Abstract
Despite its well-described role in female affiliation, the influence of oxytocin on male pairbonding is largely unknown. However, recent human studies indicate that this nonapeptide has a potent influence on male behaviors commonly associated with monogamy. Here we investigated the distribution of oxytocin receptors (OTR) throughout the forebrain of the socially monogamous male prairie vole (Microtus ochrogaster). Because males vary in both sexual and spatial fidelity, we explored the extent to which OTR predicted monogamous or non-monogamous patterns of space use, mating success and sexual fidelity in free-living males. We found that monogamous males expressed higher OTR density in the nucleus accumbens than non-monogamous males, a result that mirrors species differences in voles with different mating systems. OTR density in the posterior portion of the insula predicted mating success. Finally, OTR in the hippocampus and septohippocampal nucleus, which are nuclei associated with spatial memory, predicted patterns of space use and reproductive success within mating tactics. Our data highlight the importance of oxytocin receptor in neural structures associated with pairbonding and socio-spatial memory in male mating tactics. The role of memory in mating systems is often neglected, despite the fact that mating tactics impose an inherently spatial challenge for animals. Identifying mechanisms responsible for relating information about the social world with mechanisms mediating pairbonding and mating tactics is crucial to fully appreciate the suite of factors driving mating systems.
Keywords: nucleus accumbens, hippocampus, evolution, mating system, neurobiology, monogamy
Introduction
Oxytocin (OT) is a mammalian hormone and neuromodulator that is closely associated with female behavior. Oxytocin induces labor, facilitates milk ejection during nursing, and mediates maternal behavior and mother-infant bonding (Gimpl and Fahrenholz, 2001). In mammals in which females form bonds with male mates, oxytocin is thought to have been co-opted to facilitate female bond formation with mating partners (Ross and Young, 2009). Oxytocin is released during vaginocervical stimulation or mating (Kendrick et al., 1986; Ross et al., 2009a; Sansone et al., 2002) and during human orgasm (Carmichael et al., 1987), presumably enhancing the association between an individual and the hedonic reward elicited by mating. While females of most mammalian species readily bond with and care for offspring, male parental care and social bonding is quite rare. Moreover, the neuroendocrine control over male attachment and paternal care in species for which these behaviors are important is often attributed to arginine vasopressin (AVP). For these reasons, oxytocin and vasopressin are considered prominent mechanisms governing mammalian monogamy and ‘love’ (Zeki, 2007), and to some extent the two hormones have been distinctly associated with each sex, much in the way estrogens and androgens once were. As a result, the potential influence of OT in male monogamy is often neglected.
Monogamy is commonly characterized by selective affiliation with a mate, bi-parental care, and selective aggression in the form of territory, nest, and mate defense (Clutton-Brock, 1991; Kleiman, 1977). In this regard, prairie voles (Microtus ochrogaster) are great models of monogamy, and studies of this species have provided one of the best descriptions of mechanisms involved in mammalian monogamous behavior. Prairie voles establish long-term pairbonds in both the field (Getz et al., 1981; Getz and Hofmann, 1986; Getz et al., 1993) and laboratory (Oliveras and Novak, 1986; Solomon, 1993a; Solomon, 1993b; Thomas and Birney, 1979; Williams et al., 1992), and exhibit bi-parental care – with males contributing to all offspring needs except lactation (refs Op. cit.). Pairs are also territorial; there is minimal overlap with non-pair neighbors of both sexes, and males are selectively aggressive to intruders (Getz and Hofmann, 1986; McGuire and Getz,1991; McGuire and Getz, 1998; McGuire et al., 1990).
The influence of OT and AVP in prairie vole monogamy is most tightly associated with pairbonding and parental care (Carter et al., 1997; Winslow et al., 1993; Young et al., 1997), which are centrally mediated by the oxytocin receptor (OTR) and the vasopressin receptor subtype V1aR (refs Op. cit.). A growing body of work has led to the proposal that a ‘pairbonding neural circuit’ integrates the action of vasopressin and oxytocin with dopaminergic-mediated reward in mesolimibic structures to facilitate social bonds (Young and Wang, 2004; Young et al., 2005). This suggestion is strongly supported by experiments manipulating these nonapeptides or their receptors within several structures throughout the prairie vole forebrain (Carter and Keverne, 2002; Young and Wang, 2004; Young et al., 2005). For example, central infusion of OT or AVP facilitates partner preferences in both females and males, while antagonists for these peptides reduce the effects of OT or AVP on behavior in both sexes (Cho et al., 1999; Williams et al., 1994). However, these studies used antagonists to block the effects of exogenous nonapeptides. Central infusion of nonapeptide receptor antagonists targeting endogenous OT or AVP acting throughout the forebrain (Insel and Hulihan, 1995; Winslow et al., 1993), and structure-specific studies suggest that OT and AVP may have sex-specific effects on mammalian bonding. For instance, oxytocin receptor antagonists delivered to the prefrontal cortex (PFC) or nucleus accumbens (NAcc) blocks mating-induced partner preferences in females, but not males (Young et al., 2001). Blockade of V1aR in the ventral pallidum (VPall) inhibits the formation of partner preferences of males, but not females (Lim and Young, 2004). This has led to the belief that oxytocin mediates female bonding, while vasopressin mediates male bonding. This assumption has led to experiments focused on manipulating these hormones in either of the two sexes. For example, male prairie voles have been manipulated to over-express V1aR in the VPall (Pitkow et al., 2001), while females have been manipulated to over-express OTR in the NAcc (Ross et al., 2009b). In both cases the animals formed social preferences characteristic of pairbonds without the necessary step of mating. The role of septal AVP in males has further contributed to this divide considering that it has been closely associated with facilitating paternal care (Bamshad et al., 1993; Oliveras and Novak, 1986; Wang et al., 1994a; Wang et al., 1994b; Wideman and Murphy, 1990), and it is both necessary and sufficient to induce male pairbonding (Liu et al., 2001). Although both OT and AVP antagonists delivered to the septum eliminated male pairbonds (Liu et al., 2001), it has been suggested that this effect may be more linked with its influence in social recognition than monogamous pairbonding per se (Young and Wang, 2004; Young et al., 2005).
It has been argued that the prairie vole pairbonding neural circuit may serve as a model for understanding human mechanisms of attachment and love. While human studies have been approached very differently than those focused on the prairie vole brain, an interesting point should be highlighted: OT clearly plays a role in human male attachment. It is generally agreed that trust is an important component of human relationships, and intranasal administration of OT in both genders facilitates trust (Baumgartner et al., 2008; Kosfeld et al., 2005; Zak et al., 2005). The influence of oxytocin in humans goes beyond trust; it facilitates recognition of faces (Rimmele et al., 2009; Savaskan et al., 2008), in-group social cohesion (De Dreu et al., 2011), and preemptive punishment toward out-groups (De Dreu et al., 2010), suggesting that it may influence a form of selective aggression in men. Furthermore, OT is increased during sexual arousal and released during orgasm in men, just as it is in women (Carmichael et al., 1987). Ironically, human studies suggest that the role of OT in male prairie vole monogamy may be underappreciated.
Hormone receptors are often the targets of selection and their distributions can reveal products of evolution (Ketterson and Nolan, 1992). Here we ask how patterns of OTR density relate to monogamous behavior in male prairie voles living in outdoor enclosures. Specifically we ask if oxytocin receptor expression predicts male mating tactics and paternity. Although we examined the distribution of oxytocin receptor expression throughout the male forebrain, we place a special focus on areas of the brain that are associated with pairbonding (e.g., NAcc and PFC). We were also particularly interested in neural structures involved in spatial or social memory (e.g., hippocampus and lateral septum) because our previous results indicated that socio-spatial memory structures expressing the vasopressin receptor, V1aR, were important predictors of the most successful male mating tactics (Ophir et al., 2008b).
