Abstract
Identification of predictors of cardiovascular risk can help in the prevention of pathologic episodes and the management of patients at all stages of illness. Here, we investigated the relationships between serum levels of Hsp60 and dyslipidemia in patients with periodontitis by performing a cross-sectional study of 22 patients with mild periodontitis without any prior treatment for it (i.e., drug naïve) and 22 healthy controls, matched for age and body mass index (BMI). All subjects were evaluated for periodontal status, gingival inflammation, and oral hygiene. Levels of circulating Hsp60, C-reactive protein (CRP), and plasma lipids were measured, and small, dense low-density lipoproteins (LDL) were indirectly assessed by determining the triglycerides/high-density lipoproteins (HDL) cholesterol ratio. We also assessed by immunohistochemistry Hsp60 levels in oral mucosa of patients and controls. No difference was found in CRP levels or plasma lipids between the two groups, but subjects with periodontitis showed, in comparison to controls, higher levels of small, dense LDL (p = 0.0355) and circulating Hsp60 concentrations (p < 0.0001). However, levels of mucosal Hsp60 did not change significantly between groups. Correlation analysis revealed that circulating Hsp60 inversely correlated with HDL-cholesterol (r = −0.589, p = 0.0039), and positively with triglycerides (r = +0.877, p < 0.0001), and small, dense LDL (r = +0.925, p < 0.0001). Serum Hsp60 significantly correlated with the degree of periodontal disease (r = +0.403, p = 0.0434). In brief, untreated patients with mild periodontitis had increased small, dense LDL and serum Hsp60 concentrations, in comparison to age- and BMI-matched controls and both parameters showed a strong positive correlation. Our data indicate that atherogenic dyslipidemia and elevated circulating Hsp60 tend to be linked and associated to periodontal pathology. Thus, the road is open to investigate the potential value of elevated levels of circulating Hsp60 as predictor of risk for cardiovascular disease when associated to dyslipidemia in periodontitis patients.
Keywords: Periodontitis; Hsp60; Small, dense LDL; Risk factors; Cardiovascular disease
Introduction
Periodontitis is a chronic infectious disease commonly affecting adults, characterized by a progressive gingival inflammatory response to bacteria in dental plaques (Williams 1990). Evidence suggests that the local inflammation-infection triggers a systemic host response that is paralleled by an increased risk for cardiovascular diseases (Scannapieco et al. 2003). Periodontitis has also been associated with glucose intolerance (Saito et al. 2004), procoagulant condition (Taylor et al. 2006), endothelial dysfunction (Tonetti et al. 2007), and dyslipidemia and metabolic syndrome (D’Aiuto et al. 2008). It has recently been suggested that the heat-shock protein (Hsp) 60 kDa (Hsp60) might be the link between periodontitis and atherosclerosis (Choi et al. 2011). Hsps are produced in response to stress induced by a variety of stressors that disturb cellular homeostasis and, under physiological conditions, Hsps assist newly formed or partially denatured proteins to either fold correctly or refold, respectively (references in Macario and Conway de Macario 2005). Although Hsps were first identified as intracellular proteins, further studies showed that they are actively secreted by cells reaching the bloodstream, by which they interact with the immune system (references in Pockley and Multhoff 2008; Pockley et al. 2008; Macario et al. 2010). It has been known for over 10 years that Hsp60 can be found in the peripheral circulation (Pockley et al. 1999), but only recently it has been shown that Hsp60 can be secreted, via various pathways, from normal and tumor human cells (Gupta and Knowlton 2007; Merendino et al. 2010; reviewed in De Maio 2011).
Data from various reports show an elevated humoral immune response to Hsp60 (both human and bacterial) in patients with periodontitis (Tabeta et al. 2000; Yamazaki et al. 2002; Yamazaki et al. 2004; Choi et al. 2004; Buhlin et al. 2009). This supports the view that molecular mimicry between bacterial and human Hsp60 may play a role in immune mechanisms triggered by antigenic cross-reaction (Cappello et al. 2009; Linhares and Witkin 2010). Hsp60 has been detected on the membrane surface of stressed human endothelial cells, in which it may become an auto-antigen for anti-Hsp60 antibodies (Pfister et al. 2005), in agreement with recent work suggesting that Hsp60 is associated with atherosclerosis development and progression (Pockley et al. 2000; Knoflach et al. 2005; Zhang et al. 2008; Novo et al. 2011).
Along these lines, we investigated relationships between levels of circulating human Hsp60 and risk factors for cardiovascular diseases, particularly atherogenic dyslipidemia, in patients with periodontitis. We studied subjects with mild periodontitis but without any prior treatment for it (i.e., drug naïve), compared to healthy controls matched for age and body mass index (BMI). Although finding parallelism between certain clinical and pathological observations does not establish cause-effect relationships, it provides indicators with potential diagnostic and prognostic usefulness. For example, levels of Hsp60 in tissues and in circulation have been found elevated or decreased in relation to pathologic parameters, and the consistency of the findings encourages the measurement of the chaperone with diagnostic purposes and to assess prognosis and response to treatment. The main goal of the work presented here was to determine if the serum levels of Hsp60 paralleled dyslipidemia in subjects with periodontal inflammation-infection and could serve as another indicator of risk for cardiovascular disease.
