Abstract
Background
Little is known about patterns of kidney function decline leading up to initiation of chronic dialysis.
Study Design
Retrospective cohort study.
Setting and Participants
5,606 VA patients who initiated chronic dialysis in 2001–2003. Predictor: Trajectory of estimated glomerular filtration rate (eGFR) during the two year period before dialysis initiation.
Outcomes and Measurements
Patient characteristics and care practices before and at the time of dialysis initiation and survival after initiation.
Results
We identified four distinct trajectories of eGFR during the two year period before dialysis initiation: 62.8% of patients had persistently low levels of eGFR below 30 ml/min/1.73 m2 (mean eGFR slope 7.7 ±4.7 (SD) ml/min/1.73 m2 per year); 24.6% had progressive loss of eGFR from levels around 30–59 ml/min/1.73 m2 (mean eGFR slope 16.3 ±7.6 ml/min/1.73 m2 per year); 9.5% had accelerated loss of eGFR from levels above 60 ml/min/1.73 m2 (mean eGFR slope 32.3 ±13.4 ml/min/1.73 m2 per year); and 3.1% experienced catastrophic loss of eGFR within six months or less from levels above 60 ml/min/1.73 m2. Patients with steeper eGFR trajectories were more likely to have been hospitalized and to have an inpatient diagnosis of acute kidney injury. They were less likely to have received recommended pre-dialysis care and had a higher risk of death in the first year after dialysis initiation.
Conclusions
There is substantial heterogeneity in patterns of kidney function loss leading up to initiation of chronic dialysis, perhaps calling for a more flexible approach toward preparing for end-stage renal disease.
An understanding of illness trajectories preceding a seminal event can help to guide clinical decision-making, to shape goals of care and to anticipate service needs.1, 2 Trajectories of kidney function before initiation of chronic dialysis may help to clarify the optimal timing of preparatory interventions (e.g., nephrology referral, vascular access placement, transplant referral) and to guide treatment choices and priorities.3,4
Prospective studies among patients with non-dialysis dependent chronic kidney disease (CKD) point to substantial heterogeneity in rates of progression to end-stage renal disease (ESRD), defined as initiation of chronic dialysis or receipt of a kidney transplant.5–12 Many patients have non-progressive or slowly progressive disease and never reach ESRD.5–9 At the opposite extreme, some patients progress rapidly to ESRD as a result of an irreversible episode of acute kidney injury.10–12 Only a few studies have described patterns of kidney function loss among patients who have gone on to initiate chronic dialysis.13–15 These studies focused primarily on the frequency of acute kidney injury (AKI) at the time of dialysis initiation. To our knowledge, no prior studies have provided a detailed description of the full spectrum of different chronic trajectories of kidney function leading up to dialysis initiation.
We used trajectory modeling to describe trajectories of kidney function over the two year period before initiation of chronic dialysis among members of a large integrated healthcare system. Our goals were to estimate the frequency of different kidney function trajectories, and to define the relationship of these trajectories to patient characteristics, care practices and mortality after onset of ESRD.
METHODS
Patients and Data Sources
We used data from the VA and the United States Renal Data System (USRDS),16 to identify 6,253 patients aged 18 years and older who initiated chronic dialysis between October 1, 2001 and December 31, 2003 (more recent USRDS data are not currently available for Veteran cohorts) and had at least one serum creatinine measurement at a VA medical center within 45 days of dialysis initiation. Of these, we identified a subset of 5742 (87%) patients with at least two serum creatinine measurements distributed over at least two different quarters during the two year period before dialysis initiation. We excluded a further 136 patients who were missing information on co-morbid conditions in USRDS data sources, yielding an analytic cohort of 5,606 patients. Serum creatinine measurements were obtained from the VA Decision Support System Laboratory Results file (DSS LAR; a record of all laboratory results obtained within the VA system).
We used information from the USRDS Patients’ and Medical Evidence files to define patient characteristics and treatment modality at the time of onset of ESRD.16 Measures of healthcare utilization during the two year period before initiation of chronic dialysis were ascertained from VA administrative files (that include all episodes of inpatient and outpatient care within the VA and outside the VA if paid for by the VA) and from Medicare inpatient and outpatient claims for patients eligible for Medicare. Urine protein dipstick measurements were obtained from the VA Corporate Data Warehouse and death data were obtained from the VA Vital Status File, a comprehensive source of death data for Veterans.17
Predictor variable
As described in greater detail in the analysis section, groups of patients with similar trajectories of estimated glomerular filtration rate (eGFR) during the two year period before initiation of chronic dialysis (defined as the first ESRD service date from USRDS) were identified using trajectory modeling. In order to characterize each patient’s chronic eGFR trajectory, we modeled their median eGFR during each successive quarter preceding dialysis initiation based on all available inpatient and outpatient serum creatinine measures within the VA. Glomerular filtration rate was estimated using the four-variable Modification of Diet in Renal Disease (MDRD) Study equation based on age at the time of serum creatinine measurement, race, and gender.18
Study Outcomes
We examined the association of eGFR trajectory with patient characteristics, care practices and mortality after dialysis initiation as follows:
Patient characteristics
Patient characteristics included age, race (white, black, and other), gender, co-morbid conditions (diabetes, ischemic heart disease, congestive heart failure, stroke, peripheral arterial disease, pulmonary disease and cancer), functional status (able to transfer or ambulate), listed cause of ESRD (diabetes, hypertension, glomerulonephritis, cystic diseases, urologic, acute tubular necrosis, hematologic malignancy, “other”, and unknown), number of hospitalizations, and percentage of patients and proportion of hospitalizations with an inpatient diagnostic code for AKI and (ICD-9 codes 584.5–584.9). We also characterized each patient’s level of proteinuria based on the results of their most recent urine dipstick assessment prior to dialysis initiation.
