Abstract
In this multicenter, prospective study of 288 children (half under 2 years of age) undergoing cardiac surgery, we evaluated whether the measurement of pre- and postoperative serum cystatin C (CysC) improves the prediction of acute kidney injury (AKI) over that obtained by serum creatinine (SCr). Higher preoperative SCr-based estimated glomerular filtration rates predicted higher risk of the postoperative primary outcomes of stage 1 and 2 AKI (adjusted odds ratios (ORs) 1.5 and 1.9, respectively). Preoperative CysC was not associated with AKI. The highest quintile of postoperative (within 6 h) CysC predicted stage 1 and 2 AKI (adjusted ORs of 6 and 17.2, respectively). The highest tertile of percent change in CysC independently predicted AKI, whereas the highest tertile of SCr predicted stage 1 but not stage 2 AKI. Postoperative CysC levels independently predicted longer duration of ventilation and intensive care unit length of stay, whereas the postoperative SCr change only predicted longer intensive care unit stay. Thus, postoperative serum CysC is useful to risk-stratify patients for AKI treatment trials. More research, however, is needed to understand the relation between preoperative renal function and the risk of AKI.
Keywords: acute renal failure, cardiovascular, creatinine, epidemiology and outcomes, renal function
The development of acute kidney injury (AKI) is a risk factor for negative hospital outcomes in adults and children who undergo cardiac surgery.1–3 Intense contemporary research has been directed toward validating novel biomarkers to predict AKI earlier than is feasible with acute changes in serum creatinine (SCr).4–7 This is with the ultimate goal being to initiate potential AKI treatments in clinical trials and in clinical practice eventually.8 Currently, early AKI diagnosis may direct clinicians to apply timely conservative treatments such as avoidance of nephrotoxins and optimization of fluid status.
Early AKI diagnosis is an especially challenging task in children. In a previous single-centre retrospective study, we found that a substantial proportion of children display signs of AKI defined by acute SCr rise within hours after cardiac surgery and that most AKI occurs within the first 2–3 postoperative days.3 Therefore, preoperative and early postoperative clinical data and biological markers will offer the highest chance of capturing patients who will develop AKI or who are in the early stages of AKI. Some studies suggest that the presence of chronic kidney disease (CKD) is a risk factor for developing AKI in different settings,1,9,10 although this remains inconclusive in adults11,12 and has not been well studied in children.
Serum cystatin C (CysC) is a more accurate marker of glomerular filtration rate (GFR) than SCr in children with CKD.13,14 The benefit of using serum CysC for early AKI diagnosis in critically ill patients has been controversial.15 Because CysC is a marker of filtration, it is unclear whether it should be expressed as a percentage change from baseline levels, when measured postoperatively for AKI prediction (like SCr is), or whether raw concentration values should be used. In terms of preoperative AKI risk prediction in children, SCr concentrations are highly affected by multiple non-renal factors, including gender, age, growth, and muscle mass.14 Therefore CysC may offer a novel method for AKI risk stratification before cardiac surgery.
In this multicenter, prospective study of children undergoing cardiac surgery, we sought to compare the abilities of (1) preoperative SCr and CysC, and of (2) first postoperative serum CysC and SCr to predict cardiac surgery-associated AKI as well as secondary outcomes including length of stay and duration of mechanical ventilation. Because of lack of previous literature on CysC, we also evaluated different methods of expressing postoperative CysC for evaluating the ability of CysC to predict AKI.
RESULTS
Study population
Among 311 children in this study, 288 were available for analysis, after excluding subjects with missing biomarker data. Table 1 displays the baseline and outcome characteristics of the study cohort. Half the cohort was less than 2 years old and most subjects had normal preoperative estimated GFR (eGFR), with 27 subjects (9%) having preoperative eGFR below the 10th percentile of normative age-adjusted values (Figure 1). In all, 42% of the (n=288) cohort developed stage 1 AKI or worse (≥50% or 26.5 μmol/l SCr rise above baseline or need for dialysis) and 11% developed stage 2 AKI or worse (doubling of SCr or need for dialysis). All children who had a 26.5 mmol/l rise in SCr also had a ≥50% SCr rise and all patients who had a ≥50% SCr rise also had a 26.5 μmol/l rise in SCr from baseline. Four of the five subjects requiring dialysis had a ≥50% SCr rise from baseline at the time of dialysis initiation.
Table 1.