Materials and methods
Subjects and study protocol
Twenty-two patients with untreated mild periodontitis were studied. The patients were admitted consecutively in the Periodontology Department, University of Granada, Spain. At admission all subjects underwent a medical examination, including a blood test, and also answered a questionnaire on personal and medical items, including age, past medical history, and use of medications. The adopted procedures were in agreement with the Helsinki Declaration of 1975, as revised in 1983. The study was approved by the Ethics Committee of the University of Granada, Spain, and each subject gave written informed consent to participate in this study. Inclusion criteria were adult age, presence of periodontal disease as diagnosed according to the criteria of Arbes et al. (1999), presence of at least five teeth in the mouth, and consent to participate in the study. Exclusion criteria were: previous cardiovascular disease; occurrence of any inflammatory condition other than periodontitis; presence of renal, hepatic, or thyroid disease; use of beta blockers, hypolipidemia-inducing drugs, or any other modifier of plasma lipids and lipoproteins.
Twenty-two healthy subjects matched for age and BMI were included as controls, with the same exclusion criteria as described above, who were co-workers or family co-workers. Height and weight were recorded and BMI was calculated as kg⁄m2. The cardiovascular risk factors taken into consideration were: hypertension (systolic or diastolic blood pressure, respectively, ≥140 or ≥90 mmHg or previous pharmacological therapy with antihypertensive drugs), diabetes (fasting glucose plasma concentrations higher than 126 mg⁄dl or previous pharmacological therapy with antidiabetic drugs or insulin), and smoking addiction.
Periodontal examination
The oral examination was performed by a single dentist (RM) at the Periodontology Department of the University of Granada, Spain. The diagnosis of periodontitis was compared with that of another dentist (FM) in 13 patients, obtaining intraclass correlation coefficients (for gingival recession and pocket depth) above 0.71, which is considered substantial according to the scale of Landis and Koch (1977). The loss of periodontal attachment was measured using the PCPUNC-15 periodontal probe (Hu-Friedy, Leimen, Germany) and a number 5 non-magnifying oral mirror (SE plus) by adding the pocket depths and gingival recessions (in millimeters). Six sites per tooth (mesiobuccal, buccal, distobuccal, mesiolingual, lingual, and distolingual) were examined for all the teeth present in the oral cavity. The degree of periodontal disease was defined, as we previously reported (Cueto et al. 2005), by the percentage of sites with loss of attachment ≥3 mm as follows: 0% = absent, 0–32% = mild, 33–66% = moderate, and 67–100% = severe, according to the criteria of Arbes et al. (1999). The gingival bleeding index of Ainamo and Bay (1975) and the plaque index of O'Leary et al. (1972) were also determined in order to assess the degree of gingival inflammation and oral hygiene, respectively. All patients had mild periodontitis.
Laboratory analyses
Whole blood was collected from each subject, after 12–14-h overnight fast, in sodium-EDTA tubes and stored at −80°C until analysis. Total cholesterol, triglycerides, and high-density lipoproteins (HDL)-cholesterol were quantified by standard enzymatic-colorimetric methods (Allain et al. 1974; Nagele et al. 1984; Warnick et al. 1985). Low-density lipoproteins (LDL)-cholesterol was calculated using the Friedewald formula. Non-HDL-cholesterol was calculated as total cholesterol minus HDL-cholesterol. The triglycerides/HDL-cholesterol ratio was calculated as an indirect measure of small, dense LDL particles (Décary et al. 2010). C-reactive protein (CRP) was determined by the nephelometric method (Montagne et al. 1992).
Hsp60 determination in patients’ sera by ELISA
A blood sample from each subject was allowed to clot at room temperature for 30 minutes. After a centrifugation at 2,700 × g for 10 min, sera were collected, aliquoted and stored at −20°C until use. The levels of human circulating Hsp60 in sera were determined by Hsp60 (human) enzyme immunoassay (EIA) quantitative sandwich immunoassay (ADI EKS 600 ELISA kit, Stressgen). A mouse monoclonal antibody specific for Hsp60 is pre-coated on the wells of the Hsp60 Immunoassay Plate. All reagents were brought to 24°C and the Hsp60 standard was diluted in sample diluent to generate a standard curve with six points, ranging from 3.125 to 100 ng/mL. Sample diluent alone was used as 0 (zero) standard. To start, 100 μL of prepared standards and serum (no diluted) was added in duplicate to wells and incubated at 24°C for 1 h. After washing six times with 1× Wash Buffer, 100 μL of diluted anti-Hsp60 goat polyclonal antibody was added to each well and incubated at 24°C for 1 h. Then, 100 μL of diluted horse radish peroxidase-conjugate anti-goat IgG was added to the plate and incubated at 24°C for 30 min. After washing, 100 μL of 3,3′,5,5′-tetramethylbenzidine substrate was added and incubated for 15 min in the dark. Finally, 100 μL of Stop Solution was added and absorbance was measured at 450 nm in a microplate photometric reader (DV990BV4, GDV, Milan, Italy). Sample concentration was calculated by interpolating the sample concentrations in the standard curve. The sensitivity of the Hsp60 (human), EIA was determined to be 3.125 ng/mL. The intra-assay coefficient of variation of Hsp60 (human) EIA was determined to be <10% and the inter-assay coefficient of variation was determined to also be <10%. Hsp60 (human). EIA is specific for Hsp60 and the Hsp60 ELISA has been certified for the detection of human Hsp60.
