Abstract
Objective
To estimate differences in skeletal maturity and stature from birth to age 18 years between individuals who are overweight vs. normal weight in young adulthood.
Patients and methods
Weight, length and height, and relative skeletal age (skeletal -chronological age) were assessed annually from birth to age 18 years in 521 subjects (255 females) in the Fels Longitudinal Study who were overweight or obese (BMI >25kg/m2, n=131) or normal weight (n=390) in young adulthood (18–30 years). Generalized estimating equations were used to test for skeletal maturity and stature differences by young adult BMI status.
Results
Differences in height increased during puberty, being significant for girls at ages 10 to 12 years, and for boys at ages 11 to 13 years (p-values<0.001), with overweight or obese adults being ~3cm taller at those ages than normal weight adults. These differences then diminished so that by age 18 years, overweight or obese adults were not significantly different in stature to their normal weight peers. Differences in skeletal maturity were similar, but more pervasive; overweight or obese adults were more skeletally advanced throughout childhood. Skeletal maturity differences peaked at chronological age 12 in boys and 14 in girls (p-values<0.001), with overweight or obese adults being ~1 year more advanced than normal weight adults.
Conclusions
This descriptive study is the first to track advanced skeletal maturity and linear growth acceleration throughout infancy, childhood, and adolescence in individuals who become overweight, showing that differences occur primarily around the time of the pubertal growth spurt. Increased BMI in children on a path to becoming overweight adults precedes an advancement in skeletal development and subsequently tall stature during puberty. Further work is required to assess the predictive value of accelerated pubertal height growth for assessing obesity risk in a variety of populations.
Keywords: obesity, body height, skeletal age measurement, critical period, longitudinal studies
Introduction
Obese children present with greater heights than their normal weight peers (1), although this association is not present in adulthood (2,3). Longitudinal studies have demonstrated that taller child height is prospectively associated with greater young adult Body Mass Index (BMI) and elevated risk of obesity (4,5). Child and adult obesity are also associated with advanced pubertal development (6–8). As development advances, the relationship between linear growth and pace of maturation reverses, and those with earlier puberty display diminished subsequent height growth to reach similar adult heights as their peers (3). The relationships between obesity, linear growth, and maturation are complex, and without extensive longitudinal data it is not possible to assess the temporal order of the known associations.
Pubertal secondary sexual development scales are a popular method used to assess physical development because they are non-invasive and can be administered in large study samples at relatively low costs. Self-reported pubertal development scale data are, however, known to be unreliable (9–11), and the alternative approach of physical inspection, including palpitation of mammary tissue and orchidometry, is invasive and potentially stressful for the child and his or her parents. These approaches are, in addition, limited to the assessment of maturational differences within the age limits of pubertal development. Skeletal development, in contrast, can be assessed with a hand-wrist radiograph involving a low amount of ionizing radiation exposure to one hand-wrist that is not harmful to health (12), does not involve physical examination to yield reliable data, and provides a common measure for both sexes across the entire span of childhood and adolescence.
Studies with serial skeletal age information are quite rare. Furthermore, there are few longitudinal datasets that can be used to address the temporal relationships between linear growth, BMI change, and pace of maturation according to later BMI status, beginning in early childhood. The present study will use extensive longitudinal data to estimate differences in height, BMI, and relative skeletal age from birth to 18 years between overweight or obese and normal weight young adults. This retrospective approach will provide an estimate of when differences in linear growth and pace of maturation between overweight and normal weight young adults emerge and how they change throughout infancy, childhood, and adolescence.
Patients and methods
Sample
The analysis sample consisted of 521 apparently healthy European American individuals (266 males; 255 females) born between 1928 and 1991 in southwestern Ohio, and who were enrolled in the Fels Longitudinal Study. The Fels Longitudinal Study has been described in detail elsewhere (13), but in summary began in 1929 in Yellow Springs, Ohio as a study of normal child growth and development and continues today as a study of the early life antecedents of chronic diseases of aging. The 521 individuals in the analysis sample for the present study were selected on the basis of having serial weight, length and height, and skeletal maturity data from birth onwards, as well as having at least one measurement of BMI between 18 and 30 years of age. Birth weight, birth length, birth year, and the proportion of girls to boys were not significantly different in the analysis sample than in the entire dataset of 917 (p-values>0.40).
All protocols and informed consent documents used in the Fels Longitudinal Study were approved by the Wright State University Institutional Review Board. All adult subjects provided written consent and minors provided verbal assent, as well as written consent. In the case of infants and children under eight years of age, parents provided written consent for data collected from their offspring.
Measurements
Anthropometry
Serial weight, length, and height measurements were made using standard procedures (14) at birth and at each year of age until age 30 years. Linear growth was assessed using recumbent length from birth to three years and standing height thereafter. BMI (BMI = weight (kg)/height (m)2) was calculated when both weight and length or height data were available at the same study visit.
Skeletal maturity
Skeletal maturity was assessed using hand-wrist radiographs collected at the same study visit as the anthropometric measurements. The radiographs were scored by trained anthropometrists using the Fels method (15) to obtain a skeletal age between the possible range of zero and 18 years (i.e., full skeletal maturity is achieved at skeletal age 18 years in both boys and girls). The Fels method involves the grading of various skeletal development indicators of the hand-wrist (e.g., epiphyseal ossification and shape of the radius) selected on the basis of chronological age and sex; the maximum number of indicators necessary is 22. A computer program, with an estimating equation, is then used to calculate skeletal age. Inter-rater technical error in the Fels Longitudinal Study is low with a mean of 0.08 years. Relative skeletal age was calculated by subtracting chronological age from skeletal age; negative values indicate a slower pace of skeletal maturation while positive values indicate a faster pace of skeletal maturation. Because skeletal maturity was not assessed at birth, comparisons of differences in relative skeletal age are presented at age one year and onwards.