Characteristics and outcomes of the study cohort (n=288)
| Categorical variables: n (%) Continuous variables: mean±s.d., median [interquartile range] | |
|---|---|
| Male gender | 159 (55%) |
| Age (years) | 3.8±4.5, 2.1 [5.0] |
| ≤2 Years old | 144 (50%) |
| Height/length (cm) | 90±33, 81 [46.5] |
| Percentile | 32±32, 20 [58] |
| Standard deviation score | −0.9±2.2, −0.8 [2.2] |
| Weight (kg) | 16.4±17.3, 10.5 [12.6] |
| Percentile | 29±33, 12 [54] |
| Standard deviation score | −1.1±2.0, −1.2 [2.4] |
| Study site | |
| Cincinnati, Ohio | 197 (68%) |
| Montreal, Quebec | 57 (20%) |
| New Haven, Connecticut | 34 (12%) |
| Operative characteristics | |
| RACHS-1 score ≥3a | 129 (45%) |
| Cardiopulmonary bypass timeb | |
| <60 min | 54 (19%) |
| 61–90 min | 80 (28%) |
| 91–120 min | 60 (21%) |
| 121–180 min | 65 (23%) |
| >180 min | 29 (10%) |
| Renal function | |
| Preoperative CysC (mg/dl) | 0.8±0.2, 0.73 [0.29] |
| Preoperative estimated GFR (ml/min per 1.73 m2)c | 90.5±25.4, 87.8 [31.6] |
| Preoperative estimated GFR percentilec | 52.7±34, 56 [66] |
| First postoperative markers | |
| Hours after surgery of 1st postoperative blood sampling | 0.6±1.3, 0.5 [0.33] |
| Cys C (mg/l) | 0.77±0.32, 0.69 [0.38] |
| % SCr change from baseline | 15±20, 16 [33] |
| % CysC change from baseline | −1±27, −7 [29] |
| AKI and other outcomes | |
| Stage 1 AKId | 121 (42%) |
| Stage 2 AKId | 47 (11%) |
| Dialysis | 5 (2%) |
| Length of intensive care unit stay (days) | 4.0±6.1, 2.0 [3.0] |
| Length of hospital stay (days) | 8.0±9.2, 5.0 [4.0] |
| Duration of mechanical ventilation (days) | 1.4±1.5, 1.0 [2.0] |
| Mortality | 4 (1.4%) |
Abbreviations: AKI, acute kidney injury; CysC, cystatin C; GFR, glomerular filtration rate; SCr, serum creatinine.
Risk Adjustment in Congenital Heart Surgery (RACHS)-1 score.
Four subjects who did not receive cardiopulmonary bypass were not included in these descriptive analyses.
Estimated GFR (glomerular filtration) was calculated by the Schwartz formula.18 For percentile estimated GFR, normative values from children with normal renal function was used for percentile derivation.22
Stage 1 AKI: ≥50% or 26.5 μmol/l SCr rise from baseline or need for dialysis; stage 2 AKI: ≥doubling of SCr from baseline or need for dialysis.
Figure 1. Plotted glomerular filtration rate (GFR) percentile values for subjects less than 2 years old.
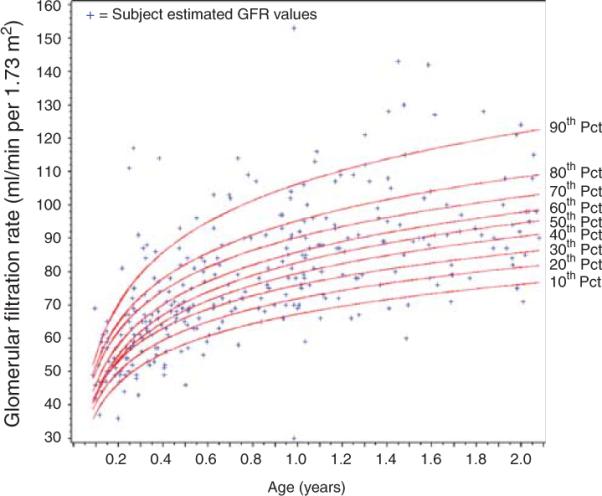
Displays the percentile curves for GFR derived from normal children from infancy to young adulthood. Individual preoperative estimated GFR (eGFR) of subjects from this study are plotted on the curves (+). To the right of the graph, the value for each percentile (Pct) curve shown.
Predicting AKI from preoperative renal function with serum creatinine and serum cystatin C
Table 2 displays the characteristics of different preoperative renal function measures for predicting postoperative stage 1 and 2 AKI in all subjects (expressed as odds ratios (OR) and area under the receiver operating characteristic curves (AUC)). In the whole cohort, stage 1 AKI or worse occurred on average 36±14 h and stage 2 AKI or worse occurred 41±12 h after the end of surgery. In unadjusted analyses, higher preoperative CysC predicted postoperative stage 1 and 2 AKI, but this association disappeared in adjusted analyses. There was no significant increase in AUC when CysC was added to the regression model to predict stage 1 or 2 AKI, compared with clinical variables alone (lower portion of Table 2). Pre-operative eGFR in ml/min per 1.73 m2 and in percentile value was significantly associated with development of postoperative stage 1 and 2 AKI in adjusted analyses (Table 2). There was a modest and statistically significant increase in AUC when preoperative eGFR was added to the predictive model, compared with using clinical variables alone (~5% AUC increase, P<0.05), Of note, the association between eGFR and AKI development was positive, indicating that higher preoperative eGFR was associated with an increased risk of AKI development.
Table 2.
