Abstract
Context
Dementia is associated with increased rates and often poorer outcomes of hospitalization, including worsening cognitive status. New evidence is needed to determine whether excess admissions in dementia might be potentially preventable.
Objective
To determine whether dementia onset is associated with higher rates or different reasons for hospitalization, particularly for ambulatory care sensitive conditions (ACSCs) for which proactive outpatient care might prevent the need for a hospital stay.
Design, Setting, and Participants
We conducted a retrospective analysis of hospitalizations among 3019 participants in Adult Changes in Thought (ACT), a longitudinal cohort study of initially non-demented adults aged 65 and older enrolled in an integrated healthcare system. Automated data were used to identify all hospitalizations from time of enrollment in ACT until death, disenrollment from the health plan, or end of follow-up, whichever came first. The study period spanned from February 1, 1994 to December 31, 2007.
Main Outcome Measures
Hospital admission rates for dementia and dementia-free groups, for all causes, by type of admission, and for ACSCs.
Results
Four hundred ninety-four cognitively normal individuals eventually developed dementia and 427 (86%) of these persons were admitted at least once; 2525 remained dementia free and 1478 (59%) were admitted at least once. The unadjusted all-cause admission rate in the dementia group was 419 admits per 1000 person-years vs. 200 admits/1000 in the dementia-free group. After adjustment for age, gender, and other potential confounders, the ratio of admission rates for all-cause admissions was 1.41 (95% confidence interval [CI], 1.23 to 1.61; P<.0001), while for ACSCs, the adjusted ratio of admission rates was 1.78 (95% CI, 1.38 to 2.31; P<.0001). Adjusted admission rates classified by body system were significantly higher in the demented group for most categories. Adjusted admission rates for all types of ACSCs, including bacterial pneumonia, congestive heart failure, dehydration, duodenal ulcer, and urinary tract infection, were significantly higher among those with dementia.
Conclusions
Among patients aged 65 years and older, incident dementia was significantly associated with increased risk of hospitalization, including hospitalization for ACSCs.
Keywords: health services for the aged, delivery of health care, outcome and process assessment (health care), dementia/economics, hospitalization/statistics and numerical data
Claims-based, retrospective studies have long reported that dementia is associated with increased hospitalizations,1–9 but empirical data to elucidate this finding are few. Suboptimal management in the outpatient setting may be a contributing factor, as evidenced by lower prescription drug costs and fewer office visits after diagnosis.4 Accomplishing adequate chronic disease management is more difficult, which may lead to hospitalization for acute exacerbation of comorbid conditions.4, 5 Non-elective hospitalization of older people, particularly those with dementia, is not a trivial event. Among non-demented older persons, hospitalization for serious illness is associated with subsequent cognitive decline,10 and frail elders, including those with dementia, are at increased risk for delirium, functional decline, and iatrogenic complications during an inpatient stay.11–13 A closer look at conditions that precipitate hospitalization of elders with dementia could focus clinical priorities on secondary and tertiary prevention in the outpatient setting and improve health care for this vulnerable and growing population. We used a unique longitudinal data set to determine whether dementia onset is associated with higher rates or different reasons for hospitalization, particularly for ambulatory care sensitive conditions (ACSCs) for which proactive outpatient care might prevent the need for a hospital stay.
METHODS
Source of Participants
Participants came from the Adult Changes in Thought (ACT) cohort. Begun in 1994, ACT is a population-based, longitudinal study of aging and the incidence of and risk factors for dementia involving over 3500 members of Group Health Cooperative (GHC), a large integrated healthcare delivery system.14, 15 Eligible persons were aged 65 years or older, cognitively intact, and not residing in a nursing home at time of enrollment in the cohort (mean age at inception was 75.3 years). Participants have been followed up every 2 years with an in-person interview that includes dementia and health status assessment. Compliance rates with follow-up visits are generally very high, as reflected in a completeness of follow-up index for the ACT study of over 95%.16 Detailed descriptions of study methods have been published previously.10, 15, 17, 18
The purpose of the bienniel examination was to identify cases of incident dementia. Participants who scored <86 on the Cognitive Abilities Screening Instrument (CASI) or had symptoms suggesting possible new onset of cognitive impairment underwent a standardized dementia diagnostic evaluation consisting of an examination by a study physician and detailed neuropsychological testing as described elsewhere.15, 17 Informants knowledgeable about participants were interviewed as part of the dementia diagnostic work-up, and Jorm and Korten Informant Interviews 19 were conducted with all informants. The results were presented at a consensus conference attended by study physicians, a neuropsychologist, research nurse, and interviewers and a consensus diagnosis was recorded based on standardized criteria (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) [Association, 1994 #2074] and Neurological and Communicative Disorders and Stroke—Alzheimer Disease and Related Disorders Association (NINCDS-ADRDA) 21 criteria). Primary care providers of ACT participants were notified by letter of a study diagnosis of dementia. Persons found not to be demented were returned to the cohort for bienniel evaluations. Participants with incident dementia underwent one annual follow-up examination for verification of dementia status and dementia type. The ACT cohort was assembled to determine the incidence of dementia; it was not designed to focus on follow-up after dementia diagnosis beyond one year.
Study Design
We used a retrospective, longitudinal cohort design to assess inpatient admission rates in individuals from the ACT study. Follow-up for each participant began at first enrollment in ACT and ended at death, health plan disenrollment, or end of study follow-up (12/31/2007), whichever came first. During follow-up, some developed dementia; most did not. Dementia was treated as a time-varying covariate so that those who developed dementia contributed time-at-risk to both the non-dementia and dementia groups. This approach avoids the bias which results when the non-dementia group is restricted to those who are dementia free through follow-up and is consistent with the design of nested case-control studies.22, 23
Eligibility Criteria
ACT participants eligible for our analyses met the following selection criteria: 1) were non-demented at the baseline ACT visit; 2) completed ≥1 ACT follow-up visit (to assess for incident dementia); and 3) were enrolled in GHC at the time of a follow-up visit (to ensure availability of hospitalization data post-baseline). This study was approved by the institutional review boards of GHC and the University of Washington. All ACT participants provided written informed consent for baseline and follow-up assessments at time of enrollment into ACT. The IRB approved a waiver of consent for the present study.