Some individuals had more than one observation of length or height, BMI, or relative skeletal age at a certain target age, and in those instances the recording closest to the target age was selected. Not all individuals were measured at all target ages, which means the total sample at any given target age varied, although, for each sex, was never less than 149 observations. Birth data were always collected within five days of birth, and yearly data were always collected within ± three months of the target age.
Young adult BMI status
For individuals who had more than one observation of BMI between 18 and 30 years, the data closest to 25 years of age were selected. The height measurement closest to 25 years of age and the height measurement from the previous visit were used to establish that all individuals had achieved a stable young adult height and were growing less the 1cm per year. Young adult overweight and obesity were defined using the cut-offs of >25kg/m2 and >30kg/m2, respectively. Due to the relatively small number of overweight and obese individuals in the study sample, a binary variable was created: 1) normal weight and 2) overweight or obese. There were no underweight individuals (i.e., BMI <18.5kg/m2) in young adulthood. A subset of individuals with BMI data at five, 10, and 15 years of age (n=439) were selected and categorized into normal weight and overweight or obese groups during childhood using Cole et al’s (16) international sex and age specific cut-off points, which are based on BMI centile curves modeled to pass through 25kg/m2 and 30kg/m2 at 18 years of age, thereby allowing us to investigate the percentage of overweight young adults who were also overweight in childhood and adolescence.
Statistical Analysis
There were three dependent variables in this analysis: serial BMI from birth to 18 years, serial length or height from birth to 18 years, and serial relative skeletal age from one to 18 years. Individuals in the analysis sample had a median number of 18 recordings for both length or height and BMI and a median number of 17 recordings for relative skeletal age. The independent variable of interest was young adult BMI status (overweight or obese vs. normal weight).
Because the dependent variables were repeat measurements, and thus violated the assumption of independence necessary for general linear regression, sex specific generalized estimating equations (GEE) were fitted using the GENMOD procedure in SAS. This approach resolves the problem of non-independence of observations by considering the structure of the correlation matrix for the repeat measurements. We tested a number of matrices (e.g., unstructured and autoregressive) and found that a banded structure, that specifies that all data a given distance apart (i.e. one, two, etc years) share the same correlation, resulted in the best fitting models. In addition to young adult BMI status, all models included target age as a categorical variable (with levels 0, 1, 2 years etc), birth year as a continuous variable, which was centered about the mean (1957), and also the difference between the target age of measurement and the actual age at measurement to account for small variations in the exact timing of the measurements relative to the target ages. This age difference variable was centered with a mean of zero. Models also included a young adult BMI status category-by-target age interaction which made it possible to estimate differences by young adult BMI status at each age. Model fit was assessed using the Quasilikelihood under the Independence model Criterion (QIC), which is analogous to the Akaike’s Information Criterion (AIC) statistic used for comparing models’ fit with likelihood based methods, and also the QICu, which adds a penalty for the number of parameters and will approximate the QIC when the GEE is correctly specified.
From each model, least-squares means (LS-means) estimates at each yearly age for each young adult BMI group were produced using the LSMEANS option. LS-means are predicted population margins that, in this instance, are very similar to the model coefficients. LS-means were used because they can be estimated for the referent groups (i.e., normal young adult weight and 0 years) as well as all other groups, whereas the generalized estimating equation does not provide coefficients for the referent groups. For height and relative skeletal age, the difference in these estimates between the overweight or obese group and the normal weight group were calculated and plotted by age. Positive differences indicate that the overweight or obese group was taller or more mature, and negative differences indicate that they were shorter or less mature.
Analyses were conducted in SAS version 9.1 (SAS Institute, Cary, North Carolina).
Results
The overall prevalence of overweight or obesity in young adulthood (ages 18 to 30 years) in the study sample of 521 individuals was approximately 25% (62 (23.3%) men and 39 (15.3%) women overweight; 12 (4.5%) men and 18 (7.1%) women obese) (Table 1). The mean birth weight of women in the overweight or obese group was significantly greater than that for women in the normal weight group, and a significantly larger number of these overweight or obese young women were born macrosomic (i.e., >4.0kg) compared to normal weight women (17.9% vs. 3.6%). These associations were not present in men. To further characterize the prevalence of overweight and obesity in the sample, of the 439 individuals with BMI data at five, 10, and 15 years of age, none were overweight or obese at age five years, 6% were overweight or obese at 10 years of age, and 15% were overweight or obese at 15 years of age. Approximately 80% of those individuals who were overweight or obese at 10 or 15 years of age remained overweight or obese as young adults.
Table 1.
