Abstract
BACKGROUND
The primary goal of this trial was to evaluate the confirmed response rate of temsirolimus (CCI-779), a mammalian target of rapamycin in patients with advanced soft tissue sarcomas (STS).
METHODS
Patients ≥18 years with measurable advanced STS, no prior chemotherapy for metastatic disease (adjuvant and neoadjuvant chemotherapy allowed), adequate organ function, and performance status of ≤2 were eligible. After premedication with an antihistamine, CCI-779 was given intravenously at 25 mg over 30 minutes on Days 1, 8, 15, and 22, repeated every 4 weeks. The primary endpoint was confirmed response rate per Response Evaluation Criteria in Solid Tumors.
RESULTS
Between June 2004 and November 2005, a total of 41 patients were enrolled and began treatment; 40 patients are evaluable for response and adverse events. The median age was 62 years (range, 28–72 years) with 56% women. Eighty percent had high-grade STS, and 22% had prior adjuvant chemotherapy. There were 2 patients (5%; 95% confidence interval [CI], 1–17) (undifferentiated fibrosarcoma and uterine leiomyosarcoma) who achieved a confirmed partial response lasting 3 and 17 months, respectively. Thirty-nine (95%) patients have progressed, with a median time to progression of 2.0 months (95% CI, 1.8–3.5). The median overall survival was 7.6 months (95% CI, 6.1–15.9). Forty-three percent experienced grade 3+ adverse events that were possibly related to therapy.
CONCLUSIONS
Temsirolimus in this patient population of STS had limited clinical activity and had moderate toxicities.
Keywords: soft tissue sarcoma, mammalian target of rapamycin, temsirolimus, toxicities
Soft tissue sarcomas (STS) are a heterogeneous group of cancers with various biologic activities. It is estimated that there will be 10,520 new cases of STS diagnosed in 2010, with an estimated mortality of 37%.1 Despite activity of certain agents for histologic-specific STS such as of imatinib and sunitinib for gastrointestinal stromal sarcomas2,3 or gemcitabine and docetaxel for uterine leiomyosarcoma,4 for the vast majority of the other STS, treatment with palliative doxorubicin- or ifosfamide-based therapy is toxic and has marginal activity.5,6
Mammalian target of rapamycin (mTOR) is a serine/threonine kinase that regulates numerous cellular functions. mTOR functions within 2 distinct signaling complexes, denoted as mTOR complex 1 (mTOR complex 1) and mTOR complex 2. In the presence of adequate nutrient and energy stores, mTOR complex 1 integrates signals from mitogenic signaling pathways and controls downstream signaling cascades that regulate translation of a subset of mRNAs with complex 5′ untranslated regions or 5′ polypyrimidine tracts. Many of these transcripts encode proteins involved in promoting cell proliferation, angiogenesis, and cell survival. Key downstream targets that modulate protein translation include eukaryotic initiation factor 4E and p70S6 kinase, the latter of which phosphorylates ribosomal S6 protein.7 Several potent inhibitors of mTOR complex 1 signaling have been developed, including sirolimus (rapamycin, Rapamune) and the related ester temsirolimus (CCI-779, sirolimus 42-ester with 2,2-bis[hydroxymethyl] propionic-acid). CCI-779 inhibition has growth inhibitory effects on a wide range of histologically diverse tumor cells, including STS.8,9 The primary goal of this phase 2 study was to determine confirmed response rate of CCI-779 in STS.
MATERIALS AND METHODS
Eligibility
Patients with histologically confirmed STS were eligible for the study. Patients had Eastern Cooperative Oncology Group (ECOG) performance status (PS) of ≤2, and life expectancies of ≥12 weeks. The inclusion criteria included: absolute neutrophil count (ANC) ≥1500/mm3, platelet count ≥100,000/mm3, hemoglobin ≥10.0 g/dL, direct bilirubin ≤1.5× the institutional upper limit of normal (ULN), aspartate aminotransferase ≤2.5× the institutional ULN or ≤5× the ULN if there were liver metastases, alanine aminotransferase ≤2.5× ULN or≤5× the ULN if there were liver metastases, creatinine ≤1.5× the institutional ULN (or creatinine clearance ≥50mL/min for patients with creatinine levels >1.5× institutional ULN), fasting serum cholesterol ≤350 mg/dL, fasting triglycerides ≤400 mg/dL, age ≥18 years, and negative pregnancy test for women of childbearing potential. All patients were required to have at least 1 lesion that could be accurately measured, with the longest diameter measuring ≥2.0 cm. Exclusion criteria included: chemotherapy for metastatic disease (exceptions: patients with gastrointestinal stromal tumors [GIST] who fail Gleevec are eligible; patients who have had adjuvant/neoadjuvant chemotherapy are also eligible), pregnancy or lactation, uncontrolled intercurrent illness, central nervous system metastases unless treated and stable symptoms for ≥1 month, history of allergic reactions attributed to compounds similar to temsirolimus (CCI779), and known human immunodeficiency virus-positive patients receiving combination antiretroviral therapy.
