Abstract
Background
The traditional paradigm of glomerular filtration rate (GFR) progression among chronic kidney disease (CKD) patients is a steady, nearly linear decline over time. We describe individual GFR progression trajectories over twelve years of follow-up among participants in the African American Study of Kidney Disease and Hypertension (AASK).
Study Design
Longitudinal, observational study
Setting & Participants
846 AASK patients with at least 3 years of follow-up and 8 GFR estimates.
Measurements
Longitudinal GFR estimates (eGFR) from creatinine-based equations.
Predictors
Patient demographic and clinical features.
Outcomes
Probability of a nonlinear trajectory and probability of a period of nonprogression, calculated for each patient from a Bayesian model of individual eGFR trajectories.
Results
Three hundred and fifty-two (41.6%) patients exhibited a greater than 0.9 probability of having either a nonlinear trajectory or a prolonged nonprogression period; in 559 (66.1%), the probability was larger than 0.5. Baseline eGFR > 40 mL/min/1.73m2 and urine protein-creatinine < 0.22 g/g were associated with a higher likelihood of a nonprogression period. Seventy-four patients (8.7%) had both a substantial period of stable or increasing eGFR and a substantial period of rapid eGFR decline.
Limitations
Clinical trial population; absence of direct GFR measurements.
Conclusions
In contrast to the traditional paradigm of steady GFR progression over time, many CKD patients have a non-linear GFR trajectory or a prolonged period of nonprogression. These findings highlight the possibility that stable kidney disease progression can accelerate, and, conversely provide hope that CKD need not be relentlessly progressive. These results should encourage researchers to identify time-dependent factors associated with periods of nonprogression and other desirable trajectories.
INDEX WORDS: Chronic kidney disease, estimated glomerular filtration rate, nonlinear progression, longitudinal cohort study, African American, slope
Chronic kidney disease (CKD) is characterized by progressive loss of kidney function, often manifested by a decline in glomerular filtration rate (GFR) over a long period of time. GFR decline has been hypothesized to follow a linear or possibly a loglinear trajectory1–3. Linearity is a convenient assumption in models of GFR progression. Slope is an intuitive measure of the “progression rate”, the model is simple and easy to interpret, and the statistical methodology and software are well developed. Physicians have used the assumption of a linear decline to counsel patients on when they might reach end stage renal disease (ESRD), to time dialysis access placement and for clinical research design. However, most studies of CKD progression have relied on data with less than 3–5 years of follow-up and under 10 longitudinal GFR measurements or estimates per subject4–6. With such data, it is difficult to determine whether GFR progression is truly linear, given the amount of measurement/estimation error and biological variation in GFR.
The question of whether CKD progression generally declines according to a linear trajectory has both clinical and methodological relevance. Knowledge of possible existence of nonlinear decline would allow appropriate counseling of patients and planning for ESRD care. If many patients’ decline in kidney function deviates from linear progression, then estimating the time of occurrence of near end stage renal disease could be significantly impacted with many clinical consequences. Deviation from linearity implies acceleration and/or deceleration of progression during the observation period and suggests that the determinants of progression may vary over time within the same patient. Identification of these determinants, particularly those which are modifiable, would be of scientific and clinical interest. If a large proportion of trajectories are nonlinear but a simplifying linear assumption is made in study design and data analysis, the result may be biased in unpredictable ways. This question becomes more relevant as more studies begin to accrue long-term follow-up data.
To our knowledge, this is the first systematic investigation of the shape of GFR trajectories in a large CKD cohort. The objective is to study the prevalence of nonlinear GFR trajectories and nonprogression periods among CKD patients, and to describe the nature of deviations from linear decline.
METHODS
Study Population
The AASK study was a multi-center, randomized clinical trial of 1094 African American individuals aged 18 to 70 year and with a GFR between 20 and 65 mL/min/1.73m2 7. The participants were randomized in a 3 × 2 factorial design to one of three antihypertensive drugs (ramipril, amlodipine, or metoprolol) and two levels of blood pressure control (mean arterial pressure ≤ 92 mmHg or 102–107 mmHg). At the completion of the trial, 787 participants were alive and not on dialysis; of these, 691 were enrolled in the subsequent AASK Cohort Study8.
Our analyses used data from both the trial and cohort studies. The study sample consisted of 846 participants with at least 3 years of follow-up and at least 8 visits in which GFR could be estimated from serum creatinine measurements. The other 248 of the 1094 randomized patients were excluded as they did not have sufficient longitudinal data to assess the trajectory pattern. Of the 248 excluded patients, 123 (50%) reached dialysis and 65 (26%) died prior to reaching dialysis. The maximum follow-up of the 846 studied patients was 12 (median, 9) years. Each patient had between 8 and 30 GFR estimates (median, 20). During follow-up, 195 (23%) of the 846 patients reached dialysis, and 111 (13%) died before reaching dialysis.
Estimation of Glomerular Filtration Rate
Serum creatinine was measured twice at baseline, less than 3 months apart, and at follow-up months 3, 6, and then every 6 months for the rest of the study. We used the estimated GFR (eGFR) based on the AASK estimating equation: eGFR = 329 × (serum creatinine)-1.096 × (age)-0.294 × (0.736 for female). This formula is similar to the more widely used MDRD Study equation, and has been used for longitudinal assessment of kidney function in AASK study9.