Description of study sample, by young adult1 BMI status and sex.
| Normal weight (n=390) | Overweight or obese2 (n=131) | Total | |||
|---|---|---|---|---|---|
| Male (n=192) | Female (n=198) | Male (n=74) | Female (n=57) | Total (n=521) | |
| Median (range) birth year | 1955 (1930–1990) | 1955 (1929–1991) | 1955 (1928–1991) | 1965 (1931–1989)a | 1957 (1928–1991) |
| Mean (SD) age of young adult BMI assessment | 23.61 (2.37) | 23.42 (2.48) | 23.68 (2.14) | 23.88 (2.69) | 23.58 (2.42) |
| Mean (SD) young adult BMI | 22.03 (1.76) | 20.78 (2.12) | 28.24 (3.41)a | 29.84 (5.19)a | 23.29 (4.31) |
| (n=186) | (n=192) | (n=73) | (n=56) | (n=507) | |
| Mean (SD) birth weight3 | 3.46 (0.49) | 3.23 (0.50) | 3.40 (0.63) | 3.39 (0.62)a | 3.36 (0.54) |
| Percent (N) macrosomic (i.e., >4.0kg) | 10.2 (19) | 3.6 (7) | 13.7 (10) | 17.9 (10)a | 9.1 (46) |
| (n=128) | (n=118) | (n=53) | (n=31) | (n=330) | |
| Mean (SD) length at birth | 50.35 (2.80) | 49.40 (2.41) | 50.71 (2.57) | 49.64 (2.65) | 50.00 (2.66) |
| (n=128) | (n=118) | (n=51) | (n=31) | (n=328) | |
| Mean (SD) BMI at birth | 13.34 (1.34) | 13.21 (1.56) | 13.14 (1.51) | 13.76 (1.58) | 13.30 (1.47) |
| (n=138) | (n=131) | (n=50) | (n=30) | (n=349) | |
| Mean (SD) relative skeletal age at one year | −0.02 (0.32) | −0.07 (0.38) | 0.00 (0.44) | 0.06 (0.33) | −0.03 (0.36) |
|
| |||||
| (n=178) | (n=177) | (n=65) | (n=39) | (n=459) | |
| Percent (N) overweight or obese4 at age five years | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Percent (N) overweight or obese at age 10 years | 0.0 | 4.0 (7) | 0.0 | 53.8 (21)a | 6.1 (28) |
| Percent (N) overweight or obese at age 15 years | 2.8 (5) | 4.0 (7) | 52.3 (34)a | 59.0 (23)a | 15.0 (69) |
Young adults were selected as those with weight and height data, and thus BMI status, between 18 and 30 years of age.
Young adult overweight and obesity were defined using internationally accepted cut-offs of >25kg/m2 and >30kg/m2, respectively
Not all individuals were measured exactly at birth or had skeletal age assessed at exactly one year. In all instance weight and length were assessed within five days of birth. For the one year measurement, skeletal age was assessed between 10 and 15 months.
Child overweight and obesity were defined using Cole et al’s (16) internationally accepted cut-off points.
Indicates a significant (P<0.05) difference in this variable by young adult BMI status. Differences were tested using independent samples t-tests for continuous variables and Chi-squared tests for categorical variables.
Table 2 shows the parameter estimates from the generalized estimating equations. In all instances, the QICu approximated the QIC and was not different by a number greater than 50, thereby demonstrating that the GEEs were correctly specified. As expected, the greater BMI of overweight or obese young adults developed incrementally, with statistically significant differences (p<0.05) observed from two years of age onward in boys and from five years of age onward in girls. The pattern for length and height and relative skeletal age was quite different, in that the parameter estimates reporting the effect of young adult overweight or obesity compared to the normal weight group increased from birth to approximately 12 years of age but then decreased to age 18 years. The largest parameter estimates indicated a height advantage of overweight or obese young adults of greater than 4cm in both sexes, and also a skeletal age advantage of 0.6 years in boys and 1.3 years in girls (p-values <0.01). To determine whether the observed differences were driven by the obese young adults within the overweight or obese group, we also performed the analysis excluding obese young adults (i.e., overweight vs. normal weight). The pattern for each of the dimensions was the same as that for the overweight or obese vs. normal weight comparison (Supplementary Table 1). In addition, the parameters estimates for BMI and relative skeletal age were generally similar to the original analysis. The length or height advantage of overweight young adults (relative to normal weight young adults) was, however, consistently greater than the height advantage of overweight or obese young adults (relative to normal weight young adults).
Table 2.
Estimates from generalized estimating equations1 for the association of young adult overweight or obesity with yearly BMI, height, and relative skeletal age for 521 Fels Longitudinal Study participants.