Treatment Administration and Evaluation
CCI-779 was administered intravenously at 25 mg over 30 minutes on Days 1, 8, 15, and 22, repeated every 4 weeks. Premedication with antihistamines was given intravenously 30 minutes before CCI-779. Doses were held for grade 3–4 hematologic and nonhematologic toxicities. Treatment was resumed at 5 mg dose reduction once the nonhematologic toxicity resolved to grade ≤2, ANC ≥1000/mm3, and platelets ≥75,000/mm3. A maximum of 3 dose modifications were allowed.
Baseline evaluations were done within 7 days of treatment, including history and physical, complete blood count (CBC), albumin, alkaline phosphatase, bicarbonate, blood urea nitrogen, calcium, chloride, creatinine, glucose, lactate dehydrogenase, phosphorus, potassium, total protein, aspartate aminotransferase, alanine aminotransferase, sodium, total and direct bilirubin, cholesterol, triglycerides, and pregnancy test. CBC were done weekly before treatment in addition to chemistry, cholesterol, and triglycerides done every other week. Adverse events were collected via National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0. Pre-treatment and post-treatment blood samples were processed to evaluate sirolimus blood levels and S6 phosphorylation to document inhibition of mTOR complex 1 signaling.
The study was approved by the institutional review board at each treating site. Toxicity stopping rules were in place. Specifically, if at any time 4 of the initial 20 patients or 20% of all patients experienced a grade 4 or 5 adverse event (at least possibly related to study treatment), then accrual to the study would have been halted for full review of the data by the study team.
Disease Assessment
Disease assessment by computed tomography scan or magnetic resonance imaging was performed within 21 days of registration. Tumor evaluations were done after 2 cycles of therapy and then every other cycle (ie, every 8 weeks). Tumor response was assessed using Response Evaluation Criteria in Solid Tumors (RECIST), with reevaluation every 8 weeks.10 Total disappearance of target lesions constituted a complete response (CR), whereas a minimum of a 30% decrease in the sum of the longest diameter of the target lesions was classified as a partial response (PR). New lesions or a 20% increase in the sum of the longest diameters of the target lesions were considered progressive disease (PD). Patients were re-evaluated for disease status 4 weeks after initial documentation of CR or PR to confirm the assessment. Similarly, stable disease (SD) was reassessed at a minimum interval of 8 weeks. Patients with global deterioration of health status requiring discontinuation of treatment without objective evidence of disease progression at that time, and not related to study treatment or other medical conditions, were considered to have PD because of symptomatic deterioration.
Duration of response was calculated from the first date of a patient’s objective status of either CR or PR to the date of PD (or last tumor assessment). Duration of SD was calculated from the date of registration to the date of PD (or last tumor assessment if no PD) for patients having achieved a best response of SD. Patients were censored for progression (survival) at their date of last assessment (last contact) if no progression (death) occurred. Time to PD was calculated from the date of registration to the date of PD. Survival or time to death was calculated from the date of registration to the date of death. All patients were followed until death or a maximum of 5 years after registration, whichever was earlier.
Laboratory Correlative Studies
Peripheral blood mononuclear cells (PBMC) were isolated from blood samples obtained before and 24 hours after the first infusion of CCI-779. Samples were processed as described in detail previously.11 Briefly, fresh PBMC were divided into aliquots, spiked with or without rapamycin, and then stimulated with phytohemagglutinin and phorbol 12-myristate 14-acetate before freezing. Frozen samples then were batch processed for Western blotting with phospho- and total-S6 antibodies. NIH Image J (http://rsbweb.nih.gov/ij/) was used to measure optical density of individual bands, and the ratio of phospho-S6 versus total S6 was calculated for each sample. The difference in the phospho-S6 intensity between the spiked and unspiked pretreatment blood sample was considered the dynamic range of S6 phosphorylation with and without effective mTOR complex 1 inhibition, respectively, and a 75% or greater reduction in this dynamic range in the post-treatment blood sample was considered effective inhibition of S6 phosphorylation. Blood samples for sirolimus serum levels 24 hours after treatment were determined in the Mayo Medical Laboratories.
