Abstract
Sarcopenia is a geriatric syndrome in which there is a decrease of muscle mass and strength with aging. In age-related loss of muscle strength, there are numerous observations supporting the assertion that neural factors mediate muscle strength. A possible contributing cause may be that aging changes systemic extracellular heat shock protein (eHsp)72 activity. The present study was designed to assess the plasma levels of eHsp72 in elderly people and to investigate its potential interaction with components of sarcopenia. A total of 665 men and women participated in an official medical health examination and an integrated health examination, including psychological and physical fitness tests. Blood samples were assayed for levels of plasma Hsp72, serum C-reactive protein, interleukin 6, tumor necrosis factor α, and regular biomedical parameters. We found that higher Hsp72 in plasma is associated with lower muscle mass, weaker grip strength, and slower walking speed, and may be a potential biomarker of sarcopenia in elderly people. This finding was supported by other results in the present study: (1) older age and shrinking body and lower hemoglobin levels, all of which characterize sarcopenia, were related to higher eHsp72 tertiles and (2) the ORs of the highest tertile of eHsp72 for the lowest tertiles of muscle mass, grip strength, and walking speed were 2.7, 2.6, and 1.8, respectively. These ORs were independent of age, sex, and the incidence of related diseases. Our results would reveal that eHsp72 in plasma is linked to sarcopenia factors and is a potential biomarker or predictor of sarcopenia.
Keywords: Sarcopenia, Geriatric syndrome, Biomarkers, Extracellular Hsp, Skeletal muscle, Grip strength
Introduction
Sarcopenia is a geriatric syndrome in which there is a decrease of muscle mass and strength with aging (Rosenberg 1997). The prevalence of sarcopenia has been estimated at 5–13% of elderly people aged 60–70 years, and the numbers increase to 11–50% for those aged 80 or above (Haehling et al. 2010). It is a normal part of aging, but if unchecked, it can lead to weakness, disability, falls, loss of independence, and frailty (Roubenoff and Hughes 2000). Recently, the European Working Group on Sarcopenia in Older People developed a definition and diagnosis of sarcopenia (Cruz-Jentoft et al. 2010), introducing quantitative assessments of muscle mass, muscle strength, and physical performance, and established cutoff points based on measurements of muscle mass, grip strength, and gait speed. The International Working Group on Sarcopenia has also proposed criteria for diagnosing sarcopenia (Fielding et al. 2011), including gait speed and muscle mass. Unfortunately, since those cutoff points were based on data from specific ethnic groups, the utilization of these cutoff points or criteria could be limited. However, we may universally assess the data of muscle mass, grip strength, and gait speed with regard to the definition of sarcopenia, respectively. Sarcopenia has many causative factors, including a sedentary lifestyle and neurological, hormonal, nutritional, and immunological determinants (Roubenoff and Hughes 2000). Sarcopenic changes in the muscles include losses in muscle fiber quality and quantity, α-motor neurons, protein synthesis rates, and anabolic and sex hormone production (Waters et al. 2010). Many mechanisms have been proposed to cause the aforementioned sarcopenic changes, but the overall etiology is still not completely understood (Narici and Maffulli 2010). In age-related loss of muscle strength, there are numerous observations supporting the assertion that neural factors mediate muscle strength, and aging adaptations may involve changes in supraspinal drive generated from the cortex, coactivation of the antagonist muscle, as well as maximal spinal cord output and muscle coordination (Clark and Manini 2008). A possible contributing cause may be that aging changes systemic extracellular heat shock protein (Hsp) 72 activity.
Hsp are highly conserved proteins that are expressed both constitutively and under stressful conditions. The major role of Hsp appears to be the protection of the proteome via their molecular chaperone function. Hsp recognize damaged proteins and either channel such proteins into the repair/refolding pathways or to the proteolytic pathway (Lindquist and Craig 1988). In terms of cell survival, Hsp allow cells to respond to damage and begin the processes required to resolve cellular insults (Kampinga et al. 1995). Among the Hsp, the Hsp70 family is intrinsic to cellular life, permitting proteins to perform essential enzymatic reactions, signaling, and structural functions within the tightly packed milieu of the cell, and working to avert the catastrophe of protein aggregation during stress (Lindquist and Craig 1988; Georgopoulos and McFarland 1993). Hps70s are induced to extremely high levels by stress along with a cohort of other Hsp through powerful transcriptional activation, mRNA stabilization, and preferential translation (Lindquist and Craig 1988). Hsp72, which is a member of the Hsp70 family, circulates in the blood (Pockley et al. 1998), where it is referred to as an extracellular Hsp (eHsp, Fleshner et al. 2003).
Aging is associated with the degeneration of Hsp expression with time and the loss of resistance to cellular oxidants (Calderwood 2008). The effects of heat shock factor (Hsf)1 and Hsp on longevity appear to be particularly mediated through their ability to protect motor neurons (Calderwood 2008). It has been shown that the presence of eHsp72 can have a protective effect against necrotic cell death of smooth muscle cells (Johnson and Tytell 1993) and against apoptosis of motor neurons (Robinson et al. 2005). On the other hand, the elderly, even when considered healthy, frequently present systemic low-grade inflammation (Ogawa et al. 2010). It has shown that interleukin (IL)-1β, IL-6, and TNF-α levels in elderly people are related to severe muscle wasting and cachexia (Roubenoff and Hughes 2000), because these inflammatory cytokines are involved in the muscle catabolic processes associated with inflammation (Degens 2010).
While eHsp72 can have a protective effect against apoptosis of motor neurons (Robinson et al. 2005) for the muscle anabolic process, based on the category of sarcopenia, it has been hypothesized that inflammatory cytokines and eHsp72 have independent effects that are potentially associated with prevalent sarcopenia. Thus, whereas inflammatory cytokines are related to muscle catabolism, eHsp72 might be related to anabolic protection of motor neurons. Investigating this hypothesis will help advance our understanding of the involvement of these two distinct components of sarcopenia mechanisms. To test this hypothesis, a cross-sectional analysis of data from the Kusatsu study was conducted, evaluating the associations between prevalent lower muscle mass, grip strength, and gait speed, individually and in combination.