Description of Variables
Outcome Measures
The primary outcome measure was rate of hospitalization, measured as mean number of admissions per year of follow-up. An admission was defined as a hospitalization requiring an overnight stay. An automated hospitalizations file was used to identify admissions during the follow-up period. Hospitalization data from GHC have been validated and used extensively for research.24
The secondary outcome measure was the rate of hospitalization by type, classified by the principal discharge diagnosis (International Classification of Diseases, 9th Revision (ICD-9) code). Large categories/groupings of discharge diagnoses were modeled after those used in the Agency for Healthcare Research and Quality’s (AHRQ) Healthcare Cost and Utilization Project (HCUP).25 Classes of ICD-9 codes comprising each category, along with examples of conditions in a category, are shown in the Appendix. We identified ACSCs among principal discharge diagnoses, to count conditions for which hospitalization may potentially be prevented with timely, evidence-driven, outpatient care.26–29 We used previously described classification schemes for ACSC to allow direct comparison with other studies.30, 31 Although not all admissions for ACSC are avoidable, and although the concept of an ACSC as originally described 26, 32, 33 addressed the full spectrum of outpatient care recipients and not specifically older persons with dementia, the construct is useful for evaluating the potential impact of dementia on patterns of care. Because all patients were enrolled in the same delivery system, which has no care pathway for persons with dementia, access to care should be comparable and unlikely to confound any group differences. Further, higher rates of hospitalization for ACSC could pinpoint areas for improvement in the quality of care.34
Covariates
Potential confounders of the dementia-hospitalization relationship, including sociodemographic characteristics, comorbid conditions, health behaviors, self-rated health, and place of residence were ascertained from self-reported data collected at the baseline visit as well as at two-year follow-up visits. Response options for self-report of race included White, Black not of Hispanic origin, Asian or Pacific Islander, American Indian or Alaska Native, Hispanic, and Other. Depression was defined as a score of ≥10 on the 10-item Center for Epidemiological Studies Depression Scale (CES-D; score range 0 to 30).35 Comorbidity burden was estimated using the RxRisk score, a case-mix measure that uses automated, outpatient pharmacy data to identify medications used to treat chronic conditions known to be associated with future healthcare cost and use.36 When the score was initially developed,37 an expert panel comprised of physicians, pharmacists, and health services researchers selected medication classes and assigned weights to signify the predicted impact on chronic disease severity. The RxRisk updates and expands the Chronic Disease Score 37 by creating a more complete drug assignment algorithm based on National Drug Codes (unique identifying numbers) 38 and expanding the set of conditions used to assess comorbidity. A regression model is used to relate medication classes to health care costs. The RxRisk score is the linear combination of an individual’s age, gender, and set of conditions for which prescription drug dispenses have been observed. The lowest possible risk score is one determined by age and gender, with scores increasing with age and with males having higher age-adjusted risk. Versions of the RxRisk have been developed for commercially insured populations,36 United States veterans,39–41 and Medicaid recipients.42 Each version of the RxRisk has been validated against case mix models using diagnostic codes and found to perform statistically similarly in terms of population mean cost as well as among individuals likely to be future high users of health care. Important to the present study, no version of the RxRisk includes medications used to treat dementia. Global cognitive function was evaluated based on scores on the CASI (scored 0–100)43 from the baseline ACT assessment. Participants with CASI scores <86 at follow-up received comprehensive evaluations for dementia.
Statistical Analyses
Demographic and health-related characteristics were compared between those who developed dementia and those who did not using t-tests (continuous variables) and chi-square tests (categorical variables). Missing baseline data were infrequent; no single covariate had more than 1.6% missing. Our primary analyses compared the admission rates in the 2 groups, with dementia handled as a time-varying covariate. Rates were computed as the total number of admissions in each group divided by the number of years of follow-up in that group. For the dementia group, only admissions following the first dementia diagnosis were used in computing admission rates. In the non-dementia group, the rate was computed as the total number of admissions among persons while free of dementia divided by the total years of dementia-free follow-up. (In the tables, rates are presented as rates per 1000 person-years.) We then computed the ratio of the admission rates for the dementia and non-dementia groups. To account for time-varying covariates (including dementia status), we divided each person’s follow-up period into a series of periods averaging approximately 2 years in length, timed to start and end with the dates of each person’s baseline and follow-up ACT visit dates. Since this analysis involved repeated observations for the same person, we used the generalized estimating equation (GEE) version of Poisson regression to account for any within-person correlation. P-values and confidence intervals for ratios were computed using empirical standard errors to account for overdispersion in Poisson regression models.44, 45 For key outcomes, we repeated the analysis using negative binomial regression; results were similar so not reported herein. In fully adjusted models including all covariates, only 5% of observations were excluded due to missing baseline or follow-up information. Only 65 (2.2%) participants were excluded entirely from the fully adjusted analysis. Given this low frequency of missing data, we did not impute missing covariates.
Three sets of Poisson regression analyses were performed for each outcome: 1) unadjusted, 2) age- and gender-adjusted, and 3) “fully” adjusted. In adjusted models, linear and quadratic terms for age for each gender were included to account for the non-linear relationship between hospitalization rates and age. All tests were two-sided, and P<.05 was considered statistically significant. To account for the possibility that moving into a nursing home could alter patterns of medical care and influence decisions regarding hospitalization, we fit a fully adjusted model adding nursing home residence at follow-up. All analyses were carried out using SAS, version 9.0 (SAS Institute, Inc., Cary, North Carolina).
