Abstract
Cortactin is an F-actin binding protein that functions as a scaffold to regulate Arp2/3 mediated actin polymerization in lamellipodia and invadopodia formation as well as functioning in cell migration and endocytosis of many different cell types. In light of the fact that regulated actin polymerization is critical for many cellular processes we launched a search for novel cortactin interactions with cellular proteins that might indicate heretofore undescribed biological activities supported by cortactin. Using a modified stable isotope labeling in cell culture (SILAC) approach in HEK293 cells and Flag-tagged cortactin (F-cortactin) as bait, we identified a limited set of cortactin interactions including several proteins which have not previously been identified as cortactin associated proteins. Among these were serine/threonine-protein phosphatase 2A subunit beta (PP2A-beta) and RCC2/TD60, a Rac guanine nucleotide exchange factor (GEF) required for completion of mitosis and cytokinesis. The interaction between cortactin and RCC2/TD60 was verified in cell lysates immunoprecitated with anti- RCC2/TD60 antibody. Furthermore, cortactin was localized by immunofluorescence in the equatorial plane of dividing HeLa cells in the region where RCC2/TD60 has previously been localized thus providing support for a complex containing cortactin and RCC2/TD60 complex that may play a functional role in cells undergoing mitosis.
Keywords: SILAC, focal adhesion, cortactin, RCC2/TD60
1. Introduction
The formation of cell edge protrusions requires cortical actin polymerization which is nucleated by the Arp2/3 complex [1]. The F-actin binding protein cortactin is a key regulator of the Arp2/3 complex and promotes dynamic actin-rich protrusion of the cell membrane, including circular dorsal ruffles, lamellipodia and invadopodia [2–7]. Cortactin is a multi-domain scaffold protein that interacts with the Arp2/3 complex via amino-terminal acidic (NTA) domain, binds F- actin via its “repeat” domain and interacts via its SH3 domain with a repertoire of cytoplasmic effector proteins. When cortactin binds the Arp3 subunit of the Arp2/3 complex there is weak stimulation of F-actin nucleation whereas in conjunction with Wiscott-Aldrich protein (N-WASP) there is a robust F-actin nucleation by the Arp2/3 complex [8–10]. Cortactin can also stabilize Arp2/3-mediated F-actin branching in vitro [11], and this activity may be critical for the stability of F-actin–rich cellular protrusions in vivo [12]. The SH3-domain of cortactin has been demonstrated to interact with a variety of proteins including: Arp2/3 stimulating N-WASP [13]; the WASP-interacting protein WIP [14]; the missing in metastasis protein, MIM [15]; the endocytic GTPase dynamin-2 [16]; the receptor endocytosis-regulator scaffold CD2AP [17]; the tight junction (TJ) protein ZO-1 [18]; the synaptic adaptor protein Shank2 [19]; the Cdc42 activator guanine nucleotide exchange factor (GEF) faciogenital dysplasia 1 (FGD1) [20]; as well as other important cellular modulating proteins. This suggests an important, central role for cortactin in a variety of biological processes related to cell proliferation, migration, invasion and endocytosis; all processes which require regulated actin polymerization [21–27].
Given cortactin’s potential for numerous interaction partners based on the diversity of binding motifs in its structure we pursued the identification of heretofore undescribed cortactin interacting proteins which might point to novel cellular activities. A variety of approaches can be found in the literature for identifying novel binding partners for proteins [28–33]. Most suffer from issues associated with nonspecific binding to the bait or matrix making it somewhat problematic to determine bona fide biologically relevant binding partners. In this study we utilized the Stable Isotope Labeled Amino Acids in Cell Culture (SILAC) technology to enhance the capability to discriminate between specific and non-specific interactions with tagged baits. Using SILAC labeling in conjunction with immunopurification and LC/MS/MS analysis of labeled proteins we identified a limited list of proteins as potential bona fide interacting partners of cortactin. One cortactin associated protein, RCC2/TD60, a Rac guanine nucleotide exchange factor (GEF) is known to be involved in completion of mitosis and cytokinesis [34]. Binding of cortactin to RCC2 was verified by reverse immunoprecipitation. Finally, we demonstrated cortactin to be localized to the equatorial plate of dividing cells; a region known to be enriched for RCC2/TD60. These observations suggest a novel role for cortactin in cells undergoing mitosis, possibly via the regulation of actin polymerization and thus warrant further studies on the role of cortactin in these key cellular processes..
2. Material and Methods
2.1 Cells and transfections
HEK 293 cells grown in MEM supplemented with 10% FBS were transfected using Polyfectin (Qiagen) and pcDNA3.1 based plasmids containing either full length Flag-tagged cortactin (pF-cortactin), or EGFP (enhanced green fluorescent protein)-labeled cortactin (pEGFP-cortactin) as described previously [35, 36]. Following transfection, G418 resistant clones were selected by limited dilution. Expression of Flag-tagged cortactin in G418 resistant cells was confirmed by immunoblot analysis of cell lysates with anti-Flag peptide monoclonal antibody M5 and in the case of the EGFP-labeled cortactin transfected cells by fluorescent microscopy of the cells. HEK293 cells were also independently transfected with plasmids pF-cortactinDD and pF-cortactinWK coding for Flag-tagged N- and C- terminal cortactin mutants that fail to interact efficiently with ARP2/3 proteins and dynamin, respectively [37].
