Abstract
Vitamin D deficiency has been linked with increased cancer risk, and vitamin D has been shown to be cytotoxic to some cancer cells in vitro. In the present study we evaluated whether vitamin D would have antiproliferative or cytotoxic effects on human pre-B acute lymphoblastic leukemia cells. Contrary to our hypotheses, calcitriol, the active form of vitamin D, had no effect on leukemia cell proliferation. Calcitriol actually had a modest effect to impair dexamethasone cytotoxicity and induction of apoptosis. Further studies are needed to evaluate the effects of vitamin D on leukemia cells in vivo.
Keywords: Vitamin D, calcitriol, acute lymphoblastic leukemia, dexamethasone
Introduction
In addition to its classical actions on intestine and bone, vitamin D exerts other diverse effects regulating cellular proliferation, differentiation, apoptosis, autophagy, hematopoiesis, immune function and angiogenesis. The vitamin D receptor which mediates a majority of these actions is expressed in several tissues including bone, kidney, prostate, breast, blood, intestine and cells of the immune system. Vitamin D deficiency has been proposed as a link between cancer and lack of sun exposure [1]. This hypothesis is supported by large prospective studies which show that vitamin D deficiency is associated with increased risk of several types of cancer [2], and by in vitro studies, which show that vitamin D and its analogues suppress the proliferation of many cancer cell types. Furthermore, calcitriol (1α,25(OH)2D3), the active form of vitamin D, potentiates the antitumor effects of several chemotherapeutic agents on various cancer types in vitro and in xenograft models [3].
Although vitamin D receptors are expressed in hematopoietic precursors and malignant T and B lymphocyte derived cell lines [4], the effects of calcitriol on the most common childhood cancer, acute lymphoblastic leukemia (ALL) have to our knowledge not been reported. Although survival rates of childhood ALL have improved dramatically over the prior decades, relapse continues to be a problem. Leukemia cell resistance to steroids is a significant problem, which likely contributes to relapse. In the present study, we hypothesized that calcitriol would: 1) suppress the growth of B precursor acute lymphoblastic leukemia (Pre B ALL) cells, and 2) act synergistically with the steroid chemotherapy dexamethasone to augment their cytotoxicity against ALL cells.
Materials and Methods
Human ALL cell lines were purchased from ATCC (RS4;11, Sup-B15) and DSMZ (SD-1, RCH-ACV), and cultured under standard conditions. Viable cells were counted at 72 hour time points using trypan blue exclusion. Apoptosis was measured using Annexin V and 7-AAD staining with flow cytometry on a Becton Dixon FACscan. Gene expression of the vitamin D receptor, glucocorticoid receptor, and Bim was measured using quantitative rtPCR. Primers used included: human β-Actin-forward (5’-ACA GAG CCT CGC CTT TGC CG-3’) and reverse (5’-CGA TGC CGT GCT CGA TGG GG-3’); human VDR-forward (5’-CGG CCG GAC CAG AAG CCT TT-3’) and reverse (5’-CCT TCC GCT TCA TGC TTC GCC T-3’); human BIM forward (5’- TGG CAA AGC AAC CTT CTG ATG-3’) and reverse (5’-GCA GGC TGC AAT TGT CTA CCT-3’); human glucocorticoid receptor forward (5’-ACC AAT TCC CGT TGG TTC CGA-3’) and reverse (5’-GTC CTT CCA CTG CTC TTT TGA AGA-3’).