Statistical Methods
The primary endpoint for this trial was the proportion of confirmed tumor responses. All eligible patients who have initiated study treatment and signed consent were considered evaluable for the primary endpoint. Confirmed tumor response to treatment is defined as a CR or PR on 2 consecutive evaluations, at least 4 weeks apart. The primary endpoint was estimated by the number of confirmed responses divided by the total number of evaluable patients. Five percent was the threshold used for clinical inactivity (Ho) in regard to confirmed tumor response rate, whereas an observed confirmed response rate of 20% was considered promising (Ha) in this population. To test these hypotheses and in recognition of the lack of beneficial treatments in this population, a single-stage phase 2 study design with a planned interim analysis was used; no suspension of accrual between stages was allowed unless there was excessive toxicity. Here, 2 confirmed responses within the initial 20 patients expanded enrollment to 50 patients. Six of 50 patients with confirmed tumor responses was considered evidence that this treatment could be recommended for further testing in subsequent studies in this patient population. This single-stage Fleming design12 yielded 91% power to detect a true confirmed response rate of at least 20%, at a .09 level of significance. Confidence intervals (CIs) for the primary endpoint were calculated by the method of Duffy and Santner.13
Summary statistics and frequency tables were used to summarize baseline patient characteristics and adverse events. Adverse events were reported as a maximum severity per patient and type, across all cycles of treatment. All attributions collected for adverse events were reported unless otherwise noted. The Kaplan-Meier14 method was used to estimate distributions of time to progression and time to death. All analyses were conducted using SAS version 9.0 (SAS Institute, Cary, NC).
Laboratory correlatives were investigated using graphical techniques and summary statistics (eg, mean, median). Serum sirolimus was measured post-treatment only, whereas all other correlates were measured at both pretreatment and post-treatment. Changes over time were assessed using percentage change from baseline, as well as categorical methods (eg, using frequency tables to identify changes in the patterns of phosphorylation intensity). Clinical characteristics (eg, sex, age) and patient outcome (eg, progression, death, response) were assessed relative to laboratory correlatives to look for possible associations (or lack thereof). These analyses were considered hypothesis generating in nature.
RESULTS
Demographics
Patient characteristics are presented in Table 1. Between June 2004 and November 2005, the study enrolled 41 patients from 4 sites (Mayo Clinic [n = 23], University of Wisconsin Comprehensive Cancer Center [n = 12], Washington University [n = 4], and Johns Hopkins [n = 2]). One patient did not return after receiving 1 dose of therapy. The median age was 62 years (range, 28–79), with 85% of patients having an ECOG performance status of 0 or 1. Seventy-two percent of patients presented with lung metastases. Eighty percent had high-grade STS based on local pathology review.
Table 1.
Patient Characteristics
| Characteristic | Frequency (%) |
|---|---|
| Age, median y (range) | 62 (28–79) |
| Sex, women | 23 (56) |
| ECOG performance status | |
| 0 | 15 (36) |
| 1 | 20 (49) |
| 2 | 6 (15) |
| Time from diagnosis to going on study, median mo (range) | 10.9 (0.9–137.1) |
| Distant metastasesa | |
| Nodal | 6 (19) |
| Subcutaneous | 2 (6) |
| Bone | 5 (16) |
| Lung | 23 (72) |
| Liver | 4 (13) |
| Abdominal | 5 (16) |
| Brain | 2 (6) |
| Otherb | 3 (9) |
| Histological type | |
| MFH | 8 (20) |
| Sarcoma, NOS | 9 (22) |
| Fibrosarcoma, NOS | 3 (7) |
| Myxosarcoma | 1 (2) |
| Liposarcoma, NOS | 5 (12) |
| Leiomyosarcoma | 9 (22) |
| Endometrial stromal sarcoma | 1 (2) |
| Synovial sarcoma | 1 (2) |
| Hemangiosarcoma/angiosarcoma | 2 (5) |
| Hemangiopericytoma, NOS | 1 (2) |
| Neurofibrosarcoma | 1 (2) |
| Sarcoma site | |
| Pelvis | 3 (8) |
| Head (skull, face) | 2 (5) |
| Extremities | 16 (40) |
| Intra-abdominal, NOS | 5 (13) |
| Genitourinary, NOS | 1 (2) |
| Vascular, NOS | 2 (5) |
| Cardiac, muscle | 1 (3) |
| Fallopian tube | 1 (3) |
| Uterus | 5 (13) |
| Skin | 1 (3) |
| Pulmonary, lung | 1 (3) |
| Truncal, chest wall | 2 (5) |
| Differentiation (grade) | |
| High (grade 3 or 4) | 33 (80) |
| Low (grade 1 or 2) | 8 (20) |
| Status of primary tumor site | |
| Resected with no residual | 13 (32) |
| Resected with known residual | 7 (17) |
| Unresected | 8 (19) |
| Recurrent | 13 (32) |
| Prior adjuvant therapy, yes | 9 (22) |
ECOG indicates Eastern Cooperative Oncology Group; MFH, malignant fibrous histiocytoma; NOS, not otherwise specified.