Methods
Subjects
A total of 665 participants aged 65–96 years living in a community setting participated in an official medical health examination for community residents administered by the local government of Kusatsu, Gunma. The total population aged 65 or over was 1,928. All of the elderly people received information on an official medical health examination for community residents by post. Therefore, the ratio of those having the medical examination was 34% (665/1,928). The sex ratio distribution of the participants was significantly different from a random distribution (male, n = 264; female, n = 356; P < 0.001), but there were no significant differences in the mean ages between the sexes [mean ± standard deviation (SD): male 73.5 ± 6.0 years, female 73.4 ± 6.3 years]. All participants were informed of the purpose and risks of the study before giving written informed consent. This study was conducted in accordance with the Declaration of Helsinki, and its protocol was approved by the ethics committee at Tokyo Metropolitan Institute of Gerontology.
Assessment of functional health status, lifestyle, and life satisfaction, and measurement of physical performance
Functional health status, lifestyle, and life satisfaction can possibly confound sarcopenia symptoms. To examine healthiness, the lack of which leads to low daily activity and low total energy expenditure, functional health status was assessed using six parameters: (1) poor hearing, (2) poor sight, (3) walking aid, (4) bathing, (5) dressing, and (6) toileting status. To examine daily activity and locomotive status, which is related to low activity and other factors that reflect on chronic diseases, lifestyle was assessed by five parameters: (1) shopping, (2) cooking, (3) frequency of outdoor activity, (4) alcohol drinking status, and (5) smoking status. To examine mental health status, which is related to low daily activity, life satisfaction was assessed by self-rated health (1–4, score 4 represents self-reported unhealthy status), Geriatric Depression Scale (0–15, a total score of 15 represents depressive moods), and Mini-Mental State Examination (0–30, a total score of 30 represents normal cognitive condition).
The physical performance test consisted of grip strength and walking speed (Suzuki et al. 2003; Studenski et al. 2011; Cooper et al. 2010). The grip strength of the preferred hand was measured two times using a handheld Smedly-type dynamometer. The higher value was adopted. In the walking test, the participant walked along a straight walkway of 11 m on a flat floor. The speed and number of steps were measured for the middle 5-m portion of the walkway. The participant took the test by walking at a preferred speed two times, and the faster speed was recorded.
Clinical history and medical examination
In the medical examinations, body height (using a body height meter), body weight, and skeletal muscle mass were measured using bioelectrical impedance analysis systems (InBody; BIOSPACE, Tokyo, Japan). During the medical examination, a blood sample was collected from the antecubital vein for routine hematological and biochemical tests, including white blood cell count, hemoglobin, albumin, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and triglycerides. Clinical histories and medical examinations were carried out by physicians.
Blood sampling for other laboratory assays
Blood samples were collected from the antecubital vein for plasma in a tube containing 30 μl of EDTA, and serum in a plain tube, and spun at 1,000×g at 4°C for 10 min, and the supernatant was stored at −80°C until analysis. For analysis of eHsp72, enzyme-linked immunosorbent assay (ELISA) kits were used to measure the plasma concentrations of Hsp72 (#ADI EKS-715; Enzo Life Sciences, Inc. NY, USA). Serum C-reactive protein (CRP), IL-6, β2-microglobulin (β2-MG), and tumor necrosis factor (TNF)-α levels were measured using ELISA and enzyme immunoassay, respectively (SRL Co., Tokyo, Japan). The interassay coefficient of variance was 3.6–11.1%. The intra-assay coefficient of variance was 4.6–9.2%.
Statistics
Average values and SD are given in the total numbers of age results. However, eHsp72 had a non-normal distribution as evaluated using the Kolmogorov–Smirnov test (P < 0.01). As a consequence, in the analysis of these parameters, nonparametric tests were used when comparing and correlating the parameters according to eHsp72 levels. Median and interquartile range (25–75th percentile) or average values ± SD are given in the “Results” section, depending on the measured level.
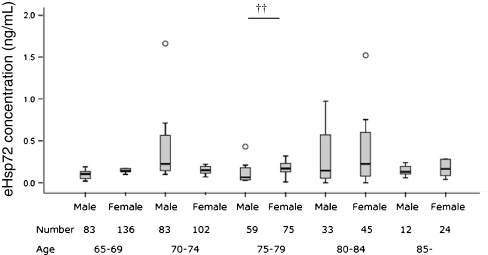
Age differences in eHsp72 were analyzed using Kruskal–Wallis analysis as shown in Fig. 1. Sex differences in eHsp72 in the same age groups were analyzed using the Mann–Whitney test as shown in Fig. 1. The subjects were classified according to tertile levels of eHsp72 shown in Table 1. We then analyzed anthropometric variables, physical fitness variables, biochemical variables, and inflammatory biomarkers using the Jonckheere–Terpstra test to compare the tertile groups. Since sex differences might have biased the relationships between physical fitness variables and other parameters, the values based on eHsp72 tertiles were expressed as totals for men and women separately. The associations between eHsp72 levels and these variables are represented by Spearman correlation coefficients shown in Table 2 and Fig. 2A–C.
Fig. 1.
Box and whisker plots on plasma levels of eHsp72 by age group and sex. Significant differences between sexes in the same age group were analyzed using Mann–Whitney analysis.††P < 0.01. Circles outliter. No differences were found between any of the age groups within the same sex, as analyzed using Kruskal–Wallis analysis
Table 1.