RESULTS
Participant Characteristics
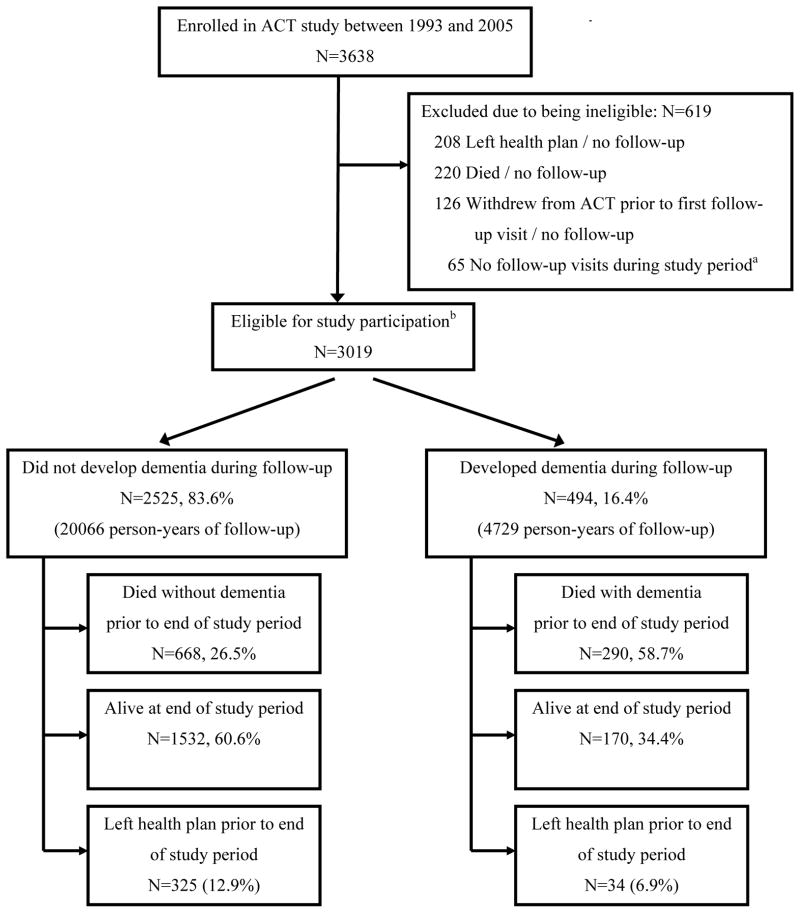
A total of 3019 ACT participants met eligibility criteria and were included in analyses (Figure). Thirty-four (6.9%) participants who developed dementia discontinued follow-up prior to the end of the study period due to disenrollment from the health plan, and 290 (58.7%) died; 325 (12.9%) of the group that remained dementia-free left the health plan, and 668 (26.5%) died prior to the end of the study (12/31/2007).
Figure 1. Participant Flow and Vital Status by End of Follow-up (12/31/2007).
a No follow-up visit during study period due either to not being yet due for follow-up or not presenting for follow-up visit when invited.
b EligibilityCriteria Met by Participants (N=3019)
- Enrolled in ACT between 1993–2005
- Free of dementia at time of ACT enrollment
- Completed baseline assessment as part of ACT study
- At least 1 follow-up visit with ACT to assess dementia status while still enrolled in health plan
Table 1 shows subject characteristics at ACT enrollment, grouped by dementia status ascertained through the entire follow-up period. Those in the eventually-demented group were older at cohort entry by about 3 years; fewer had graduated from high school; a larger percentage reported having trouble dressing; a larger percentage reported a diagnosis of depression or Parkinson’s disease, and their mean CASI score was a few points lower than for those who remained dementia free. Differences on race, RxRisk, and prior hospitalization were all due to the older age of the dementia group and were not significant after adjustment for age and gender.
Table 1.
Characteristics of Demented and Dementia-Free Cohorts at Baseline Visit for the Adult Changes in Thought Study.
| Characteristic | Developed Dementia (N=494) | Did Not Develop Dementia (N=2525) | Unadjusted P-value | Age- and Gender-Adjusted P-Value |
|---|---|---|---|---|
| Age, years, mean (SD) | 78.0 (6.0) | 74.5 (6.1) | .0001 | — |
| Age distribution, year, No. (%) | .0001 | — | ||
| 65–74 | 148 (30.0) | 1429 (56.6) | ||
| 75–84 | 274 (55.5) | 914 (36.2) | ||
| 85+ | 72 (14.5) | 182 (7.2) | ||
| Female, No. (%) | 305 (61.7) | 1493 (59.1) | .28 | — |
| High school graduate, No. (%) | 402 (81.4) | 2200 (87.1) | .0007 | .008 |
| Non-white, No. (%) | 35 (7.1) | 257 (10.2) | .03 | .18 |
| Living alone, No. (%) | 196 (39.7) | 910 (36.0) | .13 | .35 |
| Annual income, median | 23820 | 27478 | .0001 | .001 |
| Smoker, current, No. (%) | 27 (5.5) | 153 (6.1) | .79 | .60 |
| Exercise ≥3 times per week, No. (%) | 337 (68.2) | 1813 (71.8) | .10 | .32 |
| Self-rated health poor, No. (%) | 13 (2.6) | 38 (1.5) | .08 | .15 |
| ADL difficulty | ||||
| Bathing, No. (%) | 25 (5.1) | 109 (4.3) | .46 | .62 |
| Ambulating, No. (%) | 40 (8.1) | 146 (5.8) | .05 | .45 |
| Transferring, No. (%) | 91 (18.4) | 423 (16.8) | .37 | .68 |
| Dressing, No. (%) | 39 (7.9) | 106 (4.2) | .0004 | .006 |
| Feeding, No. (%) | 3 (0.6) | 23 (0.9) | .50 | .26 |
| Toileting, No. (%) | 9 (1.8) | 43 (1.7) | .85 | .62 |
| Chronic conditions | ||||
| Cancer (non-skin), No. (%) | 80 (16.2) | 444 (17.6) | .46 | .07 |
| Depression, No. (%) | 75 (15.2) | 246 (9.7) | .0003 | .003 |
| Heart disease, No. (%) | 103 (20.9) | 487 (19.3) | .42 | .85 |
| Hypertension, No. (%) | 191 (38.7) | 980 (38.8) | .95 | .40 |
| Congestive heart failure, No. (%) | 23 (4.7) | 96 (3.8) | .37 | .98 |
| Diabetes, No. (%) | 52 (10.5) | 239 (9.5) | .46 | .24 |
| Stroke, No. (%) | 21 (4.3) | 81 (3.2) | .24 | .58 |
| Parkinson’s disease, No. (%) | 10 (2.0) | 8 (0.3) | .0001 | .0004 |
| RxRisk score, $ | ||||
| Mean (SD) | 4872 (2707) | 4336 (2890) | .0001 | .18 |
| CASI score, raw, mean (SD)a | 90.3 (5.7) | 93.6 (4.4) | .0001 | .0001 |
| Hospitalized for medical condition in 2 years prior to baseline, No. (%) | 112 (22.7) | 455 (18.0) | .01 | .17 |
Abbreviations: ADL, activities of daily living; CASI, Cognitive Abilities Screening Instrument; COPD, chronic obstructive pulmonary disease.
CASI score range, 0–100.
The most frequent etiologic diagnoses in the dementia group were probable Alzheimer’s disease as a single cause (58%); vascular dementia alone (16%), and dementia of multiple etiologies (15%). Other etiologies included other medical (7%), substance-related (2%), and other/unknown (2%). The mean (SD) age at diagnosis was 84.3 (5.8) years, with 61% diagnosed in their 80’s. The mean (SD) CASI score at time of diagnosis (not time of enrollment) was 76 (10.7), consistent with mild dementia.
