Abstract
Adulthood weight gain predicts estrogen receptor-positive breast cancer. Because local estrogen excess in the breast likely contributes to cancer development, and aromatase is the key enzyme in estrogen biosynthesis, we investigated the role of local aromatase expression in weight gain-associated breast cancer risk in a humanized aromatase (Aromhum) mouse model containing the coding region and the 5′-regulatory region of the human aromatase gene. Compared with littermates on normal chow, female Aromhum mice on a high fat diet gained more weight, and had a larger mammary gland mass with elevated total human aromatase mRNA levels via promoters I.4 and II associated with increased levels of their regulators TNFα and C/EBPβ. There was no difference in total human aromatase mRNA levels in gonadal white adipose tissue. Our data suggest that diet-induced weight gain preferentially stimulates local aromatase expression in the breast, which may lead to local estrogen excess and breast cancer risk.
Keywords: aromatase, overweight, weight gain, obesity, mammary, breast cancer
1. Introduction
The prevalence of overweight (defined as a BMI between 25.0 and 29.9 kg/m2) and obesity (defined as a BMI ≥30 kg/m2) have reached epidemic proportions in many parts of the world. In the United States from 2007 to 2008, 32.2% of adult men and 35.5% of adult women were obese (Flegal et al., 2010). The rates of overweight and obesity combined were 72.3% for adult men and 64.1% for adult women (Flegal et al., 2010). Obesity is a risk factor for breast cancer in postmenopausal women. Postmenopausal obese women have 1.5 times the risk of breast cancer compared with women at healthy weights (Trentham-Dietz et al., 1997; Yoo et al., 2001). In addition, weight gain during adulthood (age >18 years) is a consistent and strong predictor of estrogen receptor (ER)-positive breast cancer risk in postmenopausal women as well as premenopausal women of Hispanic ethnicity (Huang et al., 1997; Wenten et al., 2002).
The mechanisms underlying obesity and adulthood weight gain-associated breast cancer risk are unclear. Increased circulating levels of estrogen, possibly through increased fat mass and increased aromatase expression in subcutaneous fat tissue, are believed to play a role (Key et al., 2003). However, a randomized WHI study showing a possible reduction in breast cancer risk in postmenopausal women given estrogen-only hormone replacement challenged the role of circulating estrogen in breast cancer risk (Anderson et al., 2004). Alternatively, adulthood weight gain may elevate breast cancer risk by inducing local aromatase expression in the breast.
By converting androgens to estrogens, aromatase is a key enzyme in estrogen biosynthesis (Simpson et al., 2005). In breast cancer, breast adipose fibroblasts adjacent to breast tumors display high aromatase expression, leading to high local levels of estrogen that probably further promote breast cancer development and progression. Aromatase inhibitors are by far the most effective endocrine treatment for ER-positive breast cancer in postmenopausal women (Baum et al., 2002; Bulun et al., 1993; O’Neill et al., 1988). Multiple promoters regulate the transcription of the human aromatase gene in a partially tissue-specific fashion (Bulun et al., 2005), indicated by the presence of alternatively used unique 5′-untranslated first exons immediately downstream of the different aromatase promoters. In disease-free breast adipose tissue, low activity of promoter I.4 maintains low levels of total aromatase mRNA. In adipose tissue adjacent to a breast tumor, the proximal aromatase promoters I.3 and II are coordinately activated, leading to high levels of total aromatase mRNA (Chen et al., 2009). In cultured primary human breast adipose fibroblasts, TNFα, which is highly upregulated in adipose tissue of obese humans and mice (Hotamisligil et al., 1993; Hotamisligil et al., 1995), stimulates aromatase promoter I.4 in the presence of glucocorticoids such as dexamethasone (Zhao et al., 1996). Transcription factors C/EBPβ, JunB, and JunD, among others, are essential for activation of aromatase promoters I.3/II (Chen et al., 2011; Zhou et al., 2001).
The tissue distribution patterns of aromatase expression in humans and mice are drastically distinct (Bulun et al., 2005; Zhao et al., 2009). In female mice, aromatase is expressed only in the ovaries and the brain via 2 promoters, whereas women use at least 10 distinct promoters to express aromatase in many peripheral tissues including breast fat. To create an in vivo experimental model to study aromatase as a potential link between adulthood weight gain and breast cancer risk in women, we generated a humanized aromatase (Aromhum) mouse line that harbors both the coding region and more than 78 kb of the 5′-regulatory region of the human aromatase gene encompassing aromatase promoters I.4, I.7, I.f, I.6, and I.3/II. Both female and male Aromhum mice express aromatase in a humanized pattern in their peripheral (extragonadal) tissues, such as adipose tissue, muscle, and bone. In particular, female Aromhum mice express aromatase in mammary gland using the appropriate human promoters. In this study, we examined change in human aromatase expression in mammary gland of overweight female Aromhum mice fed a high fat diet vs lean littermates on normal chow. The advantage of using this novel Aromhum mouse model is that such a change in aromatase expression is more likely to mirror that in the breast tissue of women in response to adulthood weight gain.
