Abstract
Although (GT)n repeats in heme oxygenase-1 (HO-1) promoter may modulate gene transcriptional activity, the association between (GT)n repeats polymorphism and risk of coronary heart disease (CHD) from different levels of oxidative stress (OS) is unknown. We determined the allelic frequencies of (GT)n repeats in the HO-1 gene promoter and plasma malonaldehyde (MDA) as biomarkers of OS in 2,298 pairs of CHD patients and controls in the Chinese population. Furthermore, we measured MDA in culture mediums and HO-1 expressions levels in cell lysates of endothelial cells carrying various (GT)n genotypes under different concentrations of H2O2. Compared with L/L genotype (>25 repeats) carriers, the adjusted odd ratios for S/S genotype (≤25 repeats) in subjects with different levels of OS (MDA < 1.83, 1.83–2.91, >2.91 μmol/L) were 1.06 (95%CI, 0.75 to 1.49), 0.79 (95%CI, 0.55 to 1.12), and 0.60 (95%CI, 0.44 to 0.81), respectively (Pinteraction = 0.002). In biological experiments, compared with endothelial cells carrying L/L genotype, cells with S/S genotype did not have a significantly higher HO-1 expression under 0 μmol/L H2O2, but displayed a significantly higher HO-1 expression under 50 μmol/L H2O2 (Pinteraction = 0.003). S/S genotype in HO-1 gene promoter is associated with a lower risk of CHD in subjects with higher levels of OS, because under conditions of high OS, the S/S genotype has higher levels of HO-1, an antioxidant.
Keywords: Heme oxygenase-1, Oxidative stress, Malonaldehyde, Coronary heart disease, Gene polymorphism
Introduction
Well-established cardiovascular risk factors, such as hypercholesterolemia (Duarte et al. 2009), diabetes (Giugliano et al. 1996), and hypertension (Sowers 2002), have been shown to elevate the levels of oxidative stress (OS) in vivo (Morita 2005). High levels of OS can markedly upregulate heme oxygenase-1 (HO-1) in vascular endothelial cells, vascular smooth muscle cells, and macrophages whereas HO-1 is not increased when exposed to low levels of OS (Yamaguchi et al. 1993; Ishikawa et al. 1997; Wang et al. 1998), strongly suggesting that the expression of HO-1 may depend on levels of OS in vivo (Siow et al. 1995; Anwar et al. 2005). As heat shock protein 32, HO-1 catalyzes the first and rate-controlling step of the degradation of heme into ferrous (Fe2+) iron, carbon monoxide, and biliverdin subsequently converted into bilirubin (Ponka 1999). Considerable evidence has revealed that these products are potentially anti-inflammatory, antioxidant, and antiproliferative (Djousse et al. 2001; Duckers et al. 2001; Kawamura et al. 2005; Morita 2005). Thus HO-1 has a recognized protective effect in the development of cardiovascular diseases (Idriss et al. 2008), and the induction of the HO-1 gene has been proposed as a new therapy (Stocker and Perrella 2006).
The human HO-1 gene was mapped to chromosome 22q12. In HO-1 gene promoter, there is a length polymorphism of (GT)n repeats (Kimpara et al. 1997) which may influence HO-1 expression levels. Transient-transfection assays revealed that short numbers of (GT)n repeats display much higher gene transcriptional activity than long ones when exposed to OS (Yamada et al. 2000; Chen et al. 2002; Exner et al. 2001). Two previous case–control studies in small Asian populations (Kaneda et al. 2002; Chen et al. 2008) found that the length polymorphism of (GT)n repeats was not significantly associated with risk of coronary heart disease in the general population but that this association existed in subjects with hypercholesterolemia, diabetes, hypertension, or smoking habits, all of which are related to higher levels of OS (Duarte et al. 2009; Giugliano et al. 1996; Morrow et al. 1995; Sowers 2002). We postulated that levels of OS might affect the difference in HO-1 expression levels between short and long (GT)n repeats, and thus the length polymorphism of (GT)n repeats might be associated with the susceptibility to cardiovascular diseases in subjects with high levels of OS.
To test this hypothesis we determined the allelic frequencies of (GT)n repeats in the HO-1 gene promoter and measured plasma malonaldehyde (MDA) concentrations as the biomarker of OS in 2,298 pairs of coronary heart diseases (CHD) patients and age- and sex-frequency-matched controls, and then assessed the associations between HO-1 gene promoter polymorphism and the risk of CHD by different levels of OS. To verify the result, we further investigated HO-1 expressions in cell lysates and MDA in culture mediums of human umbilical venous endothelial cells (HUVECs) carrying various (GT)n genotypes under different concentrations of H2O2.
Subjects and methods
Study population
The study population was composed of 2,298 case patients and 2,298 age- and sex-frequency-matched controls. Briefly, patients were consecutively recruited from three hospitals (Union Hospital, Tongji Hospital, and Wugang Hospital) in Wuhan (Hubei, China) between May 2004 and October 2006. The diagnostic criteria for CHD cases included one of the following: (1) the presence of a stenosis >50% in at least one of the major segments of coronary arteries (the right coronary artery, left circumflex, or left anterior descending arteries) on coronary angiography; (2) based on World Health Organization criteria in terms of elevations of cardiac enzymes, electrocardiographic changes, and clinical symptoms (nomenclature and criteria for diagnosis of ischemic heart disease. Report of the Joint International Society and Federation of Cardiology/World Health Organization task force on standardization of clinical nomenclature 1979); (3) a documented history of coronary artery bypass graft or percutaneous coronary intervention. Patients with congenital heart disease, cardiomyopathy, and vascular disease were excluded. The control subjects, residing in the same communities as the cases, were determined to be free of CHD and peripheral atherosclerotic arterial disease by medical history, clinical examinations, and electrocardiography; 2298 of them were selected to match age- and sex-frequency in cases group. Subjects with severe liver or kidney disease were excluded. Medical history, medication use, home environment, and lifestyle factors were obtained through questionnaire interviews.