Follow-up totaled 24795 person-years, including 1703 years of follow-up post-diagnosis among the 494 who developed dementia. Follow-up averaged 8 years (median=7.8 years; interquartile range [IQR], 4.3–11.9 years) among those who never developed dementia and averaged 9.6 years (median=9.9 years; IQR, 6.6–12.0 years) among those who did (6.1 years before and 3.5 years after diagnosis, on average). Admissions totaled 5328, including 714 occurring after a dementia diagnosis. During follow-up, 427 (86%) of the dementia group was hospitalized (96 once, 103 twice, and 228 3 or more times) vs. 1478 (59%) of the dementia-free group (548 once, 384 twice, and 546 3 or more times). Forty percent (196) of the dementia group had at least 1 ACSC admit (118 had 1, 46 had 2, and 32 had 3 or more) as compared to 17% (424) of the dementia-free group (266 with 1, 99 with 2, and 59 with 3 or more).
Rates and Reasons for Hospitalization
Table 2 gives the all-cause rate of hospitalization and rates by major reasons for hospitalization, according to body system, by study group. The most common reasons for hospitalization, regardless of dementia status, were circulatory, respiratory, and digestive disorders. Among participants with dementia, the average annual admission rate was 419 admits per 1000 persons, more than twice that of those without dementia, who averaged 200 admits per 1000 persons each year (crude rate ratio, 2.10, 95% CI, 1.87 to 2.35; P<.0001). After age/gender adjustment, the ratio of admission rates was 1.57 (95% CI, 1.39 to 1.78; P<.0001), and 1.41 (95% CI, 1.23 to 1.61; P<.0001) after adjusting for additional covariates. This ratio changed very minimally with adjustment for residence in a nursing home prior to hospitalization (rate ratio, 1.39, 95% CI, 1.20 to 1.40, P<.0001).
Table 2.
Hospital Admission Rates Per 1000 Person-Years, for All-Causes, by Principal Discharge Diagnosis Category, and by Ambulatory Care Sensitive (Potentially Preventable) Causes for Demented and Non-Demented Groups.
| Demented Crude Ratea (No.) | Non-Demented Crude Rate (No.) | Unadjusted Rate Ratio (95% CI) | Unadjusted P-value | Age/sex Adjusted Rate Ratiob (95% CI) | Age/sex Adjusted P-Value | Fully Adjusted Rate Ratioc (95% CI) | Fully Adjusted P-Value | |
|---|---|---|---|---|---|---|---|---|
| All admits | 419 (714) | 200 (4614) | 2.10 (1.87 to 2.35) | .0001 | 1.57 (1.39 to 1.78) | .0001 | 1.41 (1.23 to 1.61) | <.0001 |
| Circulatory | 112 (190) | 58 (1345) | 1.92 (1.60 to 2.29) | .0001 | 1.58 (1.30 to 1.93) | .0001 | 1.42 (1.16 to 1.75) | .0008 |
| Digestive | 40 (68) | 23 (529) | 1.71 (1.28 to 2.28) | .0003 | 1.21 (0.90 to 1.67) | .20 | 1.17 (0.84 to 1.61) | .35 |
| Endocrine | 10 (17) | 4 (100) | 2.31 (1.37 to 3.88) | .002 | 1.56 (0.86 to 2.72) | .15 | 1.03 (0.54 to 1.96) | .92 |
| Genitourinary | 37 (63) | 9 (209) | 4.09 (2.87 to 5.81) | .0001 | 3.00 (1.92 to 4.60) | .0001 | 2.46 (1.55 to 3.92) | .0001 |
| Infections | 15 (25) | 4 (85) | 3.99 (2.48 to 6.42) | .0001 | 2.17 (1.30 to 3.61) | .003 | 2.01 (1.15 to 3.52) | .02 |
| Musculoskeletal | 7 (11) | 20 (468) | 0.32 (0.18 to 0.58) | .0002 | 0.30 (0.16 to 0.54) | .0001 | 0.31 (0.17 to 0.57) | .0002 |
| Nervous system | 11 (18) | 2 (54) | 4.52 (2.24 to 9.12) | .0001 | 3.19 (1.49 to 6.87) | .003 | 2.91 (1.18 to 7.18) | .02 |
| Respiratory | 66 (112) | 21 (486) | 3.12 (3.13 to 2.43) | .0001 | 2.03 (1.53 to 2.69) | .0001 | 1.65 (1.21 to 2.25) | .002 |
| Otherd | 123 (210) | 58 (1328) | 2.14 (1.80 to 2.56) | .0001 | 1.57 (1.30 to 1.90) | .0001 | 1.46 (1.19 to 1.79) | .0003 |
| All ACSC admitse | 116 (198) | 37 (845) | 3.18 (2.59 to 3.90) | .0001 | 2.19 (1.74 to 2.76) | 0.0001 | 1.78 (1.38 to 2.31) | <.0001 |
| Anginaf | 0 (0) | 2 (46) | 0.0 | .0001 | N/Ag | N/Ag | N/Ag | N/Ag |
| Bacterial pneumonia | 34 (57) | 10 (225) | 3.44 (2.48 to 4.75) | .0001 | 2.16 (1.47 to 3.13) | 0.0001 | 1.88 (1.25 to 2.82) | .002 |
| Cellulitis | 4 (6) | 3 (67) | 1.21 (0.39 to 3.80) | 0.74 | 1.03 (0.34 to 3.10) | 0.95 | 0.78 0.23 to 2.61) | .69 |
| CHF exacerbation | 33 (56) | 10 (233) | 3.26 (2.29 to 4.64) | .0001 | 2.10 (1.41 to 3.10) | 0.0003 | 1.73 (1.15 to 2.60) | .01 |
| COPD exacerbation | 7 (12) | 3 (76) | 2.14 (0.98 to 4.69) | .06 | 1.43 (0.62 to 5.91) | 0.40 | 1.15 (0.49 to 2.67) | .75 |
| Dehydration | 5 (8) | 1 (21) | 5.17 (2.26 to 11.81) | .0001 | 4.56 (1.51 to 13.89) | 0.007 | N/Ag | N/Ag |
| Duodenal ulcer | 5 (8) | 1 (15) | 7.23 (3.07 to 17.04) | .0001 | 4.30 (1.63 to 11.32) | 0.003 | 4.39 | .003 |
| Gastric ulcer | 4 (6) | 1 (32) | 2.54 (0.95 to 6.82) | .06 | 2.57 (0.77 to 8.25) | 0.12 | 2.42 (0.77 to 7.65) | .13 |
| UTI | 25 (42) | 3 (76) | 7.49 (4.94 to 11.37) | .0001 | 4.31 (2.53 to 7.28) | 0.0001 | 3.38 (1.93 to 5.93) | <.0001 |
Abbreviations: ACSC, ambulatory care sensitive conditions, or conditions for which hospitalization may be preventable with adequate outpatient care; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; UTI, urinary tract infection.