Overall, 13 (32%) patients had multiple metastasis sites.
Other sites included right chest wall in rib cage, adrenal mass, and left lower thoracic paraspinal mass.
Treatment Efficacy
Forty (98%) of 41 patients were considered evaluable for the primary endpoint of confirmed tumor response. Accrual was rapid, and at the time response data were available for the first 20 evaluable patients, only 1 patient achieved a confirmed PR. This failed to meet the criteria to complete full accrual of 50 patients; thus, the trial was closed at Patient 41. Overall, 2 (5%; 95% CI, 1–17) patients achieved a confirmed PR (Table 2). A 63-year-old man, with undifferentiated fibrosarcoma of the thigh and metastases to the lung, achieved a PR after 2 cycles of therapy, which was sustained for 17 months until progression in the lung at Cycle 19. He died 10 months later of his cancer. The other PR was a 42-year-old woman, with leiomyosarcoma of the uterus and metastases in the lymph nodes, bone, and lung, achieved a PR in Cycle 4. She had progression in the lymph nodes and lung in Cycle 7 (duration of response, 3 months). She died 22 months later of her cancer.
Table 2.
Patient Outcomes (N = 41)
| Outcome | Frequency/Estimate |
|---|---|
| Response ratea | 5% (95% CI, 1–17) |
| No. of responders (PR) | 2 |
| Time to response, median mo | 2.7 mo (range, 2–4) |
| Overall survival, median mob | 7.6 mo (95% CI, 6.1–15.9) |
| 6 months | 66% (95% CI, 53–82) |
| 12 months | 39% (95% CI, 27–57) |
| 18 months | 34% (95% CI, 22–52) |
| Time to disease progression, median mob | 2.0 (95% CI, 1.8–3.5) |
| 2 months | 54% (95% CI, 41–72) |
| 3 months | 41% (95% CI, 28–60) |
| 4 months | 22% (95% CI, 12–40) |
| 6 months | 13% (95% CI, 6–31) |
CI indicates confidence interval; PR, partial response.
n = 40; response sustained for at least 2 consecutive evaluations.
Kaplan-Meier method.
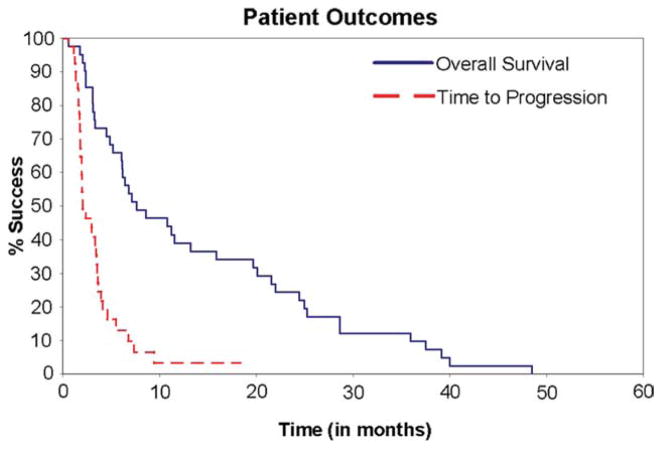
Thirty-nine (95%) patients progressed; the most common site of progression was the lung (58%). Figure 1 and Table 2 indicate time to progression (TTP) and overall survival (OS), as well as duration of response. The median time to progression was 2.0 months (95% CI, 1.8–3.5). All patients have died. The median time to death (OS) was 7.6 months (95% CI, 6.1–15.9).
Figure 1.
Kaplan-Meier survival curves are shown.
Tolerability
Forty-one patients completed a total of 143 cycles of treatment (median, 2; range, 1–19). Nine patients had dose reductions in 9 cycles for nonhematologic (6 patients) and hematologic (3 patients) toxicities. CCI-779 was held in 19 patients (Day 8, 9 patients; Day 15, 9 patients; Day 22, 18 patients). Table 3 describes the number of doses omitted per cycle. All patients have completed study treatment. Reasons for discontinuing treatment include: disease progression (85%); adverse event (7%); refusal (3%), and surgical debulking (3%).