Median and interquartile ranges of anthropometric physical fitness factors flammatory and biomarker levels in relation to tertiles of eHsp72
| Lowest (<0.13 ng/mL) | Middle (0.13–0.22 ng/mL) | Highest (0.22 < ng/mL) | Jonckheere–Terpstra | |||||
|---|---|---|---|---|---|---|---|---|
| Male/female (n) | 109/120 | 85/122 | 76/140 | Total | 270/382 | |||
| Median | IQR | Median | IQR | Median | IQR | P value | ||
| Hsp72 (ng/mL) | ||||||||
| Total | 0.08 | 0.05–0.11 | 0.16 | 0.14–0.19 | 0.53 | 0.29–0.95 | 0.000* | |
| Male | 0.09 | 0.04–0.11 | 0.16 | 0.14–0.19 | 0.54 | 0.31–0.91 | 0.000* | |
| Female | 0.07 | 0.05–0.11 | 0.16 | 0.14–0.18 | 0.52 | 0.28–1.06 | 0.000* | |
| Age (year) | ||||||||
| Total | 72 | 68–77 | 71 | 68–76 | 73 | 69–78 | 0.040** | |
| Male | 72 | 68–77 | 72 | 68–77 | 73 | 69–79 | 0.427 | |
| Female | 71 | 67–78 | 71 | 68–76 | 74 | 69–78 | 0.035** | |
| Height (cm) | ||||||||
| Total | 154 | 148–162 | 153 | 147–160 | 151 | 146–158 | 0.002* | |
| Male | 162 | 158–165 | 161 | 157–165 | 159 | 157–164 | 0.067 | |
| Female | 148 | 143–153 | 149 | 144–153 | 147 | 144–151 | 0.441 | |
| Weight (kg) | ||||||||
| Total | 56 | 49–64 | 53 | 47–61 | 53 | 46.2–59.5 | 0.005* | |
| Male | 61 | 55–68 | 60 | 53–65 | 58 | 52–66 | 0.079 | |
| Female | 51 | 44–58 | 50 | 45–55 | 50 | 44–57 | 0.611 | |
| Muscle volume (kg) | ||||||||
| Total | 21.1 | 17.6–24.7 | 19.9 | 17.4–23.6 | 18.8 | 17–22.4 | 0.000* | |
| Male | 24.7 | 22.8–27.1 | 24.3 | 22.2–26.4 | 24 | 21.5–26 | 0 0.023** | |
| Female | 17.8 | 15.6–19.9 | 18 | 16.2–19.5 | 17.8 | 15.9–18.9 | 0.222 | |
| Grip strength (kg) | ||||||||
| Total | 24.8 | 19.6–34.0 | 24.0 | 19.5–31.3 | 22.5 | 18.4–29.0 | 0.005* | |
| Male | 34.5 | 30.0–38.5 | 33.0 | 28.5–37.0 | 32.5 | 26.5–37.0 | 0.029** | |
| Female | 20.0 | 16.8–22.5 | 21.0 | 16.8–24.0 | 19.5 | 16.5–22.5 | 0.722 | |
| Walking speed (m/s) | ||||||||
| Total | 1.38 | 1.22–1.56 | 1.37 | 1.22–1.52 | 1.30 | 1.1–1.5 | 0.002* | |
| Male | 1.43 | 1.25–1.56 | 1.39 | 1.25–1.52 | 1.37 | 1.2–1.6 | 0.068 | |
| Female | 1.39 | 1.19–1.52 | 1.35 | 1.19–1.52 | 1.28 | 1.1–1.4 | 0.037** | |
| CRP (U/L) | ||||||||
| Total | 602 | 274–1,027 | 510 | 222–1,070 | 562 | 271–1,140 | 0.874 | |
| Male | 624 | 247–1,150 | 510 | 239–1,300 | 602 | 302–1,200 | 0.730 | |
| Female | 590 | 277–1,010 | 510 | 217–870 | 491 | 231–1,090 | 0.728 | |
| IL–6 (pg/mL) | ||||||||
| Total | 2.0 | 1.3–2.5 | 1.9 | 1.2–2.9 | 1.91 | 3–3.0 | 0.399 | |
| Male | 2.1 | 1.5–2.7 | 2.1 | 1.3–3.4 | 2.0 | 1.4–2.8 | 0.890 | |
| Female | 1.7 | 1.2–2.5 | 1.9 | 1.2–2.6 | 1.9 | 1.3–3.1 | 0.210 | |
| TNF-α (pg/mL) | ||||||||
| Total | 1.2 | 0.9–1.6 | 1.1 | 0.9–1.5 | 1.3 | 0.9–1.9 | 0.079 | |
| Male | 1.2 | 0.9–1.7 | 1.2 | 1.0–1.6 | 1.5 | 1.1–2.2 | 0.010** | |
| Female | 1.2 | 0.9–1.5 | 1.1 | 0.8–1.4 | 1.2 | 0.9–1.7 | 0.570 | |
| β2-MG (mg/L) | ||||||||
| Total | 1.8 | 1.6–2.1 | 1.8 | 1.5–2.0 | 1.8 | 1.6–2.3 | 0.065 | |
| Male | 1.9 | 1.6–2.1 | 1.9 | 1.6–2.2 | 1.9 | 1.6–2.3 | 0.620 | |
| Female | 1.8 | 1.5–2.1 | 1.7 | 1.5–2.0 | 1.8 | 1.6–2.3 | 0.016** | |
| WBC/mL | ||||||||
| Total | 5,400 | 4,500–6,400 | 5,500 | 4,400–6,500 | 5,300 | 4,525–6,175 | 0.579 | |
| Male | 5,500 | 4,600–6,500 | 5,600 | 4,300–6,600 | 5,400 | 4,525–6,200 | 0.900 | |
| Female | 5,300 | 4,425–6,175 | 5,450 | 4,400–6,425 | 5,200 | 4,525–6,075 | 0.620 | |
| Hb (g/dL) | ||||||||
