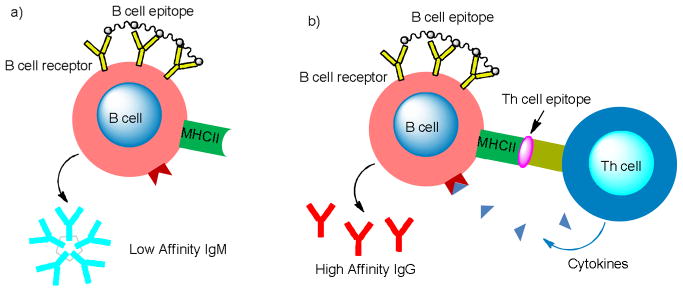
The humoral response, in contrast, is mediated by secreted antibodies, which are produced by B cells upon cross-linking the B cell receptors (BCRs) by B cell epitopes (Figure 2a). The first type of antibody generated responding to an immunological challenge is the pentameric IgM, which have relatively low affinities to antigens and last only a short period of time for a few days. In order to have long lasting humoral responses, the activated B cells must be able to bind with Th cells, receiving the stimulatory cytokine signals from Th cells (Figure 2b).[20,21] This leads to the switching of antibody subtypes to high affinity IgGs and the generation of memory cells, which can rapidly produce large amounts of high affinity IgG antibodies upon subsequent encounter with the same antigen. The antibodies can bind with the target cancer cells, marking them for destruction by either the complement system through the complement-dependent cytotoxicity (CDC) or lymphocytes (antibody dependent cell-mediated cytotoxicity ADCC).
Figure 2.
Activation of the B cells through crosslinking of the B cell receptors secrets a) IgM in the absence of Th cell binding and b) IgG in the presence of Th cell binding.
There are strong clinical evidences that anti-TACA immune responses can be beneficial to cancer treatment. In melanoma, the small population of patients with high titers of natural antibodies against GM2 was found to have up to 90% 5 year survival, compared to the expected 40% rate.[22,23] Administration of mAbs specific for TACAs such as the anti-GD3 mAb R24 led to tumor regression in human.[24,25] Therefore, if a strong immune response can be elicited towards TACAs, it can potentially exert protective effects to the host against cancer.
Despite these encouraging observations, there are significant challenges to develop active anti-tumor immunotherapy based on TACAs. Among the obstacles is the difficulty of isolating sufficient quantities of TACAs from natural sources, as cell surface carbohydrates are very heterogeneous (Table 1).[7,26] The possibility of highly active trace contaminants suggests that caution needs to be taken with naturally isolated samples.[27] Therefore, synthesis is currently the preferred method to acquire adequate amounts of TACAs in pure form.[28] In addition, synthetic chemistry can bestow great flexibility in functionalizing TACAs and enable large scale preparation. Because TACA structures are often highly complex and carbohydrate synthesis requires much experience in design and execution,[7,28] the successful development of TACA based vaccines will necessitate an interdisciplinary approach bridging immunology with carbohydrate synthetic chemistry. We refer interested readers to several reviews on state-of-the-art carbohydrate synthesis strategies.[28–30]
The main immunological challenge lies in the low immunogenicity of TACAs.[31] Although they are over-expressed on cancer cell surface, TACAs are also present in small amounts on normal cells, thus perceived as self-antigens by the immune system. When administered alone, TACAs only weakly activate the B cells. Without additional help from Th cells, low titers of the low affinity IgM antibodies are generated.[32–34] Therefore, TACAs need to be covalently linked with an immunogenic carrier,[35–37] which contain epitopes recognizable by the Th cells. Such synthetic constructs can be uptaken and processed by APCs, which will lead to the presentation of the Th cell epitopes to the Th cells, resulting in their activation and differentiation. Subsequently, the activated Th cells will specifically recognize matched B cells displaying the same Th cell epitopes, releasing powerful helper cytokines to stimulate the B cells (Figure 2b).[20,21] This will initiate the isotype switching and production of high titers of IgG by B cells as well as the crucial immuno-memory against TACAs.[38–40]
Carbohydrates as B Cell Epitopes
Carbohydrates are mainly epitopes for B cells. In order to generate IgG by B cells, two signals are required: an antigen-specific first signal delivered via cross-linking of surface BCRs and a second co-stimulatory cytokine signal delivered by Th cells (Figure 2b).[20,21] The effect of BCR cross-linking was first demonstrated by using size fractionated linear acrylamide polymers substituted with haptens, which demonstrated that greater than 20 haptens spaced by 5 to 10 nm was enough to trigger T-cell-independent B-cell activation.[41] The patterned displays of antigens were subsequently illustrated to lead to earlier B cell amplification for potent IgM responses as well as isotype switching to IgG.[42–45] Antigen organization can also have a great influence on B cell tolerance with B cells unresponsive to poorly organized antigens while responding promptly to the same antigen presented in a highly organized manner.[46] For TACA based anti-cancer vaccines, construct structure is an important factor that must be considered to generate high antibody titers.
Glycoconjugates as T Cell Epitopes
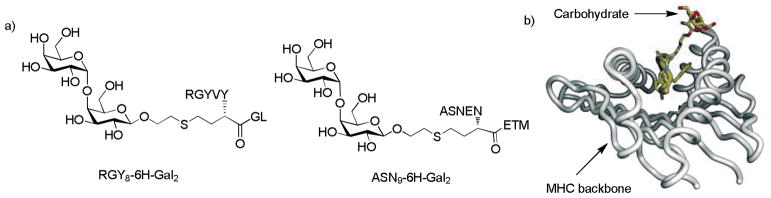
With the exception of zwitterionic polysaccharides (see section 5.3), most carbohydrate antigens are T cell independent because they do not bind with the major histocompatibility complexes (MHC) directly and cannot activate T cells by themselves. It is known that peptides are the principal epitopes of T cells through the binding with MHCs[47] (There are two types of MHCs. Class I MHCs are expressed on all nucleated cells and they present epitopes to Tc cells. Class II MHCs are expressed on APCs, which present epitopes to Th cells). As many peptides and proteins are glycosylated, the glycopeptides and glycoproteins can potentially serve as T cell epitopes as well.[48–50] In 1994, Haurum et al demonstrated that immunization with MHC class I restricted peptides conjugated with GlcNAc generated Tc cells recognizing the glycopeptides.[48] Jondal and coworkers discovered that immunization with MHC I binding glycopeptide RGY8-6H-Gal2 (Figure 3a) produced Tc cells that specifically killed cells presenting either the glycopeptide or the glycolipid with same carbohydrate moiety.[49] Furthermore, the Tc cells generated by RGY8-6H-Gal2 could also kill target cells pulsed with glycopeptide ASN9-6H-Gal2 (Figure 3a), which shared the same carbohydrate structure but contained different peptide sequences. This suggested recognition of the carbohydrate portion of the glycopeptide epitope independent of peptide-MHC complexes. Subsequent crystal structures of MHC and RGY8-6H-Gal2 complex showed that the carbohydrate moiety protruded out of the MHC groove and dominated the putative TCR/MHC I interactions (Figure 3b).[50]
Figure 3.
a) Structure of RGY8-6H-Gal2 and ASN9-6H-Gal2. The peptide sequence RGYVYQGL is a H-2Kb Tc epitope from vesicular stomatitis virus and ASNENMETM is a H-2Db Tc epitope; b) Co-crystal structure of RGY8-6H-Gal2 and H-2Kb. The carbohydrate moiety of the glycopeptide points out of MHC groove and dominates the TCR binding site[50] (Reprint with permission from Elsevier)
In addition to binding with MHC class I, many glycopeptides have been shown to bind with MHC class II. For example, two glycopeptides from CD53, CD53122–136 and CD53121–136 glycosylated with a hexasaccharide at Asn129, were shown to bind with class II MHC.[51] Furthermore, MHC class II restricted human T cell response to the bee venom allergen phospholipase A2 in allergic patients was found to be dependent on the presence of N-glycans.[52] Based on these observations, it is possible to induce activations of both B cells and T cells against carbohydrates and glyco-conjugates for cancer vaccine development.
With the general knowledge of TACAs and immune responses, in the following sections we will discuss two main strategies to boost the immunogenicity against TACAs: 1) modifications of TACAs; 2) immunogenic platforms for TACA delivery. We will focus on the immunological aspects of the TACA constructs and the clinical results will be presented when available.
4. Modification of TACAs towards Anti-cancer Vaccine Development
4.1. Vaccination with Rare or Unnatural Epitopes
An approach to break immune tolerance to TACAs is to use rare TACA derivatives or unnatural TACAs mimics. These modified TACAs may be much more immunogenic and generate robust antibody response cross-reactive to the native TACA antigens.
Ganglioside Lactones
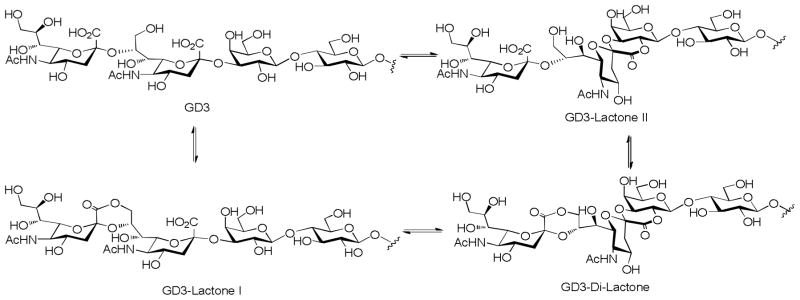
Since gangliosides are hydroxy acids, they are prone to form lactones at low pH. For gangliosides with more than one sialic acid such as GD3, various lactones can be formed[53] (Figure 4), which may affect ganglioside structures in vivo. As early as 1987, Nores et al showed that mice immunized with a GM3 lactone resulted in the formation of strikingly high number of hybridomas secreting antibodies, which recognized both GM3 and GM3 lactone.[10] Under the same condition, immunization with GM3 did not result in hydridoma formation. Inspired by this finding, Livingston and coworkers prepared GD3 lactones, GD3 amide and GD3 gangliosidol to overcome the poor immunogenicity of GD3. All the three GD3 derivatives were immunogenic in mice, and GD3 lactone induced production of antibodies that cross-reacted with both GD3 and GD3 expressing human melanoma cells.[54] During clinical trials, these GD3 derivatives absorbed on the Bacille Calmette-Guérin (BCG) carrier elicited IgM only in melanoma patients and the antibodies did not recognize GD3.[55] In the subsequent studies, the authors showed that GD3 lactone-keyhole limpet hemocyanine (KLH) conjugates with the saponin QS-21 as the adjuvant elicited both IgM and IgG antibodies, which strongly cross-reacted with GD3.[56] In contrast, the GD3-KLH failed to generate any antibodies, suggesting that the GD3 tolerance could be broken by immunizations with GD3 lactone but not with GD3 and KLH is a more suitable carrier than BCG. Similar phenomena were reported with GD2 lactone-KLH constructs in clinical trials. Two mechanisms were proposed for the higher immunogenecity associated with GD2 or GD3 lactones: 1) while the expression of GD2 or GD3 lactones is rare in normal tissues, the lower pH values in the tumor microenvironment may favor the lactone formation from these gangliosides, leading to higher concentrations of the lactones in tumor;[57] 2) the lactones have rigid and distorted conformations, thus may be better recognized by BCR.[10,58]
Figure 4.
GD3 can form various lactones under acidic conditions.
To overcome the hydrolytic instability of the lactones, Magnusson and coworkers synthesized a GM3 lactam (Figure 5) with increased stability of the ring.[59] Immunization of mice with GM3 lactam-bovine serum albumin (BSA) conjugate followed by establishment of hybridomas generated a large number of GM3 lactam specific clones. Interestingly, most of these clones recognized GM3 lactone but not GM3. Recently, Arcangeli and colleagues reported the design and synthesis of disaccharides to mimic GM3 lactone.[60] After KLH conjugation, the resulting glycoprotein was used for immunization in mice with significant IgM titers obtained against melanoma cells.
Figure 5.
GM3 lactam analog synthesized by Magnusson and coworkers
O-Acetyl GD3
Besides the GD3 lactone, O-acetylated GD3, including the 9-O-acetyl GD3 (CD60b) and 7-O-acetyl GD3 (CD60c), have been observed in several cancers including melanoma and breast cancer.[61] It is supposed that the O-acetylation may reduce or totally abolish apoptosis irrespectively of apoptosis-inducing agents.[61] Cheresh et al demonstrated that the mAb D1.1 recognized 9-O-acetyl GD3 on human melanoma cells.[62] Clinical studies by Livingston and coworkers showed that 9-O-acetyl GD3 was weakly immunogenic in humans and GD3 bearing two or more O-acetyl groups induced good immune responses, but the antibodies did not recognize human melanoma cells.[63] To use the O-acetylated GD3 as effective anti-cancer vaccines, further studies are required.
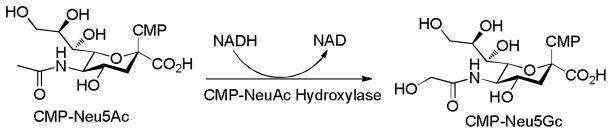
Neu5Gc GM3
The two most common forms of the non-reducing terminal carbohydrates of gangliosides are the N-acetylneuraminic acid (Neu5Ac) and its derivative N-glycolylneuraminic acid (Neu5Gc). Neu5Gc is biosynthesized from Neu5Ac catalyzed by the cytidine monophopho-N-acetylneuraminic acid hydroxylase (CMAH) (Figure 6).[64] In contrast to most mammals such as horse, sheep, and goat, human tissues normally lack Neu5Gc due to deletion of the CMAH gene.[65] Interestingly, Neu5GcGM3 is found highly expressed in several types of human cancer cells such as breast and melanoma.[66] The significance of Neu5Gc for tumor cell biology is still under investigation with a recent report indicating that Neu5GcGM3 down-modulated CD4 expression in murine and human T lymphocytes especially in non-activated T cells.[67] It is noteworthy that patients with anti-Neu5Gc antibody were found to have increased 5 year survival rates.[68]
Figure 6.
Hydroxylation of CMP-Neu5Ac produces CMP-Neu5Gc.
Since Neu5Ac GM3 is poorly immunogenic, the tumor-specific character of Neu5Gc GM3 presents a novel target for anti-cancer vaccine development. Currently, two constructs targeting Neu5Gc GM3 are undergoing clinical trials:[69] anti-idiotype murine mAb racotumomab (formerly known as 1E10) against Neu5Gc-containing gangliosides for non-small or small cell lung cancer and Neu5GcGM3-VSSP (very small size proteasome, made of out membrane proteins from Neisseria meningitides) for breast cancer or melanoma. Recent Phase Ib/IIa study of Neu5Gc GM3-VSSP for advanced melanoma patients demonstrated the vaccine construct was well tolerated.[70] All patients generated Neu5Gc GM3 specific IgM, IgG and IgA antibodies, and the IgM antibodies showed significant cross-reactivity with Neu5Ac GM3 and Neu5Ac GM2. Importantly, 9 of 22 patients survived more than 1 year with 7 patients living more than 2 years. This encouraging observation is uncommon for advanced melanoma patients.
