Abstract
The hypothalamus is a small structure located in the ventral diencephalon. Hypothalamic neurons sense changes in circulating metabolic cues (e.g.: leptin, insulin, glucose), and coordinate responses aimed at maintaining normal body weight and glucose homeostasis. Recent findings indicate that a nicotinamide adenine dinucleotide (NAD+)-dependent protein deacetylase (namely, SIRT1) expressed by hypothalamic neurons is crucial for mounting responses against diet-induced obesity and type 2 diabetes mellitus (T2DM). Here, the repercussions of these findings will be discussed and particular emphasis will be given to the potential exploitation of hypothalamic SIRT1 as a target for the treatment of the rapidly-spreading metabolic disorders of obesity and T2DM. The possible roles of hypothalamic SIRT1 on regulating metabolic ageing processes will also be addressed.
Keywords: Hypothalamus, SIRT1, aging, metabolism, obesity, diabetes, insulin, skeletal muscle
SIRT1: An ancient metabolic-sensor protein with modern value
Silent Information Regulator 2 (SIR2; also known as mating-type regulator 1) of yeast Saccharomyces cerevisae is the first sirtuin protein being discovered (1). Orthologs of SIR2 can be found in several organisms as for example mammals, plants, bacteria, worms, flies, and fish. Humans and rodents have seven orthologs of SIR2, named SIRT1 to SIRT7 (2). Mammalian sirtuins are found in virtually all tissues, yet they are selectively localized at the subcellular level. In fact, SIRT3, 4 and 5 are localized to the mitochondrion, SIRT1, 6 and 7 are nuclear, and SIRT2 (and possibly SIRT1) is cytosolic (3). Because of their broad distribution in nature, it has been proposed that sirtuins exert important protective roles aimed at guaranteeing organismal survival, hence the suggested reason for their conservation throughout evolution (3-7).
Sirtuins are enzymes able to exert deacetylation and/or mono-ADP ribosylation of their target proteins (6, 8, 9). Very recently, novel enzymatic activities have been attributed to this class of proteins, as SIRT5 was found to also have protein lysine desuccinylase and demalonylase functions (10, 11). Therefore, it is possible that these proteins carry out other yet-to-be-identified types of post-translational modifications. Of note, sirtuins use nicotinamide adenine dinucleotide (NAD+) as co-substrate (6, 8, 9). And, due to their dependence on NAD+ and the fact that targets of sirtuins include histones, transcription factors, cofactors, proteins involved in oxidative phosphorylation, circadian clock regulators and many others (3), these enzymes are thought to link the redox status to gene expression, activity, and fate of the cell. Therefore, sirtuins are to be considered bona fide metabolic-sensor proteins as for example mammalian target of rapamycin (mTOR) and AMP-activated kinase (AMPK), two proteins that also are evolutionarily well-conserved.
It must be noted however that, in addition to changes in the amounts of available NAD+, the activity of sirtuins is also influenced by other modifications as for example by changes in their association/dissociation status with other proteins (e.g.: deleted in breast cancer-1) (12) and/or following their own post-translational modification(s) (e.g.: adrenergic-meditated phosphorylation of SIRT1 has been shown to change its catalytic activity) (13). Thus, an emerging view is that variations in the activity of sirtuins could also occur independently of changes in cellular levels of NAD+; provided that the amount of NAD+ is sufficient to guaranteeing the execution of their enzymatic actions.
Owing to their metabolic-sensor status, interests on this class of proteins significantly mounted over the last decade. Indeed, it has been proposed that sirtuins are the molecular link between calorie-restriction (CR) and the improved health and longevity brought on by this feeding regimen; an idea still much debated (14, 15). Regardless to the actual roles (if any) of sirtuins on ageing, the activity of SIRT1 (the focus of this review) seems to increase in several but not all tissues following CR or fasting (16-20). In these low-energy states, SIRT1 has been suggested to be crucial for carrying out physiological adaptive responses, as for example the switch from glucose to lipid oxidation in skeletal muscle (21) and liver (22), the increase in hepatic glucose production (19), and the mobilization of lipids from adipose tissue (23). In addition, SIRT1 appears to be important for executing physiological adaptive responses to high-energy states, as for example after prolonged feeding on hypercaloric diets (24-27). The latter function is of particular relevance to modern human physiology and pathophysiology, as people often feed on hypercaloric diets that cause obesity (Box 1) and type 2 diabetes mellitus (T2DM; Box 2) (28). Thus, SIRT1 could represent an ideal molecular target for the treatment of diet-induced obesity and T2DM (see below).
Text box 1.
Obesity: disease characteristics.
Obesity is a metabolic disorder characterized by a positive energy imbalance. This defect is due to increased food intake (hyperphagia), or reduced energy expenditure (hypometabolic rate), or both. Of note, even small changes in food intake and/or energy expenditure over a long period of time could cause obesity (28). The World Health Organization indicates that an adult with a Body Mass Index (BMI = body weight in kilograms divided by the square of the height in meters) equal or greater than 30 is obese. Due to its high incidence and serious co-morbidities as for example heart disease and diabetes, obesity is a serious threat to human health. With the exception of very rare monogenic forms (e.g. leptin or leptin receptor loss-of-function mutations), the primary defects causing this metabolic disease are still incompletely understood (83). Nevertheless, a common feature of obese people is elevated levels of circulating leptin indicating that resistance to the hormone is in part involved in the etiology of the disease. This leptin resistance is very likely caused by chronic feeding on hypercaloric diets (52) and precludes the use of leptin as a therapeutic tool against obesity, as clearly indicated by the fact that obese individuals respond poorly to leptin treatment (84, 85). Thus, obesity is a very difficult disease to treat and another major roadblock for the development of effective anti-obesity drugs is the anatomical overlap between brain circuitries controlling feeding and hedonic/reward pathways. Therefore, drugs aimed at curtailing food intake often cause serious psychotropic side effects as for example increased risk of developing depression and/or suicidal thoughts (28). Another untoward effect of anti-obesity drugs is detrimental action on the cardiovascular system. For example, increased incidence of valvular heart defects were observed after administration of fenfluramine-phentermine and an increased risk of myocardial infarction and stroke were seen after treatment with sibutramine (86, 87).