Admission rate shown as mean number of admissions per year per 1000 persons. Total years of follow-up (used in computation of rates): 23092 years for dementia free and 1703 for dementia group.
Adjusted for age (age modeled using linear and quadratic terms, 1 set of males and 1 set for females), gender, calendar year, and wave (i.e., original or expansion) 10 of ACT cohort.
Adjusted for age, gender, race, education, living alone (all assessed only at baseline), and time-varying variables, including calendar year of admit, self-rating of health, count of number of activities of daily living (ADL) performed with difficulty, and RxRisk (log transformed). Models for all admits and all ACSC admits adjusted for all of the preceding covariates plus cancer, diabetes, and heart disease.
“Other” category includes liver disorders, skin disorders, mental health and substance abuse disorders, blood disorders, reproductive organ disorders, neoplasms, ear, nose, mouth, and throat disorders, eye disorders, and injuries.
ACSC admits with ≤ 20 instances omitted from table: asthma, diabetes, ear/nose/throat infection, gastroenteritis, hypertension, hypoglycemia, hypokalemia, influenza, malnutrition, peptic ulcer, and seizure disorder.
Adjusted analyses could not be run for angina due to 0 admits in the dementia group.
N/A = not applicable, because adjusted rate ratio and P-value from Poisson regression could not be computed due to too few admits.
In the fully adjusted model, admission rates for 5 types of disorders were significantly higher among participants with dementia compared to those without dementia (Table 2): 1) circulatory, 2) genitourinary, 3) infectious, 4) neurological, and 5) respiratory. Rates for the “other” category (Table 2) were also significantly higher. By contrast, those with dementia had significantly lower admission rates for musculoskeletal disorders.
Admissions considered potentially preventable with timely and appropriate ambulatory care (ACSCs) were analyzed separately.26–29 The crude admission rate for ACSCs was higher among those with dementia (116 admits/1000 person-years vs. 37 admits/1000 person-years, crude rate ratio, 3.18, 95% CI, 2.59 to 3.90; P=.0001). After full adjustment for covariates, the rate ratio was 1.78 (95% CI, 1.38 to 2.31; P<.0001). Three ACSCs accounted for 2/3 of all potentially preventable admissions – bacterial pneumonia, congestive heart failure (CHF), and urinary tract infection (UTI) – and admission rates among those with dementia were significantly higher for all 3 conditions. Admission rates for dehydration and duodenal ulcer, though low overall, were also significantly higher among those with dementia. Admissions for ACSCs accounted for 28% of all hospitalizations among those with dementia vs. only 19% of all admits among those who remained dementia-free.
Examining all-cause and ACSC admission rates by dementia etiology (Alzheimer’s disease alone vs. other etiologies) compared to the group that remained dementia free (Table 3) revealed that dementia was associated with higher rates of admission regardless of etiology.
Table 3.
Hospital Admission Rates per 1,000 Person-Years, for All-Cause and Ambulatory Care Sensitive (Potentially Preventable) Causes, by Dementia Type vs. Non-Demented Group.
| Crude Ratea | Adjusted Rate Ratiob | Adjusted P-value | |||||
|---|---|---|---|---|---|---|---|
| Dementia Type
|
Non-demented
|
AD vs. Non | Other vs. Non | AD vs. Non | Other vs. Non | ||
| AD (No.) | Otherc (No.) | (No.) | |||||
| All admits | 358 (375) | 518 (339) | 200 (4614) | 1.27d (0.93 to 1.50) | 1.61 (1.33 to 1.96) | .005 | <.0001 |
| ACSC admits | 99 (104) | 144 (94) | 37 (845) | 1.65 (1.21 to 2.24) | 1.92 (1.39 to 2.66) | .002 | <.0001 |
Abbreviation: AD, Alzheimer type dementia.
Admission rate shown as mean number of admissions per year per 1000 persons. Total years of follow-up (used in computation of rates): 23092 years for the non-demented group, 1049 years for the AD group, and 654 years for the other dementia group.
Adjusted for age, gender, race, education, living alone (all assessed only at baseline), and time-varying variables, including calendar year of admit, self-rating of health, count of number of activities of daily living (ADL) performed with difficulty, and RxRisk (log transformed), cancer, diabetes, and heart disease.
Other dementia includes non-AD etiologies and AD mixed with additional etiologies.
Test of whether the rate ratio for AD vs. Non. differs from the rate ratio for other dementia vs. Non: P-value =.07 for all admits and =.50 for ACSC admits.
Relationship Between Death and Hospitalization
To better understand to what extent the higher mortality rate among persons with dementia might explain their higher hospitalization rates, multivariate analyses were repeated, excluding follow-up periods in which a person died. These analyses revealed that the association between dementia and admission rates remained significant and was attenuated for all-cause but not ACSC admissions (fully adjusted rate ratio, 1.21, 95% CI, 1.02 to 1.43; P=.03 for all-cause hospitalizations, and 1.71, 95% CI, 1.24 to 2.34; P=.001 for ACSC hospitalizations).
DISCUSSION
We found significantly higher all-cause and ACSC admission rates for persons with dementia compared to persons without dementia. Adjusted admission rates for most disease categories were significantly higher in the demented group and also higher for all types of ACSCs, including bacterial pneumonia, CHF, dehydration, duodenal ulcer, and UTI. While higher mortality rates for persons with dementia accounted for about half of the difference in the all-cause admission rate, it accounted for very little of their higher rate of admits for ACSCs.