Comparison of preoperative CysC and preoperative eGFR as predictors of postoperative stage 1 and stage 2 AKI
| Stage 1 AKI (#events=121) |
Stage 2 AKI (#events=47) |
|||
|---|---|---|---|---|
| Association of preoperative renal function with AKIa |
||||
| Unadjusted OR (95% CI) | Adjusted OR (95% CI)b | Unadjusted OR (95% CI) | Adjusted OR (95% CI)b | |
| Pre-operative CysC (mg/l) | 5.2 (2.2, 12.2) | 1.4 (0.5, 4.4) | 5.5 (1.9, 16.2) | 2.1 (0.5, 9.0) |
| Pre-operative eGFR (ml/min per 1.73 m2) | 1.2 (0.5, 2.6) | 8.4 (2.7, 25.9) | 2.9 (0.9, 9.0) | 34.9 (7, 174.3) |
| Pre-operative eGFR percentile | 1.3 (1.1, 1.7) | 1.5 (1.2, 2.0) | 1.6 (1.1, 2.3) | 1.9 (1.2, 3.0) |
| Area under receiver operating characteristic curvea |
||||
|---|---|---|---|---|
| Unadjusted AUC (95% CI) | Adjusted AUC (95% CI)b | Unadjusted AUC (95% CI) | Adjusted AUC (95% C)b | |
| Clinical variables onlyc | NA | 0.73 (0.67, 0.80) | NA | 0.79 (0.70, 0.87) |
| Pre-operative CysC (mg/l) | 0.63 (0.57, 0.70) | 0.74 (0.68, 0.80) | 0.62 (0.53, 0.72) | 0.79 (0.70, 0.87) |
| Pre-operative eGFR (ml/min per 1.73 m2) | 0.51 (0.44, 0.58) | 0.77 (0.71, 0.82)d | 0.59 (0.50, 0.68) | 0.84 (0.77, 0.92)d |
| Pre-operative eGFR percentile | 0.66 (0.59, 0.72) | 0.76 (0.70, 0.82)d | 0.68 (0.60, 0.77) | 0.82 (0.74, 0.90)d |
Abbreviations: AKI, acute kidney injury; AUC, area under the curve; CI, confidence interval; CysC, cystatin C; eGFR, estimated GFR; GFR, glomerular filtration rate; NA, not applicable; OR, odds ratio.
Note: preoperative CysC, eGFR, and eGFR percentile were transformed to their logarithm for inclusion in logistic regression analyses.
Adjusted for age, gender, Risk Adjustment in Congenital Heart Surgery (RACHS)-1 score, cardiopulmonary bypass time, and study site.
This model includes only clinical variables (age, gender, RACHS-1 score, cardiopulmonary bypass time, and study site), to determine how accurately AKI may be predicted without the use of biomarkers.
Adding preoperative eGFR to the clinical model led to a significant increase in AUC (P<0.05), compared with clinical variables alone.
Comparison of first postoperative biomarkers (serum creatinine and cystatin C) to predict AKI
Table 3 displays the ability of first postoperative CysC and SCr to predict stage 1 or 2 AKI in patients who did not already have these events (n=241 and n=267, respectively) within the 6 h after the end of surgery. In these subjects, stage 1 AKI occurred on average 43±12 h and stage 2 AKI occurred 48±7 h after surgery. First postoperative CysC concentration in the 5th quintile (1.01–2.91 mg/l) was independently associated with development of stage 1 AKI and of stage 2 AKI (shown in the upper portion of Table 3). First postoperative CysC ≥1.16 mg/l (the cutoff derived from a previously performed study),16 was also associated with future stage 1 AKI development (adjusted OR=6.4, 95% confidence interval (CI)=1.9–22.3) and stage 2 AKI (adjusted OR=20.0, 95% CI=4.1–97.5). When CysC was expressed as percent change from preoperative value, the highest tertile of percent CysC change was independently associated with development of stage 1 and 2 AKI (Table 3). Adding CysC concentration to the model to predict AKI including clinical variables alone resulted in an increase in AUC of 7% for predicting stage 1 AKI (P<0.01 for increase in AUC) and an ~5% increase in AUC for predicting stage 2 AKI (P=0.09).
Table 3.
Postoperative stage 1 and 2 AKI prediction with CysC and SCr, in subjects with no immediate postoperative AKI event
| Stage 1 AKIa (#events=79) |
Severe AKIa (#events=30) |
|||||
|---|---|---|---|---|---|---|
| Association of postoperative renal function with AKI |
||||||
| % With stage 1 AKIb | Unadjusted OR (95% CI) | Adjusted OR (95% CI)c | % With stage 2 AKIb | Unadjusted OR (95% CI) | Adjusted OR (95% CI)c | |
| CysC value d | ||||||
| 1st Quintile | 21 | 1 (Referent) | 1 (Referent) | 4 | 1 (Referent) | 1 (Referent) |
| 2nd Quintile | 13 | 0.5 (0.2, 1.6) | 0.5 (0.2, 1.8) | 6 | 1.5 (0.2, 9.4) | 1.7 (0.2, 13.8) |
| 3rd Quintile | 28 | 1.4 (0.6, 3.5) | 1.4 (0.4, 4.5) | 6 | 1.5 (0.2, 9.4) | 2.5 (0.2, 27.3) |
| 4th Quintile | 37 | 2.2 (0.9, 5.4) | 2.0 (0.6, 6.8) | 19 | 5.8 (1.2, 28.0) | 3.5 (0.4, 33.4) |
| 5th Quintile | 59 | 5.4 (2.2, 13.2) | 6.0 (1.5, 23.3) | 23 | 7.3 (1.6, 34.6) | 17.2 (1.6, 189.3) |
| % CysC change e | ||||||
| 1st Tertile | 24 | 1 (Referent) | 1 (Referent) | 9 | 1 (Referent) | 1 (Referent) |
| 2nd Tertile | 24 | 1.0 (0.5, 2.2) | 0.9 (0.4, 2.3) | 9 | 1.0 (0.4, 2.7) | 1.6 (0.4, 6.4) |
| 3rd Tertile | 47 | 2.9 (1.5, 5.8) | 4.4 (1.8, 10.8) | 16 | 1.8 (0.7, 4.6) | 5.7 (1.4, 24.0) |
| % SCr change f | ||||||
| 1st Tertile | 11 | 1 (Referent) | 1 (Referent) | 7 | 1 (Referent) | 1 (Referent) |
| 2nd Tertile | 25 | 2.7 (0.8, 9.7) | 3.1 (0.8, 11.9) | 7 | 1.0 (0.2, 4.7) | 0.6 (0.1, 3.2) |
| 3rd Tertile | 49 | 8.2 (2.3, 28.9) | 9.6 (2.4, 39.0) | 20 | 3.3 (0.7, 15.1) | 1.4 (0.3, 8.4) |
| Area under the receiver operating characteristic curve |
||||
|---|---|---|---|---|
| Unadjusted AUC (95% CI) | Adjusted AUC (95% CI)c | Unadjusted AUC (95% CI) | Adjusted AUC (95% CI)c | |
| Clinical variables onlyg | NA | 0.77 (0.70, 0.83) | NA | 0.85 (0.76, 0.93) |
| CysC value | 0.70 (0.63, 0.78) | 0.81 (0.75, 0.88) | 0.70 (0.60, 0.81) | 0.89 (0.81, 0.97) |
| % CysC change | 0.62 (0.54, 0.70) | 0.80 (0.73, 0.86) | 0.59 (0.48, 0.70) | 0.88 (0.80, 0.96) |
| % SCr change | 0.71 (0.63, 0.78) | 0.83 (0.77, 0.90) | 0.64 (0.53, 0.75) | 0.84 (0.76, 0.93) |
Abbreviations: AKI, acute kidney injury; CI, confidence interval; CysC, cystatin C; OR, odds ratio; SCr, serum creatinine.