2.2 Affinity purification
In a typical experiment 3–5 × 107 cells expressing Flag-tagged cortactin (F-cortactin) were lysed in CSK-NP buffer (0.15 M NaCl, 0.005 M MgCl2, 0.02 M Tris-HCl pH 7.4, 10% glycerol, 1% NP40, 0.01 M Na ortho-vanadate and proteinase inhibitor cocktail (Roche). Clarified lysates (3-5mg/ml final protein concentration) were incubated with M2 agarose beads (Sigma) for 2 h at 4°C followed by 1 wash with 15-20 volumes of CSK-NP buffer and medium salt (MS) buffer (0.1 M NaCl, 2 mM MgCl2, 25 mM Tris-HCl pH 7.4). The beads were sequentially extracted with 0.3 ml of high salt (HS) buffer (1M NaCl, 2mM MgCl2, 25 mM Tris-HCl pH 7.4) for 20 min at 0-4°C and 150 ul of Flag peptide solution (200ug/ml in 0.1M Na Cl, 20 mM Tris-HCl, pH 7.5) followed by rotation at room temperature for 30 min. Both HS and Flag elution fractions were then subjected to LC/MS/MS analysis to identify proteins present in the immunocomplexes.
2.3 Western blot analysis
Samples were subjected to SDS-PAGE on 8% gels followed by electroblotting on nitrocellulose membranes (Protran). The membranes were then first stained with Ponceau to visualize proteins followed incubation with a mixture of antibodies against Flag-peptide (SIGMA), dynamin, ARP3 (Biosource) and RCC2 (Methyl) followed by standard chemiluminescent detection (ECL, Amersham).
2.4 SILAC
For SILAC labeling F-cortactin expressing HEK 293 cells were grown for 5–6 generations in “heavy” media containing 13C lysine and 13C/15N arginine (Cambridge Isotope Laboratories). Untransfected HEK 293 cells were grown in parallel in “light” media containing regular amino acids. Cell lysates were prepared in CSK-NP buffer from heavy and light cells as described above, clarified (15 min at 15,000 rpm) and mixed to yield approximately 4–5 mg of total protein in a final volume of 6 ml. The mixed lysates were incubated with M2 agarose beads, preincubated with HEK 293 cell lysate to minimize non-specific binding, followed by MS and HS washes and then eluted with Flag-peptide buffer as described above. The eluted proteins were subjected to LC/MS/MS analysis.
2.5. Sample processing and mass spectroscopic analysis
Samples were diluted six fold with 0.1 M ammonium bicarbonate pH 8.0 and then reduced using 10 μL of 10 mM dithiolthreitol in 0.1 M ammonium bicarbonate at room temperature for 0.5 h followed by alkylation with 10 μL 50 mM iodoacetamide in 0.1 M ammonium bicarbonate at room temperature for 0.5 h. Samples were then digested with 1 μg Promega trypsin in 50 mM ammonium bicarbonate overnight at room temperature. The digestion was quenched by bringing the sample to 5% acetic acid. The LC/MS/MS system consisted of a Thermo Electron LTQ-FT mass spectrometer with a Protana nanospray ion source interfaced to a self-packed 8 cm × 75 um id Phenomenex Jupiter 10 um C18 reversed-phase capillary column. 25% of the digested sample was injected and the peptides eluted from the column by an acetonitrile/0.1 M acetic acid gradient at a flow rate of 0.4 μL/min over 2 h. The nanospray ion source was operated at 2.5 kV. The digest was analyzed using the double play capability of the instrument acquiring a full scan mass spectrum (100K resolution FTICR) to determine peptide molecular masses followed by 10 product ion spectra (ion trap) to determine amino acid sequences.
The data were analyzed by database searching using the Sequest (search algorithm against NCBI NR database (downloaded August 2011) restricted to human. Parent tolerance was 8ppm and fragment tolerance was 1Da. The potential matches were first filtered by xcorr (+1>1.8, +2>2.3, +3>2.7, +4>3.7). Any peptides that passed these initial filters and demonstrated from the SILAC experiments to be likely specific cortactin binding partners were manually verified. In SILAC experiments for those proteins of interest and some control proteins (i.e. all light) the selected ion chromatograms for the individual peptides detected (light and heavy) were plotted, areas calculated and the average L:H ratio was then calculated for each of these proteins.
2.6. Proteins associated with endogenous cortactin in mitotic cells
HeLa cells were grown overnight to 70–80% confluence. Nocodazole was added to the media to a final concentration of 50ng/ml and the cells allowed to incubate for 20–24 h. Clarified cells lysates prepared in CSK-NP buffer were pre-incubated with protein A Sepharose beads for 1 hour with rotation at 4°C. The beads were pelleted and the supernatant divided into two identical aliquots that were incubated for 2 h at 4°C with either the cortactin monoclonal antibody 4F11 (Biosource) or control monoclonal antibody against a non-related protein. Antibodies were precipitated with a mix of protein G and protein A agarose beads (10% resuspended in saline buffer) followed by washings with CSK-NP and saline PBS and resuspended in SDS mercaptoethanol containing sample buffer and analyzed by immunoblotting with an anti-RCC2/TD60 rabbit polyclonal antibody.
