Abstract
Background
Randomized controlled trials have reported lower mortality among patients who adhere to placebo compared with those who do not. We explored this phenomenon by reanalyzing data from the placebo arm of the Beta Blocker Evaluation of Survival Trial (BEST), a randomized, double-blind, placebo-controlled trial of bucindolol and mortality.
Aims
Our primary aim was to measure and explain the association between adherence to placebo and total mortality among the placebo-allocated participants in the BEST trial. Secondary aims included assessment of the association between placebo adherence and cause-specific mortality.
Methods
Participants with "higher placebo adherence" were defined as having taken at least 75% of their placebo study medication over the entire course of each individual’s participation in the study, while those with “lower placebo adherence” took <75%. Primary outcome was in-study all-cause mortality. To account for confounding, we adjusted for all available modifiable, non-modifiable and psychosocial variables.
Results
Adherent participants had a significantly lower total mortality compared to less-adherent participants (HR = 0.61, 95% Confidence Interval: 0.46–0.82). Adjusting for available confounders did not change the magnitude or significance of the estimates. When considering cause-specific mortality, CVD and pump failure showed similar associations.
Conclusions
Analyses of the BEST trial data support a strong association between adherence to placebo study medication and total mortality. While probably not due to publication bias or simple confounding by healthy lifestyle factors, the underlying explanation for the association remains a mystery. Prospective examination of this association is necessary to better understand the underlying mechanism of this observation.
Keywords: Double-blind clinical trials, Placebo, Adherence
Introduction
It is common for studies to report that better adherence to study medication is associated with better outcomes, however, several studies have reported this association among participants randomized only to the placebo arms in double-blind clinical trials (1–7). Because placebo, by design, has no specific physiological effects, this finding is intriguing. The most obvious explanation for this phenomenon is publication bias – the tendency for studies that find a positive effect to report it, while studies that find no effect will not even mention it in passing. Other possible explanations include confounding by lifestyle factors (factors that contribute to a person’s propensity to take their medicine as prescribed also contribute to their outcomes), and time-dependent confounding (an underlying cause of their lowered adherence is also the underlying cause of their outcome). We sought to study this effect in clinical trials that had not previously reported an examination of this association. By restricting analyses to the placebo-allocated participants, we transform the study to an observational one, giving up the benefits of randomization, but we gain the ability to measure the effects of adherence in a setting where potential specific effects of a drug are irrelevant. This brief report is part of a series of detailed standardized analyses addressing the association of adherence itself with mortality.
Methods
We used data from the Beta Blocker Evaluation of Survival Trial (BEST), a randomized, double-blind, placebo-controlled trial of bucindolol and mortality in a cohort of men and women with heart failure (8). Eligible participants were adult men and women with New York Heart Association functional classifications III or IV who also had a left ventricular ejection fraction of 35% or lower. Participants were randomized to either bucindolol or a placebo; the starting dose was 6.25 mg, and increased weekly to a maximum dose of 100mg orally twice each day. The maximum dose varied by weight and drug tolerance. Follow-up visits occurred every 6 months for 3 years. Data for our analyses were obtained from the National Heart, Lung, and Blood Institute Data Repository of Epidemiology and Clinical Trials (9).
Our primary aim was to measure and explain the association between adherence to placebo and total mortality among the placebo-allocated participants in the BEST trial. Secondary aims included assessment of the association between placebo adherence and cause-specific morbidity and mortality. Additional details regarding the analytic methods have been published previously (10).
Participants with "higher placebo adherence" were defined as having taken at least 75% of their placebo study medication over the entire course of each individual’s participation in the study, while those with “lower placebo adherence” took <75%. We conducted a sensitivity analysis on this definition by recalculating the association between placebo adherence and total morality while varying the definition of adherence from 50% to 95%. For the primary analyses, the total in-study adherence was calculated as the total number of pills taken (as determined by the difference between number of pills dispensed and number of pills returned over the course of the study) divided by the total number of pills that should have been taken if adherence was 100% (as determined by total number of days assigned to study medication). We also calculated adherence as a cumulative adherence variable at each visit (i.e., cumulative adherence up to the study visit just prior to the current visit), and also as a simple time-dependent individual visit adherence variable (i.e., the adherence for the time between the prior and current visit only). In addition, total in-study adherence was modeled as a continuous variable in one set of analyses. No missing data were imputed.
To correct for individual visit adherence measures that exceeded 100%, those between 100% and 125% were capped at 100%. Measurements that exceeded 125% were assumed to be data-entry errors and were set to missing. For total in-study adherence calculation, we used all non-missing visit adherence data.