Tissue collection and processing
We collected, from the files of the Department of Periodontology, Oral Surgery and Pathology, University of Granada, Granada, Spain, formalin-fixed/paraffin-embedded blocks of samples of periodontal mucosa randomly from 20 patients with mild periodontitis and 20 subjects with normal oral mucosa. Immunostaining by avidin-biotin complex method (LSAB2, DAKO, Carpinteria, CA, USA) was performed on 4–5-μm sections from each case using primary antibodies against Hsp60 (monoclonal mouse, 1:300, Sigma, Italy, cat. no. H4149). Appropriate positive controls, as well as non-immune serum for negative controls, were run concurrently. Nuclear counterstaining was done using hematoxylin (DAKO). Three independent observers (FC, FR, and GB) examined the code-marked specimens in blind mode, and performed a semiquantitative analysis to evaluate the percentage of epithelial and stromal cells positive for Hsp60. All the observations were made at a magnification of ×400 and the means of triplicate counts were used for statistical analyses.
Statistical analyses
Statistical analyses were performed using Statview® 5.0 (SAS Institute Inc., Cary, NC, USA). Univariate analyses were performed using the non-parametric Mann-Whitney test for numeric variables, while the differences in the prevalence for nominal variables were analyzed by the McNemar test. Correlation analyses were performed using the Spearman rank correlation method.
Results
Clinical and laboratory characteristics of patients and controls are displayed in Table 1. No difference was found in the prevalence of main risk factors for cardiovascular pathology, such as hypertension, diabetes, and smoking addiction between patients and controls. Patients had a clear evidence of periodontal pathology compared to controls (as measured by the Arbes index, p < 0.0001) and gingival inflammation (as measured by the gingival bleeding index, p = 0.0007), while no difference was found between the two groups in oral hygiene (as measured by the plaque index). CRP levels were higher in patients than in controls but the difference did not reach statistical significance. Similar concentrations in plasma lipids were found in all investigated parameters in patients and controls, with the only exception that patients had higher levels of small, dense LDL (p = 0.0355). Serum Hsp60 concentrations were considerably higher in patients than in controls (p < 0.0001).
Table 1.
Clinical and laboratory characteristics of patients with mild periodontitis and controls
| Characteristic | Controls, (n = 22) | P value | Patients (n = 22) |
|---|---|---|---|
| Age (years) | 42 ± 6 | ns | 42 ± 5 |
| Female gender, n (%) | 13 (59) | ns | 13 (59) |
| Body-mass index (kg/m2) | 26 ± 3 | ns | 26 ± 4 |
| Periodontal pathology | 0 ± 0 | <0.0001 | 13 ± 12 |
| Gingival inflammation | 4 ± 7 | 0.0007 | 26 ± 19 |
| Plaque index | 9 ± 6 | ns | 15 ± 11 |
| Hypertension, n (%) | 1 (5) | ns | 1 (5) |
| Diabetes, n (%) | 0 (0) | ns | 0 (0) |
| Smoking, n (%) | 9 (41) | ns | 9 (41) |
| C-reactive protein (mg/L) | 0.14 ± 0.20 | ns | 0.27 ± 0.39 |
| Total cholesterol (mmol/L) | 4.6 ± 0.8 | ns | 4.8 ± 0.8 |
| Triglycerides (mmol/L) | 1.0 ± 0.5 | ns | 1.5 ± 0.8 |
| HDL-cholesterol (mmol/L) | 1.7 ± 0.4 | ns | 1.3 ± 0.4 |
| LDL-cholesterol (mmol/L) | 2.6 ± 0.7 | ns | 2.7 ± 0.8 |
| Non-HDL-cholesterol (mmol/L) | 2.9 ± 0.8 | ns | 3.2 ± 0.9 |
| Small dense LDL (=triglycerides/HDL-c ratio) | 0.6 ± 0.4 | 0.0355 | 1.3 ± 0.5 |
| Hsp60 (ng/mL) | 0.4 ± 0.3 | <0.0001 | 14 ± 21 |
Mean ± SD
ns not significant
Spearman correlation analysis in patients with periodontitis showed that BMI was significantly correlated with all lipid parameters (Table 2) and with CRP (all p < 0.05). Periodontal pathology positively correlated with levels of total, LDL, and non-HDL-cholesterol, (all p < 0.05), and correlated inversely with HDL-cholesterol concentrations (p < 0.05). Serum Hsp60 levels inversely correlated with HDL-cholesterol concentrations (p = 0.0039), and correlated positively with levels of triglycerides (p < 0.0001); this positive correlation of serum Hsp60 levels was even more evident with levels of small, dense LDL (r = +.925, p < 0.0001, Fig. 1). We have further analysed the data shown in the lower panel of Fig. 1, excluding the data pertaining to the individual exhibiting high Hsp60 and small, dense LDL values; interestingly, we still found a significant correlation (p < 0.05, data not shown). Circulating Hsp60 levels also significantly correlated with the degree of periodontal disease (p = 0.0434). Spearman correlation analysis in the group of controls did not reveal significant correlations (data not shown).
Table 2.