| Total | 13.9 | 13–14.9 | 13.6 | 12.9–14.5 | 13.6 | 12.7–14.3 | 0.005* | |
| Male | 14.9 | 14.1–15.5 | 14.5 | 13.4–15.4 | 14.4 | 13.8–15.3 | 0.040** | |
| Female | 13.1 | 12.6–13.9 | 13.3 | 12.7–13.9 | 13.2 | 12.6–13.8 | 0.530 | |
| Albumin (g/dL) | ||||||||
| Total | 4.2 | 4.0–4.4 | 4.2 | 4.0–4.4 | 4.2 | 4.0–4.3 | 0.234 | |
| Male | 4.2 | 4.0–4.4 | 4.2 | 4.0–4.3 | 4.2 | 4.0–4.4 | 0.375 | |
| Female | 4.2 | 4.0–4.3 | 4.2 | 4.1–4.4 | 4.2 | 4.0–4.3 | 0.335 | |
| LDL-Cho (mg/dL) | ||||||||
| Total | 114 | 95–135 | 114 | 95–138 | 116 | 98–138 | 0.439 | |
| Male | 109 | 90–132 | 107 | 86–128 | 114 | 97–137 | 0.290 | |
| Female | 117 | 102–141 | 123 | 101–141 | 118 | 98–138 | 0.660 | |
| Triglyceride (mg/dL) | ||||||||
| Total | 132 | 86–189 | 126 | 90–185 | 122 | 88–159 | 0.187 | |
| Male | 139 | 80–197 | 127 | 84–185 | 126 | 87–189 | 0.600 | |
| Female | 122 | 88–185 | 123 | 93–185 | 119 | 89–153 | 0.198 | |
| HDL-Cho (mg/dL) | ||||||||
| Total | 55 | 47–66 | 56 | 48–65 | 58 | 48–67 | 0.296 | |
| Male | 53 | 45–61 | 52 | 45–61 | 53 | 44–62 | 0.910 | |
| Female | 60 | 49–69 | 60 | 50–67 | 60 | 50–69 | 0.676 | |
IQR interquartile range, β2-MG β2-microgrobulin, WBC white blood cells, Hb hemoglobin, LDL-Cho low-density lipoprotein, HDL-Cho high-density lipoprotein
*P < 0.01; **P < 0.05, using Jonckheere–Terpstra
Table 2.
Significant Spearman correlations between eHsp72 and other variables
| Hsp72 | ||
|---|---|---|
| r | P value | |
| Height | ||
| Total | −0.106 | 0.008* |
| Male | −0.085 | 0.168 |
| Female | −0.023 | 0.664 |
| Weight | ||
| Total | −0.095 | 0.017** |
| Male | −0.060 | 0.336 |
| Female | −0.020 | 0.703 |
| Muscle volume | ||
| Total | −0.138 | 0.001* |
| Male | −0.104 | 0.092 |
| Female | −0.055 | 0.295 |
| Grip strength | ||
| Total | −0.111 | 0.006* |
| Male | −0.111 | 0.075 |
| Female | −0.037 | 0.485 |
| Walking speed | ||
| Total | −0.117 | 0.004* |
| Male | −0.093 | 0.140 |
| Female | −0.114 | 0.034** |
| TNF-α (pg/mL) | ||
| Total | 0.062 | 0.139 |
| Male | 0.164 | 0.012** |
| Female | 0.013 | 0.804 |
| Hb | ||
| Total | −0.090 | 0.022** |
| Male | −0.088 | 0.149 |
| Female | −0.026 | 0.619 |
Total n = 652, male n = 270, female n = 382
TNF tumor necrosis factor, Hb hemoglobin
*P < 0.01; **P < 0.05
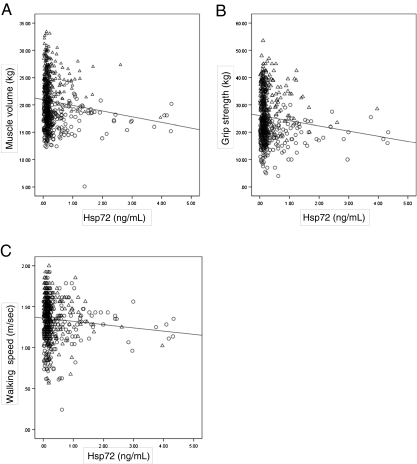
Fig. 2.
a Regression plot of Hsp72 levels in plasma and muscle volume. Circles female, triangles male. R = −0.138, P = 0.001, as analyzed using Spearman correlation. b Regression plot of Hsp72 levels in plasma and grip strength. Circles female, triangles male. R = −0.111, P = 0.006, as analyzed using Spearman correlation. c Regression plot of Hsp72 levels in plasma and walking speed. Circles female, triangles male. R = −0.117, P = 0.004, as analyzed using Spearman correlation
Previous studies have shown that diseases are known confounding factors of sarcopenia (Fried et al. 2001) and can trigger it; diseases induce lower physical activity or disuse of muscle. To test which diseases might influence eHsp72 levels, differences in eHsp72 tertiles between patients were evaluated using the Jonckheere–Terpstra test. To examine whether serious underlying diseases confounded the association of eHsp72 with sarcopenia, odds ratios were derived from multiple logistic regression analysis of eHsp72 tertiles adjusted by sex, age, and the incidence of disease (Table 3).
Table 3.