Subjects were classified as smokers and nonsmokers, and the definition has been described previously (Zhou et al. 2008). Briefly, those who had smoked less than 100 cigarettes in their lifetime were defined as nonsmokers; otherwise, they were defined as smokers. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. Subjects were considered to be hypertensive if their systolic blood pressure was ≥140 mmHg or diastolic pressure ≥90 mmHg or they were already being treated with antihypertensive drugs. Diabetes was defined either by 1999 World Health Organization criteria (Puavilai et al. 1999) or by self-report of being previously diagnosed as diabetic. All subjects gave written consent after receiving a full explanation of the study. The Ethics Committee of Tongji Medical College approved this study.
Analysis of length variability of (GT)n repeats in HO-1 gene promoter
Fasting venous blood was collected in 5-mL EDTA tubes, and genomic DNA was extracted with Puregene kit (Gentra Systems Inc), as mentioned previously (Zhou et al. 2008). The 5′-flanking region containing (GT)n repeats of the HO-1 gene was amplified. Briefly, a polymerase chain reaction (PCR) was performed using FAM-labeled sense primer, 5′-TTCTGGAACCTTCTGGGACG-3′, and an antisense primer, 5′-GGGTGGAGAGGAGCAGTCAT-3′, which were designed on the basis of the published sequence. The PCR cycle of 95°C for 45 s, 60°C for 45 s, and 72°C for 45 s was carried out for a total of 30 cycles. Determination of the sizes of PCR products were carried out by capillary electrophoresis on an automated DNA capillary sequencer (ABI Prism Genetic Analyzer 3130; Applied Biosystems, Foster City, California) as described in previous studies (Song et al. 2009). For analysis, 1 μL of polymerase chain reaction product were mixed with 9 μl of Hi-Di formamide (Applied Biosystems) containing GeneScan-500 LIZ size standard solution (size range, 50–500 base pairs) as an internal lane size standard. Mixtures were then denatured at 95°C for 5 min and snap-cooled on ice for 5 min. Subsequently electrophoresis on a polyacrylamide gel POP-7 (Applied Biosystems) in a 50-cm capillary was carried out for genetic analysis. The size of PCR product was analyzed and converted to dinucleotide repeat lengths using GeneMapper 3.5 analysis software (Applied Biosystems). The genotyping was done in 10% of the samples as blind duplicates; the concordance rate was 100%.
Determination of plasma MDA
The level of MDA in plasma is a widely used indicator of the level of oxidative stress in humans. All patients and controls were sampled similarly, and for the acute MI and stable or unstable angina, we collected the blood sample the day the patient was enrolled or the next morning after the patient was enrolled to the ward. We previously determined plasma ferritin level in some of the samples (Shi et al. 2011). The concentrations of plasma MDA were determined by the method described (Ohkawa et al. 1979). Briefly, a 100 μL aliquot of plasma was mixed with thiobarbituric acid reagent and incubated. After centrifugation, the optical density of the clear pink supernatant was read at 532 nm. Malondialdehyde bis (dimethyl acetal) was used as standard. The assay was conducted using kits purchased from Nanjing Jiancheng Bio-engineering Institute (CV = 2.0%) as in a previous study (Yang et al. 2007).
Functional experiments
HUVECs were obtained by proteolytic dissociation of the umbilical cord veins from 25 normal deliveries. DNAs were extracted from umbilical cord venous blood, and then genotypes of (GT)n repeats in HO-1 gene promoter of HUVECs were analyzed.
HUVECs were cultured on gelatin-coated culture flasks in endothelial cell medium (ScienCell) until treatment on their second passages. The cells were seeded at a density of 1 × 106 cells/ml in 12-well plates and were allowed to attach for 24 h before treatment. Subsequently the cells were exposed to different concentrations of H2O2 (0, 25, and 50 μmol/L) for 3 h. After that, cell culture supernates were collected for the determination of MDA and cells were harvested using trypsinization and then centrifuged to pellets. The following procedures completely complied with the instructions provided by the manufacturer of Elisa kits (Stressgen Bioreagents). Briefly, each cell pellet was resuspended with 0.2 ml of 1× HO-1 extraction reagent supplemented with protease inhibitors, and then incubated for 30 min on ice with occasional mixing. After that, extracts were centrifuged at 15,000×g for 10 min in a 4° refrigerated microfugation, and supernatants were collected as the cell lysates. Cell lysates were then frozen at −80° and assayed for HO-1 concentrations within a week.
Statistical analysis
All analyses were conducted using the statistical software package SPSS12.0 (SPSS Inc). Distribution of continuous variables in groups were expressed as means ± SD. Normal distribution of data was analyzed using the Kolmogorov–Smirnov normality test. Data with a normal distribution were compared by student t test or ANOVA and those without a normal distribution were analyzed by a Mann–Whitney rank sum test. Categorical values were compared by the Chi-square test. The association between the HO-1 genotype and CHD risk was estimated by computing odds ratios (ORs) and 95% confidence intervals (CIs) from the multivariate logistic regression analysis after adjustment for conventional coronary risk factors, such as age, sex, BMI, hypertension, diabetes, and smoking habits. The probability level accepted for significance was P < 0.05. In addition, overall subjects were divided into three groups based on tertiles of MDA concentrations in the control group. The significance of multiplicative interactions between the genotypes and covariates was determined by the likelihood ratio test using the logistic regression model.
Results
General characteristic of the subjects
The general characteristics of the study subjects are presented in Table 1. The traditional CHD risk factors such as hypertension, diabetes, and smoking were significantly different between the cases and controls. However, total cholesterol levels were significantly lower in cases than in controls, which might be due to the cholesterol-lowering medication in the patients. The proportion of subjects reported to have taken cholesterol-lowering medications such as a statin in the cases and controls in our study were 67.1% and 0.3%, respectively. In addition, there was a significant difference in MDA concentrations between case and control groups (P < 0.001).