2. Materials and methods
2.1. Generation and diet treatment of Aromhum mice
Aromhum mice were generated in the FVB/N background as described (see the attached unpublished manuscript). At 3 months of age, female Aromhum mice were randomized to receive a normal chow diet (Harlan 7912, 17% kcal from fat) or a high fat diet (Harlan TD 93075, 55% kcal from fat). Mice were fed these diets ad libitum, and their body weights were measured for 3 months before being sacrificed for tissue collection. The entire gonadal white adipose tissue pad and one intact fourth mammary gland from each mouse were weighed and immediately subjected to RNA extraction with Tri-reagent (Sigma, St. Louis, MO). The other intact fourth mammary gland was snap frozen in liquid nitrogen, stored at −80oC, and processed for protein extract preparation. The animal protocol was approved by the Institutional Animal Care and Use Committee at Northwestern University.
2.2. Real-time RT-PCR
Total RNA was isolated with Tri-reagent and reverse transcribed with the Superscript III cDNA synthesis system (Invitrogen, Carlsbad, CA). Taqman-based real-time PCR to quantify total and promoter I.4-specific human aromatase mRNA levels and SYBR green-based real-time PCR to quantify promoter II-specific human aromatase mRNA levels were described previously (Chen et al., 2011). For mouse GAPDH mRNA (control), the forward and reverse primers were 5′-TGT GTC CGT CGT GGA TCT GA-3′ and 5′-CCT GCT TCA CCA CCT TCT TGA-3′, and the fluorescence-labeled probe was 5′-CCG CCT GGA GAA ACC TGC CAA GTA TG-3′. Mouse TNFα, C/EBPβ, JunB, and JunD mRNA levels were quantified by SYBR green-based real-time PCR with primers purchased from QIAGEN (Valencia, CA).
2.3. Semi-quantitative PCR
Semi-quantitative PCR of aromatase promoter II- and I.4-specific transcripts used the common reverse primer 5′-CAG AGA TCC AGA CTC GCA TG-3′ and the following forward primers: promoter II: 5′-GCA ACA GGA GCT ATA GAT GAA C-3′ and promoter I.4: 5′-GTA GAA CGT GAC CAA CTG GAG-3′. The forward and reverse primers for mouse GAPDH transcript were 5′-AAC TTT GGC ATT GTG GAA GGG CTC-3′ and 5′-ACC CTG TTG CTG TAG CCG TAT TCA-3′, respectively.
2.4. Mammary gland protein extract preparation and immunoblotting
Tissue homogenates were prepared with ice-cold homogenization buffer containing 20 mM Tris-HCl (pH 7.4), 10 mM sodium vanadate, 50 mM sodium pyrophosphate, 100 mM sodium fluoride, 10 mM EDTA, 10 mM EGTA, 2 mM phenylmethylsulfonyl fluoride, 2 μM pepstatin A, 10 μg/ml aprotinin, and 10 μM leupeptin. For immunoblotting, anti-C/EBPβ (Cell Signaling Technology, Beverly, MA) and anti-actin (Sigma) antibodies were used as indicated, followed by a horseradish peroxidase-conjugated secondary antibody (Cell Signaling Technology). All immunoblots were developed with ECL reagents (Thermo Fisher Scientific, Rockford, IL).
2.5. Immunohistochemistry
Intact fourth mammary glands were removed from Aromhum mice, fixed in 4% phosphate-buffered paraformaldehyde overnight, and transferred to 70% ethanol. The mammary glands were then embedded in paraffin and sectioned at 5 μm. After deparaffinization, rehydration, and antigen retrieval by heating in antigen unmasking solution (Vector Laboratories Burlingame, CA), an anti-human aromatase antibody (1:100; AbD Serotec, Oxford, UK) was applied to the sections. After incubation at 4°C overnight, the sections were washed with PBS and incubated with a biotinylated horse-anti-mouse secondary antibody (1:600; Vector Laboratories, CA) at 37°C for 30 min followed by avidin-biotin-horseradish peroxidase complex (ABC/HRP; Vector, Laboratories, CA). Sections were examined using a Zeiss Axio Scope microscope (Zeiss, Goettingen, Germany).