Statistical Analysis
The eGFR trajectories for each of the 846 patients were estimated separately. Since the observed eGFR values include measurement error, short-term biological variation and other noise, we used a Bayesian smoothing technique10 to estimate each patient’s eGFR trajectory as a smooth curve. The smoothness of the curve was automatically determined by the data with no input from the analyst regarding the amount of deviation of the curve from linearity. For each patient, the Bayesian approach produced 3,000 Monte Carlo samples to approximate the posterior distribution of all the modeling parameters, which led to 3,000 posterior curves that quantified the uncertainty in the true trajectory given the variation in the data. Under this Bayesian approach, we estimated the “most likely” trajectory by the average of these 3,000 Monte Carlo curves, and computed pointwise 95% credible intervals (analogous to confidence intervals) based on the variation in the curves around the mean curve. As detailed below, the Bayesian approach also allowed us to compute the posterior probability that a patient’s trajectory had a particular feature of interest (e.g., a designated deviation from linearity, a prolonged period of nonprogression, etc.) as the proportion of the 3,000 Monte Carlo trajectories that exhibited this feature. Since the estimated trajectory was a smooth curve, its slope could be calculated month by month, accommodating possible change in rate of progression over time. Technical details of the Bayesian computation can be found in Item S1 (available as online supplementary material).
We defined a trajectory to be nonlinear if the mean slope for the half of follow-up months with faster decline and the mean slope for the other half with slower decline differed by more than 3 mL/min/1.73m2/year. The posterior probability that a patient’s GFR trajectory was nonlinear was then calculated as the proportion of the 3,000 Monte Carlo trajectories that satisfied this criterion. The threshold of 3 mL/min/1.73m2/year was selected somewhat arbitrarily as representing a change in slope greater than the overall mean progression rate of approximately 2 mL/min/1.73m2/year in the trial phase of AASK11.
A trajectory was considered to contain a significant nonprogression period if all of the following criteria were satisfied: (1) the length of the period was at least 4.5 years or, in the case of less than 4.5 years of follow-up, the entire follow-up period for that patient; (2) the slope was ≥ −2 mL/min/1.73m2 per year for every month of the period; and (3) the average slope over the entire period was ≥ −1 mL/min/1.73m2 per year, which corresponded roughly to the average age-related decline in GFR12–13. (Note that slopes ≥ a negative threshold include positive slopes and negative slopes which are less steep than the threshold.) The posterior probability of nonprogression for a patient was calculated as the proportion of the 3,000 Monte Carlo trajectories that satisfied the criteria above.
We compared the mean posterior probabilities of nonlinearity and nonprogression between dichotomized baseline groups using one-way analysis of variance. In order to describe the diversity and prevalence of clinically meaningful deviations from linearity, a number of nonlinear features were defined and their frequencies of occurrence among the fitted trajectories were reported.
All analyses and graphics were produced by R 2.12.2 (www.R-project.org).14
RESULTS
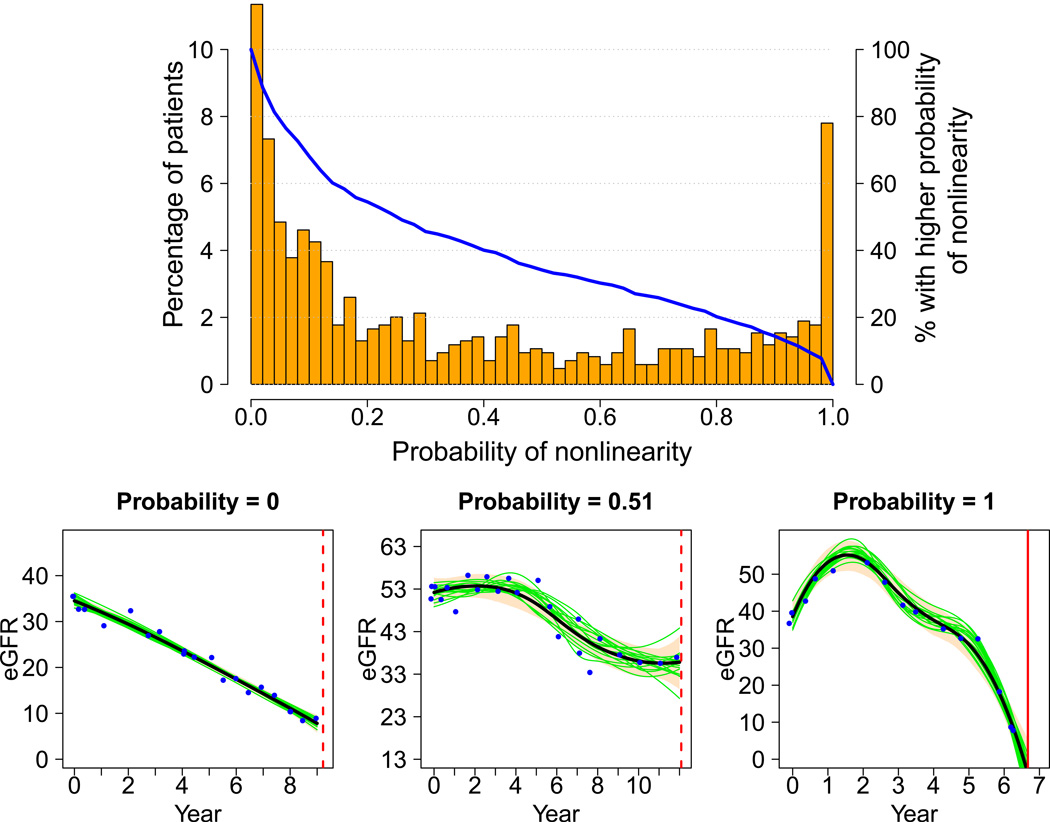
Table 1 provides summary statistics of key variables from the 846 patients in the study sample. Compared with the original AASK cohort (Table S1), these patients had higher mean baseline GFR and lower proteinuria, reflecting a lower risk of rapid progression to ESRD as they must have at least 3 years of follow-up for inclusion in this analysis. The distributions of the probability of nonlinearity and the probability of nonprogression are shown in Figures 1 and 2, along with sample trajectory plots of individual patients. The joint distribution of these two probabilities is shown in Table S2. In Figure 1, the superimposed blue curve of the cumulative proportions shows that 122 (14.4%) patients have a probability of nonlinearity > 0.9, and 289 (34.2%) patients have a probability > 0.5. Likewise, 151 (17.9%) patients have a probability of at least one occurrence of nonprogression > 0.9 and 311 (36.8%) patients have a probability > 0.5 (Figure 2).