| BMI (kg/m2) | Height (cm) | Relative skeletal age (years) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boys | Girls | Boys | Girls | Boys | Girls | |||||||
| N2 | B(SE) | N | B(SE) | N | B(SE) | N | B(SE) | N | B(SE) | N | B(SE) | |
| Birth year | −0.005 (0.006) | −0.003 (0.006) | 0.046** (0.014) | 0.013 (0.016) | 0.001 (0.002) | 0.002 (0.002) | ||||||
| Age difference3 | −0.544 (1.083) | 0.138 (0.357) | −0.644 (2.091) | 3.370 (2.599) | −0.484* (0.192) | −0.420* (0.204) | ||||||
| BMI status (referent: normal weight): | ||||||||||||
| Overweight/obese | 74/192 | −0.086 (0.323) | 57/198 | 0.533 (0.335) | 74/192 | 0.323 (0.489) | 57/198 | −0.750 (0.709) | 74/192 | 0.136 (0.087) | 57/198 | −0.173 (0.107) |
| BMI status* target age (referent: normal weight at 0 years (1 year for relative skeletal age)): | ||||||||||||
| Overweight/obese at 1 year | 66/177 | 0.462 (0.353) | 46/179 | −0.070 (0.346) | 66/177 | 0.281 (0.451) | 46/179 | 0.871 (0.715) | ||||
| Overweight/obese at 2 years | 67/178 | 0.678* (0.344) | 51/182 | 0.349 (0.347) | 67/180 | 0.567 (0.544) | 51/182 | 1.036 (0.693) | 60/162 | 0.007 (0.067) | 39/151 | 0.068 (0.060) |
| Overweight/obese at 3 years | 70/177 | 0.661* (0.331) | 51/174 | 0.470 (0.340) | 70/177 | 0.776 (0.604) | 51/174 | 0.888 (0.772) | 64/165 | 0.091 (0.088) | 41/165 | 0.101 (0.091) |
| Overweight/obese at 4 years | 64/171 | 0.869* (0.342) | 52/183 | 0.528 (0.327) | 65/171 | 0.793 (0.638) | 53/183 | 1.139 (0.769) | 63/164 | 0.162 (0.100) | 41/168 | 0.179 (0.102) |
| Overweight/obese at 5 years | 70/183 | 1.037** (0.349) | 48/190 | 0.746* (0.331) | 70/183 | 1.088 (0.704) | 49/190 | 1.222 (0.816) | 65/166 | 0.206* (0.104) | 39/163 | 0.367** (0.114) |
| Overweight/obese at 6 years | 72/182 | 1.278*** (0.384) | 50/190 | 1.066** (0.346) | 73/182 | 1.096 (0.732) | 50/190 | 1.616 (0.862) | 62/159 | 0.292* (0.123) | 37/162 | 0.485*** (0.137) |
| Overweight/obese at 7 years | 73/182 | 1.513*** (0.422) | 49/189 | 1.288*** (0.361) | 73/183 | 1.107 (0.761) | 49/189 | 2.004* (0.905) | 64/158 | 0.336** (0.127) | 38/163 | 0.692*** (0.159) |
| Overweight/obese at 8 years | 73/183 | 1.929*** (0.431) | 51/194 | 1.921*** (0.397) | 73/183 | 1.303 (0.803) | 51/194 | 2.393* (0.950) | 67/172 | 0.392** (0.139) | 47/179 | 0.795*** (0.187) |
| Overweight/obese at 9 years | 73/182 | 2.249*** (0.462) | 50/190 | 2.237*** (0.411) | 73/182 | 1.413 (0.825) | 50/190 | 2.848** (1.001) | 70/170 | 0.498** (0.151) | 47/179 | 1.031*** (0.204) |
| Overweight/obese at 10 years | 72/182 | 2.750*** (0.490) | 50/190 | 2.435*** (0.462) | 72/182 | 1.769* (0.871) | 50/190 | 3.282** (1.065) | 68/174 | 0.567*** (0.163) | 45/179 | 0.987*** (0.203) |
| Overweight/obese at 11 years | 69/180 | 3.143*** (0.509) | 47/187 | 2.762*** (0.417) | 69/180 | 2.271* (0.905) | 47/187 | 3.936*** (1.143) | 62/165 | 0.613*** (0.173) | 43/171 | 0.815*** (0.185) |
| Overweight/obese at 12 years | 70/182 | 3.672*** (0.515) | 52/186 | 3.563*** (0.416) | 70/182 | 2.650** (1.004) | 52/186 | 3.867*** (1.168) | 62/166 | 0.666*** (0.184) | 46/170 | 0.843*** (0.199) |
| Overweight/obese at 13 years | 69/179 | 4.189*** (0.538) | 48/183 | 3.771*** (0.416) | 69/181 | 3.185** (1.125) | 48/183 | 2.829** (1.046) | 64/162 | 0.455* (0.182) | 44/172 | 1.171*** (0.241) |
| Overweight/obese at 14 years | 65/179 | 4.137*** (0.550) | 48/178 | 3.805*** (0.443) | 66/179 | 2.330* (1.152) | 48/178 | 1.569 (1.025) | 62/163 | 0.249 (0.173) | 44/159 | 1.301*** (0.238) |
| Overweight/obese at 15 years | 67/170 | 4.133*** (0.535) | 45/175 | 3.845*** (0.512) | 68/176 | 1.129 (1.055) | 45/175 | 0.786 (1.071) | 62/159 | 0.194 (0.204) | 40/158 | 1.086*** (0.233) |
| Overweight/obese at 16 years | 67/176 | 4.183*** (0.532) | 41/171 | 4.174*** (0.538) | 67/176 | −0.029 (1.000) | 41/171 | 0.339 (1.081) | 63/158 | 0.151 (0.209) | 37/154 | 0.774*** (0.207) |
| Overweight/obese at 17 years | 67/165 | 4.367*** (0.518) | 43/167 | 4.406*** (0.625) | 67/165 | −0.558 (1.001) | 43/168 | 0.378 (1.063) | 61/119 | 0.080 (0.181) | 29/140 | 0.500** (0.191) |
| Overweight/obese at 18 years | 70/180 | 4.456*** (0.561) | 46/183 | 4.712*** (0.647) | 70/180 | −0.981 (1.025) | 47/183 | 0.974 (1.252) | 62/164 | −0.122 (0.164) | 36/129 | 0.314 (0.175) |
|
| ||||||||||||
| Genmod fit criteria: | ||||||||||||
| QIC4 | 4723.9 | 4370.6 | 4706.2 | 4377.3 | 4103.1 | 3658.7 | ||||||
| QICu | 4677.0 | 4348.0 | 4688.0 | 4352.0 | 4083.0 | 3654.0 | ||||||
p<0.05,
p<0.01,
p<0.001 (two tailed)
Sex specific models were fitted using the Genmod command in SAS, specifying a banded structure for the correlation matrix.
N(overweight or obese group)/N(normal weight group).
Calculated as the difference between the target age of measurement and the actual age at measurement; in all instances this age difference variable was centered to mean zero.