Total subjects in analysis differ between the stage 1 and 2 AKI events, because only subjects without the event (stage 1 AKI on the left side of the Table or stage 2 AKI on the right side of the Table) immediately postoperatively were included. There were 241 patients in the stage 1 AKI prediction analyses and 267 patients in the stage 2 AKI prediction analyses.
% Patients who developed AKI (stage 1 AKI on the left side of the Table and stage 2 AKI on the right side of the Table) within each quintile or tertile (these should not add up to 100%).
Adjusted for age, gender, Risk Adjustment in Congenital Heart Surgery (RACHS)-1 score, preoperative estimated glomerular filtration rate, cardiopulmonary bypass time, and study site.
Quintile CysC concentrations (mg/dl) for stage 1 AKI event analyses (from 1st to 5th quintiles) were 0.31–0.53, 0.53–0.64, 0.64–0.77, 0.77–1.01, 1.01–2.91; for the stage 2 AKI analyses, 1st to 5th quintiles were 0.31–0.53,0.53–0.65, 0.65–0.77, 0.77–1.00,1.00–2.91. The number of patients per quintile for the stage 1 AKI analysis: Q1 n=47, Q2 n=48, Q3 n=47, Q4 n=46, Q5 n=49 and for the stage 2 AKI analysis: Q1 n=52, Q2 n=52, Q3 n=52, Q4 n=53, Q5 n=53.
Tertiles of % CysC rise for the stage 1 AKI event analyses were −50 to −15%, −15 to 5%, 5–143%; for the stage 2 AKI analyses, 1st to 3rd tertiles were −50 to −15%, −15 to 4%, 4–143%. The number of patients per tertile for the stage 1 AKI analysis: n=79 and for the stage 2 AKI analysis n=87 except for the 3rd tertile n=88.
Tertiles of SCr % change for stage 1 AKI analyses: −33 to 0%, 0–25%, 25–50%; for stage 2 AKI analyses: −33 to 0%, 0–33%, 33–85%. The number of patients per tertile in the stage 1 AKI analysis; T1 n=28, T2 n=118, T3 n=95 and for stage 2 AKI analysis; T1 n=28, T2 n=143, T3 n=96.
This model includes only clinical variables (age, gender, RACHS-1 score, cardiopulmonary bypass time, and study site), to determine how accurately AKI may be predicted without the use of biomarkers.
First postoperative percent SCr change was also independently associated with the development of stage 1 AKI, but not of stage 2 AKI (Table 3). When percent SCr change was added to the clinical variable predictive model, AUC increased by ~5% for predicting future stage 1 AKI (P<0.01), but by only ~2% for predicting stage 2 AKI (P=0.2). When time after surgery (hours) of first postoperative blood sample was controlled for in all analyses, ORs were nearly identical except confidence intervals (CIs) were wider.
Predicting length of stay and duration of mechanical ventilation
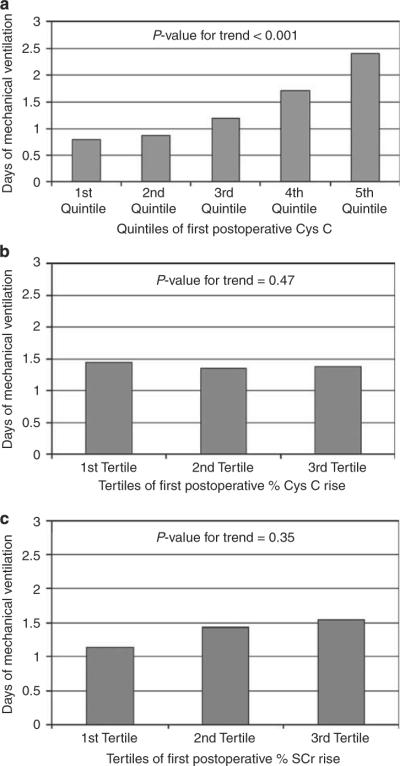
The associations between first postoperative CysC value (mg/dl), percent CysC change from baseline and percent SCr change from baseline and length of hospital or intensive care unit stay and duration of mechanical ventilation, were evaluated. In all, 288 (72%) of children were still mechanically ventilated at the time of first postoperative CysC and SCr measurement. Figure 2 displays that there was a statistically significant association between increasing first postoperative CysC value and longer duration of mechanical ventilation, in univariate analyses (P<0.001, Jonckheere-Terpstra test).