Care practices
Care practices included whether, when, and how often each patient had an outpatient visit to a nephrologist during the two year period before dialysis initiation, whether patients who initiated hemodialysis had undergone a permanent vascular access procedure (graft or fistula), whether dialysis was initiated in the hospital, and if so, whether there was a diagnostic code for AKI associated with that hospitalization, each patient’s most recent eGFR within 45 days of dialysis initiation as reported to USRDS, and first dialysis modality (center hemodialysis vs. peritoneal dialysis or home hemodialysis).
Mortality after dialysis initiation
For each group defined by trajectory modeling, we estimated the adjusted hazard for death after dialysis initiation. Death data were ascertained through July 4, 2011.
Analysis
In contrast with traditional longitudinal models which are designed to describe average effects, trajectory modeling can be used to identify distinct patterns of change within a population and to estimate each individual’s probability of assignment to each trajectory identified.1, 19, 20 We used trajectory modeling to identify distinct patterns of eGFR before dialysis initiation among cohort patients. Trajectory modeling was implemented using the SAS procedure PROC TRAJ (which fits a semi-parametric (discrete) mixture model to longitudinal data with the use of the maximum-likelihood method) using a censored normal model.19, 20 Because this procedure could not accommodate the large amount of variability in the timing and frequency of serum creatinine measurements obtained in the clinical setting, we modeled the trajectory of each patient’s median quarterly eGFR. The Bayesian information criterion (BIC) was used to test models with up to six trajectories and to determine whether each trajectory was best fit by intercept only (i.e., constant) or by linear, quadratic, cubic or higher level polynomial terms.19, 20 Among models with similar performance based on the BIC, we preferentially selected the model with the greater number of trajectories. Patients were assigned to the trajectory group for which they had the maximum estimated probability of assignment. An assignment probability of 0.9 or greater was considered to be an excellent fit and 0.7 or less a poor fit.1, 2 We assessed the face validity of trajectory assignments based on median quarterly eGFR as follows: 1) we used a repeated measures linear mixed model to estimate each patient’s eGFR slope based on all inpatient and outpatient serum creatinine measures during the two year period before dialysis initiation; 2) we fitted locally weighted scatterplot smoothing (LOWESS) curves to all eGFR measures for randomly selected patients in each trajectory group; and 3) we graphed individual trajectories based on all serum creatinine measures for randomly selected patients in each trajectory group.
We used logistic regression analysis to measure the adjusted association of trajectory group with binary patient characteristics and care practices at the time of onset of ESRD or during the preceding two year period. These analyses were adjusted for patient demographics, co-morbid conditions at dialysis initiation and listed cause of ESRD. For each trajectory group, we estimated median survival using the Kaplan-Meier product limit method. We used a Cox proportional hazards model to evaluate the association of eGFR trajectory with survival after dialysis initiation after adjustment for patient characteristics at onset of ESRD and care practices before and at this time. Hazard ratios are presented for discrete time periods during follow-up in order to accommodate a violation in the proportional hazards assumption for the primary predictor variable.
Sensitivity analyses
We performed the following sensitivity analyses to test the robustness of study findings. To determine whether differences in the frequency of serum creatinine measurements may have influenced study results, we repeated the primary analyses after stratification by number of serum creatinine measurements (by quartile). We also repeated the primary analysis among cohorts with a minimum of 3 serum creatinine measurements in 3 different quarters within one and two years before cohort entry, respectively. Analyses were performed with the use of SAS (version 9.2) and Stata (version 11.1, StataCorp, College Station, TX) statistical software.