Sarcopenia factors associated with eHsp72 levels in plasma, adjusted for age and sex
| Lowest (<0.13 ng/mL) | Middle (0.13–0.22 ng/mL) | Highest (0.22 < ng/mL) | |
|---|---|---|---|
| Male/female (n) | 109/120 | 85/122 | 76/140 |
| Reference | OR [95% CI] | OR [95% CI] | |
| Muscle mass tertile | |||
| Low | 1 | 2.379 [1.067–5.303] | 2.724 [1.205–6.157] |
| Middle | 1 | 2.143 [1.119–4.105] | 2.226 [1.143–4.335] |
| High | 1 (reference) | 1 | 1 |
| P for trend | 0.041 | 0.016 | |
| Grip strength tertile | |||
| Low | 1 | 1.076 [0.482–2.402] | 2.604 [1.168–5.805] |
| Middle | 1 | 1.375 [0.715–2.645] | 2.270 [1.181–4.362] |
| High | 1 (reference) | 1 | 1 |
| P for trend | 0.858 | 0.019 | |
| Walking speed tertile | |||
| Low | 1 | 1.322 [0.766–2.283] | 1.815 [1.029–3.202] |
| Middle | 1 | 1.459 [0.892–2.388] | 1.802 [1.068–3.042] |
| High | 1 (reference) | 1 | 1 |
| P for trend | 0.316 | 0.040 | |
Adjusted age, sex, and the incidence of other diseases
OR odds ratio, 95% CI 95% confidence interval
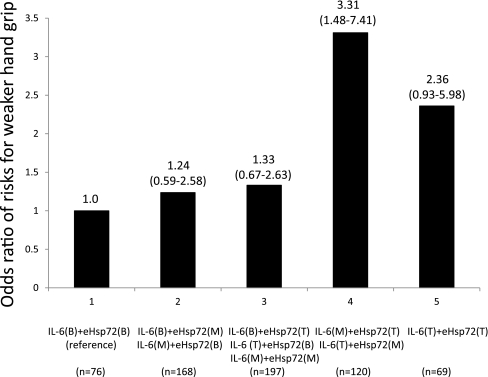
To better understand the gradient of weaker muscle strength risk associated with combined levels of IL-6 and eHsp72, differences in grip strength were explored according to the five combination groups using multiple logistic regression analysis (Leng et al. 2007; Bautmans et al. 2007). For grip strength, values were divided into two groups: a lower strength group (below one SD of the average value) and a higher strength group (above one SD of the average value) with men and women, respectively. To explore potential synergy between IL-6 and eHsp72 tertile levels, we divided five mutually exclusive groups in increasing order of severity: group 1—IL-6 bottom tertile + eHsp72 bottom tertile (the reference group); group 2—IL-6 bottom + eHsp72 middle, and IL-6 middle + eHsp72 bottom; group 3—IL-6 bottom + eHsp72 top, IL-6 top + eHsp72 bottom, and IL-6 middle + eHsp72 middle; group 4—IL-6 middle + eHsp72 top, and IL-6 top + eHsp72 middle; and group 5—IL-6 top + eHsp72 top (Leng et al. 2007). The analyses were adjusted for age and sex (Fig. 3).
Fig. 3.
Odds ratio of risks for weaker hand grip. Subjects were grouped based on their tertiles of IL-6 levels and eHsp72 levels. B bottom tertile, M middle tertile, T top tertile (95% CI)
All reported P values were two tailed, and the level of significance was set at P < 0.05. Statistical analysis was performed using IBM SPSS version 19 for Japanese (Nihon IBM Inc., Tokyo, Japan).
Results
eHsp72 profiles in relation to age and sex
The number of total subjects was 665; however, the plasma levels of eHsp72 in 13 subjects were undetectable. The plasma levels of eHsp72 with sex and age groups are shown in Fig. 1. A significant interaction in the age groups was not found using Kruskal–Wallis analysis. In the age group 75–79, eHsp72 in female subjects was higher than that in male subjects. However, there were no significant differences between the sexes in the other age groups. No differences were found between any of the age groups.
Anthropometrics, physical fitness, and biomarker profiles in relation to eHsp72 tertiles
When eHsp72 levels were divided into three equally spaced categories (tertiles), the cutoff values for eHsp72 tertiles were as follows: the lowest tertile of eHsp72 was under 0.12 ng/mL, the middle tertile was 0.13–0.22, and the highest tertile was over 0.23 ng/mL (P < 0.01). The anthropometric, physical fitness, and biomarker profiles in relation to the eHsp72 tertiles are summarized in Table 1. Using the Jonckheere–Terpstra test, we found significant higher in age (both sexes and females) stepwise from low eHsp72 to middle eHsp72 to high eHsp72 tertiles. They were significantly lower in height (both sexes), weight (both sexes), muscle volume (both sex and males), grip strength (both sexes and males), and walking speed (both sexes and females) increasing stepwise from low eHsp72 to middle eHsp72 to high eHsp72 tertiles (Table 1).
Among the biomarkers and the inflammatory markers, there was a significant decrease in hemoglobin (Hb) levels (both sexes and males) stepwise from low eHsp72 to middle eHsp72 to high eHsp72 tertiles. They were significantly higher in TNF-α levels in males increasing stepwise from low eHsp72 to middle eHsp72 to high eHsp72 tertiles. β2-MG levels in females, both in the lowest and highest tertiles of eHsp72, were higher than that in the middle tertile group (Table 1).
Correlation analysis in relation to eHsp72 concentration
Correlation coefficients are shown in Table 2 and Fig. 2a–c. Height, weight, skeletal muscle volume, grip strength, walking speed, and Hb in total subjects were associated with lower eHsp72 (negative correlation). TNF-α in male subjects was positively correlated with eHsp72 (Table 2).
Multiple logistic regression analysis
For disease history, no significant trends for eHsp72 tertiles among the specific diseases were found. Previous studies have shown that diseases are known confounding factors of sarcopenia (Fried et al. 2001) and can trigger it. Sarcopenia, including the accompanying lower physical activity, can, in turn, induce diseases. To confirm that no diseases seriously confounded the association of eHsp72 with sarcopenia, we performed logistic regression analysis of eHsp72 tertiles with additional adjustments for age, sex and the incidence of disease (e.g., the incidence of other disease). Table 3 shows the odds ratios for muscle mass, grip strength, and walking speed, adjusted sex, age, and the incidence of disease. The highest tertile of eHsp72 retained significant associations with the lowest tertile of skeletal muscle mass [odds ratio (OR), 2.72; 95% confidence interval (CI), 1.21–6.16, P < 0.01], the lowest tertile of grip strength [OR 2.60, 95% CI 1.17–5.81; P < 0.01], and the lowest tertile of walking speed [OR 1.82, 95% CI 1.03–3.20; P < 0.01] using logistic regression models (Table 3).