4.2. Synthetic Modifications of TACAs
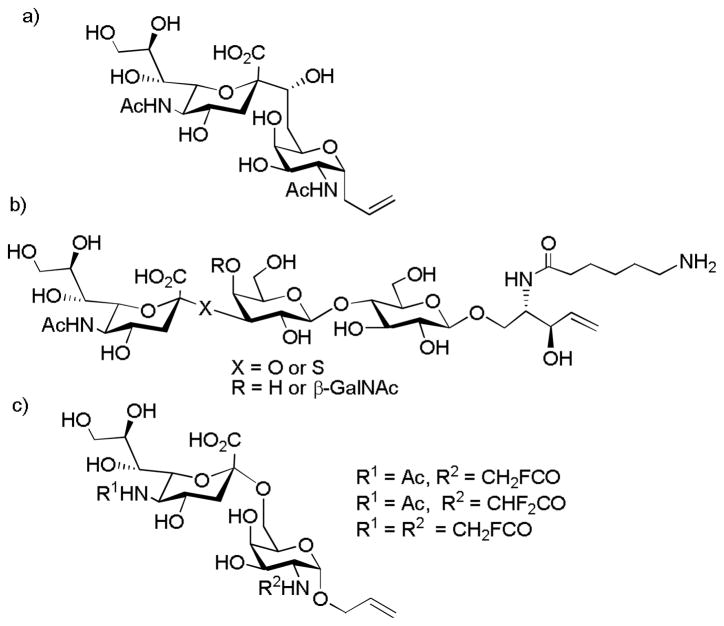
As the glycosyl linkages in TACAs are potentially susceptible to acid catalyzed hydrolysis and enzymatic degradation in vivo, to improve the stability of the antigens, C-linked STn (Figure 7a) and S-linked GM2 or GM3 (Figure 7b) have been explored as surrogates of the corresponding native O-linked TACAs by the Linhardt and Bundle groups.[71–73] Antigen specific immune responses were generated against these modified antigens with moderate cross-reactivity towards the native O-glycosides.
Figure 7.
a) C-linked STn synthesized by the Linhardt group; b) S-linked GM2 and GM3 prepared by Bundle and coworkers; c) Structures of fluorinated STn analogs that elicited high immune responses synthesized by the Ye group.
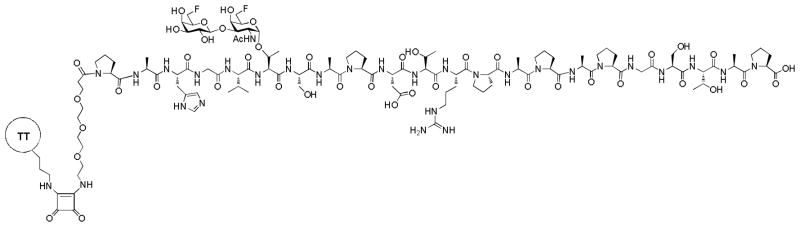
Recently, Ye and coworkers prepared 40 STn analogs and evaluated the immune responses in mice after conjugation with KLH.[74] Several fluorine containing STn compounds were found to give significant increases in antibody production and the antibodies strongly cross-reacted with native STn or STn expressing tumor cells (Figure 7c). Similarly, the Kunz group synthesized the MUC1 peptide linked with TF antigen analogs substituted at 6 and 6′ positions with fluorine atoms (Figure 8).[75] When conjugated to the tetanus toxoid (TT) as the carrier, the vaccine construct elicited strong IgG responses that recognized the native antigens present on breast cancer cell MCF-7.
Figure 8.
Structure of the MUC1 glycopeptide bearing fluorinated TF antigen synthesized by the Kunz group.
4.3. Glycoengineering-Based Cancer Vaccine
Cells constantly uptake nutrients from their environments, which enables cellular structure engineering through exogenously added compounds. The pioneering research by Reutter and coworkers disclosed that mammalian cells could uptake various N-acylmannosamine analogs from the cultural medium as biosynthesis precursors and convert them to unnatural N-acyl neuraminic acids, which were further incorporated into glycosphingolipids on cell surface.[76] Through this process, the cell surface glycoproteins and glycolipids could be engineered to bear unnatural carbohydrates, which could potentially break the tolerance and at the same time avoid generating an autoimmune response to the native TACAs.
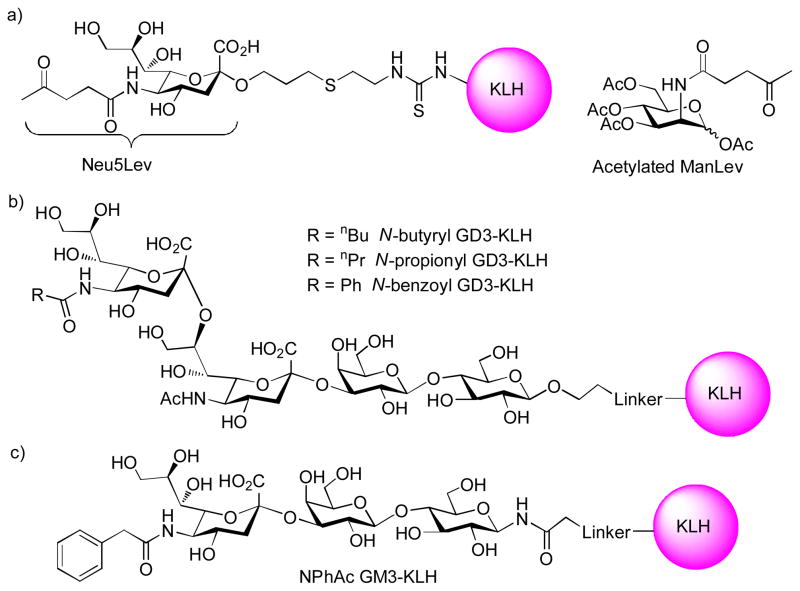
Bertozzi and coworkers demonstrated that immunization of rabbits with unnatural sialic acid bearing N-levulinoyl group (Neu5Lev) conjugated to KLH (Figure 9a) produced significant titers of antibodies specific for the modified sialic acid.[77] These antibodies recognized tumor cells incubated with the biosynthesis precursor peracetylated N-levulinoyl mannosamine (ManLev), and did not cross-react with the cells expressing the natural sialosides. Furthermore, the antibodies were capable of directing complement-mediated lysis of tumor cells expressing the unnatural sialic acids. About the same time, Jennings and coworkers reported that incubation of leukemic cells with N-propionyl mannosamine led to the substitution of N-acetyl groups of cell surface α2–8 polysalic acids with N-propionyl groups.[78] This induced the susceptibility of glyco-engineered tumor cells to cell death mediated by the mAb 13D9, which specifically recognized the α2–8 N-propionylated polysialic acid. In a mouse model, tumor metastasis was significantly reduced by feeding the mice with N-propionyl mannosamine followed by treatment with the mAb 13D9. Furthermore, the Jennings group showed that mice immunized with N-butyryl GD3-KLH conjugates elicited much higher IgG titers than N-propionyl GD3-KLH and N-benzoyl GD3-KLH (Figure 9b).[79] The antibodies did not cross-react with native GD3. Subsequent tumor challenge experiments demonstrated although this method was not effective against pre-established large solid tumor (10 mm diameter), administering monoclonal antibody 2A specific for N-butyryl GD3 in combination with the biosynthesis precursor N-butyryl mannosamine effectively protected mice from SK-MEL-28 tumor grafting before solid tumor was developed.
Figure 9.
Structures of various KLH conjugates containing unnatural sialic acids.
Instead of relying on passive immunization with exogenous mAbs, Guo and coworkers explored the possibility of generating antibodies in vivo against the glyco-engineered cancer cells using vaccine constructs containing the unnatural TACA analog. They systematically evaluated the effects of N-substituents in sialic acids of various GM3 and STn analogs.[80] The N-phenylacetyl (NPhAc) substituted GM3 (Figure 9c) and STn conjugates with KLH elicited the highest antibody titers without cross-reactivity against native GM3 or STn. The NPhAc GM3-KLH antiserum and NPhAc GM3 specific mAb exhibited strong and specific complement dependent cytotoxicity to several melanoma cell lines incubated with low μM concentration of the precursor N-phenylacetyl mannosamine.[81,82] Recently, GM3 and STn analogs bearing p-substituted phenyl acetamide moieties were found to give even higher antibody titers than the parent NPhAc containing compounds,[83,84] which are promising candidates for further study. The tumor protective efficacy of this innovative strategy needs to be established further in vivo in animal models.
5. Immunogenic Platforms for TACA delivery
5.1. TACAs Delivered by Immunogenic Protein Carriers
Since Landsteiner’s classic experiments with hapten-carrier conjugates,[85] conjugation to immunogenic proteins has been successfully applied to enhance antibody productions against a variety of antigens. The protein carriers can provide the crucial Th epitopes required to induce the isotype switching of the antibody responses to the hapten. A variety of protein carriers, which include KLH, TT, BCG, and BSA, have been utilized in the development of TACA based anti-cancer vaccines. To date, KLH has been the most commonly employed protein carrier due to its superior immunogenic properties when conjugated with TACAs. In this section, we will describe TACA conjugates with immunogenic protein carriers and the progress in evaluations of these vaccine constructs.
Globo H
Globo H is a globo-series glycolipid (Figure 1), which was first isolated by Hakomori and coworkers from breast tumor extracts.[86] Globo H is a tumor-associated antigen that is heavily expressed on a number of tumors, including breast, lung (small cell and non-small cell), colon, ovarian, pancreas, and prostate.[87] Although it is also expressed on the luminal surface of normal epithelial cells, the location precludes its access by the immune system. The restricted distribution and differential expression of Globo H renders it a promising target for anti-cancer vaccine development.
By testing the reactivity of multiple Globo H analogs with a Globo H specific mAb MBr1, the structure-activity relationship for Globo H is established as follows:[88,89] 1) the fucosyl unit (ring F, see Figure 1 for ring designation) is crucial and the binding is abolished without it; 2) the β-linkage between the C and D rings is critical as the α-linked analog was very weakly bound; 3) A and B rings do not contribute much to the binding; 4) the α-linkage between the B and C rings is not critical as the β anomer gives similar binding. Mice immunized with Globo H-KLH generated antibodies against Globo H-ceramide with higher IgM titers than IgG. The antibodies showed moderate binding with breast cancer cell MCF-7 (30%–45%). The antiserum also gave 48% lysis of MCF-7 in the presence of human complements while the maximum of CDC with mAb MBr1 was 72%. In subsequent clinical trials using the Globo H-KLH construct,[90] all five patients with progressive and recurrent prostate cancer produced strong IgM responses with two of the five patients showing significant IgG titers. All the antisera recognized synthetic and tumor-derived Globo H – ceramide as well as MCF-7 cells. Another clinical study on prostate cancer patients proved that the vaccine is safe with no significant toxicity,[91] and the antibodies responses were similar to the previous study.[90] The therapeutic effects in these patients were demonstrated by the decline of PSA concentration in one-third of the patients. Beneficial results were found in clinical trials with breast cancer patients as well.[92] Large scale clinical trials are necessary to fully establish the potential of Globo H based vaccine.
GM2
GM2 is widely expressed in most types of cancer including breast, ovary, prostate, small cell lung cancer, and melanoma.[87] It is recognized as the most immunogenic ganglioside expressed on melanoma cells.[34] A correlation between the presence of anti-GM2 natural serum antibodies and survival in stage III melanoma patients was observed.[22] Early clinical studies with BCG as the protein carrier for GM2 only induced IgM antibodies in the majority of patients. Subsequent clinical evaluation[93] of GM2 conjugated to KLH not only induced high-titer, longer-lasting IgM antibodies against GM2, but also elicited significant GM2 specific IgG antibodies in most of melanoma patients suggesting the activation of Th cells and the induction of isotype switching. Furthermore, both the IgM and IgG antibodies from all seven patients showed moderate to high reactivity against GM2 expressing human sarcoma cell line A2394. The antisera from all patients showed significant CDC (average 66%) and ADCC (average 61%) against A2394 cells. Subtype analysis indicated the IgG antibodies were primarily IgG1 and IgG3, which were strong inducers of CDC. Unfortunately, a randomized phase III study demonstrated that patients immunized with GM2-KLH showed an inferior relapse-free and overall survival compared with patients who received high-dose interferon α-2b (HDI) therapy.[94] The HDI therapy is currently the standard of care for adjuvant therapy of high-risk melanoma patients and the reference standard for evaluation of alternative therapies such as vaccines in the United States. Therefore, the study was terminated early. Recently, another phase III study of stage II melanoma patients demonstrated that GM2-KLH vaccination with saponin QS-21 as the adjuvant was ineffective and could even be detrimental to patients.[95]
Ley and KH-1
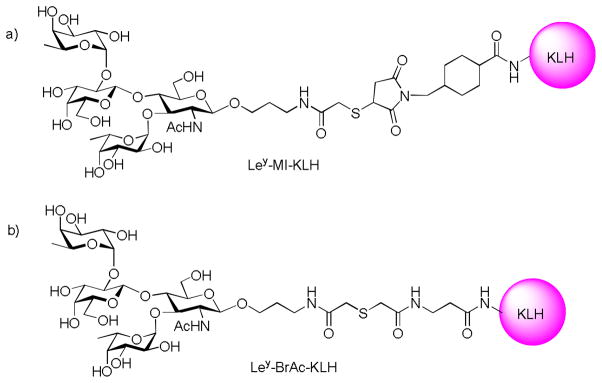
The Ley antigen, also known as CD-174, is related in structure to the ABH family of blood group antigens found on human blood cells. It can be carried by glycolipids, glycoproteins or mucins, and is highly expressed on a number of cancers of epithelial origin such as colon,[96] lung,[97] breast,[98] prostate[99], and ovary.[100] It was reported that Ley expression in ovary tumor is relatively uniform compared to other glycolipids.[101] The linker for attaching Ley to KLH was found to be crucial for high immune responses. The rigid cyclic 4-(maleimidomethyl)cyclohexane-1-carboxylate linker (Ley-MI-KLH) (Figure 10a) induced high antibody response to the linker, while the flexible acyclic 3-(bromoacetamido)propionate functionalized Ley conjugated with KLH (Ley-BrAc-KLH) (Figure 10b) helped direct the humoral responses towards the desired Ley epitope.[102]
Figure 10.
Structure of Ley-KLH constructs with different linkers.
In animal studies, mice immunized with Ley-KLH, which was prepared by conjugating a Ley pentasaccharide with KLH through a flexible linker,[103] generated high titers of IgG and IgM antibodies against natural occurring Ley-ceramide and showed moderate toxicity to MCF-7 tumor cells.[104] In clinical trials, the same Ley-KLH construct was well tolerated with no obvious side effects.[105] However, the immune responses against Ley were low. Only about 16 of 24 patients produced low to moderate IgM antibodies and 3 of 24 patients gave low titers of IgG. The antibodies were transiently generated in patients even with repeated immunizations. In contrast, very high titers of IgM and IgG responses against KLH were observed in patients suggesting that KLH dominated the immune responses.
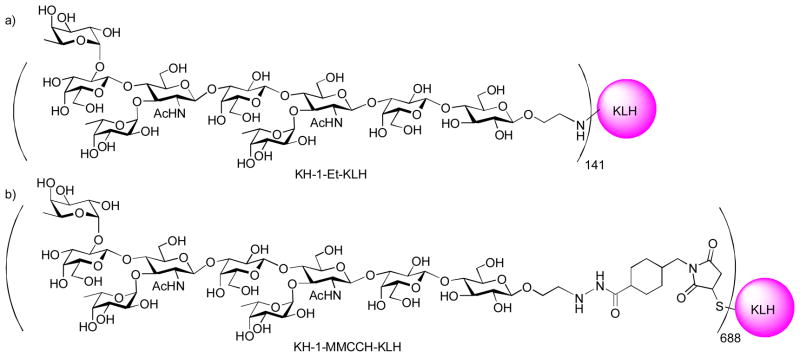
To overcome the weak immunogenicity of Ley, the Ley–Lex heterodimer (KH-1 antigen) was explored as the antigen,[106] which was originally identified with monoclonal antibodies AH6 and was found to be over-expressed in a number of cancers.[107] Mice immunized with KH-1-KLH generated high IgM and IgG antibodies against KH-1 and significant titers of IgG against Ley with little recognition of Lex. This suggested that the carbohydrates at the non-reducing end of the TACA are the major determinant in epitope recognition. Interestingly, preclinical studies[108] showed that mice immunized with KH-1-Et-KLH elicited low titer of IgM only, but the rigid linker containing KH-1-MMCCH-KLH generated significant IgG and IgM antibodies in all mice (Figure 11). This is in contrast to earlier study on Ley-KLH, where the conjugate with a flexible linker gave higher immune response.[102] The exact cause for this discrepancy is not known, which could be related to the higher antigen density of KH-1-MMCCH-KLH. The IgG antibodies showed excellent reactivity with MCF-7 cells. However, preliminary results from the clinical trial results were not encouraging. Patients with ovarian cancer immunized with KH-1-KLH generated very low IgM and undetectable IgG titers against Ley.