Table 3.
Number of Omitted Doses per Cycle
| Cycle | No. | Omits Day 8 | Omits Day 15 | Omits Day 22 |
|---|---|---|---|---|
| 1 | 41 | 2 | 4 | 6 |
| 2 | 36 | 2 | 1 | 4 |
| 3 | 17 | 2 | 3 | 5 |
| 4 | 13 | 1 | 1 | 1 |
| 5 | 7 | 1 | 0 | 1 |
| 6 | 6 | 0 | 0 | 0 |
| 7 | 5 | 0 | 0 | 0 |
| 8 | 4 | 0 | 0 | 0 |
| 9 | 3 | 0 | 0 | 1 |
Toxicity
Table 4 describes the maximum severity of treatment-related adverse events. Forty-three percent experienced grade 3+ adverse events at least possibly related to treatment during all cycles of therapy. The most common toxicities (adverse events at least possibly related to treatment) included (number of patients with grade 3–4): stomatitis (n = 3), fatigue (n = 2), and anemia (n = 1). Two patients experienced grade 4 adverse events; these included hyperglycemia, hypocalcemia, and peripheral motor neuropathy.
Table 4.
Maximum Severity of Adverse Eventsa for All Patients (N = 40)
| Body System | Toxicityb | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 3–4 |
|---|---|---|---|---|---|---|
| Constitutional symptoms | Fatigue | 15 (38%) | 9 (23%) | 2 (5%) | 0 | 2 (5%) |
| Dermatology/skin | Acne | 18 (45%) | 3 (8%) | 0 | 0 | 0 |
| Gastrointestinal | Stomatitis | 19 (48%) | 5 (13%) | 3 (8%) | 0 | 3 (8%) |
| Anorexia | 13 (33%) | 2 (5%) | 1 (3%) | 0 | 1 (3%) | |
| Diarrhea | 11 (28%) | 5 (13%) | 1 (3%) | 0 | 1 (3%) | |
| Nausea | 6 (15%) | 3 (8%) | 2 (5%) | 0 | 2 (5%) | |
| Vomiting | 4 (10%) | 0 | 2 (5%) | 0 | 2 (5%) | |
| Taste alteration | 5 (13%) | 0 | 0 | 0 | 0 | |
| Hematology | Anemia | 15 (38%) | 5 (13%) | 1 (3%) | 0 | 1 (3%) |
| Thrombocytopenia | 17 (43%) | 1 (3%) | 0 | 0 | 0 | |
| Leukopenia | 8 (20%) | 5 (13%) | 1 (3%) | 0 | 1 (3%) | |
| Neutropenia | 0 | 5 (13%) | 2 (5%) | 0 | 2 (5%) | |
| Hepatic | ALT | 13 (33%) | 5 (13%) | 2 (5%) | 0 | 2 (5%) |
| AST | 14 (35%) | 2 (5%) | 2 (5%) | 0 | 2 (5%) | |
| Hypoalbuminemia | 7 (18%) | 2 (5%) | 0 | 0 | 0 | |
| Bilirubin | 4 (10%) | 0 | 0 | 0 | 0 | |
| Metabolic/laboratory | Hypertriglyceridemia | 13 (33%) | 4 (10%) | 0 | 0 | 0 |
| Hypercholesterolemia | 13 (33%) | 3 (8%) | 0 | 0 | 0 | |
| Hyperglycemia | 10 (25%) | 3 (8%) | 1 (3%) | 1 (3%) | 2 (5%) | |
| Bicarbonate | 12 (30%) | 1 (3%) | 0 | 0 | 0 | |
| Alkaline phosphatase | 10 (25%) | 1 (3%) | 0 | 0 | 0 | |
| Hypocalcemia | 9 (23%) | 0 | 0 | 1 (3%) | 1 (3%) | |
| Hypokalemia | 4 (10%) | 0 | 2 (5%) | 0 | 2 (5%) | |
| Hypophosphatemia | 2 (5%) | 2 (5%) | 1 (3%) | 0 | 1 (3%) | |
| Hyperkalemia | 4 (10%) | 0 | 0 | 0 | 0 | |
| Hyponatremia | 4 (10%) | 0 | 0 | 0 | 0 | |
| Neurology | Neurosensory | 4 (10%) | 0 | 0 | 0 | 0 |
| Neuromotor | 0 | 0 | 0 | 1 (3%) | 1 (3%) | |
| Pulmonary | Cough | 10 (25%) | 1 (3%) | 0 | 0 | 0 |
| Dyspnea | 3 (8%) | 2 (5%) | 2 (5%) | 0 | 2 (5%) | |
| Renal/genitourinary | Creatinine | 5 (13%) | 0 | 0 | 0 | 0 |
ALT indicates alanine aminotransferase; AST, aspartate aminotransferase.