The primary outcome was total in-study mortality. Secondary outcomes included all cardiovascular disease (CVD) mortality, CVD mortality with likely prodrome, non-CVD mortality, coronary heart disease (CHD) mortality and congestive heart failure (CHF) mortality. We also examined the incidence of combined fatal or non-fatal CHD events.
Using survival analysis (11), we evaluated the association between placebo adherence and each outcome with Kaplan-Meier curves stratified by adherence (higher/lower). The statistical significance of observed differences in survival curves was determined with log-rank tests. Multivariable analyses were conducted with Cox proportional hazards models (11). For consistency among analyses, baseline covariates were classified as follows: non-modifiable (age, sex, and race), modifiable (smoking, total cholesterol, triglycerides, heart rate, body-mass index (BMI) category, and systolic blood pressure (BP)), and psychosocial (quality of life). Five types of adjusted models were examined containing the following covariates: non-modifiable risk factors, modifiable risk factors, all risk factors, psychosocial measures only, and a full model with all covariates (10). We tested the proportional hazards assumption in the main unadjusted model by testing for interaction between main effect and time.
An important potential bias in the context of adherence and mortality is time-dependent confounding. This occurs if a medical condition causes death (outcome) and is also responsible for reducing the participant's adherence (exposure) in the time period prior to death. For example, a fatal disease that is accompanied by pain and/or discomfort might cause the participant to stop taking regular study medications, resulting in a lower adherence. In order to test for this bias, we repeated the analyses for total in-study mortality after deleting each participant's last adherence measurement and last two measurements (these procedures remove the effect of the adherence measurements in the months just prior to a participant's death when they are most susceptible to the ill-effects of the underlying disease). We also repeated the time varying proportional-hazards models with lagged and twice-lagged cumulative adherence variables in order to delete the influence of the last and last two adherence measurements, respectively. Finally, we considered the possibility that these cumulative lag-adherence calculations were sensitive to amount of time in-study. That is, removing a single adherence measure from a cumulative measure early in the study would have a greater effect on the measure than the same process performed later in the study. In order to avoid this bias, we considered individual visit adherence in time varying models overall and with lagged models.
All analyses were performed with SAS v. 9.1 (12).
Results
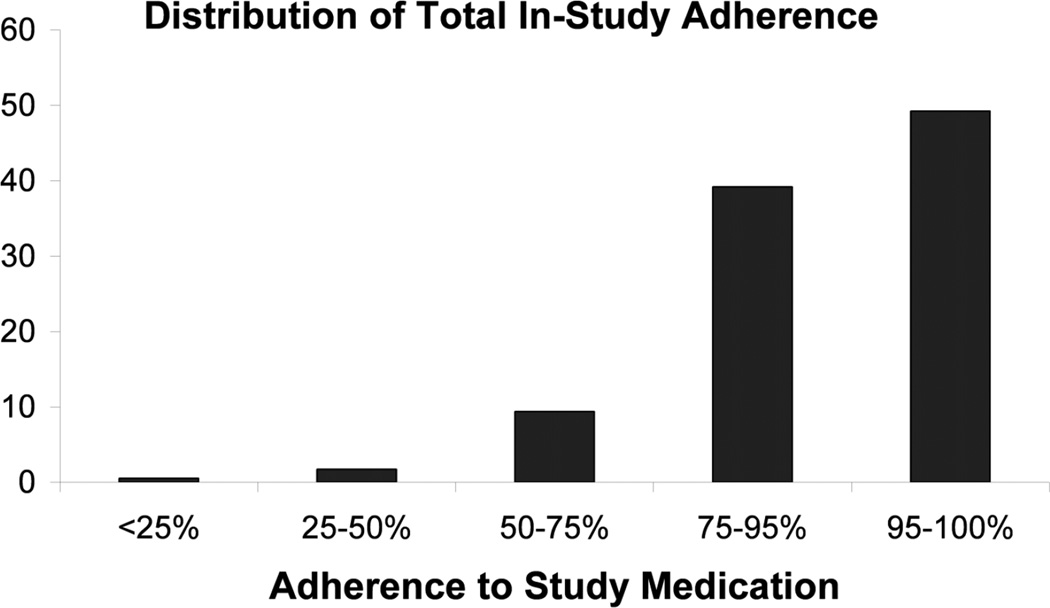
Among the 1354 placebo-allocated subjects in the BEST trial, we had non-missing adherence and outcome data on 1174(87%). Nearly half (49%) were greater than or equal to 95% compliant, and an additional 39% were between 75% and 95% compliant, leaving only 136 (12%) who took less than 75% of their prescribed study medication (Figure 1). Whites were more likely to be adherent than other races and current smokers were less likely to be adherent. Triglycerides were lower and heart rates were higher among non-adherent subjects (Table 1). Overall, there were 346 in-study deaths (Table 2) in the placebo group, with 88% attributable to cardiovascular causes and, of those, 44% were due to CHD.