The QIC (Quasilikelihood under the Independence model Criterion) is analogous to the AIC (Akaike’s Information Criterion) statistic used for comparing models fit with likelihood based methods; the model with the smaller statistics is preferred. QICu adds a penalty to the quasilikelihood for the number of parameters in the model, and will approximate the QIC when the generalized estimating equation is correctly specified.
The difference in the LS-means estimates between the overweight or obese and normal weight groups were plotted to provide a visual representation of how the differences in linear growth and pace of maturation changed from birth to 18 years of age (Figures 1 & 2). For length and height, the magnitude of the difference increased steadily from birth to nine years of age in boys and to age five years in girls, after which the linear growth of the overweight or obese group accelerated relative to the normal weight group. The peak difference was approximately 3cm, which was reached at 11 years in girls and at 13 years in boys. Differences in height then decreased, leaving the overweight or obese young adults not significantly different in stature to their peers at age 18 years. Differences in relative skeletal age between overweight or obese and normal weight young adults increased at a more constant pace and were more pervasive throughout childhood, being significant from six to 14 years of age in boys and from seven to 16 years of age in girls. At 14 years of age in girls and at 12 years of age in boys, differences in skeletal maturity reached their maximum. At that age, overweight or obese young adults were approximately one year more advanced than their normal weight peers.
Figure 1.
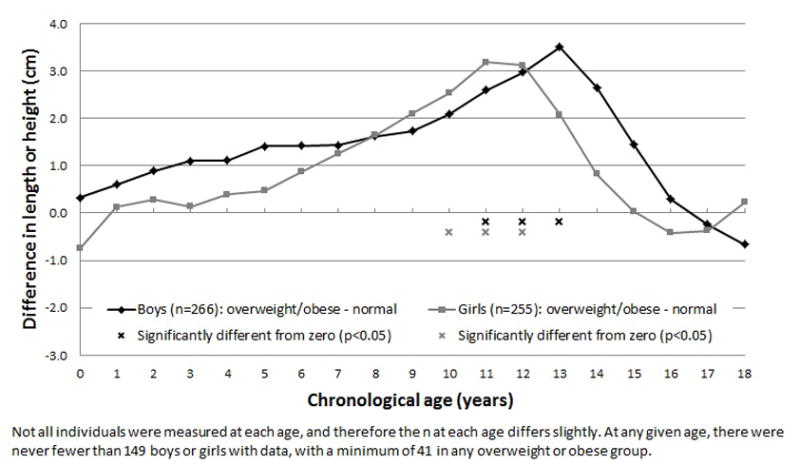
The yearly differences in height, from birth to 18 years of age, between overweight or obese and normal weight young adults, using least-squares means estimates from generalized estimating equations.
Figure 2.
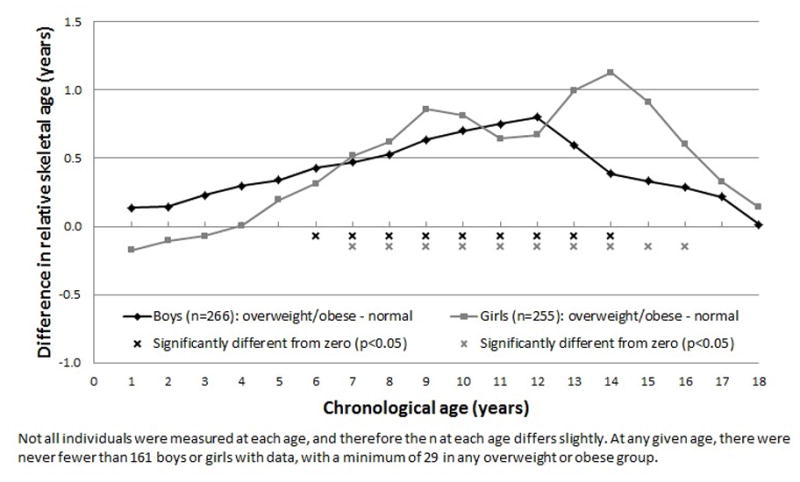
The yearly differences in relative skeletal age, from birth to 18 years of age, between overweight or obese and normal weight young adults, using least-squares means estimates from generalized estimating equations.
Discussion
This descriptive paper presents for the first time the differences in height as well as skeletal maturity between overweight or obese and normal weight young adults, throughout infancy, childhood, and adolescence. Over half of the overweight or obese young adults in the current study were also overweight or obese at age 15 years, and therefore the observed differences in growth and maturation according to young adult BMI status should not be viewed only as the result of young adult overweight or obesity but also as the result of overweight or obesity operating during puberty. Our primary finding is that skeletal maturity is more advanced in individuals who become overweight young adults compared to individuals who become normal weight young adults, starting in mid-childhood, and that these differences (and the corresponding differences in stature), are most marked during puberty.
Large differences in stature between overweight or obese and normal weight young adults during adolescence were also found by Stovitz et al (17) for 1375 boys and 1433 girls in the Child and Adolescent Trial for Cardiovascular Health (CATCH) cohort. CATCH was a cluster-randomized field trial initiated in 1991 to evaluate the effectiveness of a school classroom intervention on diet and physical activity; 96 schools across four locations (San Diego, CA; Minneapolis, MN; Austin, TX; New Orleans, LA) participated and each school was randomized to either an intervention or control arm (18). In CATCH participants, Stovitz et al found that difference in height between obese and normal weight young adults peaked at approximately 11 years of age. The larger total sample size in the Stovitz et al (17) study meant that differences between overweight and normal weight young adults, and between obese and normal weight young adults, could be investigated separately. The peak difference in height between overweight or obese vs. normal weight young adults in the current study was more comparable to the peak difference between overweight vs. normal weight young adults in the Stovitz et al study than to the difference they observed between obese vs. normal weight young adults. When we excluded the obese young adults and performed an overweight vs. normal weight young adult comparison, however, the height advantage of overweight young adults (relative to normal weight) was greater than the advantage of the overweight or obese young adults combined. In contrast, Stovitz et al found a dose response relationship in which the height advantage increased from normal to overweight to obese young adults. Perhaps a study with a larger sample is needed to fully investigate the graded effect of young adult BMI on linear growth and maturation.