2.5. Fluorescence analysis of EGFP-cortactin expressing cells
pEGFP-cortactin expressing cells were grown on fibronectin coated coverslips and treated with 100 nM nocodazole and/or 5 mM thymidine for cell cycle synchronization [38]. Cells were fixed with 4% paraformaldehyde and mounted on slides using ProLong Gold with DAPI (Invitrogen). EGFP-cortactin was visualized using a Nikon Eclipse TE2000-E confocal microscope with a 60X objective. The fluorophore was excited using a 40nW 488/514 argon ion laser.
3. Results
3.1 F-cortactin cellular complexes
To confirm the functional interactions of F-cortactin with known binding partners immune complexes were prepared from cells transiently transfected with wild type F-cortactin and two mutants of cortactin, F-cortactin-DD and F-cortactin-WK. Figure 1 shows a Ponceau staining profile following PAGE-SDS analysis and blotting onto nitrocellulose of M2 agarose affinity purified F-cortactin complexes from lysates of cells expressing F-cortactin (Panel A, lane 2) , F-cortactin DD (containing a mutation in the NTA region that abrogates Arp2/3 binding, lane 3), F-cortactin WK (containing a mutation in the C-terminal SH3 domain that abrogates the binding of dynamin and other SH3 binding proteins, lane 4), and F-FAK (FF, lane 1). Immunoblotting with specific antibodies that detect two known binding partners of cortactin, dynamin and actin related protein 3 (ARP3, panel B, lanes 2 and 3), confirmed the presence of these proteins in the F-cortactin immune complexes and not in immune complexes from F-FAK expressing cells (lane 1). The specificity of the interaction between recombinant F-cortactin and dynamin and ARP3 was confirmed by the loss of binding of these proteins by F-cortactin variants with mutations that abrogate the binding of dynamin and ARP3 (Panel B, lanes 3 and 4). These results confirm that recombinat F-cortactin expressed in transfected cells is functional in terms of its ability to interact with the its binding partners dynamin and Arp2/3 [37].
Figure 1.
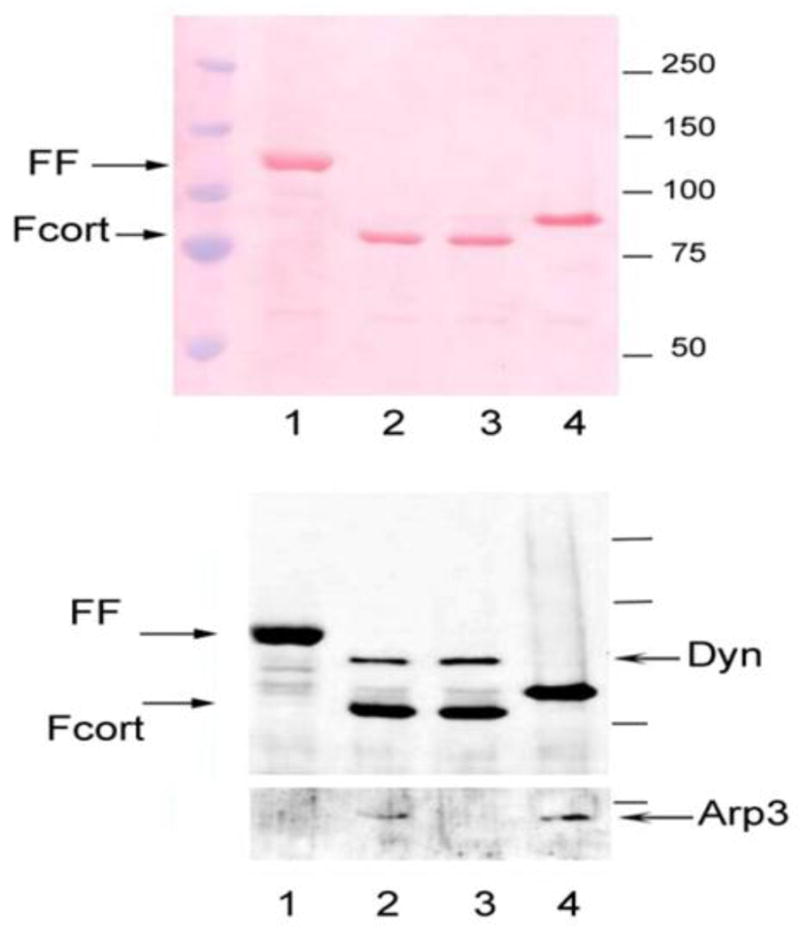
Immunodetection of cortactin binding partners in M2 agarose eluates. SDS PAGE of M2 agarose eluates. Eluates were electrophoresed in 8% PAGE-SDS gel, blotted and stained with Ponceau followed by incubation with a mixture of anti-Flag, anti-dynamin and anti-ARP3 antibodies followed by chemiluminescent detection as described in the text. Lane 1 : Flag-FAK (FF) expressing cells. Lane 2: Flag-cortactin (F-cort). Lane 3: Flag-cortactin DD mutant. Lane 4: Flag-cortactin WK mutant.