Table 1.
Summary statistics of the study sample
| Baseline characteristics | Summary statistics | |
|---|---|---|
| Age at randomization | 55.8 (47.9, 63.4) | |
| eGFR (mL/min/1.73m2)* | 50.2 (40.2, 59.0) | |
| UPCR (g/g) | 0.061 (0.027, 0.22) | |
| Year of last eGFR | 9.1 (6.2, 10.5) | |
| Gender | ||
| Male | 514 (60.8%) | |
| Female | 332 (39.2 %) | |
| AASK randomized intervention | ||
| MAP goal | ||
| ≤ 92 mmHg | 427 (50.5 %) | |
| 102–107 mmHg | 419 (49.5 %) | |
| Antihypertensive drug | ||
| Ramipril | 344 (40.7 %) | |
| Metoprolol | 342 (40.4 %) | |
| Amlodipine | 160 (18.9 %) | |
N=846.
eGFR, estimated glomerular filtration rate; UPCR, urinary protein-creatinine ratio; AASK, African American Study of Kidney Disease and Hypertension; MAP, mean arterial pressure
Continuous variables were summarized by median (25th, 75th percentile); categorical variables were summarized by count (percent).
Averaged over two baseline values, less than 3 months apart
Figure 1.
Distribution of the probability of nonlinearity, visualized by a percentage histogram (columns drawn to the scale of the vertical axis on the left), and a curve of the proportion of patients in the study sample (n=846) with higher probability (blue curve drawn to the scale of the vertical axis on the right). Below are three example trajectories with probabilities close to 0, 0.5, and 1. These were selected to illustrate the increasing oscillation as the probability of nonlinearity increases. On each trajectory plot, the horizontal axis is year since randomization, and the vertical axis is eGFR (mL/min/1.73m2). The blue dots are eGFR data, the black smooth curve is the estimated trajectory, and the bisque color band is the pointwise 95% Bayesian confidence interval. The red vertical line represents time of either censoring (dashed) or dialysis (solid). Fifteen of the 3,000 Monte Carlo trajectories are randomly selected and plotted for illustration (green curves).
Figure 2.