Table 1.
General characteristics of patients with CHD and controls
| Variables | Cases (n = 2,298) | Control (n = 2,298) | P value* |
|---|---|---|---|
| Sex, male/female, (%) | 1,675/623 (72.9/27.1) | 1,675/623 (72.9/27.1) | 1.00 |
| Age, years | 60.1 ± 10.3 | 59.9 ± 10.2 | 0.62 |
| Blood pressure, mmHg | |||
| Systolic | 135.5 ± 24.0 | 130.8 ± 24.1 | <0.001 |
| Diastolic | 81.6 ± 14.4 | 82.3 ± 25.5 | 0.01 |
| Body mass index, kg/m2 | 24.5 ± 3.6 | 24.3 ± 3.7 | 0.80 |
| Fasting glucose, mmol/L | 6.9 ± 5.7 | 5.1 ± 1.5 | <0.001 |
| Total cholesterol | 4.4 ± 1.1 | 4.8 ± 1.0 | <0.001 |
| Triglyceride, mmol/L | 1.8 ± 1.4 | 1.7 ± 1.4 | 0.24 |
| MDA, μmol/L | 3.0 ± 1.8 | 2.7 ± 1.8 | <0.001 |
| Smoking (%) | 1,373 (59.7) | 1,045 (45.5) | <0.001 |
| Past history | |||
| Hypertension (%) | 1,512 (65.8) | 809 (35.2) | <0.001 |
| Diabetes (%) | 558 (24.3) | 193 (8.4) | <0.001 |
Variables are mean ± SD or percentage
*P values were calculated using independent-sample t test, Mann–Whitney rank sum test, or Chi-square test
Allele and genotypic frequencies of HO-1 microsatellite polymorphism
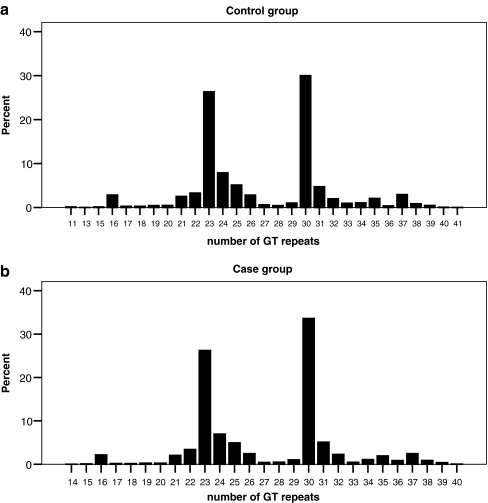
The allele frequencies of (GT)n microsatellite polymorphism in the HO-1 promoter region were highly polymorphic, ranging from 11 to 41 with (GT)23 and (GT)30 being the two most common alleles in our study population. Mann–Whitney rank sum test showed that the repeat numbers in case and control groups are significantly different (P = 0.007), whereas the distributions in the two groups are quite similar, as shown in Fig. 1. Because the proportion of allele frequencies of either ≤25 or >25 GT repeats was about 50% in control group, we classified the alleles into two subclasses: class S, standing for short repeat numbers, included alleles with ≤25 GT repeats; class L, standing for long repeat numbers, included alleles with >25 GT repeats. These patients were then classified as having an S/S, S/L, or L/L genotype according to each of their (GT)n alleles.
Fig. 1.
Frequency distribution of number of (GT)n repeats (a) control group (b) case group
Associations of HO-1 promoter gene polymorphism and CHD risk in overall population and by levels of OS
MDA, a product of lipid peroxidation, is most widely used to assess levels of OS and technically available to be measured in plasma (Stephens et al. 2009; Kotur-Stevuljevic et al. 2007). Low, intermediate, and high levels of OS were then defined by tertile for MDA concentrations in control group: <1.83, 1.83–2.91, and >2.91 μmol/L (<33.3rd percentile, 33.3rd–66.6th percentile, and >66.6th percentile).
As shown in Table 2, after adjustment for conventional CHD risk factors such as age, gender, smoking, BMI, hypertension, and diabetes, (GT)n polymorphism in HO-1 gene promoter was significantly associated with CHD risk: compared with L/L genotypes carriers, the OR for subjects carrying S/S genotype was 0.798 (95%CI 0.659 to 0.965). In the stratified analyses by levels of OS, the adjusted ORs for S/S genotype were 1.056 (95%CI 0.749 to 1.491) in subjects with low levels of OS, 0.787 (95%CI 0.551 to 1.123) in subjects with intermediate levels of OS, and 0.595 (95%CI 0.438 to 0.809) in subjects with high levels of OS (Pinteraction = 0.002).
Table 2.