The finding of accelerated linear growth in childhood, followed by reduced subsequent height gain in adolescence, has also been reported in a sample of 3650 healthy Swedish children (3). After adjusting for mid-parental height, a one unit increase in child BMI gain from two to eight years of age was associated with a 0.23 cm increase in height in boys and a 0.29 cm increase in girls over the same age period, and was also associated with an earlier age of puberty onset and diminished subsequent height growth in adolescence. The results of the He & Karlberg (3) study provide supporting evidence for the advanced linear growth and maturation of individuals who are currently, or subsequently become, overweight or obese, but do not inform of us the expected timing of the differences in growth and maturation according to BMI gain or status. Results of the present study are novel and show that height growth starts to rapidly increase in overweight or obese young adults after approximately seven years of age, and that significant differences in height emerge at approximately age 10 years and peak at age 12 years. Our results do not suggest that taller children always become overweight or obese young adults, but that in this sample, greater peri-pubertal heights were characteristic of individuals who proceeded to develop overweight or obesity as young adults. Further research in larger cohorts with long term follow-up is needed to quantify the usefulness of child height in the prediction of young adult overweight and obesity.
The differences in skeletal development observed in the current study help to interpret the height differences. Greater skeletal age in overweight or obese compared to normal weight young adults generally tracked with their height advantage, beginning with greater relative skeletal age during mid-childhood (age six to seven years), and then a gradually increasing advancement to age 12 in boys and age 14 in girls. Thus, during childhood, the overweight young adult begins exhibiting faster skeletal maturity years before height differences are observed. Indeed, the correlation between height and relative skeletal age was significant at all yearly ages between one and 14 years (data not shown), but was lower earlier in life (e.g., r=0.209 at age one year) than during adolescence (e.g., r=0.534 at age 12 years).
The finding that advanced skeletal maturity precedes excess child height in overweight individuals has not to our knowledge been documented previously, and suggests that factors involved in physical maturation generally, not just sexual development, are etiologically linked with overweight risk. It has been proposed that the decline in relative height after puberty onset in overweight or obese young adults is due to the earlier completion of growth and the fusion of the epiphyseal plates of the long bones (19–21). It is well known that obese children experience adrenarche and pubarche earlier than normal weight children (22–24). Due to missing data, it was not possible to test in this study whether measures of sexual development exhibited similar contrasts between overweight or obese and normal weight young adults as did skeletal maturity. This information could help to explain the unexpected finding that differences in relative skeletal age peaked three years later for girls than for boys. As the acceleration of height differences between overweight or obese and normal weight young adults were apparent earlier in girls than boys, it is unclear why skeletal development differences continued into later ages in girls than boys. This more persistent advanced maturation of girls who became overweight or obese young adults is not due to a “ceiling effect” caused by the maximum possible skeletal age being limited to 18 years. If a girl, for example, achieved full skeletal maturity (i.e., skeletal age of 18 years) before they reached 18 years of chronological age, their relative skeletal age would be positive indicating advanced maturity. Relative skeletal age is then going to decrease across subsequent assessments because chronological age is increasing and full skeletal maturity has already been achieved so will remain constant at 18 years. If anything, the limit on the maximum possible skeletal age may, therefore, mean that relative skeletal age at later ages is underestimated in girls who mature quickly.
In contrast to the results for height, the excess BMI of overweight or obese young adults monotonically increased from birth to 18 years of age. Greater BMI is expected during childhood in this group, but the important point here is that significant differences in BMI emerged earlier (by age 2 to 5 years) than those in height and relative skeletal age, thereby providing support for the hypothesis that greater relative weight in young children is driving faster linear growth and skeletal maturation, as opposed to advanced maturation preceding and driving greater size. Structural equation modeling and other more complex modeling of the time-varying associations of BMI, height, and skeletal development would be a reasonable next step in examination of the causal relationships among these variables.
It is important to emphasize that this paper demonstrates the effects of young adult overweight or obesity on height growth and pace of skeletal maturation from birth to 18 years of age, which may not necessarily reflect the effect of excess adiposity (25). It is well known that the BMI is correlated not only with fat mass but also with lean mass (26), and in childhood increases in BMI are attributed more to the lean component of body weight than the fat component (27). The observed differences in early life BMI between individuals who became overweight or obese young adults compared to those who became normal weight young adults may, therefore, reflect greater lean mass rather than fat mass. This may be particularly true because our sample was composed of primarily overweight young adults and not obese young adults who are more likely to have the greatest levels of adiposity. Disentangling the lean mass and fat mass components of BMI and how each contributes to difference in linear growth and pace of maturation warrants further research.