Ours may be the first study to report rates and reasons for hospitalization among persons from the time of an incident, research-based, dementia diagnosis and with follow-up of most individuals until death. Most prior studies have used claims diagnoses or registry data to construct demented and control groups and a window of 1 year or less to establish hospitalization rates.1–7, 9, 46 Many studies could not adequately adjust for comorbidities. Due to the frequency of missed and delayed dementia diagnoses in usual clinical practice,47 claims and registry data from such studies of prevalent dementia are likely to identify hospitalizations predominantly during middle and later stages of cognitive decline. Studies relying on claims data to classify cases and controls are also susceptible to misclassification bias, which our study overcame with the research evaluation for dementia conducted biennially for all participants as part of the ACT follow-up protocol. The availability of prospectively collected ACT data along with Group Health delivery system data allowed us to adjust for a number of potential confounders (age, gender, and measures of comorbidity) that have been found to be independent predictors for hospitalization among persons with Alzheimer type dementia,46 and also for place of residence, increasing confidence that the effect on hospitalizations is specific for the presence of dementia.
Our findings extend the small literature on hospitalizations in dementia that, as a whole, has not looked systematically or comprehensively at hospital discharge diagnoses.1–7 Furthermore, by examining all forms of dementia developing within a population-based sample of community-dwelling elders, evaluated prospectively using research standards for diagnosis, our work extends prior studies that have examined only certain dementia diagnoses (e.g., Alzheimer’s disease, vascular dementia) 1, 4, 5, 7 and often lack information about diagnostic reliability.
Why might dementia lead to more frequent hospitalization? The explanation is likely multifaceted. First, underlying conditions that increase the risk of dementia (e.g., stroke) or that develop in the setting of dementia (e.g., trouble swallowing, which increases the risk of pneumonia) may increase the risk of hospitalization. Second, due to its primary deleterious effects on global cognition, executive function, expressive language, symptom perception, and awareness of deficits, dementia impairs the ability to self-manage chronic conditions and to pinpoint symptoms and alert others to their presence, thereby creating substantial diagnostic and treatment challenges for primary care providers.48 Situational factors might also contribute, including a change of living situation, or the temporary or permanent absence of a caregiver familiar with the demented person’s usual habits, behaviors, and ongoing general medical management. Another potential explanation is that the threshold for hospitalizing such persons may be lower, due to the fact that dementia increases central nervous system vulnerability to the metabolic effects of acute illness, such that for a comparable severity of illness, persons with dementia are in fact sicker (e.g., more likely to develop delirium and functional impairments as a result of acute illness).49
Three ACSCs – pneumonia, CHF, and UTI – accounted for 2/3 of all potentially preventable admissions among persons with dementia. Knowledge of the ACSCs most likely to lead to hospitalization is important, as this information may help care providers focus their differential diagnostic considerations and thereby permit proactive, early management for these conditions among patients with dementia. Early detection and outpatient management of acute illness when it is still in its early phases might minimize the need for hospitalization for these conditions, and help healthcare organizations reduce their rates of ACSC admissions and associated costs.
The excess dementia-associated hospitalization rates in our study are somewhat lower than reported previously 50 but still considerable from the standpoint of burdened healthcare systems. Our results may reflect methodological refinements over prior work, including earlier, more reliable dementia diagnosis and comprehensive adjustment for confounders, including time-varying covariates (e.g., comorbidities) assessed regularly during follow-up along with non-dementia factors known to be associated with ACSC hospitalizations (e.g., advanced age, impaired ADL functioning, etc.).30 In addition, our longitudinal design more accurately represents the chronic, multi-year course of dementing diseases than do studies assessing admissions over 1 or 2 years. From a health systems planning perspective, our estimates of the impact of dementia on hospitalizations can probably be considered a lower bound of risk.
Prior studies that have used claims diagnoses have found higher hospitalization rates for persons with “other” dementias compared to Alzheimer type dementia.4 By contrast, in our study, rates were not significantly different across these groups for either all-cause or ACSC admissions. The explanation for this discrepant finding is uncertain but may result from differences in how dementia etiology was ascertained.
Our work has some limitations. The study included only consenting GHC enrollees; ACT participants may have been younger and healthier at enrollment than the general population, indicated by their willingness to participate. As an integrated health plan and healthcare delivery system, GHC takes a proactive approach to healthcare in order to manage risk, and existing programs seek to anticipate and manage complications of chronic diseases.51 Therefore, our observed rate of ACSC hospitalizations is likely to be lower than that in less integrated, fee-for-service environments. In a study of ACSC hospitalizations in the Medicare+Choice (managed care) population,31 a somewhat higher unadjusted rate (47 ACSC admits/1000) than ours (42 ACSC admits/1000) was observed; although the distribution of ACSCs was similar, dementia status and impact were not evaluated. In this study, we were not able to determine how many of the individual ACSCs were actually preventable. Verification of preventability would have required chart review and adjudication of the preventability of each admission, activities that were beyond the scope of our study. Lastly, we did not examine discharge diagnoses according to other potentially relevant groupings (e.g., medical vs. surgical diagnoses; elective vs. emergency admissions).
These limitations not withstanding, this study has several noteworthy strengths: Incident, research-based, dementia diagnosis; follow-up of most participants until death; a large sample size; a control group from the same population-based, longitudinal cohort of community dwelling elders as dementia cases; average follow-up of over 8 years and a relatively long duration of follow-up (>3 years, on average) post-dementia diagnosis; complete capture of hospitalizations through GHC’s automated data warehouse 24; a comprehensive assessment of discharge diagnoses across the spectrum of diagnostic codes, which permits comparisons with other studies 25; a complete spectrum of dementia types, rather than a single etiology; and high completeness of follow-up for the overall cohort, which minimizes misclassification bias.
In summary, our findings that persons with dementia have higher rates of hospitalizations for most categories of medical illness and for ACSCs suggest that there may be important opportunities for improving care of demented older persons, including developing better strategies for delivering anticipatory, proactive primary care to this population. The characteristic feature of late life dementia cognitive impairment in the face of multiple other comorbidities – presents a special challenge not currently addressed in models of chronic disease care.
Supplementary Material
Acknowledgments
Funding/Support: This research was conducted while Dr. Phelan was a K23 recipient from the National Institute on Aging and a Paul Beeson Physician Faculty Scholars in Aging Research Award recipient. Dr. Borson received support from the University of Washington Alzheimer’s Disease Research Center (NIA P50 AG 005136). The Adult Changes in Thought study is supported by NIA UO1 AG 06781 (PI: Dr. Larson).
Funders’ Role: The funding agencies had no role in the design or conduct of the study, in the collection, management, analysis, or interpretation of the data, or in the preparation, review, or approval of the manuscript.
Footnotes
Conflicts of Interest: None of the authors has any financial or other conflicts of interest.