3. Results
3.1. High fat diet induces weight gain and increases mammary gland mass in female Aromhum mice
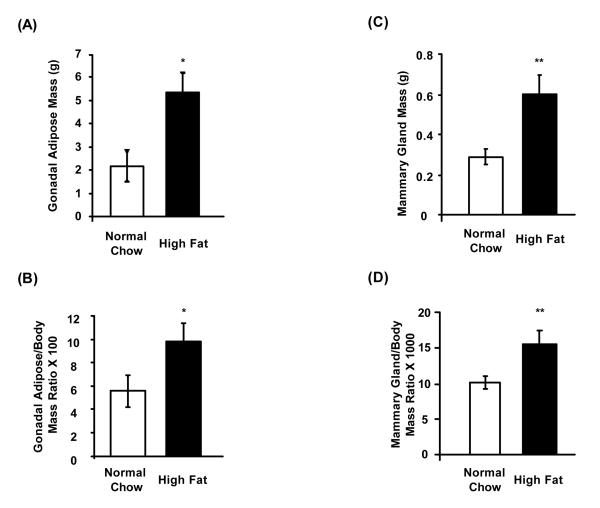
We fed 3-month old female Aromhum mice either a normal chow diet or a high fat diet for 3 months, and monitored body weight (Fig. 1). As expected, Aromhum mice on a high fat diet significantly gained more weight than their littermates on a normal chow diet. In addition, Aromhum mice on a high fat diet became hyperglycemic, with their fed glucose levels significantly higher than those on a normal chow diet (325.3±30.7 vs 214.3±11.3 mg/dl, mean±SE, P<0.05), and had larger absolute and relative gonadal white adipose tissue mass normalized to body mass (Fig. 2A and 2B, P<0.05). More importantly, female Aromhum mice on a high fat diet had a 2-fold higher mammary gland mass that was also disproportionately higher than their whole body weight gain when compared with littermates fed the normal chow diet (Fig. 2C and 2D, P<0.01). These data demonstrate the effect of high fat diet on fat deposition in gonad and mammary gland.
Figure 1.
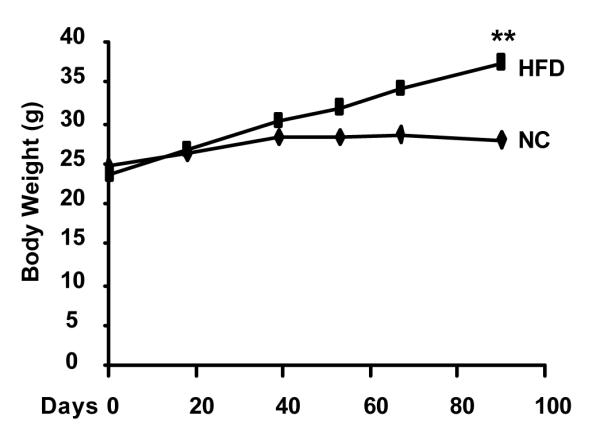
High fat diet induces weight gain in female Aromhum mice. Three-month-old female Aromhum mice were fed a normal chow (NC, n=8) diet or a high fat diet (HFD, n=7) for 3 months, and their body weights were measured on days 0, 18, 39, 53, 67, and 90. **, P<0.01 (paired t test).
Figure 2.
High fat diet increases gonadal white adipose tissue and mammary gland mass disproportionate to weight gain in female Aromhum mice. At the end of the 3-month diet treatment period, female Aromhum mice were sacrificed and their intact gonadal adipose tissue and fourth mammary glands were removed and weighed. (A) Average absolute mass of gonadal white adipose tissue. (B) Average mass of gonadal white adipose tissue relative to total body mass. Normal chow group, n=4; high fat group, n=4. *, P<0.05 (paired t test). (C) Average absolute mass of mammary glands. (D) Average mass of mammary glands relative to total body mass. Normal chow group, n=8; high fat group, n=7. **, P<0.01 (paired t test).
3.2. High fat diet-fed, overweight female Aromhum mice exhibit increased human aromatase mRNA levels in mammary gland
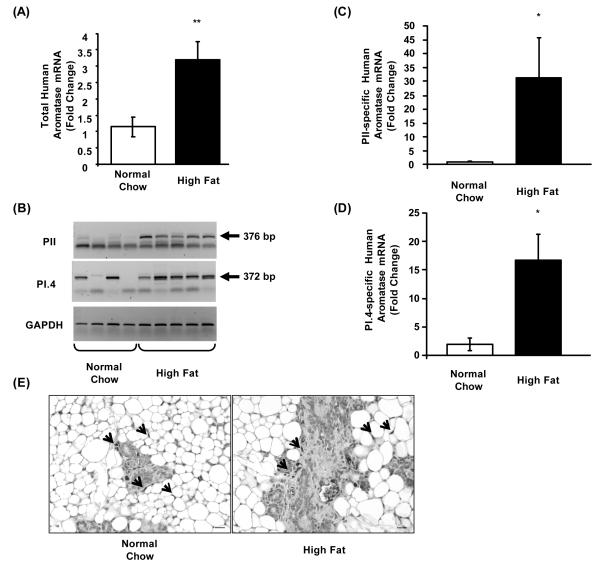
Using real-time PCR, we observed 3-fold higher human aromatase mRNA levels in the mammary gland of female Aromhum mice fed a high fat diet vs. normal chow (Fig. 3A, P<0.01). The mammary gland of female Aromhum mice did not express measurable mouse aromatase mRNA (unpublished observation).
