Abstract
Among its many roles in body and brain, oxytocin influences social behavior. Understanding the precise nature of this influence is crucial, both within the broader theoretical context of neurobiology, social neuroscience and brain evolution, but also within a clinical context of disorders such as anxiety, schizophrenia, and autism. Research exploring oxytocin’s role in human social behavior is difficult owing to its release in both body and brain and its interactive effects with other hormones and neuromodulators. Additional difficulties are due to the intricacies of the blood-brain barrier and oxytocin’s instability, which creates measurement issues. Questions concerning how to interpret behavioral results of human experiments manipulating oxytocin are thus made all the more pressing. The current paper discusses several such questions. We highlight unresolved fundamental issues about what exactly happens when oxytocin is administered intranasally, whether such oxytocin does in fact reach appropriate receptors in brain, and whether central or peripheral influences account for the observed behavioral effects. We also highlight the deeper conceptual issue of whether the human data should be narrowly interpreted as implicating a specific role for oxytocin in complex social cognition, such a generosity, trust, or mentalizing, or more broadly interpreted as implicating a lower-level general effect on general states and dispositions, such as anxiety and social motivation. Using several influential studies, we show how seemingly specific, higher-level social-cognitive effects can emerge via a process by which oxytocin’s broad influence is channeled into a specific social behavior in a context of an appropriate social and research setting.
Keywords: Oxytocin, social cognition, social neuroscience, blood-brain barrier, central effects, peripheral effects, anxiety, trust, generosity, schizophrenia, mentalizing, parsimony
1. Introduction
Not long ago, interest in oxytocin (OXT) was largely confined to its role in female reproduction; more specifically, in milk ejection during lactation and in the smooth muscle contraction of the uterus during parturition. With the groundbreaking discovery by neuroendocrinologists that injection of OXT into the brain of female rats brought on full maternal behavior toward foster pups (Pedersen and Prange, 1979), and the subsequent discovery of the role of OXT in mate attachment in prairie voles (Carter, 1998; Williams et al., 1994), and social recognition in mice (Ferguson et al., 2000), OXT and its role in social behavior has become a target of a large number of research projects. While many of these new developments are both important and intriguing, our brief is to step back a little and raise some questions, especially those with an interpretational flavor, that may usefully be considered in moving this field forward (see also (Bartz et al., 2011; Meyer-Lindenberg et al., 2011).
As an overview, in the next section we examine the complicated relationship between peripheral and central OXT, and wonder how exactly intranasal OXT administration influences physiology and social behavior. In the following section we examine interpretations that characterize OXT as influencing specific, higher-order social cognitive processes, as opposed to having more general lower level effects that can modify the profile of higher level functions. The central message emerging from both sections is that biology is nothing if not alarmingly complex, and the simplicity of the roles sometimes attributed to oxytocin (e.g. “the moral molecule”) may mask the true biological intricacy of causal interactions in social contexts. (Orgel’s Third Law: biology is more complicated than you imagine, even when you take Orgel’s Third Law into account.) Needless to say, it is no part of our intent to rain on the parade, but merely to draw attention to matters where confusion, uncertainty and misinterpretation may crop up. It is also fair to mention that since we did not have access to papers in this special issue of Hormones and Behavior before preparing our commentary, it should not be assumed that these papers provoked our concerns.
2. Pharmacokinetics of Oxytocin and Vasopressin
2A. Oxytocin In the Periphery
As is well known, OXT is produced in the brain. Less well appreciated is the production of OXT in the body, namely in the gastrointestinal tract, heart, testes, uterus, corpus luteum, placenta and amnion. OXT is also present in the kidney, pancreas, thymus and in adipocytes (Kiss and Mikkelsen, 2005). There are receptors for oxytocin in gut, which among other things cause contraction of smooth muscle (Klein et al., 2011). Peripheral OXT has a role in follicle lutenization and ovarian steroidogensis. Whether these peripheral sources of OXT are of behavioral significance remains largely unknown.
Why should we care about peripheral OXT? To begin with, the presence of peripheral OXT is relevant to behavioral experiments that involve no exogenous administration of OXT, but rather monitor peripheral OXT concentrations following social stimuli. One highly publicized paradigm uses a psychological manipulation, such as a positive social interaction or viewing a tragic video, where increased plasma levels of OXT after the experimental manipulation are reported (Barraza and Zak, 2009). Behavioral correlations, such as an increase in generosity in the ultimatum game, have also been reported following a positive social interaction in humans (Morhenn et al., 2008). A prevailing assumption is that the stimulus causes OXT releases in the brain, which then directly modifies activity of socially-relevant brain circuitry. The plasma level OXT concentrations are assumed to strongly correlate with the relevant brain levels of the peptide.
In considering the source of reported elevated plasma levels, we must first ask what is known about whether OXT, of either endogenous or exogenous sources, crosses the blood-brain barrier (BBB). Endocrinologists believe that OXT has poor BBB penetration. Small, lipophilic molecules readily cross the BBB into the CSF; hydrophilic molecules do not (see Figure 1). AVP and OXT are relatively large, hydrophilic molecules (McEwen, 2004). The CSF-to-blood transfer of AVP appears to be achieved by carrier-mediated transport, and the carriers are saturable (inhibited by excess amounts), with a half-time efflux of about 12.4 minutes. The rate for OXT is about 19.1 minutes, and transport of OXT involves a different saturable carrier (McEwen, 2004). Incidentally, BBB penetrability to AVP and OXT can be affected by such things as hypertension, stress, and disease.