Author Contributions:
Lou Grothaus had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design: Borson, Grothaus, Phelan
Acquisition of data: Larson
Analysis and interpretation of data: Balch, Borson, Grothaus, Larson, Phelan
Drafting of the manuscript: Phelan
Critical review of the manuscript: Balch, Borson, Grothaus, Larson
Statistical analysis: Balch, Grothaus
Obtained funding: Larson, Phelan
Supervision: Borson, Larson
Contributor Information
Soo Borson, Email: soob@u.washington.edu.
Louis Grothaus, Email: grothaus.l@ghc.org.
Steven Balch, Email: balch.s@ghc.org.
Eric B. Larson, Email: larson.e@ghc.org.
References
- 1.Albert SM, Costa R, Merchant C, Small S, Jenders RA, Stern Y. Hospitalization and Alzheimer’s disease: results from a community-based study. J Gerontol A Biol Sci Med Sci. 1999;54(5):M267–271. doi: 10.1093/gerona/54.5.m267. [DOI] [PubMed] [Google Scholar]
- 2.Lyketsos CG, Sheppard JM, Rabins PV. Dementia in elderly persons in a general hospital. Am J Psychiatry. 2000;157(5):704–707. doi: 10.1176/appi.ajp.157.5.704. [DOI] [PubMed] [Google Scholar]
- 3.Bynum JP, Rabins PV, Weller W, Niefeld M, Anderson GF, Wu AW. The relationship between a dementia diagnosis, chronic illness, medicare expenditures, and hospital use. J Am Geriatr Soc. 2004;52(2):187–194. doi: 10.1111/j.1532-5415.2004.52054.x. [DOI] [PubMed] [Google Scholar]
- 4.Hill J, Fillit H, Shah SN, del Valle MC, Futterman R. Patterns of healthcare utilization and costs for vascular dementia in a community-dwelling population. J Alzheimers Dis. 2005;8(1):43–50. doi: 10.3233/jad-2005-8105. [DOI] [PubMed] [Google Scholar]
- 5.Zhao Y, Kuo TC, Weir S, Kramer MS, Ash AS. Healthcare costs and utilization for Medicare beneficiaries with Alzheimer’s. BMC Health Serv Res. 2008;8:108. doi: 10.1186/1472-6963-8-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Natalwala A, Potluri R, Uppal H, Heun R. Reasons for hospital admissions in dementia patients in Birmingham, UK, during 2002–2007. Dement Geriatr Cogn Disord. 2008;26(6):499–505. doi: 10.1159/000171044. [DOI] [PubMed] [Google Scholar]
- 7.Malone DC, McLaughlin TP, Wahl PM, et al. Burden of Alzheimer’s disease and association with negative health outcomes. Am J Manag Care. 2009;15(8):481–488. [PubMed] [Google Scholar]
- 8.Gutterman EM, Markowitz JS, Lewis B, Fillit H. Cost of Alzheimer’s disease and related dementia in managed-Medicare. J Am Geriatr Soc. 1999;47(9):1065–1071. doi: 10.1111/j.1532-5415.1999.tb05228.x. [DOI] [PubMed] [Google Scholar]
- 9.Tuppin P, Kusnik-Joinville O, Weill A, Ricordeau P, Allemand H. Primary health care use and reasons for hospital admissions in dementia patients in France: database study for 2007. Dement Geriatr Cogn Disord. 2009;28(3):225–232. doi: 10.1159/000238394. [DOI] [PubMed] [Google Scholar]
- 10.Ehlenbach WJ, Hough CL, Crane PK, et al. Association between acute care and critical illness hospitalization and cognitive function in older adults. JAMA. 2010;303(8):763–770. doi: 10.1001/jama.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inouye SK. Delirium in older persons. N Engl J Med. 2006;354(11):1157–1165. doi: 10.1056/NEJMra052321. [DOI] [PubMed] [Google Scholar]
- 12.Sager MA, Rudberg MA, Jalaluddin M, et al. Hospital admission risk profile (HARP): identifying older patients at risk for functional decline following acute medical illness and hospitalization. J Am Geriatr Soc. 1996;44(3):251–257. doi: 10.1111/j.1532-5415.1996.tb00910.x. [DOI] [PubMed] [Google Scholar]
- 13.Lefevre F, Feinglass J, Potts S, et al. Iatrogenic complications in high-risk, elderly patients. Arch Intern Med. 1992;152(10):2074–2080. [PubMed] [Google Scholar]
- 14.Larson EB, Kukull WA, Teri L, et al. University of Washington Alzheimer’s Disease Patient Registry (ADPR): 1987–1988. Aging (Milano) 1990;2(4):404–408. [PubMed] [Google Scholar]
- 15.Kukull WA, Higdon R, Bowen JD, et al. Dementia and Alzheimer’s disease incidence: a prospective cohort study. Arch Neurol. 2002;59(11):1737–1746. doi: 10.1001/archneur.59.11.1737. [DOI] [PubMed] [Google Scholar]
- 16.Clark TG, Altman DG, De Stavola BL. Quantification of the completeness of follow-up. Lancet. 2002;359(9314):1309–1310. doi: 10.1016/s0140-6736(02)08272-7. [DOI] [PubMed] [Google Scholar]
- 17.Larson EB, Wang L, Bowen JD, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144(2):73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- 18.Breitner JC, Haneuse SJ, Walker R, et al. Risk of dementia and AD with prior exposure to NSAIDs in an elderly community-based cohort. Neurology. 2009;72(22):1899–1905. doi: 10.1212/WNL.0b013e3181a18691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jorm AF, Korten AE. Assessment of cognitive decline in the elderly by informant interview. Br J Psychiatry. 1988;152(2):209–213. doi: 10.1192/bjp.152.2.209. [DOI] [PubMed] [Google Scholar]
- 20.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 21.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 22.Breslow NE. Statistics in epidemiology: the case-control study. J Am Stat Assoc. 1996;91(433):14–28. doi: 10.1080/01621459.1996.10476660. [DOI] [PubMed] [Google Scholar]
- 23.Breslow NE, Lubin JH, Marek P, Langholz B. Multiplicative models and cohort analysis. J Am Stat Assoc. 1983;78(381):1–12. [Google Scholar]
- 24.Saunders KW, Davis RL, Stergachis A. Group Health Cooperative. In: Pharmacoepidemiology. 4. Strom BL, editor. Hoboken, NJ: John Wiley and Sons; 2005. pp. 223–240. [Google Scholar]
- 25.Russo CA, Elixhauser A. Hospitalizations in the Elderly Population, 2003. [Accessed July 21, 2006.];Statistical Brief #6. 2006 May; http://www.hcup-us.ahrq.gov/reports/statbriefs/sb6.pdf. [PubMed]