Figure 11.
Structures of KH-1-KLH conjugates with different linkers
Fucosyl-GM1
Fucosy1-GM1 (Fuc-GM1) was first isolated from bovine thyroid gland.[109] It was subsequently identified as a unique antigen present on most small cell lung cancer (SCLC) cells,[110] and it has the most restricted distribution on normal tissues among all the gangliosides. Preclinical study in mice using Fuc-GM1-KLH gave high titers of IgG and IgM against Fuc-GM1-ceramide.[111] The antibodies did not cross-react with GM1, which is widely expressed in normal tissues such as brain. Interestingly, patients immunized with bovine-derived Fuc-GM1 vaccine generated IgM and IgG responses while those immunized with vaccine constructs derived from synthetic Fuc-GM1 gave mainly IgM responses.[112,113] This difference could be due to the fact that the ceramide lipid is part of the bovine-derived Fuc-GM1 structure. The IgM antibodies generated from both constructs showed significant CDC against SCLC cells. The clinical effectiveness of the Fuc-GM1-KLH construct against cancer is yet to be established.
MUC-1 Glycopeptide
Mucin MUC1 is a membrane-bound glycoprotein with a large extracellular domain consisting of between 30 and 100 copies of the 20 amino acid HGVTSAPDTRPAPGSTAPPA tandem repeat.[114] The serine and threonine residues within the tandem repeat can be glycosylated. In many cancers of epithelial origins such as breast, ovarian, pancreatic, and prostate, the O-glycans of MUC1 are limited to short carbohydrate chains such as Tn, TF, and the corresponding sialylated STn and STF.[115] These short carbohydrates are not only TACAs themselves but also expose previously masked peptide backbone, thus rendering MUC1 glycopeptides targets for anti-cancer vaccine development.
The Kunz group has prepared a series of MUC1 glycopeptide constructs for immunological evaluations.[116] In their latest work, two MUC1 glycopeptides bearing a STn disaccharide at separate locations were synthesized and conjugated with TT at the carrier protein. Very strong antibody responses (mainly IgG1 subtype) were obtained upon immunization of mice with these two constructs. The binding of the antiserum with MCF-7 cell was very strong, which could be inhibited by competitive binding with the free glycopeptide suggesting the specificity of the biological recognition. The antisera also exhibited selectivities towards native tumor tissues, as the advanced tumor tissue but not the barely dedifferentiated one was stained strongly by the post-immune antiserum.
Sorensen et al reported that 60 amino acid MUC1 peptide (containing three tandem repeats) fully substituted with Tn or STn conjugated with KLH elicited stronger IgG antibody responses than the partially glycosylated peptide-KLH construct in both wide type and MUC1 transgenic mice.[117] The antiserum only recognized the full glycopeptide with little cross-reactivity with the unglycosylated peptide or carbohydrate antigen alone (Tn or STn). This is in contrast to Livingston’s observations,[118] but it should be mentioned different adjuvants and different length of the glycopeptides were used in these two studies. In subsequent in vivo studies,[119] the 60mer MUC1 peptide fully substituted with STn coupled with KLH did not show consistent protection of MUC1 transgenic mice from tumor challenge.
Monomeric cluster Vaccine
As TACAs are over-expressed on cancer cell surface, another potential strategy to enhance the anti-TACA immune response is to increase local concentrations of TACAs through clustering of the epitope in the vaccine construct. This is supported by the observations that there are tandem repeat units in mucins containing multiple threonine and serine residues, the potential glycosylation sites.
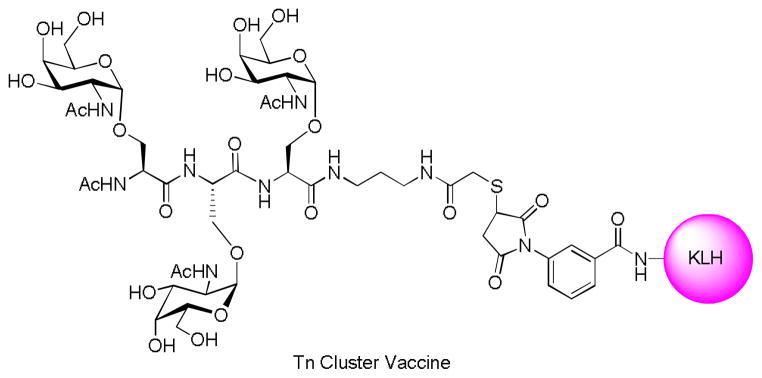
The cluster effect was demonstrated by Livingston and coworkers using the Tn antigen.[118] Mice immunized with Tn-KLH raised little antibodies against Tn cluster (Tn(c)) conjugated to human serum albumin (HSA) or desialylated ovine submaxillary mucin (dOSM) –a natural source of Tn, although the loading of Tn in KLH was as high as 1,330 copies of Tn per KLH. In addition, the antiserum showed very little binding to Tn expressing tumor cell LSC. In contrast, the Tn(c)-KLH (Figure 12) generated high IgG titers towards both Tn(c)-HSA and dOSM with moderate recognition of LSC. In phase I clinical trials with prostate cancer patients,[120] Tn(c)-KLH elicited significant titers of IgM and IgG. Although the antisera showed modest binding with LSC cells and complement mediated cell lysis, vaccination induced declines of the slopes of prostate specific antigen in several patients.
Figure 12.
Tn(c)-KLH vaccine
In contrast to Tn antigen, no significant differences were observed between STn antigen and clustered STn (STn(c)).[121] Mice immunized with STn-KLH and STn(c)-KLH could elicit significant titers of IgG and IgM with good recognition of OSM that is a nature source of STn. There were little cross-reactivity as the antiserum from STn-KLH immunization reacted with STn-HSA but weakly with STn(c)-HSA, while antiserum from STn(c)-KLH immunization reacted with STn(c)-HSA but weakly with STn-HSA. Both antisera reacted with STn-positive human tumor cell LSC not with STn-negative LSB cells. In subsequent clinical studies, both STn-KLH and STn(c)-KLH constructs elicited IgG and IgM antibodies in most patients against the synthetic antigens, but only IgM exhibited moderate binding to OSM and LSC cells. Similar to STn, TF and Ley antigens and their respective clusters did not show substantial differences in antibody titers.[16,122] The differential behavior of Tn is most likely due to its small size compared to other TACAs, thus requiring clusters of antigens for immuno-recognition.
Polyvalent Vaccine (Pooled Monomeric Vaccines)
As discussed above, many cancer cells express more than one TACA. Therefore, it is reasonable to target multiple TACAs associated with a particular cancer type so as to elicit a stronger anti-cancer immune response and prevent the cells from escaping the immuno-surveillance. One approach to accomplish this is the polyvalent vaccine strategy combining various monomeric TACA-KLH constructs in the same vaccination.
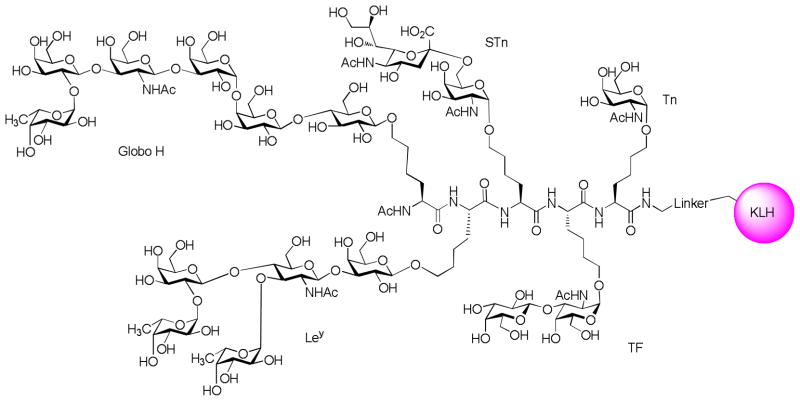
A preclinical study with heptavalent KLH-conjugate vaccine consisting of seven TACAs, including GM2, Globo H, Ley, TF(c), Tn(c), STn(c) and glycosylated MUC1, demonstrated that mice immunized with such vaccine generated antibodies against all TACAs except GM2.[123] High titers of both IgM and IgG were observed against five antigens except Ley that induced IgM antibody only. In addition, the antibodies generated recognized tumor cells well. In clinical trials,[124] the heptavalent vaccine plus QS21 adjuvant was tested in 9 patients with epithelial ovarian, fallopian tube, or peritoneal cancer in second or greater complete clinical remission. Serologic responses were primarily IgM types except for Tn-MUC1 which induced both IgM and IgG. Ley and GM2 were the least immunogenic ones among the seven TACAs tested, as only fewer than two patients had immune responses towards these two antigens. Eight out of the nine patients generated antibodies against at least three of seven antigens. However, the medium antibody titers for all antigens were significantly reduced compared to vaccination with individual constructs.
Unimolecular Polyvalent Vaccine
One possible reason for the lower titers encountered in the pooled monomeric vaccine strategy is the large amounts of carrier protein KLH administered with the seven TACA constructs. The limited number of B cells available in the host could be directed towards KLH, thus lowering anti-TACA responses. To reduce the undesired anti-carrier immune responses yet still target multiple TACAs, the unimolecular polyvalent vaccine strategy was explored in which multiple types of antigens are incorporated into a single peptide backbone.
A unimolecular pentavalent construct (Figure 13) consisting of five prostate and breast cancer associated carbohydrate antigens, Globo H, GM2, STn, TF and Tn, was synthesized and conjugated to KLH by Danishefsky and coworkers.[125] Immunization of mice with this complex construct showed that superior IgM and IgG antibodies were generated against Globo-H, while the antibody titers against Tn were inferior compared with a non-covalent mixture of the monovalent constructs and Ley did not elicit any immune responses. This suggests that the positioning of the antigen on the multivalent construct may play an important role in determining its immunogenicity and further design and development are necessary to create an effective construct against all the components. Other examples of unimolecular pentavalent constructs include clustered Gb3-MUC5AC-KLH[126] and MUC1 glycopeptide-KLH conjugates.[127]
Figure 13.
A unimolecular pentavalent vaccine construct
In conclusion, immunogenic protein carriers such as KLH have been shown to significantly enhance the immune responses towards TACAs. In spite of the significant progresses, there are still serious limitations associated with the immunogenic carriers studied so far, as exemplified by the failure of GM2-KLH[94] and Theratope vaccine (STn-KLH)[128] in clinical trials. There are several possible reasons for the lack of clinical success for KLH: 1) KLH is itself a glycoprotein, displaying a variety of carbohydrates including high mannose-type glycans and GalNAc on the surface.[129] These endogenous carbohydrates on KLH may divert the anti-carbohydrate immune response away from the desired TACAs; 2) the quality and quantity of antibodies generated with KLH conjugates may not be sufficient. To address these issues, a variety of other vaccine delivery platforms have been studied, which will be discussed in the following sections.
5.2. Virus like Particles (VLPs) Based TACA Delivery
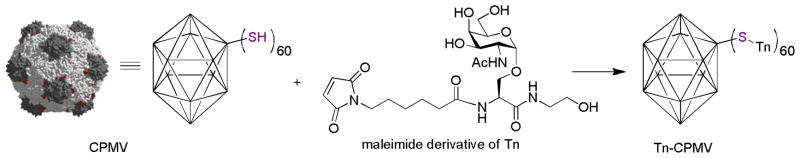
VLPs are the envelopes or capsids from viruses, which are comprised of structural proteins self-assembled in a highly ordered manner.[130] For example, the capsids of Cowpea Mosaic Virus (CPMV) are made up of 60 copies of identical subunits arranged in an icosahedral geometry.[131] As discussed in section 3, B cells respond strongly to highly organized antigens.[46] Therefore, it was hypothesized that TACAs displayed on VLPs can efficiently cross-link BCRs leading to potent B cell activation.
The Huang, Wang and Finn groups tested the application of CPMV as the immunogenic TACA carrier.[132] CPMV capsid has superior stability, which can tolerate up to 50% organic solvents such as DMSO for at least two days.[133–135] This enables its ready functionalization by TACAs without losing the capsid integrity. As Tn is a weak antigen and no IgG responses were generated using the Tn-KLH construct, Tn-CPMV conjugate was prepared to test whether organized display of Tn could significantly boost its immunogenecity. A Tn derivative bearing a maleimide moiety was synthesized and conjugated to a cysteine mutant of CPMV (Figure 14). CPMV does not contain endogenous carbohydrates and each Tn-CPMV bore an average of 60 copies of Tn suggesting that complete functionalization of CMPV was achieved. Mice immunized with Tn-CPMV elicited high titers of both IgM and IgG against Tn. The antisera showed strong reactivity towards the multidrug resistant ovarian cancer cell line NCI-ADR RES, indicating TACAs based vaccines can be a potentially novel therapeutic towards drug resistant cancer. In a separate study,[136] a variety of carbohydrates including Globo H, sialyl LewisX, and blood group A tetrasaccharides were immobilized onto CPMV. Inoculation of chickens with these constructs generated gram quantities of antiglycan IgY antibodies, which are the functional equivalent of mammalian IgGs. When screened against a microarray bearing over 200 glycans, these IgY antibodies displayed excellent specificity and avidity in binding the carbohydrate antigens from which they were generated. Therefore, VLPs are showing great promises as TACA carriers, the full potential of which will need to be established.
Figure 14.
Functionalization of a VLP CPMV bearing Tn antigen.
5.3. Zwitterionic Polysaccharides (ZPS) Based TACA Delivery
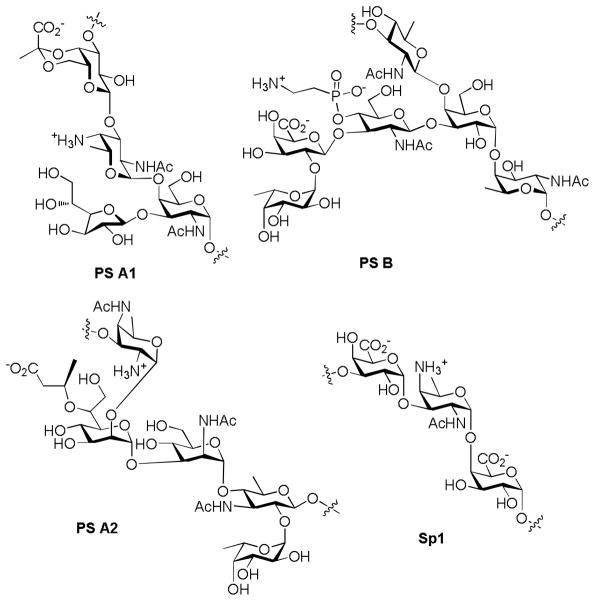
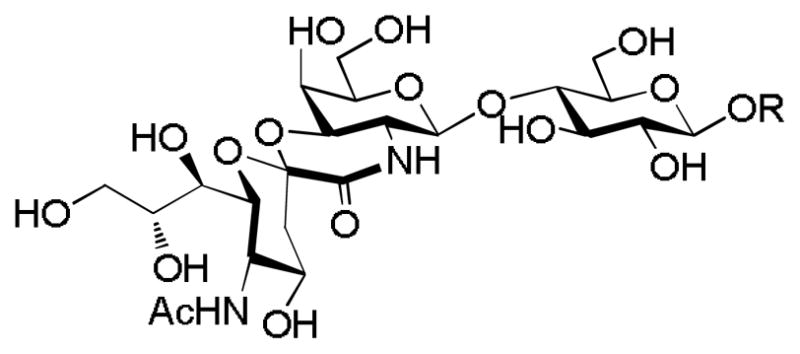
Although the majority of carbohydrate antigens are T-cell independent, i.e., they elicit immune responses independently of T cells, a novel discovery was made that ZPSs from the capsules of some bacteria can stimulate potent Th cell responses in vitro and in vivo.[137] Such immunomodulatory ZPSs have been isolated from different bacterial species, including PS A1 and PS B from Bacteroides fragilis strain 9343, PS A2 from B. fragilis 638, and Sp1 from type 1 Streptococcus pneumonia (Figure 15).[138] These ZPSs share an important structure motif -alternative positive and negative charges with high density, which are required for their binding to MHC class II and presentation to Th cells. Recently, PS A1 was identified as a TLR2 agonist. TLR-2 is a receptor playing fundamental roles in pathogen recognition and activation of innate immunity, which links the innate and adaptive immune systems.[139]
Figure 15.
