Introduction
Mammalian cells have developed a range of adaptations to survive against acute and prolonged (but not lethal) stresses (Beckmann et al. 1992). Among these adaptations, the heat shock response is the most conserved, being found in all prokaryotes and eukaryotes (Locke and Noble 1995). Heat shock proteins (HSPs) are considered part of a family of proteins known as “stress proteins” since their expression is induced by a wide range of stressors, such as oxidative stress (Krause et al. 2007), thermal stress (Yang et al. 1996), ischaemia (Richard et al. 1996), exercise (Krause et al. 2007), metabolic stress (Beckmann et al. 1992) and many others. The genes encoding Hsps are highly conserved, and many of these genes and their protein products can be assigned to different families on the basis of their typical molecular weight (kDa): HSP110 (or officially named HSPH), HSP90 (or HSPC), HSP70 (or HSPA), HSP60 (or HSPD1), HSP40 (or DNAJ) and small hsp families (HSPB) (Kampinga et al. 2009). In eukaryotes, many families comprise multiple members that differ in inducibility, intracellular localization and function (Feder and Hofmann 1999).
HSP72 (or HSPA1A) is the inducible and the most abundant of all HSPs, accounting for 1–2% of cellular protein and being rapidly induced during cell stress (Noble et al. 2008), especially in the skeletal muscle cells (Madden et al. 2008). As molecular chaperones, the intracellular HSP70 protein (iHSP72) can interact with other proteins (unfolded, in non-native state and/or stress-denatured conformations) to avoid inappropriate interactions, formation of protein aggregates and degradation of damaged proteins, as well as helping the correct refolding of proteins (Madden et al. 2008). Other HSP functions include protein translocation (Chirico et al. 1988), anti-apoptosis (Garrido et al. 2001) and anti-inflammatory responses (Homem de Bittencourt et al. 2007). The anti-inflammatory role of the iHSP72 is mediated by its interaction with the proteins involved in the activation of the nuclear factor κ-B (NF-κB), blocking its translocation to the nucleus and inducing the cessation of the inflammatory process (Homem de Bittencourt et al. 2007; Silveira et al. 2007). More recently, the HSP roles have been expanded to include control of cell signalling (Calderwood et al. 2007), modulation of immune response (Johnson and Fleshner 2006) and for chronic diseases conditions (Kampinga et al. 2007), such as diabetes, control of obesity and insulin resistance (Chung et al. 2008; Krause and Rodrigues-Krause Jda 2011).
Recent data has indicated that a lack of iHSP72 response to stress can be linked to the levels of insulin resistance in skeletal muscle cells (Chung et al. 2008; Kurucz et al. 2002). Patients with T2DM have been shown to have reduced iHSP72 gene expression, which has been correlated with reduced insulin sensitivity (Kurucz et al. 2002). Furthermore, earlier studies in patients with T2DM reported that hot tub therapy improved glycaemic control (Bernstein 2000; Bathaie et al. 2010; Hooper 1999), additionally showing an inverse correlation between iHSP72 messenger RNA (mRNA) expression and the degree of T2DM (Kurucz et al. 2002). The underlying mechanisms behind the lower induction of iHSP72 expression in diabetic patients is not fully understood, but appears to be connected to the activation of proteins involved on inflammatory response (and sensitive to changes in redox state), such as the c-jun amino terminal kinase (JNK), the inhibitor of IκB (IKK), the tumor necrosis factor alpha (TNF-α) and nuclear factor kappa b (NF-κB) (Chung et al. 2008). Activation of these proteins appears to inhibit the major inductor of HSP72, the heat shock factor 1 (HSF-1), leading to a low iHSP72 expression and induction of the stress response (Chung et al. 2008). Currently, several HSP-inducing drugs are under investigation or in clinical trials for diabetic neuropathy, neurodegenerative diseases (Westerheide and Morimoto 2005; Kurthy et al. 2002) and for the prevention of insulin resistance and treatment of impaired glycaemic control (Kurucz et al. 2002; Gupte et al. 2009a; Literati-Nagy et al. 2009; Vitai et al. 2009; Kavanagh et al. 2009). Considering that skeletal muscle is the major tissue responsible for whole body insulin-mediated glucose uptake, disturbances in skeletal muscle, such as oxidative stress and inflammatory processes, can easily progress to insulin resistance and diabetes (Newsholme et al. 2009). Physical exercise is a known inducer of glucose uptake and is also a substantial inductor of iHSP72 expression (Krause et al. 2007). This may explain, in part, the beneficial effects of exercise in diabetic patients. Therefore, strategies to increase skeletal muscle iHSP72 expression (or over-expression) could result in improved glycaemic control, reduced insulin resistance and avoidance of T2DM.
Heat shock proteins were long thought to be exclusive cytoplasmic proteins with functions restricted to the intracellular compartment. However, an increasing number of observations have shown that they may be released into the extracellular space (eHSP72) and have a wide variety of effects on other cells (Tytell 2005). The eHSP72 function is in general associated with the activation of the immune system (Whitham and Fortes 2008). For example, eHSP72 has been reported as an inductor of neutrophils microbicidal capacity (Ortega et al. 2006) and chemotaxis (Ortega et al. 2009), recruitment of NK (natural killer) cells (Horn et al. 2007) as well as cytokine production in immune cells (Asea et al. 2000; Johnson and Fleshner 2006). Besides that, eHSP72 was recently shown to be involved in the inducement of neural cell protection under stress conditions (Krause and Rodrigues-Krause Jda 2011).
In addition, hyperglycaemia is known to be involved in inflammation and vascular complications associated with diabetes, arising from reactive oxygen species generation and action (Wei et al. 2009; Wright et al. 2006). As oxidative stress is a powerful inductor of iHSP72 (Krause et al. 2007), it is expected that during inflammatory and oxidative stress states in diabetes, the levels of these proteins in the extracellular medium (plasma and serum) may be higher in diabetic than non-diabetic participants. Indeed, type 1 (Oglesbee et al. 2005) and T2DM patients have higher levels of eHSP72, and this response has been related to the duration of the disease (Nakhjavani et al. 2010). In addition, serum eHSP72 concentrations are positively correlated with markers of inflammation, such as C-reactive proteins, monocyte count, and TNF-α (Mayer and Bukau 2005; Njemini et al. 2004).