Figure 1.
Distribution of total in-study adherence among 1174 placebo-allocated participants in the BEST study
Table 1.
Characteristics of placebo-allocated participants, overall and by adherence level.
| Baseline Characteristic | Overall N=1174 | Higher Adherence* N=1038 (88%) | Lower Adherence N=136 (12%) | p-value |
|---|---|---|---|---|
| Demographics | ||||
| Age in years, mean (SD) | 60.5 (11.9) | 60.5 (11.8) | 60.4 (12.8) | 0.961 |
| Race, N (%) | <0.001 | |||
| White | 830 (71) | 763 (74) | 67 (49) | |
| African-American | 260 (22) | 206 (20) | 54 (40) | |
| Hispanic | 68 (6) | 57 (5) | 11 (8) | |
| Other | 16 (1) | 12 (1) | 4 (3) | |
| Sex - Female, N (%) | 258 (22) | 232 (22) | 26 (19) | 0.392 |
| Clinical Characteristics | ||||
| Systolic Blood Pressure, mmHg, mean (SD) | 119.4 (18.9) | 119.5 (18.5) | 118.5 (22.3) | 0.619 |
| Heart Rate, bpm, mean (SD) | 81.7 (13.0) | 81.4 (12.9) | 83.9 (13.4) | 0.037 |
| Diabetes, N (%) | 408 (35) | 358 (34) | 50 (37) | 0.600 |
| Body mass index, N(%) | 0.419 | |||
| ≤25 | 386 (33) | 340 (33) | 46 (34) | |
| 25 – 30 | 422 (36) | 368 (35) | 54 (40) | |
| ≥ 30 | 366 (31) | 330 (32) | 36 (26) | |
| Smoker N (%) | 0.025 | |||
| Current | 189 (16) | 161 (16) | 28 (21) | |
| Past | 654 (56) | 593 (57) | 61 (45) | |
| Laboratory Parameters | ||||
| Total cholesterol (mg/dl), mean (SD) | 196.3 (46.3) | 193 (45.1) | 196.6 (45.5) | 0.507 |
| Triglycerides (mg/dl), mean (SD) | 219.2 (188.5) | 223.1 (191.4) | 188.0 (160.6) | 0.029 |
| Health Characteristics/Status | ||||
| Well being (now compared to 3 months ago) | 0.521 | |||
| Much worse | 89 (8) | 81 (8) | 8 (6) | |
| Somewhat worse | 221 (19) | 196 (19) | 25 (18) | |
| Same | 265 (23) | 233 (22) | 32 (24) | |
| Somewhat better | 271 (23) | 245 (24) | 26 (19) | |
| Better | 327 (28) | 282 (27) | 45 (33) |
“Higher Adherence” defined as >= 75% total in-study placebo medication adherence
Table 2.