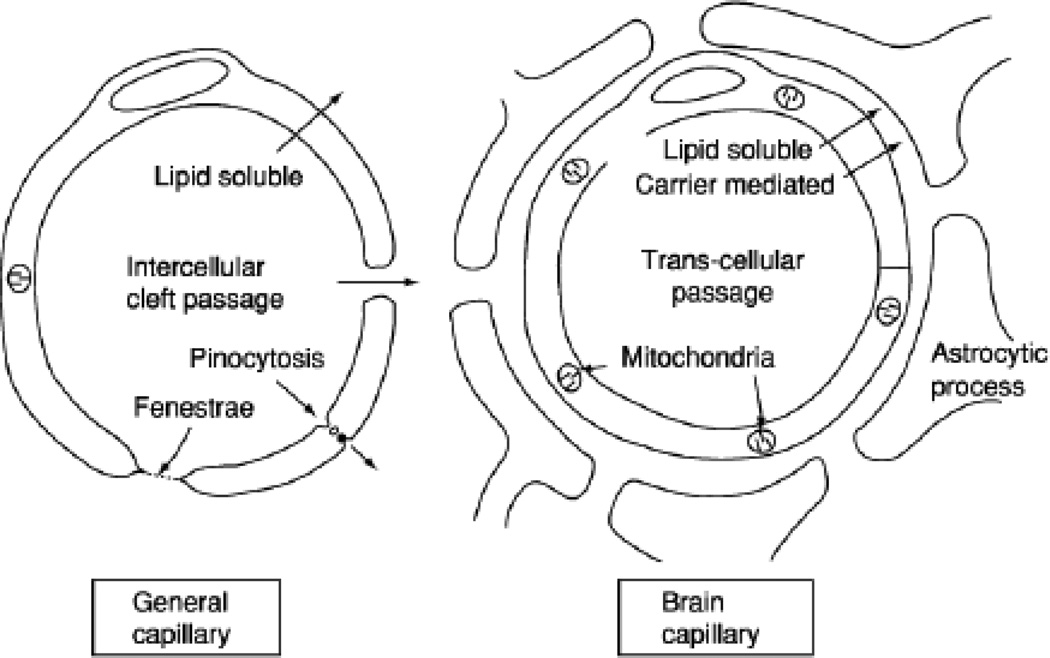
Figure 1.
A schematic diagram of the major differences between general capillaries and brain capillaries. The main point is that the endothelium of brain capillaries has very tight junctions between the cells, and lacks intercellular clefts, fenestrations (holes), pinocytes (membrane vesicles that fuse with the membrane, engulf the extracellular molecules, invaginate and then transport the extracellular molecules into the brain). The feet of the adjacent astrocytes are believed not to contribute to the barrier but to provide structural support. With permission, from Oldendorf, 1977 (Fig. 2, p. 179). Copyright 1977 by Academic Press.
Interestingly, under physiological conditions there is often a concentration difference between the plasma and CSF OXT levels, implying that concentration equivalences are not automatically restored. Both OXT and AVP levels exhibit circadian rhythms in the CSF but not in the plasma in many species of mammals including humans, and this is seen in both sexes. Different pacemakers for each appear to control release in the brain (McEwen, 2004).
Differences in concentrations or patterns of release of OXT and AVP in bodily fluids, including CSF versus plasma, could be affected by different sources of peptides. It is also likely, however, that metabolism in plasma differs from that in CSF (where metabolic enzymes may be less available and thus the peptides may accumulate). Assuming the aforementioned differences in metabolism, the frequency of sampling could also influence conclusions regarding concentrations of, or patterns of release of, peptides. Since fairly large volumes of samples are necessary to assay these peptides, practical considerations have limited the number of samples in most studies.
It is well known that in the brain, the neurohypophysis (posterior pituitary) is the major contributor to plasma levels of OXT at times of biological significance – mating, parturition, suckling and milk let-down. AVP is released from the pituitary following high sodium intake. This release does not involve the BBB, since hormones can be directly released from magnoceullar circuitry into capillaries.
During mating, there appears to be coordinated release in female prairie voles of OXT into the plasma as well as centrally (Ross et al., 2009). It is not known whether similar coordinated release occurs in human studies where subjects have a positive social experience since the stimulus is rather different. Plasma OXT probably does not reflect leakage out of the brain through the BBB, but could reflect coordinated release by magnocellular cells. Since the neurons that project to the pituitary are known to also release somatodendritically, then in the context of social stimuli, whether physical or emotional, magnocellular OXT neurons might be activated. Such activation would result in release from terminals in the pituitary as well as from some dendrites or collaterals that project into the brain. It will be important for investigators to determine whether in certain contexts central OXT concentrations parallel peripheral concentrations owing to coordinated release. This might best be done in primate studies using microdialysis to monitor central release.
In understanding the relation between social stimuli and elevated plasma levels of OXT, other factors may need to be disentangled. When human subjects receive social stimuli, it is likely that this causes reactions in the viscera (especially heart and gut) and the heart and the gut may themselves contribute to OXT plasma levels (Yu et al., 2011). More significantly, visceral signals will be picked up by the afferent branches of the vagus nerve and other visceral afferents, and will register as emotional signals in the brain. Moreover, both OXT and AVP are released in the brain following vagal stimulation (McEwen, 2004). The motor vagus may then respond with signals to the viscera as well as to the face, in a positive feedback loop. Evidence indicates that the autonomic nervous system is influenced by both endogenous and exogenous OXT (Porges, 2011).
Finally, OXT has a short half-life in the blood, estimated between about 3–9 minutes. This means that precisely when measurements are taken is extremely important. In a therapeutic context the short half-life, metabolic instability and poor BBB penetrability of OXT are problematic (consequently, neuropharmacologists are looking for robust BBB-penetrable synthetic compounds that can act as OXT agonists in clinical contexts, Ring et al., 2010). In an experimental context, these properties of OXT make life a little more complicated. At a minimum, it would be helpful if data on timing of blood draws for each subject were included in the reports. In sum, owing to the many uncertainties surrounding the significance of measures of plasma OXT, strong conclusions from these types of measures need to be qualified accordingly.