Figure 3.
High fat diet induces human aromatase mRNA expression via activation of promoters II and I.4 in mammary gland of overweight female Aromhum mice. After the 3-month diet treatment, mammary glands of female Aromhum mice were removed and subjected to RNA extraction, followed by (A) real-time RT-PCR to quantify total human aromatase mRNA levels, (B) semi-quantitative PCR to quantify promoter II (PII) and I.4 (PI.4)-specific human aromatase and mouse GAPDH mRNA levels, and real-time RT-PCR to verify PII (C) and PI.4-specific (D) human aromatase mRNA levels. The arrows indicate the specific PCR product bands with the expected sizes shown. Normal chow group, n=4; high fat diet group, n=5. *, P<0.05; **, P<0.01 (paired t test). (E) Immunohistochemical localization of human aromatase in the mammary glands of Aromhum mice on normal chow or a high fat diet. The images shown are representative of 3 mice in each group. Arrows indicate human aromatase positive cells.
To determine which human breast adipose-specific aromatase promoters were activated in the high fat diet-fed mice, we performed first exon-specific semi-quantitative PCR. Promoter II-specific aromatase mRNA levels were barely detectable in the mammary gland of Aromhum mice fed a normal chow diet, but were markedly increased in the mammary gland of Aromhum mice on a high fat diet (Fig. 3B). Likewise, promoter-I.4 specific aromatase mRNA levels were strikingly higher in the mammary gland of Aromhum mice fed a high fat diet compared with mice fed normal chow (Fig. 3B). Real-time RT-PCR revealed that the differences in promoters II and I.4-driven mRNA levels were statistically significant (Fig. 3C and 3D, P<0.05). We were able to occasionally detect the presence of promoter I.3-specific aromatase mRNA in Aromhum mammary gland on a normal chow diet, and its levels were not different in the mammary gland of Aromhum mice on a high fat diet (data not shown). Additionally, we performed immunohistochemistry to localize human aromatase expression within the mammary gland of Aromhum mice (Fig. 3E). We detected human aromatase immunoreactivity in undifferentiated adipose fibroblasts and myoepithelial cells. However, we discerned no apparent difference in the intensity of human aromatase staining between the normal chow and high fat diet-fed Aromhum mouse groups. Taken together, these data indicate that high fat diet-induced increase in mammary gland mass is accompanied by a significant increase in human aromatase mRNA via activation of promoters II and I.4 in Aromhum mice.
3.3. High fat diet-fed, overweight Aromhum mice exhibit increased TNFα and C/EBPβ expression in mammary gland
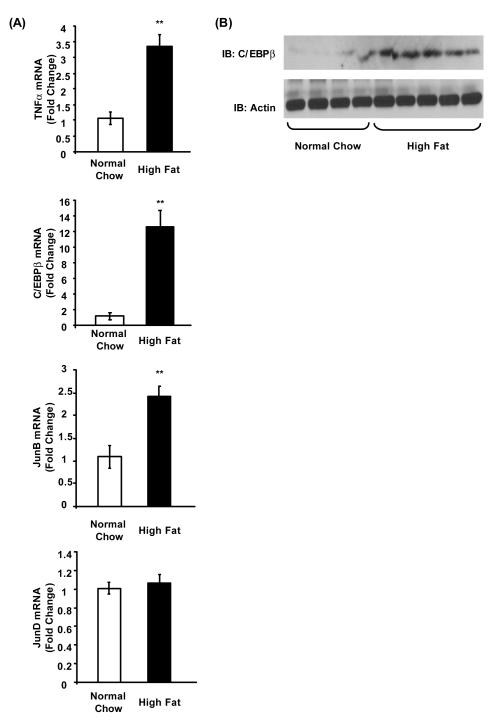
TNFα, in the presence of dexamethasone, stimulates aromatase promoter I.4 in human breast adipose fibroblasts, and its expression is generally higher in adipose tissue of obese vs. normal weight humans and mice (Hotamisligil et al., 1993; Hotamisligil et al., 1995; Zhao et al., 1996). Thus, we compared the levels of TNFα mRNA in mammary gland of Aromhum mice fed a high fat diet vs. a normal chow diet. Mammary gland TNFα levels in Aromhum mice on a high fat diet were 3.5-times those found in Aromhum mice on a normal chow diet (Fig. 4A, P<0.01).