Association of HO-1 promoter genotypes with the risk of CHD at three levels of oxidative stress
| Levels of oxidative stress | Case | Control | Age-adjusted OR | P value | Adjusted ORa | P value |
|---|---|---|---|---|---|---|
| No. (%) | No. (%) | |||||
| Overall | 2,298 (100.0) | 2,298 (100.0) | ||||
| Genotype | ||||||
| L/L | 594 (25.8) | 563 (24.5) | 1.00 | – | 1.00 | – |
| S/L | 1,268 (55.2) | 1,187 (51.7) | 1.046 (0.930–1.176) | 0.457 | 1.07 (0.913–1.248) | 0.412 |
| S/S | 436 (19.0) | 548 (23.8) | 0.844 (0.731–0.975) | 0.021 | 0.798 (0.659–0.965)* | 0.020 |
| Alleles | ||||||
| L | 2,456 | 2,313 | 1.00 | – | ||
| S | 2,140 | 2,283 | 0.88 (0.81–0.96) | – | ||
| Pbinteraction | 0.002* | |||||
| Low level | ||||||
| Genotype | ||||||
| L/L | 155 (24.6) | 200 (26.1) | 1.00 | – | 1.00 | – |
| S/L | 328 (52.1) | 394 (51.4) | 1.069 (0.827–1.813) | 0.611 | 1.148 (0.860–1.532) | 0.349 |
| S/S | 146 (23.2) | 173 (22.6) | 1.086 (0.800–1.473) | 0.597 | 1.056 (0.749–1.491) | 0.755 |
| Alleles | ||||||
| L | 638 | 794 | 1.00 | – | – | |
| S | 620 | 740 | 1.04 (0.89–1.20) | – | – | |
| Intermediate level | ||||||
| Genotype | ||||||
| L/L | 193 (27.1) | 205 (26.8) | 1.00 | – | 1.00 | – |
| S/L | 412 (57.9) | 401 (52.4) | 1.087 (0.855–1.382) | 0.494 | 1.114 (0.847–1.466) | 0.441 |
| S/S | 107 (15.0) | 159 (20.8) | 0.715 (0.522–0.979) | 0.036* | 0.787 (0.551–1.123) | 0.186 |
| Alleles | ||||||
| L | 798 | 811 | 1.00 | – | – | – |
| S | 626 | 719 | 0.88 (0.77–1.02) | – | – | – |
| High level | ||||||
| Genotype | ||||||
| L/L | 245 (25.6) | 158 (19.9) | 1.00 | – | 1.00 | – |
| S/L | 524 (54.8) | 393 (51.3) | 0.858 (0.675–1.090) | 0.210 | 0.912 (0.702–1.185) | 0.491 |
| S/S | 188 (19.6) | 215 (28.5) | 0.563 (0.425–0.745) | <0.001* | 0.595 (0.438–0.809) | 0.001* |
| Alleles | ||||||
| L | 1,014 | 709 | 1.00 | – | – | – |
| S | 900 | 823 | 0.75 (0.66–0.86) | – | – | – |
*P < 0.05
aAdjusted for age, gender, smoking, hypertension, BMI, and diabetes
bValue is P interaction between HO-1 gene promoter genotypes and levels of oxidative stress
Stratified analysis for the gene polymorphism indicated that the inverse association between the S/S genotype and CHD risk was stronger in males than females, among smokers than nonsmokers, and among diabetics than non-diabetics (Table 3). We then conducted further stratified analysis by levels of OS. Table 4 demonstrates that the significant association between the gene polymorphism and risk of CHD was more pronounced in groups with high levels of OS in the subgroups. Some subjects are missing data on one of the covariates in the questionnaires, and they are not included in this analysis.
Table 3.
Stratification analysis for association between (GT)n genotypes and risk of CHDa
| (GT)n genotype | Pbinteraction | |||
|---|---|---|---|---|
| L/L | L/S | S/S | ||
| Gender | 0.407 | |||
| Female | 1.00 | 1.184 (0.874–1.604) | 0.981 (0.677–1.421) | |
| Male | 1.00 | 1.036 (0.863–1.244) | 0.743 (0.594–0.928)* | |
| Age, years | 0.533 | |||
| ≤60 | 1.00 | 1.006 (0.803–1.261) | 0.718 (0.546–0.943)* | |
| >60 | 1.00 | 1.122 (0.902–1.394) | 0.880 (0.673–1.151) | |
| Smoke status | 0.239 | |||
| Nonsmokers | 1.00 | 1.172 (0.930–1.476) | 0.949 (0.717–1.256) | |
| Smokers | 1.00 | 0.995 (0.803–1.234) | 0.705 (0.543–0.915)* | |
| BMI, kg/m2 | 0.473 | |||
| <25 | 1.00 | 1.006 (0.819–1.236) | 0.742 (0.580–0.950)* | |
| ≥25 | 1.00 | 1.177 (0.923–1.501) | 0.910 (0.672–1.231) | |
| Hypertension | 0.095 | |||
| No | 1.00 | 1.083 (0.867–1.353) | 0.675 (0.510–0.894)* | |
| Yes | 1.00 | 1.051 (0.844–1.308) | 0.924 (0.708–1.205) | |
| Diabetes | 0.251 | |||
| No | 1.00 | 1.071 (0.906–1.268) | 0.843 (0.687–1.035) | |
| Yes | 1.00 | 1.165 (0.762–1.783) | 0.595 (0.361–0.982)* | |
Values are odds ratios (95% CI) of genotype S/S compared to genotype L/L
*P < 0.05
aAdjusted for age, gender, smoking, hypertension, BMI, and diabetes
bP interaction was calculated by binary logistic regression model
Table 4.