Materials and methods
Test animals
In total, we used 48 male and 48 female prairie voles to investigate how individual differences in brain phenotype related to space use and sexual fidelity. The methods described below were in accordance with the guidelines set and approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Memphis (project number 0012). At weaning, we grouped all animals in same-sex sibling groups and maintained them under a 14:10 L:D cycle. Food and water were provided ad libitum and temperature was maintained at 21 ± 2°C.
Animals were distributed into eight groups, each consisting of six nulliparous females and six adult, sexually mature males. All individuals were of similar age and weight and before introduction to field enclosures they were ear-tagged, weighed, and a tail clipping was taken. Further details on field and paternity methods can be found in Ophir et al. (2008a).
The study was conducted in four field enclosures located in Shelby Co. TN (for details see Mahady and Wolff, 2002; Ophir et al., 2007; Ophir et al., 2008a). Each enclosure measured 20 × 30 m. Densities were within the range of natural densities reported elsewhere (Getz et al., 1993; Taitt and Krebs, 1985).
Radio telemetry and trapping
We outfitted each vole with a 1.9g transmitter and collar (BD-2C, Holohil Systems Ltd.; Carp, Ontario) two days prior to introduction to the field. Animals were tracked with an LA12 Radio Telemetry Receiver (AVM Instruments Co, Ltd.; Livermore, CA) to within 1 m of their actual location.
To initiate a trial, we placed all twelve animals (six females and six males) in an enclosure. At the start of each trial, animals were standardized for age and body mass across enclosures. We ran a series of four trial blocks each consisting of two simultaneous trials over the breeding season.
Telemetry readings were taken twice daily for at minimum of 12 days, varying time of day and enclosure order. We began trapping and removing animals from the enclosure on day 18. By initiating trapping on this schedule we ensured that no females would give birth before trapping (gestation is 21 days), enabling us to know the identity of mothers with 100% confidence and increasing our ability to assign paternity to embryos. We collected tissue from all animals and the embryos for genetic parentage analysis. Further details are reported in Ophir et al. (2008a).
Home range size, space use, and pair determination
We used RANGES V (Anatrack Ltd.; Dorset, UK) to estimate the size of each core home range by calculating minimum convex polygons (MCP) with 75% fixes from the assembled X and Y coordinates. We focused on the central 75% of data points to estimate the core home ranges without resorting to more complex statistical kernel methods (Row and Blouin-Demers, 2006; White and Garrott, 1990; see Ophir et al., 2008a for more discussion). From these MCPs, we calculated the percent of home-range overlap between pairs of individuals. Home range overlap was used to estimate encounter rates between each pair of individuals to determine which animals were paired ‘residents’ and which were single ‘wanderers’ (Ophir et al., 2008a; Ophir et al., 2008b). Ultimately, we defined a pair as males and females for which both animals encountered each other more than all other opposite-sexed individuals combined.
Tissue extraction and autoradiography
Once trapped, animals were immediately returned to the lab, and euthanized with CO2. Brains were then extracted, frozen on powdered dry ice, and stored at −70°C. We cryosectioned brains coronally into four sets 20μm thick at 100μm intervals, mounted them on Superfrost slides (Fisher Scientific; Pittsburgh, PA), and stored them at −80°C. We used standard protocols for autoradiography using 125I-ornithine vasotocin ([125I]-OVTA, PerkinElmer; Waltham, MA) to visualize OTR (Insel et al., 1994; Young et al., 1997) on one of these sets.
To process tissues, we lightly fixed sections in 0.1% paraformaldehyde for 2 min, washed them in 1X Tris for 20 min, incubated them with 40pM [125I]-OVTA for 60 min, washed them again in 1X Tris with MgCl2 for 50 min, and finally rapidly air-dried them. We then exposed the radioactive sections to film for 72 h alongside 125I labeled radiographic standards (American Radiolabeled Chemicals; St. Louis, MO).
High expression of receptor results in high binding of radioactive ligand and can be measured by optical density of film exposed to the tissue sections. To investigate the forebrain binding of OTR, we digitized films on a Microtek ArtixScan M1 and then quantified receptor density on the standardized scans using NIH ImageJ software. Optical density was converted to disintegrations per minute (dpm) per mg of standardized rat neural tissue equivalence (TE) using a log function to fit curves generated by radiographic standards. We measured OTR in the prefrontal cortex (PFC), anterior insular cortex (ICa), nucleus accumbens (NAcc), septohippocampal nucleus (SHi), lateral septum (LS), caudate-putamen (CPu), posterior insular cortex (ICp), central amygdala (CeA), basolateral amygdala (BLA), intermedial dorsal thalamic nucleus (IMD), centromedial thalamic nucleus (CM), centrolateral thalamic nucleus (CL), and hippocampus (Hi) (Fig. 1). Specific binding was calculated by subtracting nonspecific binding from the total binding for each area. Nonspecific binding was estimated from background levels of fiber track binding (e.g., corpus callosum) on the same sections to correct for slight variations in section thickness or quality.
Figure 1.
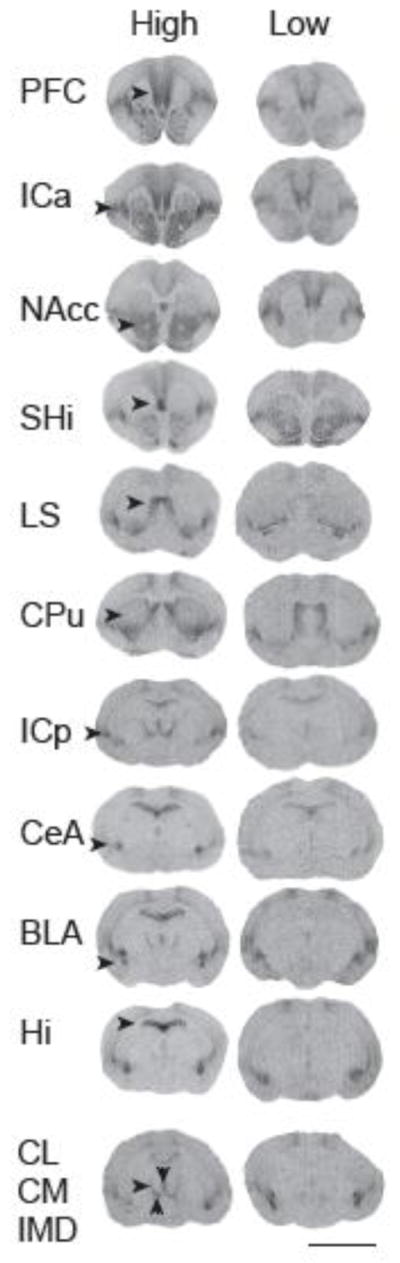
Autoradiograms of OTR ([125I]-OVTA specific binding) across the forebrain for individuals representing the upper (High) and lower (Low) quartile of OTR density for all animals. Arrows indicate the prefrontal cortex (PFC), anterior insular cortex (ICa), nucleus accumbens (NAcc), septohippocampal nucleus (SHi), lateral septum (LS), caudate-putamen (CPu), posterior insular cortex (ICp), central amygdala (CeA), basolateral amygdala (BLA), hippocampus (Hi), centrolateral thalamic nucleus (CL, horizontal arrow), centromedial thalamic nucleus (CM, vertical upward arrow), and intermedial dorsal thalamic nucleus (IMD, vertical downward arrow). Scale bar = 5 mm.
Statistics and analysis
Analyses of space use (home range size, and conspecific home range overlap) and sexual fidelity (animals who mated with a partner [intra-pair fertilizations; IPF] or those who mated with a female who was not a partner [extra-pair fertilizations; EPF]) are reported in Ophir et al. (2008b). As reported in Ophir et al. (2008b), we found no significant enclosure effects on male pairing (G=6.18, p=0.78) or EPF/IPF frequencies (G=11.42, p=0.30), and thus pooled data across enclosures. We used one-tailed t-tests to compare OTR expression between individuals producing EPFs and IPFs. One-tailed tests were chosen because we expected phenotypes of EPF-producing males to resemble those of promiscuous species and phenotypes of IPF-producing males to resemble the ‘typical’ phenotype for monogamous species.