Text box 2.
Type 2 diabetes mellitus: disease characteristics.
Type 2 diabetes mellitus (T2DM) is an illness characterized by insulin resistance and elevated blood levels of glucose (hyperglycemia), insulin (hyperinsulinemia) and lipids (hypertriglyceridemia) (88). Due to its high incidence and serious co-morbidities as for example heart disease, higher risk of developing cancer (e.g.: pancreatic and hepatic tumors), retinopathy, and nephropathy, T2DM is a serious threat to human health. Several treatments are available to patients with T2DM. Probably, the most widely prescribed anti-T2DM drug is metformin, a biguanide that suppresses glucose output from the liver (89, 90). Other anti-T2DM drugs include thiazolidinediones, synthetic ligands to the nuclear receptor/transcription factor peroxisome proliferator-activated receptor gamma (PPARγ) known to increase insulin sensitivity (91), as well as insulin and insulin secretagogues (e.g.: sulphonylureas). Although metformin does not cause major side effects, thiazolidinediones have been shown to cause harmful effects on the heart while insulin and sulphonylureas are prone to induce hypoglycemia. Furthermore, it is common that the dose of each of the current anti-T2DM drugs (alone or in combination) tend to increase with the progression of the disease hence causing an increase in the likelihood of inducing side effects (92).
Metabolic diseases and the hypothalamus
We are in the midst of an epidemic of metabolic diseases; indeed it is estimated that hundreds of million people are affected by either obesity, or T2DM, or both. This is most likely one of the worst man-made epidemics and the result of the introduction of low-cost, easily-accessible calorie-rich foods, combined with reduced needs to performing physical activity. It must be noted however that some, albeit rare, forms of obesity and/or T2DM have a strong genetic underlying origin, as most clearly illustrated by the phenotypes displayed by rodents and humans homozygous for loss-of-function mutation in the leptin or the leptin receptor gene (29-33).
To effectively diminish the incidence of obesity and/or T2DM, changes in feeding habits and lifestyles could be all is needed. However, this seemingly simple approach appears to be much harder to accomplish than anticipated, likely because of socio-economical, environmental, and cultural reasons. Thus, understanding the endogenous mechanisms that prevent the insurgence of increased body adiposity and impaired glucose homeostasis induced by chronic feeding on hypercaloric foods (hereafter referred to as diet-induced metabolic imbalance), and the reasons why these defense pathways are going awry in nowadays environment, is of paramount medical importance.
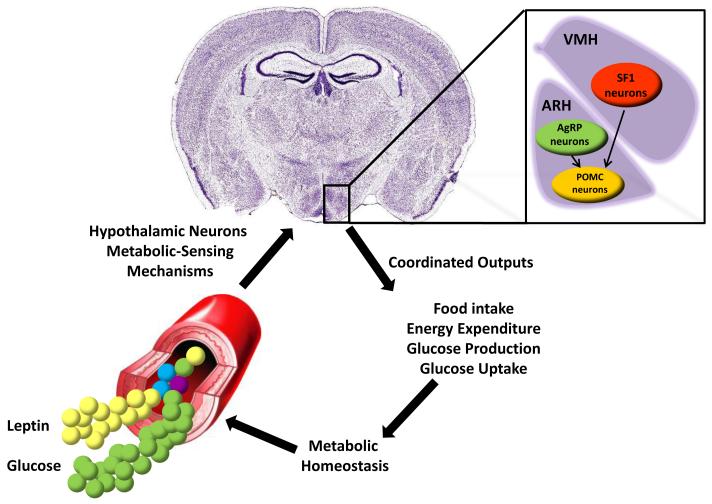
A critical anatomical site in which cells able to monitor changes in energy status of the body and trigger responses aimed at maintaining metabolic homeostasis are found is the hypothalamus (Figure 1). Located in the ventral diencephalon, the hypothalamus borders with the optic chiasm (rostrally), the optic tracts and cerebral peduncles (laterally) and the mammilary bodies (caudally). Numerous types of cells and neurons populate the hypothalamus. Some of these hypothalamic neurons secreted peptide that stimulate food intake (orexigenic neurons) whereas others produce appetite suppressant molecules (anorectic neurons). One classical example of neuronal types that are morphologically very similar and anatomically located in the same hypothalamic site but exert opposite effects on feeding behavior is represented by the pro-opiomelanocortin (POMC) and the agouti-related peptide (AgRP) neurons. Indeed, the peptide α-melanocyte stimulating hormone (α-MSH) secreted from POMC neurons activates whereas AgRP inhibits melanocortin 3 and 4 receptors (MC3R and MC4R). As a result, α-MSH suppresses feeding whereas AgRP promotes food intake (34-37). Of note, POMC and AgRP neurons are oppositely regulated by circulating metabolic cues as for example the adipose-tissue-secreted hormone leptin that activates POMC neurons (38) and inhibits AgRP neurons (39-41). In addition to POMC and AgRP neurons that reside in the arcuate nucleus of the hypothalamus (ARH), neurons in other hypothalamic nuclei are also crucial for maintaining normal metabolic homeostasis. Another example of these metabolically-relevant cells is represented by neurons expressing steroidogenic factor 1 (SF1). SF1 neurons are present in the ventromedial hypothalamic nucleus (VMH) and known to respond to changes in circulating leptin and other metabolic cues (42). As AgRP and VMH neurons project to POMC neurons (43), it is likely that VMH and ARH neurons communicate to each other as part of an elaborated neuronal circuitry underlying normal metabolic homeostasis (Figure 1).
Figure 1. A model depicting hypothalamic-mediated control of metabolic homeostasis.
AgRP and POMC neurons in the hypothalamic arcuate nucleus (ARH) and SF1 neurons in the ventromedial hypothalamic nucleus (VMH) are able to detect fluctuations in circulating levels of metabolic cues (e.g.: leptin and glucose) and to coordinate responses (e.g.: changes in food intake, energy expenditure, glucose production from the liver and glucose uptake by the skeletal muscle) aimed at maintaining normal body weight and glucose balance. Depicted are also projections from AgRP and VMH neurons to POMC neurons.