Figure 2. Association of postoperative serum creatinine (SCr) and cystatin C (CysC) with duration of ventilation.
Displays mean duration of mechanical ventilation (days) for the (a) quintiles of first postoperative CysC concentrations, (b) tertiles of first postoperative percent CystC change and (c) tertiles of first postoperative percent SCr change from baseline. Standard errors were extremely low (ranging from 0.01 to 0.04, and are not displayed). Univariate P-values for test of linear trend are displayed.
In multiple linear regression analyses, adjusting for clinical factors, only first postoperative percent CysC change from baseline (P = 0.04) and first postoperative CysC value (P<0.001), but not percent SCr change from baseline, were independently associated with longer duration of mechanical ventilation. Only first postoperative CysC value was independently associated with longer length of hospital stay (P = 0.04). First postoperative percent SCr change from baseline (P = 0.02) and first postoperative CysC value (P = 0.002), but not percent CysC change from baseline, were associated with longer length of intensive care unit stay.
DISCUSSION
In this multicenter study of children undergoing cardiac surgery, higher CysC concentrations obtained within the first 6 h of surgery strongly predicted stage 2 (more severe) AKI development in the subsequent days, as well as longer duration of mechanical ventilation and longer length of stay. We found that preoperative CysC did not predict the development of postoperative AKI. However, higher pre-operative eGFR and percent SCr change in the first 6 h after surgery predicted the development of postoperative stage 1 (or mild) AKI. This is the first study to directly compare two markers of GFR, SCr and CysC, for early prediction of AKI in children.
Preoperative CysC concentrations were not associated with development of postoperative AKI, when adjusted for other clinical factors, such as cardiopulmonary bypass time, demographics, and surgical severity score. Interestingly, we found that higher and not lower preoperative eGFR was associated with the development of AKI, even when we meticulously adjusted for age-associated differences in GFR by deriving GFR percentile values and adjusting for age in multivariate analyses. These two findings appear to refute our underlying hypothesis that preoperative CKD is a risk factor for AKI, as has been suggested by adult studies11 and in one study of AKI in children treated with aminoglycosides.17 A potential explanation is that children who are malnourished with lower muscle mass and consequently lower SCr concentrations (resulting in higher eGFR), are at increased risk for AKI development. This highlights the limitations of using SCr for GFR estimations. However, it is also possible that because few subjects had preoperative CKD, we may not have been able to detect a CKD–AKI association. Another possible confounding factor is that the Schwartz formula, which was derived from children greater than 2 years of age with established CKD,18 overestimates GFR, particularly in infants or children with fairly preserved renal function. This is supported by the fact that the median preoperative eGFR percentile value for children under 2 years old was the 81st percentile, compared with the 41st percentile in older children (shown in Figure 1, for children less than 2 years old), suggesting that eGFR is overestimated in younger children. We did not estimate GFR using published CysC equations, as none of them have been validated in infants. These results provide a serendipitous indicator that future studies should attempt to derive equations from SCr and CysC specific to younger children, as well as to children with GFR approaching the normal range, which may help better identify mild CKD as a risk factor for postoperative AKI. Finally, it is possible that the use of a SCr-based AKI definition is an imperfect, yet only available, reference standard, which is currently a challenge for all AKI studies.
From our previous work, we discovered that almost all postoperative cardiac surgery-associated AKI occurs within the first 3 days after surgery.3 Therefore, AKI therapeutic trials in this population will need to provide an intervention as early as possible during or after surgery. We found that postoperative serum CysC concentrations in the 5th quintile (>1.01 mg/dl), obtained very early after surgery, independently predicted development of stage 2 AKI. The cut-point of 1.16 mg/dl from a recent single-centre study was strongly associated with AKI severity.16 Thus, early postoperative CysC may be helpful for future clinical trials aimed at reducing the incidence of SCr doubling and dialysis need or other clinical outcomes such as duration of ventilation. We also found that clinical variables that are easily determinable immediately after surgery provided fairly good prediction of postoperative AKI development. It is likely that using a combination of clinical and early postoperative biomarker data will provide the best method for determining eligibility into future AKI clinical trials in children undergoing cardiac surgery, to optimize sensitivity and specificity.
Although we have demonstrated that CysC may have a role in early stage 2 AKI identification for trials, it is still unclear to what extent CysC should be used in clinical care. CysC currently costs significantly more to measure than SCr does, though cost will hopefully become less of a hindrance in time. Second, as we currently do not have specific treatments for AKI in children, whether or not incorporating CysC into clinical care will improve outcome will remain unknown until trials are performed. Therefore, we propose that future AKI clinical trials should incorporate measurement of CysC and cost analyses as outcomes. Trials examining currently available measures of managing AKI (such as early dialysis initiation or early goal-directed fluid management) should examine if knowledge of early postoperative CysC concentrations would lead to changes in outcomes such as duration of mechanical ventilation or management of fluid balance and oxygenation. The results of our study justify thorough evaluations of incorporating CysC into clinical care of this population.