Results
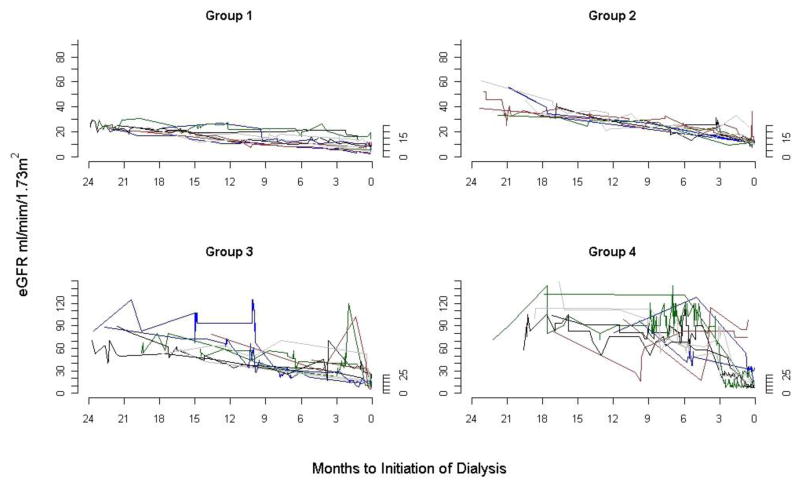
Among the 5,606 cohort members, there were a median of 20 (25th–75th percentile, 12–34) serum creatinine measurements during the two year period before dialysis initiation. On average, patients had at least one serum creatinine measurement in 5 of the 8 quarters before dialysis initiation. The median eGFR slope for all cohort members was 10 (25th–75th percentile, 5.8 to 16.6) ml/min/1.73 m2 per year. We identified four distinct trajectories of eGFR among cohort members (Figure 1, Table 1). Most patients (62.8%) had persistently low levels of eGFR below 30 ml/min/1.73 m2 (Group 1). These patients had a mean eGFR slope of 7.7 ±4.7 (SD) ml/min/1.73 m2 per year. A further 24.6% of patients had progressive loss of eGFR from levels around 30–59 ml/min/1.73 m2 (Group 2). These patients had a mean eGFR slope of 16.3 ±7.6 ml/min/1.73 m2 per year. A further 9.5% of patients had accelerated loss of eGFR from levels above 60 ml/min/1.73 m2 (Group 3). These patients had a mean eGFR slope of 32.3 ±13.4 ml/min/1.73 m2 per year. Finally, 3.1% experienced catastrophic loss of eGFR from levels above 60 ml/min/1.73 m2 within six months or less and had a mean eGFR slope of 50.7 ±30.4 ml/min/1.73/m2 per year (Group 4). Trajectories of median quarterly eGFR corresponded well with LOWESS curves generated using all eGFR values from a random sample of patients in each trajectory group (Figure 2) and with eGFR trajectories for individual patients (Figure 3). There was substantial intraindividual variability in eGFR values around long-term eGFR slopes. This was especially true for those with the most rapid loss of eGFR, even when comparing patients with similar numbers of serum creatinine measurements (results not shown).
Figure 1. eGFR trajectories and 95% confidence intervals defined by trajectory modeling. Dotted lines denote 95% confidence intervals.
Trajectory Group 1 (persistently low levels of eGFR): 63% of patients with a mean probability of assignment 0.88 (±0.24)
Trajectory Group 2 (progressive loss of eGFR): 25% of patients with a mean probability of assignment 0.86 (±0.27)
Trajectory Group 3 (accelerated loss of eGFR): 9% of patients with a mean probability of assignment 0.91 (±0.25)
Trajectory Group 4 (catastrophic loss of eGFR): 3% of patients with a mean probability of assignment 0.99 (±0.11)
Table 1.
Information on kidney function during the two year period before cohort entry
| Parameter | All patients (n=5,606) | Trajectory group | |||
|---|---|---|---|---|---|
| Persistently low eGFR (Group 1, n=3,520) | Progressive eGFR loss (Group 2, n=1,379) | Accelerated eGFR loss (Group 3, n=535) | Catastrophic eGFR loss (Group 4, n=172) | ||
| eGFR slope + | 10.0 (5.8, 16.6) | 7.2 (4.5, 10.5) | 16.0 (11.6, 20.9) | 31.7 (23.8, 40.2) | 47.7 (29.1, 64.6) |
| eGFR slope+ | 13.5±13.1 | 7.8±4.7 | 16.3±7.6 | 32.3±13.4 | 50.7±30.4 |
| SCr measures, median ++ | |||||
| All | 20 (12, 34) | 18 (12, 30) | 24 (11, 46) | 24 (12, 44) | 25 (8, 42) |
| Outpatient | 12 (7, 18) | 13 (7, 19) | 8 (5, 14) | 6 (3, 10) | 6 (3, 10) |
| Inpatient | 6 (0, 17) | 4 (0, 12) | 9 (1, 23) | 14 (3, 32) | 15 (2, 33) |
| Quarters with >=1 SCr measure ++ | 5.4±2 | 5.6 ±2.1 | 5.5±1.8 | 4.8±1.8 | 4.1±1.9 |
Values shown are median (25th, 75th percentile) or mean ± SD.
Abbreviations: SCr, serum creatinine; eGFR estimated glomerular filtration rate
Based on all inpatient and outpatient serum creatinine measures during the two year period before dialysis initiation
During the two year period (8 quarters) before dialysis initiation
Figure 2.
Scatter plots of all eGFR measurements during the two year period before dialysis initiation for 25 randomly selected members of each trajectory group
Figure 3.
Individual eGFR trajectories for 10 randomly selected members of each trajectory group
Patients with more rapid loss of eGFR were younger but there were not large differences in the racial and gender composition of trajectory groups (Table 2). The prevalence of all co-morbid conditions except cancer was highest for Group 2. During the two year period before initiation, patients with more rapid loss of eGFR were hospitalized more often and were more likely to have a diagnosis of AKI while hospitalized. Patients with accelerated or catastrophic loss of eGFR (Groups 3 & 4) were less likely than the other groups to have ESRD due to diabetes or hypertension, and more likely to have ESRD due to acute tubular necrosis, multiple myeloma/light chain nephropathy, “other” or unknown causes. Heavy proteinuria was most common among patients with slower loss of eGFR, but was common in all groups.
Table 2.