2B. Intranasal Administration of OXT
Many recent studies investigating the behavioral effects of administration of OXT have used intranasal spray, a highly convenient and noninvasive method of delivering exogenous OXT. Despite the clear appeal of this method, the following questions arise: does nasal OXT get into the brain, and if so, how? And if it does get into the brain, does it reach OXT receptor sites? Does the exogenously administered OXT lead to elevated levels of OXT in the brain, or does intranasal OXT stimulate neurons to release OXT, or both? Much uncertainty surrounds all these questions, though it is commonly assumed that “intranasal delivery provides a direct pathway to the brain” (MacDonald and MacDonald, 2010, p.4). So far as we know, no one has reported a direct measure of how much nasal OXT or nasal AVP of a given dose reaches and affects appropriate receptor sites. Perhaps labeling nasal OXT might be one way to address these questions in rodents or primates.
An important 2002 study in humans is frequently cited in favor of the hypothesis that OXT does get into the brain (Born et al., 2002). Note, however, that this study measured changes in the level in the ventricular cerebrospinal fluid (CSF), not the brain areas where OXT receptors reside, as their samples were obtained via spinal tap. Additionally, the peptide administered intranasally was arginine vasopressin (AVP). To be sure, OXT and AVP are closely related peptides, but they are different and have different targets.
Where exactly does AVP go after it is puffed into the nose? The answer is not precisely known, but some likely enters the blood through the nasal mucosa. Unless the nasal epithelium has a compromised BBB, the poor penetration of OXT across the BBB suggests that direct entry via the nasal capillaries is not the main route into the brain. Born et al. (2002) point out that intranasal vasopressin probably reaches the brain either intraneuronally or extraneuronally. They are doubtful of the efficacy of the first route on grounds that transport into olfactory bulb neurons followed by axonal transport to target regions not only would run the risk of proteolysis (degradation), it would also be exceedingly slow. The time course matters, since some researchers report effects within minutes of the nasal puff. Additionally, the intraneuronal route would likely be highly variable, both within subjects and across subjects over time.
Born et al suggest that extraneuronal transport is the more probable option, with AVP going into the subarachnoid space. Where does it go then? The Born et al results, along with the known anatomy, suggest that at least some of the intranasally administered AVP goes into the ventricular CSF, perhaps then entering the extra-cellular space (ECS) of the brain, or some may simply cross the pia and enter the ECS of the brain, or both. (Incidentally, the arachnoid membrane on the roof of the subarachnoid space stands between the blood and the CSF, and as such, constitutes a blood-brain barrier). Figure 2 shows some of the relevant structures.
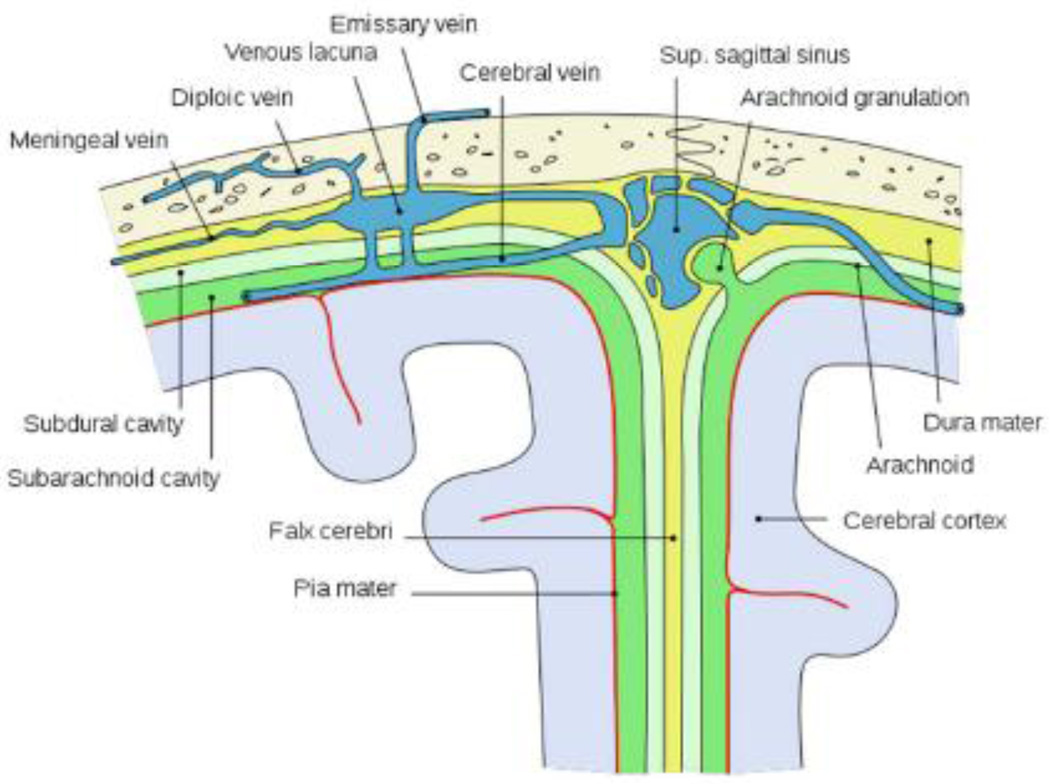
Figure 2.
Schematic depiction of the main structures located between the outer skull and the cerebral cortex. Source: Wikipedia, open source, public domain.
The key question concerns diffusion of AVP once it gets into the ECS of the brain. In physiological conditions OXT is released in the hypothalamus and binds to receptors in the amygdala and nucleus accumbens (Ludwig and Leng, 2006). Consequently, it is reasonable to assume effective distribution in physiological conditions. Even so, assuming the nasal AVP travels from the subarachnoid space to the ventricular CSF and then into the ECS of brain, the question remains: using nasal puffs, do sufficient amounts diffuse to the brain regions containing AVP receptors and thus where the peptide could be effective in altering behavior? Ditto for OXT. Thus it would be ideal for studies to be performed in non-human primates in which extracellular OXT concentrations in the brain can be monitored following intranasal OXT delivery.