Stratification analysis for association between (GT)n genotypes and risk of CHD according to different levels of oxidative stressa
| Levels of oxidative stress | ||||||
|---|---|---|---|---|---|---|
| Low levels | Intermediate levels | High levels | ||||
| No | ORb | No | ORb | No | ORb | |
| Gender | ||||||
| Female | 370 | 1.49 (0.77–2.90) | 396 | 1.10 (0.53–2.25) | 480 | 0.61 (0.34–1.11) |
| Male | 1,019 | 0.96 (0.64–1.44) | 1,088 | 0.68 (0.45–1.03) | 1,243 | 0.60 (0.42–0.85)* |
| Age, years | ||||||
| ≤60 | 691 | 0.95 (0.58–1.56) | 742 | 0.87 (0.53–1.43) | 854 | 0.40 (0.25–0.64)* |
| >60 | 698 | 1.17 (0.71–1.91) | 742 | 0.68 (0.41–1.13) | 869 | 0.81 (0.53–1.24) |
| Smoke status | ||||||
| Nonsmokers | 676 | 1.09 (0.66–1.81) | 710 | 0.94 (0.56–1.60) | 780 | 0.78 (0.50–1.21) |
| Smokers | 710 | 1.09 (0.68–1.76) | 770 | 0.66 (0.40–1.06) | 938 | 0.48 (0.31–0.74) |
| BMI, kg/m2 | ||||||
| <25 | 817 | 0.99 (0.63–1.54) | 834 | 0.65 (0.41–1.02) | 915 | 0.59 (0.39–0.88)* |
| ≥25 | 505 | 1.31 (0.75–2.29) | 553 | 1.03 (0.59–1.81) | 714 | 0.62 (0.38–0.99)* |
| Hypertension | ||||||
| No | 721 | 0.90 (0.55–1.49) | 736 | 0.52 (0.30–0.90)* | 801 | 0.53 (0.34–0.83)* |
| Yes | 664 | 1.23 (0.76–2.00) | 745 | 1.08 (0.66–1.76) | 912 | 0.67 (0.44–1.02) |
| Diabetes | ||||||
| No | 1,158 | 1.17 (0.82–1.68) | 1,251 | 0.76 (0.53–1.10) | 1,410 | 0.61 (0.44–0.84)* |
| Yes | 227 | 0.47 (0.20–1.13) | 226 | 0.85 (0.30–2.43) | 298 | 0.61 (0.28–1.34) |
*P < 0.05
aAdjusted for age, gender, smoking, hypertension, BMI, and diabetes
bValues are odds ratios (95% CI) of genotype S/S compared to genotype L/L
Functional effect of the HO-1 gene polymorphism
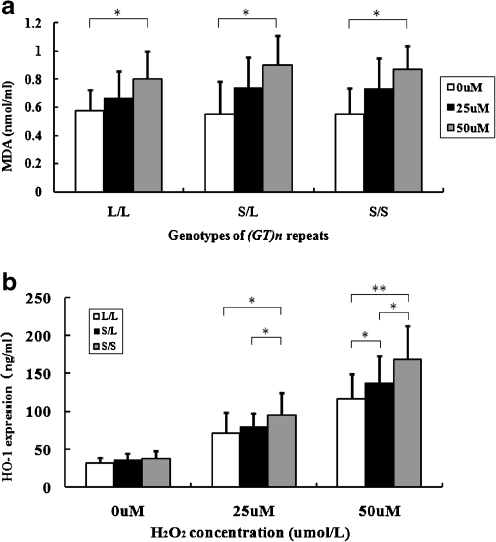
Analysis on the (GT)n repeats in HO-1 gene promoter of the 25 HUVECs revealed that there were 5 cell samples carrying L/L genotype, 13 cell samples carrying L/S genotype, and 7 cell samples carrying S/S genotype. H2O2, a well-recognized oxidant, was used to induce different levels of OS. After HUVECs were exposed to different concentrations of H2O2 (0, 25, and 50 μmol/L) for 3 h, there was a consistent trend in all three genotypes that MDA concentrations in culture mediums elevated as H2O2 concentration increased (Fig. 2a). Subsequently we determined HO-1 expression levels in cell lysates of HUVECs. As shown in Fig. 2b, under 0 μmol/L H2O2, there were no significant differences in HO-1 expression levels among HUVECs with different genotypes; however, under 25 and 50 μmol/L H2O2, HUVECs carrying genotype S/S had significantly higher levels of HO-1 expressions than L/L carriers (P = 0.019 and P < 0.001, respectively). In addition, there was a significant interaction between (GT)n genotypes and H2O2 concentrations on HO-1 expression levels (Pinteraction = 0.003).
Fig. 2.
MDA concentrations in culture medium and HO-1 expression in cell lysates of HUVECs carrying three genotypes under different levels of OS. HUVECs were freshly isolated from umbilical cord veins (S/S [n = 7], S/L [n = 13] or L/L [n = 5], respectively), and exposed to 0, 25, 50µmol/L of H2O2 for 3 hours. (a) Values are means ± S.D. *Ptrend < 0.001 (b) Values are means ± S.D. Under 25µmol/L H2O2, HUVECs carrying S/S had significantly higher HO-1 expression levels than L/L and S/L(*P < 0.05), whereas there was no significant difference between S/L and S/S. Under 50µmol/L H2O2, HUVECs carrying S/L had significantly higher HO-1 expression levels than L/L (*P < 0.05); HUVECs carrying S/S had significantly higher HO-1 expression levels than S/L (*P < 0.05) and L/L (**P < 0.001)
Discussion
In the present study, we found that the association between (GT)n genotypes in HO-1 gene promoter and CHD susceptibility was modified by levels of OS. In particular, the genotype was not associated with CHD in subjects with low or intermediate levels of OS, but as expected, S/S genotype carriers had significantly lower risk of CHD in subjects with high levels of OS. Our functional experiment on HUVECs revealed that (GT)n genotypes were not associated with HO-1 expression levels in endothelial cells under low levels of OS; however, endothelial cells carrying S/S genotype displayed a significantly higher HO-1 expression under high levels of OS.
In two previous studies conducted in Japan (279 controls and 298 CHD patients) (Kaneda et al. 2002) and Taiwan (322 controls and 664 CHD patients) (Chen et al. 2008), significant associations were found between HO-1 gene promoter polymorphism and CHD risk in subjects with one of the CHD risk factors; another study (Bai et al. 2010) also found that (GT)n repeats in HO-1 gene promoter may affect cerebral ischemic risk in dyslipidemia patients. Our study is broadly consistent with these results, however, we further demonstrated for the first time that this association may be attributed to the interaction between HO-1 gene promoter and OS. Moreover, HO-1 expression levels of short and long (GT)n repeats shown in the present study is consistent with their modulatory effect on gene transcriptional activity (Chen et al. 2002). Nevertheless, our study showed for the first time that the effect of heterozygote S/L on levels of HO-1 expression might be closer to the L/L genotype than to the S/S genotype.