3.2 SILAC Experiments
SILAC labeling was used to differentiate between bona fide partners of cortactin and non-specific binding of cellular proteins to the antibody/bead matrix during the purification. LC/MS/MS analysis identified 1776 peptides representing 154 proteins. Of these peptides, 1010 (57%) were ‘light” and 766 (43%) were “heavy” labeled (see Appendix). Table 1 shows a list of the proteins identified that showed exclusively either a “light” or “heavy” peptide profile in MS/MS analysis. Only 2 % of the total peptides (43 out of the total 1776 above) representing 19 proteins displayed an “all heavy” profile indicating that a very minor proportion of the eluted proteins were exclusively generated in the heavy labeled lysate. Among them were members of the ARP2/3 complex (i.e., actin related protein 2, actin related protein 2/3 complex subunits 1A, 3 and 4) as well as dynamin confirming the effectiveness of F-cortactin bait as well as also validating the SILAC strategy for enhanced identification of specific binders to cortactin. Several proteins were identified which had not been previously reported as cortactin binding partners including: RCC2/TD60; and PP2A, a serine/threonine-protein phosphatase that localizes at centromeres and the spindle poles during mitosis [39]. Keratins, among other proteins showed a “light” only peptide profile, indicating that they are most likely non-specific binding proteins generated during the purification process.
Table I.
Distribution of exclusively heavy or light labeled peptides eluted from M2-agarose beads from SILAC experiments
| Exclusively “Light” | Exclusively “Heavy” | ||||
|---|---|---|---|---|---|
| Protein name | Accession number | # Peptides | Protein name | Accession number | # Peptides |
| Keratin, type II cytoskeletal 1 | IPI00220327 | 27 | 60S ribosomal protein L30 | IPI00219156 | 4 |
| Keratin, type I cytoskeletal 9 | IPI00019359 | 21 | Isoform Long of Splicing factor, proline- and glutamine-rich | IPI00010740 | 5 |
| Keratin, type II cytoskeletal 2 epidermal | IPI00021304 | 15 | 60S ribosomal protein L6 | IPI00790342 | 3 |
| Keratin, type I cytoskeletal 14 | IPI00384444 | 6 | 60S ribosomal protein L7a | IPI00299573 | 4 |
| Isoform 1 of Gelsolin | IPI00026314 | 10 | Fructose-bisphosphate aldolase A | IPI00465439 | 3 |
| Keratin, type I cytoskeletal 10 | IPI00009865 | 19 | Nucleosome assembly protein 1-like 1 | IPI00023860 | 3 |
| Keratin, type II cytoskeletal 5 | IPI00009867 | 3 | Isoform 1 of Eukaryotic translation initiation factor 3 subunit B | IPI00396370 | 3 |
| D-3-phosphoglycerate dehydrogenase | IPI00011200 | 3 | 40S ribosomal protein S25 | IPI00012750 | 3 |
| Keratin, type II cytoskeletal 1 | IPI00220327 | 18 | 60 kDa heat shock protein, mitochondrial | IPI00784154 | 6 |
| Keratin, type I cytoskeletal 9 | IPI00019359 | 16 | cDNA FLJ59211, highly similar to Glucosidase 2 subunit beta | IPI00026154 | 3 |
| Probable serine carboxypeptidase CPVL | IPI00301395 | 3 | Isoform 1 of Dynamin-2 | IPI00033022 | 5 |
| Isoform 1 of Transcription intermediary factor 1-beta | IPI00438229 | 4 | 40S ribosomal protein S16 | IPI00221092 | 5 |
| Isoform 3 of Spectrin alpha chain, brain | IPI00843765 | 5 | 60S ribosomal protein L23a | IPI00021266 | 3 |
| Keratin, type I cytoskeletal 10 | IPI00009865 | 10 | Actin-related protein 2 | IPI00005159 | 6 |
| Isoform Short of Heterogeneous nuclear ribonucleoprotein U | IPI00479217 | 4 | Serine/threonine-protein phosphatase 2A catalytic subunit alpha | IPI00008380 | 4 |
| Heterogeneous nuclear ribonucleoprotein H | IPI00013881 | 4 | Actin-related protein 2/3 complex subunit 1A | IPI00333068 | 7 |
| Profilin-1 | IPI00216691 | 4 | Protein RCC2 | IPI00465044 | 4 |
| Myristoylated alanine-rich C-kinase substrate | IPI00219301 | 4 | Actin-related protein 2/3 complex subunit 3 | IPI00005162 | 3 |
| 60S acidic ribosomal protein P2 | IPI00008529 | 4 | Actin-related protein 2/3 complex subunit 4 | IPI00554811 | 6 |
| Vimentin | IPI00418471 | 6 | |||
| T-complex protein 1 subunit alpha | IPI00290566 | 3 | |||
| Importin subunit beta-1 | IPI00001639 | 4 | |||
| Isoform 1 of E3 ubiquitin-protein ligase TRIM21 | IPI00018971 | 3 | |||
| Peptidyl-prolyl cis-trans isomerase FKBP10 | IPI00303300 | 3 | |||
| Isoform 1 of Myosin-9 | IPI00019502 | 10 | |||
|
| |||||
| 25 proteins identified from 209 peptides | 19 proteins indentified from 80 peptides | ||||
3.3 Verification of RCC2/TD60 as a Cortactin Associated Protein
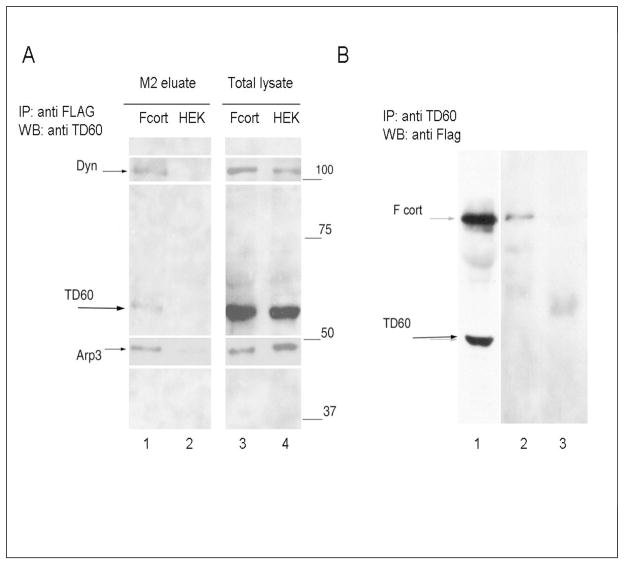
To validate the association of RCC2/TD60 with cortactin, western blot analysis of complexes purified from either F-cortactin and control mock transfected cells were carried out. As shown in Figure 2A RCC2/TD60 as well as ARP3 and dynamin were detected in F-cortactin complexes but not in the control eluate from HEK293 cell lysates. Immunoprecipitation of lysates from F-cortactin expressing cells with anti-RCC2/TD60 antibody yielded F-cortactin as shown in Figure 4B (lane 2), supporting the interaction of cortactin and RCC2/TD60
Figure 2.