Unadjusted hazard ratios and 95% confidence intervals for all outcomes; comparing those with total adherence of 75% or greater to those with lesser adherence
| Number of events | ||||
|---|---|---|---|---|
| Outcome | Higher-Adherent Participants1 (N=1038) N(%) | Lower-Adherent Participants2 (N=136) N(%) | HR | 95% CI |
| Total Mortality | 291(28) | 55(42) | 0.61 | 0.46 – 0.82 |
| CVD Mortality | 256(25) | 47(36) | 0.63 | 0.46 – 0.86 |
| Non-CVD Mortality | 27(3) | 6(5) | 0.52 | 0.21 – 1.26 |
| CVD Mortality with Sx | 130(12) | 31(24) | 0.48 | 0.33 – 0.71 |
| CHD Mortality | 117(11) | 15(11) | 0.91 | 0.53 – 1.56 |
| Pump Failure | 96(9) | 21(16) | 0.53 | 0.33 – 0.85 |
| CHD Morbidity/Mortality | 136(13) | 16(12) | 1.00 | 0.60 – 1.69 |
defined as total adherence of at least 75%
defined as total adherence less than 75%
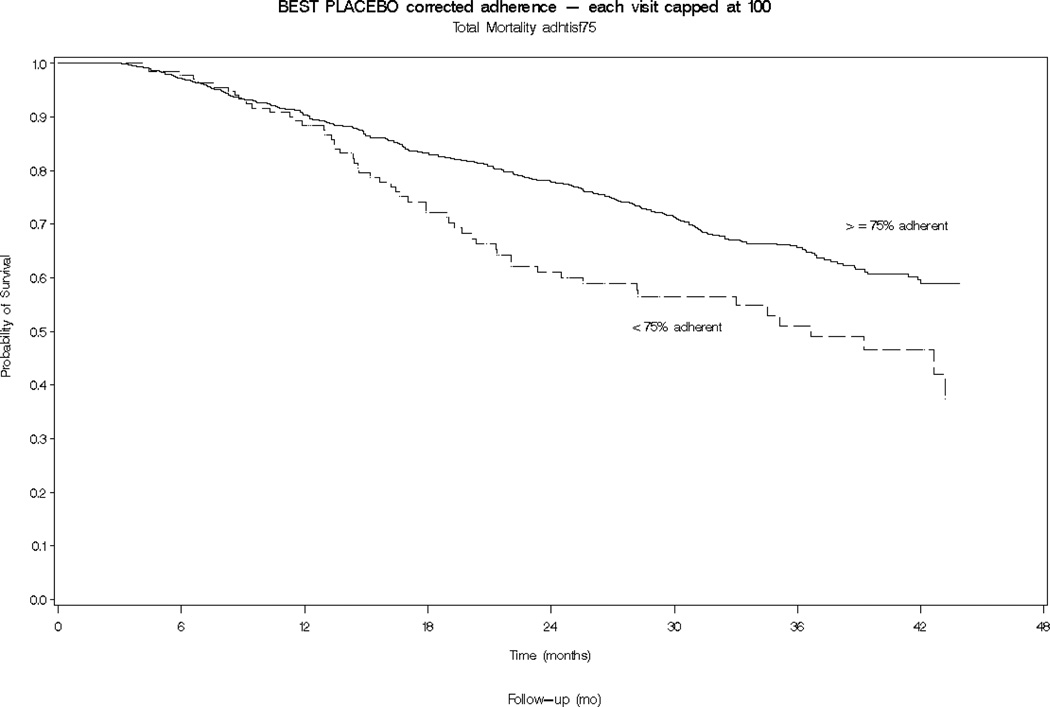
Participants who were at least 75% adherent to their placebo study medication had a significantly lower total mortality compared to less-adherent participants (HR = 0.61, 95% Confidence Interval (CI): 0.46 to 0.82; Table 2 and Figure 2). When considering cause-specific mortality, the association was found to be statistically significant for CVD mortality and pump failure, but was attenuated and non-significant for CHD. For non-CVD mortality, the effect was of similar magnitude (HR = 0.52 CI: 0.21 to 1.26) but statistical significance was not achieved, possibly due to the small number of events.
Figure 2.
Kaplan Meier curves of cumulative survival for higher-adherent (≥ 75%, blue line) and lower-adherent (< 75%, red line) placebo-allocated participants in the BEST study
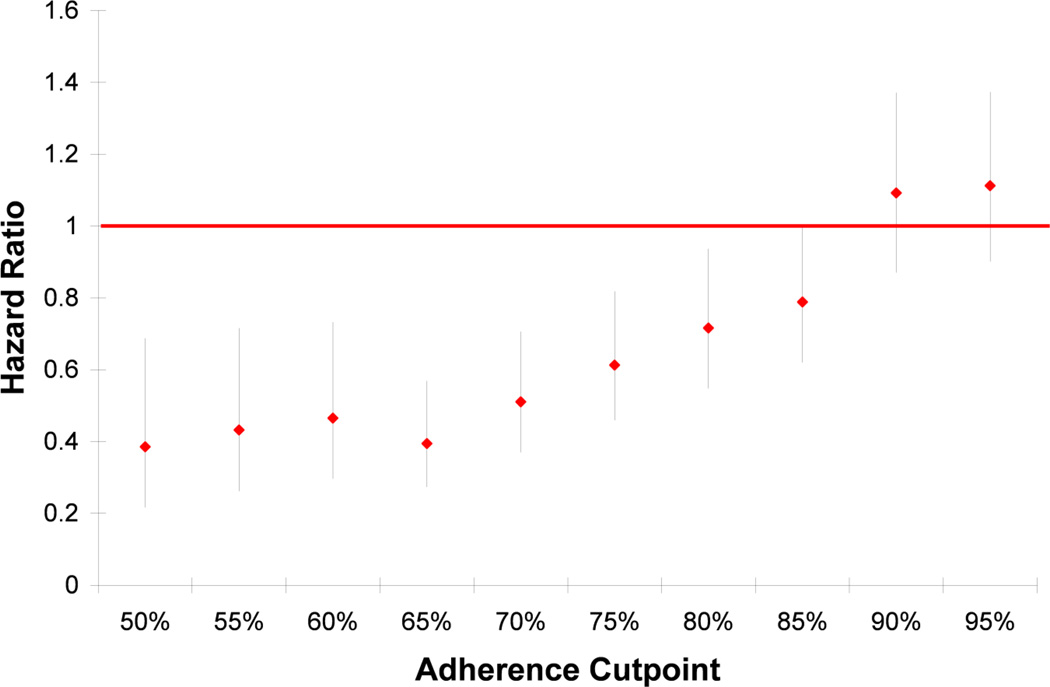
The association between placebo adherence and total mortality remained when adherence was treated as a continuous measurement (HR = 0.45, 95% CI: 0.42–0.49 for every 10% increase in adherence). When adherence was calculated as a mean cumulative adherence variable, the association lessened, but remained significant (HR = 0.72, 95% CI: 0.52 to 0.99). However, the association attenuated and was no longer significant when individual visit adherence was used as a time-varying covariate (HR = 0.89, 95% CI: 0.58 to 1.39, for ≥75% adherence versus less adherent). The value of the total mean adherence for which the association with mortality was strongest was found to be at 50% adherence (HR = 0.39, 95% CI: 0.22 to 0.69), and the relationship of cut-point to hazard ratio appeared to be linear (Figure 3).
Figure 3.