A key homeostatic behavioral adaptation against diet-induced metabolic imbalance is reduced amounts of ingested food; an effect that is in part mediated by leptin action on SF1-expressing neurons (42). Other defense mechanisms include increased energy expenditure; a homeostatic autonomic adaptation triggered by POMC- and SF1-expressing neurons (24, 25, 42, 44). By engaging the sympathetic nervous system, these hypothalamic neurons enhance amount and activity of the calorie-burning brown adipocytes in several fat depots (25, 44), including the interscapular brown adipose tissue (iBAT; a prominent tissue located between the shoulder blades in rodents) and visceral and subcutaneous depots previously thought to be only made of calorie-stockpiling white adipocytes (45-49). Unfortunately, in modern societies, the search for calorie-rich foods induced by stress and/or hedonic/reward pathways may override homeostatic behavioral adaptation, thus shift the energy balance towards the accruing arm, and hence favor the development of obesity and/or T2DM (50). Also, chronic feeding on a hypercaloric diet leads to altered metabolic-sensing (e.g.: glucose- and leptin-sensing) mechanisms in hypothalamic neurons responsible for safeguarding normal energy and glucose homeostasis; another defect that likely contribute to the impairment of the aforementioned homeostatic behavioral and autonomic adaptations hence leading to obesity and T2DM (51-53).
Hypothalamic SIRT1: An endogenous weapon in the arsenal against metabolic imbalance
Because obesity and T2DM probably emerged long time after sirtuins first appeared in nature (3, 7), it seems that is by serendipity that the ancient protein SIRT1 exerts protecting actions against diet-induced metabolic imbalance (24-26). Interestingly, the metabolic effects of SIRT1 are cell-type-specific and environment dependent. Indeed, the reported roles of SIRT1 on body weight are disparate. For example, it was recently shown that SIRT1 deletion or overexpression in selected hypothalamic neurons such as POMC or SF1 neurons does not bring about any changes on body weight or adiposity in mice that are fed on a regular chow diet, while it impacts these parameters in the hypercaloric diet feeding context (24, 25). Specifically, these data indicate that in POMC and SF1 neurons, SIRT1 is a key molecule that selectively and properly controls homeostatic autonomic (energy expenditure) adaptations against diet-induced obesity (24, 25). Interestingly, while SIRT1 in POMC neurons is required for normal BAT-like remodeling of the perigonadal fat depot, SIRT1 in SF1 neurons seems to induce futile cycles in skeletal muscle (24, 25). These results also indicate that SIRT1 in POMC and SF1 neurons does not regulate food intake in either normal or hypercaloric diet feeding contexts (24, 25). SIRT1 in other, non-POMC and non-SF1 hypothalamic neurons, may however affect feeding behavior. Indeed, Cakir and colleagues showed that reduced hypothalamic SIRT1 contents suppresses food intake in rodents fed a regular diet (54), an effect likely due to diminished SIRT1 activity in AgRP neurons (55). Thus, from a therapeutic perspective, the aforementioned results would suggest that SIRT1-activating compounds effective in restraining body weight gain must target only SF1 and POMC neurons, while avoiding the AgRP-expressing neuronal group. Another possible advantage brought on by avoiding harnessing SIRT1-dependent pathways in AgRP neurons is to help reduce the risks of developing psychotropic side effects that are typical untoward actions of anorectic anti-obesity drugs (28) (Box 1).
In addition to protecting against dietary obesity, hypothalamic SIRT1 is also important for mounting homeostatic responses against dietary diabetes. In fact, mice overexpressing SIRT1 only in SF1 neurons are protected from developing diet-induced insulin resistance in skeletal muscle and hyperglycemia whereas mice lacking SIRT1 in these same neurons are more prone to develop dietary diabetes (24). Furthermore, intracerebroventricular delivery of resveratrol, a natural polyphenolic molecule known to activate SIRT1 (56, 57), improves dietary diabetes in rodents (58) via mechanisms that require the presence of SIRT1 in hypothalamus (59). Collectively, these results indicate that the “ancient” SIRT1 in hypothalamic neurons is crucial for triggering effective defense responses against the development of diet-induced metabolic imbalance (Figure 2).
Figure 2. SIRT1 in POMC and SF1 neurons is a crucial molecule for triggering efficacious responses against diet-induced metabolic imbalance.
Hypothalamic arcuate nucleus (ARH) POMC neurons selectively govern brown adipose tissue (BAT)-like remodeling of perigonadal fat while ventromedial hypothalamic nucleus (VMH) SF1 neurons selectively control skeletal muscle insulin sensitivity. The proper execution of these hypothalamic-mediated actions requires the presence of SIRT1 in POMC and SF1 neurons.