Our study had several unique strengths. This is the first multicenter study of AKI and new AKI biomarkers in children, and to our knowledge the first study to examine specifically the role of preoperative renal function for AKI prediction in children. Because the timing of the most likely renal injurious event was known (cardiopulmonary bypass surgery), we were able to identify a clinically reproducible time point on which to evaluate CysC for early AKI prediction. Our use of eGFR percentiles for expressing preoperative renal function is novel and likely more valid for expressing renal function in younger children who are known to have an expected age- and development-associated lower raw eGFR value compared with older children. Another strength was our careful exclusion of subjects who already had clear evidence of AKI immediately postoperatively, when evaluating the ability of CysC to truly predict AKI earlier than SCr can. We evaluated doubling of SCr or need for dialysis (stage 2 AKI) as one of our primary outcomes. Thus, our findings will be useful for future clinical trials for AKI, which are likely to use a similar clinically meaningful outcome.
A limitation of this study was the somewhat restricted sample size, which did not allow us to adequately evaluate for effect modification and led to poor precision around some estimates, with wide CIs. Although the low prevalence of significant preoperative CKD was a limitation in being able to assess the CKD–AKI relation, our finding that few children having complex heart surgery have baseline CKD was important to demonstrate for future studies and understanding this population. Our study was limited to children undergoing cardiac surgery; therefore, the findings may not be immediately generalizable to all other hospitalized children and similarly rigorous studies in other non-cardiac pediatric populations are warranted.
In conclusion, the use of clinical risk factors may have an important role in predicting postoperative AKI in children who have had cardiac surgery. Early postoperative CysC is a useful marker to predict development of AKI but preoperative CysC does not contribute significantly. More research is required to better understand how to estimate preoperative renal function in young children and in children with GFR in the normal to mildly low range.
MATERIALS AND METHODS
Study design and study population
The Translational Research Investigating Biomarker Endpoints in AKI (TRIBE-AKI) study was a prospective USA–Canada, multi-center cohort study of patients undergoing cardiac surgery, with the objective to validate biomarkers of AKI. Three pediatric centers were included: Cincinnati Children's Hospital Medical Center (Cincinnati), Yale Children's Hospital (Yale), and Montreal Children's Hospital (Montreal). All children undergoing cardio-pulmonary bypass surgery for congenital cardiac surgery repairs were eligible. Patients with end stage renal disease or overt preoperative AKI (known SCr increase of ≥44.2 μmol/l from baseline before surgery) were excluded. For this analysis, we excluded subjects who were less than 1-month-old (due to challenges and controversies in defining AKI in the neonatal population19). We also excluded patients who did not have preoperative SCr, CysC, and height as well as postoperative SCr and CysC available. All centers obtained institutional internal review or research ethics board approval before study initiation and parental informed consent was obtained from all subjects.
Study procedure
Procedure aspects pertinent to this study are reported. Subjects were recruited preoperatively within 1 month of surgery. Blood samples were obtained during routine phlebotomy at the time of preoperative evaluation or on anesthesia induction, for SCr and CysC measurement. On admission to the intensive care unit, additional blood was drawn for CysC measurement within 6 h of surgery and was concurrent with the first planned routine blood testing. SCr was measured routinely by the treating team, from shortly after surgery (at the same time as the first postoperative CysC measurement) and every morning thereafter during intensive care unit and hospital stay. Blood for CysC measurement was centrifuged and the plasma aliquoted for storage at −80 °C until shipped for measurement. Each individual subject had pre- and postoperative SCr measured using the same SCr assay at the same laboratory. CysC was measured using a nephelo-meter (Siemens BN-II, Siemens, AG; www.Siemens.com) at the Cincinnati Children's Hospital Biomarker Laboratory (coefficient of variation=1.1%). Individuals performing the SCr and CysC assays were blinded to the clinical data.
Clinical data collection
The following clinical variables were collected: age, gender, study site, cardiopulmonary bypass time, and the Risk Adjustment in Congenital Heart Surgery (RACHS-1) score.20 RACHS-1 contains six categories designed to differentiate surgical risk for mortality on the basis of procedure. Height was recorded to calculate preoperative eGFR.
Pre-operative renal function assessment
One of our objectives was to evaluate the ability of preoperative renal function to predict postoperative AKI. Pre-operative renal function was assessed by preoperative CysC and SCr concentrations. Pre-operative CysC was expressed as actual concentration values (mg/dl). Pre-operative SCr was expressed as estimated GFR (eGFR, by the Schwartz formula 36.5×height in cm/SCr in μmol/l),18 because of the dependence of SCr concentrations on muscle mass. We noted a methodological challenge of eGFR interpretation in younger children. Children less than 2 years old have a physiologically lower GFR than older children,21 making it difficult to use raw eGFR as a risk factor of AKI when studying children aged 1 month to 18 years old. One previous study of children with normal renal function from ages 0.1 to 19 years old reported measurement of nuclear medicine scan GFR in 651 individuals.22 We generated percentile value GFR norms according to age, using the data provided by the corresponding author. We plotted our subjects' preoperative eGFR percentiles using these normative values (shown in Figure 1). To understand how the utilization of raw versus percentile preoperative eGFR values affected our results, we analyzed both methods of preoperative eGFR expression (eGFR in ml/min per 1.73 m2 and eGFR percentile value).