Hazard ratios (95% CI) of total mortality for adherent versus non-adherent by cut-point of adherence in the BEST study. Sensitivity analysis of cut-point for defining higher and lower adherence.
Adjustment for potential confounders did not result in any meaningful change in the association for any of the outcomes (Table 3). Adjustment for modifiable and non-modifiable CVD risk factors, and psychosocial variables, had little effect on the hazard ratios (Table 3).
Table 3.
Adjusted Hazard Ratios and 95% CIs comparing those with total adherence of 75% or greater to those with lesser adherence for five different adjusted models
| Outcome | Non- modifiable Risk Factors1 HR (95% CI) |
Modifiable Risk Factors2 HR (95% CI) |
All Risk Factors3 HR (95% CI) |
Psychosocial Measures4 HR (95% CI) |
Full Model5 HR (95% CI) |
|---|---|---|---|---|---|
| Total Mortality | 0.58 (0.43,0.78) | 0.57 (0.43,0.77) | 0.56 (0.42,0.77) | 0.61 (0.46,0.82) | 0.57 (0.42,0.77) |
| CVD Mortality | 0.58 (0.42,0.80) | 0.59 (0.43,0.81) | 0.56 (0.40,0.78) | 0.63 (0.46,0.86) | 0.56 (0.40,0.78) |
| Non-CVD Mortality | 0.57 (0.23,1.42) | 0.44 (0.16,1.18) | 0.57 (0.20,1.62) | 0.52 (0.21,1.26) | 0.57 (0.20,1.62) |
| CVD Mortality with Sx | 0.43 (0.29,0.65) | 0.47 (0.31,0.70) | 0.44 0.29,0.67) | 0.48 (0.33,0.71) | 0.44 (0.29,0.67) |
| CHD Mortality | 0.87 (0.50,1.50) | 0.80 (0.46,1.38) | 0.77 (0.44,1.36) | 0.91 (0.53,1.55) | 0.78 (0.44,1.37) |
| Pump Failure | 0.47 (0.29,0.77) | 0.51 (0.32,0.83) | 0.49 (0.30,0.82) | 0.52 (0.33,0.84) | 0.50 (0.30,0.83) |
| CHD Morbidity/Mortality | 0.96 (0.57,1.63) | 0.88 (0.52,1.49) | 0.86 (0.50,1.48) | 1.00 (0.59,1.67) | 0.87 (0.57,1.49) |
Age, race, sex
Smoking status, Total Cholesterol, Triglycerides, Heart Rate, BMI category, Systolic BP, Hx of Diabetes
1,2
Quality of life - Perceived health status
All
To test for time-dependent confounding, we used several analytic methods. First, we estimated the association after eliminating the last and the last two adherence measurements; this procedure resulted in very little attenuation in the association (HR = 0.66, 95% CI: 0.48 to 0.91 and HR = 0.61, 95% CI: 0.44 to 0.86). Next, we lagged the adherence variable in the survival models with cumulative adherence by one and by two measurements (HRs = 0.77, 95% CI: 0.54 to 1.10 and HR = 0.77, 95% CI: 0.53 to 1.12, respectively). Finally, when adherence was entered as a time-dependent variable, the association became stronger with one lag and weaker with 2 lags. (HR = 0.89, 95% CI: 0.58 to 1.39 for no lag; HR = 0.64, 95% CI: 0.42 to 0.98 for one lag; and HR = 0.80, 95% CI: 0.49 to 1.30 for two lags).