Metabolic actions of SIRT1 in peripheral tissues
In addition to the brain, SIRT1 is expressed in virtually every peripheral tissue of the mammalian body. And, a large body of literature has established peripheral SIRT1 as an important regulator of metabolic function. Interestingly, the reported roles of peripheral SIRT1 on glucose homeostasis appear to be distinct and tissue-specific. For example, several studies indicate that hepatic SIRT1 is a main molecule for triggering gluconeogenic function. In fact, hepatocytes overexpressing SIRT1 have higher contents of mRNAs whose product are enzymes of the gluconeogenic pathway, compared to hepatocytes containing normal SIRT1 levels (26). These findings are in agreement with data shown by Shulman and colleagues, who reported that rats affected by T2DM and bearing reduced levels of liver SIRT1 have lower hepatic glucose production and improved diabetes, compared to controls (60). Also, SIRT1 is known to deacetylate and thus activate peroxisome proliferator activated receptor γ (PPARγ) coactivator 1α (PGC-1α) that stimulates the expression of gluconeogenic genes (19). Therefore, at a first glance, the results that either pharmacologically- or genetically-induced whole-body SIRT1 activation protects from dietary diabetes appear at odds with data indicating SIRT1 as a positive regulator of hepatic gluconeogenic function (26, 27, 61, 62). One possible explanation for this apparent conundrum is that SIRT1 actually does not activate but rather inhibits hepatic gluconeogenesis, as suggested by Montminy and collaborators (63). However, in case SIRT1 is indeed a positive regulator of the gluconeogenic program, its activation in liver should be avoided in diabetic subjects who frequently have increased hepatic gluconeogenesis. On the other hand, considering the fact that this enzyme in myocytes has been suggested to positively regulate fatty acid oxidation, enhance O2 consumption (21, 64), and potentiate the insulin sensitizing actions of adiponectin (65), SIRT1 activation in skeletal muscle is expected to be beneficial for the treatment of diabetes. The roles of SIRT1 in adipocytes on glucose metabolism are currently uncertain. SIRT1 suppresses the secretion of the insulin sensitizer hormone adiponectin from adipocytes (66). Also, by inhibiting the activity of PPARγ (a key regulator of adipogenesis) SIRT1 exerts an inhibitory effect on adipocyte differentiation and fat tissue accrual (23). Reduced fat mass is usually accompanied with reduced levels of leptin that is a positive regulator of glucose homeostasis as clearly indicated by the remarkable beneficial results of leptin therapy on glycemic control in lipodystrophic humans (67, 68). Therefore, based on the aforementioned results, activation of SIRT1 in adipocytes is expected to cause the very negative combination of reduced leptin and adiponectin levels that should bring about increased insulin resistance and hyperglycemia; an effect that is clearly unwanted in diabetic subjects. Future investigation directed to assess the real contribution of SIRT1 in liver, skeletal muscle, and adipose tissue in mediating the anti-diabetic actions of resveratrol and other SIRT1-activating compounds are therefore warranted.
The roles SIRT1 exerts on body lipid metabolism are also peculiar. Recently, Qiang and colleagues reported that addition of an extra copy of Sirt1 gene engenders a predisposition to developing more severe aortic atherosclerotic lesions in mice fed on a cholesterol-rich diet; an effect probably mediated by increased hepatic lipid synthesis and secretion (69). These results are in line with previous findings showing that SIRT1 deletion causes reduced hepatic and circulating lipid levels (70) but are at odds with results of Pfluger and collaborators indicating that high-fat-diet-induced hepatic lipid accumulation is diminished in a different SIRT1 overexpressor mouse model (62). Differential SIRT1 overexpression in various tissues could explain the diverse outcomes observed by Qiang and colleagues and Pfluger and collaborators. Interestingly, SIRT1 overexpression only in endothelial cells has been suggested to protect against atherosclerotic lesions (71). Therefore, it appears as if SIRT1-activating compounds should not target the liver but perhaps the endothelium, if they are to exert positive actions against diet-induced atherosclerosis.
Does hypothalamic SIRT1 regulate ageing processes?
The answer to this question may depend on the environment the organism lives in. For example, the amount and type of food ingested greatly impacts the ageing process, as clearly demonstrated by the fact that CR prolongs lifespan in organisms from yeasts to monkeys (72-77) and by inference likely in humans as well. In contrast, hypercaloric feeding causes metabolic imbalance, accelerates the pace of ageing thus causing shorten mammalian lifespan (27). Because a significant proportion of the human population currently lives in obesogenic and diabetogenic environments in which calorie-rich foods are easily and inexpensively available (28), the roles of hypothalamic SIRT1 on ageing should be investigated in the context of hypercaloric feeding if these studies are to be relevant to the physiology and pathophysiology of nowadays humans.
Ageing processes are multiple and heterogeneous. For example, an age-dependent tendency to a decline in BAT content and activity in fat depots, and reduced insulin sensitivity, are components of the metabolic aging process. These defects are thought to increase the probability of developing diseases in which age is a risk factor as for example obesity and T2DM (78). Because SIRT1 is known to affect metabolic function and several groups have shown that brain-mediated mechanisms link changes in energy intake to lifespan (79-81), hypothalamic SIRT1 seems to be perfectly placed to coordinate metabolic ageing processes. Indeed, studies indicate that at least two facets of metabolic ageing are controlled by hypothalamic SIRT1 in the obesogenic and diabetogenic hypercaloric feeding environment. As mentioned above, BAT content and activity in humans varies with age; they are both high early-on in life, while they tend to decrease by age (46, 82). This effect has also been observed in mice (78). Interestingly, young-adult mice lacking SIRT1 only in POMC neurons have reduced BAT in perigonadal fat, and as such possess an aged-like perigonadal fat at a young age (25). This defect is physiologically relevant as it predisposes to developing obesity (25). Impaired insulin sensitivity is also associated with ageing and a process that appears also to be influenced by hypothalamic SIRT1. In fact, young-adult mice lacking SIRT1 only in SF1 neurons have reduced skeletal muscle insulin sensitivity, and as such possess aged-like skeletal muscle at a young age (24). Conversely, aged mice overexpressing SIRT1 only in SF1 neurons have enhanced skeletal muscle insulin sensitivity and therefore display young-like skeletal muscle at an old age (24). This SIRT1-in-SF1-neuron-skeletal-muscle circuitry is physiologically relevant as it protects against the development of diet-induced T2DM (24).
Therefore, despite the recent controversy pertinent to the putative role of sirtuins on promoting longevity (14), the abovementioned results would suggest that, in mammals, the metabolic-sensor protein SIRT1 in metabolic-sensing hypothalamic neurons is a crucial component of the mechanisms underlying mammalian lifespan; at least in obesogenic and diabetogenic environments. Future studies will be required to establish the role (if any) of hypothalamic sirtuins on longevity in mammalian organisms. These studies will need to include measurements of lifespan in mice bearing POMC- and/or SF1-neuron-specific loss- or gain-of-function mutations in Sirt1.