Figure 4.
High fat diet induces TNFα and C/EBPβ expression in mammary gland of overweight female Aromhum mice. After the 3-month diet treatments, mammary glands of female Aromhum mice were removed and subjected to (A) RNA extraction and real-time RT-PCR to quantify mouse TNFα, C/EBPβ, JunB, and JunD mRNA levels, and (B) tissue protein extract preparation and immunoblotting with anti-C/EBPβ and anti-actin antibodies. Normal chow group, n=4; high fat diet group, n=5. **, P<0.01 (paired t test).
Multiple transcription factors, including C/EBPβ, JunB, and JunD are reported to be involved in activation of human aromatase promoter II in human breast adipose fibroblasts (Chen et al., 2011; Zhou et al., 2001). We found that there was a marked, 11-fold increase in C/EBPβ mRNA (P<0.01); a slight, but statistically significant, 1-fold increase in JunB mRNA (P<0.01); and no change in JunD mRNA in the mammary gland of Aromhum mice fed a high fat diet compared with the mammary gland from littermates on a normal chow diet (Fig. 4A). By immunoblot, we also observed a marked increase in C/EBPβ protein levels (Fig. 4B), but not in JunB levels (data not shown) in the mammary gland of Aromhum mice fed a high fat diet vs. a normal chow diet. Taken together, our data indicate that weight gain-induced increase in human aromatase mRNA levels via promoters I.4 and II is associated with increased levels of their respective regulators TNFα and C/EBPβ.
3.4. High fat diet-fed, overweight female Aromhum mice exhibit no change in human aromatase mRNA level in gonadal white adipose tissue
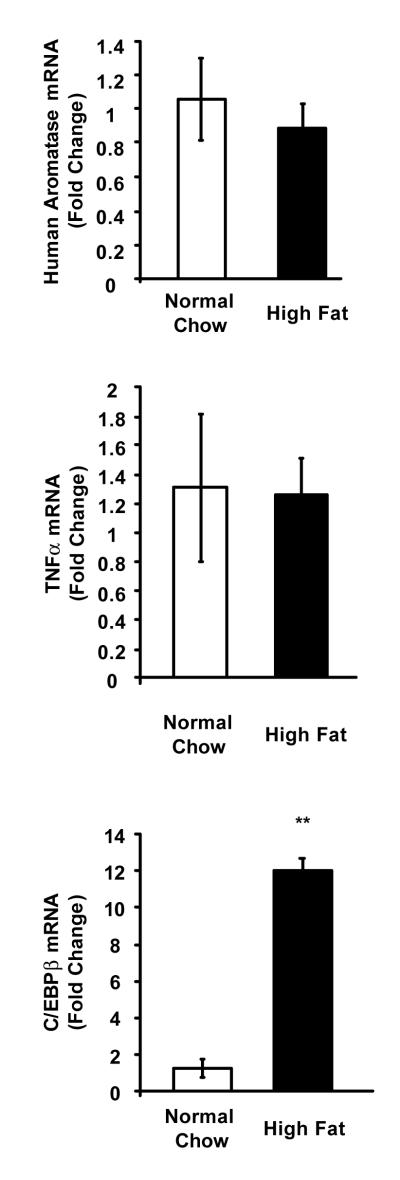
To determine whether high fat diet-fed overweight female Aromhum mice also had increased aromatase mRNA levels in adipose tissue outside the mammary gland, we isolated gonadal white adipose tissue from Aromhum mice on a normal chow diet or a high fat diet, and likewise quantified mRNA levels of human aromatase, mouse TNFα and C/EBPβ. There was no difference in aromatase or TNFα mRNA levels, but C/EBPβ mRNA levels were significantly higher (P<0.01) in overweight Aromhum mice fed a high fat diet compared to mice fed normal chow (Fig. 5). These data suggest that increased C/EBPβ expression is not sufficient to induce aromatase expression, and that human aromatase gene expression in response to high fat diet-induced weight gain is distinct between mammary/breast fat and fat tissue at other body sites.
Figure 5.
High fat diet does not increase human aromatase mRNA levels in gonadal white adipose tissue of overweight female Aromhum mice. After the 3-month diet treatments, gonadal white adipose tissue of female Aromhum mice was removed and subjected to real-time RT-PCR to quantify total human aromatase, mouse TNFα, and mouse C/EBPβ mRNA levels. Normal chow group, n=3; high fat diet group, n=3. **, P<0.01 (paired t test).