HO-1 might play a protective role in the development of CHD due to the antiatherogenic function of its enzymatic products (Chen et al. 2003; Exner et al. 2004). Although HO-1 may have an antioxidant effect, the fact that HO-1 has been found to be colocalized with oxidized phospholipids in all atherosclerotic lesions (Ishikawa et al. 2001a; Ishikawa et al. 2001b) including early oxidized lesions (Wang et al. 1998) suggests that the OS may be the cause of HO-1 induction rather than a consequence (Morita 2005). In response to OS, the stimulation of HO-1 gene is primarily controlled at the transcriptional level by responsive elements localized in the promoter 5′-flanking region of the HO-1 gene (Alam and Den 1992; Dalton et al. 1994; Lavrovsky et al. 1994). On the other hand, (GT)n repeats in HO-1 gene promoter have been demonstrated to modify the transcriptional activity. Transient-transfection assays in various cell lines (Yamada et al. 2000; Chen et al. 2002; Exner et al. 2001), such as A549, Hep3B, and rat aortic smooth muscle cells, showed that cells carrying short (GT)n repeats in HO-1 gene promoter have a significantly higher transcriptional activity when exposed to OS. Up to now, the exact molecular mechanisms to explain the (GT)n repeats’ modulatory effect on HO-1 promoter activity are unclear. Focus has been placed on possible conformational changes since no transcription factors have been reported to bind to the (GT)n repeats region (Lavrovsky et al. 1994). The different lengths in (GT)n repeats may alter the HO-1 promoter structure, which brings change to other adjacent biologically crucial regulatory elements, such as TATA boxes or activator protein 1 binding sites (Chora et al. 2007; Rueda et al. 2007). Therefore, it is probable that at low levels of OS, responsive elements localized in the promoter region are suppressed and thus different lengths in (GT)n repeats’ modulatory effect on gene transcriptional activity might be limited and fail to result in significant difference in HO-1 expression levels; on the contrary, at higher levels of OS, responsive elements are activated and different lengths in (GT)n repeats will influence HO-1 expression levels. Our functional experiment lends support to this hypothesis, which may also explain the result of our case–control study.
This study has several strengths. First, we described for the first time that the association between (GT)n repeats in HO-1 gene promoter and risk of CHD was modulated by patients’ levels of OS. The relatively large number of CHD cases and controls provide statistical power to detect the multiplicative interaction between the genotypes and OS levels on CHD risk. Second, the consistent results from biological experiment on HUVECs which are similar to target cells of CHD provided solid evidence for our epidemiologic findings.
Several limitations should also be acknowledged. First, MDA concentrations of CHD patients were evaluated after diagnosis and thus there may be bias caused by treatments. However, adjustment for medication use did not substantially alter our results. Second, we selected controls only according to electrocardiograms and history of CHD, but without performing coronary angiography on them. Although we could not exclude the possibility that some of them were affected by CHD, recent result from a Chinese cohort (Wu et al. 2006) showed that the incidence of CHD in normal Chinese subjects is very low and thus the bias caused by any misclassification should be limited.
In conclusion, our study provides evidence that the S/S genotype of (GT)n repeats in HO-1 gene promoter is associated with lower risk of CHD in subjects with high levels of OS as endothelial cells carrying S/S genotype have higher HO-1 expression only at higher levels of OS.
Acknowledgments
We are particularly grateful to all volunteers for participating in the present study and to the medical personnel of Union Hospital, Tongji Hospital, Wugang Hospital in Wuhan city, Hubei Province, China, for their kind assistance in collecting the data and samples. This study is supported by research funds from the National Natural Scientific Foundation of China (NSFC 30525031 and 30711120579 to Tangchun Wu).
Glossary
- OS
Oxidative stress
- HO-1
Heme oxygenase-1
- MDA
Malonaldehyde
- HUVECs
Human umbilical venous endothelial cells
- BMI
Body mass index
- ORs
Odds ratios
- CIs
Confidence intervals
References
- Alam J, Den Z. Distal AP-1 binding sites mediate basal level enhancement and TPA induction of the mouse heme oxygenase-1 gene. J Biol Chem. 1992;267(30):21894–21900. [PubMed] [Google Scholar]
- Anwar AA, Li FY, Leake DS, Ishii T, Mann GE, Siow RC. Induction of heme oxygenase 1 by moderately oxidized low-density lipoproteins in human vascular smooth muscle cells: role of mitogen-activated protein kinases and Nrf2. Free Radic Biol Med. 2005;39(2):227–236. doi: 10.1016/j.freeradbiomed.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Bai CH, Chen JR, Chiu HC, Chou CC, Chau LY, Pan WH. Shorter GT repeat polymorphism in the heme oxygenase-1 gene promoter has protective effect on ischemic stroke in dyslipidemia patients. J Biomed Sci. 2010;17:12. doi: 10.1186/1423-0127-17-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YH, Chau LY, Chen JW, Lin SJ. Serum bilirubin and ferritin levels link heme oxygenase-1 gene promoter polymorphism and susceptibility to coronary artery disease in diabetic patients. Diabetes Care. 2008;31(8):1615–1620. doi: 10.2337/dc07-2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YH, Lin SJ, Lin MW, Tsai HL, Kuo SS, Chen JW, Charng MJ, Wu TC, Chen LC, Ding YA, Pan WH, Jou YS, Chau LY. Microsatellite polymorphism in promoter of heme oxygenase-1 gene is associated with susceptibility to coronary artery disease in type 2 diabetic patients. Hum Genet. 2002;111(1):1–8. doi: 10.1007/s00439-002-0769-4. [DOI] [PubMed] [Google Scholar]