Distribution of the probability of nonprogression. The layout is similar to Figure 1. The three example trajectories were selected to illustrate the increasing upward trend as the probability of nonprogression increases.
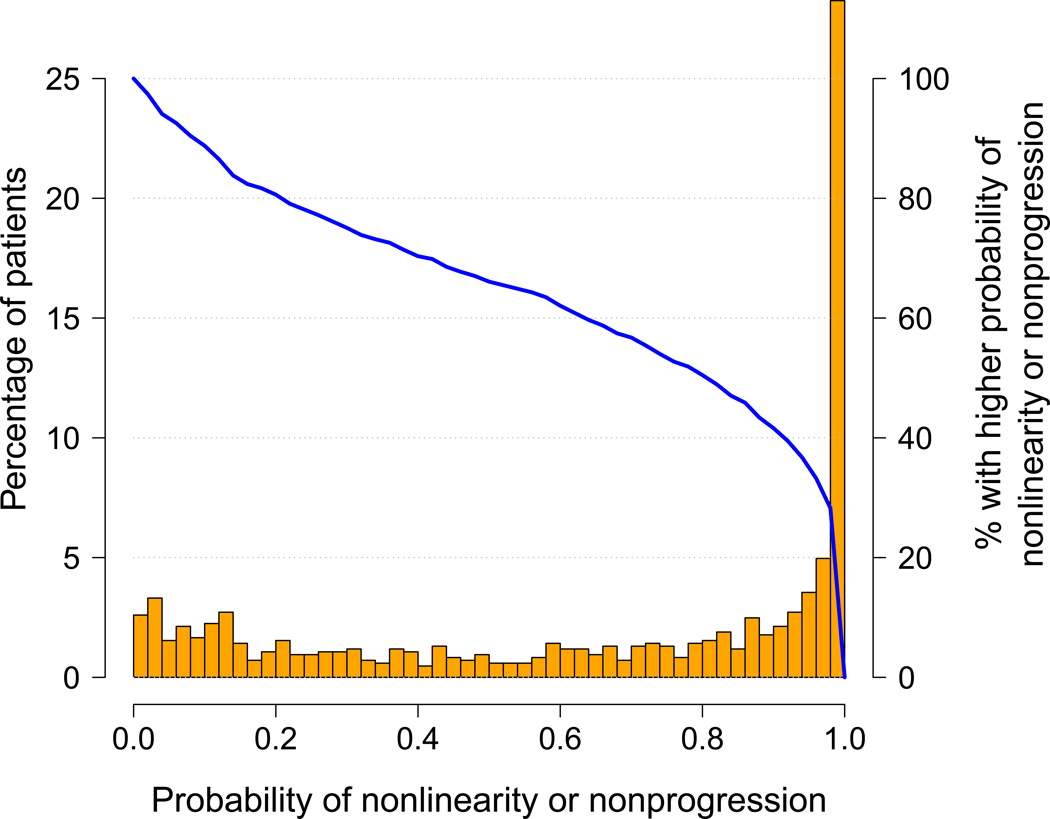
Deviation from the classical paradigm of linear decline can be characterized by a trajectory that is either nonlinear or nonprogressive. Figure 3 shows the histogram of the probability of either nonlinearity or nonprogression. A substantial proportion of patients demonstrated deviation from the “classical paradigm”: 352 (41.6%) patients have a joint probability of nonlinearity or nonprogression > 0.9 and 559 (66.1%) have a joint probability > 0.5. Note that although for each patient, the combined probability of nonlinearity or nonprogression never exceeds the sum of the respective probabilities of nonlinearity and nonprogression, the proportion of patients in the study sample with their combined probabilities of nonlinearity or nonprogression exceeding a certain level may be either larger or smaller than the sum of the corresponding proportions for the two respective probabilities.
Figure 3.
Distribution of the probability of either nonlinearity or nonprogression. The layout is similar to Figures 1 and 2, but without the sample trajectory plots.
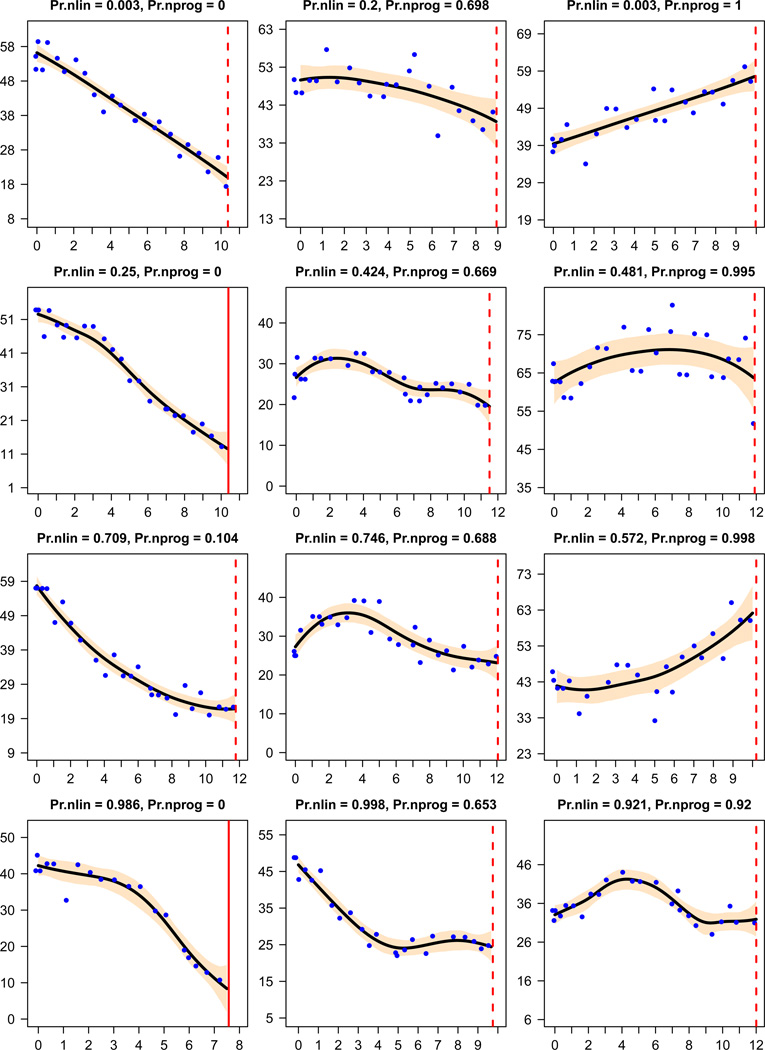
Figure 4 presents twelve selected patients to illustrate the diversity of trajectories. The plots are organized such that the probability of nonlinearity increases from the top row to the bottom, as the curves demonstrate more oscillation, and the probability of nonprogression increases from the left column to the right, as the upward trend in certain parts of the trajectory becomes more apparent.
Figure 4.
GFR Trajectories of twelve patients and their probabilities of nonlinearity (Pr.nlin) and nonprogression (Pr.nprog). The setup of each trajectory plot is similar to those in Figure 1.