We used measures of paternity assessment to determine whether males successfully sired offspring, and measures of space use to define male mating tactics. We used 2-factor analysis of variance (ANOVA) to investigate differences in male OTR expression with respect to these factors. Thus, our factors were: mating tactic (paired residents or single wanderers), and mating success (successful or unsuccessful males). Sample sizes were unequal (successful residents, N=22; successful wanderers, N=3; unsuccessful residents, N=7; unsuccessful wanderers, N=7). The unbalanced design, for which we had no a priori control, violated the assumption of orthogonality and our ability to obtain an additive partition of the sum of squares necessary to run a parametric test. Therefore our statistics package (Aabel 2.4.4, Gigawiz Ltd.; Tulsa, OK) used the methods of van Belle et al. (2004) to satisfy these assumptions, enabling the use of the 2-factor ANOVA. We used Tukey-Kramer post hoc tests to compare across groups.
Lastly, we investigated the correlations of OTR density expression across the forebrain. Here, we corrected all correlation p-values for multiple comparisons (FDR α ≤ 0.013; Benjamini and Hochberg, 1995).
Results and Discussion
Throughout the forebrain we found extensive individual variation in OTR expression. For example, the septohippocampal nucleus (SHi), the hippocampus (Hi), and the thalamic structures showed relatively high degrees of individual variation, whereas other areas, such as the prefrontal cortex (PFC), the lateral septum (LS), and the nucleus accumbens (NAcc), were less variable (see Fig. 1, and Supplementary Material, table S1).
To ask if OTR expression relates to sexual fidelity, we compared OTR expression across the forebrain between males that sired offspring exclusively with their partners (IPF) or males that sired offspring with a female that was not their partner (EPF). Our results showed that OTR expression patterns did not predict differences between males producing IPF or EPFs (Student’s t-test; all t’s ≤ 1.47, all one-tailed p’s ≥ 0.08). Sexual fidelity, therefore, does not relate to oxytocin receptor density.
Next we asked if OTR expression relates to mating tactics or mating success, and if OTR density correlated across neural structures in the forebrain. Although most areas of the brain expressing OTR did not show any patterns that related to mating tactics or mating success (table 1), there were some important and notable exceptions.
Table 1.
Two-factor ANOVA results, adjusted for unequal sample sizes, for OTR expression ([125I]-OVTA specific binding disintegrations per minute [dpm] per milligram in tissue equivalence [TE]) across the forebrain for reproductively successful and unsuccessful resident and wandering male prairie voles.
| Mating Tactic (MT) | Breeding Success (BS) | Interaction (MT × BS) | ||||
|---|---|---|---|---|---|---|
| F(1, 35) | p | F(1, 35) | p | F(1, 35) | p | |
| PFC | 0.02 | > 0.50 | 0.60 | 0.44 | 0.04 | > 0.50 |
| ICa | 2.89 | 0.10 | 0.55 | > 0.46 | 0.87 | 0.36 |
| NAcc | 5.11 | 0.03 | 0.27 | > 0.50 | 1.49 | 0.23 |
| SHi | 0.08 | > 0.50 | 0.60 | 0.44 | 4.82 | 0.04 |
| LS | 0.31 | > 0.50 | 0.31 | > 0.50 | 0.03 | > 0.50 |
| CPu | 0.03 | > 0.50 | 1.99 | 0.17 | 0.02 | > 0.50 |
| ICp | 2.61 | 0.12 | 4.02 | 0.05 | 1.13 | 0.30 |
| CeA | 1.46 | 0.23 | 0.22 | > 0.50 | 0.09 | > 0.50 |
| BLA | 0.04 | > 0.50 | 0.83 | 0.37 | 0.01 | > 0.50 |
| Hi | 0.12 | > 0.50 | 0.78 | 0.38 | 6.47 | 0.02 |
| IMD | 2.16 | 0.15 | 0.20 | > 0.50 | 0.32 | > 0.50 |
| CM | 0.29 | > 0.50 | 0.56 | 0.46 | 0.20 | > 0.50 |
| CL | 0.02 | > 0.50 | 2.32 | 0.14 | 2.18 | 0.15 |
Abbreviations are defined in text and Fig. 1. Successful residents, N = 22; Unsuccessful residents, N = 7; Successful wanderers, N = 3; Unsuccessful wanderers, N = 7. Significant p-values are in bold.
Nucleus accumbens and mating tactics
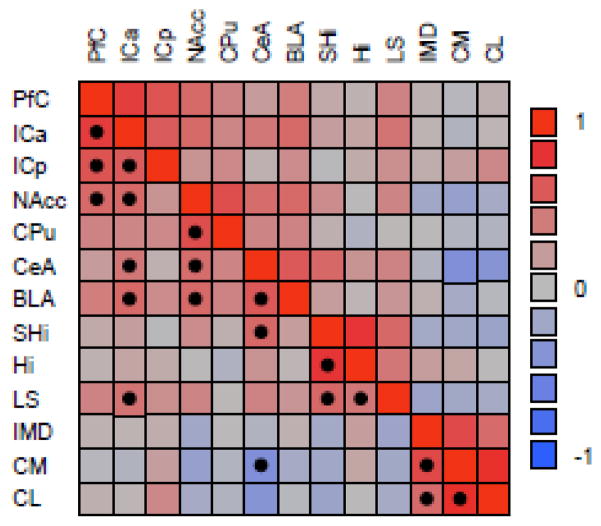
First, we found that paired (resident) males had more OTR expression in the NAcc than single (wandering) males (p = 0.03; Fig. 2). This is interesting because not only is the nucleus accumbens an integral component of the prairie vole pairbonding neural circuit, it is often more generally touted as the reward center of the brain and it regulates hedonic reward during mating. The other OTR expressing component of the pairbonding neural circuit, the prefrontal cortex, did not differ between residents and wanderers, or any other measure we investigated (table 1). Nevertheless, oxytocin receptor density in the PFC was positively correlated with the NAcc (p < 0.001; Fig. 3). Furthermore, accumbal OTR was correlated with OTR density in the anterior portion of the insula (p = 0.001), the striatum (p < 0.0001), and both the central (p < 0.002) and basolateral (p = 0.001) amygdala (see Fig. 3).
Figure 2.
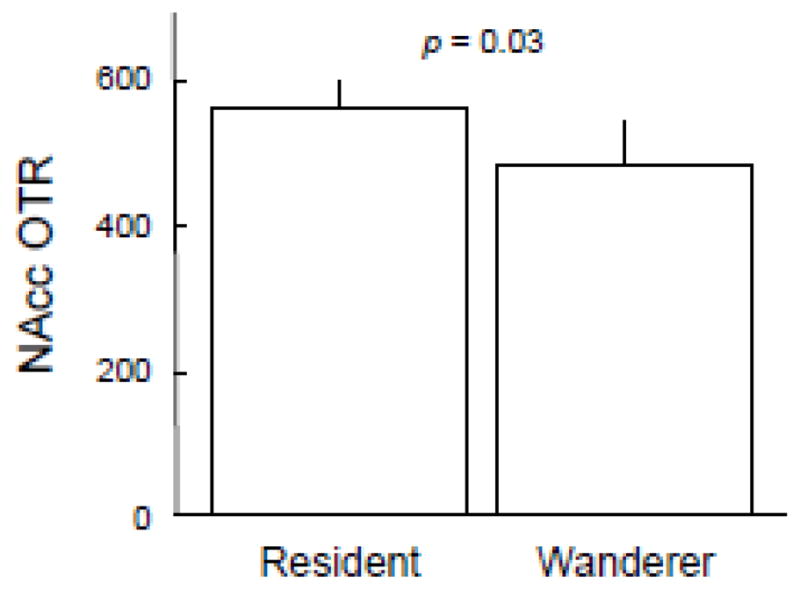
OTR expression in the nucleus accumbens (NAcc) predicts male mating tactic. Mean (± SEM) OTR expression measured as tissue equivalent disintegrations per minute/mg (dpm/mg TE) of [125I]-OVTA specific binding for paired ‘residents’ (N = 29) and single ‘wanderers’ (N = 10).