Several publications have discussed including factors beyond child BMI to predict risk for adult obesity, such as maternal smoking during pregnancy, bottle feeding during infancy, and child sleep patterns (28–30). The United States does not have a national growth monitoring program, but there is increasing evidence that it is possible to identify those who are growing into obesity in schools, at ages and in an environment where intervention may be more effective (31,32). Monitoring for tall adolescent stature, particularly greater than that expected from parental stature, in combination with advanced maturation may provide additional useful information for the prediction of young adult obesity. The predictive ability of the adolescent growth and maturation traits presented in the current paper for young adulthood obesity may deserve investigation, but the health relevance of any obesity prediction tool will ultimately rely on the effectiveness of the subsequent intervention.
There are a number of limitations in this analysis. The study is descriptive of the phenomena of greater stature and advanced skeletal maturation in children who become overweight or obese young adults, and we did not address the myriad causal factors that may have led to the observed growth and maturation differences. There is a clear need to examine the possible maternal environmental factors (e.g., maternal obesity, maternal dietary fat and hyperglycemia), in addition to child diet, hormone, and adiposity differences that may interact with genetic variation to explain the accelerated pace of child growth and maturation of overweight individuals. There were too few obese young adults in the sample to allow examination of differences between obese and overweight young adults, which would have provided evidence for a dose-response relationship between young adult BMI status and child growth patterns; nonetheless, our results were still similar in nature to those previously documented for overweight compared to normal weight young adults. The individuals in this study were all of European-American ancestry, and were born over a broad period of the 20th century. The results, therefore, may not be representative of other race-ethnic groups, nor necessarily of children born today. However, in that regard, it is expected that since rates of child overweight are higher in the United States today than in this sample, the taller stature and advancement of skeletal maturity may be even greater in more contemporary samples than was observed in this analysis.
In conclusion, this study provides novel information on the timing of advanced skeletal maturation, as well as taller stature, in children on a path to young adult overweight and obesity. Further work to establish the precise temporal sequence of increased weight, developmental pace, and taller stature, as well as work aimed at translating the present findings into a useful clinical tool are both required. Nonetheless, the findings demonstrate that increased BMI in children on a path to becoming overweight young adults precedes an advancement in skeletal development and subsequently tall stature during puberty, which then resolves in later adolescence.
Supplementary Material
Acknowledgments
This study was supported by National Institutes of Health grants R01-HD012252 and R01-HD053685. We acknowledge the life-long contributions of the Fels Longitudinal Study participants, and the study staff members, without whose commitment and enthusiasm the work of the study could never have been completed. In particular, we would like to thank Frances Tyleshevski for her help in the creation of the dataset, Carol Cottom for the skeletal age assessments, and the past and present Lifespan Health Research Center data collection team for their contributions. We also acknowledge Peter Hannan from the University of Minnesota for providing statistical consultation and advice.
Footnotes
Conflict of interest
The authors have no conflicts of interest to declare.
Supplementary information is available at the International Journal of Obesity’s website.
Financial disclosure: This study was supported by grants from the National Institutes of Health: R01-HD012252 and R01-HD053685.
References
- 1.Freedman DS, Thornton JC, Mei Z, Wang J, Dietz WH, Pierson RN, Jr, et al. Height and adiposity among children. Obes Res. 2004;12(5):846–853. doi: 10.1038/oby.2004.102. [DOI] [PubMed] [Google Scholar]
- 2.Freedman DS, Khan LK, Serdula MK, Dietz WH, Srinivasan SR, Berenson GS. Inter-relationships among childhood BMI, childhood height, and adult obesity: the Bogalusa Heart Study. Int J Obes Relat Metab Disord. 2004;28(1):10–16. doi: 10.1038/sj.ijo.0802544. [DOI] [PubMed] [Google Scholar]
- 3.He Q, Karlberg J. Bmi in childhood and its association with height gain, timing of puberty, and final height. Pediatr Res. 49(2):244–251. doi: 10.1203/00006450-200102000-00019. 200. [DOI] [PubMed] [Google Scholar]
- 4.Stovitz SD, Pereira MA, Vazquez G, Lytle LA, Himes JH. The interaction of childhood height and childhood BMI in the prediction of young adult BMI. Obesity. 2008;16(10):2336–2341. doi: 10.1038/oby.2008.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stovitz SD, Hannan PJ, Lytle LA, Demerath EW, Pereira MA, Himes JH. Child height and the risk of young-adult obesity. Am J Prev Med. 2010;38(1):74–77. doi: 10.1016/j.amepre.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aksglaede L, Juul A, Olsen LW, Sorensen TI. Age at puberty and the emerging obesity epidemic. PLoS One. 2009;4(12):e8450. doi: 10.1371/journal.pone.0008450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freedman DS, Khan LK, Serdula MK, Dietz WH, Srinivasan SR, Berenson GS, et al. The relation of menarcheal age to obesity in childhood and adulthood: the Bogalusa heart study. BMC Pediatr. 2003;3:3. doi: 10.1186/1471-2431-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denzer C, Weibel A, Muche R, Karges B, Sorgo W, Wabitsch M. Pubertal development in obese children and adolescents. Int J Obes. 2007;31(10):1509–1519. doi: 10.1038/sj.ijo.0803691. [DOI] [PubMed] [Google Scholar]