These have an attribution of possible, probable, or definite.
National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0.
Translational Correlates
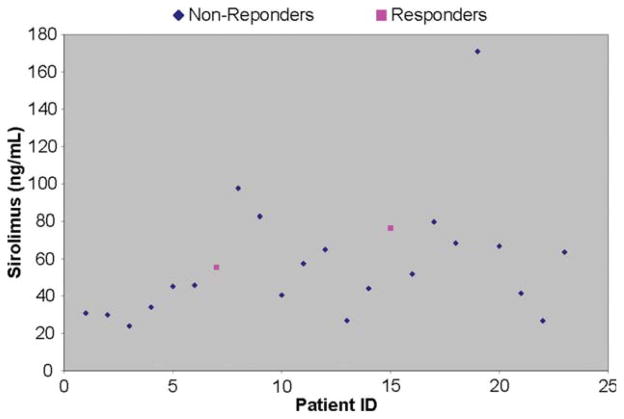
Sample collection was optional for this study. Samples for sirolimus testing were available for 23 patients; samples for PBMC testing were available for 37 patients. Sirolimus is the active metabolite of temsirolimus, and as related in Table 5, 23 patients had sirolimus levels ranging from 23.9 to 171 ng/mL, with a median serum level of 51.8 ng/mL (Fig. 2). Although the limited pharmacokinetic sampling limits the interpretation of the drug-level data, the therapeutic range for effective mTOR complex 1 suppression in the transplant setting is trough levels of 4 to 12 ng/mL sirolimus, and thus, all patients had drug levels above this range 24 hours after the first infusion of CCI-779. Inhibition of mTOR complex 1 signaling was assessed in PBMC samples by comparing phosphorylation levels of ribosomal protein S6 in pretreatment and post-treatment PBMC. Pretreatment and post-treatment blood samples were available from 32 of 37 patients and were processed in this assay. Of these 32, results from 10 patients were inconclusive (no P-S6 and/or total S6 signal for at least 1 sample), 1 patient’s samples were insensitive to the rapamycin spike, and 1 patient had equivocal results. Of the remaining 20 interpretable paired patient samples, 16 (80%) of 20 patients had robust suppression of S6 phosphorylation exceeding a 75% reduction in PBMC P-S6 signal. Thus, potentially therapeutic drug levels were achieved in all patients, and mTOR signaling in PBMC was effectively suppressed in approximately 2/3 of patients. Both sirolimus and PBMC samples were available for 18 patients. Patients experiencing robust suppression of S6 phosphorylation (median, 50.6; range, 23.9–171) did not have significantly different sirolimus values from those who did not (median, 62.9; range, 41.5–76.4; Wilcoxon rank sum test P = .71). In addition, the patient having a 17-month duration of response demonstrated P-S6 inhibition, showing a 75% drop at 24 hours. The other patient having a 3-month response showed only a 36% drop at 24 hours (ie, no P-S6 inhibition).
Table 5.
Translational Results
| Correlative | Frequency/Estimate |
|---|---|
| PBMC, inhibited | 16 (73%) |
| No. of samples | 22 |
| Sirolimus, therapeutic levels | 23 (100%) |
| No. of samples | 23 |
| Median (range) | 51.8 (23.9–171) |
PBMC indicates peripheral blood mononuclear cells.
Figure 2.
Sirolimus values are shown by patient.