Results
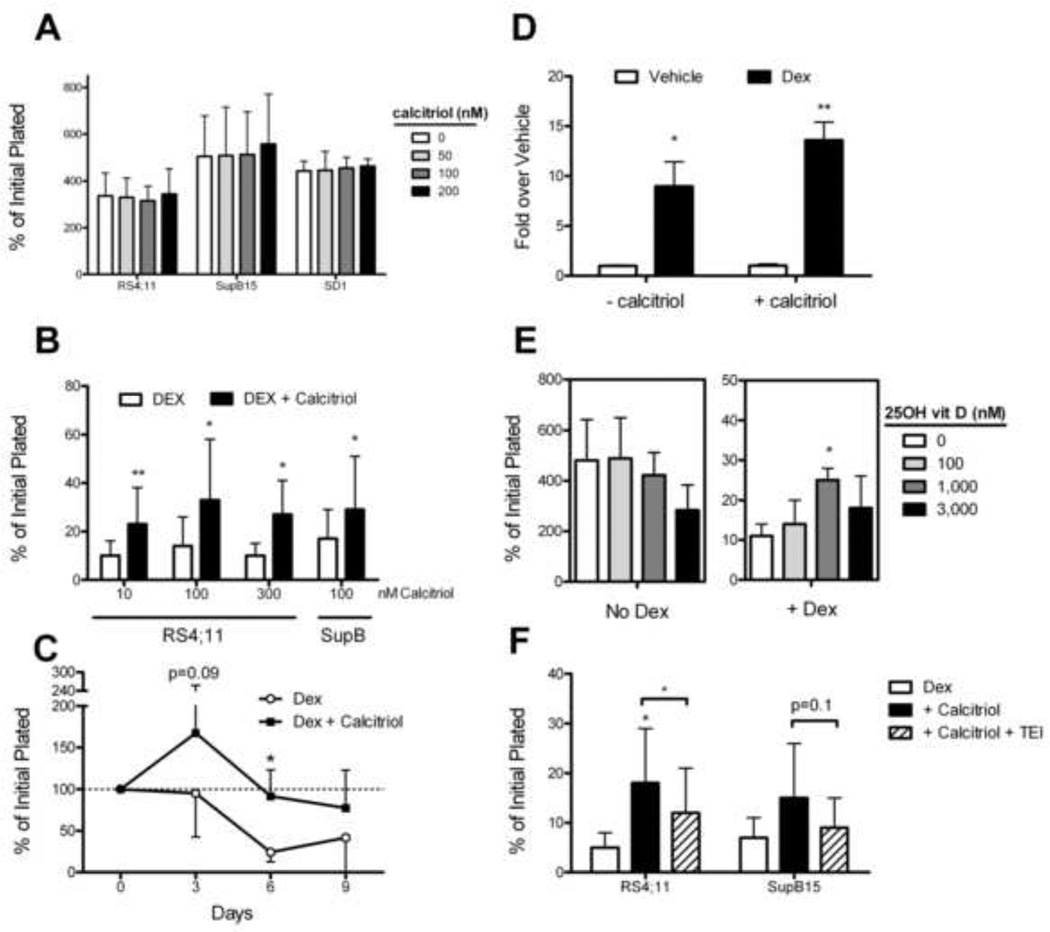
Contrary to our first hypothesis, we found that various doses of calcitriol (Sigma) had no effect on proliferation of four different human ALL cell lines in vitro (Fig 1A). To see whether calcitriol would augment the cytotoxic effect of the steroids on ALL, we cultured two dexamethasone-sensitive ALL cell lines with 100 nM (RS4;11) or 60nM (Sup-B15) dexamethasone for 72 hrs, with and without calcitriol. Again contrary to our hypothesis, we found that calcitriol had a small but consistent effect to protect the ALL cells from dexamethasone (Fig 1B). This protective effect of calcitriol was confirmed with an alternative source of the compound (Cayman Chemicals, not shown), and was present at concentrations as low as 10 nM. Calcitriol continued to protect RS4;11 ALL cells from a low concentration of dexamethasone (20 nM) for at least nine days (Fig 1C).
Figure 1. Calcitriol protects human ALL cells from dexamethasone.
A. Proliferation of human ALL cell lines after 72 hours of culture with calcitriol (n=4 experiments). B. Percent of remaining, viable cells after 72 exposure to dexamethasone, with and without calcitriol (see text for details, n=4). C: Percent of remaining viable RS4;11 cells after longer term culture with 20 nM dexamethasone ± 100 nM Calcitriol. Cells were washed and replated in fresh media and drugs every 72 hours. D: Expression of the VDR in RS4;11 cells by rtPCR after 24 hours exposure to dexamethasone (100 nM), with and without exposure to calcitriol (100 nM, n=3). E. Effect of 25(OH) vitamin D on RS4;11 cells in culture for 72 hours (left panel), and after dexamethasone treatment (right panel, n=3). F. Percent of viable human ALL cells after 72 hour exposure to dexamethasone (100 nM for RS4;11, 60 nM for Sup-B15) in the presence of 100 nM calcitriol, with and without the VDR antagonist, TEI-9647 (1,000 nM, n=4). *p<0.05, **p<0.01 vs. Dex alone.