A further puzzle concerns what constitutes a sufficient dose to ensure a behavioral effect. Born et al report that they had to use large doses of AVP to get increases in the CSF. They administered either 40 IU or 80 IU in total, and they puffed each nostril of subjects every 30–45 sec. over about five minutes, with a total of 8 puffs. A pertinent question, therefore, is whether either the doses of intranasal OXT or the time schedules for puffs used in social behavior experiments are comparable to those used to Born et al. and whether they need to be.
In some papers we analyzed, lower values for OXT were used (e.g. 24 IU) and frequently the number of puffs was not reported, though some specify that only a few puffs were administered. For example, De Dreu et al. (2010) used 3 puffs per nostril for a total of 24 IU. Petrovic et al., (2008) used 4 puffs per nostril for a total of 32 IU. At those values, Born et al did see a modest elevation in the CSF, but whether these lower doses allow for sufficient OXT to reach receptors to yield behavioral effect is even less clear. Spacing puffs matters because you do not want to risk having the OXT just run down the throat. Timing and frequency of treatment may also be critical if indeed there is release of endogenous peptide under some treatment conditions and not others. This suggests that future studies should examine the effects of intranasal delivery parameters on brain concentrations.
Additionally, most studies of the behavioral effects of OXT involve testing at 30–60 minutes or more after administration. It is possible that the reported effects are based on changes in the peptide pathways that are triggered by a “pulse” of OXT or AVP (Porges and Carter, 2011). It is possible, at least for OXT, that treatment with exogenous peptide is capable of stimulating a “feed forward” release of endogenous peptide, which is well known from mechanisms such as the Ferguson reflex, in which stimulation of the uterus or breast can facilitate the subsequent release of OXT. Even if nasal OXT does get into the brain, it remains unclear whether it interacts with other substances such as stress hormones, endogenous OXT, endogenous opiods and nitric oxide to achieve the reported behavioral effects. For example, in rat studies on pain, injecting OXT into the periaqueductal gray appears to stimulate release of a whole host of endogenous opiods when the animal experiences pain (Yang et al., 2011). This result provokes us to wonder whether in humans the onset of emotional pain initiates a cascade that has a roughly comparable profile.
Our questions concerning the travels of nasal AVP and OXT may strike some as nitpicking, and with luck they will turn out to be so. Nit picking or not, from our perspective, answers to these questions would help us better evaluate the results reported in nasal OXT experiments. Some results appear to be exceptionally dramatic and some may have translational significance. Consequently, answers concerning the travels of nasal AVP and OXT might guide us and others concerning when our skeptical circuitry should be up-regulated and when not (see also Meyer-Lindenberg et al., 2011). Finally, developing standardized methods would make cross-study comparisons much more meaningful. This is especially important in the context of some dramatic data interpretations and some unorthodox uses of statistics (for a particular critique, see Conlisk, 2011).
3. Oxytocin and specialized social cognition
The uncertainties regarding central vs. peripheral role of OXT are compounded by interpretational complexities due to the many psychological mechanisms that may mediate OXT effects on social behavior. One critical question is whether OXT effects on social behavior reflect its influences on specialized high-order social cognitive processes (e.g., trust, generosity, suspiciousness, mentalizing) or relatively broad states and orientations (e.g., general anxiety, affiliative motivation, global saliency of social cues). One reason to raise this question is the issue of explanatory parsimony. But this question is also important from the perspective of what cognitive science and psychology typically assume about the mind. On these standard assumptions, one would expect neurotransmitters and hormones, especially those acting peripherally, to work via relatively broad modulation, rather than via qualitative changes of computations on specific, higher-order content. Yet, many interpretations in human research on OXT and social behavior, and some of the public appeal of this research, rest on the possibility that this hormone and neurotransmitter selectively targets “the social brain” and has qualitatively distinct effects on complex, higher-order social-cognitive processes.
In what follows, we will argue that one such broad factor, anxiety, may account for a good chunk of the seemingly specific OXT effects on social cognition. We should say at the outset that, as outsiders to the field of OXT research but eager consumers of its findings, we find this possibility important not because of our particular interest in anxiety. Rather, we consider this possibility because it represents a genuine puzzle about the nature of the relationships between OXT and social cognition, and, more generally, the selectivity of mechanisms by which hormones and neurotransmitters influence higher-order mental processes.
3A. Anxiolytic Properties of Oxytocin
As is well known, anxiolytic effects of OXT have been demonstrated in a variety of species. These effects occur both after exogenous OXT administration and after endogenous release (Neumann, 2008). It is likely that some mechanisms involve primarily central routes. For example, amygdala is rich in OXT receptors (Huber et al., 2005). Some anxiolytic effects likely involve primarily peripheral routes. For example, OXT suppresses the “classic” stress hormones of the hypothalamic–pituitary–adrenal axis (Heinrichs et al., 2006). Most likely, however, anxiolytic effects reflect a complex interaction of central and peripheral mechanisms. For example, OXT alters cardiovascular reactivity – a peripheral effect. However, OXT achieves this effect not only via its actions on the heart itself, but also centrally – via nucleus of the solitary tract (NTS), which integrates and relays incoming peripheral visceral inputs with central influences (Norman et al., 2011). Further, though intranasal OXT administration reduces amygdala activity (Kirsch et al., 2005), this “central” effect may involve peripheral influences (e.g., via vagal stimulation, Hassert et al., 2004). In short, it is clear that a reduction of physiological and psychological reactivity to stressors is a common consequence of OXT, with many pathways leading to this outcome. These effects are what have motivated Sue Carter to describe chronic exposure to oxytocin as a “physiological metaphor for safety” (Carter, personal communication 2011).