Combined IL-6 and eHsp72 levels and weaker hand grip
Figure 3 shows the odds ratio of risks for weaker hand grip with combined levels of IL-6 and eHsp72 level. We divided five mutually exclusive groups in increasing order of severity: group 1—76 participants IL-6 bottom tertile + eHsp72 bottom tertile (the reference group); group 2—168 participants IL-6 bottom + eHsp72 middle, and IL-6 middle + eHsp72 bottom; group 3—197 participants IL-6 bottom + eHsp72 top, IL-6 top + eHsp72 bottom, and IL-6 middle + eHsp72 middle; group 4—120 participants IL-6 middle + eHsp72 top, and IL-6 top + eHsp72 middle; and group 5—69 participants IL-6 top + eHsp72 top. For weaker hand grip strength, only those in the group 4 (IL-6 middle + eHsp72 top, and IL-6 top + eHsp72 middle) had significantly higher risk for being weaker hand grip strength [OR 3.31, 95% CI 1.48–7.41], adjusted age, and sex.
Discussion
We focused on the biological significance of eHsp72 in elderly people. We demonstrated that higher Hsp72 in plasma is associated with lower muscle mass, weaker grip strength, and slower walking speed, and is a potential biomarker of sarcopenia in elderly people. This finding was supported by other results in the present study: (1) older age and shrinking body (i.e., shorter height and lighter body weight) and lower hemoglobin levels, all of which characterize sarcopenia, were related to higher eHsp72 tertiles and (2) the ORs of the highest tertile of eHsp72 for the lowest tertiles of muscle mass, grip strength, and walking speed were 2.7, 2.6, and 1.8, respectively. These ORs were independent of age, sex, and the incidence of related diseases (Table 3).
We also found that inflammatory cytokines and eHsp72 were independent and potentially associated with prevalent sarcopenia. Group 4 (IL-6 middle + eHsp72 top, and IL-6 top + eHsp72 middle) had a significantly higher risk for being in the weaker grip strength group (R 3.31, 95% CI 1.48–7.41; P = 0.004) compared to group 5 (IL-6 top + eHsp72 top). One possible explanation is that IL-6 and eHsp72 negate each other. These results might imply that eHsp72 reflects the opposite status of inflammation. Bautmans et al. (2008) investigated the effect of IL-6 and eHsp72 on muscle endurance in elderly nursing home residents, and demonstrated that subjects with both high serum levels of IL-6 and eHsp72 had worse muscle endurance compared to those with high IL-6 and low eHsp72, or with low IL-6 and high eHsp72. They pointed out the possibility that the response of eHsp72 to exercise might reflect the anti-inflammatory status of elderly people, which might support our present results. Clark and Manini (2008) suggested the term “dynapenia” to specifically describe the age-associated loss of muscle strength. They argued that (1) longitudinal aging studies indicate a disassociation between the loss of muscle mass and strength and (2) the changes in muscle mass and the changes in strength resulting from alterations in physical activity levels (i.e., exercise training or disuse) do not follow the same time course, suggesting that the human neuromuscular system must be involved in the regulation of strength (Clark and Manini 2008). Since eHsp72 (Asea et al. 2000) is involved in the inflammatory cytokine cascade yielding IL-6 and is known to function as a cytokine, this has prompted us to consider it as an inflammatory factor; however, it also plays a role as an anti-inflammatory factor. Accordingly, it might be that inflammatory cytokines are related to muscle mass changes per se (i.e., sarcopenia), including muscle catabolism and synthesis, whereas eHsp72 is related to muscle strength for the protection of motor neurons (i.e., dynapenia).
Interestingly, in the nervous system, as in other tissues, the induction of Hsps not only serves as a marker for stress but has a protective effect as well (Tidwell et al. 2004). It is generally assumed that a cell must produce its own proteins (e.g., Hsps) to be protected by them (Lasek et al. 1977). Robinson et al. demonstrated that not only expressed Hsp72 but also extracellular Hsp72 plays a role after stress to promote the maintenance of survival pathways and/or inhibit the activation of cell death-specific events in motoneurons (Robinson et al. 2005). They claimed that considering the size of and metabolic demands on motoneurons, it is possible that these cells are only capable of synthesizing amounts of Hsc70 or Hsp70 necessary for the maintenance of cell function and survival. The cells do not appear able to increase production in response to the greater demands of stressful stimuli. The extracellular Hsp72 derived from other cell types may compensate for this deficit (Robinson et al. 2005).
Acute bouts of aerobic exercise induce eHsp72 elevation (Walsh et al. 2001). This elevation is transient; once the stressor (i.e., exercise) is removed, the levels of eHsp72 return to normal. At the resting level in young humans, Hsp72 concentrations in cerebrospinal fluid (CSF) are threefold higher than in plasma (Steensberg et al. 2006), suggesting enhanced neuronal stress tolerance (Guzhova et al. 2001). However, whether acute exercise contributes to the system or not remains unknown, because the CSF levels of Hsp72 are not affected by 2 h of exhausting exercise (Steensberg et al. 2006). However, a study has shown that a 12-week resistance training program induces a reduction of eHsp72 in elderly women (Ogawa et al. 2010), and centenarians are an exception in that they have decreased eHsp72, suggesting that lower eHsp72 leads to healthy outcomes later in life (Terry et al. 2004). Accordingly, higher eHsp72 may relate to unhealthier conditions in aged people, as our results implied. On the other hand, a number of studies have reported reduced levels of circulating Hsp70 in the elderly, and some more historic data have indicated that the ability to mount a stress response is decreased with aging (Rea et al. 2001). Results from cultured cells suggest that the age-related decline in Hsp70 expression is constitutive and is due to decreased binding of the heat shock factor (Hsf) 1 to the heat shock element (Hse) and diminished Hsp 70 transcription (Horowitz and Robinson 2007). Alternatively, there may be an age-associated increase in abnormal or denatured proteins that could interfere with Hsf biding to Hse (Munro and Pelham 1985). Since mitochondria in old animals are more vulnerable to incurring and less able to repair oxidative damage that occurs in response to a physiologically relevant heat stress, an increase in free radical production and oxidative damage with aging might induce a decrease in stress tolerance at both cellular and whole-organism levels (Haak et al. 2009). In vivo, severe ATP depletion can cause destabilization and aggregation of many proteins (Kabakov et al. 2002). If Hsp70, a known ATP-dependent chaperone induced by the appearance of denatured proteins within a cell following stress, is not being properly induced in old rats following heat stress (Fargnoli et al. 1990), the resultant subcellular stress caused by the toxic accumulation of protein aggregates could be significant enough to cause damage to mitochondria, peroxisomes, rough endoplasmic reticulum, and membrane lipids (Oberley et al. 2008). Interestingly, decreased ATP levels in old rats compared with young throughout most of recovery time course indicated an overall decreased ability of senescent animals to compensate for a loss in energy metabolism (Oberley et al. 2008). Mechanisms for an attenuated stress response in aging remain complex and unknown, and this warrants further investigation.