Figure 3.
Correlation matrix color map clustered on the correlations for OTR density across the forebrain (N = 39). Hue reflects strength of the correlation, color indicates the direction (red = positive, blue = negative). Significant correlations, adjusted for multiple comparisons (FDR α ≤ 0.013; Benjamini and Hochberg, 1995), are indicated with a solid dot. Abbreviations are defined in text and Fig. 1.
Insular cortex and mating success
Sires expressed more OTR in the posterior region of the insular cortex (ICp) than did reproductively unsuccessful males (p = 0.05; Fig. 4). This difference, however, was not seen in the anterior insular cortex. Oxytocin receptor in both the anterior and posterior regions of the insular cortex were significantly and positively correlated with each other and with OTR in the PFC (all p’s < 0.0001; Fig. 3). Interoceptive information (i.e., an individual’s assessment of its current body condition) is processed by the insular cortex (Craig, 2002), and may be used to inform mating decisions and promote mating behavior within chosen mating tactics. The PFC is commonly used for planning and emotional processing. Such mating behavior should require some degree of planning, and the correlation between OTR in the PFC and ICp suggests that oxytocin may act to mediate or coordinate planning of general mating behavior in males based on the evaluation of their current body condition.
Figure 4.
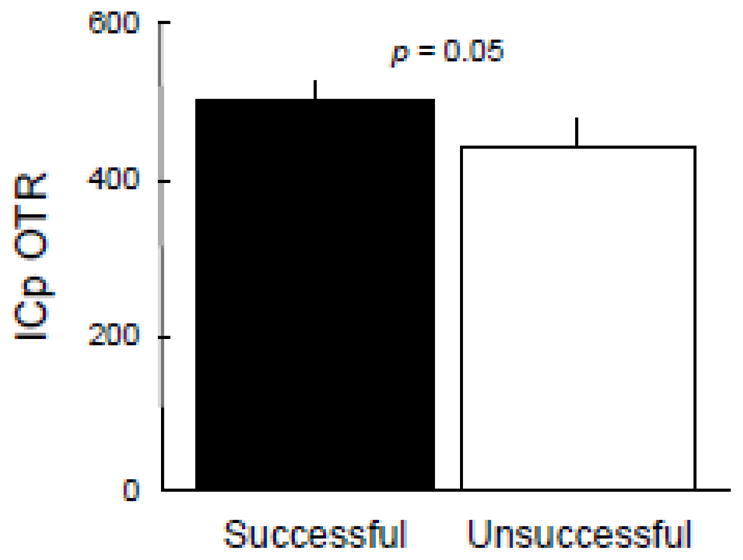
OTR expression in the posterior region of the insular cortex (ICp) predicts male breeding success. Mean (± SEM) OTR expression (dpm/mg TE) of [125I]-OVTA specific binding for males that sired offspring (successful, black, N = 29) and those that did not (unsuccessful, white, N = 10).
Septo-hippocampal complex and socio-spatial memory
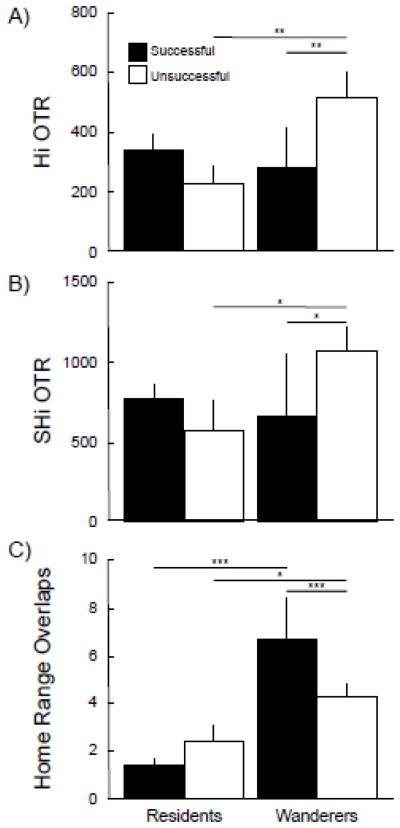
We found a particularly interesting cluster of correlations among the lateral septum, the hippocampus, and the septohipocampal nucleus. Oxytocin receptor density across all of these structures was positively correlated (all p’s ≤ 0.007; Fig. 3). The lateral septum, among other functions, governs social recognition. The hippocampus is best known for its involvement in regulating spatial memory. The septohippocampal nucleus acts as a relay between the LS and the Hi. Although the LS exhibited no significant differences in expression of OTR with regard to mating tactic or mating success, OTR in both the Hi (p < 0.02) and the SHi (p < 0.05) showed significant interactions between these two factors (see table 1, and Fig. 5). Generally, residents that sired offspring had more hippocampal and septohippocampal OTR than residents that did not produce offspring, and wanderers that sired offspring had less OTR than unsuccessful wanderers. In other words, OTR density in these structures predicted opposite patterns of mating success for each reproductive tactic.
Figure 5.
OTR expression in spatial memory areas predicts space use and paternity. Mean (± SEM) OTR expression (dpm/mg TE) for animals varying in reproductive tactic (paired ‘residents’ or single ‘wanderers’) and mating success (successful [black] or unsuccessful [white]) in the hippocampus (Hi, panel A) and the septohippocampal nucleus (SHi, panel B). Significant ANOVA and post-hoc test results are reported within the panels and in table 1. Panel C presents mean (± SEM) number of conspecific home ranges that overlapped residents and wanderers who were successful (black) or unsuccessful (white) at siring young. Resident males’ partners were excluded from the analysis. Panel C represents a modified analysis of data reported in Ophir et al. (2008b). Successful residents, N = 22; unsuccessful residents, N = 7; successful wanders, N = 3; unsuccessful wanders, N = 7. * ≡ p ≤ 0.05; ** ≡ p ≤ 0.02; *** ≡ p ≤ 0.001.
We previously reported that patterns of space use mirror V1aR density in areas associated with spatial memory (Ophir et al., 2008b). Here, we re-visit these data on space use to assess the extent to which the patterns of OTR density in the Hi and SHi predicted patterns of space use and reproductive success among resident and wandering males. Unlike Ophir et al. (2008b), which analyzed male and female overlaps separately, we combined both male and female overlaps of male territories. We excluded the partners of resident males from the analysis, since these are animals that territorial males should not exclude. We found a main effect of mating tactic (F(1, 35) = 120.18, p < 0.001); residents have fewer home range overlaps than wandering males. This further supports the notion that resident males defend their territories from intrusions (Getz and Hofmann, 1986; McGuire and Getz, 1991; McGuire and Getz, 1998; McGuire et al., 1990; Ophir et al., 2008a). A significant interaction between mating tactic and mating success (F(1, 35) = 12.93, p < 0.001, Fig. 5) indicates that reproductively successful residents had even fewer home range overlaps than unsuccessful residents, while the opposite was true for wandering males, a result we have reported elsewhere (Ophir et al., 2008b). Most importantly, like V1aR (Ophir et al., 2008b), this pattern of space use mirrors the significant interactions demonstrated in OTR density reported above. Taken together, these patterns of home range intrusions and OTR density in spatial memory areas of the brain suggest that resident males that best restricted intrusions into their territories and wandering males that intruded the most, maximized their reproductive success; OTR expression in spatial memory areas may promote these behaviors within each tactic.