Patient characteristics at initiation of chronic dialysis by eGFR trajectory group
| Characteristic | All patients (n=5,606) | Trajectory group | |||
|---|---|---|---|---|---|
| Persistently low eGFR (Group 1, n=3,520) | Progressive eGFR loss (Group 2, n=1,379) | Accelerated eGFR loss (Group 3, n=535) | Catastrophic eGFR loss (Group 4, n=172) | ||
| Age (years) | 66.2 ±11.5 | 66.8 ±11.4 | 65.7±11.6 | 64.1±11.4 | 64.1±11.0 |
| Age category | |||||
| <50 y | 8.2 | 7.5 | 9.0 | 10.8 | 9.3 |
| 50–59 y | 25.0 | 23.1 | 27.5 | 29.5 | 29.7 |
| 60–74 y | 40.2 | 40.9 | 38.1 | 39.1 | 44.8 |
| ≥ 75 y | 26.6 | 28.5 | 25.4 | 20.6 | 16.3 |
| Race | |||||
| White | 63.0 | 63.4 | 62.4 | 60.6 | 66.5 |
| Black | 34.3 | 33.6 | 35.4 | 37.2 | 32.3 |
| Other | 2.7 | 3.0 | 2.2 | 2.2 | 1.2 |
| Female | 1.6 | 1.8 | 1.5 | 1.3 | 0.6 |
| Diabetes | 50.0 | 49.9 | 56.5 | 42.6 | 22.7 |
| Ischemic heart disease | 30.9 | 30.3 | 33.9 | 28.8 | 26.2 |
| Heart failure | 29.0 | 26.1 | 36.2 | 31.8 | 24.4 |
| Stroke | 11.6 | 11.5 | 13.2 | 10.5 | 5.8 |
| Peripheral vascular disease | 16.2 | 15.0 | 19.7 | 16.5 | 11.6 |
| Pulmonary disease | 10.4 | 9.4 | 12.3 | 12.0 | 12.2 |
| Cancer | 8.6 | 8.0 | 8.6 | 11.2 | 14.0 |
| Unable to ambulate or transfer | 5.1 | 3.8 | 6.4 | 9.2 | 8.1 |
| Cause of ESRD | |||||
| Diabetes | 49.8 | 52.1 | 53.7 | 35.5 | 16.9 |
| Hypertension | 27.3 | 29.0 | 25.7 | 24.1 | 15.1 |
| Glomerulonephritis | 6.5 | 6.4 | 5.9 | 7.7 | 9.3 |
| Cystic disease | 1.6 | 2.5 | 0.2 | 0.2 | 0 |
| Urologic cause | 2.5 | 2.3 | 2.5 | 3.7 | 4.1 |
| Acute tubular necrosis | 2.5 | 0.5 | 2.7 | 9.2 | 20.4 |
| Hematologic malignancy+ | 1.9 | 0.8 | 2.0 | 6.4 | 10.5 |
| Other | 4.1 | 3.3 | 3.3 | 8.6 | 14.0 |
| Unknown | 3.8 | 3.3 | 4.1 | 4.7 | 9.9 |
| Urine protein++ | |||||
| Negative or trace | 12 | 8.7 | 16.5 | 16.5 | 27.9 |
| 1or 2+ | 14.4 | 15. | 12.47 | 15.1 | 14.0 |
| 3 or 4+ | 61.2 | 63.1 | 59.32 | 55.7 | 41.3 |
| missing | 12.4 | 12.4 | 11.8 | 12.7 | 16.9 |
| Hospitalized+ | 88.3 | 85.4 | 92.5 | 94.8 | 94.2 |
| Admissions+++ | 2 (1, 4) | 2 (1, 4) | 3 (2, 5) | 3 (2, 5) | 3 (1, 5) |
| Inpatient diagnosis of AKI+++ | 52.9 | 43.3 | 64.3 | 72.4 | 79.6 |
| Proportion of hospitalizations with diagnosis of AKI+++ | 30.4 | 24.8 | 33.9 | 45.2 | 58.8 |
Continuous variables are given as mean ± SD or median (25th, 75th percentile); categorical variables as percentage.
Abbreviations: AKI Acute kidney injury; eGFR estimated glomerular filtration rate; ESRD, end-stage renal disease
includes myeloma, light chain deposition disease, lymphoma of the kidney, complication of bone marrow transplant, amyloid
Most recent urine dipstick within two years before dialysis initiation
Among patients hospitalized at least once within two years before initiation.
Nephrology care practices differed substantially by trajectory group (Table 3). Patients with more rapid loss of eGFR were less likely to have seen an outpatient nephrologist within two years before dialysis initiation, less likely to have a permanent vascular access, more likely to initiate dialysis in the hospital, more likely to have an eGFR ≥ 15 ml/min/1.73 m2 and to have an inpatient diagnosis of AKI at initiation, and more likely to receive in-center hemodialysis as their first modality. In analyses adjusted for patient characteristics, there were statistically significant trends across trajectory groups in age ≥75 years, heart failure, pulmonary disease, inability to ambulate or transfer, diabetes or hypertension as cause of ESRD, heavy proteinuria, hospitalization, AKI as well as all care practices examined (Table 4).
Table 3.