Spearman correlations between clinical and biochemical parameters in patients with periodontitis
| Parameter | Age | BMI | Periodontal pathology | Plaque index | Gingival bleeding index | Hsp60 |
|---|---|---|---|---|---|---|
| Total cholesterol | 0.292 | 0.575** | 0.432* | −0.302 | 0.221 | 0.258 |
| Triglycerides | 0.245 | 0.408* | 0.278 | −0.213 | −0.119 | 0.877*** |
| HDL-cholesterol | −0.295 | −0.584** | −0.485* | 0.164 | −0.185 | −0.589** |
| LDL-cholesterol | 0.257 | 0.550* | 0.456* | −0.223 | 0.279 | 0.030 |
| Non-HDL-cholesterol | 0.333 | 0.655*** | 0.504* | −0.302 | 0.240 | 0.233 |
| Small, dense LDL | 0.208 | 0.245 | 0.265 | −0.216 | −0.131 | 0.925*** |
| C-reactive protein | 0.005 | 0.536* | −0.105 | −0.144 | 0.124 | −0.153 |
| Hsp60 | 0.241 | 0.199 | 0.403* | −0.027 | −0.101 | – |
In bold the relationships that reached statistical significance
*p < 0.05; **p < 0.005; ***p ≤ 0.0005
Fig. 1.
Correlations between Hsp60 levels in blood and clinical parameters. Spearman correlations between Hsp60 and degree of periodontal pathology (upper panel) and between Hsp60 and small, dense LDL, as indirectly assessed by the triglycerides/HDL-cholesterol ratio (bottom panel), in patients with mild periodontitis
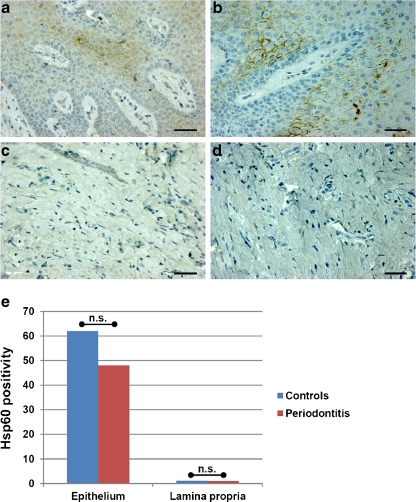
Our immunohistochemical analyses of oral mucosa to determine tissue levels of Hsp60 in patients with mild periodontitis and matched controls did not reveal significant differences between the two groups (Fig. 2). The levels of Hsp60 were higher in epithelium than in lamina propria. Likewise, Hsp60 levels tended to be higher (although not statistically significant, p > 0.05) in the periodontal mucosa of healthy controls than in that of patients with periodontitis.
Fig. 2.
Immunostaining for Hsp60 in periodontal mucosa. The non-parametric Mann–Whitney test showed the absence of significant differences in Hsp60 levels assessed immunohistochemically between patients with mild periodontitis and healthy controls in both epithelium and lamina propria. The images are representative of Hsp60 levels in epithelium (a and b) and lamina propria (c and d) in healthy subjects (a and c) and patients with mild periodontitis (b and d). Bar: 50 microns. e Histogram showing results of semiquantitative evaluation of Hsp60 immunostaining. Vertical axis, semiquantitative scale measuring amount of Hsp60 positivity in the tissue; n.s. not significant
Discussion
Dyslipidemia is common in subjects with periodontitis and may be manifested as increased plasma LDL-cholesterol and triglyceride levels as well as reduced HDL-cholesterol concentrations (Cutler and Iacopino 2003). However, the exact type and extent of atherogenic dyslipidemia in periodontitis is still largely unknown and probably involves alterations in lipoproteins, beyond those in plasma lipids (Rufail et al 2005). In the present study, small alterations in all plasma lipid parameters were found but they did not reach statistical significance. In contrast, a significant difference was observed in the triglycerides/HDL-cholesterol ratio, which is widely used as an indirect measure of small, dense LDL (Décary et al. 2010). The quality, and not only the quantity, of LDL seem to be associated with risk for cardiovascular disease (Wierzbicki 2007). LDL comprises multiple distinct subclasses that differ in size, density, chemical composition, metabolic functions, and atherogenicity (Rizzo and Berneis 2006). LDL particles are an important predictor of cardiovascular pathological events and progression of coronary artery disease, and the predominance of small, dense LDL has been accepted as an emerging risk factor for cardiovascular pathology by the National Cholesterol Education Program Adult Treatment Panel III. In addition, it has been shown in recent years that there is a linear correlation between the concentration of small, dense LDL particles and the risk for the development of cardiovascular pathology (Gardner et al. 1996; St Pierre et al. 2005). Therefore, screening for the presence of small, dense LDL may potentially identify subjects with higher risk for cardiovascular disease and help in establishing prevention strategies. Patients with periodontitis have a higher risk for cardiovascular pathology than the general population (Scannapieco et al. 2003) and also have lipid and lipoproteins alterations (Cutler and Iacopino 2003; Rufail et al. 2005).
In the present study, patients with mild periodontitis did not show alterations of statistical significance in plasma lipids, including LDL-cholesterol but, interestingly, our patients did show increased levels of small, dense LDL. In brief, our patients had no quantitative abnormality in the overall levels of plasma lipids but did show qualitative modifications, manifested as increased levels of small, dense LDL. These findings are consistent with previous reports, showing elevated levels of pro-atherogenic lipoproteins in periodontitis (Rufail et al. 2005; Kallio et al. 2008; Bengtsson et al. 2008). Our findings extend those previous observations to a population of patients with periodontitis free of treatment prior and during the study, namely patients that had not been modified by medication. It has been hypothesized that oxidative stress may be the link between atherosclerosis and periodontal infection (Bullon et al. 2009); this view implies that oxidative modifications of LDL represent an early stage of atherosclerosis, and small, dense LDL are more susceptible to oxidation than larger, more buoyant subspecies (Tribble et al. 2001). Recent evidence further suggests that oxidative stress and atherogenic dyslipidemia have a synergistic impact on atherosclerosis and cardiovascular diseases (reviewed in Rizzo et al. 2009).