General Discussion
Oxytocin and vasopressin are increasingly considered to be major influences in mammalian social behavior. Furthermore, social behaviors in other animals are frequently attributed to non-mammalian homologs of these hormones. For instance, comparisons of estrildid finches indicate that social grouping may be regulated by the action of vasotocin and mesotocin on V1a-like and OT-like receptors in the forebrain (Goodson et al., 2009; Kelly et al., 2011).
A major focus of OT and AVP in mammalian social behavior has been directed toward understanding social bonding, particularly in voles. Pairbonding is central to monogamous bonds, and monogamous and non-monogamous species often differ dramatically in the expression patterns of these nonapeptides and their receptors (but see Turner et al., 2010). Consequently, the influences of OT and AVP on pairbonding are routinely equated with monogamous mating tactics. Our results indicate that the natural variation between pairbonded males that have adopted monogamous mating tactics express more OTR in the nucleus accumbens, one of the major nodes in the prairie vole ‘pairbonding neural circuit’. Strangely, this difference was not found in the other major OTR-expressing area associated with pairbonding, the prefrontal cortex.
What might PFC and NAcc oxytocin receptors contribute to male pairbonding and monogamy? Pairbonded male prairie voles produce more offspring, indicating that selection should favor males that form pairbonds (Ophir et al., 2008a). Ophir et al. (2008b) found that V1aR expression across the pairbonding neural circuit did not vary with respect to monogamous mating tactics, sexual fidelity, or mating success, and argued that selection may have eliminated this variation to predispose all males to form pairbonds when such opportunities arise. Here we see the same patterns in OTR expression in the prefrontal cortex (table 1). This leads to two possible interpretations: either OTR in the PFC does not influence male pairbonding, or its influence is so important that selection has eliminated standing variation in OTR density. Studies manipulating oxytocin in male PFC can clearly distinguish between these alternatives.
In contrast to the PFC, OTR in the NAcc was strongly associated with male pairbonding. The NAcc is the major reward center in the brain, and our results support the hypothesis that pairbonds emerge out of associations between the hedonic reward of mating and the social identity of the partner (Young et al., 2005). The influences of OT and other neuroactive substances (e.g., dopamine) in the nucleus accumbens are critical for successful pairbond formation (Aragona et al., 2006; Young and Wang, 2004), and OTR density may effectively bias males to either form pairbonds or remain single. Indeed, oxytocin is a neuromodulator and could act in the NAcc to exaggerate the association between the dopaminergic-driven reward derived during mating with social identity (Young and Wang, 2004; Young et al., 2005). Species differences in voles support the notion that differences in NAcc OTR is important in pairbonding; monogamous species have higher densities of OTR in the nucleus accumbens than do non-monogamous species (Insel and Shapiro, 1992; Insel et al., 1994). We have observed that (a) mating tactics vary across individuals and (b) that differences in accumbal OTR neural phenotype predict differences between residents and wanderers. Combining the aforementioned species differences with these observations supports the hypothesis that sensitivity of males to OT release influences the probability that they will adopt a monogamous mating tactic. However, laboratory studies indicating OT is unnecessary for partner preference formation (e.g. Winslow et al., 1993) suggest that either the behavioral context shapes OTR phenotype (see below), or that the influence of accumbal OT in male pairbonding is complex and may only become obvious under more natural circumstances.
If OTR in the accumbens proves to be important for the initiation of male bonds, the data raise a challenging question: How does variation in accumbal OTR persist, given the fitness benefits associated with pairing? The persistence of variation suggests either we have not identified the source of selection on accumbal OTR variation, or that accumbal OTR is not driving variation in pairbonding. The latter explanation seems unlikely given prior studies that have demonstrated that accumbal OTR is so central to this behavior in females (Ross et al., 2009b; Young et al., 2001; but see Winslow et al., 1993). So what sources might drive the variation in male accumbal OTR? Some answers to this question may be found by considering whether OTR density in the NAcc is a cause or consequence of pairbonding. If OTR expression in the NAcc is a ‘fixed’ phenotype, then such variation may indicate that some males are predisposed to form bonds, while others are not. If this were true, it would suggest that there are conflicting sources of stabilizing and disruptive selection on accumbal OTR abundance. Alternatively, pairbonding or some other aspect of the social context prior to pairbonding could increase accumbal OTR. Indeed, accumbal dopamine receptor density changes after pairbonding; pairbonds are initially facilitated and then exclusively maintained by the relative densities of dopamine receptors in the NAcc (Aragona et al., 2006). Like dopamine receptors, OTR density could be plastic, changing as a function of the social experience. If this were true, then it may be useful to view accumbal OTR expression as a means of weighting the intensity of reward to facilitate pairbond reinforcement during or just prior to the early stages of bonding. In this view, a ‘dynamic’ phenotype of accumbal OTR might act as a ‘knob’ (Young and Hammock, 2007) to enhance or curtail the rewarding experience associated with a particular individual, thereby altering the probability of pairbond formation. The former alternative suggests that accumbal OTR predisposes animals toward bonding independently of the social landscape, the latter suggests that social influences are important in initiating bonds. Unfortunately our data are unable to resolve whether accumbal OTR represents a fixed or dynamic phenotype. In either case, accumbal OTR is a remarkably strong predictor of male mating behavior, suggesting a previously unappreciated role for oxytocin in regulating male pairing.
Mating success across tactics
Convention in behavioral ecology dictates that the decision to mate for males is usually quite simple: mate whenever possible. Socially monogamous males should account for a few important factors before engaging in such behavior, including their resource holding potential, the social environment, and their current body condition (Emlen and Oring, 1977). Considering this, our results indicating that OTR in the posterior region of the insular cortex (ICp) predicted mating success (Fig. 4) were unanticipated, somewhat surprising, and particularly intriguing. This is because the insular cortex facilitates self-assessment of current body condition (Craig, 2002), modulates human emotion (Gallese et al., 2008; Lamm and Singer, 2010) and affects social affiliation (Caruana et al., 2011). Taken together, such information may be used to inform mating decisions and promote mating behavior within chosen mating tactics. Moreover, the prefrontal cortex is commonly used for planning and emotional processing, and our results demonstrated that OTR in the PFC was positively correlated with OTR in both the anterior and posterior regions of the insular cortex (Fig. 3). The insula has reciprocal connections to limbic regions such as the prefrontal cortex, cingulate cortex, medial amygdala, and ventral striatum (Augustine, 1996). It is plausible that OTR in the insula of voles may modulate incoming information about body condition to influence mating decisions and promote the urge to engage in sexual behavior when conditions are maximal for such behavior, regardless of whether an individual is pairbonded. Whether or not the insula modifies the decision to mate remains to be tested. Nevertheless, the insula, and any other areas that vary by mating success, may provide a fruitful area of investigation to understand tactic-independent mating decisions.
Socio-spatial memory
Establishing pairbonds is without question a pivotal component of monogamy, but social bonds are clearly not the whole story. Animals require a larger set of cognitive abilities to properly express and maintain monogamy. Rarely are spatial or social memory considered to be constituents of monogamy or other mating systems. However, monogamous and non-monogamous animals use space differently (Ophir et al., 2008a; Ophir et al., 2008b), making space use an important component of mating tactics. For instance, when formulating the decision whether or not to mate monogamously, a male must consider the resources located within a given territory that he is able to defend and the number of mating partners he is capable of monopolizing (Emlen and Oring, 1977). To successfully mate-guard, he must also be proficient at defending those resources while monitoring the activity of his mate (or mates). Similarly, a male must monitor the activity of his neighbors. For example monogamous males must assess which individuals threaten their fitness through cuckoldry or infanticide, and which may boost their fitness through pairing opportunities or chance extra-pair mating. While the need for monogamous individuals to patrol and defend territorial boundaries from neighbors should place a significant reliance on socio-spatial memory, larger home ranges associated with non-monogamous mating tactics might also demand proficient socio-spatial memory to navigate large areas and track the estrous cycles of neighboring females. Selective pressures stemming from a species’ natural history, the general form of mating system in the population, and other behavioral or ecological factors will play an important role in shaping the different forms such memory might take for a given tactic, and which neural mechanisms are involved in governing the different necessary aspects of socio-spatial cognition. Either way, an inherent factor in a male’s decision to adopt monogamy or other mating tactics may have much to do with associations between using space (spatial memory), distinguishing the identities of neighbors (social memory), and accounting for the spatial distribution of those neighbors (socio-spatial memory).