- 9.Bonat S, Pathomvanich A, Keil MF, Field AE, Yanovski JA. Self-assessment of pubertal stage in overweight children. Pediatrics. 2002;110(4):743–747. doi: 10.1542/peds.110.4.743. [DOI] [PubMed] [Google Scholar]
- 10.Raman A, Lustig RH, Fitch M, Fleming SE. Accuracy of self-assessed Tanner staging against hormonal assessment of sexual maturation in overweight African-American children. J Pediatr Endocrinol Metab. 2009;22(7):609–622. doi: 10.1515/jpem.2009.22.7.609. [DOI] [PubMed] [Google Scholar]
- 11.Schlossberger NM, Turner RA, Irwin CE. Validity of self-report of pubertal maturation in early adolescents. J Adolesc Health. 1992;13(2):109–113. doi: 10.1016/1054-139x(92)90075-m. [DOI] [PubMed] [Google Scholar]
- 12.Cameron N. Assessment of maturation: Bone age and pubertal assessment. In: Glorieux FH, Pettifor JH, Juppner H, editors. Pediatric bone: Biology and diseases. Academic Press; London, UK: 2003. pp. 325–338. [Google Scholar]
- 13.Roche AF. Growth, maturation and body composition: The Fels Longitudinal Study 1929–1991. Cambridge: Cambridge University Press; 1992. [Google Scholar]
- 14.Lohman T, Roche A, Martorell R. Anthropometric standardization reference manual. Champaign, IL: Human Kinetics Publishers; 1998. [Google Scholar]
- 15.Roche A, Chumlea W, Thissen D. Assessing the skeletal maturity of the hand-wrist. Springfield, IL: Charles C Thomas; 1988. [Google Scholar]
- 16.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320(7244):1240–1243. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stovitz SD, Demerath EW, Hannan PJ, Lytle LA. Growing into obesity: Patterns of height growth in those who become normal weight, overweight or obese as young adults. Am J Hum Biol. doi: 10.1002/ajhb.21191. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perry CL, Stone EJ, Parcel GS, Ellison RC, Nader PR, Webber LS, et al. School-based cardiovascular health promotion: the child and adolescent trial for cardiovascular health (CATCH) J Sch Health. 60(8):406–413. doi: 10.1111/j.1746-1561.1990.tb05960.x. [DOI] [PubMed] [Google Scholar]
- 19.Leonard MB, Shults J, Wilson BA, Tershakovec AM, Zemel BS. Obesity during childhood and adolescence augments bone mass and bone dimensions. Am J Clin Nutr. 2004;80(2):514–523. doi: 10.1093/ajcn/80.2.514. [DOI] [PubMed] [Google Scholar]
- 20.Minuto F, Barreca A, Del Monte P, Fortini P, Resentini M, Morabito F, et al. Spontaneous growth hormone and somatomedin-C/insulin-like growth factor-I secretion in obese subjects during puberty. J Endocrinol Invest. 1988;11(7):489–495. doi: 10.1007/BF03350166. [DOI] [PubMed] [Google Scholar]
- 21.Russell DL, Keil MF, Bonat SH, Uwaifo GI, Nicholson JC, McDuffie JR, et al. The relation between skeletal maturation and adiposity in African American and Caucasian children. J Pediatr. 2001 Dec;139(6):844–848. doi: 10.1067/mpd.2001.119446. [DOI] [PubMed] [Google Scholar]
- 22.Heger S, Korner A, Meigen C, Gausche R, Keller A, Keller E, et al. Impact of weight status on the onset and parameters of puberty: analysis of three representative cohorts from central Europe. J Pediatr Endocrinol Metab. 2008 Sep;21(9):865–877. doi: 10.1515/JPEM.2008.21.9.865. [DOI] [PubMed] [Google Scholar]
- 23.Neville KA, Walker JL. Precocious pubarche is associated with SGA, prematurity, weight gain, and obesity. Arch Dis Child. 2005;90(3):258–261. doi: 10.1136/adc.2004.053959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Remer T. Adrenarche and nutritional status. J Pediatr Endocrinol Metab. 2000;13 (Suppl 5):1253–1255. [PubMed] [Google Scholar]
- 25.Prentice AM, Jebb SA. Beyond body mass index. Obes Rev. 2001;2(3):141–147. doi: 10.1046/j.1467-789x.2001.00031.x. [DOI] [PubMed] [Google Scholar]
- 26.Wells JC, Chomtho S, Fewtrell MS. Programming of body composition by early growth and nutrition. Proc Nutr Soc. 2007;66(3):423–434. doi: 10.1017/S0029665107005691. [DOI] [PubMed] [Google Scholar]
- 27.Maynard LM, Wisemandle W, Roche AF, Chumlea WC, Guo SS, Siervogel RM. Childhood body composition in relation to body mass index. Pediatrics. 2001;107(2):344–350. doi: 10.1542/peds.107.2.344. [DOI] [PubMed] [Google Scholar]
- 28.Cameron N, Pettifor J, De Wet T, Norris S. The relationship of rapid weight gain in infancy to obesity and skeletal maturity in childhood. Obes Res. 2003;11(3):457–460. doi: 10.1038/oby.2003.62. [DOI] [PubMed] [Google Scholar]
- 29.Dennison BA, Edmunds LS, Stratton HH, Pruzek RM. Rapid infant weight gain predicts childhood overweight. Obesity. 2006;14(3):491–499. doi: 10.1038/oby.2006.64. [DOI] [PubMed] [Google Scholar]
- 30.Ong KK, Ahmed ML, Emmett PM, Preece MA, Dunger DB. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. BMJ. 2000;320(7240):967–971. doi: 10.1136/bmj.320.7240.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dahly D, Rudolf M. Identifying obesity risk in the early years: Report on the completion of a project funded by the Cross Government Obesity Unit. 2010 ( http://www.noo.org.uk/)
- 32.Ihmels MA, Welk GJ, Eisenmann JC, Nusser SM, Myers EF. Prediction of BMI change in young children with the family nutrition and physical activity (FNPA) screening tool. Ann Behav Med. 2009;38(1):60–68. doi: 10.1007/s12160-009-9126-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