The mean probability of nonlinearity and nonprogression between baseline subgroups are compared in Table 2. The patient characteristics of these subgroups are presented in Tables S3–S4. Consistent with previous reports15, patients with higher baseline eGFR, and lower baseline urine protein-creatinine ratio more likely had a period with stable or increasing eGFR, i.e. a period of nonprogression. However, the proportion of patients exhibiting nonlinearity does not differ significantly by baseline eGFR or urine protein-creatinine ratio, but is significantly elevated among patients ≤ 55 years of age compared to older patients. Patients receiving amlodipine on average have higher probability of nonlinearity, possibly due to the temporary elevation of GFR at the beginning of the follow-up. A sensitivity analysis addressing this issue is at the end of this section. The likelihood of nonlinearity or a nonprogression period is inherently related to follow-up time, as there is more opportunity for these features to occur and to be detected for patients with longer follow-up. As baseline eGFR and urine protein-creatinine ratio are strongly associated with end stage renal disease and death, the length of follow-up is associated with baseline eGFR (median follow-up of 6.9 years if eGFR < 40, vs. 9.4 years if eGFR ≥ 40) and baseline urine protein-creatinine ratio (median follow-up of 9.4 years if ratio ≤ 0.22 vs. 7.3 years if ratio > 0.22). The contrasts between subgroups with different levels of baseline proteinuria and eGFR should be interpreted in the context of these differences in follow-up time. There is little association between baseline age or gender with length of follow-up.
Table 2.
Comparison of baseline subgroups on posterior probabilities of nonlinearity and nonprogression
| Comparison Groups | Probability of | Years of follow-up* | ||
|---|---|---|---|---|
| Nonlinearity | Nonprogression | |||
| eGFR | ||||
| < 40 mL/min/173m2 (n=208) | 0.36 | 0.28 | 6.9 (4.1, 9.3) | |
| ≥ 40 mL/min/1.73m2 (n=638) | 0.39 | 0.40 | 9.4 (7.2, 10.7) | |
| p-value | 0.4 | < 0.001 | ||
| UPCR | ||||
| ≤ 0.22 g/g (n=632) | 0.39 | 0.44 | 9.4 (7.1, 10.7) | |
| > 0.22 g/g (n=214) | 0.35 | 0.17 | 7.3 (4.6, 9.4) | |
| p-value | 0.09 | < 0.001 | ||
| Age at randomization | ||||
| ≤ 55 y (n=405) | 0.44 | 0.36 | 9.0 (6.1, 10.5) | |
| > 55 y (n=441) | 0.33 | 0.39 | 9.2 (6.2, 10.6) | |
| p-value | < 0.001 | 0.3 | ||
| Gender | ||||
| Male (n=514) | 0.39 | 0.37 | 9.1 (6.3, 10.6) | |
| Female (n=332) | 0.37 | 0.37 | 9.0 (5.9, 10.3) | |
| p-value | 0.3 | 0.9 | ||
| Blood pressure goal | ||||
| Lower (n=427) | 0.37 | 0.40 | 9.2 (6.5, 10.5) | |
| Usual (n=419) | 0.39 | 0.35 | 9.0 (5.9, 10.5) | |
| p-value | 0.4 | 0.04 | ||
| Antihypertensive drug | ||||
| Ramipril (n=344) | 0.37 | 0.41 | 9.1 (6.4, 10.4) | |
| Metoprolol (n=342) | 0.35 | 0.34 | 9.0 (6.2, 10.5) | |
| Amlodipine (n=160) | 0.48 | 0.36 | 9.1 (5.5, 10.6) | |
| p-value | < 0.001 | 0.1 | ||
eGFR, estimated glomerular filtration rate; UPCR, urinary protein-creatinine ratio
The mean posterior probabilities are reported for each subgroup, along with a p-value from one-way analysis of variance for the null hypothesis that the mean probability of nonlinearity (or nonprogression) is equal between subgroups.
Given as median (25th, 75th percentile).