Our data have demonstrated that differential patterns of space use are the best predictors of reproductive success among monogamous residents and non-monogamous wanderers (Ophir et al., 2008a; Ophir et al., 2008b). Patterns of OTR expression in the Hi and SHi bolster the hypothesis that monogamy is an inherently spatial task for prairie voles. In addition to V1aR expression in the posterior cingulate cortex, and lateral dorsal thalamic nucleus, OTR expression in the Hi and SHi directly mirror patterns of space use among free living prairie voles (Ophir et al., 2008b, see Fig. 5). Like V1aR, OTR in neural structures that govern socio-spatial memory among residents was generally greater in those that demonstrated reproductive success, whereas reproductively successful wanderers expressed significantly less OTR than wanderers that did not breed. All four of these neural structures are deeply involved in social and spatial memory processing (Giovannini et al., 1994; Good, 2002; Marighetto et al., 1994; Mizumori et al., 1994; van Groen et al., 2002; van Groen et al., 2004), and AVP and OT are well known for their influences in modulating various forms of memory (Caldwell et al., 2008; Lee et al., 2009; McEwen, 2004). Connections among these structures and with the lateral septum (Desmedt et al., 1999; Giovannini et al., 1994; Jaffard et al., 1996; Marighetto et al., 1994) suggest that they form a coordinated network of memory-related structures that could be used to meet the cognitive demands necessary to succeed at various mating tactics within a social system. Estimating the socio-spatial landscape should be heavily influenced by neural structures involved in socio-spatial memory and in particular those affected by nonapeptides. Differential neural phenotypes within these structures could drive individual behavioral tactics and the associated decision rules therein (Phelps and Ophir, 2009). Reproductively successful resident and wandering male prairie voles may therefore differentially rely on socio-spatial memory, and the action of nonapeptides in socio-spatial structures may shape reproductive decisions by assisting evaluation of the social context.
Concluding remarks
Our data indicate that oxytocin receptor densities in the nucleus accumbens, the reward center of the brain and an area central to pairbonding, differed between paired and single males. The prefrontal cortex, an OTR dense area typically associated with pairbonding, did not show this pattern. Similarly, the posterior portion of the insular cortex demonstrated different OTR expression profiles among males that sired offspring and those that did not. Finally, our data indicated that oxytocin receptor densities in the hippocampus and the septohippocampal nucleus predict patterns of behavior in the field for the most successful monogamous residents and non-monogamous wanderers.
In most nodes of the pairbonding neural circuit that express nonapeptide receptors, selection appears to have eliminated natural variation implicating this neural circuit as a critical component of social monogamy. The one exception to this is the nucleus accumbens, for which OTR density differed by male mating tactic. While our data indicate that OTR expression seems intimately related to males’ chosen mating tactics, we cannot address how or why they might be related. We speculate that the NAcc may bias the probability of forming pairbonds. The nucleus accumbens receives strong projections from the Hi and LS, and it sends afferents to the LS and other limbic structures (Groenewegen and Russchen, 1984; Kelly and Domesick, 1982; Powell and Leman, 1976; Swanson and Cowan, 1977), suggesting that it is well positioned to bridge between other neural structures that enable monogamous bonds to form and socio-spatial neural structures that predict monogamous mating tactics.
The ability to navigate space and relate that ability to social interactions is something that has been relatively unappreciated in discussions of mating system. Our data indicate that these behaviors are integral to successful monogamy and appear to each be influenced by the neuromodulatory action of oxytocin in important ways. The fact that these data originated from male brains provokes a reevaluation of the commonly held belief that oxytocin is crucial for female monogamous behavior, but is less important in males. In reality, the role of oxytocin in mating tactics and its influence over mating decisions is probably much more nuanced and widespread than the oversimplified view that it serves as the ‘female monogamy hormone’.
Supplementary Material
Highlights.
Monogamous males express more OTR than non-monogamous males in the nucleus accumbens.
OTR density in the posterior portion of the insula predicts mating success.
Hippocampal OTR predicts space use and reproductive success within mating tactics.
Acknowledgments
This work was supported by funding from the National Science Foundation under grant numbers 0316631 and 0316451, by the Eunice Kennedy Schriver National Institute of Child Health and Human Development (HD065604-01), the Oklahoma State University College of Arts and Sciences, and the Oklahoma State University Wentz Research Scholars Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, Insel TR, Wang Z. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nat Neurosci. 2006;9:133–139. doi: 10.1038/nn1613. [DOI] [PubMed] [Google Scholar]
- Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Rev. 1996;22:229–244. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- Bamshad M, Novak MA, DeVries GJ. Sex and species differences in the vasopressin innervation of sexually naïve and parental prairie voles, Microtus ochrogaster and meadow voles, Microtus pennsylvanicus. J Neuroendocrinol. 1993;5:247–255. doi: 10.1111/j.1365-2826.1993.tb00480.x. [DOI] [PubMed] [Google Scholar]
- Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron. 2008;58:639–650. doi: 10.1016/j.neuron.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57:289–300. [Google Scholar]
- Caldwell HK, Lee H-J, Macbeth AH, Young WS., 3rd Vasopressin: Behavioral roles of an “original” neuropeptide. Prog Neurobiol. 2008;84:1–24. doi: 10.1016/j.pneurobio.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael MS, Humbert R, Dixen J, Palmisano G, Greenleaf W, Davidson JM. Plasma oxytocin increases in the human sexual response. J Clin Endocriniol Metab. 1987;64:27–31. doi: 10.1210/jcem-64-1-27. [DOI] [PubMed] [Google Scholar]
- Carter CS, DeVries AC, Taymans SE, Roberts RL, Williams JR, Getz LL. Peptides, steroids, and pair bonding. Ann NY Acad Sci. 1997;807:260–272. doi: 10.1111/j.1749-6632.1997.tb51925.x. [DOI] [PubMed] [Google Scholar]
- Carter CS, Keverne EB. The neurobiology of social affiliation and pair bonding. In: Pfaff D, editor. Hormones, Brain and Behavior. Academic Press; San Diego: 2002. pp. 299–337. [Google Scholar]
- Caruana F, Jezzini A, Sbriscia-Fioretti B, Rizzolatti G, Gallese V. Emotional and social behaivors elicited by electrical stimulation of the insula in the macaque monkey. Curr Biol. 2011;21:1–5. doi: 10.1016/j.cub.2010.12.042. [DOI] [PubMed] [Google Scholar]
- Cho MM, DeVries AC, Williams JR, Carter CS. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster) Behav Neurosci. 1999;113:1071–1079. doi: 10.1037//0735-7044.113.5.1071. [DOI] [PubMed] [Google Scholar]