We next sought to investigate the potential role of the vitamin D receptor (VDR) in the calcitriol-induced protection from dexamethasone. Both RS4;11 and SupB15 cells expressed the VDR; while exposure to calcitriol (100 nM) had little effect on VDR expression, dexamethasone treatment (100 nM) increased expression of the VDR several fold in 24 hours (Fig 1D). The physiological precursor of calcitriol, 25(OH) Cholecalciferol (25OH Vitamin D, Sigma), which has an ~1 000 fold lower affinity for VDR, did not enhance RS4;11 proliferation rate, but did cause a partial dexamethasone resistance at doses over 1,000 nM (Fig 1E). To confirm that the protective effect of calcitriol was mediated by VDR, we utilized the VDR antagonist TEI-9647 (23S-25-dehydro-1a-hydroxyvitamin D3-26, 23-lactone, kindly provided by Teijin Pharma, Japan, [5]). By itself, 1000 nM TEI-9647 had no effect on proliferation of RS4;11 cells over 72 hours (1.58±0.45 vs. 1.75±0.40×106, vehicle vs. TEI-9647, p=n.s.), nor on SupB15 cells (not shown). However, this dose of TEI-9647 partially reversed the calcitriol-mediated protection from dexamethasone in RS4;11 cells, although this did not reach statistical significance in SupB15 cells (100 nM dex was used for RS4;11, 60 nM used for Sup-B15 cells, Fig 1F).
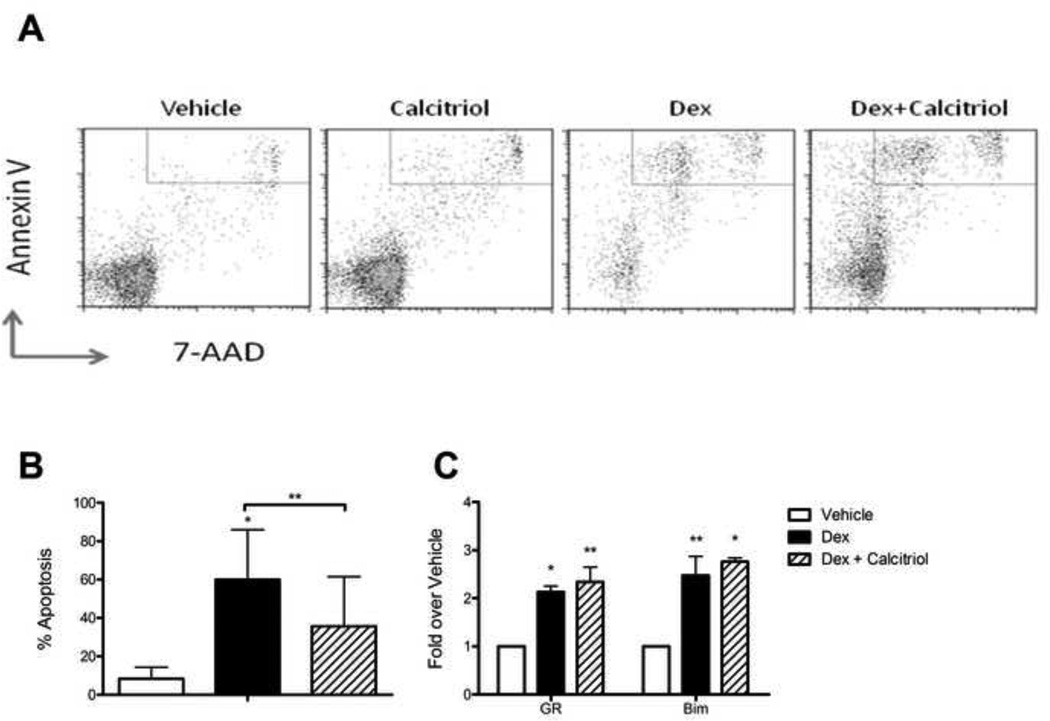
While dexamethasone induced significant apoptosis in RS4;11 cells, this apoptosis was partially prevented by calcitriol (Fig 2A & B). Since dexamethasone-induced apoptosis has been shown to depend on induction of both the glucocorticoid receptor (GR, [6]) and the pro-apoptotic signal, BIM [7], we measured expression of these genes in RS4;11 cells after exposure to these two drugs. Calcitriol (100 nM) did not alter dexamethasone (100 nM) induction of either GR or BIM after 5 hours (Fig 2C), implying that calcitriol does not impair dexamethasone binding to the GR receptor, translocation to the nucleus, or GR binding to response elements.