- Chen YH, Yet SF, Perrella MA. Role of heme oxygenase-1 in the regulation of blood pressure and cardiac function. Exp Biol Med (Maywood) 2003;228(5):447–453. doi: 10.1177/15353702-0322805-03. [DOI] [PubMed] [Google Scholar]
- Chora AA, Fontoura P, Cunha A, Pais TF, Cardoso S, Ho PP, Lee LY, Sobel RA, Steinman L, Soares MP. Heme oxygenase-1 and carbon monoxide suppress autoimmune neuroinflammation. J Clin Invest. 2007;117(2):438–447. doi: 10.1172/JCI28844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton T, Palmiter RD, Andrews GK. Transcriptional induction of the mouse metallothionein-I gene in hydrogen peroxide-treated Hepa cells involves a composite major late transcription factor/antioxidant response element and metal response promoter elements. Nucleic Acids Res. 1994;22(23):5016–5023. doi: 10.1093/nar/22.23.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djousse L, Levy D, Cupples LA, Evans JC, D'Agostino RB, Ellison RC. Total serum bilirubin and risk of cardiovascular disease in the Framingham offspring study. Am J Cardiol. 2001;87(10):1196–1200. doi: 10.1016/S0002-9149(01)01494-1. [DOI] [PubMed] [Google Scholar]
- Duarte MM, Rocha JB, Moresco RN, Duarte T, Cruz IB, Loro VL, Schetinger MR. Association between ischemia-modified albumin, lipids and inflammation biomarkers in patients with hypercholesterolemia. Clin Biochem. 2009;42(7–8):666–671. doi: 10.1016/j.clinbiochem.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Duckers HJ, Boehm M, True AL, Yet SF, San H, Park JL, Clinton Webb R, Lee ME, Nabel GJ, Nabel EG. Heme oxygenase-1 protects against vascular constriction and proliferation. Nat Med. 2001;7(6):693–698. doi: 10.1038/89068. [DOI] [PubMed] [Google Scholar]
- Exner M, Minar E, Wagner O, Schillinger M. The role of heme oxygenase-1 promoter polymorphisms in human disease. Free Radic Biol Med. 2004;37(8):1097–1104. doi: 10.1016/j.freeradbiomed.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Exner M, Schillinger M, Minar E, Mlekusch W, Schlerka G, Haumer M, Mannhalter C, Wagner O. Heme oxygenase-1 gene promoter microsatellite polymorphism is associated with restenosis after percutaneous transluminal angioplasty. J Endovasc Ther. 2001;8(5):433–440. doi: 10.1583/1545-1550(2001)008<0433:HOGPMP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Giugliano D, Ceriello A, Paolisso G. Oxidative stress and diabetic vascular complications. Diabetes Care. 1996;19(3):257–267. doi: 10.2337/diacare.19.3.257. [DOI] [PubMed] [Google Scholar]
- Idriss NK, Blann AD, Lip GY. Hemoxygenase-1 in cardiovascular disease. J Am Coll Cardiol. 2008;52(12):971–978. doi: 10.1016/j.jacc.2008.06.019. [DOI] [PubMed] [Google Scholar]
- Ishikawa K, Navab M, Leitinger N, Fogelman AM, Lusis AJ. Induction of heme oxygenase-1 inhibits the monocyte transmigration induced by mildly oxidized LDL. J Clin Invest. 1997;100(5):1209–1216. doi: 10.1172/JCI119634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa K, Sugawara D, Goto J, Watanabe Y, Kawamura K, Shiomi M, Itabe H, Maruyama Y. Heme oxygenase-1 inhibits atherogenesis in Watanabe heritable hyperlipidemic rabbits. Circulation. 2001;104(15):1831–1836. doi: 10.1161/hc3901.095897. [DOI] [PubMed] [Google Scholar]
- Ishikawa K, Sugawara D, Wang X, Suzuki K, Itabe H, Maruyama Y, Lusis AJ. Heme oxygenase-1 inhibits atherosclerotic lesion formation in ldl-receptor knockout mice. Circ Res. 2001;88(5):506–512. doi: 10.1161/01.res.88.5.506. [DOI] [PubMed] [Google Scholar]
- Kaneda H, Ohno M, Taguchi J, Togo M, Hashimoto H, Ogasawara K, Aizawa T, Ishizaka N, Nagai R. Heme oxygenase-1 gene promoter polymorphism is associated with coronary artery disease in Japanese patients with coronary risk factors. Arterioscler Thromb Vasc Biol. 2002;22(10):1680–1685. doi: 10.1161/01.ATV.0000033515.96747.6F. [DOI] [PubMed] [Google Scholar]
- Kawamura K, Ishikawa K, Wada Y, Kimura S, Matsumoto H, Kohro T, Itabe H, Kodama T, Maruyama Y. Bilirubin from heme oxygenase-1 attenuates vascular endothelial activation and dysfunction. Arterioscler Thromb Vasc Biol. 2005;25(1):155–160. doi: 10.1161/01.ATV.0000148405.18071.6a. [DOI] [PubMed] [Google Scholar]
- Kimpara T, Takeda A, Watanabe K, Itoyama Y, Ikawa S, Watanabe M, Arai H, Sasaki H, Higuchi S, Okita N, Takase S, Saito H, Takahashi K, Shibahara S. Microsatellite polymorphism in the human heme oxygenase-1 gene promoter and its application in association studies with Alzheimer and Parkinson disease. Hum Genet. 1997;100(1):145–147. doi: 10.1007/s004390050480. [DOI] [PubMed] [Google Scholar]