Concluding remarks
Recent findings have indicated that SIRT1 in POMC and SF1 neurons protects against dietary metabolic defects such as obesity and T2DM. The roles of SIRT1 in peripheral tissues on metabolic regulation have also been investigated. However, while some findings support, others counter, the idea that SIRT1 activation in specific peripheral tissues is beneficial in the context of metabolic diseases. Based on the findings discussed above, it seems reasonable that ways to delivering SIRT1 agonists selectively in specific cells (e.g.: POMC- and SF1-expressing neurons, myocytes and endothelial cells) should be developed. The cellular selectivity of these compounds would in theory guarantee that only the beneficial effects of SIRT1 activation, for example improved body adiposity and diabetes in obesogenic and diabetogenic environments, are achieved, while the deleterious ones, such as changes in food intake, increases in glucose and lipids outputs from the liver, are avoided. Unfortunately, cell-selective SIRT1-activating drugs are not yet available. Future efforts should be made to find means by which these drugs are tailored in a cell-specific manner if only the beneficial effects of SIRT1 activation are to be achieved.
To better understand the biology and physiological relevance of mammalian sirtuins, several outstanding questions will also need to be addressed. These include the following: i) what are the intracellular mechanisms by which SIRT1 in hypothalamic neurons promotes anti-obesity and -T2DM actions? Some data are already available and indicate that this protein regulates the sensitivity of hypothalamic neurons to hormones (e.g. leptin) and neuropeptides (e.g.: orexin) (24, 25). However, much more needs to be done to fully elucidate the intracellular pathways regulated by SIRT1 in hypothalamic neurons; ii) how is the activity of hypothalamic SIRT1 regulated by hypercaloric feeding? It is indeed interesting and somewhat counterintuitive that a protein known to be activated in the low-energy state is key for preventing diseases induced in the high-energy state (a condition in which the activity of SIRT1 is predicted to be reduced). One possible explanation for this apparent conundrum is that the activity of SIRT1 in the hypothalamic neurons that have been studied is actually increased following high-fat diet feeding. This counterintuitive regulation of SIRT1 could be due to the fact that high-fat diet feeding leads to an increase in NAD+ in those neurons and/or to hormonal changes that bring about post-translation modification(s) of SIRT1 that ultimately lead to enhanced activity. Alternatively, SIRT1 activity is suppressed in those neurons following high-fat diet feeding; yet the residual SIRT1 activity is required for mounting appropriate responses against diet-induced obesity and diabetes. As mentioned above, no data are currently available to support of reject either hypotheses; thus, future experiments will be required to address this important question; iii) why the effects of genetic manipulation of hypothalamic SIRT1 are mainly observed in the high-energy state? As discussed above, one possible explanation is that SIRT1 in hypothalamic neurons is more active following high-fat diet feeding. Other possibilities include the following: in the chow-fed state compensatory mechanisms are able to counterbalance the defects brought on by lack of SIRT1 in hypothalamic neurons. This phenomenon could explain the lack of metabolic imbalance in mutants fed on a chow diet. However, in the context of high-fat diet feeding these compensatory mechanisms may not be able to prevent the whole-body metabolic effects caused by lack of SIRT1 in hypothalamic neurons. Supporting this notion are data indicating that chow-fed mice lacking SIRT1 only in SF1 neurons have reduced insulin sensitivity in skeletal muscle; yet they display normal glycemia. This is explained (at least in part) by the fact that insulin sensitivity is, on the other hand, enhanced in the liver; an aberrancy that may well be part of compensatory mechanisms aimed at maintaining blood glucose levels normal in chow-fed mutants. In the high-fat-feeding state, insulin sensitivity is reduced in the skeletal muscle but not up-regulated in liver, hence the manifestation of hyperglycemia in mice lacking SIRT1 only in SF1 neurons fed on a high-fat diet (24). Because the available data are not sufficient to support of reject these hypotheses, additional experiments will be required to address this important question; iv) does hypothalamic SIRT1 regulate lifespan? Experiments aimed at determining if the lifespan of mice lacking or overexpressing SIRT1 in discrete hypothalamic neuronal groups are already in process. While we wait for these experiments to be concluded, the idea that the metabolic-sensor protein SIRT1 in metabolic-sensing POMC and/or SF1 neurons is a crucial component of the mechanisms underlying mammalian lifespan seems to be an exciting possibility.
In summary, regardless to whether hypothalamic SIRT1 governs lifespan or not, current data pinpoint this enzyme in hypothalamic POMC and SF1 neurons as an attractive molecular target for drugs aimed at treating the rapidly-spreading metabolic disorders of obesity and T2DM.
Outstanding Questions box.
What are the intracellular mechanisms by which SIRT1 in hypothalamic neurons promotes anti-obesity and -T2DM actions?
How is the activity of hypothalamic SIRT1 regulated by hypercaloric feeding?
Why the effects of genetic manipulation of hypothalamic SIRT1 are mainly observed in the high-energy state?
Does hypothalamic SIRT1 regulate lifespan?
Acknowledgements
This work was supported by the American Heart Association (Scientist Development Grant to R.C.) and the National Institutes of Health (DK080836 to R.C.). I thank Drs. Giorgio Ramadori and Claudia R. Vianna at The University of Texas Southwestern Medical Center, Dallas, TX, USA for suggestions and critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The author of this manuscript has no conflict of interests to declare.