3B. OXT Effects on Specific, Higher-Order Social Cognitive Processes
Given the anxiolytic properties of OXT, it is reasonable to wonder, as researchers have long wondered, to what extent specific higher-order social-cognitive effects observed in humans are due to OXT’s general anxiolytic effect. Consider some well-known findings. After intranasal OXT administration, participants display more trust in economic game involving allocating money to a stranger, with an anticipation of receiving greater returns (Kosfeld et al., 2005), rate strangers’ faces higher on trustworthiness (Theodoridou et al., 2009), receive higher “mind-reading” scores in a task that involves interpreting strangers eyes (Domes et al., 2007), and show more in-group favoritism in a prisoner-dilemma task that involves allocation of money between arbitrarily assigned ‘in-group’ or “out-group” (De Dreu et al., 2010). Note that all these effects involve high-order psychological processes (“trust”, “mentalizing”, “cooperation”) and are typically interpreted as suggesting that OXT selectively targets circuitry involved in sophisticated social-cognitive computations.
Findings suggesting that OXT influences complex social-cognitive circuitry are encountered also in translational research into mental health issues. Several studies now report that OXT administration positively influences complex psychiatric disorders such as autism and schizophrenia (for review, see Meyer-Lindenberg et al., 2011). For example, in one study, schizophrenic patients received either 3 weeks of daily intranasal OXT (40 IU twice a day) or a placebo adjunctive to prescribed antipsychotics (Feifel et al., 2010). Compared with the placebo + antipsychotics medications, the OXT group showed greater reduction of positive symptoms and, less robustly, negative symptoms of schizophrenia, as measured by standard scale PANSS, (Kay et al., 1987). The positive symptoms in this scale include things like hallucinations, delusions, suspiciousness (paranoia), thoughts disorders, excitement, and hostility, whereas negative symptoms include flat affect, poor rapport, social withdrawal, etc. Endogenous peripheral OXT levels also have been correlated with severity of symptoms on the PANSS, especially in women (Rubin et al., 2010). These results are particularly intriguing, as they imply that OXT may influences the very core mechanisms of belief formation, perhaps allowing one to reconceptualize schizophrenia as partially a disorder of “social trust”.
So, yes, on first glance, these findings clearly suggest that OXT modulates complex social cognitive circuitry. Nevertheless, might non-specific reduction in anxiety contribute in a nontrivial way to all those effects? Let us look at some studies in more detail in light of this possibility, and discuss some counterarguments.
3C. But Oxytocin Works on Things that have Nothing to do with Anxiety
An understandable response to the challenge that anxiety reduction plays a major role in the results of administering nasal OXT to human subjects is to attack the very plausibility of such challenge. After all, it will be argued, the dependent measures in the above OXT studies seem far removed from anything to do with anxiety -- trust, generosity, mental state attributions, autism, or schizophrenia. On one view, the core feature connecting these constructs is the willingness or capacity to make inferences, especially favorable inferences, about other people’s dispositions and intentions. For example, such social inferences underlie subjects’ expectation that their partner will return, rather than pocket their money (i.e., trust, Kosfeld et al., 2005); subjects’ interpretation of mental state from a stranger’s eyes (i.e., mentalizing, Domes et al., 2005), or suspiciousness about other people’s goals in schizophrenia (Feifel et al., 2010). Why should these social-cognitive capacities be anxiety-sensitive?
One rebuttal takes the following form. In general, any social inference, and especially positive inference, is preconditioned on the subject’s willingness to engage in social interaction, and this willingness is anxiety-sensitive. That is, changes in anxiety levels may influence broad preconditions for many complex social computations, from basic social perception to understanding to greeting to cooperating to mating. As a result, though the initial cause might be quite simple and general, the downstream effects may be a quite specific, involving complex cognition and behavior. Consistent with this idea, evidence suggest that the effects observed in the trust studies do indeed appear to involve fairly general mechanisms of anxiety down-regulation, as mediated by the amygdala (Baumgartner et al., 2008). Other recent work also suggests that complex mental state inferences in the mind-in-the-eye tasks depend on the general willingness to look into strangers’ eyes, which in turn appear anxiety-dependent (Evans et al., 2010).
Our rebuttal also applies to the schizophrenia studies. As mentioned, early interpretations proposed that the improvements in positive symptoms were due to selective changes in trust. In practice, however, the assessment of these positive symptoms is hard to separate from the patients’ general willingness to engage with others. Notice that the rating of positive symptoms of schizophrenia on a scale like PANSS (Kay et al., 1987) is performed by a psychiatrist who asks the patient questions about his social interactions (e.g., “do you talk to other people?”) and observes his/her interactions with others (e.g., does the patient show expressions of anger, resentment, sarcasm?). If a patient is less anxious, and thus more socially outgoing, he may show improvements on the index of positive symptoms, even with any reduction in the very core aspects of schizophrenia (e.g., auditory hallucinations, disorganized thought systems). Indeed, it’s well known that improvements in interpersonal engagement can occur even with low doses of standard antianxiety medications, or SSRIs (Knutson et al., 1998). Consistent with our perspective, more recent and comprehensive interpretations of OXT effects on schizophrenia and autism tend to emphasize OXT’s broader effects on anxiety and affiliative motivation (Meyer-Lindenberg et al., 2011).
3D. But We Did Control for Anxiety
Several researchers worry enough about the potential role of anxiety that they try to control for it, typically using a questionnaire. For example, in the work on trust (Kay et al., 1987) and in the work on mentalizing (Domes et al., 2007) researchers have found no significant effects of OXT on subjective experience of anxiety, as assessed by a German-language questionnaire called MDBF -- Multidimensional Mood State Questionnaire (Steyer et al., 1997), available in English translation here: http://www.metheval.uni-jena.de/mdbf.php. Accordingly, the researchers argued that anxiety reduction cannot be the underlying mechanisms for the reported high-order social cognitive effects. A closer look suggests there are several problems with this argument and the deployed methods.