First postoperative SCr and CysC evaluation
Our goal was to provide a comparison of the ability of first postoperative SCr versus first postoperative CysC concentrations to predict development of the AKI outcomes, to elucidate to what extent utilizing CysC would lead to improved AKI prediction over the current standard biomarker, SCr. We expressed the first postoperative SCr value as a percentage change from preoperative concentrations (first postoperative SCr/preoperative SCr×100). Because there was little prior research on use and interpretation of CysC in the intensive care unit, we expressed the first postoperative CysC concentrations in 2 ways: (1) as raw first postoperative CysC concentration (mg/dl) and (2) as a percentage change from preoperative concentrations, similar to how SCr was expressed (first postoperative CysC/preoperative CysC×100). We also evaluated the presence of first postoperative CysC value of < versus ≥1.16 mg/dl, based on a significant postoperative CysC cutoff found to be predictive of postoperative cardiac surgery-associated AKI, in a previously published single-centre pediatric study.16
Definition of AKI
AKI was defined according to the Acute Kidney Injury Network (AKIN) staging.23 We evaluated two primary postoperative AKI outcomes: (1) `mild AKI' or stage 1 AKI according to the AKIN staging (≥50% SCr rise from baseline or a 26.5 mmol/l SCr rise from baseline or need for dialysis) and (2) `severe AKI' or stage 2 AKI according to the AKIN staging (doubling of SCr from baseline or need for dialysis).
Other outcomes
We evaluated length of intensive care unit and hospital stay and duration (days) of mechanical ventilation as secondary outcomes. There were only four deaths, therefore we could not evaluate mortality as an outcome.
Statistical analysis
Continuous variables were expressed as mean±standard deviation and categorical variables as proportions (n, %). To calculate preoperative eGFR percentiles a quantile regression model using intercept and log-transformed age, in children < and ≥2 years old, was fitted using data from the normal renal function pediatric patient study by Piepsz et al.22 The proc quantreg procedure of the SAS 9.2 statistical software (Cary, NC) was used for this analysis.
To evaluate the association between preoperative renal function (preoperative CysC, eGFR, and eGFR percentile) and postoperative stage 1 AKI or stage 2 AKI, multiple logistic regression was used, controlling for age (< or ≥2 years old), gender, RACHS-1 score (1 or 2 versus 3 versus 4), study site (as a fixed effect), and cardiopulmonary bypass time (≤60, 61–90, 91–120, 121–180, or ≥181 min). For these analyses, preoperative CysC, eGFR, and eGFR percentile were expressed in the logarithmic scale to allow for similar scaling between all three measures and to account for skewed distribution. For pre-op eGFR percentile, we added 1 to all values before logarithmic transformation as some percentile values were at or near zero.
To evaluate the association between first postoperative percent SCr change from baseline, percent CysC change from baseline or first postoperative CysC value and development of stage 1 or 2 AKI, we used multiple logistic regression, controlling for age (< or ≥2 years old), gender, RACHS-1 score (1 or 2 versus 3 versus 4), study site (as a fixed effect), cardiopulmonary bypass time (≤60, 61–90, 91–120, 121–180, or ≥181 min), as well as preoperative eGFR percentile. We also evaluated whether the time (hours) of first postoperative blood sampling affected results. For these analyses, we expressed first postoperative percent SCr change and percent CysC change from baseline into tertile categories and first postoperative CysC values into quintile categories to adjust for non-normal distribution of these values and also to enhance interpretation of the derived ORs. Of note, we only included subjects in these analyses who did not already have the AKI event on the first postoperative blood sampling to avoid overestimating the predictive association of CysC and already-diagnosable AKI. AUC for first postoperative percent SCr and CysC change from baseline and for first postoperative CysC value to predict stage 1 or 2 AKI was also calculated. All AUC's and OR's were expressed as point estimates and associated 95% CI. Differences in calculated AUC's were tested for significance using the test developed by DeLong et al.24
To evaluate the association between first postoperative SCr and CysC to predict length of stay and duration of mechanical ventilation, multiple linear regression was used, controlling for age (< or ≥2 years old), gender, RACHS-1 score (1 or 2 versus 3 versus 4), study site, cardiopulmonary bypass time (≤60, 61–90, 91–120, 121–180, or ≥181 min), preoperative eGFR percentile, and postoperative time (hours) of first postoperative blood sampling. Because of non-normal distribution of length of stay and of duration of mechanical ventilation, we log-transformed these variables for inclusion in regression analyses. Statistical software R version 2.10.1 and SAS 9.2 statistical software were used to perform all analyses.
ACKNOWLEDGMENTS
The study was supported by the NIH grant to CRP (RO1-HL085757). MZ received research support from the Kidney Research Core Education and National Training Program, the Montreal Children's Hospital Research Institute and the Fondation de Recherche en Sante du Quebec. AXG was supported by a Clinician Scientist Award from the Canadian Institutes of Health Research. We thank the study site research co-ordinators who meticulously performed the study procedures and data entry and the hospital staff for obtaining preoperative blood specimens. We sincerely thank A Piepsz (Brussels, Belgium) for sharing her data on nuclear medicine technology-measured GFR in children with normal kidney function, which we used to generate our GFR percentile values.