Detection of TD60 by western blot following immunoprecipitation F-cortactin expressing HEK 293 cell lysates. A) Eluant of M2 agarose beads incubated with either F-cortactin expressing cell lysate (Fcort) or mock transfected HEK 293 cell lysate (HEK). Lanes 1 to 4 show the staining obtained by 2 sequential incubations of the blot beginning with anti-RCC2/TD60, followed by a mixture of mouse monoclonal antibodies specific for dynamin and Arp3. Time of exposure for each antibody was adjusted to produce the composed image shown here. B) Detection of A clarified lysate from approximately 2 × 107 F-cortactin expressing cells was divided in two and treated with either a 1/200 final dilution of anti RCC2/TD60 rabbit antibody (track 2) or control rabbit serum (track 3) immunoprecipitated and analyzed in PAGE SDS and blotted with anti Flag antibody as described in Materials and Methods.
3.4 Visualization of cortactin in dividing cells
In light of reports of RCC2/TD60 playing a role in cell division [34] HEK 293 cells expressing EGFP-cortactin were visualized 6h after release from nocodazole to enrich for cells under going mitosis. Although EGFR-cortactin was visualized throughout the cytoplasm, a significant enrichment of EGFP-cortactin was observed at the juncture of the dividing cells (Figure 3A, arrows). Similarly, immunoprecipitation of cortactin from nocodazole synchronized HeLa cells with an anti-cortactin antibody followed by western blot analysis using anti-TD60 antibody further confirmed the present of RCC2/TD60 in complex with cortactin (Figure 3B).
Figure 3.
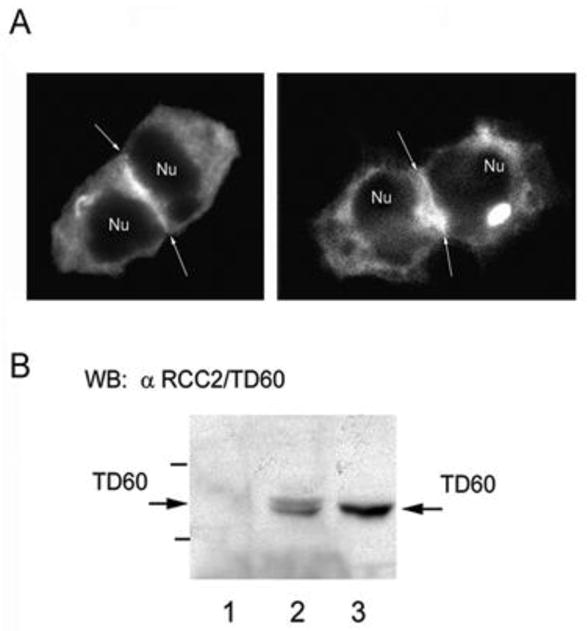
A) Distribution of EGFP cortactin in diving HEK 293 cells. Two examples of EGFP-cortactin expressing HEK 293 cells 6 h after release from nocodazole driven cell cycle arrest. Arrows point to zones of marked intensity of cortactin statining in the equatorial zone of the dividing pairs of cells. B) Co-immmunoprecipitation of endogenous cortactin and RCC2/TD60 from lysates of nocodazole synchronized Hela cells. Endogenous cortactin was immunoprecipitated with monocloncal antibody 4F11 followed by western blot analysis with anti- RCC2/TD60 polyclonal antibody as described in the text. Lanes 1 and 2 corresponds to a control non-related monoclonal antibody and anti-cortactin antibody 4F11, respectively. In Lane 3 is seen an aliquot of the total cell lysate.
4. Discussion
In this investigation we utilized the Stable Isotope Labeled Amino Acids in Cell Culture (SILAC) approach to discriminate between specific and non-specific association of cellular proteins with Flag-tagged cortactin and consequently generated evidence suggesting that key elements of the mitotic machinery of the cell are members of the cortactin interactome. Among a limited list of potential bona fide cortactin interacting proteins we identified RCC2/TD60, a Rac guanine nucleotide exchange factor (GEF) known to be involved in completion of mitosis and cytokinesis [34]. Whether RCC2/TD60 exhibits direct binding to cortactin remains to be investigated. We also note that several known interacting proteins were not detected in this analysis possibly because they were not expressed in our system or their abundance was too low to detect by MS, emphasizing a limitation of this methodology.