References
- 1.Klar AJ, Fogel S, Macleod K. MAR1-a Regulator of the HMa and HMalpha Loci in SACCHAROMYCES CEREVISIAE. Genetics. 1979;93:37–50. doi: 10.1093/genetics/93.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frye RA. Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem Biophys Res Commun. 2000;273:793–798. doi: 10.1006/bbrc.2000.3000. [DOI] [PubMed] [Google Scholar]
- 3.Ramadori G, Coppari R. Pharmacological manipulations of CNS sirtuins: potential effects on metabolic homeostasis. Pharmacol Res. 2010;62:48–54. doi: 10.1016/j.phrs.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rogina B, Helfand SL, Frankel S. Longevity regulation by Drosophila Rpd3 deacetylase and caloric restriction. Science. 2002;298:1745. doi: 10.1126/science.1078986. [DOI] [PubMed] [Google Scholar]
- 5.Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci U S A. 2004;101:15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- 7.Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol. 2010;5:253–295. doi: 10.1146/annurev.pathol.4.110807.092250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liszt G, Ford E, Kurtev M, Guarente L. Mouse Sir2 homolog SIRT6 is a nuclear ADP-ribosyltransferase. J Biol Chem. 2005;280:21313–21320. doi: 10.1074/jbc.M413296200. [DOI] [PubMed] [Google Scholar]
- 9.North BJ, Marshall BL, Borra MT, Denu JM, Verdin E. The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol Cell. 2003;11:437–444. doi: 10.1016/s1097-2765(03)00038-8. [DOI] [PubMed] [Google Scholar]
- 10.Du J, Zhou Y, Su X, Yu JJ, Khan S, Jiang H, Kim J, Woo J, Kim JH, Choi BH, et al. Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science. 2011;334:806–809. doi: 10.1126/science.1207861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng C, Lu Z, Xie Z, Cheng Z, Chen Y, Tan M, Luo H, Zhang Y, He W, Yang K, et al. The first identification of lysine malonylation substrates and its regulatory enzyme. Mol Cell Proteomics. 2011 doi: 10.1074/mcp.M111.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Escande C, Chini CC, Nin V, Dykhouse KM, Novak CM, Levine J, van Deursen J, Gores GJ, Chen J, Lou Z, et al. Deleted in breast cancer-1 regulates SIRT1 activity and contributes to high-fat diet-induced liver steatosis in mice. J Clin Invest. 2010;120:545–558. doi: 10.1172/JCI39319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerhart-Hines Z, Dominy JE, Jr., Blattler SM, Jedrychowski MP, Banks AS, Lim JH, Chim H, Gygi SP, Puigserver P. The cAMP/PKA Pathway Rapidly Activates SIRT1 to Promote Fatty Acid Oxidation Independently of Changes in NAD(+) Mol Cell. 2011;44:851–863. doi: 10.1016/j.molcel.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burnett C, Valentini S, Cabreiro F, Goss M, Somogyvari M, Piper MD, Hoddinott M, Sutphin GL, Leko V, McElwee JJ, et al. Absence of effects of Sir2 overexpression on lifespan in C. elegans and Drosophila. Nature. 2011;477:482–485. doi: 10.1038/nature10296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guarente L. SIR2 and aging--the exception that proves the rule. Trends Genet. 2001;17:391–392. doi: 10.1016/s0168-9525(01)02339-3. [DOI] [PubMed] [Google Scholar]
- 16.Ramadori G, Lee CE, Bookout AL, Lee S, Williams KW, Anderson J, Elmquist JK, Coppari R. Brain SIRT1: anatomical distribution and regulation by energy availability. J Neurosci. 2008;28:9989–9996. doi: 10.1523/JNEUROSCI.3257-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- 18.Satoh A, Brace CS, Ben-Josef G, West T, Wozniak DF, Holtzman DM, Herzog ED, Imai S. SIRT1 promotes the central adaptive response to diet restriction through activation of the dorsomedial and lateral nuclei of the hypothalamus. J Neurosci. 2010;30:10220–10232. doi: 10.1523/JNEUROSCI.1385-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 20.Chen D, Bruno J, Easlon E, Lin SJ, Cheng HL, Alt FW, Guarente L. Tissue-specific regulation of SIRT1 by calorie restriction. Genes Dev. 2008;22:1753–1757. doi: 10.1101/gad.1650608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, Alt FW, Wu Z, Puigserver P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. Embo J. 2007;26:1913–1923. doi: 10.1038/sj.emboj.7601633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Purushotham A, Schug TT, Xu Q, Surapureddi S, Guo X, Li X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009;9:327–338. doi: 10.1016/j.cmet.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R, Leid M, McBurney MW, Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramadori G, Fujikawa T, Anderson J, Berglund ED, Frazao R, Michan S, Vianna CR, Sinclair DA, Elias CF, Coppari R. SIRT1 Deacetylase in SF1 Neurons Protects against Metabolic Imbalance. Cell Metab. 2011;14:301–312. doi: 10.1016/j.cmet.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramadori G, Fujikawa T, Fukuda M, Anderson J, Morgan DA, Mostoslavsky R, Stuart RC, Perello M, Vianna CR, Nillni EA, et al. SIRT1 deacetylase in POMC neurons is required for homeostatic defenses against diet-induced obesity. Cell Metab. 2010;12:78–87. doi: 10.1016/j.cmet.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banks AS, Kon N, Knight C, Matsumoto M, Gutierrez-Juarez R, Rossetti L, Gu W, Accili D. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab. 2008;8:333–341. doi: 10.1016/j.cmet.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vianna CR, Coppari R. A treasure trove of hypothalamic neurocircuitries governing body weight homeostasis. Endocrinology. 2011;152:11–18. doi: 10.1210/en.2010-0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coppari R, Ichinose M, Lee CE, Pullen AE, Kenny CD, McGovern RA, Tang V, Liu SM, Ludwig T, Chua SC, Jr., et al. The hypothalamic arcuate nucleus: a key site for mediating leptin’s effects on glucose homeostasis and locomotor activity. Cell Metab. 2005;1:63–72. doi: 10.1016/j.cmet.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, Hughes IA, McCamish MA, O’Rahilly S. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med. 1999;341:879–884. doi: 10.1056/NEJM199909163411204. [DOI] [PubMed] [Google Scholar]