The present investigated the biological significance of eHsp72 in an elderly population. Our results would reveal that eHsp72 in plasma is linked to sarcopenia factors and is a potential biomarker or predictor of sarcopenia. Geriatric syndromes have a biological basis and are considered to be highly prevalent and carry a high risk for adverse health outcomes. The present results could lead to the development of methods for screening those who require effective, targeted care.
Acknowledgments
We are grateful to our elderly subjects in Kusatu-machi, Dr. Shoji Shinkai, Dr. Ryutaro Takahashi, and Dr. Hideki Ito, Tokyo Metropolitan Institute of Gerontology. We wish to acknowledge the contribution of the management of Kusatu-machi Health Center in Gunma and the Research Team for Social Participation and Health Promotion, Tokyo Metropolitan Institute of Gerontology. This study was supported by a Grant-in-Aid for the Scientist (1403 B: 21300261) of the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6(4):435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- Bautmans I, Gorus E, Njemini R, Mets T. Handgrip performance in relation to self-perceived fatigue, physical functioning and circulating IL-6 in elderly persons without inflammation. BMC Geriatr. 2007;7:5. doi: 10.1186/1471-2318-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautmans I, Njemini R, Predom H, Lemper JC, Mets T. Muscle endurance in elderly nursing home residents is related to fatigue perception, mobility, and circulating tumor necrosis factor-alpha, interleukin-6, and heat shock protein 70. J Am Geriatr Soc. 2008;56(3):389–396. doi: 10.1111/j.1532-5415.2007.01571.x. [DOI] [PubMed] [Google Scholar]
- Calderwood SK. Protein quality control and heat shock protein gene expression in the nervous system. In: Asea AAA, Brown IR, editors. Heat shock protein and the brain: implications for neurodegenerative diseases and neuroprotection. Dordrecht: Springer; 2008. pp. 349–364. [Google Scholar]
- Clark BC, Manini TM. Sarcopenia =/= dynapenia. J Gerontol A Biol Sci Med Sci. 2008;63A(8):829–834. doi: 10.1093/gerona/63.8.829. [DOI] [PubMed] [Google Scholar]
- Cooper R, Kuh D, Hardy R, Mortality Review Group. FALCon and HALCyon Study Teams Objectively measured physical capability levels and mortality: systematic review and meta-analysis. BMJ. 2010;341:c4467. doi: 10.1136/bmj.c4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M, European Working Group on Sarcopenia in Older People Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degens H. The role of systemic inflammation in age-related muscle weakness and wasting. Scand J Med Sci Sports. 2010;20(1):28–38. doi: 10.1111/j.1600-0838.2009.01018.x. [DOI] [PubMed] [Google Scholar]
- Fargnoli J, Kunisada T, Fornace AJ, Jr, Schneider EL, Holbrook NJ. Decreased expression of heat shock protein 70 mRNA and protein after heat treatment in cells of aged rats. Proc Natl Acad Sci U S A. 1990;87(2):846–850. doi: 10.1073/pnas.87.2.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, AbellanvanKan G, Andrieu S, Bauer J, Breuille D, Cederholm T, Chandler J, Meynard C, Donini L, Harris T, Kannt A, Keime Guibert F, Onder G, Papanicolaou D, Rolland Y, Rooks D, Sieber C, Souhami E, Verlaan S, Zamboni M. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12(4):249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleshner M, Campisi J, Johnson JD. Can exercise stress facilitate innate immunity? A functional role for stress-induced extracellular Hsp72. Exerc Immunol Rev. 2003;9:6–24. [PubMed] [Google Scholar]
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA, Cardiovascular Health Study Collaborative Research Group Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C, McFarland H. Heat shock proteins in multiple sclerosis and other autoimmune diseases. Immunol Today. 1993;14(8):373–375. doi: 10.1016/0167-5699(93)90135-8. [DOI] [PubMed] [Google Scholar]
- Guzhova I, Kislyakova K, Moskaliova O, Fridlanskaya I, Tytell M, Cheetham M, Margulis B. In vitro studies show that Hsp70 can be released by glia and that exogenous Hsp70 can enhance neuronal stress tolerance. Brain Res. 2001;914(1–2):66–73. doi: 10.1016/S0006-8993(01)02774-3. [DOI] [PubMed] [Google Scholar]
- Haak JL, Buettner GR, Spitz DR, Kregel KC. Aging augments mitochondrial susceptibility to heat stress. Am J Physiol Regul Integr Comp Physiol. 2009;296(3):R812–R820. doi: 10.1152/ajpregu.90708.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haehling S, Morley JE, Anker SD. An overview of sarcopenia: facts and numbers on prevalence and clinical impact. J Cachex Sarcopenia Muscle. 2010;1:129–133. doi: 10.1007/s13539-010-0014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz M, Robinson SD. Heat shock proteins and the heat shock response during hyperthermia and its modulation by altered physiological conditions. Prog Brain Res. 2007;162:433–446. doi: 10.1016/S0079-6123(06)62021-9. [DOI] [PubMed] [Google Scholar]