Cortactin has been recently detected both in centrosomes [40] and in invadopodias, subcellular structures involved in both regulation of chromosome condensation and the invasive machinery of metastatic cancer cells, suggesting that cortactin could be playing a critical role linking cell division and cell migration processes [40]. The fact that dynamin, a major cortactin partner also found in invadopodia, plays a major role in centrosome cohesion [42], is compatible with this hypothesis.
Protein RCC2/TD60, is a member of the chromosomal passenger complex (CPC) together with Aurora B kinase and INCENP but its function during the mitotic cycle is not well understood. RCC2/TD60 localizes in the equatorial mid zone of dividing cells during telophase and has been postulated to be a Rac-GEF [43] and a regulator of Rac1 and Arf6 [44]. It has been recently shown that tyrosine 357 of RCC2/TD60 is phosphorylated in cells overexpressing Src, a major kinase responsible for cortactin tyrosine phosphorylation [23, 45]. Given this, it is therefore tempting to speculate that cortactin – RCC2/TD60 interactions and tyrosine phosphorylation are linked events with a potential impact on the mitosis/cytokinesis process. Furthermore, it is worth noting the heterogeneous nature of RCC2/TD60 immunoprecipitated with MAb 4F11 (Fig 3B, lane 2) suggesting increased phosphorylation in the subpopulation of RCC2/TD60 that is associated with intracellular cortactin. Rebloting with an anti-phospho tyrosine monoclona antibody 4G10 confirmed that the RCC2/TD60 is tyrosine phosphorylated (data not shown). The fact that EGFP-tagged cortactin is detected at the juncture of cells released from nocodazole-thymidime blockade (Figure 5a) suggests a colocalization with RCC2/TD60 and possibly other passenger proteins which is compatible with the hypothesis that these proteins interact during the cell division process.
In summary we have demonstrated that the use of a SILAC protocol, complemented with clasic immunodetection techniques, allowed for the identification of novel binding partners of cortactin including some with a relevant role in the process of cell division. Our results also demonstrate that this is an effective strategy to minimize false positives in this approach to identify proteins that exhibit functional associations within the subcellular compartments.
Acknowledgments
We acknowledge the support of NIH 1U54 GM64346 (J.T.P. and J.W.F) and support from NCI CA40042 (JTP) for this project.
Appendix
Representative data from the SILAC experiments can be downloaded from: http://proteus.achs.virginia.edu/proteus; User ParsonsPub1; Password 1221. Click files tab and download files of interest. To view Scaffold files you will need to download free viewer and install before opening .sf3 files (http://www.proteomesoftware.com/Proteome_software_prod_Scaffold3_download-main.html).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pollard TD. Regulation of actin filament assembly by Arp2/3 complex and formins. Annu Rev Biophys Biomol Struct. 2007;36:451–77. doi: 10.1146/annurev.biophys.35.040405.101936. [DOI] [PubMed] [Google Scholar]
- 2.Weed SA, Du Y, Parsons JT. Translocation of cortactin to the cell periphery is mediated by the small GTPase Rac1. J Cell Sci. 1998;111:2433–43. doi: 10.1242/jcs.111.16.2433. [DOI] [PubMed] [Google Scholar]
- 3.Bowden ET, Barth M, Thomas D, Glazer RI, Mueller SC. An invasion-related complex of cortactin, paxillin and PKCmu associates with invadopodia at sites of extracellular matrix degradation. Oncogene. 1999;18:4440–9. doi: 10.1038/sj.onc.1202827. [DOI] [PubMed] [Google Scholar]
- 4.McNiven MA, Kim L, Krueger EW, Orth JD, Cao H, Wong TW. Regulated interactions between dynamin and the actin-binding protein cortactin modulate cell shape. J Cell Biol. 2000;151:187–98. doi: 10.1083/jcb.151.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Head JA, Jiang D, Li M, Zorn LJ, Schaefer EM, Parsons JT, Weed SA. Cortactin tyrosine phosphorylation requires Rac1 activity and association with the cortical actin cytoskeleton. Mol Biol Cell. 2003;14:3216–29. doi: 10.1091/mbc.E02-11-0753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bryce NS, Clark ES, Leysath JL, Currie JD, Webb DJ, Weaver AM. Cortactin promotes cell motility by enhancing lamellipodial persistence. Curr Biol. 2005;15:1276–85. doi: 10.1016/j.cub.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 7.Boyle SN, Michaud GA, Schweitzer B, Predki PF, Koleske AJ. A critical role for cortactin phosphorylation by Abl-family kinases in PDGF-induced dorsal-wave formation. Curr Biol. 2007;17:445–51. doi: 10.1016/j.cub.2007.01.057. [DOI] [PubMed] [Google Scholar]