In summary, while intracellular levels of iHSP72 are decreased in T2DM and correlated with insulin resistance, extracellular eHSP72 levels are increased and correlated with oxidative damage and stress. To date, no study has investigated both intracellular and extracellular levels of HSP72 in T2DM patients simultaneously. Herein, we aimed to determine the levels of HSP72, intracellularly (skeletal muscle) and extracellularly (blood plasma) in three groups of patients: obese without T2DM and obese with T2DM and non-obese with T2DM. We also determined HSF-1 skeletal muscle expression for all groups.
Materials and methods
Participant characteristics
Fifteen sedentary non-smoking male participants (54 ± 9 years old) volunteered for this study (7 obese controls vs. 5 obese T2DM and 3 non-obese T2DM). Participants were selected according to their BMI (>30 kg/m2 for obese and 23–27 kg/m2 for non-obese participants). Informed consent form was obtained from all participants prior to beginning the study. Research assessments and protocols were approved by the UCD Human Research Ethics Committee—Sciences (reference, LS-08-106) and Institute of Technology Tallaght Research Ethics Committee (reference, REC-A6-09).
Participants were free of clear diabetic complications such as retinopathy, neuropathy, nephropathy or vascular disease at the time of the recruitment. All participants were assessed for biochemical variables such as fasted glycaemia, lipid profile [low-density lipoprotein (LDL), high-density lipoprotein (HDL), total cholesterol and triglycerides) and HbA1c (glycated haemoglobin). Participants’ characteristics are listed on Table 1. Subject’s years of diagnosis and medications taken are individually listed in Table 2.
Table 1.
Subjects’ anthropometric, biochemical and physiological characteristics
| Obese controls (n = 7) | Non-obese diabetics (n = 3) | Obese diabetic (n = 5) | |
|---|---|---|---|
| Age (years) | 51.6 ± 9.6 | 62 ± 7.9 | 56.6 ± 7.84 |
| BMI (kg/m2) | 30.5 ± 3.2 | 24.7 ± 1.2*,** | 30.8 ± 5.6 |
| Body fat (%) | 34.1 ± 4,2 | 21.9 ± 3.1*,** | 39.6 ± 3.6 |
| SBP (mmHg) | 134 ± 22.3 | 136 ± 5.3 | 142.8 ± 13.2 |
| DBP (mmHg) | 83.1 ± 8.7 | 75.3 ± 7.4** | 93.5 ± 7.5 |
| CRP (μg/mL) | 2.4 ± 0.52‡ | 1.66 ± 0.21 | 1.74 ± 0.41 |
| Fast glycaemia (mmol/l) | 5.2 ± 1.2 | 6.3 ± 1.1 | 7.5 ± 0.4* |
| HbA1C (%) | 6.0 ± 0.2 | 7.1 ± 0.6* | 7.3 ± 0.8* |
| Total cholesterol (mg/dl) | 178.1 ± 56.3 | 145.4 ±34.3 | 181.2 ± 55.1 |
| LDL (mg/dl) | 111.29 ± 59 | 89.22 ± 54 | 132.1 ± 85 |
| HDL (mg/dl) | 43.3 ± 7.5 | 43.3 ± 12.7 | 31.4 ± 8 |
| Triglycerides (mg/dl) | 119.5 ± 57.6 | 64.8 ± 19.7 | 92.5 ± 24.3 |
| Serum Insulin (pmol/l) | 66.78 ± 32.4 | 42.96 ± 1.43 | 185.2 ± 75.5*‡ |
| HOMA-IR | 1.25 ± 0.66 | 1 ± 0.06** | 3.2 ± 1.4*‡ |
SBP systolic blood pressure, DBP diastolic blood pressure
*P < 0.05 when compared with obese control group, **P < 0.05 when compared with obese diabetic group
Table 2.
Subjects’ years of diagnosis and medications used
| Subject | Time of diagnose (years) | Medications used |
|---|---|---|
| Obese control 1 | – | – |
| Obese control 2 | – | – |
| Obese control 3 | – | – |
| Obese control 4 | – | – |
| Obese control 5 | – | – |
| Obese control 6 | – | – |
| Obese control 7 | – | – |
| Non-obese T2DM 1 | 10 | Metformin (5 × 500 mg daily), Diamicron (4 × 30 mg daily), Pioglitazone (1 × 8 mg daily), Aspirin (1 × 75 mg daily) |
| Non-obese T2DM 2 | 6 | Metformin 1500 mg daily, Dutasteride 0.5 mg daily |
| Non-obese T2DM 3 | 1 | Metformin 2 g/day |
| Obese T2DM 1 | 1 | Liraglutide 1.8/day, Diamicron 120 mg/day, Perindopril, Rosuvastatin, Escitalopram, Modafinil |
| Obese T2DM 2 | 12 | Pioglitazone 30 mg/day, Lisenopril 20 mg/day, Amlodipine 10 mg/day, Doxazosin mesylate 8 mg/day, Sitagliptin 50 mg (2 per day), Aspirin 1/day |
| Obese T2DM 3 | 2 | Metformin 1000 × 3 per day, Liraglutide 1.8 × 1 per day, Perindopril 2.5 mg/day |
| Obese T2DM 4 | 10 | – |
| Obese T2DM 5 | 12 | Metformin 1,000 × 3/day, Liraglutide 1.8 × 1/day, Perindopril 2.5 mg/day |
Anthropometric measurements and body composition
Standing height was measured using a stainless steel Harpenden Stadiometer (Holtain Ltd, Pembrokeshire, UK), with the participants’ shoes off and head at the Frankfort horizontal plane. Body mass was measured using a Seca 888 weighting scale, Birmingham, UK). Body composition was assessed using dual energy X-ray absorptiometry (Lunar iDXA, GE Healthcare, Buckinghamshire, UK).
BP and biochemistry
Systemic arterial blood pressure (BP) was measured in the brachial artery using an Omron M5-1 fully automated BP monitor (HEM 907, Omron Healthcare, Kyoto, Japan). The mean of two measurements taken at 2-min intervals of supine rest was recorded. Venous blood samples were taken from an antecubital vein in heparin coated and gel-clot VacutainerTM tubes using standard aseptic techniques. Samples were immediately centrifuged (at 4°C and 1,000×g for 15 min), after which plasma and serum was removed and stored at −80°C for further analysis.