- Johnson AD, Tytell M. Exogenous HSP70 becomes cell associated, but not internalized, by stressed arterial smooth muscle cells. In Vitro Cell Dev Biol Anim. 1993;29A(10):807–812. doi: 10.1007/BF02634348. [DOI] [PubMed] [Google Scholar]
- Kabakov AE, Budagova KR, Latchman DS, Kampinga HH. Stressful preconditioning and HSP70 overexpression attenuate proteotoxicity of cellular ATP depletion. Am J Physiol Cell Physiol. 2002;283(2):C521–C534. doi: 10.1152/ajpcell.00503.2001. [DOI] [PubMed] [Google Scholar]
- Kampinga HH, Brunsting JF, Stege GJ, Burgman PW, Konings AW. Thermal protein denaturation and protein aggregation in cells made thermotolerant by various chemicals: role of heat shock proteins. Exp Cell Res. 1995;219(2):536–546. doi: 10.1006/excr.1995.1262. [DOI] [PubMed] [Google Scholar]
- Lasek RJ, Gainer H, Barker JL. Cell-to-cell transfer of glial proteins to the squid giant axon. The glia-neuron protein transfer hypothesis. J Cell Biol. 1977;74(2):501–523. doi: 10.1083/jcb.74.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng SX, Xue QL, Tian J, Walston JD, Fried LP. Inflammation and frailty in older women. J Am Geriatr Soc. 2007;55(6):864–871. doi: 10.1111/j.1532-5415.2007.01186.x. [DOI] [PubMed] [Google Scholar]
- Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Munro S, Pelham H. What turns on heat shock genes? Nature. 1985;317(6037):477–478. doi: 10.1038/317477a0. [DOI] [PubMed] [Google Scholar]
- Narici MV, Maffulli N. Sarcopenia: characteristics, mechanisms and functional significance. Br Med Bull. 2010;95:139–159. doi: 10.1093/bmb/ldq008. [DOI] [PubMed] [Google Scholar]
- Oberley TD, Swanlund JM, Zhang HJ, Kregel KC. Aging results in increased autophagy of mitochondria and protein nitration in rat hepatocytes following heat stress. J Histochem Cytochem. 2008;56(6):615–627. doi: 10.1369/jhc.2008.950873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa K, Sanada K, Machida S, Okutsu M, Suzuki K. Resistance exercise training-induced muscle hypertrophy was associated with reduction of inflammatory markers in elderly women. Mediators Inflamm. 2010;171023:1–6. doi: 10.1155/2010/171023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pockley AG, Shepherd J, Corton JM. Detection of heat shock protein 70 (Hsp70) and anti-Hsp70 antibodies in the serum of normal individuals. Immunol Invest. 1998;27(6):367–377. doi: 10.3109/08820139809022710. [DOI] [PubMed] [Google Scholar]
- Rea IM, McNerlan S, Pockley AG. Serum heat shock protein and anti-heat shock protein antibody levels in aging. Exp Gerontol. 2001;36(2):341–352. doi: 10.1016/S0531-5565(00)00215-1. [DOI] [PubMed] [Google Scholar]
- Robinson MB, Tidwell JL, Gould T, Taylor AR, Newbern JM, Graves J, Tytell M, Milligan CE. Extracellular heat shock protein 70: a critical component for motoneuron survival. J Neurosci. 2005;25(42):9735–9745. doi: 10.1523/JNEUROSCI.1912-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg IH. Sarcopenia: original and clinical relevance. J Nutr. 1997;127(Suppl):S990–S991. doi: 10.1093/jn/127.5.990S. [DOI] [PubMed] [Google Scholar]
- Roubenoff R, Hughes VA. Sarcopenia: current concepts. J Gerontol A Biol Sci Med Sci. 2000;55(12):M716–M724. doi: 10.1093/gerona/55.12.M716. [DOI] [PubMed] [Google Scholar]
- Steensberg A, Dalsgaard MK, Secher NH, Pedersen BK. Cerebrospinal fluid IL-6, HSP72, and TNF-alpha in exercising humans. Brain Behav Immun. 2006;20(6):585–589. doi: 10.1016/j.bbi.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, Nevitt M, Visser M, Kritchevsky S, Badinelli S, Harris T, Newman AB, Cauley J, Ferrucci L, Guralnik J. Gait speed and survival in older adults. JAMA. 2011;305(1):50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Yoshida H, Kim H, Yukawa H, Sugiura M, Furuna T, Nishizawa S, Kumagai S, Shinkai S, Ishizaki T, Watanabe S, Shibata H. Walking speed as a good predictor for maintenance of I-ADL among the rural community elderly in Japan: a 5-year follow-up study from TMIG-LISA. Geriatr Gerontol Int. 2003;3:S6–S14. doi: 10.1111/j.1444-0594.2003.00090.x. [DOI] [Google Scholar]
- Terry DF, McCormick M, Andersen S, Pennington J, Schoenhofen E, Palaima E, Bausero M, Ogawa K, Perls TT, Asea A. Cardiovascular disease delay in centenarian offspring: role of heat shock proteins. Ann N Y Acad Sci. 2004;1019:502–505. doi: 10.1196/annals.1297.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidwell JL, Houenou LJ, Tytell M. Administration of Hsp70 in vivo inhibits motor and sensory neuron degeneration. Cell Stress Chaperones. 2004;9(1):88–98. doi: 10.1379/CSC-9R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh RC, Koukoulas I, Garnham A, Moseley PL, Hargreaves M, Febbraio MA. Exercise increases serum Hsp72 in humans. Cell Stress Chaperones. 2001;6(4):386–393. doi: 10.1379/1466-1268(2001)006<0386:EISHIH>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters DL, Baumgartner RN, Garry PJ, Vellas B. Advantages of dietary, exercise-related, and therapeutic interventions to prevent and treat sarcopenia in adult patients: an update. Clin Interv Aging. 2010;5:259–270. doi: 10.2147/CIA.S6920. [DOI] [PMC free article] [PubMed] [Google Scholar]