- 8.Weaver AM, Heuser JE, Karginov AV, Lee WL, Parsons JT, Cooper JA. Interaction of cortactin and N-WASp with Arp2/3 complex. Curr Biol. 2002;12(15):1270–8. doi: 10.1016/s0960-9822(02)01035-7. [DOI] [PubMed] [Google Scholar]
- 9.Uruno T, Liu J, Zhang P, Fan Yx, Egile C, Li R, Mueller SC, Zhan X. Activation of Arp2/3 complex-mediated actin polymerization by cortactin. Nat Cell Biol. 2001;3:259–66. doi: 10.1038/35060051. [DOI] [PubMed] [Google Scholar]
- 10.Kowalski JR, Egile C, Gil S, Snapper SB, Li R, Thomas SM. Contacting regulates cell migration through activation of N-WASP. J Cell Sci. 2005;118:79–87. doi: 10.1242/jcs.01586. [DOI] [PubMed] [Google Scholar]
- 11.Weaver AM, Karginov AV, Kinley AW, Weed SA, Li Y, Parsons JT, Cooper JA. Contacting promotes and stabilizes Arp2/3-induced actin filament network formation. Curr Biol. 2001;11:370–4. doi: 10.1016/s0960-9822(01)00098-7. [DOI] [PubMed] [Google Scholar]
- 12.Bryce NS, Clark ES, Leysath JL, Currie JD, Webb DJ, Weaver AM. Cortactin promotes cell motility by enhancing lamellipodial persistence. Curr Biol. 2005;15:1276–85. doi: 10.1016/j.cub.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 13.Novak N, Bar V, Sabanay H, Frechter S, Jaegle M, Snapper SB, Meijer D, Peles E. N-WASP is required for membrane wrapping and myelination by Schwann cells. J Cell Biol. 2011;192:243–50. doi: 10.1083/jcb.201010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bañón-Rodríguez I, Monypenny J, Ragazzini C, Franco A, Calle Y, Jones GE, Antón IM. The cortactin-binding domain of WIP is essential for podosome formation and extracellular matrix degradation by murine dendritic cells. Eur J Cell Biol. 2011;90:213–23. doi: 10.1016/j.ejcb.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Lin J, Liu J, Wang Y, Zhu J, Zhou K, Smith N, Zhan X. Differential regulation of cortactin and N-WASP-mediated actin polymerization by missing in metastasis (MIM) protein. Oncogene. 2005;24:2059–66. doi: 10.1038/sj.onc.1208412. [DOI] [PubMed] [Google Scholar]
- 16.Segev N, Semin G. GTPases in intracellular trafficking: An overview. Cell Dev Biol. 2011;22:1–2. doi: 10.1016/j.semcdb.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lynch DK, Winata SC, Lyons RJ, Hughes WE, Lehrbach GM, Wasinger V, Corthals G, Cordwell S, Daly RJ. A Cortactin-CD2-associated protein (CD2AP) complex provides a novel link between epidermal growth factor receptor endocytosis and the actin cytoskeleton. J Biol Chem. 2003;278:21805–13. doi: 10.1074/jbc.M211407200. [DOI] [PubMed] [Google Scholar]
- 18.Katsube T, Takahisa M, Ueda R, Hashimoto N, Kobayashi M, Togashi S. Cortactin associates with the cell-cell junction protein ZO-1 in both Drosophila and mouse. J Biol Chem. 1998;273:29672–7. doi: 10.1074/jbc.273.45.29672. [DOI] [PubMed] [Google Scholar]
- 19.Berkel S, Marshall CR, Weiss B, Howe J, Roeth R, Moog U, Endris V, Roberts W, Szatmari P, Pinto D, Bonin M, Riess A, Engels H, Sprengel R, Scherer SW, Rappold GA. Mutations in the SHANK2 synaptic scaffolding gene in autism spectrum disorder and mental retardation. Nat Genet. 2010;42:489–91. doi: 10.1038/ng.589. [DOI] [PubMed] [Google Scholar]
- 20.Hou P, Estrada L, Kinley AW, Parsons JT, Vojtek AB, Gorski JL. Fgd1, the Cdc42 GEF responsible for Faciogenital Dysplasia, directly interacts with cortactin and mAbp1 to modulate cell shape. Mol Genet. 2003;12 (16):1981–1993. doi: 10.1093/hmg/ddg209. [DOI] [PubMed] [Google Scholar]
- 21.Daly RJ. Cortactin signalling and dynamic actin networks. Biochem J. 2004 Aug 15;382(Pt 1):13–25. doi: 10.1042/BJ20040737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cosen-Binker LI, Kapus A. Cortactin: the gray eminence of the cytoskeleton. Physiology (Bethesda) 2006 Oct;21:352–61. doi: 10.1152/physiol.00012.2006. [DOI] [PubMed] [Google Scholar]
- 23.Orth JD, McNiven MA. Get off my back! Rapid receptor internalization through circular dorsal ruffles. Cancer Res. 2006;66:11094–6. doi: 10.1158/0008-5472.CAN-06-3397. [DOI] [PubMed] [Google Scholar]
- 24.Weaver AM. Invadopodia: specialized cell structures for cancer invasion. Clin Exp Met. 2006;23:97–105. doi: 10.1007/s10585-006-9014-1. [DOI] [PubMed] [Google Scholar]
- 25.Buday L, Downward J. Roles of cortactin in tumor pathogenesis. Biochim Biophys Acta. 2007;1775:263–273. doi: 10.1016/j.bbcan.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Yamaguchi, Condeelis H, Yamaguchi, Condeelis J. Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim Biophys Acta. 2007;1773:642–652. doi: 10.1016/j.bbamcr.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clarka ES, Weaver AM. A new role for cortactin in invadopodia: Regulation of protease secretion. E J Cell Biol. 2008;35:581–590. doi: 10.1016/j.ejcb.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gavin AC, Bosche M, Krause R, Grandi P, Marzioch M, Bauer A, et al. Functional organization of the yeast proteome by systematic analysis of protein complexes. Nature. 2002;415:141–147. doi: 10.1038/415141a. [DOI] [PubMed] [Google Scholar]