- Clutton-Brock TH. The evolution of parental care. University of Cambridge Press; Cambridge: 1991. [Google Scholar]
- Craig AD. How do you feel? Introception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- De Dreu CKW, Greer LL, Handgraaf MJJ, Shalvi S, Van Kleef GA, Baas M, Ten Velden FS, Van Dijk E, Feith SWW. The neuropeptide oxytocin regulates parochial altruism in intergroup conflict among humans. Science. 2010;328:1408–1411. doi: 10.1126/science.1189047. [DOI] [PubMed] [Google Scholar]
- De Dreu CKW, Greer LL, Van Kleef GA, Shalvi S, Handgraaf MJJ. Oxytocin promotes human ethnocentrism. PNAS. 2011;108:1262–1266. doi: 10.1073/pnas.1015316108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmedt A, Garcia R, Jaffard R. Vasopresin in the lateral septum promotes elemental conditioning to the detriment of contextual fear conditioning in mice. Eur J Neurosci. 1999;11:3913–3921. doi: 10.1046/j.1460-9568.1999.00815.x. [DOI] [PubMed] [Google Scholar]
- Emlen ST, Oring LW. Ecology, sexual selection, and evolution of mating systems. Science. 1977;197:215–223. doi: 10.1126/science.327542. [DOI] [PubMed] [Google Scholar]
- Gallese V, Keysers C, Rizzolatti G. A unifying view of the basis of social cognition. Trends Cogn Sci. 2008;8:296–403. doi: 10.1016/j.tics.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Getz LL, Carter CS, Gavish L. The mating system by the prairie vole, Microtus ochrogaster. Behav Ecol Sociobiol. 1981;8:189–194. [Google Scholar]
- Getz LL, Hofmann JE. Social organization in free-living prairie voles, Microtus ochrogaster. Behav Ecol Sociobiol. 1986;18:275–282. [Google Scholar]
- Getz LL, McGuire B, Pizzuto T, Hofmann J, Frase B. Social organization of the prairie vole (Microtus ochrogaster) J Mammal. 1993;74:44–58. [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: Structure, function, and regulation. Physiol Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Giovannini MG, Mutolo D, Blanchi L, Michelassi A, Pepeu G. NMDA receptor antagonists decrease GABA outflow from the septum and increase acetylcholine outflow from the hippocampus: A microdialysis study. J Neurosci. 1994;14:1358–1365. doi: 10.1523/JNEUROSCI.14-03-01358.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good M. Spatial memory and hippocampal function: Where are we now? Psicologica. 2002;23:109–138. [Google Scholar]
- Goodson JL, Schrock SE, Klatt JD, Kabelik D, Kingsbury MA. Mesotocin and nonapeptide receptors promote estrildid flocking behavior. Science. 2009;325:862–866. doi: 10.1126/science.1174929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Russchen FT. Organization of the efferent projections of the nucleus accumbens to pallidal, hypothalamic, and mesencephalic structures: A tracing and immunohistochemical study in the cat. J Comp Neurol. 1984;223:347–367. doi: 10.1002/cne.902230303. [DOI] [PubMed] [Google Scholar]
- Insel TR, Hulihan TJ. A gender-specific mechanism for pair bonding: Oxytocin and partner preference formation in monogamous voles. Behav Neurosci. 1995;109:782–789. doi: 10.1037//0735-7044.109.4.782. [DOI] [PubMed] [Google Scholar]
- Insel TR, Shapiro LE. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. PNAS. 1992;89:5981–5985. doi: 10.1073/pnas.89.13.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Wang ZX, Ferris CF. Patterns of brain vasopressin receptor distribution associated with social organization in microtine rodents. J Neurosci. 1994;14:5381–5392. doi: 10.1523/JNEUROSCI.14-09-05381.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffard R, Vouimba RM, Marighetto A, Garcia R. Long term potentiation and long term depression in the lateral septum in spatial working and reference memory. J Physiol (Paris) 1996;90:339–341. doi: 10.1016/s0928-4257(97)87915-6. [DOI] [PubMed] [Google Scholar]
- Kelly AE, Domesick VB. The distribution of the projection from the hippocampal formation to the nucleus accumbens in the rat: An anteriograde- and retrograde-horseradish peroxidase study. Neuroscience. 1982;7:2321–2335. doi: 10.1016/0306-4522(82)90198-1. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Kingsbury MA, Hoffbuhr K, Schrock SE, Waxman B, Kabelik D, Thompson RR, Goodson JL. Vacotocin and septal V1a-like receptors potentially modulate songbird flocking and responses to novelty. Horm Behav. 2011;60:12–21. doi: 10.1016/j.yhbeh.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick KM, Keverne EB, Baldwin BA, Sharman DF. Cerebrospinal fluid levles of acetylcholinesterase, monoamines and oxytocin during labour, parturition, vaginocervical stimulation, lamb separation and suckling in sheep. Neuroendocriniol. 1986;44:149–156. doi: 10.1159/000124638. [DOI] [PubMed] [Google Scholar]
- Ketterson ED, Nolan V. Hormones and life history: An integrative approach. Am Nat. 1992;140:S33–S62. doi: 10.1086/285396. Supplement: Behavioral mechanisms in evolutionary ecology. [DOI] [PubMed] [Google Scholar]
- Kleiman DG. Monogamy in mammals. Q Rev Biol. 1977;52:39–69. doi: 10.1086/409721. [DOI] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Lamm C, Singer T. The role of anterior insular cortex in social emotions. Brain Struct Funct. 2010;214:579–591. doi: 10.1007/s00429-010-0251-3. [DOI] [PubMed] [Google Scholar]
- Lee H-J, Macbeth AH, Pagani JH, Young WS., 3rd Oxytocin: The great facilitator of life. Prog Neurobiol. 2009;88:127–151. doi: 10.1016/j.pneurobio.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MM, Young LJ. Vasopressin-dependent neural circuits underlying pair bond formation in the monogamous prairie vole. Neuroscience. 2004;125:35–45. doi: 10.1016/j.neuroscience.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Liu Y, Curtis JT, Wang ZX. Vasopressin in the lateral septum regulates pair bond formation in male prairie voles (Microtus ochrogaster) Behav Neurosci. 2001;115:910–919. doi: 10.1037//0735-7044.115.4.910. [DOI] [PubMed] [Google Scholar]
- Mahady SJ, Wolff JO. A field test of the Bruce effect in the monogamous prairie vole (Microtus ochrogaster) Behav Ecol Sociobiol. 2002;52:31–37. [Google Scholar]
- Marighetto A, Micheau J, Jaffard R. Effects of intraseptally injected glutamatergic drugs on hippocampal sodium-dependent high-affinity choline uptake in ‘naive’ and ‘trained’ mice. Pharmacol Biochem Behav. 1994;49:689–699. doi: 10.1016/0091-3057(94)90089-2. [DOI] [PubMed] [Google Scholar]
- McEwen B. The roles of vasopressin and oxytocin in memory processing. Elsevier Academic Press; San Diego: 2004. [DOI] [PubMed] [Google Scholar]
- McGuire B, Getz LL. Response of young female prairie voles (Microtus ochrogaster) to nonresident males: Implications for population regulation. Can J Zool. 1991;69:1348–1355. [Google Scholar]
- McGuire B, Getz LL. The nature and frequency of social interactions among free-living prairie voles (Microtus ochrogaster) Behav Ecol Sociobiol. 1998;43:271–279. [Google Scholar]
- McGuire B, Pizzuto T, Getz LL. Potential for social interaction in a natural population of prairie voles, Microtus ochrogaster. Can J Zool. 1990;68:391–398. [Google Scholar]
- Mizumori SJY, Miya DY, Ward KE. Reversible inactivation of the lateral dorsal thalamus disrupts hippocampla place representation and impairs spatial learning. Brain Res. 1994;644:168–174. doi: 10.1016/0006-8993(94)90361-1. [DOI] [PubMed] [Google Scholar]