Neither formal testing for proportionality nor visual inspection of survival curves for the primary model suggested violation of the proportionality assumption.
Discussion
Similar to our prior analysis of the SOLVD CHF trial (10), we found that adherence to placebo study medication was strongly associated with mortality. Total in-study mortality was reduced by nearly 40% among those participants who were at least 75% adherent to their study medication relative to those who were less adherent. This association was consistent for cardiovascular mortality and CHF-related mortality, though not for non-cardiovascular or CHD mortality. Adjustment for numerous baseline risk factors did not substantially attenuate the relationship.
There is no clear explanation for why better adherence to an inert pill should be associated with a 39% decrease in one’s mortality risk. However, the magnitude of this relationship is very similar to that found in several other clinical trials that have explored this association (1–5, 7, 13, 14). Because the relationship is unlikely to be causal, an underlying explanation likely exists. Using data from the original BEST trial, and restricting our analyses to the placebo arm, we had a unique opportunity to study this perplexing association, and to try to determine the underlying mechanism.
We addressed several theories that might explain this observed association between placebo adherence and decreased mortality. The first and most obvious is that of publication bias. Trials that observe this provocative effect will tend to report it, while those that do not find this effect are much less likely to do so. Publication bias, however, seems unlikely as we have now identified the effect in four trials, none of which reported this in any prior publications. These trials include the two SOLVD studies (10), and a third trial, Hormone and Estrogen Replacement Study (HERS), for which the manuscript is in process. Our results are consistent with several other studies that have examined this issue, though not all studies have found similar observations (15–17). Of course, there are many more trials than those examined and the possibility of publication bias cannot be entirely ruled out.
Next, we tested different outcomes to see if the association was driven by a particular cause of mortality. Unlike our previous experience with cause-specific mortality models (10), we found that, despite a relatively large number of events, the association with placebo adherence in the BEST trial was present for overall and cardiovascular mortality but did not persist among the smaller subgroup of CHD outcomes. It is not clear why CHD mortality behaved differently from other cardiovascular mortality. Certainly, reduced statistical power in this subgroup may have played a role as the number of events was much reduced in this subgroup. Type II statistical error is possible, particularly since this dichotomy has not been observed in other analyses.
Another possibility is that lifestyle variables are responsible for a person’s desire and ability to be adherent to study medications, and that these factors are also associated with decreased mortality. In order to test this theory, we created five sets of adjusted models: non-modifiable risk factors, modifiable risk factors, all risk factors, psychosocial factors, and all covariates. Effect estimates did not change appreciably for any of the models for any of the outcomes. Probably due to sample size constraints in some of the less-common outcomes, some effects were of similar magnitude, but were not statistically significant.
The next concept we tested was that of time-dependent confounding. We approached these analyses from several angles. To start, we used total in-study adherence as a predictor, first dropping the last visit from the calculation, and then dropping the last two visits. In calculating adherence this way, we removed the effect of the closest adherence measure to the event. If there is an underlying process that is causing both decreased adherence and death, the adherence ought to drop in the visits immediately preceding the event. However, we found that the reduction in mortality was unchanged when we excluded last and second-to-last visit adherences from the total in-study adherence calculations. Next, we considered cumulative adherence up to the time of event modeled as a time-varying covariate, and used a lag of one and two visits to test for changes just prior to the event. Though these estimates started a bit higher than their total in-study counterparts, (HR = 0.77, p>0.05) the effect sizes changed very little when we lagged the calculations.