Chemical structures of the repeating units of several ZPSs.
Based on their ability to activate Th cells, ZPSs were explored as a novel carrier for TACA based anti-cancer vaccine development. The Andreana group selectively oxidized the vicinal hydroxyl groups in D-galactofuranose of PS A1.[140] The resulting aldehyde was then conjugated with a GalNAc through an oxime bond (Figure 16). Immunization without exogenous adjuvant stimulated strong antibodies against the construct, possibly due to immuno-potentiation by PS A1 as a Toll-like receptor-2 (TLR-2) agonist. Addition of Titermax Gold adjuvant for vaccination helped further boost the antibody titers. Subtyping of the IgG by ELISA revealed that only significant amounts of IgG3 were observed, which was viewed as an anti-carbohydrate response. Further studies are necessary to illustrate the full potential of ZPSs as TACA carriers.
Figure 16.
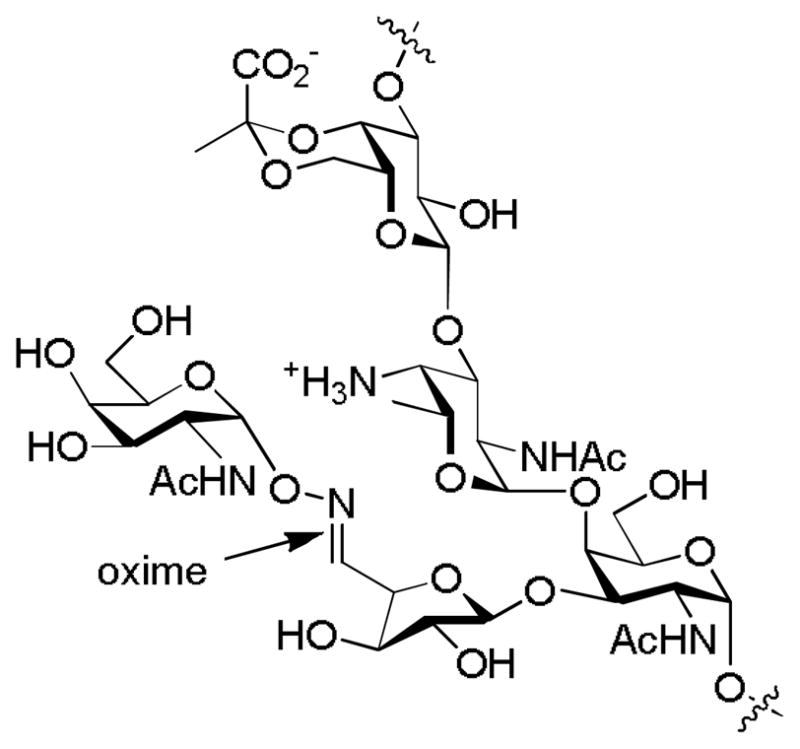
Structure of the GalNAc-PS A1 vaccine construct
5.4. Fully Synthetic Carbohydrate Cancer Vaccine
The conjugation of carbohydrate antigens to an immunogenic carrier represents a classic approach for vaccine design. One potential drawback of this approach is that the carrier proteins themselves are highly immunogenic and can induce strong antibody response.[141,142] This can possibly lead to immuno-suppression to epitopes displayed on carriers, especially the weakly immunogenic TACAs. For example, Tn antigen covalently linked to KLH gave low antibody titers against Tn but elicited strong anti-KLH responses.[118] To address this issue, fully synthetic vaccines, which contain the exact Th peptide epitope and the TACA B cell epitope, have been developed in the past two decades.
5.4.1. Two Component Vaccine: Multiple Antigen Glycopeptide (MAG)
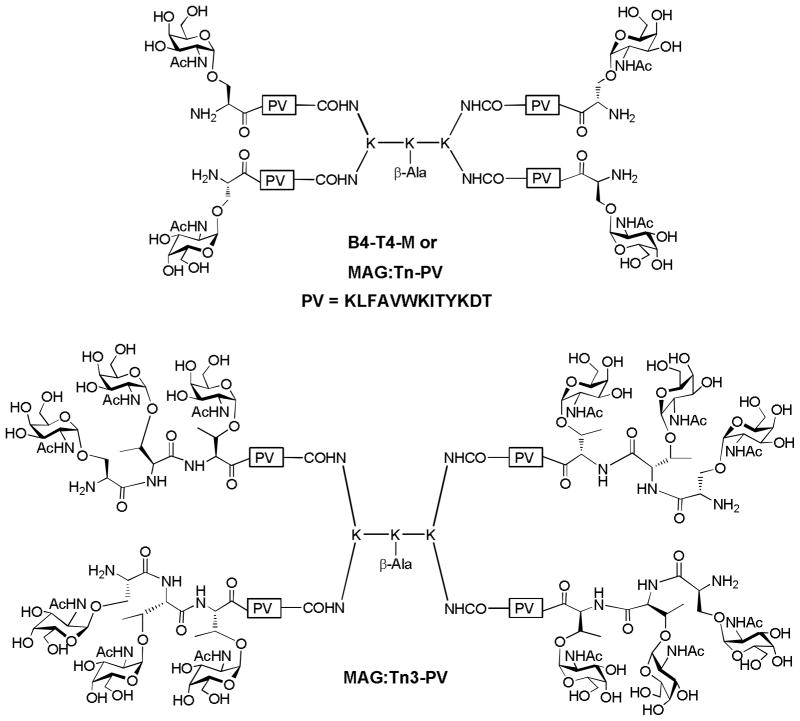
In 1988, Tam and coworkers described a new method for assembly of immunogenic peptide antigen, referred to as multiple antigen peptide, in which high density of peptide epitopes were attached to the amino groups in oligomeric branched polylysine.[143] This concept was extended by Lo-man and coworkers to overcome the low immunogenicity of Tn antigen. They prepared the first MAG construct B4-T4-M (also known as MAG:Tn-PV, Figure 17), a tetrameric structure composed of four copies of Tn and a mouse Th cell peptide epitope derived from poliovirus (PV).[144] Mice immunized with MAG:Tn-PV in the presence of alum as the adjuvant generated high IgG titers. T cell stimulation was enhanced by 1000 times with MAG:Tn-PV, suggesting that the incorporation of Tn in the construct dramatically increased the binding of PV peptide by MHC class II molecules. To further improve the immune responses, trimeric Tn cluster was introduced into the MAG (Figure 17).[145] Immunization with the resulting MAG:Tn3-PV induced higher antibody titers (by several hundred thousand) and longer-lasting responses than MAG:Tn-PV.[145] The antibody titers remained stable even five months after the final immunization, and the antisera showed strong reactivity against Jurkat and TA3/Ha cells. The capability of MAG:Tn3-PV immunization to protect mouse from the highly tumorigenic TA3/Ha adenocarcinoma was tested first in the prophylactic setting. The mice were immunized with the MAG:Tn3-PV construct, which was followed by injection of TA3/Ha cells. While all the untreated mice died from the cancer within 30 days, 80% of the animals vaccinated with MAG:Tn3-PV survived. The control construct MAP:PV without the Tn antigens only protected 10% mice from tumor induced death, thus highlighting the importance of TACA targeting. The efficacy of the vaccine construct was further tested in a therapeutic model against pre-established cancer. TA3/Ha cells were injected into mice first to allow for tumor growth. If left untreated, all mice died within 25 days. In contrast, close to 40% mice receiving the MAG:Tn3-PV survived after 100 days.
Figure 17.
Structures of MAG:Tn-Pv and MAG:Tn3-PV
Inspired by these results, the authors further investigated the effect of amino acid sequence of the backbone of Tn cluster on the immune response.[146] The glycocluster of three α-GalNAc on the Ser-Thr-Thr tripeptide backbone sequence with each amino acid residue was found to be most antigenic and the immune responses were much stronger than the corresponding Tn(c)-KLH conjugate. The MAG:Tn3 construct was able to induce anti-Tn IgG antibodies not only in mice but also in two nonhuman primate species. The antisera were able to induce CDC or ADCC against human tumor cells, thus opening the way for a new generation of anticancer vaccine design based on fully synthetic glycopeptides.
Recently, Freire et al reported that even in the absence of an adjuvant, mice intradermally immunized with MAG:Tn3-PV generated high titers of IgG1.[147] As some skin residing DCs have galactose-type lectin (MGL; CD301) receptors, the Tn modified glycopeptide MAG:Tn3-PV was much more efficiently captured and presented to Th cells by the MGL+ DCs than the non-glycosylated conjugates. This study helped to explain the earlier observation that the presence of Tn in MAG:Tn3-PV increased Th cell simulation by 1000 times.[144] Although the recognition of Tn antigen by MGL is known, this study showed for the first time that Tn antigen could play dual roles: tumor associate antigen and ligand for MGL. Hence, Tn clusters can not only strengthen the immune responses, but also enhance the uptake and presentation of vaccine constructs.
5.4.2. Self-adjuvanting multiple component vaccine
Although great progress has been made for two component vaccines, adjuvants are still needed to boost immune responses in many cases. Of the adjuvants currently available, only aluminum salts (alum) is approved for human usage and others such as Freund’s adjuvant can cause severe side effects. To address this issue, the fully synthetic self-adjuvant multiple component vaccines are pursued.
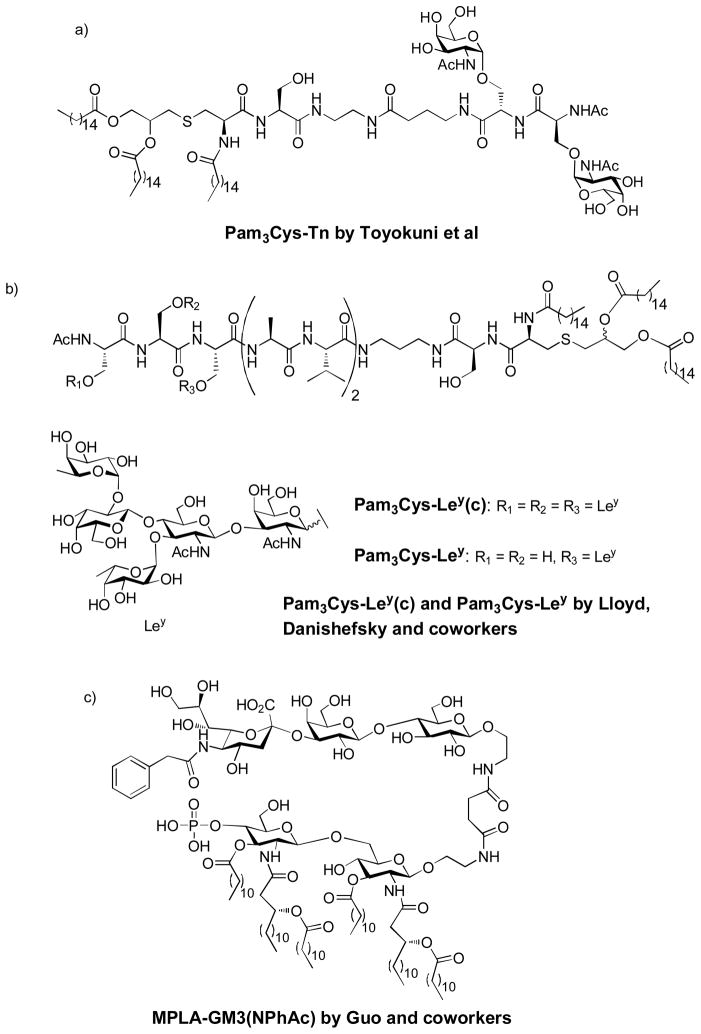
The pioneering research by Deres et al showed that synthetic viral peptides covalently linked to tripalmitoyl-S-glycerylcysteinyl-seryl-serine (Pam3Cys), which was part of the immunologically active N-terminal sequence of the principle lipoprotein of Escherichia coli, also known as Braun’s lipoprotein, can efficiently prime influenza-virus-specific Tc cells in vivo.[148] Later on, the first fully synthetic carbohydrate vaccine was described by Toyokuni et al, who covalently linked a Tn dimer to Pam3Cys (Figure 18a).[149] Mice immunized with this vaccine construct showed significant IgM but low IgG response. This study showed for the first time that a short synthetic glycolipid could elicit specific immune response against TACAs without exogenous adjuvant and carrier protein.
Figure 18.
Chemical structures of several self-adjuvanting vaccine constructs
Instead of the Tn antigen, Lloyd, Danishefsky and coworkers synthesized the monomeric Ley and various trimetric Ley cluster (Ley(C)) and conjugated them to peptide-Pam3Cys, to examine the effects of epitope clustering, carrier structure, and adjuvant on antibody production (Figure 18b).[16] In the absence of adjuvant, all the glyco-lipopeptide gave IgM only, with clustered-epitope constructs more superior than monomeric one. A significant but low titer of IgG was elicited in the presence of QS-21 adjuvant. The antibodies reacted strongly with Ley-positive ovarian cancer cell line OVCAR-3.
Recently, the Guo group reported that conjugating the adjuvant monophosphoryl lipid A (MPLA) with the N-phenylacetyl analog of GM3 (Figure 18c). Interestingly, although this construct lacks a peptide Th epitope, robust IgG3 antibody responses were observed, which reacted strongly with glycoengineered SKMEL-28 cancer cells.[150] The addition of an external adjuvant, i.e., Titermax Gold, greatly reduced the immunological activity of the conjugates possibly due to the interaction between MLPA and Titermax Gold.
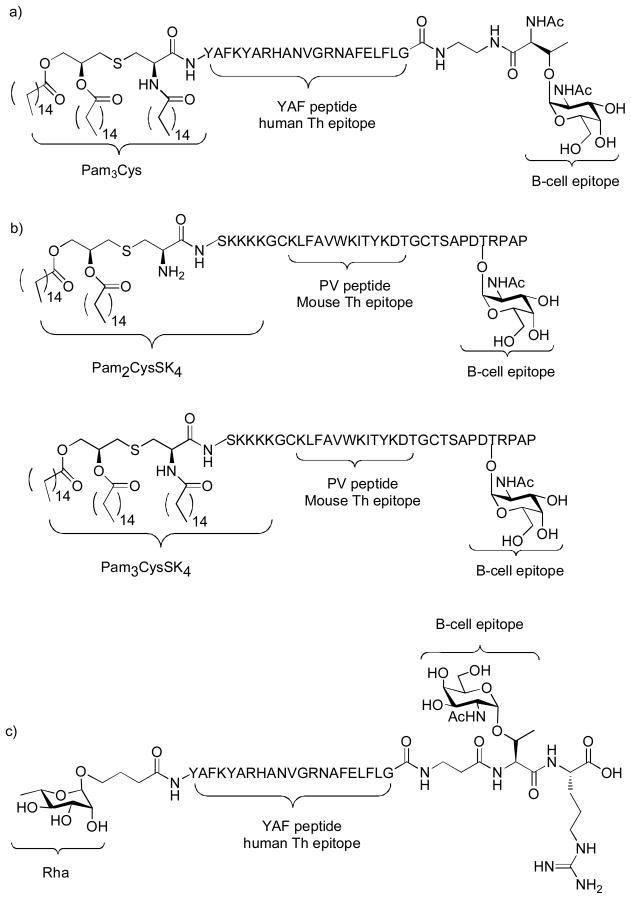
To further boost the immune responses, Boons and coworkers introduced a three component vaccine construct, which is composed of the lipopeptide adjuvant Pam3Cys, the B cell epitope Tn antigen, and the YAF peptide (Figure 19a).[151] The YAF peptide, derived from an outer-membrane protein of Neisseria meningitides, has been identified as a human MCH class II ligand, thus serving as a Th epitope. Mice immunized with the glycolipopeptide with QS21 as the adjuvant generated modest IgM and IgG antibodies against the Tn antigen. In a subsequent study, the three component construct was improved by substituting the human Th epitope YAF peptide with the mouse Th epitope derived from the PV. For the B cell epitope, instead of just the Tn antigen, Tn containing glycopeptide MUC1 was incorporated. Two variations of the Pam3Cys, i.e., Pam2CysSK4 or Pam3CysSK4 were used as the built-in adjuvant (Figure 19b).[152] Mice immunized with the three component Pam3CysSK4 glycopeptide elicited exceptionally higher titers (several hundred thousands) of anti-MUC1 IgG antibodies, which displayed strong recognition of MUC1 expressing tumor cells (MCF-7). Importantly, the antibody titer against the Th peptide was much lower compared to that towards MUC1, suggesting the humoral response elicited was focused against the desired epitope. The immune response was further boosted with the co-administration of an exogenous adjuvant QS-21 with the tri-component vaccine construct.