We found that patients with periodontitis had higher concentrations of circulating Hsp60 than controls. This result contrasts with that of previous works in which no difference was found in the levels of circulating Hsp60 between patients with periodontitis and controls (Buhlin et al. 2003; Shamaei-Tousi et al. 2007). However, periodontitis patients had higher intermediate levels of Hsp60 (Shamaei-Tousi et al. 2007). It has to be emphasized that the methods used to measure circulating Hsp60 in the two previous works just cited and by us were not the same, which makes comparisons between these studies if not impossible, at least of limited value. Another difference between the aforementioned studies and ours that impedes comparisons stems from the selection of periodontitis cases examined, since our study is the only one to focus on mild periodontitis.
Our immunohistochemical analysis not only aimed at assessing levels of Hsp60 in the periodontal mucosa of patients in comparison to that of the controls but also at obtaining clues about the origins of the circulating chaperonin. Do pathological tissues produce elevated quantities of Hsp60 in response to stressors, e.g., reactive oxygen species or bacterial products, and the excess intracellular chaperonin is secreted out of the cells and reaches the circulating blood? The answer to this question seems to be negative, at least in what pertains to the pathological periodontal tissue we examined, since no difference was found in the levels of Hsp60 between the mucosa of patients and that of controls. Thus, the increased quantities of circulating Hsp60 we detected in patients with mild periodontitis must originate not in the pathological periodontal mucosa but somewhere else.
We extended the previous observations mentioned earlier by assessing the relationships between circulating Hsp60 levels and risk factors for cardiovascular disease, particularly atherogenic dyslipidemia. We found very strong correlations between Hsp60 and small, dense LDL and periodontal pathology. However, the parallelism between levels of Hsp60, small dense LDL, and periodontitis does not provide evidence for cause–effect relationships between the parameters investigated. Could it be that extracellular Hsp60 is involved in a cross talk between lipoproteins and immune cells? Some recent reports would tend to favour this view, for example: (a) the lectin-like oxidized low-density lipoprotein receptor-1 of macrophages was suggested to be a specific receptor for Hsp60 that binds the chaperonin via its C terminus (Xie et al. 2010); (b) immunization with a combination of Hsp60 and apolipoprotein-B peptide antigen significantly reduced early atherosclerotic lesions in a mouse model (Lu et al. 2010); (c) it has been postulated that cariogenic and periodontal pathogens can accelerate atherosclerosis in spontaneously hyperlipidemic mice by initiating inflammation (Zhang et al. 2010); and (d) Hsp60 was found to regulate some functions of oxidized LDL such as modulation of F-actin capping proteins involved in actin polymerization and macrophage motility (Dupont et al. 2008). These reports tend to coincide with our observations in as much as that circulating Hsp60 could play a role in chronic inflammation and could become target for therapeutic measures aiming at reducing the risk of atherogenic dyslipidemia and its complications. In this regard, our work shows that measuring the two blood parameters, i.e., Hsp60 and small dense LDL, along with periodontitis, is a promising strategy to predict risk for cardiovascular pathology. The data encourage further research on the possible role of Hsp60 and of small, dense LDL in the pathogenesis of cardiovascular disease. The data also imply that treatment of periodontitis (and by extension other mouth and throat infections) may have beneficial effects in what regards development of cardiovascular pathology.
Finally, although CRP levels were elevated in the patients group, they remained well below the levels that are considered to be indicators of a risk of cardiovascular disease. Also, we did not found any significant correlation between CRP levels and periodontal pathology, plaque index, or gingival bleeding index, suggesting a limited value of CRP levels in the prediction of periodontitis in our patients. No significant correlation was found between CRP and Hsp60 levels, either. In conclusion, we found that patients with mild periodontitis undisturbed by any previous medication show increased small, dense LDL and circulating Hsp60 concentrations as compared to matched controls. Correlation analysis revealed a very strong relationship between these two parameters, which were associated with periodontal pathology. Thus, our study opens the road for further investigation on the value of elevated levels of circulating Hsp60 as predictor of risk for cardiovascular disease when associated with dyslipidemia in periodontitis patients.
Acknowledgments
This work has been supported by University of Palermo (funds MR and FC) and IEMEST (Istituto Euro-Mediterraneo di Scienza e Tecnologia; funds FC and AJLM).
Footnotes
Manfredi Rizzo and Francesco Cappello contributed equally to the present work.