4. Discussion
In this study we described that, in mammary gland of overweight female Aromhum mice fed a high fat diet compared with lean littermates fed normal chow, human aromatase mRNA levels were increased via activation of promoters I.4 and II associated with increased levels of their respective regulators TNFα and C/EBPβ. The sites of human aromatase expression in the mammary glands of lean and overweight Aromhum mice were identical, and included undifferentiated adipose fibroblasts and myoepithelial cells. Our findings suggest that aromatase may be a molecular link between adulthood weight gain and increased breast cancer risk among pre- and postmenopausal women.
Subbaramaiah et al. (2011) recently reported increased endogenous aromatase expression in the mammary gland of C57BL/6J mice fed a high fat diet as compared to mice fed a low fat diet, but did not identify the mouse aromatase promoters that were activated (Subbaramaiah et al., 2011). Although we were not able to detect endogenous aromatase expression in adipose tissue and mammary gland of female Aromhum mice (unpublished observations), possibly due to difference in the mouse strains used (FNB/N vs. C57BL/6J), our results are consistent with their findings. Moreover, because the female Aromhum mouse model has similar human aromatase tissue distribution as women, our results support a causal relationship between body weight gain and breast cancer risk through local aromatase in humans. One indication of this relationship may be the effect of obesity on the clinical benefit from aromatase inhibitors in estrogen receptor (ER)-positive breast cancer treatment. Sestak et al (2010) demonstrated that women with a higher BMI had more recurrences of breast cancer after treatment with an aromatase inhibitor (Sestak et al., 2010). The mechanism for this important clinical observartion may be complex and is unclear at the moment. Our findings suggest that high breast tissue aromatase expression associated with overweight and obesity may account, at least in part, for the lower efficacy of aromatase inhibitor treatment in high BMI women. Thus, higher doses or more potent aromatase inhibitors may be required for overweight or obese women with breast cancer.
By real-time PCR, we found that overweight female Aromhum mice had 3-fold higher human aromatase mRNA levels in their mammary gland compared with their lean littermates. The levels of human aromatase mRNA quantified were relative amounts per unit mass of mRNA, normalized to mRNA levels of the endogenous housekeeping gene GAPDH. Because overweight female Aromhum mice had twice the mammary gland mass as lean controls, in absolute terms, the overweight Aromhum mice could have up to 6-fold higher human aromatase mRNA in their mammary gland compared with lean counterparts. However, immunohistochemistry did not show a difference in human aromatase protein level between the mammary glands of normal chow and high fat diet-fed Aromhum mice, likely due to its limited quantification power.
Both semi-quantitative and quantitative real-time PCRs revealed that promoters I.4 and II were activated in mammary gland of overweight female Aromhum mice, correlating with marked increases in TNFα and C/EBPβ expression levels. In the presence of dexamethasone, TNFα stimulates aromatase promoter I.4 in primary human breast adipose fibroblasts (Zhao et al., 1996). Additionally, Ghosh et al. (2009) found that high cell density of primary human adipose fibroblasts increases the expression of C/EBPβ, which in turn mediates density-induced aromatase promoter II activation, suggesting that C/EBPβ may play a role in mammographic density- and obesity-associated breast cancer risk by stimulating aromatase expression (Ghosh et al., 2009).
The higher fold increases in promoter I.4- and II-specific aromatase mRNA than that in total aromatase mRNA suggests a possibility that the promoter I.4- and II-specific transcripts make up a minority of total aromatase transcripts present in the mammary gland of high fat diet-fed Aromhum mice, i.e. that additional aromatase promoters may be activated, although the cellular sites of human aromatase expression remain unchanged. However, such a direct comparison among the results of these disparate real-time PCRs needs caution, because of the differences in the assay methodology used (Taqman-based for total and promoter I.4-specific aromatase mRNA vs. SYBR green-based for promoter II-specific aromatase mRNA) and assay sensitivity affected by the real-time PCR mechanism, the primer pair and probe selections, and the absolute amount of target mRNA species. Absolute real-time quantification may overcome at least some of the above limitations, however, the internal standards it requires for constructing standard curves are not available.