Figure 19.
Structures of self-adjuvanting three component vaccines from the Boons and Sucheck groups.
It was shown that the covalent conjugation of Pam3CysSK4 to glycopeptide is crucial for excellent antibody response because mixing the Pam3CysSK4 and glycopeptide gave little antibodies. However, this finding perhaps was not unexpected since the glycopeptide could not insert into liposomes and form the multivalent constructs for B cell activation. Therefore, in another study, the authors synthesized the same glycopeptide linked with an immunosilent lipid anchor to further examine the effect of formulation on immune responses.[153] When co-administrated with Pam3CysSK4 or MPLA, similar IgG titers were observed compared to Pam3CysSK4 linked glycopeptide. However, the abilities of the antisera to react with cancer cells were impaired. Covalent linkage of the Th epitope with the B cell epitope was found to be essential for high immune responses. This is consistent with the notion that potent B cell activation requires the stimulation by matched Th cells as discussed in section 3.
Instead of relying on lipopeptides as the built-in adjuvant, Sucheck and coworkers explored the possibility of using pre-existing antibodies to boost the immuno-recognition of TACA constructs. They synthesized a novel three component vaccine (Rha-YAF-Tn) containing the B cell epitope Tn antigen, Th epitope YAF peptide, and a rhamnose moiety (Figure 19c).[154] Human serum has been reported to contain large amounts of naturally occurring anti-Rha antibodies. To mimic this condition, mice were immunized first with a Rha-OVA conjugate to induce high titers of anti-Rha antibodies in mice, which generated >100 fold higher anti-Rha antibodies than the control group. The mice producing anti-Rha antibodies were then administered the three component Rha-YAF-Tn construct. The anti-Rha antibodies would recognize the Rha-YAF-Tn, thus enhancing the recognition of the construct as well as antigen processing and presentation by APCs. ELISA results showed that mice pre-immunized with Rha-OVA generated higher anti-Tn titers relative to those without pre-immunization. T cells in the presence of anti-Rha antibodies could be activated with much lower concentration of Rha-YAF-Tn, thus demonstrating the immuno-recognition of the Tn construct was enhanced.
Kunz and coworkers recently reported a fully synthetic vaccine consisting of the TLR2 ligand Pam3CysK4 and the 20 amino acid tandem repeat domain of MUC1 bearing Tn or TF antigens at various positions.[155] However, the authors did not show the self-adjuvanting ability of the vaccine construct. Instead, the vaccine was co-administered with the Freund’s adjuvant to mice. MUC1 specific antibodies were elicited, but the titers were not as high as those from the corresponding MUC1-TT conjugates. This was perhaps due to the lack of additional Th epitopes in the construct.
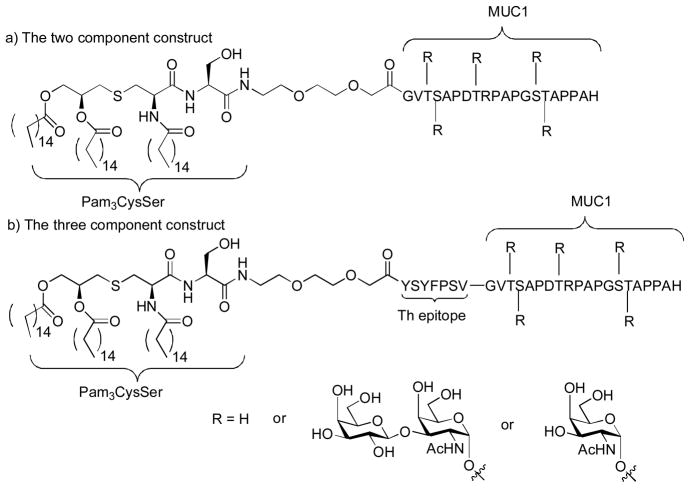
Payne and coworkers reported the synthesis of a series of lipoglycopeptides containing Pam3CysSer as the built-in adjuvant, MUC1 tandem repeating domains glycosylated with either Tn or TF with (three component construct) or without (two component construct) a Th epitope (Figure 20).[156,157] The corresponding lipopeptides without glycosylation in the MUC1 were prepared for comparison. Full glycosylation of MUC1 greatly reduced the responses against the two component vaccines in mice, which may be due to the diminished Th response from the glycosylated MUC1 peptide. The tri-component construct gave much higher antibody titers than the corresponding di-component one, confirming the importance of Th epitope incorporation. Glycosylation of the lipopeptides has great effects on specificity of the humoral response. Mice immunized with the TF substituted lipoglycopeptide elicited significant IgG antibodies while the one substituted with Tn generated lower IgG response. The serum from the unglycosylated three component vaccine showed significant recognition to MCF-7 (47.6%) and the fully glycosylated ones exhibited lower binding (22.1%–34.1%). Importantly, the antisera from the three component vaccines did not bind to MUC1 isolated from normal human breast milk, presumably due to the more extensive glycosylation on MUC1 isolated from normal tissues. This suggests that the antibodies raised were selective against tumor.
Figure 20.
Payne’s multi-component self-adjuvanting vaccine constructs
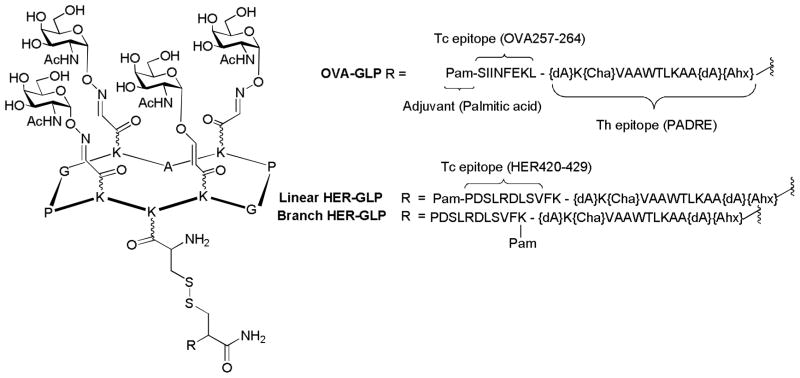
Building on the success of three component vaccine, Dumy, BenMohamed and coworkers extended it to four components incorporating an additional peptide epitope for Tc cells.[158,159] As illustrated in Figure 21, the vaccine construct OVA-GLP included: 1) a cluster of GalNAc as B cell epitope linked to a functionalized template RAFT, which is a cyclic decapeptide consisting of lysine (K), proline (P), glycine (G) and alanine (A); 2) one universal Th helper epitope known as PADRE (Pan HLA DR-binding Epitope). PADRE is a synthetic, non-natural peptide that binds to 15 of the 16 most common types of MHC class II molecules HLA-DR in human as well as those in mice. It can help overcome the extreme polymorphism of HLR-DR encountered in the human population; 3) one Tc epitope OVA(257–264), a peptide from chicken egg ovalbumin recognized by MHC class I in mice; 4) one palmitic acid moiety serving as a built-in adjuvant. The resulting construct is water-soluble, which enabled in vivo delivery in adjuvant-free saline. Upon administration in mice, significant levels of IgG and IgM specific to the four component construct were elicited, which were able to recognize the Tn-antigen expressing human breast tumor cells MCF-7. The palmitic acid was shown to be crucial for antibody production. The vaccine also generated strong PADRE-specific Th cells and OVA(257–264) specific Tc cell responses. Importantly, in a therapeutic setting using the MO5/BALB/c tumor mouse model, the vaccinated group achieved 100% host survival 45 days after tumor challenge, while all the mice died in the control group with mock treatment.
Figure 21.
Four component vaccine constructs by Dumy and coworkers. dA: L-alanine; Cha: cyclohexyl alanine; Ahx: L-2-aminohexanoic acid.
In another study,[160] instead of the OVA antigen, the authors used human tumor specific Tc epitope HER-2 and two vaccine constructs were designed with palmitic acid attached either at the N-terminal (linear HER-GLP, Figure 21) or between the Th and Tc cell epitopes (branch HER-GLP, Figure 21). Immunization of mice showed that the linear HER-GLP elicited more potent HER specific IFN-γ producing Tc cell responses, while the branched HER-GLP generated a stronger tumor-specific IgG response. It was supposed that the position of the lipid moiety greatly affected the uptake and cross-presentation pathway resulting in the modulation of the magnitude of induced B and T-cell responses. Therapeutic immunization with the linear HER-GLP was more effective in reducing tumor progression as compared to the branched HER-GLP. Interestingly, the combination of branched HER-GLP and linear HER-GLP led to higher tumor therapeutic efficiencies than individual ones.
The excellent immune responses produced by the fully synthetic multi-component vaccine constructs demonstrated great promises for this approach. As it does not have any unnecessary antigenic components, the immune responses can be focused on the desired TACA epitopes. The TLR ligands present in the construct can facilitate the uptake by APCs and assist the full activation of APCs. However, the broad applicability of this approach to other TACAs will still need to be established as the Tn antigen has been the primary focus so far. The synthetic construct often contains only one Th epitope. As human MHC class II molecules are highly polymorphic, multiple Th epitopes may be required in a construct to elicit a powerful response. In addition, with more and more components built into the constructs, sophisticated synthetic methodologies will need to be developed to access these increasingly complex molecules.
6. Conclusions
Great progress has been made in the past decades for carbohydrate based anti-cancer vaccine development. Many of the studies have examined the antibody titers and subtypes generated, which yield useful information on the type and strength of the immune responses. However, to advance the field further, the efficacy of the constructs in tumor protection and treatment should be established. Moreover, immune responses encompassing both the humoral arm and the cellular arm especially the Tc cell activation will be highly desired to combat cancer. More studies are necessary to understand how to create a powerful antigenic construct to elicit an effective multi-faceted response. With the collective efforts from chemists and immunologists, perhaps in the near future, we will see TACA-based anti-cancer vaccines in clinical use for cancer prevention and treatment.
Acknowledgments
We would like to thank the National Cancer Institute for generous financial support of our work (R01CA149451-01A1).