Glycaemic control and blood lipids
Plasma glucose concentration, total cholesterol and HDL cholesterol were measured using a portable Cardiochek Analyser (Polymer Technology Systems, Indianapolis, IN, USA). Triglyceride (TG) concentration was quantified using a commercially available colorimetric assay (Tryglicerides Liquicolor, Wiebsbaden, Germany). Estimates of LDL cholesterol concentration were calculated using the Friedewald formula (Friedewald et al. 1972). Glycosylated haemoglobin (% HbA1c) was assayed using DCA Vantage Serum Analyser (Siemens, Dublin, Ireland). A highly sensitive, enzyme-linked immunosorbent assay (EIA) method was used to determine the plasma concentration of insulin (Mercodia, Catalogue 10-1113-01, Uppsala, Sweden).
Plasma HSP72 (eHSP72) quantification
A highly sensitive, EIA method (EKS-715 Stressgen, Victoria, BC, Canada) was used to determine the expression of HSP72 protein in serum as previously described (Walsh et al. 2001). Absorbance was measured at 450 nm, and a standard curve constructed from known dilutions of HSP72 protein to allow quantitative assessment of HSP72 concentration. Quantification was made using a microplate reader (Molecular Devices SpectraMax Plus 384, Sunnyvale, CA, USA). Intra-assay coefficient of variation was identified as being <2%.
High-sensitivity C-reactive protein
Serum high-sensitivity C-reactive protein (CRP) was assayed using a CRP high sensitivity assay kit (Cayman, Ireland). Intra-assay coefficient of variation was identified as being <1.8%.
Skeletal muscle microbiopsy
Muscle biopsies were conducted using a spring-loaded and reusable instrument (MG1522; Bard, Dublin, Ireland). This technique has been assessed in terms of patient tolerance, and it is reported as causing minimal or no discomfort (Hayot et al. 2005). The samples (∼15 μg) were collected from the vastus lateralis muscle. After local anaesthesia with Lidocaine 2%, the skin was punctured with a cannula (14 G disposable biopsy needle, Bard, MC 1410) perpendicular to the muscle until the fascia was pierced. The biopsy needle was inserted through the cannula and muscle samples were obtained by the activation of a trigger button. The samples obtained from each biopsy were immediately frozen in liquid nitrogen and stored at −80°C. Muscle biopsy samples were analysed for expression of intracellular HSP72, HSF-1 and GAPDH (as housekeeping protein).
Proteins extraction and Western blot analysis
After removing from −80°C storage, tissue samples were thawed and homogenized in lysis buffer (20 mM Tris–HCl, 5 mM EDTA, 10 mM Na-pyrophosphate, 100 mM NaF, 2 mM Na3VO4, 10 μg/ml Aprotinin, 10 μg/ml leupeptin, 3 mM benzamidine and 1 mM phenylmethylsulfonyl fluoride) using an automated homogeniser (TissueLyser LT, Qiagen, Dublin Ireland) . The homogenate was rotated at 4°C for 60 min, followed by centrifugation at 13,000 rpm at 4°C for 10 min, after which the supernatant collected. Cellular protein concentration was determined using a BCA protein Assay (Pierce, Rockford, IL, USA, catalog no. 23225). Protein samples (15 μg) were denatured in sample buffer and separated by 10% sodium dodecyl sulphate polyacrylamide gel electrophoresis. The proteins were transferred onto a nitrocellulose membrane (Amersham Biosciences, Ireland), blocked in 5% (BSA) and probed with the appropriate polyclonal antibodies; anti-HSF-1 (1:10,000 dilution, Cell Signalling Technologies, USA), anti-HSP72 (1:25,000 dilution, Sigma-Aldrich, Ireland) and anti-GAPDH (1:10,000 dilution, Cell Signalling technologies, USA) over night. Following over night incubation membranes were washed and incubated for 60 min at room temperature with horseradish peroxidase-conjugated secondary antibodies (1:30,000 dilutuion, Cell Signalling technologies, USA). The blots were washed and visualized with a horseradish peroxidase-based Supersignal West Pico chemiluminescent substrate (Pierce). Results of digitalized images were expressed as means ± SD using anti-GAPDH as an expression control. The densities of the bands were quantified using ImageJ version 1.44p (National Institutes of Health, USA).
Statistical analysis
Data are described in mean and SD. Shapiro–Wilk normality test was applied previously to all analyses. As the data did not present normal distribution, Kruskal–Wallis test was used to evaluate the differences among groups, followed by Dunn’s test for multiple comparisons. Spearman’s correlation test was performed to verify relationships between eHSP72 and CRP. Alpha level was set at p < 0.05.
Results
General blood biochemistry measurements and body composition
As expected, fasting glucose (C = 5.2 ± 1.2 vs. T2DM = 7.5 ± 0.4 mmol/l) and HbA1c (C = 6 ± 0.2 vs. T2DM = 7.3 ± 0.5%) were higher in the obese T2DM group compared to control. In non-obese T2DM participants, glycaemia (6.3 ± 1.1 mmol/l) did not differ from the obese controls. No differences were found in blood pressure values (Table 1). Curiously, in our subjects, CRP concentration was lower in the T2DM groups; however, these values were all at normal levels for health subjects (Hayashida et al. 2005; Kume et al. 2010). Body fat content was lower in the non-obese group (21.9 ± 3.1%) when compared with obese controls (34.1 ± 4.1%) or obese T2DM (39.6 ± 3.6%). Despite no statistical differences were found regarding lipid profile, non-obese patients with diabetes tend to have lower LDL and triglycerides (Table 1). Insulin levels were decreased in non-obese T2DM subjects. In addition, HOMA-IR indicates that the non-obese T2DM group have lower insulin resistant state when compared with the obese T2DM group (Table 1).