Figure 2. Calcitriol reduces Dex-induced apoptosis in RS4;11 cells.
A: Representative dot plots of RS4;11 cells after 72 hours of exposure to 50 nM Dex ± 100 nM calcitriol and staining with Annexin V and 7-AAD. B: Percentage of apoptotic (Annexin V and 7-AAD double positive) cells averaged from 3 experiments. C: Gene expression of the glucocorticoid receptor (GR) and the pro-apoptotic signal, BIM after 5 hours exposure to Dex ± calcitriol (both at 100 nM, n=3). *p<0.05, **p<0.01 vs. vehicle
Discussion
A number of epidemiological studies have found that calcitriol has a protective role against many types of cancer [2]. In this study we found that calcitriol, different from its effect on other types of cancer cells, does not impair the proliferation/survival of ALL cells. In fact, calcitriol had a small but consistent effect to protect ALL cells from dexamethasone. Although this was a small effect in vitro, it raises the concern that very high doses of vitamin D could potentially have adverse effects on leukemia treatment. Since children with leukemia often have low vitamin D levels (<30 ng/mL, [8]), and suffer from osteopenia and fractures, it is likely that leukemia patients are being prescribed vitamin D supplementation. In addition, calcitriol has been proposed as a therapeutic adjuvant in the treatment of many cancers [3]. Thus, clinicians should use caution before administering high doses of vitamin D in patients with leukemia, until these effects are more fully explored in vivo and in patients.
Acknowledgements
This study was supported in part by a grant from the National Institutes of Health National Cancer Institute (CA139060).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
RA designed and performed the experiments and prepared the manuscript; RA, XS, EAE, EN, RP, and BI performed experiments and collected data; LK performed and interpreted the FACS analysis; SDM designed experiments, analyzed results, and prepared the manuscript.
Conflict of Interest
The authors declare no conflict of interest
References
- 1.Kricker A, Armstrong B. Does sunlight have a beneficial influence on certain cancers? Prog Biophys Mol Biol. 2006;92:132–139. doi: 10.1016/j.pbiomolbio.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Wei MY, Giovannucci EL. Vitamin D and multiple health outcomes in the Harvard cohorts. Mol Nutr Food Res. 2010;54:1114–1126. doi: 10.1002/mnfr.200900574. [DOI] [PubMed] [Google Scholar]
- 3.Trump DL, Deeb KK, Johnson CS. Vitamin D: considerations in the continued development as an agent for cancer prevention and therapy. Cancer J. 2010;16:1–9. doi: 10.1097/PPO.0b013e3181c51ee6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983;221:1181–1183. doi: 10.1126/science.6310748. [DOI] [PubMed] [Google Scholar]
- 5.Ozono K, Saito M, Miura D, Michigami T, Nakajima S, Ishizuka S. Analysis of the molecular mechanism for the antagonistic action of a novel 1alpha,25-dihydroxyvitamin D(3) analogue toward vitamin D receptor function. J Biol Chem. 1999;274:32376–32381. doi: 10.1074/jbc.274.45.32376. [DOI] [PubMed] [Google Scholar]
- 6.Ramdas J, Liu W, Harmon JM. Glucocorticoid-induced cell death requires autoinduction of glucocorticoid receptor expression in human leukemic T cells. Cancer Res. 1999;59:1378–1385. [PubMed] [Google Scholar]
- 7.Jiang N, Koh GS, Lim JY, Kham SK, Ariffin H, Chew FT, et al. BIM is a prognostic biomarker for early prednisolone response in pediatric acute lymphoblastic leukemia. Exp Hematol. 2011;39:321–329. doi: 10.1016/j.exphem.2010.11.009. 329. [DOI] [PubMed] [Google Scholar]
- 8.Halton JM, Atkinson SA, Fraher L, Webber C, Gill GJ, Dawson S, et al. Altered mineral metabolism and bone mass in children during treatment for acute lymphoblastic leukemia. J Bone Miner Res. 1996;11:1774–1783. doi: 10.1002/jbmr.5650111122. [DOI] [PubMed] [Google Scholar]