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012 doi: 10.3322/caac.20141. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Coley WB. The classic - the treatment of malignant-tumors by repeated inoculations of erysipelas - with a report of 10 original cases. Clin Orthop Relat Res. 1991;262:3–11. [PubMed] [Google Scholar]
- 4.Burnet M. Cancer-a biological approach. Brit Med J. 1957;1:841–847. doi: 10.1136/bmj.1.5023.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grillo-Lopez AJ, Hedrick E, Rashford M, Benyunes M. Rituximab: Ongoing and future clinical development. Semin Oncol. 2002;29:105–12. doi: 10.1053/sonc.2002.30145. [DOI] [PubMed] [Google Scholar]
- 6.Weiner LM, Surana R, Wang S. Monoclonal antibodies: Versatile platforms for cancer immunotherapy. Nat Rev Immunol. 2010;10:317–27. doi: 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danishefsky SJ, Allen JR. From the laboratory to the clinic: A retrospective on fully synthetic carbohydrate-based anticancer vaccines. Angew Chem Int Ed. 2000;39:836–863. doi: 10.1002/(sici)1521-3773(20000303)39:5<836::aid-anie836>3.0.co;2-i. and references cited therein. [DOI] [PubMed] [Google Scholar]
- 8.Hakomori S, Zhang Y. Glycosphingolipid antigens and cancer therapy. Chem Biol. 1997;4:97–104. doi: 10.1016/s1074-5521(97)90253-2. [DOI] [PubMed] [Google Scholar]
- 9.Hakomori S. Tumor malignancy defined by aberrant glycosylation and sphingo(glyco)lipid metabolism. Cancer Res. 1996;56:5309–5318. [PubMed] [Google Scholar]
- 10.Nores GA, Dohi T, Taniguchi M, Hakomori S. Density-dependent recognition of cell surface GM3 by a certain anti-melanoma antibody, and GM3 lactone as a possible immunogen: Requirements for tumor-associated antigen and immunogen. J Immunol. 1987;139:3171–3176. [PubMed] [Google Scholar]
- 11.Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, Mellman I, Prindiville SA, Viner JL, Weiner LM, Matrisian LM. The prioritization of cancer antigens: A national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15:5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hakomori S. Tumor-associated carbohydrate antigens defining tumor malignancy: Basis for development of anti-cancer vaccines. Adv Exp Med Biol. 2001;491:369–402. doi: 10.1007/978-1-4615-1267-7_24. [DOI] [PubMed] [Google Scholar]
- 13.Livingston PO, Zhang S, Lloyd KO. Carbohydrate vaccines that induce antibodies against cancer. 1. Rationale. Cancer Immunol Immunother. 1997;45:1–9. doi: 10.1007/s002620050394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamilton WB, Helling F, Lloyd KO, Livingston PO. Ganglioside expression on human malignant melanoma assessed by quantitative immune thin-layer chromatography. Int J Cancer. 1993;53:566–573. doi: 10.1002/ijc.2910530407. [DOI] [PubMed] [Google Scholar]
- 15.Huang C-Y, Thayer DA, Chang AY, Best MD, Hoffmann J, Head S, Wong CH. Carbohydrate microarray for profiling the antibodies interacting with Globo H tumor antigen. Proc Nat Acad Sci USA. 2006;103:15–20. doi: 10.1073/pnas.0509693102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kudryashov V, Glunz PW, Williams LJ, Hintermann S, Danishefsky SJ, Lloyd KO. Toward optimized carbohydrate-based anticancer vaccines: Epitope clustering, carrier structure, and adjuvant all influence antibody responses to Lewisy conjugates in mice. Proc Nat Acad Sci, USA. 2001;98:3264–3268. doi: 10.1073/pnas.051623598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou BB, Zhang H, Damelin M, Geles KG, Grindley JC, Dirks PB. Tumour-initiating cells: Challenges and opportunities for anticancer drug discovery. Nat Rev Drug Discov. 2009;8:806–23. doi: 10.1038/nrd2137. [DOI] [PubMed] [Google Scholar]
- 18.Lin W-M, Karsten U, Goletz S, Cheng R-C, Cao Y. Co-expression of CD173 (H2) and CD174 (Lewis y) with CD44 suggests that fucosylated histo-blood group antigens are markers of breast cancer-initiating cells. Vischow Arch. 2010;456:403–409. doi: 10.1007/s00428-010-0897-5. [DOI] [PubMed] [Google Scholar]
- 19.Chang W-W, Lee CH, Lee P, Lin J, Hsu C-W, Hung J-T, Lin J-J, Yu J-C, Shao L-e, Yu J, Wong C-H, Yu AL. Expression of Globo H and SSEA3 in breast cancer stem cells and the involvement of fucosyl transferases 1 and 2 in Globo H synthesis. Proc Natl Acad Sci, USA. 2008;105:11667–11672. doi: 10.1073/pnas.0804979105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldsby RA, Kindt TJ, Osborne BA. Kuby Immunology. Freeman; New York: 2000. pp. 275–285. [Google Scholar]
- 21.Bretscher P, Cohn M. A theory of self-nonself discrimination: Paralysis and induction involve the recognition of one and two determinants on an antigen, respectively. Science. 1970;169:1042–1049. doi: 10.1126/science.169.3950.1042. [DOI] [PubMed] [Google Scholar]
- 22.Livingston PO, Wong GYC, Adluri S, Tao Y, Padavan M, Parente R, Hanlon C, Calves MJ, Helling F, Ritter G, Oettgen HF, Old LJ. Improved survival in stage III melanoma patients with GM2 antibodies: A randomized trial of adjuvant vaccination with GM2 ganglioside. J Clin Oncol. 1994;12:1036–1044. doi: 10.1200/JCO.1994.12.5.1036. [DOI] [PubMed] [Google Scholar]
- 23.Jones PC, Sze LL, Liu PY, Morton DL, Irie RF. Prolonged survival for melanoma patients with elevated igm antibody to oncofetal antigen. J Nat Cancer Inst. 1981;66:249–254. [PubMed] [Google Scholar]
- 24.Vadhan-Raj S, Cordon-Cardo C, Carswell E, Mintzer D, Dantis L, Duteau C, Templeton MA, Oettgen HF, Old LJ, Houghton AN. Phase I trial of a mouse monoclonal antibody against GD3 ganglioside in patients with melanoma: Induction of inflammatory responses at tumor sites. J Clin Oncol. 1988;6:1636–48. doi: 10.1200/JCO.1988.6.10.1636. [DOI] [PubMed] [Google Scholar]
- 25.Nasi ML, Meyers M, Livingston PO, Houghton AN, Chapman PB. Anti-melanoma effects of r24, a monoclonal antibody against GD3 ganglioside. Melanoma Res. 1997;7:S155–S162. [PubMed] [Google Scholar]
- 26.Kuberan B, Linhardt RJ. Carbohydrate based vaccines. Curr Org Chem. 2000;4:635–677. [Google Scholar]
- 27.Filion MC, Phillips NC. Pro-inflammatory activity of contaminating DNA in hyaluronic acid preparations. J Pharm Pharmacol. 2001;53:555–561. doi: 10.1211/0022357011775677. [DOI] [PubMed] [Google Scholar]
- 28.Galonic DP, Gin DY. Chemical glycosylation in the synthesis of glycoconjugate antitumor vaccines. Nature. 2007;446:1000–1007. doi: 10.1038/nature05813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsu C-H, Hung S-C, Wu C-Y, Wong C-H. Toward automated oligosaccharide synthesis. Angew Chem Int Ed. 2011;50:11872–11923. doi: 10.1002/anie.201100125. [DOI] [PubMed] [Google Scholar]
- 30.Wang Z, Huang X. Strategies in oligosaccharide synthesis. In: Kamerling JP, editor. Comprehensive glycoscience from chemistry to systems biology. Vol. 1. Elsevier; 2007. pp. 379–413. [Google Scholar]
- 31.Icart LP, Fernandez-Santana V, Veloso RC, Carmenate T, Sirois S, Roy R, Bencomo VV. T-cell immunity of carbohydrates. In: Roy R, editor. Carbohydrate-based vaccines (ACS symposium series, 989) American Chemical Society; Washington, D.C: 2008. pp. 1–19. [Google Scholar]
- 32.Helling F, Shang A, Calves M, Zhang S, Ren S, Yu RK, Oettgen HE, Livingston PO. GD3 vaccines for melanoma: Superior immunogenicity of keyhole limpet hemocyanin conjugate vaccines. Cancer Res. 1994;54:197–203. [PubMed] [Google Scholar]
- 33.Livingston PO, Ritter G, Srivastava P, Padavan M, Calves MJ, Oettgen HE, Old LJ. Characterization of IgG and IgM antibodies induced in melanoma patients by immunization with purified GM2 ganglioside. Cancer Res. 1989;49:7045–7050. [PubMed] [Google Scholar]
- 34.Livingston PO, Natoli EJJ, Calves MJ, Stockert E, Oettgen HFO, LJ Vaccines containing purified GM2 ganglioside elicit GM2 antibodies in melanoma patients. Proc Natl Acad Sci USA. 1987;84:2911–2915. doi: 10.1073/pnas.84.9.2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vliegenthart JFG. Carbohydrate based vaccines. FEBS Lett. 2006;580:2945–2950. doi: 10.1016/j.febslet.2006.03.053. [DOI] [PubMed] [Google Scholar]
- 36.Freire T, Bay S, Vichier-Guerre S, Lo-Man R, Leclerc C. Carbohydrate antigens: Synthesis aspects and immunological applications in cancer. Mini-Rev Med Chem. 2006;6:1357–1373. doi: 10.2174/138955706778992996. [DOI] [PubMed] [Google Scholar]
- 37.Roy R. New trends in carbohydrate-based vaccines. Drug Disc Today: Technologies. 2004;1:327–336. doi: 10.1016/j.ddtec.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 38.Liakatos A, Kunz H. Synthetic glycopeptides for the development of cancer vaccines. Curr Opin Mol Therap. 2007;9:35–44. [PubMed] [Google Scholar]
- 39.Livingston PO, Ragupathi G. Carbohydrate vaccines that induce antibodies against cancer. 2. Previous experience and future plans. Cancer Immunol Immunother. 1997;45:10–19. doi: 10.1007/s002620050395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Germain RN. MHC-dependent antigen processing and peptide presentation: Providing ligands for T lymphocyte activation. Cell. 1994;76:287–299. doi: 10.1016/0092-8674(94)90336-0. [DOI] [PubMed] [Google Scholar]
- 41.Dintzis HM, Dintzis RZ, Vogelstein B. Molecular determinants of immunogenicity: The immuno model of immune response. Proc Nat Acad Sci USA. 1976;73:3671–3675. doi: 10.1073/pnas.73.10.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Denis J, Majeau N, Acosta-Ramirez E, Savard C, Bedard M-C, Simard S, Lecours K, Bolduc M, Pare C, Willems B, Shoukry N, Tessier P, Lacasse P, Lamarre A, Lapointe R, Lopez Macias C, Leclerc D. Immunogenicity of papaya mosaic virus-like particles fused to a hepatitis C virus epitope: Evidence for the critical function of multimerization. Virology. 2007;363:59–68. doi: 10.1016/j.virol.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 43.Jegerlehner A, Storni T, Lipowsky G, Schmid M, Pumpens P, Bachmann MF. Regulation of IgG antibody responses by epitope density and CD21-mediated costimulation. Eur J Immunol. 2002;32:3305–3314. doi: 10.1002/1521-4141(200211)32:11<3305::AID-IMMU3305>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 44.Bachmann MF, Hengartner H, Zinkernagel RM. T helper cell-independent neutralizing B cell response against vesicular stomatitis virus: Role of antigen patterns in B cell induction? Eur J Immunol. 1995;25:3445–3451. doi: 10.1002/eji.1830251236. [DOI] [PubMed] [Google Scholar]
- 45.Bachmann MF, Zinkernagel RM. Neutralizing antiviral B cell responses. Annu Rev Immunol. 1997;15:235–270. doi: 10.1146/annurev.immunol.15.1.235. [DOI] [PubMed] [Google Scholar]
- 46.Bachmann MF, Rohrer UH, Kundig TM, Burki K, Hengartner H, Zinkernagel RM. The influence of antigen organization on B cell responsiveness. Science. 1993;262:1448–1451. doi: 10.1126/science.8248784. [DOI] [PubMed] [Google Scholar]
- 47.Germain RN, Margulies DH. The biochemistry and cell biology of antigen processing and presentation. Annu Rev Immunol. 1993;11:403–50. doi: 10.1146/annurev.iy.11.040193.002155. [DOI] [PubMed] [Google Scholar]
- 48.Haurum JS, Arsequell G, Lellouch AC, Wong SY, Dwek RA, McMichael AJ, Elliott T. Recognition of carbohydrate by major histocompatibility complex class I-restricted, glycopeptide-specific cytotoxic t lymphocytes. J Exp Med. 1994;180:739–44. doi: 10.1084/jem.180.2.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abdel-Motal UM, Berg L, Rosen A, Bengtsson M, Thorpe CJ, Kihlberg J, Dahmen J, Magnusson G, Karlsson KA, Jondal M. Immunization with glycosylated Kb-binding peptides generates carbohydrate-specific, unrestricted cytotoxic t cells. Eur J Immunol. 1996;26:544–451. doi: 10.1002/eji.1830260307. [DOI] [PubMed] [Google Scholar]
- 50.Speir JA, Abdel-Motal UM, Jondal M, Wilson IA. Crystal structure of an MHC class I presented glycopeptide that generates carbohydrate-specific CTL. Immunity. 1999;10:51–61. doi: 10.1016/s1074-7613(00)80006-0. [DOI] [PubMed] [Google Scholar]
- 51.Dengjel J, Rammensee HG, Stevanovic S. Glycan side chains on naturally presented MHC class II ligands. J Mass Spectrom. 2005;40:100–4. doi: 10.1002/jms.780. [DOI] [PubMed] [Google Scholar]
- 52.Dudler T, Altmann F, Carballido JM, Blaser K. Carbohydrate-dependent, HLA class II-restricted, human T cell response to the bee venom allergen phospholipase A2 in allergic patients. Eur J Immunol. 1995;25:538–42. doi: 10.1002/eji.1830250235. [DOI] [PubMed] [Google Scholar]
- 53.Ando S, Yu RK, Scarsdale JN, Kusunoki S, Prestegard JH. High resolution proton NMR studies of gangliosides. Structure of two types of GD3 lactones and their reactivity with monoclonal antibody R24. J Biol Chem. 1989;264:3478–83. [PubMed] [Google Scholar]
- 54.Ritter G, Boosfeld E, Calves MJ, Oettgen HF, Old LJ, Livingston PO. Antibody response to immunization with purified GD3 ganglioside and GD3 derivatives (lactones, amide and gangliosidol) in the mouse. Immunobiology. 1990;182:32–43. doi: 10.1016/S0171-2985(11)80581-4. [DOI] [PubMed] [Google Scholar]
- 55.Ritter G, Boosfeld E, Adluri R, Calves M, Oettgen HF, Old LJ, Livingston P. Antibody response to immunization with ganglioside GD3 and GD3 congeners (lactones, amide and gangliosidol) in patients with malignant melanoma. Int J Cancer. 1991;48:379–385. doi: 10.1002/ijc.2910480312. [DOI] [PubMed] [Google Scholar]
- 56.Ragupathi G, Meyers M, Adluri S, Howard L, Musselli C, Livingston PO. Induction of antibodies against GD3 ganglioside in melanoma patients by vaccination with GD3-lactone-klh conjugate plus immunological adjuvant QS-21. Int J Cancer. 2000;85:659–666. doi: 10.1002/(sici)1097-0215(20000301)85:5<659::aid-ijc11>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 57.Kawashima I, Kotani M, Ozawa H, Suzuki M, Tai T. Generation of monoclonal antibodies specific for ganglioside lactones: Evidence of the expression of lactone on human melanoma cells. Int J Cancer. 1994;58:263–8. doi: 10.1002/ijc.2910580220. [DOI] [PubMed] [Google Scholar]
- 58.Maggio B, Ariga T, Yu RK. Ganglioside GD3 lactones: Polar head group mediated control of the intermolecular organization. Biochemistry. 1990;29:8729–34. doi: 10.1021/bi00489a032. [DOI] [PubMed] [Google Scholar]
- 59.Ding K, Rosen A, Ray AK, Magnusson G. Anti-GM3-lactam monoclonal antibodies of the IgG type recognize natural GM3-ganglioside lactone but not GM3-ganglioside. Glycoconjugate J. 1992;9:303–6. doi: 10.1007/BF00731090. [DOI] [PubMed] [Google Scholar]
- 60.Arcangeli A, Toma L, Contiero L, Crociani O, Legnani L, Lunghi C, Nesti E, Moneti G, Richichi B, Nativi C. Stable GM3 lactone mimetic raises antibodies specific for the antigens expressed on melanoma cells. Bioconjugate Chem. 2010;21:1432–1438. doi: 10.1021/bc900557v. [DOI] [PubMed] [Google Scholar]
- 61.Kohla G, Stockfleth E, Schauer R. Gangliosides with O-acetylated sialic acids in tumors of neuroectodermal origin. Neurochem Res. 2002;27:583–592. doi: 10.1023/a:1020211714104. [DOI] [PubMed] [Google Scholar]