Plasma eHSP72 concentration
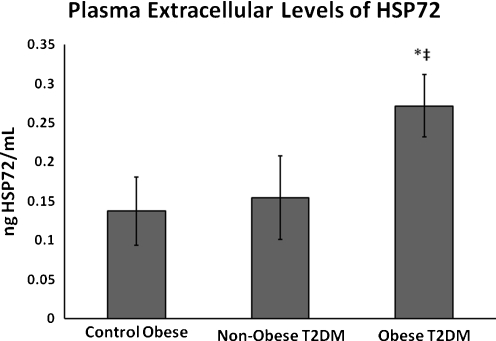
In the obese T2DM group, a higher level of eHSP70 was observed (0.227 ± 0.03 ng/ml) when compared with obese controls (0.13 ± 0.04 ng/ml) and non-obese T2DM (0.154 ± 0.05 ng/ml) (Fig. 1, p < 0.05). No correlation between eHSP72 and CRP was found (P = 0.7878; rs = −0.07921).
Fig. 1.
Plasma concentration of eHSP72. *P < 0.05 when compared diabetic with obese control group. ‡P < 0.05 when compared with obese diabetic group
Skeletal muscle iHSP72 and HSF-1 expression
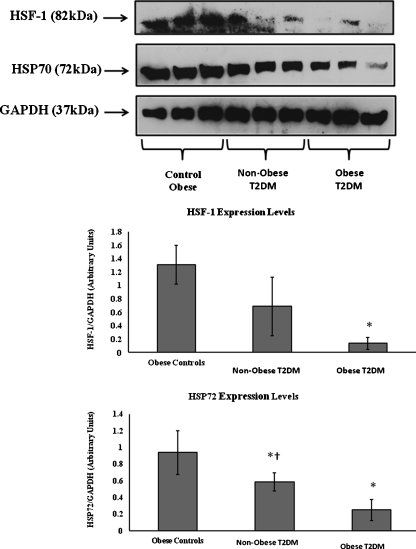
Skeletal muscle iHSP72 (0.94 ± 0.2 for obese controls vs. 0.58 ± 0.1 for non-obese T2DM vs. 0.25 ± 0.12 for obese T2DM—arbitrary units) and skeletal muscle HSF-1 (1.3 ± 0.28 for obese controls vs. 0.68 ± 0.43 for non-obese T2DM vs. 0.13 ± 0.08 for obese T2DM—arbitrary units) were both reduced in T2DM. However, this reduction was lower in the non-obese group with T2DM than the obese with T2DM group. (Fig. 2; P < 0.05).
Fig. 2.
Representative Western blot of human skeletal muscle biopsies for HSF-1 and IHSP72. *P < 0.05 when compared diabetic with obese control group. †P < 0.05 when compared with obese diabetic group
Discussion
The main finding of this study was that the concentration of HSP72 differed between intra- and extracellular compartments in T2DM participants. The fact that body composition seems to influence iHSP72 content and release (eHSP72) in diabetic patients is an additional relevant finding. We report here that iHSP72 and HSF-1 is higher in participants without diabetes and is decreased in T2DM subjects. Interestingly, this condition is improved in non-obese T2DM where the decreased level of these proteins was less evident (Fig. 2). The opposite was found for eHSP72, where the content of the plasma eHSP72 was increased only in obese diabetic participants.
Induction of HSP expression is regulated by an interaction between the heat shock transcription factors (HSF) and the regulatory heat shock elements (HSE, specific sequences of DNA on the promoter region of the Hsp gene) in the nucleus (Krause and Rodrigues-Krause Jda 2011). At rest (without stress), HSF is inactive in a monomeric state bonded with the cytosolic HSP70s, possessing no capacity for DNA binding. Under stress conditions and in the presence of unfolded proteins, HSP70 releases the HSF and binds to the denatured proteins (acting as chaperones), releasing the inhibitory effect from the HSF. Furthermore, specific serine phosphorylations followed by the trimerisation of the HSF induce a high DNA-binding affinity for the HSE. The binding of the trimeric HSF to HSE initiates the transcription of the Hsp mRNA (Feder and Hofmann 1999). Any sub-lethal exposure to a specific stressor can rapidly induce the appearance of the iHSP72 on the cytoplasm (Krause and Rodrigues-Krause Jda 2011).
Even though an increase in the HSP level is essential for any stress adaptation, the absence and/or the inhibition of iHSP70 expression has been shown to result in increased vulnerability of the cells to stress (Krause and Rodrigues-Krause Jda 2011; Lindquist and Craig 1988). The ability of a cell to properly sense and initiate this response is critical for its survival. In some conditions and chronic neurodegenerative disorders, such as amyotrophic lateral sclerosis, Parkinson’s disease, Alzheimer’s disease, and polyglutamine (polyQ) diseases, insulin resistance and diabetes, the inability to activate an appropriated heat shock response causes an abnormal accumulation and inclusions of proteins, which is a commonly observed characteristic (Hooper and Hooper 2005). Under pathological conditions, the level of such misfolded proteins may exceed the protective machinery of the cell to either maintain them in a soluble form or degrade them, resulting in their accumulation, cell dysfunction and death (Adachi et al. 2009; Krause and Rodrigues-Krause Jda 2011).
Because iHSP72 is a cytoprotective chaperone protein, it is of relevance to T2DM subjects, as both skeletal muscle iHSP72 gene (Patti et al. 2003; Kurucz et al. 2002; Bruce et al. 2003) and protein expression are lower in these patients compared with healthy individuals (Chung et al. 2008). Previous studies have shown that there is a decreased expression of the hsp-72 mRNA in skeletal muscle from patients with T2DM and that the level of hsp-72 mRNA correlates with the rate of insulin-stimulated glucose and lipid turnover and glucose tolerance (Chung et al. 2008; Kurucz et al. 2002). Our work confirms the previous findings that patients with T2DM have lower expression of HSP72. In addition, we found that the degree of reduction of iHSP72 in the skeletal muscle was reduced in diabetic patients with lower adiposity. Henstridge et al. (2010), by measuring the levels of HSP72 in young healthy human population free of hyperglycemia, demonstrated that iHSP72 protein expression in skeletal muscle is inversely correlated with percentage of body fat mass. These findings are consistent with rodent data suggesting that iHSP72 could be involved on the stimulation of fat oxidation (Gupte et al. 2009b).