In contrast to mammary tissue, gonadal white adipose tissue of overweight female Aromhum mice exhibited no difference in total human aromatase or TNFα mRNA levels compared with those of their lean littermates, despite increased C/EBPβ mRNA levels, suggesting that C/EBPβ is not sufficient for aromatase induction. Hotamisligil et al (1993) showed that TNFα was strongly induced in epididymal white adipose tissue of many obese male rodent models, such as db/db, ob/ob, and tub/tub mice and fa/fa rats, with the exception of monosodium glutamate (MSG)-induced obese mice, likely because obesity in the MSG model was less severe than that of the genetic models as the authors concluded (Hotamisligil et al., 1993). Thus, the absence of TNFα induction in the gonadal white adipose tissue of our high fat diet-fed Aromhum mice is not too surprising, because the mice were only moderately obese, and more precisely, overweight, as compared to the lean littermates on normal chow; at 6 months of age when they were sacrificed, the high fat diet-fed female Aromhum mice weighed 37.3±6.2 g (mean±SD), whereas their littermates on normal chow weighed 28.0±4.0 g (mean±SD) (Fig. 1). The body weight differential between the two groups was 9.3 g, or 2.3 SDs of the control group. In comparison, according to data from the Jackson Laboratory, female db/db mice weighed 52.8±4.7 g (mean±SD) at 5 months (the oldest age when the body weight was recorded), whereas wild type littermates of the same age weighed 25.1±1.9 g (mean±SD). The body weight differential increased to 27.7 g, or 14.6 SDs of the control group.
The increase in human aromatase expression in mammary gland was relatively modest in our high fat diet-fed, ovary-intact Aromhum mice, which mimic overweight, premenopausal women. Normal to slightly elevated circulating levels of estrogen in the mice probably suppress aromatase expression in the mammary gland via feedback inhibition (see the attached unpublished manuscript). Subbaramaiah et al. (2011) observed more severe body weight gain, higher levels of TNFα, and stronger induction of aromatase in mammary gland of ovariectomized wild type C57BL/6J mice fed a high fat diet than in ovary-intact littermates fed the same high fat diet (Subbaramaiah et al., 2011). This may explain why adulthood weight gain-associated breast cancer risk is more pronounced and more prevalent in postmenopausal women of all ethnicities (Huang et al., 1997; Wenten et al., 2002). Therefore, participation in diet and weight loss programs may benefit premenopausal overweight women by reducing breast cancer risk via a reduction in local aromatase production in the breast, and aromatase inhibitors may be particularly effective in breast cancer prevention among postmenopausal overweight and obese women.
Highlights.
Compared with littermates on normal chow, female humanized aromatase (Aromhum) mice on a high fat diet
gained more weight and had a larger mammary gland mass
had elevated human aromatase mRNA levels via promoters I.4 and II in mammary gland
expressed human aromatase at same sites: adipose fibroblasts & myoepithelial cells
had increased TNFα and C/EBPβ expression in mammary gland
exhibited no change in human aromatase mRNA levels in gonadal white adipose tissue
Acknowledgments
Funding This work was supported by grants from NIH (CA67167) and the Lynn Sage Cancer Research Foundation of Northwestern Memorial Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Summary: The authors have nothing to disclose.
References
- Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O’Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Van Horn L, Wactawski-Wende J, Wallace R, Wassertheil-Smoller S. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. Jama. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- Baum M, Budzar AU, Cuzick J, Forbes J, Houghton JH, Klijn JG, Sahmoud T. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet. 2002;359:2131–2139. doi: 10.1016/s0140-6736(02)09088-8. [DOI] [PubMed] [Google Scholar]
- Bulun SE, Price TM, Aitken J, Mahendroo MS, Simpson ER. A link between breast cancer and local estrogen biosynthesis suggested by quantification of breast adipose tissue aromatase cytochrome P450 transcripts using competitive polymerase chain reaction after reverse transcription. J Clin Endocrinol Metab. 1993;77:1622–1628. doi: 10.1210/jcem.77.6.8117355. [DOI] [PubMed] [Google Scholar]
- Bulun SE, Lin Z, Imir G, Amin S, Demura M, Yilmaz B, Martin R, Utsunomiya H, Thung S, Gurates B, Tamura M, Langoi D, Deb S. Regulation of aromatase expression in estrogen-responsive breast and uterine disease: from bench to treatment. Pharmacol Rev. 2005;57:359–383. doi: 10.1124/pr.57.3.6. [DOI] [PubMed] [Google Scholar]