First, the null findings in these particular behavioral experiments are puzzling given that other papers do report anxiolytic effects of OXT, even with similar doses and similar dependent measures. For example, in the context of stress-inducing situation (giving a public speech), OXT administration reduces self-reported anxiety using the MDBF questionnaire (Heinrichs et al., 2003). A second and related issue concerns validity, or how accurately these measures reflect the relevant state. For example, the MDBF questionnaire employed to control for potential effects on anxiety in the trust research does not contain any question that specifically asks about anxiety, but rather assesses a general state of “calmness.” A third issue is that broad mood questionnaires, like the MDBF, tend to be rather insensitive, especially when it comes to relatively mild states. For example, in the English-speaking world, a questionnaire most similar to MDBF is PANAS (Watson et al., 1988). This broad questionnaire is rather insensitive to mild states, qualitatively differentiated states, and also fails to accurately detect some important negative states, such as anger (Harmon-Jones et al., 2009). Fourth is the tricky possibility that changes in low-level affective states do not always lead to changes in conscious experience. As a result, such states do not manifest on a questionnaire, even though they do manifest in behavior as when participants respond differently to relevant cues (Winkielman and Berridge, 2004). For example, it is well known in psychiatry that anti-anxiety or anti-depressive medication can sometimes improve patients’ social behavior (e.g., greater sociability), before it improves self-reported affective experience (e.g., reduction in subjective feelings of sadness or anxiety).
To some extent, these problems can be addressed by using physiological measures that more directly tap into biological mechanisms of anxiety. One such measure, for example, is affective startle modulation, where startle responses are assessed in the presence of an emotionally-relevant stimulus. Fittingly, a recent study on rats showed that fear-potentiated startle response is reduced after peripheral administration of OXT (Ayers et al., 2011; Missig et al., 2010). Relevant to our concerns, in these studies the specific aspect of anxiety that was influenced was background anxiety. It would be thus important to know whether the specific OXT effects on trust, mental inferences from the eyes, or schizophrenia would hold when statistically controlling for the effects on such sensitive anxiety measures.
3E. But Oxytocin Does Work Selectively
The issue of non-specific anxiety may seem initially irrelevant for studies which report OXT effects that are interactive, i.e., selectively influence the targeted behavior but not unrelated behavior. For example, in the trust studies, research showed OXT effects on social trust, measured by amount invested, in a condition when participants played with another person, but not on nonsocial “trust”, in a condition when participants played against a computer (Kosfeld et al., 2005). Another recent study showed that OXT increases variables related to ingroup-favoritism, but no reduction in variables related to outgroup derogation (De Dreu et al., 2010). Importantly, these selective effects were pivotal for both papers’ argument against a more parsimonious, general-state interpretation.
Appealing as dissociations are, any argument from a dissociation has to meet high standards, as has long been appreciated (Teuber, 1955). For the argument to succeed it is necessary that both measures, or both conditions have reasonably equal sensitivity and that subjects are equally attentive and motivated to respond on both measures or in both conditions. These are fairly steep requirements. A dissociation argument is weakened if the measure or condition where the OT was absent was perceived by subject to be less important, more boring, more confusing, etc. Note therefore that in the Kosfeld et al study, the comparison was human risk vs. computer risk – conditions that clearly differ in their interest potential and anxiety-inducing properties. The DeDreu et al study, has been reinterpreted to explain the observed dissociation in the ingroup/outgroup behavior results in terms of exactly such non-specific factors (Chen et al., 2011).
More generally, both biological and psychological factors can easily turn a relatively broad physiological effect into what may deceivingly appear to be a narrow behavioral effect. This hazard is, indeed, “old news”, well appreciated at least since the famous studies on two-factor theory of emotion showed that enhancement of relatively non-specific arousal (via epinephrine injection) can channel into a variety of emotion-related behaviors and experiences based on subtle contextual cues (Schacter and Singer, 1962). A more recent, and more relevant example comes from a recent study on lactating human mothers, which presumably have higher levels of OXT (Hahn-Holbrook et al., 2011). Compared to controls, lactating mothers were found to be simultaneously less stressed, but at the same time more aggressive. The proposed explanation is that fear usually has aggression-constraining properties, so, paradoxically, OXT-related fear reduction, in proper context may lead to greater aggressive behavior. The role of psychological situational factors in channeling OXT effects was recently emphasized by a comprehensive review that pointed out the many inconsistent findings in the human OXT literature can be at least partly understood as reflecting contextual influences (Bartz et al., 2011).
3F. Anxiolytics as Control
Given the above, it is unfortunate that human OXT studies rarely control for anxiety, and when they do, these controls are weak. It is also surprising that, so far as we know, very few human studies, if any, include anti-anxiety substances as a control. This is regrettable since rat studies show that the effects of oxytocin and benzodiazepines, for example, can be quite similar (Neumann, 2008). One interesting control in human studies would be different medications for treatment of anxiety disorders. In the context of the earlier section (2), it would be especially interesting to contrast medications known to work peripherally (e.g., beta-blockers, like Propranolol) and centrally (e.g., Lorazepam). Finally, it would be critical to include measures that differentiate between social and non-social effect of such interventions.
3G. Parsimony, specification, and discriminative validity
Perhaps the core issue discussed in this section is an instance of the more general scientific problem of phenomenon description: specificity versus parsimony and consilience with the rest of the relevant science. To put it another way, how in science should we select a characterization for a phenomenon to avoid misspecification and instead maximize accuracy and specificity (construct and discriminative validity), along with its heuristic value and fit across different levels of explanations? For example, is dopamine best characterized as a “pleasure molecule” or is there a deeper and better characterization? (Berridge, 2007). Are we getting at the core features of the phenomenon? Is the actual effect narrow, or are we making a general effect appear highly specific as a consequence of using a narrow set of dependent variables? Are we including conditions and measures that ensure sufficient discriminative validity?