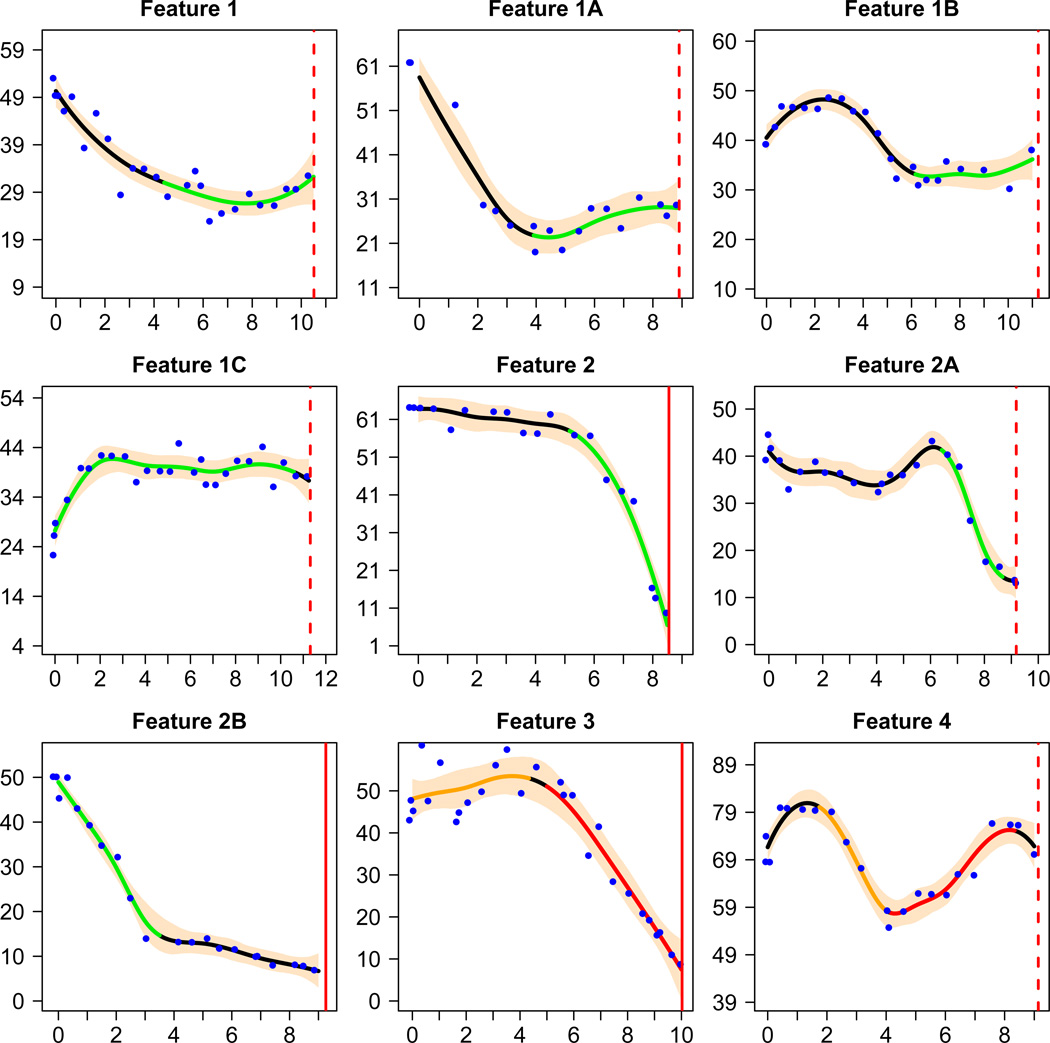
Table 3 provides the prevalence of selected features of the estimated trajectories as defined in the left hand column of the table. For simplicity, the table summarizes the proportions of patients with estimated trajectories satisfying the indicated definitions rather than the posterior probabilities satisfying the definitions. Feature 1 was defined to study how many patients, at least for a period of time, exhibited stable or increasing kidney function. It was defined to be at least 3 years in order to filter out short-term upward trends; the eGFR must either increase, or progress at a rate no faster than criteria (2) of our definition of a nonprogression period. Since the definition was somewhat arbitrary, in order to assess the sensitivity of results to this definition, we varied the definition in Features 1A–1C, and found that a period with stable or increasing eGFR remained in a considerable proportion of CKD patients. Feature 2 was designed to study a pattern opposite to Feature 1: fast deterioration of kidney function, and it was observed in a considerable proportion of patients. Patients with Features 3 or 4 (a total of 74 patients, 8.7%) experienced both a significant period with stable or increasing eGFR and a significant period with rapidly declining eGFR. Figure 5 shows sample trajectory plots of individual patients that correspond to each feature in Table 3. In Item S2, we relate the features with the probability of nonlinearity (nonprogression) by comparing the average probability of nonlinearity (nonprogression) among patients with and without the features.
Table 3.
The number and percentage of patients with pre-defined features
| Feature | There exists a period of time during which the estimated eGFR trajectory satisfies the following criteria |
count (%) |
|
|---|---|---|---|
| 1. stable or increasing | (1) the period is at least 3 years (2) the trajectory either increases or declines slowly, i.e., at a rate of less than 2 mL/min/1.73m2 per year throughout (3) the total decrease in eGFR, if any, is no more than 4.5 mL/min/1.73m2 | 491 (58.0) | |
| 1A | The same as feature 1 except that the total decrease in eGFR, if any, is no more than 3 mL/min/1.73m2 | 430 (50.8) | |
| 1B | The same as feature 1 except that the total decrease in eGFR, if any, is no more than 1.5 mL/min/1.73m2 | 365 (43.1) | |
| 1C | The same as feature 1 except that the period must be at least 4 years and there is no decrease in eGFR at the end of the period compared with the beginning | 240 (28.4) | |
| 2. fast decline | (1) the trajectory decreases at a rate of at least 4 mL/min/1.73m2 per year during the entire period (2) the total decline is at least 8 mL/min/1.73m2 | 271 (32.0) | |
| 2A | The same as feature 2 except that the total decline is at least 12 mL/min/1.73m2 | 225 (26.6) | |
| 2B | The same as feature 2 except that the total decline is at least 16 mL/min/1.73m2 | 195 (23.1) | |
| 3. stable or increasing followed by fast decline | See 1 and 2 above | 45 (5.3) | |
| 4. fast decline followed by stable or increasing | See 2 and 1 above | 29 (3.4) | |
eGFR, estimated glomerular filtration rate;
Figure 5.