- 31.Farooqi IS, Wangensteen T, Collins S, Kimber W, Matarese G, Keogh JM, Lank E, Bottomley B, Lopez-Fernandez J, Ferraz-Amaro I, et al. Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N Engl J Med. 2007;356:237–247. doi: 10.1056/NEJMoa063988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ingalls AM, Dickie MM, Snell GD. Obese, a new mutation in the house mouse. J Hered. 1950;41:317–318. doi: 10.1093/oxfordjournals.jhered.a106073. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 34.Coll AP, Farooqi IS, Challis BG, Yeo GS, O’Rahilly S. Proopiomelanocortin and energy balance: insights from human and murine genetics. J Clin Endocrinol Metab. 2004;89:2557–2562. doi: 10.1210/jc.2004-0428. [DOI] [PubMed] [Google Scholar]
- 35.Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 36.Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- 37.Cone RD, Lu D, Koppula S, Vage DI, Klungland H, Boston B, Chen W, Orth DN, Pouton C, Kesterson RA. The melanocortin receptors: agonists, antagonists, and the hormonal control of pigmentation. Recent Prog Horm Res. 1996;51:287–317. discussion 318. [PubMed] [Google Scholar]
- 38.Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 39.Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz MW, Baskin DG, Bukowski TR, Kuijper JL, Foster D, Lasser G, Prunkard DE, Porte D, Jr., Woods SC, Seeley RJ, et al. Specificity of leptin action on elevated blood glucose levels and hypothalamic neuropeptide Y gene expression in ob/ob mice. Diabetes. 1996;45:531–535. doi: 10.2337/diab.45.4.531. [DOI] [PubMed] [Google Scholar]
- 41.Stephens TW, Basinski M, Bristow PK, Bue-Valleskey JM, Burgett SG, Craft L, Hale J, Hoffmann J, Hsiung HM, Kriauciunas A, et al. The role of neuropeptide Y in the antiobesity action of the obese gene product. Nature. 1995;377:530–532. doi: 10.1038/377530a0. [DOI] [PubMed] [Google Scholar]
- 42.Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 43.Sternson SM, Shepherd GM, Friedman JM. Topographic mapping of VMH --> arcuate nucleus microcircuits and their reorganization by fasting. Nat Neurosci. 2005;8:1356–1363. doi: 10.1038/nn1550. [DOI] [PubMed] [Google Scholar]
- 44.Plum L, Rother E, Munzberg H, Wunderlich FT, Morgan DA, Hampel B, Shanabrough M, Janoschek R, Konner AC, Alber J, et al. Enhanced leptin-stimulated Pi3k activation in the CNS promotes white adipose tissue transdifferentiation. Cell Metab. 2007;6:431–445. doi: 10.1016/j.cmet.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 45.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Enerback S. Brown adipose tissue in humans. Int J Obes (Lond) 2010;34(Suppl 1):S43–46. doi: 10.1038/ijo.2010.183. [DOI] [PubMed] [Google Scholar]
- 47.Spiegelman BM, Enerback S. The adipocyte: a multifunctional cell. Cell Metab. 2006;4:425–427. doi: 10.1016/j.cmet.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 48.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 49.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 50.Chuang JC, Perello M, Sakata I, Osborne-Lawrence S, Savitt JM, Lutter M, Zigman JM. Ghrelin mediates stress-induced food-reward behavior in mice. J Clin Invest. 2011;121:2684–2692. doi: 10.1172/JCI57660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Zhang CY, Xu C, Vianna CR, Balthasar N, Lee CE, et al. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature. 2007;449:228–232. doi: 10.1038/nature06098. [DOI] [PubMed] [Google Scholar]
- 52.Munzberg H, Flier JS, Bjorbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology. 2004;145:4880–4889. doi: 10.1210/en.2004-0726. [DOI] [PubMed] [Google Scholar]
- 53.Enriori PJ, Evans AE, Sinnayah P, Jobst EE, Tonelli-Lemos L, Billes SK, Glavas MM, Grayson BE, Perello M, Nillni EA, et al. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab. 2007;5:181–194. doi: 10.1016/j.cmet.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 54.Cakir I, Perello M, Lansari O, Messier NJ, Vaslet CA, Nillni EA. Hypothalamic Sirt1 regulates food intake in a rodent model system. PLoS One. 2009;4:e8322. doi: 10.1371/journal.pone.0008322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dietrich MO, Antunes C, Geliang G, Liu ZW, Borok E, Nie Y, Xu AW, Souza DO, Gao Q, Diano S, et al. Agrp neurons mediate Sirt1’s action on the melanocortin system and energy balance: roles for Sirt1 in neuronal firing and synaptic plasticity. J Neurosci. 2010;30:11815–11825. doi: 10.1523/JNEUROSCI.2234-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J. 2007;404:1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 58.Ramadori G, Gautron L, Fujikawa T, Vianna CR, Elmquist JK, Coppari R. Central Administration of Resveratrol Improves Diet-Induced Diabetes. Endocrinology. 2009 doi: 10.1210/en.2009-0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Knight CM, Gutierrez-Juarez R, Lam TK, Arrieta-Cruz I, Huang L, Schwartz G, Barzilai N, Rossetti L. Mediobasal Hypothalamic SIRT1 Is Essential for Resveratrol’s Effects on Insulin Action in Rats. Diabetes. 2011;60:2691–2700. doi: 10.2337/db10-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Erion DM, Yonemitsu S, Nie Y, Nagai Y, Gillum MP, Hsiao JJ, Iwasaki T, Stark R, Weismann D, Yu XX, et al. SirT1 knockdown in liver decreases basal hepatic glucose production and increases hepatic insulin responsiveness in diabetic rats. Proc Natl Acad Sci U S A. 2009;106:11288–11293. doi: 10.1073/pnas.0812931106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 62.Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschop MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci U S A. 2008;105:9793–9798. doi: 10.1073/pnas.0802917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu Y, Dentin R, Chen D, Hedrick S, Ravnskjaer K, Schenk S, Milne J, Meyers DJ, Cole P, Yates J, 3rd, et al. A fasting inducible switch modulates gluconeogenesis via activator/coactivator exchange. Nature. 2008;456:269–273. doi: 10.1038/nature07349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Iwabu M, Yamauchi T, Okada-Iwabu M, Sato K, Nakagawa T, Funata M, Yamaguchi M, Namiki S, Nakayama R, Tabata M, et al. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca(2+) and AMPK/SIRT1. Nature. 2010;464:1313–1319. doi: 10.1038/nature08991. [DOI] [PubMed] [Google Scholar]