Both these methods of correcting for time-dependent confounding are subject to bias caused by length of time in study. The longer a subject is in the study, the greater the number of adherence measures available for the calculation of total or cumulative adherence, and the lesser the influence of a single measure. In a study in which everyone has complete data, this impact is non-differential. However, if those who are less adherent tend to drop out or die at a greater rate (the very concept we are testing) than those who are more adherent, then the impact can create an artificial association. Those who terminate early (deaths and drop-outs) have fewer adherence measures and therefore experience a bigger impact from the removal of a single measure. To avoid this artifact, we used individual visit adherence as a time-varying covariate. This yielded perplexing results. For the adherence immediately prior to the risk set, the hazard ratio, 0.89, was not significant. However with a single lag period, the hazard ratio, 0.64, was significant and similar to that from the total in-study model. With two lag periods, this effect attenuated and was no longer significant, HR= 0.80. Of course, this procedure introduces its own uncertainty in that all prior adherence measurements are ignored in this calculation.
The results of these analyses, viewed in light of prior studies of this question, appear to generally support the presence of an association between higher adherence levels to placebo and reduced mortality. As noted by Simpson, et al., the great majority of studies that have examined this issue have found associations of a similar strength (1). Importantly, we determined a priori to publish the results of these analyses, regardless of the outcome, in order to avoid contributing to any possibility of publication bias.
It is becoming increasingly clear that this association is not due to simple confounding: as in several other studies (2–5, 7), adjustment for numerous risk factors for mortality (including blood pressure, smoking status, lipid levels, diabetes, etc.) has no appreciable effect on the presence of the association. While it would seem intuitive that high adherence levels are associated with healthier lifestyles, in general, adherence levels are independent of such factors.
The possibility of time-dependent confounding remains a particularly difficult construct for which to control. We used several different models in an attempt to better understand how such a phenomenon, sometimes referred to as “effect-cause” (18) might explain the association between placebo adherence and mortality (including treating placebo adherence as a pure time-varying covariate and lagging the adherence variable in the total and cumulative-adherence models). None of the models is entirely satisfactory in addressing this problem and, while the results tended not to support time-dependent confounding as an important factor, the results were not fully consistent and this possibility cannot be rejected.
In the BEST trial, the strength of the unadjusted relationship, a 39% reduction in total mortality, was strong and of similar magnitude to effects we and others have previously reported. In the pair of SOLVD trials we found that both the treatment and the prevention trials exhibited a 48% reduction in total mortality in unadjusted models (10). In addition, several independent studies have reported the same or even stronger effects. In a meta-analysis summarizing many of these reported associations, Simpson, et al., calculated an overall effect size of HR = 0.56 (95% CI: 0.43 – 0.74) (1). Our analyses of the BEST trial further support the presence of this association.
While the BEST data provided an excellent opportunity to gain deeper insight into the association between placebo adherence and mortality, several limitations also exist. The data contained relatively little information about potentially important lifestyle factors such as exercise, diet, and psychological states; hence, residual confounding may be present despite the extensive multivariate modeling. In addition, study of placebo adherence was never an intent of the original investigators, so the measurement of adherence may be imperfect. In fact this trial had no objective measures of adherence, such as smart pill bottles or blister packs. Finally, this trial was focused on patients with CHF and may not generalize well to other populations.
Looking to the future, we plan to repeat our analyses in other completed studies to continue exploring the ideas presented in this manuscript. However, the best way to study this perplexing effect is to build the analyses into the design of a new clinical trial. Among the trials in which this effect has been noted, none has adequately measured a comprehensive list of clinical and care-related variables. Building the adherence-mortality association question into the design of a new trial will enable us to carefully measure other potential confounders of the relationship, such as exercise, spirituality and other lifestyle factors. We will also be able to test non-fatal outcomes, and we can design the trial to collect the best possible measures of adherence.
Conclusions
In summary, extensive analyses of the BEST trial data support a strong association between adherence to placebo study medication and total mortality. While probably not due to publication bias or simple confounding by healthy lifestyle factors, the underlying explanation for the association remains enigmatic. Time-dependent confounding may contribute to this association, and strong unmeasured predictors of mortality may also play an important role. It is likely that only carefully planned prospective examinations of this association will be sufficiently reliable to better understand the existence and explanations for this fascinating observation.