Footnotes
DISCLOSURE PD is a co-inventor on patents pertaining to neutrophil gelatinase-associated lipocalin for the diagnosis of renal failure, and is a consultant to Abbott Diagnostics and Biosite. CRP is a co-inventor on patents pertaining to IL-18 for AKI and consultant to Abbott Diagnostics.
REFERENCES
- 1.D'Onofrio A, Cruz D, Bolgan I, et al. RIFLE criteria for cardiac surgery-associated acute kidney injury: risk factors and outcomes. Congest Heart Fail. 2010;16(Suppl 1):S32–S36. doi: 10.1111/j.1751-7133.2010.00170.x. [DOI] [PubMed] [Google Scholar]
- 2.Li SY, Chen JY, Yang WC, et al. Acute kidney injury network classification predicts in-hospital and long-term mortality in patients undergoing elective coronary artery bypass grafting surgery. Eur J Cardiothorac Surg. 2011;39:323–328. doi: 10.1016/j.ejcts.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 3.Zappitelli M, Bernier PL, Saczkowski RS, et al. A small postoperative rise in serum creatinine predicts acute kidney injury in children undergoing cardiac surgery. Kidney Int. 2009;76:885–892. doi: 10.1038/ki.2009.270. [DOI] [PubMed] [Google Scholar]
- 4.Coca SG, Parikh CR. Urinary biomarkers for acute kidney injury: perspectives on translation. Clin J Am Soc Nephrol. 2008;3:481–490. doi: 10.2215/CJN.03520807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haase M, Bellomo R, Devarajan P, et al. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;54:1012–1024. doi: 10.1053/j.ajkd.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 6.Ichimura T, Hung CC, Yang SA, et al. Kidney injury molecule-1: a tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am J Physiol Renal Physiol. 2004;286:F552–F563. doi: 10.1152/ajprenal.00285.2002. [DOI] [PubMed] [Google Scholar]
- 7.Mishra J, Ma Q, Prada A, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534–2543. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 8.American Society of Nephrology American Society of Nephrology Renal Research Report. J Am Soc Nephrol. 2005;16:1886–1903. doi: 10.1681/ASN.2005030285. [DOI] [PubMed] [Google Scholar]
- 9.Pakfetrat M, Nikoo MH, Malekmakan L, et al. Comparison of risk factors for contrast-induced acute kidney injury between patients with and without diabetes. Hemodial Int. 2010;4:387–392. doi: 10.1111/j.1542-4758.2010.00469.x. [DOI] [PubMed] [Google Scholar]
- 10.Skott M, Norregaard R, Sorensen HB, et al. Pre-existing renal failure worsens the outcome after intestinal ischaemia and reperfusion in rats. Nephrol Dial Transplant. 2010;11:3509–3517. doi: 10.1093/ndt/gfq281. [DOI] [PubMed] [Google Scholar]
- 11.Singh P, Rifkin DE, Blantz RC. Chronic kidney disease: an inherent risk factor for acute kidney injury? Clin J Am Soc Nephrol. 2010;9:1690–1695. doi: 10.2215/CJN.00830110. [DOI] [PubMed] [Google Scholar]
- 12.Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 13.Zappitelli M, Parvex P, Joseph L, et al. Derivation and validation of cystatin C-based prediction equations for GFR in children. Am J Kidney Dis. 2006;48:221–230. doi: 10.1053/j.ajkd.2006.04.085. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz GJ, Work DF. Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol. 2009;4:1832–1843. doi: 10.2215/CJN.01640309. [DOI] [PubMed] [Google Scholar]
- 15.Bagshaw SM, Bellomo R. Cystatin C in acute kidney injury. Curr Opin Crit Care. 2010 doi: 10.1097/MCC.0b013e32833e8412. e-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 16.Krawczeski CD, Vandevoorde RG, Kathman T, et al. Serum cystatin C is an early predictive biomarker of acute kidney injury after pediatric cardiopulmonary bypass. Clin J Am Soc Nephrol. 2010;5:1552–1557. doi: 10.2215/CJN.02040310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zappitelli M, Moffett BS, Hyder A, et al. Acute kidney injury in non-critically ill children treated with aminoglycoside antibiotics in a tertiary healthcare centre: a retrospective cohort study. Nephrol Dial Transplant. 2011;1:144–150. doi: 10.1093/ndt/gfq375. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz GJ, Munoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Askenazi DJ, Griffin R, McGwin G, et al. Acute kidney injury is independently associated with mortality in very low birthweight infants: a matched case-control analysis. Pediatr Nephrol. 2009;24:991–997. doi: 10.1007/s00467-009-1133-x. [DOI] [PubMed] [Google Scholar]
- 20.Jenkins KJ, Gauvreau K, Newburger JW, et al. Consensus-based method for risk adjustment for surgery for congenital heart disease. J Thorac Cardiovasc Surg. 2002;123:110–118. doi: 10.1067/mtc.2002.119064. [DOI] [PubMed] [Google Scholar]
- 21.Atiyeh BA, Dabbagh SS, Gruskin AB. Evaluation of renal function during childhood. Pediatr Rev. 1996;17:175–180. doi: 10.1542/pir.17-5-175. [DOI] [PubMed] [Google Scholar]
- 22.Piepsz A, Tondeur M, Ham H. Revisiting normal (51)Crethylenediaminetetraacetic acid clearance values in children. Eur J Nucl Med Mol Imaging. 2006;33:1477–1482. doi: 10.1007/s00259-006-0179-2. [DOI] [PubMed] [Google Scholar]
- 23.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]