Fair enough, there is no perfect algorithm for finding the optimal characterization, only scientific judgment. Nonetheless, sensitivity to the possibilities of mis-characterization may guard against premature fixation of a description. Naturally enough, the media prefer fetching characterizations that capture the public’s imagination, but in the long run, indulging these whims can embarrass the science. They also hurt science by covering interesting complexity, since after all, nonapeptides, like OXT, may not have a “function”, and may exert different and even opposing influences on behavior, based upon the sophisticated pattern of neuromodulation in the brain and a particular social arrangement (Goodson and Kabelik, 2009).
4. Conclusions
In conclusion, despite all the challenges, caveats and cavils, we agree that the findings on OXT are both fascinating and important. However, depending on future research progress, some of the richer interpretations encountered in social neuroscience research may require some revision. After all, it is rather unlikely that any widely acting hormone or neurotransmitter will be narrowly funneled to modulate complex, high-order mental processes that are specific to social cognition. Thus, explanations in terms of more general mechanisms, whether referring to anxiety, affiliative motivation, or social saliency may be more justified and more productive.
Likewise, in translational research, it may be less captivating, but more accurate, to refrain so far from describing OXT effects as targeting the core features of “autism” or “schizophrenia.” Especially, because it may turn out that the best clinical use of intranasal OXT is primarily as an effective (and perhaps non-addictive) anti-anxiety medication which may indirectly impact some (important) symptoms of these disorders. In some way, calling OXT a schizophrenia drug or an autism drug may be a bit like calling aspirin a heart attack drug. Aspirin may be a very important tool in a cardiologist toolbox, but it acts broadly and is also used for many other purposes -- from reducing inflammation in arthritis to lowering temperature in a fever to reducing pain of a headache.
Highlights.
Understanding oxytocin’s precise role in social behavior is crucial.
Central and peripheral mechanisms contribute to oxytocin’s effects.
Blood-brain barrier and hormonal/neurotransmitter interactions complicate picture.
It is unclear how intranasal oxytocin administrations lead to behavioral effects.
It is doubtful that oxytocin directly influences complex social cognition in humans.
Acknowledgements
We thank Jan Born, Sue Carter, Paul Churchland, Jim Goodson, Michael Gorman, Barry Keverne, Martin Paulus, Shlomi Sher, and Larry Young for advice and corrections, but hasten to emphasize that they are not responsible for remaining errors and omissions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ayers LW, Missig G, Schulkin J, Rosen JB. Oxytocin Reduces Background Anxiety in a Fear-Potentiated Startle Paradigm: Peripheral vs Central Administration. Neuropsychopharmacol. 2011;36:2488–2497. doi: 10.1038/npp.2011.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraza JA, Zak PJ. Empathy toward strangers triggers oxytocin release and subsequent generosity. Annals of the New York Academy of Sciences. 2009;1167:182–189. doi: 10.1111/j.1749-6632.2009.04504.x. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends Cogn Sci. 2011;15:301–309. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron. 2008;58:639–650. doi: 10.1016/j.neuron.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology. 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci. 2002;5:514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23:779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- Chen FS, Kumsta R, Heinrichs M. Oxytocin and intergroup relations: goodwill is not a fixed pie. Proc Natl Acad Sci U S A. 2011;108:E45. doi: 10.1073/pnas.1101633108. author reply E46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlisk J. Professor Zak's empirical studies on trust and oxytocin. J Econ Behav Organ. 2011;78:160–166. [Google Scholar]
- De Dreu CK, Greer LL, Handgraaf MJ, Shalvi S, Van Kleef GA, Baas M, Ten Velden FS, Van Dijk E, Feith SW. The neuropeptide oxytocin regulates parochial altruism in intergroup conflict among humans. Science. 2010;328:1408–1411. doi: 10.1126/science.1189047. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves "mind-reading" in humans. Biological psychiatry. 2007;61:731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Evans S, Shergill SS, Averbeck BB. Oxytocin decreases aversion to angry faces in an associative learning task. Neuropsychopharmacol. 2010;35:2502–2509. doi: 10.1038/npp.2010.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feifel D, Macdonald K, Nguyen A, Cobb P, Warlan H, Galangue B, Minassian A, Becker O, Cooper J, Perry W, Lefebvre M, Gonzales J, Hadley A. Adjunctive intranasal oxytocin reduces symptoms in schizophrenia patients. Biological psychiatry. 2010;68:678–680. doi: 10.1016/j.biopsych.2010.04.039. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nature genetics. 2000;25:284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Kabelik D. Dynamic limbic networks and social diversity in vertebrates: from neural context to neuromodulatory patterning. Front Neuroendocrinol. 2009;30:429–441. doi: 10.1016/j.yfrne.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn-Holbrook J, Holt-Lunstad J, Holbrook C, Coyne SM, Lawson ET. Maternal defense: breast feeding increases aggression by reducing stress. Psychological science. 2011;22:1288–1295. doi: 10.1177/0956797611420729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon-Jones E, Harmon-Jones C, Abramson L, Peterson CK. PANAS positive activation is associated with anger. Emotion. 2009;9:183–196. doi: 10.1037/a0014959. [DOI] [PubMed] [Google Scholar]