References
- Ainamo J, Bay I. Problems and proposal for recording gingivitis and placa. Dent J. 1975;25:229–235. [PubMed] [Google Scholar]
- Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–475. [PubMed] [Google Scholar]
- Arbes SJ, Slade GD, Beck JD. Association between extent of periodontal attachment loss and self-reported history of heart attack: an analysis of NHANES lll data. J Dent Res. 1999;78:1777–1782. doi: 10.1177/00220345990780120301. [DOI] [PubMed] [Google Scholar]
- Bengtsson T, Karlsson H, Gunnarsson P, Skoglund C, Elison C, Leanderson P, Lindahl M. The periodontal pathogen Porphyromonas gingivalis cleaves apoB-100 and increases the expression of apoM in LDL in whole blood leading to cell proliferation. J Intern Med. 2008;263:558–571. doi: 10.1111/j.1365-2796.2007.01917.x. [DOI] [PubMed] [Google Scholar]
- Buhlin K, Gustafsson A, Pockley AG, Frostegård J, Klinge B. Risk factors for cardiovascular disease in patients with periodontitis. Eur Heart J. 2003;24:2099–2107. doi: 10.1016/j.ehj.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Buhlin K, Hultin M, Norderyd O, Persson L, Pockley AG, Rabe P, Klinge B, Gustafsson A. Risk factors for atherosclerosis in cases with severe periodontitis. J Clin Periodontol. 2009;36:541–549. doi: 10.1111/j.1600-051X.2009.01430.x. [DOI] [PubMed] [Google Scholar]
- Bullon P, Morillo JM, Ramirez-Tortosa MC, Quiles JL, Newman HN, Battino M. Metabolic syndrome and periodontitis: is oxidative stress a common link? J Dent Res. 2009;88:503–518. doi: 10.1177/0022034509337479. [DOI] [PubMed] [Google Scholar]
- Cappello F, Conway de Macario E, Felice V, Zummo G, Macario AJL. Chlamydia trachomatis infection and anti-Hsp60 immunity: the two sides of the coin. PLoS Pathog. 2009;5:e1000552. doi: 10.1371/journal.ppat.1000552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JI, Chung SW, Kang HS, Rhim BY, Park YM, Kim US, Kim SJ. Epitope mapping of Porphyromonas gingivalis heat-shock protein and human heat-shock protein in human atherosclerosis. J Dent Res. 2004;83:936–940. doi: 10.1177/154405910408301209. [DOI] [PubMed] [Google Scholar]
- Choi J, Lee SY, Kim K, Choi BK. Identification of immunoreactive epitopes of the Porphyromonas gingivalis heat shock protein in periodontitis and atherosclerosis. J Periodontal Res. 2011;46:240–245. doi: 10.1111/j.1600-0765.2010.01339.x. [DOI] [PubMed] [Google Scholar]
- Cueto A, Mesa F, Bravo M, Ocaña-Riola R. Periodontitis as risk factor for acute myocardial infarction. A case control study of Spanish adults. J Periodontal Res. 2005;40:36–42. doi: 10.1111/j.1600-0765.2004.00766.x. [DOI] [PubMed] [Google Scholar]
- Cutler CW, Iacopino AM. Periodontal disease: links with serum lipid/triglyceride levels? Review and new data. J Int Acad Periodontol. 2003;5:47–51. [PubMed] [Google Scholar]
- D’Aiuto F, Sabbah W, Netuveli G, Donos N, Hingorani AD, Deanfield J, Tsakos G. Association of the metabolic syndrome with severe periodontitis in a large U.S. population-based survey. J Clin Endocrinol Metab. 2008;93:3989–3994. doi: 10.1210/jc.2007-2522. [DOI] [PubMed] [Google Scholar]
- Maio A. Extracellular heat shock proteins, cellular export vesicles, and the Stress Observation System: a form of communication during injury, infection, and cell damage. It is never known how far a controversial finding will go! Dedicated to Ferruccio Ritossa. Cell Stress Chaperones. 2011;16:235–249. doi: 10.1007/s12192-010-0236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Décary S, Dumont G, Lamarche B, Hogue JC, Tremblay AJ, Bergeron J, Couture P. Assessment of the validity of the frequently used lipid indices for predicting LDL peak particle diameter in a large cohort of 1955 normal and dyslipidemic subjects. Clin Biochem. 2010;43:401–406. doi: 10.1016/j.clinbiochem.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Dupont A, Chwastyniak M, Beseme O, Guihot AL, Drobecq H, Amouyel P, Pinet F. Application of saturation dye 2D-DIGE proteomics to characterize proteins modulated by oxidized low density lipoprotein treatment of human macrophages. J Proteome Res. 2008;7:3572–3582. doi: 10.1021/pr700683s. [DOI] [PubMed] [Google Scholar]
- Gardner CD, Fortmann SP, Krauss RM. Association of small low density lipoprotein particles with the incidence of coronary artery disease in men and women. JAMA. 1996;276:875–881. doi: 10.1001/jama.1996.03540110029028. [DOI] [PubMed] [Google Scholar]
- Gupta S, Knowlton AA. HSP60 trafficking in adult cardiac myocytes: role of the exosomal pathway. Am J Physiol Heart Circ Physiol. 2007;292:H3052–H3056. doi: 10.1152/ajpheart.01355.2006. [DOI] [PubMed] [Google Scholar]