The mechanisms behind the donwregulation of iHSP72 in the skeletal muscle of T2DM patients are not completely understood. Obesity is linked to a chronic pro-inflammatory state, since the adipose tissue expansion results in the release of several cytokines such as TNF-α, which leads to the activation of serine threonine kinases, JNK and the inhibitor of IκB, IKK (Chung et al. 2008). It is known that both JNK and IKK phosphorylate IRS-1 on Ser-307, leading to the inactivation of the insulin receptor (Chung et al. 2008). In addition, lipid oversupply and hyperglycemia can lead to increased deposition of lipid species such as diacylglycerol and ceramide, which can also activate JNK and IKK in liver and/or skeletal muscle, leading to insulin resistance (Watt et al. 2006). The activation of these proteins seems to inhibit the major inductor of iHSP72, the heat shock factor 1 (HSF-1), leading to a low iHSP72 expression and stress response (Chung et al. 2008). The importance of iHSP72 for normal insulin sensitivity could be connected with its anti-inflammatory role, inhibiting factors such as NF-κB translocation to the nucleus, resulting in the cessation of the inflammatory stimulus (Krause and Rodrigues-Krause Jda 2011). The mechanisms underlying the lower HSF-1 and iHSP72levels in the skeletal muscle from our diabetic subjects may be related to their insulin response characteristics. For example, the non-obese subjects are not insulin resistant, unlike the obese T2DM group (Table 1). Taking these characteristic into account, the obese group would suffer from the lack of insulin signalling while the non-obese from insulin insufficiency, both conditions likely to reduce HSF-1 activity and then iHSP72 expression (Hooper 2009).
In addition to ceramide accumulation, excessive reactive oxygen species are also known activators of JNK and IKK (Kaneto et al. 2005). Excessive levels of reactive oxygen species not only directly damage cells by oxidizing DNA, protein and lipids but indirectly damage cells by activating a variety of stress-sensitive intracellular signalling pathways such as NF-kB, p38 MAPK, JNK/SAPK, hexosamine and others. Activation of these pathways results in increased expression of numerous gene products that may cause cellular damage and play a major role in the etiology of late complications in type 2 diabetics. In addition, recent data in vitro and in vivo suggest that activation of the same or similar stress pathways results in insulin resistance and impaired insulin secretion (Krause et al. 2011). Accordingly, it has been proposed that links exist between the hyperglycemia- and FFA-induced increases in reactive oxygen species and oxidative stress, activation of stress-sensitive pathways and the eventual development of not only diabetes late complications but also insulin resistance and β-cell dysfunction (Newsholme et al. 2009). Activation of these pathways could culminate also in down-regulation of HSF-1 and iHSP72 in skeletal muscle. In this paper, we have reported that HSF-1 expression is reduced in both diabetic groups but that this reduction was less evident in the non-obese group (not statistically significant), suggesting that adiposity reduction could result in lower inhibition of HSF-1 activation. This could explain, at least in part, the higher levels of iHSP72 in the non-obese group of T2DM compared with the obese group (Fig. 2). These results indicate that adiposity control in T2DM is an essential strategy for the improvement of the disease.
To date, only a limited number of studies have investigated the serum levels of eHSP72 in diabetes (Gruden et al. 2009; Nakhjavani et al. 2010; Oglesbee et al. 2005). However, these studies did not explore the variation of eHSP72 levels with body fat composition. What is known is that circulating levels of eHSP702 increases along with the duration of diabetes and is associated with the chronicity of the disease (Nakhjavani et al. 2010). Additionally, some sex differences were found in the literature indicating that serum eHSP72 level is increased in women with long-standing diabetes compared with men (Nakhjavani et al. 2011). In addition, eHSP72 did not decrease after glucose lowering therapy in women with newly diagnosed diabetes, but it did decrease in men (Nakhjavani et al. 2011); however, in the present work, we did not evaluate female subjects. Our data agree with previous studies showing that extracellular (plasma or serum) eHSP72 is increased in T2DM (Gruden et al. 2009; Nakhjavani et al. 2010; Oglesbee et al. 2005). Most important was the fact that there was no significant difference between obese controls and non-obese T2DM regarding the plasma eHSP72 (Fig. 1). This could indicate that (i) as mentioned before, this response is connected to the nature of diabetes (insulin resistance or insulin deficiency) and/or (ii) that the levels of eHSP72 found in diabetic patients are connected to adipose tissue expansion and associated complications, rather than to diabetes itself. Because our preliminary study lacks an additional lean control group, our suggestion for an association between eHSP72 and adipose tissue expansion is still speculative. In addition, since the HSP response seems to be reduced in diabetes, the divergences between iHSP72 and eHSP72 in the T2DM groups indicate that the source of eHSP72 is still functional or overactive. As was previously suggested, the source of eHSP72 is likely to be the immunological cells (Calderwood et al. 2007; Calderwood et al. 2005; Nakhjavani et al. 2011), especially under pro-inflammatory conditions such as diabetes (Njemini et al. 2011).
Due to the fact that oxidative damage and protein glycation are the major causes of diabetic complications, the elevation of chaperone capacity (i.e. eHSP72) in diabetic patients seems to be an important strategy for protection of proteins against these harmful changes and conservation of their native molecule structures. Thus, eHSP72 increases may serve to protect the structure of proteins, leading to the observed beneficial effects. Since we found that non-obese T2DM participants have less eHSP72, we speculate that in those patients, the levels of protein damage and oxidative stress were lower than in obese diabetic participants. If this is the case, then eHSP72 could also serve as a new marker for the progression of the disease. The authors recognize that the small sample size and the absence of a non-obese control group are limitations of the study design; however, our findings were significant and indicate that adiposity control could be the major goal to induce oxidative stress protection and improvement of skeletal muscle defences.
Conclusions and perspectives
In conclusion, we found that iHSP72 and HSF-1 protein levels in skeletal muscle were decreased in T2DM participants, but this observation was less evident in non-obese diabetic patients. This suggests that iHSP72 protein expression may be related to the level of adiposity and perhaps with the pro-inflammatory state. Interestingly, for eHSP72, our results indicate that, in obese diabetic participants, the content of the plasma eHSP72 increased. Since eHSP72 function is attributed to protein damage and oxidative stress levels that occur along with the time course of the disease, we believe that obesity and all its complications could be the major causes for eHSP72 elevation. Considering that non-obese T2DM present a better general metabolic state, our data also suggests that eHSP72 may have a novel biomarker potential in diabetes.