DISCUSSION
This phase 2 study of CCI-779 demonstrated possible activity in fibrosarcoma and leiomyosarcoma, but showed no activity in the other histologies of STS treated in our study. grade 3+ toxicities related to therapy occurred in 43% of patients. The most common grade 3+ symptom possibly related to therapy was stomatitis, which occurred in 3 patients, followed by fatigue, nausea, vomiting, and dyspnea, which each occurred in 2 patients. This toxicity is similar to that found in studies using CCI-779 in other patient populations.15–18
STS are a rare heterogenous group of cancers, each with its own natural history and response to chemotherapy. In the past several years, systemic treatment for STS has been tailored more to the histological subtype of sarcoma rather than broad-based chemotherapy. For instance, histology-specific options are available for GIST with imatinib and sunitinib2,3; for uterine leiomyosarcoma with docetaxel and gemcitabine4 as well as dacarbazine-based regimens19,20; for angiosarcoma with taxanes21; and for myxoid round cell liposarcoma with trabectedin.22–24 Despite these histology-specific options, the vast majority of the sarcoma subtypes do not have effective specific therapies, and broad-based treatment with single-agent doxorubicin or combination with ifosfamide is still the standard chemotherapy option.
mTOR inhibition is an attractive target for cancer and especially for STS, as many of the signal transduction networks in STS are affected by mTOR. In our study, we tested CCI-779 in a broad variety of STS in the first-line metastatic setting. We chose to use standard confirmed response rate by RECIST as our primary endpoint. Other endpoints such Choi criteria25 and progression-free survival26 were just being developed during the inception of this trial. Moreover, Choi criteria were developed for GIST and have not been validated in all STS.25 We also performed ancillary testing to confirm that we were obtaining inhibition of mTOR signaling.
Although CCI-779 was overall well tolerated, we only had 2 (5%) confirmed responses, with a response duration of 3 and 17 months. The median TTP was short at 2 months, and the median OS was 7.6 months. Inhibition of pS6 was achieved in 80% of the cases, and therapeutic levels of sirolimus were seen all cases tested. The relationship between TTP with the inhibition of pS6 and sirolimus was explored; results were not significant. According to the European Organization for Research and Treatment of Cancer, progression-free rates for first-line therapy at 6 months of 30% to 56% would suggest an active compound. Our 6-month progression-free rate of 13% would suggest that CCI-779 is not an active agent.
Our patient population would be considered a standard STS cohort. They had a good ECOG PS (85% with a PS of 0 or 1), had mainly lung metastasis (72%), had high-grade STS (80%), had common histologies of malignant fibrous histiocytoma (pleomorphic sarcoma), liposarcoma, leiomyosarcoma, and sarcoma not otherwise specified along with some less common histologies, and received the planned targeted dose of therapy (CCI-779 >80% of the time).
The use of CCI-779 in the first-line setting is not justified based on this study. However, further study in fibro-sarcoma and leiomyosarcoma histologies could be considered, given the PRs observed in our study. It is unknown what role the other mTOR inhibitors have in STS or what role the mTOR inhibitors have in maintaining response rates, and results from ongoing studies are awaited.
Acknowledgments
We thank Kristina Laumann for her statistical support and Ann Mladek for expert analysis of S6 phosphorylation in peripheral blood mononuclear cell samples.
Footnotes
Presented at the 42nd American Society of Clinical Oncology Annual Meeting, Atlanta, Georgia, June 2–6, 2006, abstract 9504.
CONFLICT OF INTEREST DISCLOSURES
Supported by N01-CM62205 and CA15083.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at 2 dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26:626–632. doi: 10.1200/JCO.2007.13.4452. [DOI] [PubMed] [Google Scholar]
- 3.Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368:1329–1338. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]
- 4.Hensley ML, Blessing JA, Mannel R, Rose PG. Fixed-dose rate gemcitabine plus docetaxel as first-line therapy for metastatic uterine leiomyosarcoma: a Gynecologic Oncology Group phase II trial. Gynecol Oncol. 2008;109:329–334. doi: 10.1016/j.ygyno.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maurel J, Lopez-Pousa A, de Las Penas R, et al. Efficacy of sequential high-dose doxorubicin and ifosfamide compared with standard-dose doxorubicin in patients with advanced soft tissue sarcoma: an open-label randomized phase II study of the Spanish group for research on sarcomas. J Clin Oncol. 2009;27:1893–1898. doi: 10.1200/JCO.2008.19.2930. [DOI] [PubMed] [Google Scholar]