Nephrology care practices by eGFR trajectory group
| Care practices | All patients (n=5,606) | Trajectory group | |||
|---|---|---|---|---|---|
| Persistently low eGFR (Group 1, n=3,520) | Progressive eGFR loss (Group 2, n=1,379) | Accelerated eGFR loss (Group 3, n=535) | Catastrophic eGFR loss (Group 4, n=172) | ||
| Outpatient visit to a nephrologist+ | 75.2 | 86.2 | 68.8 | 39.8 | 12.2 |
| Nephrology visits++ | 6 (3,10) | 6 (3,11) | 2 (1,4) | 1 (1,2) | 1 (1,3) |
| Time from first nephrology visit to dialysis initiation (d)++ | 386 (179, 585) | 444 (243, 625) | 276 (116, 463) | 125 (47, 270) | 23 (9, 68) |
| Graft or fistula placement before initiation +++ | 34.4 | 41.2 | 27.9 | 15.3 | 5.8 |
| Inpatient initiation | 63.8 | 57.7 | 71.1 | 79.4 | 80.8 |
| Diagnosis of AKI during hospitalization when dialysis was initiated++++ | 35.6 | 23.8 | 45.6 | 56.7 | 71.9 |
| eGFR at initiation ≥ 15 ml/min/1.73 m2+++++ | 11.2 | 6.4 | 19.9 | 17.0 | 22.1 |
| In-center hemodialysis as initial modality | 95.5 | 94.2 | 97.4 | 98.5 | 98.8 |
Continuous variables are given as median (25th, 75th percentile); categorical variables as percentage.
Abbreviations: AKI Acute kidney injury; eGFR estimated glomerular filtration rate
During the two year period before dialysis initiation
Among the subset of patients with an outpatient nephrology encounter during the two year period before dialysis initiation
Among patients who initiated hemodialysis and during the two year period before dialysis initiation
Among patients admitted to the hospital at the time of dialysis initiation
Based on the eGFR within 45 days of dialysis initiation reported to USRDS
Table 4.
Adjusted association of patient characteristics and care practices with eGFR trajectory group
| Patient characteristics | Trajectory group | P for trend | ||
|---|---|---|---|---|
| Progressive eGFR loss (Group 2, n=1,379) | Accelerated eGFR loss (Group 3, n=535) | Catastrophic eGFR loss (Group 4, n=172) | ||
| Age ≥ 75 years | 0.75 (0.64–0.88) | 0.48 (0.38–0.62) | 0.30 (0.19–0.47) | <0.001 |
| White race vs. black or other | 0.93 (0.81–1.10) | 0.86 (0.70–1.11) | 0.96 (0.67–1.37) | 0.2 |
| Female | 0.83 (0.49–1.38) | 0.62 (0.27–1.40) | 0.20 (0.03–1.56) | 0.06 |
| Diabetes | 1.29 (1.10–1.52) | 1.04 (0.81–1.32) | 0.56 (0.36–0.88) | 0.9 |
| Ischemic heart disease | 1.02 (0.88–1.18) | 0.91 (0.72–1.13) | 0.88 (0.60–1.30) | 0.4 |
| Heart failure | 1.52 (1.31–1.76) | 1.53 (1.22–1.92) | 1.25 (0.84–1.86) | <0.001 |
| Stroke | 1.13 (0.93–1.37) | 0.92 (0.67–1.27) | 0.57 (0.29–1.12) | 0.5 |
| Peripheral vascular disease | 1.19 (0.99–1.42) | 1.16 (0.88–1.53) | 0.99 (0.59–1.67) | 0.2 |
| Pulmonary disease | 1.23 (1.00–1.52) | 1.35 (0.99–1.84) | 1.41 (0.38–2.38) | 0.01 |
| Cancer | 1.10 (0.85–1.64) | 1.18 (0.85–1.64) | 1.25 (0.75–2.08) | 0.2 |
| Unable to ambulate or transfer | 1.51 (1.13–2.01) | 2.27 (1.56–3.32) | 2.17 (1.14–4.11) | <0.001 |
| Diabetes or hypertension as a cause of ESRD | 0.79 (0.67–0.94) | 0.37 (0.30–0.46) | 0.16 (0.11–0.23) | <0.001 |
| Heavy proteinuria+ | 0.73 (0.63–0.85) | 0.66 (0.54–0.82) | 0.47 (0.33–0.65) | <0.001 |
| Hospitalized within two years before initiation | 1.91 (1.52–2.39) | 2.77 (1.85–4.13) | 2.41 (1.23–4.69) | <0.001 |
| Inpatient diagnosis of AKI++ | 2.26 (1.97–2.60) | 3.08 (2.47–3.83) | 4.18 (2.78–6.30) | <0.001 |
| Care practices at or before initiation | ||||
| Outpatient visit to a nephrologist+++ | 0.36 (0.31–0.42) | 0.12 (0.09–0.14) | 0.03 (0.02–0.05) | <0.001 |
| Vascular access placement ++++ | 0.52 (0.45–0.60) | 0.26 (0.20–0.33) | 0.10 (0.05–0.19) | <0.001 |
| Inpatient dialysis initiation | 1.76 (1.53–2.02) | 2.71 (2.16–3.41) | 3.12 (2.05–4.73) | <0.001 |
| Inpatient diagnosis of AKI at initiation+++++ | 2.61 (2.21–3.09) | 3.76 (2.99–4.74) | 7.11 (4.70–10.76) | <0.001 |
| eGFR at initiation ≥ 15 ml/min/1.73 m2 | 3.34 (2.74–4.08) | 3.09 (2.32–4.10) | 4.41 (2.87–6.75) | <0.001 |
| Center hemodialysis as initial modality | 2.28 (1.80–3.49) | 4.13 (2.00–8.53) | 5.33 (1.28–22.25) | <0.001 |
Values shown are dds ratio (95% confidence interval); referenct group is persistently low eGFR (Group 1, n=3,520). Analyses are adjusted for patient demographic characteristics, co-morbid conditions, ability to ambulate and transfer, primary cause of ESRD and level of proteinuria (negative, trace,1+, 2+, 3+, 4+ or missing)
AKI, acute kidney injury; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease
Based on 3+ or higher proteinuria (vs. negative, trace 1+ or 2+) on most recent urine dipstick prior to dialysis initiation (available for 4,909 patients)
Among patients hospitalized at least once during the two year period before dialysis initiation
Within two years before dialysis initiation
Among patients who initiated hemodialysis
Among patients who initiated dialysis during an inpatient admission
By the end of follow-up in July 2011, 4,820 (86%) members of this cohort had died. Median survival after dialysis initiation for the overall cohort was 2.7 (25th–75th percentile, 0.97–5.7) years, ranging from 0.9 (25th–75th percentile, 0.3–4.3) years for patients with catastrophic loss of eGFR (Group 4) to 3.1 (25th–75th percentile, 1.3–6.1) years for those with persistently low levels of eGFR (Group 1) (Figure 4). After adjustment for patient characteristics and care practices, patients with more rapid loss of eGFR before dialysis initiation were at increased risk of death during the first year after initiation, but not over longer periods of time (Table 5). Results were similar in all sensitivity analyses.
Figure 4.
Kaplan-Meier curves showing survival after dialysis initiation by trajectory group
Table 5.
Adjusted risk of death over different time periods after dialysis initiation by trajectory group
| Follow-up time | Trajectory group | P for trend | ||
|---|---|---|---|---|
| Progressive eGFR loss (Group 2, n=1,379) | Accelerated eGFR loss (Group 3, n=535) | Catastrophic eGFR loss (Group 4, n=172) | ||
| 0–6 mo | 1.40 (1.18, 1.65) | 1.50 (1.19, 1.89) | 2.34 (1.70.3.21) | <0.001 |
| 6 –12 mo | 1.31 (1.07, 1.61) | 1.30 (0.96, 1.75) | 1.95 (1.25, 3.03) | 0.002 |
| 1–2 y | 1.19 (1.01, 1.41) | 1.18 (0.91, 1.53) | 0.99 (0.60, 1.65) | 0.2 |
| 2–5 y | 1.02 (0.90, 1.16) | 1.01 (0.83, 1.24) | 0.69 (0.46, 1.04) | 0.5 |
Values shown are djusted hazard for death (95% confidence interval); referent group is Persistently low eGFR (Group 1, n=3,520). EGFR, estimated glomerular filtration rate.
Not available
Adjusted for demographic characteristics, co-morbid conditions, whether the patient could ambulate and transfer, listed cause of ESRD, level of proteinuria (negative, trace, 1+, 2+, 3+, 4+ or missing), whether the patient was hospitalized during the two year period before initiation and whether or not there was a diagnostic code for AKI during one or more admissions, whether the patient was seen by a nephrologist during the two year period before dialysis initiation, whether they underwent a permanent vascular access procedure, whether they initiated dialysis in the hospital, whether they had a diagnosis of AKI at the time of initiation, whether they had an eGFR of 15 ml/min/1.73 m2 or higher at initiation, and whether they received center hemodialysis as their initial modality.
Discussion
Among members of a large integrated health system, there was a substantial degree of heterogeneity in patterns of eGFR loss during the two year period leading up to initiation of chronic dialysis. Trajectories of eGFR before dialysis initiation were strongly associated with care practices at and before the time of initiation, and with mortality during the first year after initiation.
To our knowledge this is the first study to comprehensively describe patterns of eGFR loss preceding initiation of chronic dialysis. Most prior studies have focused on the frequency of AKI at the time of ESRD onset, and suggest that approximately 10–20% of patients present at this time with acute or acute-on-chronic kidney injury.13–15 Other studies have described a higher risk of subsequent ESRD in patients who survive an episode of AKI, particularly among those with pre-existing chronic kidney disease.12, 21–23 We observed substantial heterogeneity in pre-dialysis eGFR trajectories among members of this cohort of incident chronic dialysis patients. Two thirds of cohort members had eGFR levels that were under 30 ml/min/1.73 m2 for at least two years before initiation, one in four had progressive loss of eGFR from levels around 30–59 ml/min/1.73 m2, one in ten had accelerated loss of eGFR from levels above 60 ml/min/1.73 m2 and 3% had catastrophic loss of eGFR within six months or less from levels above 60 ml/min/1.73 m2. In many instances, chronic eGFR trajectories were punctuated by one or more inpatient diagnoses of AKI occurring both at and before the time of ESRD onset. While more common in patients with more rapid loss of eGFR, inpatient diagnoses of AKI were also common among patients with slower rates of eGFR decline.