- Oliveras D, Novak MA. A comparison of paternal behaviour in the meadow vole (Microtus pennsylvanicus), the pine vole (Microtus pinetorum), and the prairie vole (Microtus ochrogaster) Anim Behav. 1986;34:519–526. [Google Scholar]
- Ophir AG, Phelps SM, Sorin AB, Wolff JO. Morphological, genetic, and behavioral comparisons of two prairie vole populations in the field and laboratory. J Mammal. 2007;88:989–999. [Google Scholar]
- Ophir AG, Phelps SM, Sorin AB, Wolff JO. Social but not genetic monogamy is associated with greater breeding success in prairie voles. Anim Behav. 2008a;75:1143–1154. [Google Scholar]
- Ophir AG, Wolff JO, Phelps SM. Variation in neural V1aR predicts sexual fidelity and space use among prairie voles in semi-natural settings. PNAS. 2008b;105:1249–1254. doi: 10.1073/pnas.0709116105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps SM, Ophir AG. Monogamous brains and alternative tactics: Neuronal V1aR, space use and sexual infidelity among male prairie voles. In: Dukas R, Ratcliffe JM, editors. Cognitive Ecology II. University of Chicago Press; Chicago: 2009. pp. 156–176. [Google Scholar]
- Pitkow LJ, Sharer CA, Ren X, Insel TR, Terwilliger EF, Young LJ. Facilitation of affiliation and pair-bond formation by vasopressin receptor gene transfer into the ventral forebrain of a monogamous vole. J Neurosci. 2001;21:7392–7396. doi: 10.1523/JNEUROSCI.21-18-07392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell EW, Leman RB. Connections of the nucleus accumbens. Brain Res. 1976;105:389–403. doi: 10.1016/0006-8993(76)90589-8. [DOI] [PubMed] [Google Scholar]
- Rimmele U, Hediger K, Heinrichs M, Klaver P. Oxytocin makes a face in memory familiar. J Neurosci. 2009;29:38–42. doi: 10.1523/JNEUROSCI.4260-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Cole CD, Smith Y, Neumann ID, Landgraf R, Murphy AZ, Young LJ. Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience. 2009a;162:892–903. doi: 10.1016/j.neuroscience.2009.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Freeman SM, Spiegel LL, Ren X, Terwilliger EF, Young LJ. Variation in oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviors in monogamous and polygamous voles. J Neurosci. 2009b;29:1312–1328. doi: 10.1523/JNEUROSCI.5039-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol. 2009;30:534–547. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Row JR, Blouin-Demers G. Kernels are not accurate estimators of home-range size for herpetofauna. Copeia. 2006;2006:797–802. [Google Scholar]
- Sansone GR, Gerdes CA, Steinman JL, Winslow JT, Ottenweller JE, Komisaruk BR, Insel TR. Vaginocervical stimulation releases oxytocin within the spinal chord in rats. Neuroendocriniol. 2002;75:306–315. doi: 10.1159/000057340. [DOI] [PubMed] [Google Scholar]
- Savaskan E, Ehrhardt R, Schulz A, Walter M, Schachinger H. Post-learning intranasal oxytocin modulates human memory for facial identity. Psychoneuroendocrinol. 2008;33:368–374. doi: 10.1016/j.psyneuen.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Solomon NG. Body size and social preference of male and female prairie voles, Microtus ochrogaster. Anim Behav. 1993a;45:1031–1033. [Google Scholar]
- Solomon NG. Comparison of parental behavior in male and female prairie voles (Microtus ochrogaster) Can J Zool. 1993b;71:434–437. [Google Scholar]
- Swanson LW, Cowan WM. An autoradiographic study of the organization of the efferent connections of the hippocampal formation in the rat. J Comp Neurol. 1977;172:49–84. doi: 10.1002/cne.901720104. [DOI] [PubMed] [Google Scholar]
- Taitt MJ, Krebs CJ. Population dynamics and cycles. In: Tamarin RH, editor. Biology of new world Microtus. Special publication No. 8. American Society of Mammalogists; Provo: 1985. pp. 567–620. [Google Scholar]
- Thomas JA, Birney EC. Parental care and mating system of the prairie vole, Microtus ochrogaster. Behav Ecol Sociobiol. 1979;5:171–186. [Google Scholar]
- Turner LM, Young AY, Rompler H, Schoneberg T, Phelps SM, Hoekstra HE. Monogamy evolves through multiple mechanisms: Evidence from V1aR in deer mice. Molec Biol Evol. 2010;27:1269–1278. doi: 10.1093/molbev/msq013. [DOI] [PubMed] [Google Scholar]
- van Belle G, Fisher LD, Heagerty PJ, Lumley T. Biostatistics: A methodology for the health sciences. John Wiley and Sons, Inc; Hoboken: 2004. [Google Scholar]
- van Groen T, Kadish I, Wyss JM. The role of the laterodorsal nucleus of the thalamus in spatial learning and memory in the rat. Behav Brain Res. 2002;136:329–337. doi: 10.1016/s0166-4328(02)00199-7. [DOI] [PubMed] [Google Scholar]
- van Groen T, Kadish I, Wyss JM. Retrosplenial cortex lesions of area Rgb (but not of area Rga) impair spatial learning and memory in the rat. Behav Brain Res. 2004;154:483–491. doi: 10.1016/j.bbr.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Wang ZX, Ferris CF, Devries GJ. Role of septal vasopressin innervation in paternal behavior in prairie voles (Microtus ochrogaster) PNAS. 1994a;91:400–404. doi: 10.1073/pnas.91.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZX, Smith W, Major DE, DeVries GJ. Sex and species differences in the effects of cohabitation on vasopressin messenger RNA expression in the bed nucleus of the stria terminalis in prairie voles (Microtus ochrogaster) and meadow voles (Microtus pennsylvanicus) Brain Res. 1994b;650:212–218. doi: 10.1016/0006-8993(94)91784-1. [DOI] [PubMed] [Google Scholar]
- White GC, Garrott RA. Analysis of wildlife radio-tracking data. Academic Press; New York: 1990. [Google Scholar]
- Wideman CH, Murphy HM. Vasopressin, maternal behavior, and pup well-being. Curr Psychol Res Rev. 1990;9:285–295. [Google Scholar]
- Williams JR, Catania KC, Carter CS. Development of partner preference in female prairie voles (Microtus ochrogaster): The role of social and sexual experience. Horm Behav. 1992;26:339–349. doi: 10.1016/0018-506x(92)90004-f. [DOI] [PubMed] [Google Scholar]
- Williams JR, Insel TR, Harbaugh CR, Carter CS. Oxytocin administered centrally facilitates formation of a partner preference in female prairie voles. J Neuroendocrinol. 1994;6:247–250. doi: 10.1111/j.1365-2826.1994.tb00579.x. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Young LJ, Hammock EAD. On switches and knobs, microsatellites and monogamy. Trends Genet. 2007;23:209–212. doi: 10.1016/j.tig.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Young LJ, Lim MM, Gingrich B, Insel TR. Cellular mechanisms of social attachment. Horm Behav. 2001;40:133–138. doi: 10.1006/hbeh.2001.1691. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang ZX. The neurobiology of pair bonding. Nat Neurosci. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- Young LJ, Winslow JT, Nilsen R, Insel TR. Species differences in V1a receptor gene expression in monogamous and nonmonogamous voles: Behavioral consequences. Behav Neurosci. 1997;111:599–605. doi: 10.1037//0735-7044.111.3.599. [DOI] [PubMed] [Google Scholar]
- Young LJ, Young AZM, Hammock EAD. Anatomy and neurochemistry of the pair bond. J Comp Neurol. 2005;493:51–57. doi: 10.1002/cne.20771. [DOI] [PubMed] [Google Scholar]
- Zak PJ, Kurzban R, Matzner WT. Oxytocin is associated with human trustworthiness. Horm Behav. 2005;48:522–527. doi: 10.1016/j.yhbeh.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Zeki S. The neurobiology of love. Fed Eur Biocham Soc Let. 2007;581:2575–2579. doi: 10.1016/j.febslet.2007.03.094. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.