- Hassert DL, Miyashita T, Williams CL. The effects of peripheral vagal nerve stimulation at a memory-modulating intensity on norepinephrine output in the basolateral amygdala. Behavioral neuroscience. 2004;118:79–88. doi: 10.1037/0735-7044.118.1.79. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biological psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Soravia LM, Neumann ID, Stangier U, De Quervain DJ, Ehlert U. Effects of oxytocin on social phobia. Neuropsychopharmacol. 2006;31:S10–S10. [Google Scholar]
- Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308:245–248. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia bulletin. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, Gruppe H, Mattay VS, Gallhofer B, Meyer-Lindenberg A. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss A, Mikkelsen JD. Oxytocin--anatomy and functional assignments: a minireview. Endocr Regul. 2005;39:97–105. [PubMed] [Google Scholar]
- Klein BY, Tamir H, Welch MG. PI3K/Akt responses to oxytocin stimulation in Caco2BB gut cells. J Cell Biochem. 2011;112:3216–3226. doi: 10.1002/jcb.23243. [DOI] [PubMed] [Google Scholar]
- Knutson B, Wolkowitz OM, Cole SW, Chan T, Moore EA, Johnson RC, Terpstra J, Turner RA, Reus VI. Selective alteration of personality and social behavior by serotonergic intervention. The American journal of psychiatry. 1998;155:373–379. doi: 10.1176/ajp.155.3.373. [DOI] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci. 2006;7:126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- McEwen BB. Brain-fluid barriers: relevance for theoretical controversies regarding vasopressin and oxytocin memory research. Adv Pharmacol. 2004;50592:531. 655–708. doi: 10.1016/S1054-3589(04)50014-5. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci. 2011;12:524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- Missig G, Ayers LW, Schulkin J, Rosen JB. Oxytocin reduces background anxiety in a fear-potentiated startle paradigm. Neuropsychopharmacol. 2010;35:2607–2616. doi: 10.1038/npp.2010.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morhenn VB, Park JW, Piper E, Zak P. Monetary sacrifice among strangers is mediated by endogenous oxytocin release after physical contact. Evolution and Human Behavior. 2008;29:375–383. [Google Scholar]
- Neumann ID. Brain oxytocin: A key regulator of emotional and social behaviours in both females and males. J Neuroendocrinol. 2008;20:858–865. doi: 10.1111/j.1365-2826.2008.01726.x. [DOI] [PubMed] [Google Scholar]
- Norman GJ, Hawkley LC, Cole SW, Berntson GG, Cacioppo JT. Social neuroscience: The social brain, oxytocin, and health. Social neuroscience. 2011 doi: 10.1080/17470919.2011.568702. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Prange AJ. Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proc Natl Acad Sci U S A. 1979;76:6661–6665. doi: 10.1073/pnas.76.12.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic P, Kalisch R, Singer T, Dolan RJ. Oxytocin attenuates affective evaluations of conditioned faces and amygdala activity. J Neurosci. 2008;28:6607–6615. doi: 10.1523/JNEUROSCI.4572-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW. The polyvagal theory : neurophysiological foundations of emotions, attachment, communication, and self-regulation. 1st ed. New York: W. W. Norton; 2011. [Google Scholar]
- Porges SW, Carter CS. Neurobiology and evolution: Mechanisms, mediators, and adaptive consequences of caregiving. In: Brown SL, Brown RM, Penner LA, editors. Self Interest and Beyond: Toward a New Understanding of Human Caregiving. New York: Oxford University Press; 2011. pp. 53–71. [Google Scholar]
- Ring RH, Schechter LE, Leonard SK, Dwyer JM, Platt BJ, Graf R, Grauer S, Pulicicchio C, Resnick L, Rahman Z, Sukoff Rizzo SJ, Luo B, Beyer CE, Logue SF, Marquis KL, Hughes ZA, Rosenzweig-Lipson S. Receptor and behavioral pharmacology of WAY-267464, a non-peptide oxytocin receptor agonist. Neuropharmacology. 2010;58:69–77. doi: 10.1016/j.neuropharm.2009.07.016. [DOI] [PubMed] [Google Scholar]
- Ross HE, Cole CD, Smith Y, Neumann ID, Landgraf R, Murphy AZ, Young LJ. Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience. 2009;162:892–903. doi: 10.1016/j.neuroscience.2009.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LH, Carter CS, Drogos L, Pournajafi-Nazarloo H, Sweeney JA, Maki PM. Peripheral oxytocin is associated with reduced symptom severity in schizophrenia. Schizophrenia research. 2010;124:13–21. doi: 10.1016/j.schres.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter S, Singer JE. Cognitive, social, and physiological determinants of emotional state. Psychol Rev. 1962;69:379–399. doi: 10.1037/h0046234. [DOI] [PubMed] [Google Scholar]
- Steyer R, Schwenkmezger P, Notz P, Eid M. Der Mehrdimensionale Befindlichkeitsfragebogen (MDBF) [Multidimensional mood questionnaire] Goettingen: Hogrefe; 1997. [Google Scholar]
- Teuber HL. Physiological psychology. Annual review of psychology. 1955;6:267–296. doi: 10.1146/annurev.ps.06.020155.001411. [DOI] [PubMed] [Google Scholar]
- Theodoridou A, Rowe AC, Penton-Voak IS, Rogers PJ. Oxytocin and social perception: oxytocin increases perceived facial trustworthiness and attractiveness. Hormones and behavior. 2009;56:128–132. doi: 10.1016/j.yhbeh.2009.03.019. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of personality and social psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Williams JR, Insel TR, Harbaugh CR, Carter CS. Oxytocin administered centrally facilitates formation of a partner preference in female prairie voles (Microtus ochrogaster) J Neuroendocrinol. 1994;6:247–250. doi: 10.1111/j.1365-2826.1994.tb00579.x. [DOI] [PubMed] [Google Scholar]
- Winkielman P, Berridge KC. Unconscious emotion. Curr Dir Psychol Sci. 2004;13:120–123. [Google Scholar]
- Yang J, Liang JY, Li P, Pan YJ, Qiu PY, Zhang J, Hao F, Wang DX. Oxytocin in the periaqueductal gray participates in pain modulation in the rat by influencing endogenous opiate peptides. Peptides. 2011;32:1255–1261. doi: 10.1016/j.peptides.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Yu Q, Ji R, Gao X, Fu J, Guo W, Song X, Zhao X, Burnstock G, Shi X, He C, Xiang Z. Oxytocin is expressed by both intrinsic sensory and secretomotor neurons in the enteric nervous system of guinea pig. Cell Tissue Res. 2011;344:227–237. doi: 10.1007/s00441-011-1155-0. [DOI] [PubMed] [Google Scholar]