- 26.Bindman AB, Grumbach K, Osmond D, et al. Preventable hospitalizations and access to health care. JAMA. 1995;274(4):305–311. [PubMed] [Google Scholar]
- 27.Oster A, Bindman AB. Emergency department visits for ambulatory care sensitive conditions: insights into preventable hospitalizations. Med Care. 2003;41(2):198–207. doi: 10.1097/01.MLR.0000045021.70297.9F. [DOI] [PubMed] [Google Scholar]
- 28.Zhan C, Miller MR, Wong H, Meyer GS. The effects of HMO penetration on preventable hospitalizations. Health Serv Res. 2004;39(2):345–361. doi: 10.1111/j.1475-6773.2004.00231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saha S, Solotaroff R, Oster A, Bindman AB. Are preventable hospitalizations sensitive to changes in access to primary care? the case of the Oregon Health Plan. Med Care. 2007;45(8):712–719. doi: 10.1097/MLR.0b013e318053717c. [DOI] [PubMed] [Google Scholar]
- 30.Culler SD, Parchman ML, Przybylski M. Factors related to potentially preventable hospitalizations among the elderly. Med Care. 1998;36(6):804–817. doi: 10.1097/00005650-199806000-00004. [DOI] [PubMed] [Google Scholar]
- 31.McCall N, Harlow J, Dayhoff D. Rates of hospitalization for ambulatory care sensitive conditions in the Medicare+Choice population. Health Care Financ Rev. 2001;22(3):127–145. [PMC free article] [PubMed] [Google Scholar]
- 32.Billings J, Zeitel L, Lukomnik J, Carey TS, Blank AE, Newman L. Impact of socioeconomic status on hospital use in New York City. Health Aff (Millwood) 1993;12(1):162–173. doi: 10.1377/hlthaff.12.1.162. [DOI] [PubMed] [Google Scholar]
- 33.Weissman JS, Gatsonis C, Epstein AM. Rates of avoidable hospitalization by insurance status in Massachusetts and Maryland. JAMA. 1992;268(17):2388–2394. [PubMed] [Google Scholar]
- 34.Kruzikas DT, Jiang HJ, Remus D, Barrett ML, Coffey RM, Andrews R. [Accessed January 28, 2011.];Preventable Hospitalizations: A Window into Primary and Preventive Care. 2000 http://www.ahrq.gov/data/hcup/factbk5/
- 35.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10(2):77–84. [PubMed] [Google Scholar]
- 36.Fishman PA, Goodman MJ, Hornbrook MC, Meenan RT, Bachman DJ, O’Keeffe Rosetti MC. Risk adjustment using automated ambulatory pharmacy data: the RxRisk model. Med Care. 2003;41(1):84–99. doi: 10.1097/00005650-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 37.Clark DO, Von Korff M, Saunders K, Baluch WM, Simon GE. A chronic disease score with empirically derived weights. Med Care. 1995;33(8):783–795. doi: 10.1097/00005650-199508000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Food and Drug Administration. [Accessed November 26, 2011.];National Drug Code directory webpage. http://www.fda.gov/cder/ndc.
- 39.Sloan KL, Sales AE, Liu CF, et al. Construction and characteristics of the RxRisk-V: a VA-adapted pharmacy-based case-mix instrument. Med Care. 2003;41(6):761–774. doi: 10.1097/01.MLR.0000064641.84967.B7. [DOI] [PubMed] [Google Scholar]
- 40.Sales AE, Liu CF, Sloan KL, et al. Predicting costs of care using a pharmacy-based measure risk adjustment in a veteran population. Med Care. 2003;41(6):753–760. doi: 10.1097/01.MLR.0000069502.75914.DD. [DOI] [PubMed] [Google Scholar]
- 41.Liu CF, Sales AE, Sharp ND, et al. Case-mix adjusting performance measures in a veteran population: pharmacy- and diagnosis-based approaches. Health Serv Res. 2003;38(5):1319–1337. doi: 10.1111/1475-6773.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gilmer T, Kronick R, Fishman P, Ganiats TG. The Medicaid Rx model: pharmacy-based risk adjustment for public programs. Med Care. 2001;39(11):1188–1202. doi: 10.1097/00005650-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Teng EL, Hasegawa K, Homma A, et al. The Cognitive Abilities Screening Instrument (CASI): a practical test for cross-cultural epidemiological studies of dementia. Int Psychogeriatr. 1994;6(1):45–58. doi: 10.1017/s1041610294001602. [DOI] [PubMed] [Google Scholar]
- 44.Hilbe J. Negative Binomial Regression. New York, NY: Cambridge University Press; 2007. [Google Scholar]
- 45.Davison AC. Statistical Models. New York, NY: Cambridge University Press; 2003. [Google Scholar]
- 46.Rudolph JL, Zanin NM, Jones RN, et al. Hospitalization in community-dwelling persons with Alzheimer’s disease: frequency and causes. J Am Geriatr Soc. 2010;58(8):1542–1548. doi: 10.1111/j.1532-5415.2010.02924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bradford A, Kunik ME, Schulz P, Williams SP, Singh H. Missed and delayed diagnosis of dementia in primary care: prevalence and contributing factors. Alzheimer Dis Assoc Disord. 2009;23(4):306–314. doi: 10.1097/WAD.0b013e3181a6bebc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harris DP, Chodosh J, Vassar SD, Vickrey BG, Shapiro MF. Primary care providers’ views of challenges and rewards of dementia care relative to other conditions. J Am Geriatr Soc. 2009;57(12):2209–2216. doi: 10.1111/j.1532-5415.2009.02572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Inouye SK, Bogardus ST, Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669–676. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 50.Taylor DH, Jr, Sloan FA, Doraiswamy PM. Marked increase in Alzheimer’s disease identified in medicare claims records between 1991 and 1999. J Gerontol A Biol Sci Med Sci. 2004;59(7):762–766. doi: 10.1093/gerona/59.7.m762. [DOI] [PubMed] [Google Scholar]
- 51.Wagner EH. Population-based management of diabetes care. Patient Educ Couns. 1995;26(1–3):225–230. doi: 10.1016/0738-3991(95)00761-n. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.