- Kallio KA, Buhlin K, Jauhiainen M, Keva R, Tuomainen AM, Klinge B, Gustafsson A, Pussinen PJ. Lipopolysaccharide associates with pro-atherogenic lipoproteins in periodontitis patients. Innate Immun. 2008;14:247–253. doi: 10.1177/1753425908095130. [DOI] [PubMed] [Google Scholar]
- Knoflach M, Bernhard D, Wick G. Anti-HSP60 immunity is already associated with atherosclerosis early in life. Ann N Y Acad Sci. 2005;1051:323–331. doi: 10.1196/annals.1361.074. [DOI] [PubMed] [Google Scholar]
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- Linhares IM, Witkin SS. Immunopathogenic consequences of Chlamydia trachomatis 60 kDa heat shock protein expression in the female reproductive tract. Cell Stress Chaperones. 2010;15:467–473. doi: 10.1007/s12192-010-0171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Chen D, Endresz V, Xia M, Faludi I, Burian K, Szabo A, Csanadi A, Miczak A, Gonczol E, Kakkar V. Immunization with a combination of ApoB and HSP60 epitopes significantly reduces early atherosclerotic lesion in Apobtm2SgyLdlrtm1Her/J mice. Atherosclerosis. 2010;212:472–480. doi: 10.1016/j.atherosclerosis.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Macario AJL, Conway de Macario E. Sick chaperones, cellular stress, and disease. N Engl J Med. 2005;353:1489–1501. doi: 10.1056/NEJMra050111. [DOI] [PubMed] [Google Scholar]
- Macario AJL, Cappello F, Zummo G, Conway de Macario E. Chaperonopathies of senescence and the scrambling of interactions between the chaperoning and the immune systems. Ann NY Acad Sci. 2010;1197:85–93. doi: 10.1111/j.1749-6632.2010.05187.x. [DOI] [PubMed] [Google Scholar]
- Merendino AM, Bucchieri F, Campanella C, Marcianò V, Ribbene A, David S, Zummo G, Burgio G, Corona DF, Conway de Macario E, Macario AJL, Cappello F. Hsp60 is actively secreted by human tumor cells. PLoS One. 2010;5:e9247. doi: 10.1371/journal.pone.0009247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne P, Laroche P, Cuilliere ML, Varcin P, Duheille J. Microparticleenhanced nephelometric immunoassay for human C- reactive protein. J Clin Lab Anal. 1992;1:24–29. doi: 10.1002/jcla.1860060106. [DOI] [PubMed] [Google Scholar]
- Nagele U, Hagele EO, Sauer G, Wiedemann E, Lehmann P, Wahlefeldet AW, et al. Reagent for the enzymatic determination of serum total triglycerides with improved lipolytic efficiency. J Clin Chem Clin Biochem. 1984;22:165–174. doi: 10.1515/cclm.1984.22.2.165. [DOI] [PubMed] [Google Scholar]
- Novo G, Cappello F, Rizzo M, Fazio G, Zambuto S, Tortorici E, Marino Gammazza A, Corrao S, Zummo G, Conway de Macario E, Macario AJL, Assennato P, Novo S, Li Volti G. Hsp60 and Heme Oxygenase-1 (Hsp32) in acute myocardial infarction. Transl Res. 2011;157:285–292. doi: 10.1016/j.trsl.2011.01.003. [DOI] [PubMed] [Google Scholar]
- O'Leary TJ, Drake RB, Naylor JE. The plaque control record. J Periodontol. 1972;43:38–40. doi: 10.1902/jop.1972.43.1.38. [DOI] [PubMed] [Google Scholar]
- Pfister G, Stroh CM, Perschinka H, Kind M, Knoflach M, Hinterdorfer P, Wick G. Detection of HSP60 on the membrane surface of stressed human endothelial cells by atomic force and confocal microscopy. J Cell Sci. 2005;118:1587–1594. doi: 10.1242/jcs.02292. [DOI] [PubMed] [Google Scholar]
- Pockley AG, Multhoff G. Cell stress proteins in extracellular fluids: friend or foe? Novartis Found Symp. 2008;291:86–95. doi: 10.1002/9780470754030.ch7. [DOI] [PubMed] [Google Scholar]
- Pockley AG, Bulmer J, Hanks BM, Wright BH. Identification of human heat shock protein 60 (Hsp60) and anti-Hsp60 antibodies in the peripheral circulation of normal individuals. Cell Stress Chaperones. 1999;4:29–35. doi: 10.1379/1466-1268(1999)004<0029:IOHHSP>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pockley AG, Wu R, Lemne C, Kiessling R, Faire U, Frostegård J. Circulating heat shock protein 60 is associated with early cardiovascular disease. Hypertension. 2000;36:303–307. doi: 10.1161/01.hyp.36.2.303. [DOI] [PubMed] [Google Scholar]
- Pockley AG, Muthana M, Calderwood SK. The dual immunoregulatory roles of stress proteins. Trends Biochem Sci. 2008;33:71–79. doi: 10.1016/j.tibs.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Rizzo M, Berneis K. Low-density-lipoproteins size and cardiovascular risk assessment. QJM—Int J Med. 2006;99:1–14. doi: 10.1093/qjmed/hci154. [DOI] [PubMed] [Google Scholar]
- Rizzo M, Kotur-Stevuljevic J, Berneis K, Spinas GA, Rini GB, Jelic-Ivanovic Z, Spasojevic-Kalimanovska V, Vekic J. Atherogenic dyslipidemia and oxidative stress: a new look. Transl Res. 2009;153:217–223. doi: 10.1016/j.trsl.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Rufail ML, Schenkein HA, Barbour SE, Tew JG, Antwerpen R. Altered lipoprotein subclass distribution and PAF-AH activity in subjects with generalized aggressive periodontitis. J Lipid Res. 2005;46:2752–2760. doi: 10.1194/jlr.M500389-JLR200. [DOI] [PubMed] [Google Scholar]