Rates of recommended pre-ESRD care (e.g., nephrology referral, vascular access placement) were substantially lower for patients who progressed more rapidly. That this pattern was not limited to those patients who presented with catastrophic loss of eGFR seems to suggest that rapid loss of kidney function prior to dialysis initiation (and/or conditions and circumstances contributing to more rapid loss of kidney function) may represent a significant barrier to optimal ESRD preparation among patients with advanced kidney disease. At present, the timing of most clinical interventions for patients approaching the need for chronic dialysis is guided primarily by level of eGFR.4, 24 This approach -- which assumes that patients will lose kidney function at a similar and predictable rate over time and will require equivalent periods of time to prepare for ESRD -- does not account for the substantial heterogeneity in rates of progression to ESRD observed among members of this cohort. For example, even if we exclude patients with catastrophic loss of eGFR (for whom pre-ESRD planning may not be feasible), the time between arrival at an eGFR of 20 ml/min/1.73 m2 -- the eGFR threshold below which patients can accrue time on the kidney transplant wait list – and dialysis initiation was approximately 18 months for patients with persistently low levels of eGFR (Group 1), six months for patients with progressive loss of eGFR (Group 2), and less than two months for patients with accelerated loss of eGFR (Group 3). eGFR levels approximately six months before dialysis initiation -- the minimum time period recommended for AV fistula placement and maturation -- was < 15 ml/min/1.73 m2 for patients with persistently low levels of eGFR (Group 1), around 20 ml/min/1.73 m2 for patients with progressive loss of eGFR (Group 2), and above 30 ml/min/1.73 m2 for patients with accelerated loss of eGFR. These findings, if confirmed, suggest the need for greater flexibility in timing of ESRD preparation, depending on eGFR trajectory.
At the same time, our study also provides insights into some of the challenges involved in preparing patients with CKD for ESRD. While long-term eGFR trajectories may be clear with hindsight, high rates of hospitalized AKI and substantial variability in eGFR around these trajectories may make it difficult to discern long-term trends in eGFR prospectively. Predicting whether and when patients will initiate chronic dialysis is further complicated because many patients with kidney disease will die before reaching ESRD, particularly those with more rapid loss of eGFR.25, 26 Such survivor bias might explain some of the observed differences between cohort members with differing rates of eGFR loss. For example, patients who experienced more rapid loss of eGFR were younger and had fewer chronic conditions compared with patients who progressed more slowly. We suspect that patients who were sick enough to be losing kidney function this rapidly would have been less likely to survive to the point of initiating chronic dialysis if they were older and had a greater burden of co-morbidity. Finally, high rates of hospitalization during the time leading up to dialysis initiation suggest that many patients may face competing health priorities related to acute illness or deteriorating health status at a time when preparation for dialysis would ordinarily be recommended.
Our study has several limitations. First, results may not be generalizable to groups not well represented in our population, particularly women. Second, information on co-morbid conditions, dialysis modality and listed cause of ESRD were obtained from USRDS as these data elements are prospectively and systematically ascertained at onset of ESRD. However, it should be noted that while these data are specific, they are a relatively insensitive source of information about co-morbid conditions.27 Third, because more recent data linkage to the USRDS registry are not available for Veteran cohorts, we were not able describe eGFR trajectories for patients who started chronic dialysis in more recent years. We also were unable to examine pre-dialysis eGFR trajectories beyond two years before initiation as serum creatinine measures are not available in national VA data sources prior to October 1, 1999.
In conclusion, we observed substantial heterogeneity in patterns of eGFR loss during the two year period before initiation of chronic dialysis. Patients who progressed more rapidly were more likely to have a hospital diagnosis of acute kidney injury and were less likely to have received recommended pre-ESRD care. These findings spotlight the substantial challenges involved in optimally preparing patients for ESRD, and perhaps suggest the need for a more flexible approach toward ESRD planning.
Acknowledgments
We thank Ms. Whitney Showalter of the VA Puget Sound Healthcare System (VAPSHCS) for providing study coordination and administrative support for this project.
Support: This work was supported by an Inter-agency Agreement between the Centers for Disease Control and Prevention and VAPSHCS (07FED69212), the VA Puget Sound HSR&D Center of Excellence, and a Beeson Career Development Award from the National Institute on Aging to Dr. O’Hare (K 1K23AG28980). The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the Centers for Disease Control and Prevention.