Acknowledgements
The Beta Blocker Evaluation of Survival Trial (BEST) was conducted and supported by the NHLBI in collaboration with the BEST Study Investigators. This manuscript was prepared using a limited-access dataset obtained from the NHLBI and does not necessarily reflect the opinions or views of the BEST or the NHLBI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Simpson SH, Eurich DT, Majumdar SR, Padwal RS, Tsuyuki RT, Varney J, et al. A meta-analysis of the association between adherence to drug therapy and mortality. BMJ. 2006 Jul 1;333(7557):15. doi: 10.1136/bmj.38875.675486.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Irvine J, Baker B, Smith J, Jandciu S, Paquette M, Cairns J, et al. Poor adherence to placebo or amiodarone therapy predicts mortality: Results from the CAMIAT study. arrhythmia trial. Psychosom Med. 1999 Jul–Aug;61(4):566–575. doi: 10.1097/00006842-199907000-00023. [DOI] [PubMed] [Google Scholar]
- 3.Granger BB, Swedberg K, Ekman I, Granger CB, Olofsson B, McMurray JJ, et al. Adherence to candesartan and placebo and outcomes in chronic heart failure in the CHARM programme: Double-blind, randomised, controlled clinical trial. Lancet. 2005 Dec 10;366(9502):2005–2011. doi: 10.1016/S0140-6736(05)67760-4. [DOI] [PubMed] [Google Scholar]
- 4.Gallagher EJ, Viscoli CM, Horwitz RI. The relationship of treatment adherence to the risk of death after myocardial infarction in women. Jama. 1993;270(6):742–744. [PubMed] [Google Scholar]
- 5.Horwitz RI, Viscoli CM, Berkman L, Donaldson RM, Horwitz SM, Murray CJ, et al. Treatment adherence and risk of death after a myocardial infarction. Lancet. 1990 Sep 1;336(8714):542–545. doi: 10.1016/0140-6736(90)92095-y. [DOI] [PubMed] [Google Scholar]
- 6.Walker AS, Ford D, Mulenga V, Thomason MJ, Nunn A, Chintu C, et al. Adherence to both cotrimoxazole and placebo is associated with improved survival among HIV-infected zambian children. AIDS Behav. 2009 Feb;13(1):33–41. doi: 10.1007/s10461-008-9382-4. [DOI] [PubMed] [Google Scholar]
- 7.Anonymous. Influence of adherence to treatment and response of cholesterol on mortality in the coronary drug project. N Engl J Med. 1980;303(18):1038–1041. doi: 10.1056/NEJM198010303031804. [DOI] [PubMed] [Google Scholar]
- 8.The BEST Steering Committee. Design of the beta-blocker evaluation survival trial (BEST) Am J Cardiol. 1995 Jun 15;75(17):1220–1223. doi: 10.1016/s0002-9149(99)80766-8. [DOI] [PubMed] [Google Scholar]
- 9.National heart lung and blood data repository - biologic specimen and data repository information coordinating center(BioLINCC) [Internet] Bethesda, Maryland: NHLBI; 2011. [cited May 20, 2011]. Available from: https://biolincc.nhlbi.nih.gov/home/. [Google Scholar]
- 10.Avins AL, Pressman A, Ackerson L, Rudd P, Neuhaus J, Vittinghoff E. Placebo adherence and its association with morbidity and mortality in the studies of left ventricular dysfunction. J Gen Intern Med. 2010 Dec;25(12):1275–1281. doi: 10.1007/s11606-010-1477-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kleinbaum DG, Klein M. Survival analysis. 2nd ed. New York: Springer; 2005. [Google Scholar]
- 12.SAS Institute I. SAS statistical software. 2008. 9.2. [Google Scholar]
- 13.Czajkowski SM, Chesney MA, Smith AW. Adherence and the placebo effect. In: Shumaker SA, Schron E, Ockene JK, editors. The handbook of health behavior change. New York: Springer Publishing Company, Inc.; 1990. pp. 409–423. [Google Scholar]
- 14.Horwitz RI, Horwitz SM. Adherence to treatment and health outcomes. Arch Intern Med. 1993;153(16):1863–1868. [PubMed] [Google Scholar]
- 15.Obias-Manno D, Friedmann E, Brooks MM, Thomas SA, Haakenson C, Morris M, et al. Adherence and arrhythmic mortality in the cardiac arrhythmia suppression trial (CAST) Ann Epidemiol. 1996 Mar;6(2):93–101. doi: 10.1016/1047-2797(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 16.The Lipid Research Clinics Program. The lipid research clinics coronary primary prevention trial results: I. reduction in incidence of coronary heart disease. JAMA. 1984;251:351–364. doi: 10.1001/jama.1984.03340270029025. [DOI] [PubMed] [Google Scholar]
- 17.The Lipid Research Clinics Program. The lipid research clinics coronary primary prevention trial results: II. the relationship of reduction in incidence of coronary heart disease of cholesterol lowering. JAMA. 1984;251:365–374. [PubMed] [Google Scholar]
- 18.Hulley SB, R CS, Browner WS, Grady D, Newman TB. Designing clinical research. 3rd ed. Philadelphia: Lippincott, Williams, and Wilkins; 2007. [Google Scholar]