Electronic supplementary material
Acknowledgements
We thank the Department of Science, Institute of Technology Tallaght, Dublin, Ireland, UCD School of Biomolecular and Biomedical Science, and UCD Institute for Sport and Health and the TSR: Strand III—Core Research Strengths Enhancement Scheme (Ireland) for supporting this work.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work was supported by the Institute of Technology Technological Sector Research (TSR): Strand III – Core Research Strengths Enhancement Scheme (Ireland).
References
- Adachi H, Katsuno M, Waza M, Minamiyama M, Tanaka F, Sobue G. Heat shock proteins in neurodegenerative diseases: pathogenic roles and therapeutic implications. Int J Hyperthermia. 2009;25(8):647–654. doi: 10.3109/02656730903315823. [DOI] [PubMed] [Google Scholar]
- Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6(4):435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- Bathaie SZ, Jafarnejad A, Hosseinkhani S, Nakhjavani M. The effect of hot-tub therapy on serum Hsp70 level and its benefit on diabetic rats: a preliminary report. Int J Hyperthermia. 2010;26(6):577–585. doi: 10.3109/02656736.2010.485594. [DOI] [PubMed] [Google Scholar]
- Beckmann RP, Lovett M, Welch WJ. Examining the function and regulation of hsp 70 in cells subjected to metabolic stress. J Cell Biol. 1992;117(6):1137–1150. doi: 10.1083/jcb.117.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein RK. Hot-tub therapy for type 2 diabetes mellitus. N Engl J Med. 2000;342(3):218. doi: 10.1056/NEJM200001203420318. [DOI] [PubMed] [Google Scholar]
- Bruce CR, Carey AL, Hawley JA, Febbraio MA. Intramuscular heat shock protein 72 and heme oxygenase-1 mRNA are reduced in patients with type 2 diabetes: evidence that insulin resistance is associated with a disturbed antioxidant defense mechanism. Diabetes. 2003;52(9):2338–2345. doi: 10.2337/diabetes.52.9.2338. [DOI] [PubMed] [Google Scholar]
- Calderwood SK, Theriault JR, Gong J. Message in a bottle: role of the 70-kDa heat shock protein family in anti-tumor immunity. Eur J Immunol. 2005;35(9):2518–2527. doi: 10.1002/eji.200535002. [DOI] [PubMed] [Google Scholar]
- Calderwood SK, Mambula SS, Gray PJ, Jr, Theriault JR. Extracellular heat shock proteins in cell signaling. FEBS Lett. 2007;581(19):3689–3694. doi: 10.1016/j.febslet.2007.04.044. [DOI] [PubMed] [Google Scholar]
- Chirico WJ, Waters MG, Blobel G. 70K heat shock related proteins stimulate protein translocation into microsomes. Nature. 1988;332(6167):805–810. doi: 10.1038/332805a0. [DOI] [PubMed] [Google Scholar]
- Chung J, Nguyen AK, Henstridge DC, Holmes AG, Chan MH, Mesa JL, Lancaster GI, Southgate RJ, Bruce CR, Duffy SJ, Horvath I, Mestril R, Watt MJ, Hooper PL, Kingwell BA, Vigh L, Hevener A, Febbraio MA. HSP72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci U S A. 2008;105(5):1739–1744. doi: 10.1073/pnas.0705799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol. 1999;61:243–282. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- Garrido C, Gurbuxani S, Ravagnan L, Kroemer G. Heat shock proteins: endogenous modulators of apoptotic cell death. Biochem Biophys Res Commun. 2001;286(3):433–442. doi: 10.1006/bbrc.2001.5427. [DOI] [PubMed] [Google Scholar]
- Gruden G, Bruno G, Chaturvedi N, Burt D, Pinach S, Schalkwijk C, Stehouwer CD, Witte DR, Fuller JH, Cavallo-Perin P. ANTI-HSP60 and ANTI-HSP70 antibody levels and micro/macrovascular complications in type 1 diabetes: the EURODIAB Study. J Intern Med. 2009;266(6):527–536. doi: 10.1111/j.1365-2796.2009.02129.x. [DOI] [PubMed] [Google Scholar]
- Gupte AA, Bomhoff GL, Morris JK, Gorres BK, Geiger PC. Lipoic acid increases heat shock protein expression and inhibits stress kinase activation to improve insulin signaling in skeletal muscle from high-fat-fed rats. J Appl Physiol. 2009;106(4):1425–1434. doi: 10.1152/japplphysiol.91210.2008. [DOI] [PubMed] [Google Scholar]
- Gupte AA, Bomhoff GL, Swerdlow RH, Geiger PC. Heat treatment improves glucose tolerance and prevents skeletal muscle insulin resistance in rats fed a high-fat diet. Diabetes. 2009;58(3):567–578. doi: 10.2337/db08-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida K, Kume N, Murase T, Minami M, Nakagawa D, Inada T, Tanaka M, Ueda A, Kominami G, Kambara H, Kimura T, Kita T. Serum soluble lectin-like oxidized low-density lipoprotein receptor-1 levels are elevated in acute coronary syndrome: a novel marker for early diagnosis. Circulation. 2005;112(6):812–818. doi: 10.1161/CIRCULATIONAHA.104.468397. [DOI] [PubMed] [Google Scholar]
- Hayot M, Michaud A, Koechlin C, Caron MA, Leblanc P, Prefaut C, Maltais F. Skeletal muscle microbiopsy: a validation study of a minimally invasive technique. Eur Respir J. 2005;25(3):431–440. doi: 10.1183/09031936.05.00053404. [DOI] [PubMed] [Google Scholar]
- Henstridge DC, Forbes JM, Penfold SA, Formosa MF, Dougherty S, Gasser A, Courten MP, Cooper ME, Kingwell BA, Courten B. The relationship between heat shock protein 72 expression in skeletal muscle and insulin sensitivity is dependent on adiposity. Metabolism. 2010;59(11):1556–1561. doi: 10.1016/j.metabol.2010.01.027. [DOI] [PubMed] [Google Scholar]