- 66.Qiang L, Wang H, Farmer SR. Adiponectin secretion is regulated by SIRT1 and the endoplasmic reticulum oxidoreductase Ero1-L alpha. Mol Cell Biol. 2007;27:4698–4707. doi: 10.1128/MCB.02279-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shimomura I, Hammer RE, Ikemoto S, Brown MS, Goldstein JL. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature. 1999;401:73–76. doi: 10.1038/43448. [DOI] [PubMed] [Google Scholar]
- 68.Oral EA, Simha V, Ruiz E, Andewelt A, Premkumar A, Snell P, Wagner AJ, DePaoli AM, Reitman ML, Taylor SI, et al. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346:570–578. doi: 10.1056/NEJMoa012437. [DOI] [PubMed] [Google Scholar]
- 69.Qiang L, Lin HV, Kim-Muller JY, Welch CL, Gu W, Accili D. Proatherogenic Abnormalities of Lipid Metabolism in SirT1 Transgenic Mice Are Mediated through Creb Deacetylation. Cell Metab. 2011 doi: 10.1016/j.cmet.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li X, Zhang S, Blander G, Tse JG, Krieger M, Guarente L. SIRT1 deacetylates and positively regulates the nuclear receptor LXR. Mol Cell. 2007;28:91–106. doi: 10.1016/j.molcel.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 71.Zhang QJ, Wang Z, Chen HZ, Zhou S, Zheng W, Liu G, Wei YS, Cai H, Liu DP, Liang CC. Endothelium-specific overexpression of class III deacetylase SIRT1 decreases atherosclerosis in apolipoprotein E-deficient mice. Cardiovasc Res. 2008;80:191–199. doi: 10.1093/cvr/cvn224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lin SJ, Kaeberlein M, Andalis AA, Sturtz LA, Defossez PA, Culotta VC, Fink GR, Guarente L. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature. 2002;418:344–348. doi: 10.1038/nature00829. [DOI] [PubMed] [Google Scholar]
- 73.Klass MR. Aging in the nematode Caenorhabditis elegans: major biological and environmental factors influencing life span. Mech Ageing Dev. 1977;6:413–429. doi: 10.1016/0047-6374(77)90043-4. [DOI] [PubMed] [Google Scholar]
- 74.Bross TG, Rogina B, Helfand SL. Behavioral, physical, and demographic changes in Drosophila populations through dietary restriction. Aging Cell. 2005;4:309–317. doi: 10.1111/j.1474-9726.2005.00181.x. [DOI] [PubMed] [Google Scholar]
- 75.Terzibasi E, Lefrancois C, Domenici P, Hartmann N, Graf M, Cellerino A. Effects of dietary restriction on mortality and age-related phenotypes in the short-lived fish Nothobranchius furzeri. Aging Cell. 2009;8:88–99. doi: 10.1111/j.1474-9726.2009.00455.x. [DOI] [PubMed] [Google Scholar]
- 76.McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of life span and upon the ultimate body size. Nutrition. 1989;5:155–171. 1935. discussion 172. [PubMed] [Google Scholar]
- 77.Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ramadori G, Coppari R. Does hypothalamic SIRT1 regulate aging? Aging (Albany NY) 2011;3:325–328. doi: 10.18632/aging.100311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Durieux J, Wolff S, Dillin A. The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell. 2011;144:79–91. doi: 10.1016/j.cell.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bishop NA, Guarente L. Genetic links between diet and lifespan: shared mechanisms from yeast to humans. Nat Rev Genet. 2007;8:835–844. doi: 10.1038/nrg2188. [DOI] [PubMed] [Google Scholar]
- 81.Fridell YW, Sanchez-Blanco A, Silvia BA, Helfand SL. Targeted expression of the human uncoupling protein 2 (hUCP2) to adult neurons extends life span in the fly. Cell Metab. 2005;1:145–152. doi: 10.1016/j.cmet.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 82.Horan MA, Little RA, Rothwell NJ, Stock MJ. Changes in body composition, brown adipose tissue activity and thermogenic capacity in BN/BiRij rats undergoing senescence. Exp Gerontol. 1988;23:455–461. doi: 10.1016/0531-5565(88)90057-5. [DOI] [PubMed] [Google Scholar]
- 83.O’Rahilly S. Human genetics illuminates the paths to metabolic disease. Nature. 2009;462:307–314. doi: 10.1038/nature08532. [DOI] [PubMed] [Google Scholar]
- 84.Heymsfield SB, Greenberg AS, Fujioka K, Dixon RM, Kushner R, Hunt T, Lubina JA, Patane J, Self B, Hunt P, et al. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA. 1999;282:1568–1575. doi: 10.1001/jama.282.16.1568. [DOI] [PubMed] [Google Scholar]
- 85.Hukshorn CJ, Saris WH, Westerterp-Plantenga MS, Farid AR, Smith FJ, Campfield LA. Weekly subcutaneous pegylated recombinant native human leptin (PEG-OB) administration in obese men. J Clin Endocrinol Metab. 2000;85:4003–4009. doi: 10.1210/jcem.85.11.6955. [DOI] [PubMed] [Google Scholar]
- 86.James WP, Caterson ID, Coutinho W, Finer N, Van Gaal LF, Maggioni AP, Torp-Pedersen C, Sharma AM, Shepherd GM, Rode RA, et al. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med. 2010;363:905–917. doi: 10.1056/NEJMoa1003114. [DOI] [PubMed] [Google Scholar]
- 87.Connolly HM, Crary JL, McGoon MD, Hensrud DD, Edwards BS, Edwards WD, Schaff HV. Valvular heart disease associated with fenfluramine-phentermine. N Engl J Med. 1997;337:581–588. doi: 10.1056/NEJM199708283370901. [DOI] [PubMed] [Google Scholar]
- 88.Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 2008;7:95–96. doi: 10.1016/j.cmet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 89.Mihaylova MM, Vasquez DS, Ravnskjaer K, Denechaud PD, Yu RT, Alvarez JG, Downes M, Evans RM, Montminy M, Shaw RJ. Class IIa Histone Deacetylases Are Hormone-Activated Regulators of FOXO and Mammalian Glucose Homeostasis. Cell. 2011;145:607–621. doi: 10.1016/j.cell.2011.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, Cantley LC. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Choi JH, Banks AS, Estall JL, Kajimura S, Bostrom P, Laznik D, Ruas JL, Chalmers MJ, Kamenecka TM, Bluher M, et al. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARgamma by Cdk5. Nature. 2010;466:451–456. doi: 10.1038/nature09291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lebovitz HE. Type 2 diabetes mellitus-current therapies and the emergence of surgical options. Nat Rev Endocrinol. 2011;7:408–419. doi: 10.1038/nrendo.2011.10. [DOI] [PubMed] [Google Scholar]