Footnotes
Financial Disclosure: Drs. O’Hare and Larson receive royalties from UpToDate. The remaining authors declare that they have no other relevant financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gill TM, Gahbauer EA, Han L, Allore HG. Trajectories of disability in the last year of life. N Engl J Med. 2010 Apr 1;362(13):1173–1180. doi: 10.1056/NEJMoa0909087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lunney JR, Lynn J, Foley DJ, Lipson S, Guralnik JM. Patterns of functional decline at the end of life. JAMA. 2003 May 14;289(18):2387–2392. doi: 10.1001/jama.289.18.2387. [DOI] [PubMed] [Google Scholar]
- 3.Murtagh FE, Murphy E, Sheerin NS. Illness trajectories: an important concept in the management of kidney failure. Nephrol Dial Transplant. 2008 Dec;23(12):3746–3748. doi: 10.1093/ndt/gfn532. [DOI] [PubMed] [Google Scholar]
- 4.Rosansky S. Early dialysis initiation and renal function trajectory. J Intern Med. 2011 Mar;269(3):275–277. doi: 10.1111/j.1365-2796.2010.02307.x. [DOI] [PubMed] [Google Scholar]
- 5.Levin A, Djurdjev O, Beaulieu M, Er L. Variability and risk factors for kidney disease progression and death following attainment of stage 4 CKD in a referred cohort. Am J Kidney Dis. 2008 Oct;52(4):661–671. doi: 10.1053/j.ajkd.2008.06.023. [DOI] [PubMed] [Google Scholar]
- 6.Hemmelgarn BR, Manns BJ, Lloyd A, et al. Relation between kidney function, proteinuria, and adverse outcomes. JAMA. 2010 Feb 3;303(5):423–429. doi: 10.1001/jama.2010.39. [DOI] [PubMed] [Google Scholar]
- 7.O’Hare AM, Choi AI, Bertenthal D, et al. Age affects outcomes in chronic kidney disease. J Am Soc Nephrol. 2007 Oct;18(10):2758–2765. doi: 10.1681/ASN.2007040422. [DOI] [PubMed] [Google Scholar]
- 8.Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med. 2004 Mar 22;164(6):659–663. doi: 10.1001/archinte.164.6.659. [DOI] [PubMed] [Google Scholar]
- 9.Hemmelgarn BR, Zhang J, Manns BJ, et al. Progression of kidney dysfunction in the community-dwelling elderly. Kidney Int. 2006 Jun;69(12):2155–2161. doi: 10.1038/sj.ki.5000270. [DOI] [PubMed] [Google Scholar]
- 10.Prescott GJ, Metcalfe W, Baharani J, et al. A prospective national study of acute renal failure treated with RRT: incidence, aetiology and outcomes. Nephrol Dial Transplant. 2007 Sep;22(9):2513–2519. doi: 10.1093/ndt/gfm264. [DOI] [PubMed] [Google Scholar]
- 11.Metcalfe W, Simpson M, Khan IH, et al. Acute renal failure requiring renal replacement therapy: incidence and outcome. QJM. 2002 Sep;95(9):579–583. doi: 10.1093/qjmed/95.9.579. [DOI] [PubMed] [Google Scholar]
- 12.Hsu CY, Chertow GM, McCulloch CE, Fan D, Ordonez JD, Go AS. Nonrecovery of kidney function and death after acute on chronic renal failure. Clin J Am Soc Nephrol. 2009 May;4(5):891–898. doi: 10.2215/CJN.05571008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Metcalfe W, Khan IH, Prescott GJ, Simpson K, Macleod AM. Hospitalization in the first year of renal replacement therapy for end-stage renal disease. QJM. 2003 Dec;96(12):899–909. doi: 10.1093/qjmed/hcg155. [DOI] [PubMed] [Google Scholar]
- 14.Lee P, Johansen K, Hsu CY. End-stage renal disease preceded by rapid declines in kidney function: a case series. BMC Nephrol. 2011;12:5. doi: 10.1186/1471-2369-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Onuigbo MA. Syndrome of rapid-onset end-stage renal disease: a new unrecognized pattern of CKD progression to ESRD. Ren Fail. 2010;32(8):954–958. doi: 10.3109/0886022X.2010.502608. [DOI] [PubMed] [Google Scholar]
- 16.The United States Renal Data System. [Accessed 7/12/2010]; http:\\www.usrds.org.
- 17.Sohn MW, Arnold N, Maynard C, Hynes DM. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr. 2006;4:2. doi: 10.1186/1478-7954-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999 Mar 16;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 19.Jones BNDS. Advances in group-based trajectory modeling and a SAS procedure for estimating them. Sociol Methods Res. 2007;35:542–571. [Google Scholar]
- 20.Jones BNDS, Roeder K. SAS Procedure based on mixture models for estimating developmental trajectories. Sociol Methods Res. 2001;29:374–393. [Google Scholar]
- 21.Wald R, Quinn RR, Luo J, et al. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA. 2009 Sep 16;302(11):1179–1185. doi: 10.1001/jama.2009.1322. [DOI] [PubMed] [Google Scholar]
- 22.Ishani A, Xue JL, Himmelfarb J, et al. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol. 2009 Jan;20(1):223–228. doi: 10.1681/ASN.2007080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lo LJ, Go AS, Chertow GM, et al. Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int. 2009 Oct;76(8):893–899. doi: 10.1038/ki.2009.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002 Feb;39(2 Suppl 1):S1–266. [PubMed] [Google Scholar]
- 25.Rifkin DE, Shlipak MG, Katz R, et al. Rapid kidney function decline and mortality risk in older adults. Arch Intern Med. 2008 Nov 10;168(20):2212–2218. doi: 10.1001/archinte.168.20.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Aly Z, Zeringue A, Fu J, et al. Rate of kidney function decline associates with mortality. J Am Soc Nephrol. 2010 Nov;21(11):1961–1969. doi: 10.1681/ASN.2009121210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Longenecker JC, Coresh J, Klag MJ, et al. Validation of comorbid conditions on the end-stage renal disease medical evidence report: the CHOICE study. Choices for Healthy Outcomes in Caring for ESRD. J Am Soc Nephrol. 2000 Mar;11(3):520–529. doi: 10.1681/ASN.V113520. [DOI] [PubMed] [Google Scholar]