- 6.Lorigan P, Verweij J, Papai Z, et al. Phase III trial of two investigational schedules of ifosfamide compared with standard-dose doxorubicin in advanced or metastatic soft tissue sarcoma: a European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol. 2007;25:3144–3150. doi: 10.1200/JCO.2006.09.7717. [DOI] [PubMed] [Google Scholar]
- 7.Dancey JE. Clinical development of mammalian target of rapamycin inhibitors. Hematol Oncol Clin North Am. 2002;16:1101–1114. doi: 10.1016/s0889-8588(02)00051-5. [DOI] [PubMed] [Google Scholar]
- 8.Raymond E, Alexandre J, Faivre S, et al. Safety and pharmacokinetics of escalated doses of weekly intravenous infusion of CCI-779, a novel mTOR inhibitor, in patients with cancer. J Clin Oncol. 2004;22:2336–2347. doi: 10.1200/JCO.2004.08.116. [DOI] [PubMed] [Google Scholar]
- 9.Mita MM, Tolcher AW. The role of mTOR inhibitors for treatment of sarcomas. Curr Oncol Rep. 2007;9:316–322. doi: 10.1007/s11912-007-0039-7. [DOI] [PubMed] [Google Scholar]
- 10.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 11.Sarkaria JN, Schwingler P, Schild SE, et al. Phase I trial of sirolimus combined with radiation and cisplatin in non-small cell lung cancer. J Thorac Oncol. 2007;2:751–757. doi: 10.1097/JTO.0b013e3180cc2587. [DOI] [PubMed] [Google Scholar]
- 12.Fleming TR. One-sample multiple testing procedure for phase II clinical trials. Biometrics. 1982;38:143–151. [PubMed] [Google Scholar]
- 13.Duffy D, Santner T. Confidence intervals for a binomial parameter based on multistage tests. Biometrics. 1987;43:81–93. [Google Scholar]
- 14.Kaplan E, Meier P. Nonparametric estimation for incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 15.Bellmunt J, Szczylik C, Feingold J, Strahs A, Berkenblit A. Temsirolimus safety profile and management of toxic effects in patients with advanced renal cell carcinoma and poor prognostic features. Ann Oncol. 2008;19:1387–1392. doi: 10.1093/annonc/mdn066. [DOI] [PubMed] [Google Scholar]
- 16.Motzer RJ, Hudes GR, Curti BD, et al. Phase I/II trial of temsirolimus combined with interferon alfa for advanced renal cell carcinoma. J Clin Oncol. 2007;25:3958–3964. doi: 10.1200/JCO.2006.10.5916. [DOI] [PubMed] [Google Scholar]
- 17.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 18.Galanis E, Buckner JC, Maurer MJ, et al. Phase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: a North Central Cancer Treatment Group Study. J Clin Oncol. 2005;23:5294–5304. doi: 10.1200/JCO.2005.23.622. [DOI] [PubMed] [Google Scholar]
- 19.Anderson S, Aghajanian C. Temozolomide in uterine leiomyosarcomas. Gynecol Oncol. 2005;98:99–103. doi: 10.1016/j.ygyno.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 20.Long HJ, III, Blessing JA, Sorosky J. Phase II trial of dacarbazine, mitomycin, doxorubicin, and cisplatin with sargramostim in uterine leiomyosarcoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2005;99:339–342. doi: 10.1016/j.ygyno.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Penel N, Lansiaux A, Adenis A. Angiosarcomas and taxanes. Curr Treat Options Oncol. 2007;8:428–434. doi: 10.1007/s11864-007-0042-0. [DOI] [PubMed] [Google Scholar]
- 22.Schoffski P, Dumez H, Wolter P, et al. Clinical impact of trabectedin (ecteinascidin-743) in advanced/metastatic soft tissue sarcoma. Expert Opin Pharmacother. 2008;9:1609–1618. doi: 10.1517/14656566.9.9.1609. [DOI] [PubMed] [Google Scholar]
- 23.Carter NJ, Keam SJ. Trabectedin: a review of its use in the management of soft tissue sarcoma and ovarian cancer. Drugs. 2007;67:2257–2276. doi: 10.2165/00003495-200767150-00009. [DOI] [PubMed] [Google Scholar]
- 24.Schoffski P, Wolter P, Clement P, et al. Trabectedin (ET-743): evaluation of its use in advanced soft-tissue sarcoma. Future Oncol. 2007;3:381–392. doi: 10.2217/14796694.3.4.381. [DOI] [PubMed] [Google Scholar]
- 25.Benjamin RS, Choi H, Macapinlac HA, et al. We should desist using RECIST, at least in GIST. J Clin Oncol. 2007;25:1760–1764. doi: 10.1200/JCO.2006.07.3411. [DOI] [PubMed] [Google Scholar]
- 26.Van Glabbeke M, Verweij J, Judson I, Nielsen OS EORTC Soft Tissue and Bone Sarcoma Group. Progression-free rate as the principal end-point for phase II trials in soft-tissue sarcomas. Eur J Cancer. 2002;38:543–549. doi: 10.1016/s0959-8049(01)00398-7. [DOI] [PubMed] [Google Scholar]