- Saito T, Shimazaki Y, Kiyohara Y, Kato I, Kubo M, Iida M, Koga T. The severity of periodontal disease is associated with the development of glucose intolerance in non-diabetics: the Hisayama study. J Dent Res. 2004;83:485–490. doi: 10.1177/154405910408300610. [DOI] [PubMed] [Google Scholar]
- Scannapieco FA, Bush RB, Paju S. Associations between periodontal disease and risk for atherosclerosis, cardiovascular disease, and stroke. A systematic review. Ann Periodontol. 2003;8:38–53. doi: 10.1902/annals.2003.8.1.38. [DOI] [PubMed] [Google Scholar]
- Shamaei-Tousi A, D'Aiuto F, Nibali L, Steptoe A, Coates AR, Parkar M, Donos N, Henderson B. Differential regulation of circulating levels of molecular chaperones in patients undergoing treatment for periodontal disease. PLoS One. 2007;2:e1198. doi: 10.1371/journal.pone.0001198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Pierre AC, Cantin B, Dagenais GR, Mauriege P, Bernard PM, Despres JP, et al. Low-density lipoprotein subfractions and the long term risk of ischemic heart disease in men: 13-year follow-up data from the Quebec Cardiovascular Study. Arterioscler Thromb Vasc Biol. 2005;25:553–559. doi: 10.1161/01.ATV.0000154144.73236.f4. [DOI] [PubMed] [Google Scholar]
- Tabeta K, Yamazaki K, Hotokezaka H, Yoshie H, Hara K. Elevated humoral immune response to heat shock protein 60 (hsp60) family in periodontitis patients. Clin Exp Immunol. 2000;120:285–293. doi: 10.1046/j.1365-2249.2000.01216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor BA, Tofler GH, Carey HM, Morel-Kopp MC, Philcox S, Carter TR, Elliott MJ, Kull AD, Ward C, Schenck K. Full-mouth tooth extraction lowers systemic inflammatory and thrombotic markers of cardiovascular risk. J Dent Res. 2006;85:74–78. doi: 10.1177/154405910608500113. [DOI] [PubMed] [Google Scholar]
- Tonetti MS, D’Aiuto F, Nibali L, Donald A, Storry C, Parkar M, Suvan J, Hingorani AD, Vallance P, Deanfield J. Treatment of periodontitis and endothelial function. N Engl J Med. 2007;356:911–920. doi: 10.1056/NEJMoa063186. [DOI] [PubMed] [Google Scholar]
- Tribble DL, Rizzo M, Chait A, Lewis DM, Blanche PJ, Krauss RM. Enhanced oxidative susceptibility and reduced antioxidant content of metabolic precursors of small dense low-density lipoproteins. Am J Med. 2001;110:103–110. doi: 10.1016/S0002-9343(00)00700-2. [DOI] [PubMed] [Google Scholar]
- Warnick GR, Nguyen T, Albers JJ. Comparison of improved precipitation methods for quantification of high density lipoprotein cholesterol. Clin Chem. 1985;31:217–222. [PubMed] [Google Scholar]
- Wierzbicki AS. Quality as well as quantity? Beyond low-density lipoprotein-cholesterol – the role of particle size. Int J Clin Pract. 2007;61:1780–1782. doi: 10.1111/j.1742-1241.2007.01571.x. [DOI] [PubMed] [Google Scholar]
- Williams RC. Periodontal disease. N Engl J Med. 1990;322:373–382. doi: 10.1056/NEJM199002083220606. [DOI] [PubMed] [Google Scholar]
- Xie J, Zhu H, Guo L, Ruan Y, Wang L, Sun L, Zhou L, Wu W, Yun X, Shen A, Gu J. Lectin-like oxidized low-density lipoprotein receptor-1 delivers heat shock protein 60-fused antigen into the MHC class I presentation pathway. J Immunol. 2010;185:2306–2313. doi: 10.4049/jimmunol.0903214. [DOI] [PubMed] [Google Scholar]
- Yamazaki K, Ohsawa Y, Tabeta K, Ito H, Ueki K, Oda T, Yoshie H, Seymour GJ. Accumulation of human heat shock protein 60-reactive T cells in the gingival tissues of periodontitis patients. Infect Immun. 2002;70:2492–2501. doi: 10.1128/IAI.70.5.2492-2501.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki K, Ohsawa Y, Itoh H, Ueki K, Tabeta K, Oda T, Nakajima T, Yoshie H, Saito S, Oguma F, Kodama M, Aizawa Y, Seymour GJ. T-cell clonality to Porphyromonas gingivalis and human heat shock protein 60s in patients with atherosclerosis and periodontitis. Oral Microbiol Immunol. 2004;19:160–167. doi: 10.1111/j.0902-0055.2004.00134.x. [DOI] [PubMed] [Google Scholar]
- Zhang X, He M, Cheng L, Chen Y, Zhou L, Zeng H, Pockley AG, Hu FB, Wu T. Elevated heat shock protein 60 levels are associated with higher risk of coronary heart disease in Chinese. Circulation. 2008;118:2687–2693. doi: 10.1161/CIRCULATIONAHA.108.781856. [DOI] [PubMed] [Google Scholar]
- Zhang T, Kurita-Ochiai T, Hashizume T, Du Y, Oguchi S, Yamamoto M. Aggregatibacter actinomycetemcomitans accelerates atherosclerosis with an increase in atherogenic factors in spontaneously hyperlipidemic mice. FEMS Immunol Med Microbiol. 2010;59:143–151. doi: 10.1111/j.1574-695X.2010.00674.x. [DOI] [PubMed] [Google Scholar]