GFR Trajectories of nine patients, each having one of the features in Table 3. The setup of each trajectory plot is similar to those in Figure 1, except that the green, yellow or red segments highlight periods with the corresponding feature in Table 3.
One-hundred and sixty (19%) study patients were initially randomized to a dihydropyridine calcium channel blocker (amlodipine 5–10 mg/d). Previous research has shown that this treatment may cause temporary elevation in GFR for the first few months after the randomization11, and hence potentially increasing the probability of observing nonlinearity. In our data, 69 out of the 160 patients (43%) in the amlodipine treatment group had increasing trajectory for at least 6 months after randomization, while only 27% of the remaining 686 patients possessed this feature. However, exclusion of amlodipine patients had little impact on the above reported percentages of patients exhibiting nonlinearity or nonprogression over the full follow-up period. For example, with the 160 amlodipine patients excluded, the proportion of patients in the study sample with their joint probability of nonlinearity or nonprogression exceeding 0.9 changed from 41.6% to 40.5%.
DISCUSSION
In CKD research, the classical paradigm is to model GFR progression linearly. In clinical practice, it is common for physicians to make prognostic evaluation of a patient’s future progression based on a few recent GFR or serum creatinine measurements. In this article, we present evidence from the AASK study that the classic paradigm of a steady, linear decline does not apply to a large number of CKD patients. Specifically, we demonstrated that while a linear, progressive trajectory occurs in many CKD patients, nonlinear patterns and extended periods of nonprogression are also quite common.
The findings in this paper have implications for future research on CKD. First, analytical approaches more sophisticated than those based on linear models may be needed to study the CKD progression, especially over long-term follow-up period. Second, identification of nonlinearity in the trajectory suggests a new approach to study design, specifically, within-person designs that investigate the association of time-varying risk factors with changes in renal function. After identifying patients with periods of both rapid declining and stable or increasing GFR (features 3 and 4, Table 3), one can compare time-varying risk factors, such as prognostic biomarkers, medication use, and medical events, between the two periods within the same subject. The effects of subject-specific confounders, both measured and unmeasured, are easily eliminated in such an approach.
This research also has implications for our understanding of kidney disease progression. It is highly likely that the diverse patterns of trajectories are due to a combination of chronic and acute factors. For example, a patient with a nonprogressive or slowly progressive trajectory may have a clinical event resulting in acute kidney injury which not only abruptly drops the GFR off the projected trajectory but due to a critical loss of nephron mass, may change the future trajectory from the new baseline GFR as well. Conversely, periods of rapid GFR decline may be followed by periods of stable or increasing GFR, suggesting that the determinants of the GFR decline may sometimes be ameliorated over extended periods. Patients should be counseled that current trajectories or rates of loss of kidney function may not reflect future rates of loss of kidney function. Furthermore, in current nationwide efforts to place vascular access and allow for maturation prior to dialysis, identifying factors that cause precipitous declines in kidney function would allow more timely access placement. These findings may also open new avenues of research to identify more acute causes of rapid decreases in kidney function.
This research has several strengths, particularly the extended length of follow-up period with frequent measurements of eGFR, typically every obtained every 6 months. With up to 12 years of follow-up and up to 30 eGFRs per patient, we are able to observe and model both non-linear trajectories and prolonged periods of nonprogression. Most published studies have shorter follow-up and less frequent GFR measurements or estimates, making it difficult to confirm whether the trajectory is linear, given the random variability in eGFR. Another strength is the use of modern Bayesian statistical methods to develop rigorous probability statements for clinically relevant definitions of nonlinearity and nonprogression. Typical statistical procedures diagnose nonlinearity by residuals or goodness-of-fit statistics, which do not have a direct clinical interpretation. By combining clinically relevant definitions of nonlinearity (nonprogression) and Bayesian techniques, we are able to achieve both proper clinical interpretation and statistical rigor.
There are a number of limitations to this investigation. First, the study population consists exclusively of African Americans with hypertensive CKD who satisfied the trial’s inclusion criteria. The findings of this analysis may not apply to other patient populations. In addition, our findings must be interpreted conditionally on survival for 3 years without ESRD, due to our exclusion of 248 (23%) patients with a shorter follow-up time or ≤ 8 eGFRs. This restriction was necessary because the concept of nonlinearity under investigation requires sufficient follow-up periods to define and identify sustained changes in slope over time. Second, we used eGFR instead of direct measurements. The accuracy of the GFR estimating equation may affect the results. However, since the definitions of nonlinear features in this paper reflect relatively large changes over extended follow-up, a bias if present is unlikely to substantially change the results. In a separate publication, we showed that stable or increasing eGFR over the 3–6 year clinical trial phase of the study coincides closely with stable or increasing iothalamate GFR over the same period 16. Third, when the underlying true trajectory is highly frequently oscillating, the spline model may have non-negligible bias unless there are a lot of eGFR data to identify the curvature. We believe that the CKD progression can be reasonably assumed to follow a smooth, albeit sometimes nonlinear, trajectory without too much oscillation, and therefore such bias should not substantially change the conclusions. Finally, the definition of nonlinearity or nonprogression is somewhat subjective. We used conservative definitions, and provided sensitivity analyses in Table 3 and Item S3. Even when very stringent criteria are applied, our results suggest that considerable nonlinearity and nonprogression exist.