- Homem de Bittencourt PI, Jr, Lagranha DJ, Maslinkiewicz A, Senna SM, Tavares AM, Baldissera LP, Janner DR, Peralta JS, Bock PM, Gutierrez LL, Scola G, Heck TG, Krause MS, Cruz LA, Abdalla DS, Lagranha CJ, Lima T, Curi R. LipoCardium: endothelium-directed cyclopentenone prostaglandin-based liposome formulation that completely reverses atherosclerotic lesions. Atherosclerosis. 2007;193(2):245–258. doi: 10.1016/j.atherosclerosis.2006.08.049. [DOI] [PubMed] [Google Scholar]
- Hooper PL. Hot-tub therapy for type 2 diabetes mellitus. N Engl J Med. 1999;341(12):924–925. doi: 10.1056/NEJM199909163411216. [DOI] [PubMed] [Google Scholar]
- Hooper PL. Inflammation, heat shock proteins, and type 2 diabetes. Cell Stress Chaperones. 2009;14(2):113–115. doi: 10.1007/s12192-008-0073-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper PL, Hooper JJ. Loss of defense against stress: diabetes and heat shock proteins. Diabetes Technol Ther. 2005;7(1):204–208. doi: 10.1089/dia.2005.7.204. [DOI] [PubMed] [Google Scholar]
- Horn P, Kalz A, Lim CL, Pyne D, Saunders P, Mackinnon L, Peake J, Suzuki K. Exercise-recruited NK cells display exercise-associated eHSP-70. Exerc Immunol Rev. 2007;13:100–111. [PubMed] [Google Scholar]
- Johnson JD, Fleshner M. Releasing signals, secretory pathways, and immune function of endogenous extracellular heat shock protein 72. J Leukoc Biol. 2006;79(3):425–434. doi: 10.1189/jlb.0905523. [DOI] [PubMed] [Google Scholar]
- Kampinga HH, Henning RH, Gelder IC, Brundel BJ. Heat shock proteins and atrial fibrillation. Cell Stress Chaperones. 2007;12(2):97–100. doi: 10.1379/CSC-285.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampinga HH, Hageman J, Vos MJ, Kubota H, Tanguay RM, Bruford EA, Cheetham ME, Chen B, Hightower LE. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones. 2009;14(1):105–111. doi: 10.1007/s12192-008-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneto H, Matsuoka TA, Nakatani Y, Kawamori D, Miyatsuka T, Matsuhisa M, Yamasaki Y. Oxidative stress, ER stress, and the JNK pathway in type 2 diabetes. J Mol Med (Berl) 2005;83(6):429–439. doi: 10.1007/s00109-005-0640-x. [DOI] [PubMed] [Google Scholar]
- Kavanagh K, Flynn DM, Jenkins KA, Zhang L, Wagner JD. Restoring HSP70 deficiencies improves glucose tolerance in diabetic monkeys. Am J Physiol Endocrinol Metab. 2009;300(5):E894–901. doi: 10.1152/ajpendo.00699.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause M, Rodrigues-Krause Jda C. Extracellular heat shock proteins (eHSP70) in exercise: possible targets outside the immune system and their role for neurodegenerative disorders treatment. Med Hypotheses. 2011;76(2):286–290. doi: 10.1016/j.mehy.2010.10.025. [DOI] [PubMed] [Google Scholar]
- Krause MS, Oliveira LP, Jr, Silveira EM, Vianna DR, Rossato JS, Almeida BS, Rodrigues MF, Fernandes AJ, Costa JA, Curi R, Bittencourt PI., Jr MRP1/GS-X pump ATPase expression: is this the explanation for the cytoprotection of the heart against oxidative stress-induced redox imbalance in comparison to skeletal muscle cells? Cell Biochem Funct. 2007;25(1):23–32. doi: 10.1002/cbf.1343. [DOI] [PubMed] [Google Scholar]
- Krause M, McClenaghan N, Flatt PR, Homem de Bittencourt PI, Murphy C, Newsholme P (2011) L-arginine is essential for pancreatic beta-cell functional integrity, metabolism and defence from inflammatory challenge. J Endocrinol. doi:10.1530/JOE-11-0236 [DOI] [PubMed]
- Kume N, Mitsuoka H, Hayashida K, Tanaka M, Kominami G, Kita T. Soluble lectin-like oxidized LDL receptor-1 (sLOX-1) as a sensitive and specific biomarker for acute coronary syndrome—comparison with other biomarkers. J Cardiol. 2010;56(2):159–165. doi: 10.1016/j.jjcc.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Kurthy M, Mogyorosi T, Nagy K, Kukorelli T, Jednakovits A, Talosi L, Biro K. Effect of BRX-220 against peripheral neuropathy and insulin resistance in diabetic rat models. Ann N Y Acad Sci. 2002;967:482–489. doi: 10.1111/j.1749-6632.2002.tb04306.x. [DOI] [PubMed] [Google Scholar]
- Kurucz I, Morva A, Vaag A, Eriksson KF, Huang X, Groop L, Koranyi L. Decreased expression of heat shock protein 72 in skeletal muscle of patients with type 2 diabetes correlates with insulin resistance. Diabetes. 2002;51(4):1102–1109. doi: 10.2337/diabetes.51.4.1102. [DOI] [PubMed] [Google Scholar]
- Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Literati-Nagy B, Kulcsar E, Literati-Nagy Z, Buday B, Peterfai E, Horvath T, Tory K, Kolonics A, Fleming A, Mandl J, Koranyi L. Improvement of insulin sensitivity by a novel drug, BGP-15, in insulin-resistant patients: a proof of concept randomized double-blind clinical trial. Horm Metab Res. 2009;41(5):374–380. doi: 10.1055/s-0028-1128142. [DOI] [PubMed] [Google Scholar]
- Locke M, Noble EG. Stress proteins: the exercise response. Can J Appl Physiol. 1995;20(2):155–167. doi: 10.1139/h95-011. [DOI] [PubMed] [Google Scholar]
- Madden LA, Sandstrom ME, Lovell RJ, McNaughton L. Inducible heat shock protein 70 and its role in preconditioning and exercise. Amino Acids. 2008;34(4):511–516. doi: 10.1007/s00726-007-0004-7. [DOI] [PubMed] [Google Scholar]
- Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci. 2005;62(6):670–684. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhjavani M, Morteza A, Khajeali L, Esteghamati A, Khalilzadeh O, Asgarani F, Outeiro TF. Increased serum HSP70 levels are associated with the duration of diabetes. Cell Stress Chaperones. 2010;15(6):959–964. doi: 10.1007/s12192-010-0204-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhjavani M, Morteza A, Meysamie A, Esteghamati A, Khalilzadeh O, Esfahanian F, Khajeali L, Feiz F. Serum heat shock protein 70 and oxidized LDL in patients with type 2 diabetes: does sex matter? Cell Stress Chaperones. 2011;16(2):195–201. doi: 10.1007/s12192-010-0232-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsholme P, Homem De Bittencourt PI, O' Hagan C, Vito G, Murphy C, Krause MS. Exercise and possible molecular mechanisms of protection from vascular disease and diabetes: the central role of ROS and nitric oxide. Clin Sci (Lond) 2009;118(5):341–349. doi: 10.1042/CS20090433. [DOI] [PubMed] [Google Scholar]
- Njemini R, Demanet C, Mets T. Inflammatory status as an important determinant of heat shock protein 70 serum concentrations during aging. Biogerontology. 2004;5(1):31–38. doi: 10.1023/B:BGEN.0000017684.15626.29. [DOI] [PubMed] [Google Scholar]
- Njemini R, Bautmans I, Onyema OO, Puyvelde K, Demanet C, Mets T. Circulating heat shock protein 70 in health, aging and disease. BMC Immunol. 2011;12:24. doi: 10.1186/1471-2172-12-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble EG, Milne KJ, Melling CW. Heat shock proteins and exercise: a primer. Appl Physiol Nutr Metab. 2008;33(5):1050–1065. doi: 10.1139/H08-069. [DOI] [PubMed] [Google Scholar]
- Oglesbee MJ, Herdman AV, Passmore GG, Hoffman WH. Diabetic ketoacidosis increases extracellular levels of the major inducible 70-kDa heat shock protein. Clin Biochem. 2005;38(10):900–904. doi: 10.1016/j.clinbiochem.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Ortega E, Giraldo E, Hinchado MD, Martinez M, Ibanez S, Cidoncha A, Collazos ME, Garcia JJ. Role of Hsp72 and norepinephrine in the moderate exercise-induced stimulation of neutrophils' microbicide capacity. Eur J Appl Physiol. 2006;98(3):250–255. doi: 10.1007/s00421-006-0269-7. [DOI] [PubMed] [Google Scholar]
- Ortega E, Hinchado MD, Martin-Cordero L, Asea A. The effect of stress-inducible extracellular Hsp72 on human neutrophil chemotaxis: a role during acute intense exercise. Stress. 2009;12(3):240–249. doi: 10.1080/10253890802309853. [DOI] [PubMed] [Google Scholar]
- Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci U S A. 2003;100(14):8466–8471. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard V, Kaeffer N, Thuillez C. Delayed protection of the ischemic heart—from pathophysiology to therapeutic applications. Fundam Clin Pharmacol. 1996;10(5):409–415. doi: 10.1111/j.1472-8206.1996.tb00595.x. [DOI] [PubMed] [Google Scholar]
- Silveira EM, Rodrigues MF, Krause MS, Vianna DR, Almeida BS, Rossato JS, Oliveira LP, Jr, Curi R, Bittencourt PI., Jr Acute exercise stimulates macrophage function: possible role of NF-kappaB pathways. Cell Biochem Funct. 2007;25(1):63–73. doi: 10.1002/cbf.1365. [DOI] [PubMed] [Google Scholar]
- Tytell M. Release of heat shock proteins (Hsps) and the effects of extracellular Hsps on neural cells and tissues. Int J Hyperthermia. 2005;21(5):445–455. doi: 10.1080/02656730500041921. [DOI] [PubMed] [Google Scholar]
- Vitai M, Buday B, Kulcsar E, Literati-Nagy B, Vecsei I, Bezzegh K, Peterfai E, Kurucz I, Koranyi L. Occurrence of GRB10 (+11275 G > A) polymorphism in Hungarian population and its relationship to glucose metabolism. Orv Hetil. 2009;150(40):1845–1851. doi: 10.1556/OH.2009.28729. [DOI] [PubMed] [Google Scholar]
- Walsh RC, Koukoulas I, Garnham A, Moseley PL, Hargreaves M, Febbraio MA. Exercise increases serum Hsp72 in humans. Cell Stress Chaperones. 2001;6(4):386–393. doi: 10.1379/1466-1268(2001)006<0386:EISHIH>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt MJ, Hevener A, Lancaster GI, Febbraio MA. Ciliary neurotrophic factor prevents acute lipid-induced insulin resistance by attenuating ceramide accumulation and phosphorylation of c-Jun N-terminal kinase in peripheral tissues. Endocrinology. 2006;147(5):2077–2085. doi: 10.1210/en.2005-1074. [DOI] [PubMed] [Google Scholar]
- Wei W, Liu Q, Tan Y, Liu L, Li X, Cai L. Oxidative stress, diabetes, and diabetic complications. Hemoglobin. 2009;33(5):370–377. doi: 10.3109/03630260903212175. [DOI] [PubMed] [Google Scholar]
- Westerheide SD, Morimoto RI. Heat shock response modulators as therapeutic tools for diseases of protein conformation. J Biol Chem. 2005;280(39):33097–33100. doi: 10.1074/jbc.R500010200. [DOI] [PubMed] [Google Scholar]
- Whitham M, Fortes MB. Heat shock protein 72: release and biological significance during exercise. Front Biosci. 2008;13:1328–1339. doi: 10.2741/2765. [DOI] [PubMed] [Google Scholar]
- Wright E, Jr, Scism-Bacon JL, Glass LC. Oxidative stress in type 2 diabetes: the role of fasting and postprandial glycaemia. Int J Clin Pract. 2006;60(3):308–314. doi: 10.1111/j.1368-5031.2006.00825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XM, Baxter GF, Heads RJ, Yellon DM, Downey JM, Cohen MV. Infarct limitation of the second window of protection in a conscious rabbit model. Cardiovasc Res. 1996;31(5):777–783. doi: 10.1016/0008-6363(96)00026-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.