- Kotur-Stevuljevic J, Memon L, Stefanovic A, Spasic S, Spasojevic-Kalimanovska V, Bogavac-Stanojevic N, Kalimanovska-Ostric D, Jelic-Ivanovic Z, Zunic G. Correlation of oxidative stress parameters and inflammatory markers in coronary artery disease patients. Clin Biochem. 2007;40(3–4):181–187. doi: 10.1016/j.clinbiochem.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Lavrovsky Y, Schwartzman ML, Levere RD, Kappas A, Abraham NG. Identification of binding sites for transcription factors NF-kappa B and AP-2 in the promoter region of the human heme oxygenase 1 gene. Proc Natl Acad Sci U S A. 1994;91(13):5987–5991. doi: 10.1073/pnas.91.13.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita T. Heme oxygenase and atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25(9):1786–1795. doi: 10.1161/01.ATV.0000178169.95781.49. [DOI] [PubMed] [Google Scholar]
- Morrow JD, Frei B, Longmire AW, Gaziano JM, Lynch SM, Shyr Y, Strauss WE, Oates JA, Roberts LJ., 2nd Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers. Smoking as a cause of oxidative damage. The New England journal of medicine. 1995;332(18):1198–1203. doi: 10.1056/NEJM199505043321804. [DOI] [PubMed] [Google Scholar]
- Nomenclature and criteria for diagnosis of ischemic heart disease. Report of the Joint International Society and Federation of Cardiology/World Health Organization task force on standardization of clinical nomenclature (1979). Circulation 59 (3):607–609 [DOI] [PubMed]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Ponka P. Cell biology of heme. Am J Med Sci. 1999;318(4):241–256. doi: 10.1097/00000441-199910000-00004. [DOI] [PubMed] [Google Scholar]
- Puavilai G, Chanprasertyotin S, Sriphrapradaeng A. Diagnostic criteria for diabetes mellitus and other categories of glucose intolerance: 1997 criteria by the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus (ADA), 1998 WHO consultation criteria, and 1985 WHO criteria. World Health Organization. Diabetes research and clinical practice. 1999;44(1):21–26. doi: 10.1016/S0168-8227(99)00008-X. [DOI] [PubMed] [Google Scholar]
- Rueda B, Oliver J, Robledo G, Lopez-Nevot MA, Balsa A, Pascual-Salcedo D, Gonzalez-Gay MA, Gonzalez-Escribano MF, Martin J. HO-1 promoter polymorphism associated with rheumatoid arthritis. Arthritis Rheum. 2007;56(12):3953–3958. doi: 10.1002/art.23048. [DOI] [PubMed] [Google Scholar]
- Siow RC, Ishii T, Sato H, Taketani S, Leake DS, Sweiry JH, Pearson JD, Bannai S, Mann GE. Induction of the antioxidant stress proteins heme oxygenase-1 and MSP23 by stress agents and oxidised LDL in cultured vascular smooth muscle cells. FEBS Lett. 1995;368(2):239–242. doi: 10.1016/0014-5793(95)00650-X. [DOI] [PubMed] [Google Scholar]
- Song F, Li X, Zhang M, Yao P, Yang N, Sun X, Hu FB, Liu L. Association between heme oxygenase-1 gene promoter polymorphisms and type 2 diabetes in a Chinese population. Am J Epidemiol. 2009;170(6):747–756. doi: 10.1093/aje/kwp196. [DOI] [PubMed] [Google Scholar]
- Sowers JR. Hypertension, angiotensin II, and oxidative stress. N Engl J Med. 2002;346(25):1999–2001. doi: 10.1056/NEJMe020054. [DOI] [PubMed] [Google Scholar]
- Stephens JW, Khanolkar MP, Bain SC. The biological relevance and measurement of plasma markers of oxidative stress in diabetes and cardiovascular disease. Atherosclerosis. 2009;202(2):321–329. doi: 10.1016/j.atherosclerosis.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Stocker R, Perrella MA. Heme oxygenase-1: a novel drug target for atherosclerotic diseases? Circulation. 2006;114(20):2178–2189. doi: 10.1161/CIRCULATIONAHA.105.598698. [DOI] [PubMed] [Google Scholar]
- Wang LJ, Lee TS, Lee FY, Pai RC, Chau LY. Expression of heme oxygenase-1 in atherosclerotic lesions. Am J Pathol. 1998;152(3):711–720. [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Liu X, Li X, Li Y, Zhao L, Chen Z, Li Y, Rao X, Zhou B, Detrano R, Liu K. Estimation of 10-year risk of fatal and nonfatal ischemic cardiovascular diseases in Chinese adults. Circulation. 2006;114(21):2217–2225. doi: 10.1161/CIRCULATIONAHA.105.607499. [DOI] [PubMed] [Google Scholar]
- Yamada N, Yamaya M, Okinaga S, Nakayama K, Sekizawa K, Shibahara S, Sasaki H. Microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with susceptibility to emphysema. Am J Hum Genet. 2000;66(1):187–195. doi: 10.1086/302729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M, Sato H, Bannai S. Induction of stress proteins in mouse peritoneal macrophages by oxidized low-density lipoprotein. Biochem Biophys Res Commun. 1993;193(3):1198–1201. doi: 10.1006/bbrc.1993.1752. [DOI] [PubMed] [Google Scholar]
- Yang X, Zheng J, Bai Y, Tian F, Yuan J, Sun J, Liang H, Guo L, Tan H, Chen W, Tanguay RM, Wu T. Using lymphocyte and plasma Hsp70 as biomarkers for assessing coke oven exposure among steel workers. Environ Health Perspect. 2007;115(11):1573–1577. doi: 10.1289/ehp.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Zhang X, He M, Cheng L, Chen Y, Hu FB, Wu T. Associations between single nucleotide polymorphisms on chromosome 9p21 and risk of coronary heart disease in Chinese Han population. Arterioscler Thromb Vasc Biol. 2008;28(11):2085–2089. doi: 10.1161/ATVBAHA.108.176065. [DOI] [PubMed] [Google Scholar]
- Shi Y, Zhou L, Huang LH, Lian YT, Zhang XM, Guo H, Wu TC, Cheng LX, He MA (2011) Plasma ferritin levels, genetic variations in HFE gene, and coronary heart disease in Chinese: a case-control study. Atherosclerosis. doi:10.1016/j.atherosclerosis.2011.05.040 [DOI] [PubMed]