In summary, this paper demonstrated that in contrast to the traditional paradigm of steady GFR progression over time, many CKD patients have a non-linear GFR trajectory or a prolonged period of nonprogression. These findings highlight the possibility that stable kidney disease progression can accelerate, and conversely, provide hope that CKD need not be relentlessly progressive. These results should encourage researchers to identify time-dependent factors associated with periods of nonprogression and other desirable trajectories.
Supplementary Material
Acknowledgements
Support: This research is sponsored by the following grants from the National Institutes of Health: 5U01DK048648, 1R01DK090046-01.
Financial Disclosure: Dr Lewis reports research funding from Keryx, Lilly, Pharmanet, Astra Zeneca; honoraria from Amira and Astra Zeneca. Dr Appel reports honoraria from Unilever, Culinary Institute of America, and the American Heart Association. Dr Toto has consultancy agreements with Lilly, Abbott, Daiichi Sankyo, Novartis, Takeda; research funding from Novartis, Reata; honoraria from Amgen, Abbott, Novartis, Daiichi Snkyo, Takeda; and is a scientific advisor to Boehringer Ingelheim and Amgen. Dr Wright has consultancy agreements with Take Care Health and CVRx; honoraria from Takeda; and is a scientific advisor or member of the NHLBI JNC-8 Panel, Board of Directors for Northeast Ohio Neighborhood Health and Assoc of Black Cardiologists, Editorial Board for Journal of Hypertension. Dr Greene has consultancy agreements with Amgen, Cormedix, Nephrogenex, Lilly, Keryx; receives honoraria from Amgen, Cormedix, Nephrogenex, Lilly, and Keryx.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Liang Li, Cleveland Clinic.
Brad C. Astor, University of Wisconsin-Madison.
Julia Lewis, Vanderbilt University.
Bo Hu, Cleveland Clinic.
Lawrence J. Appel, Johns Hopkins University.
Michael S. Lipkowitz, Georgetown University.
Robert D. Toto, University of Texas Southwestern Medical Center.
Xuelei Wang, Case Western Reserve University.
Jackson T. Wright, Jr, Case Western Reserve University.
Tom H. Greene, University of Utah.
REFERENCES
- 1.Mitch W, Walser M, Buffington G, Lemann J. A simple method of estimating progression of chronic renal failure. Lancet. 1976;2(7999):1326–1328. doi: 10.1016/s0140-6736(76)91974-7. [DOI] [PubMed] [Google Scholar]
- 2.Levey A, Perrone R, Madias N. Serum Creatinine and Renal Function. Annual Review of Medicine. 1988;39:465–490. doi: 10.1146/annurev.me.39.020188.002341. [DOI] [PubMed] [Google Scholar]
- 3.Hunsicker LG, Adler S, Caggiula A, et al. Predictors of the progression of renal disease in the Modification of Diet in Renal Disease Study. Kidney Int. 1997;51(6):1908–1919. doi: 10.1038/ki.1997.260. [DOI] [PubMed] [Google Scholar]
- 4.Klahr S, Levey AS, Beck GJ, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. N Engl J Med. 1994;330(13):877–884. doi: 10.1056/NEJM199403313301301. [DOI] [PubMed] [Google Scholar]
- 5.Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 6.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 7.Gassman JJ, Greene T, Wright JT, Jr, et al. Design and statistical aspects of the African American Study of Kidney Disease and Hypertension (AASK) J Am Soc Nephrol. 2003;14(7 Suppl 2):S154–S165. doi: 10.1097/01.asn.0000070080.21680.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Appel LJ, Middleton J, Miller ER, 3rd, et al. The rationale and design of the AASK cohort study. J Am Soc Nephrol. 2003;14(7 Suppl 2):S166–S172. doi: 10.1097/01.asn.0000070081.15137.c0. [DOI] [PubMed] [Google Scholar]
- 9.Lewis J, Agodoa L, Cheek D, et al. Comparison of cross-sectional renal function measurements in African Americans with hypertensive nephrosclerosis and of primary formulas to estimate glomerular filtration rate. Am J Kidney Dis. 2001;38(4):744–753. doi: 10.1053/ajkd.2001.27691. [DOI] [PubMed] [Google Scholar]
- 10.Crainiceanu CM, Ruppert D, Wand MP. Bayesian analysis for penalized spline regression using WinBUGS. Journal of Statistical Software. 2005;14(14) [Google Scholar]
- 11.Wright JT, Jr, Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288(19):2421–2431. doi: 10.1001/jama.288.19.2421. [DOI] [PubMed] [Google Scholar]
- 12.Lindeman RD, Tobin J, Shock NW. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc. 1985;33(4):278–285. doi: 10.1111/j.1532-5415.1985.tb07117.x. [DOI] [PubMed] [Google Scholar]
- 13.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–S266. [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2011. ISBN 3-900051-07-0, URL. [Google Scholar]
- 15.Norris KC, Greene T, Kopple J, et al. Baseline predictors of renal disease progression in the African American Study of Hypertension and Kidney Disease. J Am Soc Nephrol. 2006;17(10):2928–2936. doi: 10.1681/ASN.2005101101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu B, Gadegbeku C, Lipkowitz MS, et al. Kidney function can improve in patients with hypertensive chronic kidney disease: results from the African American Study of Kidney Disease and Hypertension (AASK) Clin J Am Soc Nephrol. In press, [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.