- Chen D, Reierstad S, Lu M, Lin Z, Ishikawa H, Bulun SE. Regulation of breast cancer-associated aromatase promoters. Cancer Lett. 2009;273:15–27. doi: 10.1016/j.canlet.2008.05.038. [DOI] [PubMed] [Google Scholar]
- Chen D, Reierstad S, Fang F, Bulun SE. JunD and JunB integrate prostaglandin E2 activation of breast cancer-associated proximal aromatase promoters. Mol Endocrinol. 2011;25:767–775. doi: 10.1210/me.2010-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. Jama. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Choudary A, Musi N, Hu Y, Li R. IKKbeta mediates cell shape-induced aromatase expression and estrogen biosynthesis in adipose stromal cells. Mol Endocrinol. 2009;23:662–670. doi: 10.1210/me.2008-0468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Hankinson SE, Colditz GA, Stampfer MJ, Hunter DJ, Manson JE, Hennekens CH, Rosner B, Speizer FE, Willett WC. Dual effects of weight and weight gain on breast cancer risk. Jama. 1997;278:1407–1411. [PubMed] [Google Scholar]
- Key TJ, Appleby PN, Reeves GK, Roddam A, Dorgan JF, Longcope C, Stanczyk FZ, Stephenson HE, Jr., Falk RT, Miller R, Schatzkin A, Allen DS, Fentiman IS, Wang DY, Dowsett M, Thomas HV, Hankinson SE, Toniolo P, Akhmedkhanov A, Koenig K, Shore RE, Zeleniuch-Jacquotte A, Berrino F, Muti P, Micheli A, Krogh V, Sieri S, Pala V, Venturelli E, Secreto G, Barrett-Connor E, Laughlin GA, Kabuto M, Akiba S, Stevens RG, Neriishi K, Land CE, Cauley JA, Kuller LH, Cummings SR, Helzlsouer KJ, Alberg AJ, Bush TL, Comstock GW, Gordon GB, Miller SR. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst. 2003;95:1218–1226. doi: 10.1093/jnci/djg022. [DOI] [PubMed] [Google Scholar]
- O’Neill JS, Elton RA, Miller WR. Aromatase activity in adipose tissue from breast quadrants: a link with tumour site. Br Med J (Clin Res Ed) 1988;296:741–743. doi: 10.1136/bmj.296.6624.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestak I, Distler W, Forbes JF, Dowsett M, Howell A, Cuzick J. Effect of body mass index on recurrences in tamoxifen and anastrozole treated women: an exploratory analysis from the ATAC trial. J Clin Oncol. 2010;28:3411–3415. doi: 10.1200/JCO.2009.27.2021. [DOI] [PubMed] [Google Scholar]
- Simpson ER, Misso M, Hewitt KN, Hill RA, Boon WC, Jones ME, Kovacic A, Zhou J, Clyne CD. Estrogen--the good, the bad, and the unexpected. Endocr Rev. 2005;26:322–330. doi: 10.1210/er.2004-0020. [DOI] [PubMed] [Google Scholar]
- Subbaramaiah K, Howe LR, Bhardwaj P, Du B, Gravaghi C, Yantiss RK, Zhou XK, Blaho VA, Hla T, Yang P, Kopelovich L, Hudis CA, Dannenberg AJ. Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prev Res (Phila) 2011;4:329–346. doi: 10.1158/1940-6207.CAPR-10-0381. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Trentham-Dietz A, Newcomb PA, Storer BE, Longnecker MP, Baron J, Greenberg ER, Willett WC. Body size and risk of breast cancer. Am J Epidemiol. 1997;145:1011–1019. doi: 10.1093/oxfordjournals.aje.a009057. [DOI] [PubMed] [Google Scholar]
- Wenten M, Gilliland FD, Baumgartner K, Samet JM. Associations of weight, weight change, and body mass with breast cancer risk in Hispanic and non-Hispanic white women. Ann Epidemiol. 2002;12:435–444. doi: 10.1016/s1047-2797(01)00293-9. [DOI] [PubMed] [Google Scholar]
- Yoo K, Tajima K, Park S, Kang D, Kim S, Hirose K, Takeuchi T, Miura S. Postmenopausal obesity as a breast cancer risk factor according to estrogen and progesterone receptor status (Japan) Cancer Lett. 2001;167:57–63. doi: 10.1016/s0304-3835(01)00463-3. [DOI] [PubMed] [Google Scholar]
- Zhao H, Innes J, Brooks DC, Reierstad S, Yilmaz MB, Lin Z, Bulun SE. A novel promoter controls Cyp19a1 gene expression in mouse adipose tissue. Reprod Biol Endocrinol. 2009;7:37. doi: 10.1186/1477-7827-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Nichols JE, Valdez R, Mendelson CR, Simpson ER. Tumor necrosis factor-alpha stimulates aromatase gene expression in human adipose stromal cells through use of an activating protein-1 binding site upstream of promoter 1.4. Mol Endocrinol. 1996;10:1350–1357. doi: 10.1210/mend.10.11.8923461. [DOI] [PubMed] [Google Scholar]
- Zhou J, Gurates B, Yang S, Sebastian S, Bulun SE. Malignant breast epithelial cells stimulate aromatase expression via promoter II in human adipose fibroblasts: an epithelial-stromal interaction in breast tumors mediated by CCAAT/enhancer binding protein beta. Cancer Res. 2001;61:2328–2334. [PubMed] [Google Scholar]