- 62.Cheresh DA, Reisfeld RA, Varki AP. O-Acetylation of disialoganglioside GD3 by human melanoma cells creates a unique antigenic determinant. Science. 1984;225:844–6. doi: 10.1126/science.6206564. [DOI] [PubMed] [Google Scholar]
- 63.Ritter G, Ritter-Boosfeld E, Adluri R, Calves M, Ren S, Yu RK, Oettgen HF, Old LJ, Livingston PO. Analysis of the antibody response to immunization with purified O-acetyl GD3 gangliosides in patients with malignant melanoma. Int J Cancer. 1995;62:668–672. doi: 10.1002/ijc.2910620604. [DOI] [PubMed] [Google Scholar]
- 64.Schauer R. Achievements and challenges of sialic acid research. Glycoconjugate J. 2000;17:485–499. doi: 10.1023/A:1011062223612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Irie A, Koyama S, Kozutsumi Y, Kawasaki T, Suzuki A. The molecular basis for the absence of N-glycolylneuraminic acid in humans. J Biol Chem. 1998;273:15866–71. doi: 10.1074/jbc.273.25.15866. [DOI] [PubMed] [Google Scholar]
- 66.Malykh YN, Schauer R, Shaw L. N-Glycolylneuraminic acid in human tumours. Biochimie. 2001;83:623–34. doi: 10.1016/s0300-9084(01)01303-7. [DOI] [PubMed] [Google Scholar]
- 67.de Leon J, Fernandez A, Mesa C, Clavel M, Fernandez LE. Role of tumour-associated N-glycolylated variant of GM3 ganglioside in cancer progression: Effect over CD4 expression on T cells. Cancer Immunol Immunother. 2006;55:443–450. doi: 10.1007/s00262-005-0041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakarai H, Chandler PJ, Kano K, Morton DL, Irie RF. Hanganutziu-deicher antigen as a possible target for immunotherapy of melanoma. Int Arch Allergy Appl Immunol. 1990;91:323–328. doi: 10.1159/000235135. [DOI] [PubMed] [Google Scholar]
- 69.Fernandez LE, Alonso DF, Gomez DE, Vazquez AM. Ganglioside-based vaccines and anti-idiotype antibodies for active immunotherapy against cancer. Expert Rev Vaccines. 2003;2:817–823. doi: 10.1586/14760584.2.6.817. [DOI] [PubMed] [Google Scholar]
- 70.Osorio M, Gracia E, Rodriguez E, Saurez G, Arango Mdel C, Noris E, Torriella A, Joan A, Gomez E, Anasagasti L, Gonzalez JL, Melgares Mde L, Torres I, Gonzalez J, Alonso D, Rengifo E, Carr A, Perez R, Fernandez LE. Heterophilic NeuGcGM3 ganglioside cancer vaccine in advanced melanoma patients: Results of a phase Ib/IIa study. Cancer Biol Ther. 2008;7:488–95. doi: 10.4161/cbt.7.4.5476. [DOI] [PubMed] [Google Scholar]
- 71.Weïwer M, Huang F, Chen C-C, Yuan X, Tokuzaki K, Tomiyama H, Linhardt RJ. Synthesis and evaluation of anticancer vaccine candidates, c-glycoside analogs of STn and PSA. In: Chen X, Halcomb RL, Wang PG, editors. Chemical glycobiology. American Chemical Society; Washington, DC: 2008. pp. 216–238. [Google Scholar]
- 72.Bundle DR, Rich JR, Jacques S, Yu HN, Nitz M, Ling C-C. Thiooligosaccharide conjugate vaccines evoke antibodies specific for native antigens. Angew Chem Int Ed. 2005;44:7725–7729. doi: 10.1002/anie.200502179. [DOI] [PubMed] [Google Scholar]
- 73.Kuberan B, Sikkander SA, Tomiyama H, Linhardt RJ. Synthesis of a C-glycoside analogue of STn: An HIV- and tumor-associated antigen. Angew Chem Int Ed. 2003;42:2073–5. doi: 10.1002/anie.200351099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang F, Zheng XJ, Huo CX, Wang Y, Zhang Y, Ye XS. Enhancement of the immunogenicity of synthetic carbohydrate vaccines by chemical modifications of stn antigen. ACS Chem Biol. 2011;6:252–259. doi: 10.1021/cb100287q. [DOI] [PubMed] [Google Scholar]
- 75.Hoffmann-Röder A, Kaiser A, Wagner S, Gaidzik N, Kowalczyk D, Westerlind U, Gerlitzki B, Schmitt E, Kunz H. Synthetic antitumor vaccines from tetanus toxoid conjugates of MUC1 glycopeptides with the Thomsen-Friedenreich antigen and a fluorine-substituted analogue. Angew Chem Int Ed. 2010;49:8498–8503. doi: 10.1002/anie.201003810. [DOI] [PubMed] [Google Scholar]
- 76.Kayser H, Zeitler R, Kannicht C, Grunow D, Nuck R, Reutter W. Biosynthesis of a nonphysiological sialic acid in different rat organs, using N-propanoyl-D-hexosamines as precursors. J Biol Chem. 1992;267:16934–16938. [PubMed] [Google Scholar]
- 77.Lemieux GA, Bertozzi CR. Modulating cell surface immunoreactivity by metabolic induction of unnatural carbohydrate antigens. Chem Biol. 2001;8:265–275. doi: 10.1016/s1074-5521(01)00008-4. [DOI] [PubMed] [Google Scholar]
- 78.Liu T, Guo Z, Yang Q, Sad S, Jennings HJ. Biochemical engineering of surface a2–8 polysialic acid for immunotargeting tumor cells. J Biol Chem. 2000;275:32832–32836. doi: 10.1074/jbc.C000573200. [DOI] [PubMed] [Google Scholar]
- 79.Zou W, Borrelli S, Gilbert M, Liu T, Pon RA, Jennings HJ. Bioengineering of surface GD3 ganglioside for immunotargeting human melanoma cells. J Biol Chem. 2004;279:25390–25399. doi: 10.1074/jbc.M402787200. [DOI] [PubMed] [Google Scholar]
- 80.Pan Y, Chefalo P, Nagy N, Harding C, Guo Z. Synthesis and immunological properties of N-modified GM3 antigens as therapeutic cancer vaccines. J Med Chem. 2005;48:875–883. doi: 10.1021/jm0494422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chefalo P, Pan Y, Nagy N, Guo Z, Harding CV. Efficient metabolic engineering of GM3 on tumor cells by N-phenylacetyl-D-mannosamine. Biochemistry. 2006;45:3733–3739. doi: 10.1021/bi052161r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang Q, Zhang J, Guo Z. Efficient glycoengineering of GM3 on melanoma cell and monoclonal antibody-mediated selective killing of the glycoengineered cancer cell. Bioorg Med Chem. 2007;15:7561–7567. doi: 10.1016/j.bmc.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yu S, Wang Q, Zhang J, Wu Q, Guo Z. Synthesis and evaluation of protein conjugates of GM3 derivatives carrying modified sialic acids as highly immunogenic cancer vaccine candidates. Medchemcomm. 2011;2:524–530. doi: 10.1039/C1MD00033K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang Q, Guo Z. Synthetic and immunological studies of STn derivatives carrying 5-N-(p-substituted phenylacetyl)sialic acid for the development of effective cancer vaccines. ACS Med Chem Lett. 2011;2:373–378. doi: 10.1021/ml100313d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lansteiner K, Chase MW. Experiments on transfer of cutaneous sensitivity to simple compounds. Proc Soc Exp Biol Med. 1942;49:688–690. [Google Scholar]
- 86.Bremer EG, Levery SB, Sonnino S, Ghidoni R, Canevari S, Kannagi R, Hakomori S. Characterization of a glycosphingolipid antigen defined by the monoclonal antibody MBr1 expressed in normal and neoplastic epithelial cells of human mammary gland. J Biol Chem. 1984;259:14773–14777. [PubMed] [Google Scholar]
- 87.Zhang S, Cordon-Cardo C, Zhang HS, Reuter VE, Adluri S, Hamilton WB, Lloyd KO, Livingston PO. Selection of tumor antigens as targets for immune attack using immunohistochemistry: I. Focus on gangliosides. Int J Cancer. 1997;73:42–9. doi: 10.1002/(sici)1097-0215(19970926)73:1<42::aid-ijc8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 88.Ragupathi G, Park TK, Zhang S, Kim IJ, Graber L, Adluri S, Lloyd KO, Danishefsky SJ, Livingston PO. Immunization of mice with a fully synthetic Globo H antigen results in antibodies against human cancer cells: A combined chemical—immunological approach to the fashioning of an anticancer vaccine. Angew Chem Int Ed. 1997;36:125–128. [Google Scholar]
- 89.Park TY, Kim IJ, Hu S, Bilodeau MT, Randolph JT, Kwon O, Danishefsky SJ. Total synthesis and proof of structure of a human breast tumor (Globo H) antigen. J Am Chem Soc. 1996;118:11488–11500. [Google Scholar]
- 90.Ragupathi G, Slovin SF, Adluri S, Sames D, Kim IJ, Kim HM, Spassova M, Bornmann WG, Lloyd KO, Scher HI, Livingston PO, Danishefsky SJ. A fully synthetic Globo H carbohydrate vaccine induces a focused humoral response in prostate cancer patients: A proof of principle. Angew Chem Int Ed. 1999;38:563–566. doi: 10.1002/(SICI)1521-3773(19990215)38:4<563::AID-ANIE563>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 91.Slovin SF, Ragupathi G, Adluri S, Ungers G, Terry K, Kim S, Spassova M, Bornmann WG, Fazzari M, Dantis L, Olkiewicz K, Lloyd KO, Livingston PO, Danishefsky SJ, Scher HI. Carbohydrate vaccines in cancer: Immunogenicity of a fully synthetic Globo H hexasaccharide conjugate in man. Proc Natl Acad Sci USA. 1999;96:5710–5715. doi: 10.1073/pnas.96.10.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gilewski T, Ragupathi G, Bhuta S, Williams LJ, Musselli C, Zhang X-F, Bencsath KP, Panageas KS, Chin J, Hudis CA, Norton L, Houghton AN, Livingston PO, Danishefsky SJ. Immunization of metastatic breast cancer patients with a fully synthetic Globo H conjugate: A phase I trial. Proc Nat Acad Sci USA. 2001;98:3270–3275. doi: 10.1073/pnas.051626298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Livingston P, Zhang S, Adluri S, Yao TJ, Graeber L, Ragupathi G, Helling F, Fleisher M. Tumor cell reactivity mediated by IgM antibodies in sera from melanoma patients vaccinated with GM2 ganglioside covalently linked to KLH is increased by IgG antibodies. Cancer Immunol Immunother. 1997;43:324–330. doi: 10.1007/s002620050340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kirkwood JM, Ibrahim JG, Sosman JA, Sondak VK, Agarwala SS, Ernstoff MS, Rao U. High-dose interferon alfa-2b significantly prolongs relapse-free and overall survival compared with the GM2-KLH/QS-21 vaccine in patients with resected stage IIb-III melanoma: Results of intergroup trial E1694/S9512/C509801. J Clin Oncol. 2001;19:2370–2380. doi: 10.1200/JCO.2001.19.9.2370. [DOI] [PubMed] [Google Scholar]
- 95.Eggermont AM, Suciu S, Rutkowski P, Marsden J, Testori A, Corrie P, Aamdal S, Ascierto PA, Spatz A, Group EM. Randomized phase III trial comparing postoperative adjuvant ganglioside GM2-KLH/QS-21 vaccination versus observation in stage II (T3-T4N0M0) melanoma: Final results of study EORTC 18961. J Clin Oncol. 2010;28(15_suppl):8505. [Google Scholar]
- 96.Itzkowitz SH. Blood group-related carbohydrate antigen expression in malignant and premalignant colonic neoplasms. J Cell Biochem Suppl. 1992;16G:97–101. doi: 10.1002/jcb.240501118. [DOI] [PubMed] [Google Scholar]
- 97.Fukushima K. Expression of Lewis(x), sialylated Lewis(x), Lewis(a), and sialylated Lewis(a) antigens in human lung carcinoma. Tohoku J Exp Med. 1991;163:17–30. doi: 10.1620/tjem.163.17. [DOI] [PubMed] [Google Scholar]
- 98.Larrain MI, Demichelis S, Crespo M, Lacunza E, Barbera A, Creton A, Terrier F, Segal-Eiras A, Croce MV. Breast cancer humoral immune response: Involvement of lewis y through the detection of circulating immune complexes and association with mucin 1 (MUC1) J Exp Clin Cancer Res. 2009;28:121. doi: 10.1186/1756-9966-28-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Myers RB, Srivastava S, Grizzle WE. Lewis y antigen as detected by the monoclonal antibody br96 is expressed strongly in prostatic adenocarcinoma. J Urol. 1995;153:1572–1574. [PubMed] [Google Scholar]
- 100.Yin BW, Finstad CL, Kitamura K, Federici MG, Welshinger M, Kudryashov V, Hoskins WJ, Welt S, Lloyd KO. Serological and immunochemical analysis of Lewis y (Ley) blood group antigen expression in epithelial ovarian cancer. Int J Cancer. 1996;65:406–12. doi: 10.1002/(SICI)1097-0215(19960208)65:4<406::AID-IJC2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 101.Federici MF, Kudryashov V, Saigo PE, Finstad CL, Lloyd KO. Selection of carbohydrate antigens in human epithelial ovarian cancers as targets for immunotherapy: Serous and mucinous tumors exhibit distinctive patterns of expression. Int J Cancer. 1999;81:193–8. doi: 10.1002/(sici)1097-0215(19990412)81:2<193::aid-ijc5>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 102.Buskas T, Li Y, Boons G-J. The immunogenicity of the tumor-associated antigen lewisy may be suppressed by a bifunctional cross-linker required for coupling to a carrier protein. Chem Eur J. 2004;10:3517–3524. doi: 10.1002/chem.200400074. [DOI] [PubMed] [Google Scholar]
- 103.Danishefsky SJ, Behar V, Randolph JT, Lloyd K. Application of the glycal assembly method to the concise synthesis of neoglycoconjugates of Ley and Leb blood group determinants and of H-type I and H-type II oligosaccharides. J Am Chem Soc. 1995;117:5701–5711. [Google Scholar]
- 104.Kudryashov V, Kim HM, Ragupathi G, Danishefsky SJ, Livingston PO, Lloyd KO. Immunogenicity of synthetic conjugates of Lewis(y) oligosaccharide with proteins in mice: Towards the design of anticancer vaccines. Cancer Immunol Immunother. 1998;45:281–286. doi: 10.1007/s002620050444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sabbatini PJ, Kudryashov V, Ragupathi G, Danishefsky SJ, Livingston PO, Bornmann W, Spassova M, Zatorski A, Spriggs D, Aghajanian C, Soignet S, Peyton M, O’Flaherty C, Curtin J, Lloyd KO. Immunization of ovarian cancer patients with a synthetic Lewis(y)-protein conjugate vaccine: A phase 1 trial. Int J Cancer. 2000;87:79–85. [PubMed] [Google Scholar]
- 106.Buskas T, Li Y, Boons GJ. Synthesis of a dimeric lewis antigen and the evaluation of the epitope specificity of antibodies elicited in mice. Chem Eur J. 2005;11:5457–67. doi: 10.1002/chem.200500412. [DOI] [PubMed] [Google Scholar]
- 107.Kim YS, Yuan M, Itzkowitz SH, Sun QB, Kaizu T, Palekar A, Trump BF, Hakomori S. Expression of Ley and extended Ley blood group-related antigens in human malignant, premalignant, and nonmalignant colonic tissues. Cancer Res. 1986;46:5985–5992. [PubMed] [Google Scholar]
- 108.Ragupathi G, Deshpande PP, Coltart DM, Kim HM, Williams LJ, Danishefsky SJ, Livingston PO. Constructing an adenocarcinoma vaccine: Immunization of mice with synthetic KH-1 nonasaccharide stimulates anti-KH-1 and anti-Le(y) antibodies. Int J Cancer. 2002;99:207–12. doi: 10.1002/ijc.10305. [DOI] [PubMed] [Google Scholar]
- 109.Macher BA, Pacuszka T, Mullin BR, Sweeley CC, Brady RO, Fishman PH. Isolation and identification of a fucose-containing ganglioside from bovine thyroid gland. Biochim Biophys Acta. 1979;588:35–43. doi: 10.1016/0304-4165(79)90368-4. [DOI] [PubMed] [Google Scholar]
- 110.Nilsson O, Brezicka FT, Holmgren J, Sorenson S, Svennerholm L, Yngvason F, Lindholm L. Detection of a ganglioside antigen associated with small cell lung carcinomas using monoclonal antibodies directed against fucosyl-GM1. Cancer Res. 1986;46:1403–1407. [PubMed] [Google Scholar]
- 111.Cappello S, Liu NX, Musselli C, Brezicka FT, Livingston PO, Ragupathi G. Immunization of mice with fucosyl-GM1 conjugated with keyhole limpet hemocyanin results in antibodies against human small-cell lung cancer cells. Cancer Immunol Immunother. 1999;48:483–92. doi: 10.1007/s002620050596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dickler MN, Ragupathi G, Liu NX, Musselli C, Martino DJ, Miller VA, Kris MG, Brezicka FT, Livingston PO, Grant SC. Immunogenicity of a fucosyl-GM1-keyhole limpet hemocyanin conjugate vaccine in patients with small cell lung cancer. Clin Cancer Res. 1999;5:2773–9. [PubMed] [Google Scholar]