- 30.Knuesel M, Wan Y, Xiao Z, Holinger E, Lowe N, Wang W, Liu X. Identification of novel protein – protein interactions using a versatile mammalian tandem affinity purification expression system. Mol Cell Proteomics. 2003;2:1225–1233. doi: 10.1074/mcp.T300007-MCP200. [DOI] [PubMed] [Google Scholar]
- 31.Bertwistle D, Sugimoto M, Sherr CJ. Physical and functional interactions of the Arf tumor suppressor protein with nucleophosmin/B23. Mol Cell Biol. 2004;24:985–996. doi: 10.1128/MCB.24.3.985-996.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mayhew MW, Webb DJ, Kovalenko M, Whitmore L, Fox JW, Horwitz AF. Identification of protein networks associated with the PAK1-betaPIX-GIT1-paxillin signaling complex by mass spectrometry. J Proteome Res. 2006;5:2417–23. doi: 10.1021/pr060140t. [DOI] [PubMed] [Google Scholar]
- 33.Goldfinger LE, Ptak C, Jeffery ED, Shabanowitz J, Han J, Haling JR, Sherman NE, Fox JW, Hunt DF, Ginsberg MH. An experimentally derived database of candidate Ras-interacting proteins. J Proteome Res. 2007;6:1806–11. doi: 10.1021/pr060630l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mollinari C, Reynaud C, Martineau-Thuillier S, Monier S, Kieffer S, Garin J, Andreassen PR, Boulet A, Goud B, Kleman J-P, Margolis RL. The mammalian passenger protein TD-60 is an RCC1 family member with an essential role in prometaphase to metaphase progression. Dev Cell. 2003;5:295–307. doi: 10.1016/s1534-5807(03)00205-3. [DOI] [PubMed] [Google Scholar]
- 35.Grigera PR, Jeffery ED, Martin KH, Shabanowitz J, Hunt DF, Parsons JT. FAK phosphorylation sites mapped by mass spectrometry. J Cell Sci. 2005;118:4931–4935. doi: 10.1242/jcs.02696. [DOI] [PubMed] [Google Scholar]
- 36.Martin KH, Jeffery ED, Grigera PR, Shabanowitz J, Hunt DF, Parsons JT. Cortactin phosphorylation sites mapped by mass spectrometry. J Cell Sc. 2006;119:2851–3. doi: 10.1242/jcs.03034. [DOI] [PubMed] [Google Scholar]
- 37.Webb BA, Eves R, Mak AS. Cortactin regulates podosome formation: Roles of the protein interaction domains. Exp Cell Res. 2006;312:760–9. doi: 10.1016/j.yexcr.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 38.Heintz N, Sive HL, Roeder RG. Regulation of human histone gene expression: kinetics of accumulation and changes in the rate of synthesis and in the half-lives of individual histone mRNAs during the HeLa cell cycle. Mol Cell Biol. 1983;3:539–550. doi: 10.1128/mcb.3.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lahoz A, Alcaide-Gavilán M, Daga RR, Jimenez J. Antagonistic Roles of PP2A-Pab1 and Etd1 in the Control of Cytokinesis in Fission Yeast. Genetics. 2010;186:1261–1270. doi: 10.1534/genetics.110.121368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang W, Chen L, Ding Y, Jin J, Liao K. Centrosome separation driven by actinmicrofilaments during mitosis is mediated by centrosome-associated tyrosine phosphorylated cortactin. J Cell Sci. 2008;121:1334–43. doi: 10.1242/jcs.018176. [DOI] [PubMed] [Google Scholar]
- 42.McHugh B, Krause SA, Yu B, Deans AM, Heasman S, McLaughlin P, Heck MM. Invadolysin: a novel, conserved metalloprotease links mitotic structural rearrangements with cell migration. J Cell Biol. 2004;167:673–686. doi: 10.1083/jcb.200405155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thompson HM, Cao H, Chen J, Euteneuer U, McNiven MA. Dynamin 2 binds gamma-tubulin and participates in centrosome cohesion. Nat Cell Biol. 2004;6:335–342. doi: 10.1038/ncb1112. [DOI] [PubMed] [Google Scholar]
- 44.Mollinari C, Reynaud C, Martineau-Thuillier S, Monier S, Kieffer S, Garin J, Andreassen PR, Boulet A, Goud B, Kleman JP, Margolis RL. The mammalian passenger protein TD-60 is an RCC1 family member with an essential role in prometaphase to metaphase progression. Dev Cell. 2003;2:295–307. doi: 10.1016/s1534-5807(03)00205-3. [DOI] [PubMed] [Google Scholar]
- 45.Humphries JD, Byron A, Bass MD, Craig SE, Pinney JW, Knight D, Humphries MJ. Proteomic analysis of integrin-associated complexes identifies RCC2 as a dual regulator of Rac1 and Arf6. Sci Signal. 2009;8:2. doi: 10.1126/scisignal.2000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luo W, Slebos RJ, Hill S, Li M, Brábek J, Amanchy R, Chaerkady R, Pandey A, Ham AJ, Hanks SK. Global Impact of Oncogenic Src on a Phosphotyrosine Proteome. J Proteome Res. 2008;7:3447–3460. doi: 10.1021/pr800187n. [DOI] [PMC free article] [PubMed] [Google Scholar]