- 113.Krug LM, Ragupathi G, Hood C, Kris MG, Miller VA, Allen JR, Keding SJ, Danishefsky SJ, Gomez J, Tyson L, Pizzo B, Baez V, Livingston PO. Vaccination of patients with small-cell lung cancer with synthetic fucosyl GM-1 conjugated to keyhole limpet hemocyanin. Clin Cancer Res. 2004;10:6094–100. doi: 10.1158/1078-0432.CCR-04-0482. [DOI] [PubMed] [Google Scholar]
- 114.Gendler SJ, Lancaster CA, Taylor-Papadimitriou J, Duhig T, Peat N, Burchell J, Pemberton L, Lalani EN, Wilson D. Molecular cloning and expression of human tumor-associated polymorphic epithelial mucin. J Biol Chem. 1990;265:15286–93. [PubMed] [Google Scholar]
- 115.Hanisch FG, Ninkovic T. Immunology of O-glycosylated proteins: Approaches to the design of a MUC1 glycopeptide-based tumor vaccine. Curr Protein Pept Sci. 2006;7:307–315. doi: 10.2174/138920306778018034. [DOI] [PubMed] [Google Scholar]
- 116.Gaidzik N, Kaiser A, Kowalczyk D, Westerlind U, Gerlitzki B, Sinn HP, Schmitt E, Kunz H. Synthetic antitumor vaccines containing MUC1 glycopeptides with two immunodominant domains-induction of a strong immune response against breast tumor tissues. Angew Chem Int Ed. 2011;50:9977–9981. doi: 10.1002/anie.201104529. and references cited therein. [DOI] [PubMed] [Google Scholar]
- 117.Sorensen AL, Reis CA, Tarp MA, Mandel U, Ramachandran K, Sankaranarayanan V, Schwientek T, Graham R, Taylor-Papadimitriou J, Hollingsworth MA, Burchell J, Clausen H. Chemoenzymatically synthesized multimeric Tn/STn MUC1 glycopeptides elicit cancer-specific anti-MUC1 antibody responses and override tolerance. Glycobiology. 2006;16:96–107. doi: 10.1093/glycob/cwj044. [DOI] [PubMed] [Google Scholar]
- 118.Kagan E, Ragupathi G, Yi SS, Reis CA, Gildersleeve J, Kahne D, Clausen H, Danishefsky SJ, Livingston PO. Comparison of antigen constructs and carrier molecules for augmenting the immunogenicity of the monosaccharide epithelial cancer antigen Tn. Cancer Immunol Immunother. 2005;54:424–430. doi: 10.1007/s00262-004-0584-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Julien S, Picco G, Sewell R, Vercoutter-Edouart AS, Tarp M, Miles D, Clausen H, Taylor-Papadimitriou J, Burchell JM. Sialyl-Tn vaccine induces antibody-mediated tumour protection in a relevant murine model. Br J Cancer. 2009;100:1746–1754. doi: 10.1038/sj.bjc.6605083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Slovin SF, Ragupathi G, Musselli C, Olkiewicz K, Verbel D, Kuduk SD, Schwarz JB, Sames D, Danishefsky S, Livingston PO, Scher HI. Fully synthetic carbohydrate-based vaccines in biochemically relapsed prostate cancer: Clinical trial results with a-N-acetylgalactosamine-O-serine/threonine conjugate vaccine. J Clin Oncol. 2003;21:4292–4298. doi: 10.1200/JCO.2003.04.112. [DOI] [PubMed] [Google Scholar]
- 121.Ragupathi G, Howard L, Cappello S, Koganty RR, Qiu D, Longenecker BM, Reddish MA, Lloyd KO, Livingston PO. Vaccines prepared with sialyl-Tn and sialyl-Tn trimers using the 4-(4-maleimidomethyl)cyclohexane-1-carboxyl hydrazide linker group result in optimal antibody titers against ovine submaxillary mucin and sialyl-Tn-positive tumor cells. Cancer Immunol Immunother. 1999;48:1–8. doi: 10.1007/s002620050542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Slovin SF, Ragupathi G, Musselli C, Fernandez C, Diani M, Verbel D, Danishefsky S, Livingston P, Scher HI. Thomsen-friedenreich (TF) antigen as a target for prostate cancer vaccine: Clinical trial results with TF cluster (c)-KLH plus QS21 conjugate vaccine in patients with biochemically relapsed prostate cancer. Cancer Immunol Immunother. 2005;54:694–702. doi: 10.1007/s00262-004-0598-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ragupathi G, Koide F, Sathyan N, Kagan E, Spassova M, Bornmann W, Gregor P, Reis CA, Clausen H, Danishefsky SJ, Livingston PO. A preclinical study comparing approaches for augmenting the immunogenicity of a heptavalent KLH-conjugate vaccine against epithelial cancers. Cancer Immunol Immunother. 2003;52:608–616. doi: 10.1007/s00262-003-0399-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sabbatini PJ, Ragupathi G, Hood C, Aghajanian CA, Juretzka M, Iasonos A, Hensley ML, Spassova MK, Ouerfelli O, Spriggs DR, Tew WP, Konner J, Clausen H, Abu Rustum N, Dansihefsky SJ, Livingston PO. Pilot study of a heptavalent vaccine-keyhole limpet hemocyanin conjugate plus QS21 in patients with epithelial ovarian, fallopian tube, or peritoneal cancer. Clin Cancer Res. 2007;13:4170–4177. doi: 10.1158/1078-0432.CCR-06-2949. [DOI] [PubMed] [Google Scholar]
- 125.Ragupathi G, Koide F, Livingston PO, Cho YS, Endo A, Wan Q, Spassova MK, Keding SJ, Allen J, Ouerfelli O, Wilson RM, Danishefsky SJ. Preparation and evaluation of unimolecular pentavalent and hexavalent antigenic constructs targeting prostate and breast cancer: A synthetic route to anticancer vaccine candidates. J Am Chem Soc. 2006;128:2715–2725. doi: 10.1021/ja057244+. [DOI] [PubMed] [Google Scholar]
- 126.Zhu J, Wan Q, Ragupathi G, George CM, Livingston PO, Danishefsky SJ. Biologics through chemistry: Total synthesis of a proposed dual-acting vaccine targeting ovarian cancer by orchestration of oligosaccharide and polypeptide domains. J Am Chem Soc. 2009;131:4151–4158. doi: 10.1021/ja810147j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lee D, Danishefsky SJ. “Biologic” Level structures through chemistry: A total synthesis of a unimolecular pentavalent MUC1 glycopeptide construct. Tetrahedron Lett. 2009;50:2167–2170. doi: 10.1016/j.tetlet.2009.02.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Goldman B, DeFrancesco L. The cancer vaccine roller coaster. Nature Biotech. 2009;27:129–139. doi: 10.1038/nbt0209-129. [DOI] [PubMed] [Google Scholar]
- 129.Geyer H, Wuhrer M, Kurokawa T, Geyer R. Characterization of keyhole limpet hemocyanin (KLH) glycans sharing a carbohydrate epitope with schistosoma mansoni glycoconjugates. Micron. 2004;35:105–106. doi: 10.1016/j.micron.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 130.Roldao A, Mellado MC, Castilho LR, Carrondo MJ, Alves PM. Virus-like particles in vaccine development. Expert Rev Vaccines. 2010;9:1149–1176. doi: 10.1586/erv.10.115. [DOI] [PubMed] [Google Scholar]
- 131.Lin T, Chen Z, Usha R, Stauffacher CV, Dai J-B, Schmidt T, Johnson JE. The refined crystal structure of cowpea mosaic virus at 2.8 Å resolution. Virology. 1999;265:20–34. doi: 10.1006/viro.1999.0038. [DOI] [PubMed] [Google Scholar]
- 132.Miermont A, Barnhill H, Strable E, Lu X, Wall KA, Wang Q, Finn MG, Huang X. Cowpea mosaic virus capsid: A promising carrier for the development of carbohydrate based antitumor vaccines. Chem Eur J. 2008;14:4939–4947. doi: 10.1002/chem.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gupta SS, Kuzelka J, Singh P, Lewis WG, Manchester M, Finn MG. Accelerated bioorthogonal conjugation: A practical method for the ligation of diverse functional molecules to a polyvalent virus scaffold. Bioconjugate Chem. 2005;16:1572–1579. doi: 10.1021/bc050147l. [DOI] [PubMed] [Google Scholar]
- 134.Chatterji A, Ochoa W, Shamieh L, Salakian SP, Wong SM, Clinton G, Ghosh P, Lin T, Johnson JE. Chemical conjugation of heterologous proteins on the surface of cowpea mosaic virus. Bioconjugate Chem. 2004;15:807–813. doi: 10.1021/bc0402888. [DOI] [PubMed] [Google Scholar]
- 135.Chatterji A, Ochoa WF, Paine M, Ratna BR, Johnson JE, Lin T. New addresses on an addressable virus nanoblock: Uniquely reactive lys residues on cowpea mosaic virus. Chem Biol. 2004;11:855–863. doi: 10.1016/j.chembiol.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 136.Kaltgrad E, Sen Gupta S, Punna S, Huang CY, Chang A, Wong CH, Finn MG, Blixt O. Anti-carbohydrate antibodies elicited by polyvalent display on a viral scaffold. ChemBiochem. 2007;8:1455–1462. doi: 10.1002/cbic.200700225. [DOI] [PubMed] [Google Scholar]
- 137.Cobb BA, Kasper DL. Zwitterionic capsular polysaccharides: The new MHC II- dependent antigens. Cell Microbiol. 2005;7:1398–1403. doi: 10.1111/j.1462-5822.2005.00591.x. [DOI] [PubMed] [Google Scholar]
- 138.Tzianabos A, Wang JY, Kasper DL. Biological chemistry of immunomodulation by zwitterionic polysaccharides. Carbohydr Res. 2003;338:2531–8. doi: 10.1016/j.carres.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 139.Wang Q, McLoughlin RM, Cobb BA, Charrel-Dennis M, Zaleski KJ, Golenbock D, Tzianabos AO, Kasper DL. A bacterial carbohydrate links innate and adaptive responses through Toll-like receptor 2. J Exp Med. 2006;203:2853–63. doi: 10.1084/jem.20062008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.De Silva RA, Wang Q, Chidley T, Appulage DK, Andreana PR. Immunological response from an entirely carbohydrate antigen: Design of synthetic vaccines based on Tn-PSA1 conjugates. J Am Chem Soc. 2009;131:9622–9623. doi: 10.1021/ja902607a. [DOI] [PubMed] [Google Scholar]
- 141.Herzenberg LA, Tokuhisa T, Herzenberg LA. Carrier-priming leads to hapten-specific suppression. Nature. 1980;285:664–667. doi: 10.1038/285664a0. [DOI] [PubMed] [Google Scholar]
- 142.Schutze MP, Leclerc C, Jolivet M, Audibert F, Chedid L. Carrier-induced epitopic suppression, a major issue for future synthetic vaccines. J Immunol. 1985;135:2319–2322. [PubMed] [Google Scholar]
- 143.Posnett DN, McGrath H, Tam JP. A novel method for producing anti-peptide antibodies. Production of site-specific antibodies to the T cell antigen receptor beta-chain. J Biol Chem. 1988;263:1719–1725. [PubMed] [Google Scholar]
- 144.Bay S, Lo-Man R, Osinaga E, Nakada H, Leclerc C, Cantacuzene D. Preparation of a multiple antigen glycopeptide (MAG) carrying the Tn antigen. A possible approach to a synthetic carbohydrate vaccine. J Pept Res. 1997;49:620–625. doi: 10.1111/j.1399-3011.1997.tb01171.x. [DOI] [PubMed] [Google Scholar]
- 145.Lo-Man R, Vichier-Guerre S, Bay S, Deriaud E, Cantacuzene D, Leclerc C. Anti-tumor immunity provided by a synthetic multiple antigenic glycopeptide displaying a tri-Tn glycotope. J Immunol. 2001;166:2849–2854. doi: 10.4049/jimmunol.166.4.2849. [DOI] [PubMed] [Google Scholar]
- 146.Lo-Man R, Vichier-Guerre S, Perraut R, Deriaud E, Huteau V, BenMohamed L, Diop OM, Livingston PO, Bay S, Leclerc C. A fully synthetic therapeutic vaccine candidate targeting carcinoma-associated Tn carbohydrate antigen induces tumor-specific antibodies in nonhuman primates. Cancer Res. 2004;64:4987–4994. doi: 10.1158/0008-5472.CAN-04-0252. [DOI] [PubMed] [Google Scholar]
- 147.Freire T, Zhang X, Deriaud E, Ganneau C, Vichier-Guerre S, Azria E, Launay O, Lo-Man R, Bay S, Leclerc C. Glycosidic Tn-based vaccines targeting dermal dendritic cells favor germinal center B-cell development and potent antibody response in the absence of adjuvant. Blood. 2010;116:3526–3536. doi: 10.1182/blood-2010-04-279133. [DOI] [PubMed] [Google Scholar]
- 148.Deres K, Schild H, Wiesmuller KH, Jung G, Rammensee HG. In vivo priming of virus-specific cytotoxic T lymphocytes with synthetic lipopeptide vaccine. Nature. 1989;342:561–564. doi: 10.1038/342561a0. [DOI] [PubMed] [Google Scholar]
- 149.Toyokuni T, Dean B, Cai S, Boivin D, Hakomori S, Singhal AK. Synthetic vaccines: Synthesis of a dimeric Tn antigen-lipopeptide conjugate that elicits immune responses against Tn-expressing glycoproteins. J Am Chem Soc. 1994;116:395–396. [Google Scholar]
- 150.Wang Q, Zhou Z, Tang S, Guo Z. Carbohydrate-monophosphoryl lipid a conjugates are fully synthetic self-adjuvanting cancer vaccines eliciting robust immune responses in the mouse. ACS Chem Biol. 2012 doi: 10.1021/cb200358r. published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Buskas T, Ingale S, Boons G-J. Towards a fully synthetic carbohydrate-based anticancer vaccine: Synthesis and immunological evaluation of a lipidated glycopeptide containing the tumor-associated tn antigen. Angew Chem Int Ed. 2005;44:5985–5988. doi: 10.1002/anie.200501818. [DOI] [PubMed] [Google Scholar]
- 152.Ingale S, Wolfert MA, Gaekwad J, Buskas T, Boons G-J. Robust immune responses elicited by a fully synthetic three-component vaccine. Nature Chem Biol. 2007;3:663–667. doi: 10.1038/nchembio.2007.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ingale S, Wolfert MA, Buskas T, Boons G-J. Increasing the antigenicity of synthetic tumor-associated carbohydrate antigens by targeting Toll-like receptors. ChemBiochem. 2009;10:455–463. doi: 10.1002/cbic.200800596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sarkar S, Lombardo SA, Herner DN, Talan RS, Wall KA, Sucheck SJ. Synthesis of a single molecule L-rhamnose-containing three component vaccine and evaluation of antigenicity in the presence of anti-L-rhamnose antibodies. J Am Chem Soc. 2010;132:17236–17246. doi: 10.1021/ja107029z. [DOI] [PubMed] [Google Scholar]
- 155.Kaiser A, Gaidzik N, Becker T, Menge C, Groh K, Cai H, Li YM, Gerlitzki B, Schmitt E, Kunz H. Fully synthetic vaccines consisting of tumor-associated MUC1 glycopeptides and a lipopeptide ligand of the Toll-like receptor 2. Angew Chem Int Ed. 2010;49:3688–3692. doi: 10.1002/anie.201000462. [DOI] [PubMed] [Google Scholar]
- 156.Wilkinson BL, Malins LR, Chun CK, Payne RJ. Synthesis of MUC1-lipopeptide chimeras. Chem Commun. 2010;46:6249–6251. doi: 10.1039/c0cc01360a. [DOI] [PubMed] [Google Scholar]
- 157.Wilkinson BL, Day S, Malins LR, Apostolopoulos V, Payne RJ. Self-adjuvanting multicomponent cancer vaccine candidates combining per-glycosylated MUC1 glycopeptides and the Toll-like receptor 2 agonist Pam3CysSer. Angew Chem Int Ed. 2011;50:1635–1639. doi: 10.1002/anie.201006115. [DOI] [PubMed] [Google Scholar]
- 158.Renaudet O, BenMohamed L, Dasgupta G, Bettahi I, Dumy P. Towards a self-adjuvanting multivalent B and T cell epitope containing synthetic glycolipopeptide cancer vaccine. ChemMedChem. 2008;3:737–741. doi: 10.1002/cmdc.200700315. [DOI] [PubMed] [Google Scholar]
- 159.Bettahi I, Dasgupta G, Renaudet O, Chentoufi AA, Zhang X, Carpenter D, Yoon S, Dumy P, BenMohamed L. Antitumor activity of a self-adjuvanting glyco-lipopeptide vaccine bearing B cell, CD4+ and CD8+ T cell epitopes. Cancer Immunol Immunother. 2009;58:187–200. doi: 10.1007/s00262-008-0537-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Renaudet O, Dasgupta G, Bettahi I, Shi A, Nesburn AB, Dumy P, BenMohamed L. Linear and branched glyco-lipopeptide vaccines follow distinct cross-presentation pathways and generate different magnitudes of antitumor immunity. PLoS One. 2010;5:e11216. doi: 10.1371/journal.pone.0011216. [DOI] [PMC free article] [PubMed] [Google Scholar]