Abstract
Background
To study the association between functional single nucleotide polymorphisms (SNPs) in candidate genes from oxidative stress pathways, and risk of radiation pneumonitis (RP) in patients treated with thoracic radiation therapy (RT) for locally advanced lung cancer (LC).
Methods
We reviewed 136 patients treated with RT for LC between 2001 and 2007, and had prior genotyping of functional SNPs in oxidative stress genes including superoxide dismutase 2 (SOD2; rs4880) and methylenetetrahydrofolate reductase (MTHFR; rs1801131, rs1801133). RP events were retrospectively scored using the Common Terminology Criteria for Adverse Events, version 4.0. Cox proportional hazard regression was performed to identify clinical variables and genotypes associated with risk of grade ≥2 and grade ≥3 RP on univariate and multivariate analysis. P-values were corrected for multiple hypothesis testing.
Results
With a median follow-up of 21.4 months, the incidence of ≥grade 2 RP was 29% and ≥grade 3 RP was 14%. On multivariate analysis, after adjusting for clinical factors such as concurrent chemotherapy, and consolidation docetaxel, and lung dosimetric parameters such as V20 and mean lung dose, MTHFR genotype (rs1801131; AA versus AC/CC) was significantly associated with risk of ≥grade 2 RP (Hazard ratio [HR]: 0.37; 95% confidence interval [CI]: 0.18-0.76; p=0.006, corrected p=0.018) and ≥grade 3 RP (HR: 0.21; 95% CI: 0.06-0.70; p=0.01; corrected p=0.03). SOD2 genotype was not associated with RP.
Conclusions
Our study showed an association between MTHFR genotype and risk of clinically significant RP. Further study of MTHFR-related pathways may provide insight into the mechanisms behind RP.
Keywords: Radiation pneumonitis, single nucleotide polymorphisms, lung cancer, oxidative stress, MTHFR
Introduction
Radiation pneumonitis (RP) remains a significant barrier to radiation dose escalation to achieve adequate local control in the treatment of locally advanced lung cancer (LC). While technological advances in the delivery of thoracic radiation therapy (RT) including intensity-modulated radiation therapy (IMRT) has allowed gradual dose escalation with potential reduction in toxicity1, rates of clinically-evident locoregional recurrence remain relatively high at 20-50% in randomized trials of definitive radiation with concurrent chemotherapy in patients with non-small cell lung cancer (NSCLC)2-4. Further dose escalation may be limited by the increasing risk of normal tissue toxicity, including RP.
In contemporary studies, the risk of symptomatic (grade 2-5) RP ranges from 10-30%5-9, and may be associated with radiation dose, concurrent chemotherapy regimens, use of consolidation docetaxel4, 10, and other clinical factors. Radiation dose to specific lung volumes have been correlated with risk of pneumonitis including volume of lungs receiving > 20 Gray (Gy; V20), > 5 Gy (V5), and mean lung dose (MLD)5, 8, 9. While these studies have helped establish a series of clinically useful dosimetric cutoffs, there may be a continuum of risk of normal tissue toxicity, which may be determined by the interaction between the physical distribution of radiation dose in the lungs and underlying biological factors in the patient.
The pathogenesis of radiation-induced lung injury is not completely understood, but may be due to a combination of direct radiation cytotoxicity to normal lung tissue and secondary inflammatory changes and fibrotic remodeling11. Thus, genetic variation in key genes in DNA repair, inflammation and oxidative stress pathways may ameliorate or exacerbate the effects of a given radiation dose to the lungs. Prior retrospective candidate gene studies in patients treated with RT for LC have shown associations between risk of RP and single nucleotide polymorphisms (SNPs) in the ataxia-telangiectasia mutated (ATM)12, XRCC1, APEX113, and p5314 genes, key components in cellular signaling and repair response to ionizing radiation, and transforming growth factor-beta (TGFB1), a cytokine involved in fibrotic remodeling15. Another important class of genes that may play a role in the pathogenesis of RP is oxidative stress pathways genes including superoxide dismutase 2, which is involved in free radical scavenging, and methylenetetrahydrofolate reductase, which is a key regulator of folate, homocysteine, thiol, methylation, and thymidine metabolism.
In this study, we build upon the existing literature by screening for an association between risk of RP and functional SNPs in candidate genes from oxidative stress pathways in a retrospective cohort of patients treated with thoracic RT for LC.
Methods
Patients
As part of an institutional review board-approved prospective molecular epidemiology protocol, peripheral blood was collected from patients diagnosed with either non-small cell lung cancer (NSCLC) or small cell lung cancer (SCLC) who were seen at the Massachusetts General Hospital from 1992 to present. The peripheral blood was collected as part of successive candidate gene epidemiological studies to examine associations between functional SNPs and LC risk and outcomes16-18. Patient and demographic information were collected at the time of recruitment, and informed consent was obtained to collect follow-up data. More than 85% of eligible patients were recruited in this cohort.
Out of approximately 3982 patients who consented to the protocol between 1992 and 2009, 2334 had prior genotyping of functional SNPs in two candidate genes involved in oxidative stress pathways as listed below. We limited our study to patients treated after 2001, when our department and the hospital adopted widespread utilization of an electronic medical record system that allowed more accurate retrospective collection of clinical data. Out of this group, we retrospectively identified 243 patients who were treated at MGH between 2001 and 2007 with thoracic RT with a minimum dose of 40 Gy. Of these 243 patients, we excluded non-white patients (n=8), patients who were not treated with 3-dimensional conformal RT (3D-CRT) or IMRT and did not have radiation dosimetric data for the lungs (n=57), patients with stage I disease (n=18), patients who were lost to follow-up (<1 month of follow-up) after treatment (n=7), and patients withinsufficient peripheral blood for genotyping (n=10). The remaining 136 patients constituted our sample set for this RP and candidate gene SNP association study.
Genotyping
Blood samples were collected from all study participants at the time of recruitment. Germline DNA was isolated from the peripheral blood of each patient using Autopure blood purification kits (Qiagen Sciences, Inc., Germantown, MD, USA). Genotyping was performed using the 5′-nuclease assay (TaqMan) assay and the ABI Prism 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA ,USA) on known functional SNPs for candidate genes in oxidative stress pathways including: 1) superoxide dismutase 2 (SOD2; 5482C>T, Val16Ala, rs4880); 2) methylenetetrahydrofolate reductase (MTHFR; 1298A>C, Glu429Ala, rs1801131; and 677C>T, Ala222Val, rs1801133). Genotyping was performed by laboratory personnel blinded to patient status, and a random 5% of the samples were repeated to validate genotyping procedures. Hardy-Weinberg equilibrium was tested using the χ2 test. Due to the small sample sizes and small number of patients with the homozygous variant genotypes, genotypes were primarily analyzed using the dominant effects model (SOD2, rs4880: CC vs. CT/TT; MTHFR, rs1801131: AA vs. AC/CC; MTHFR, rs1801133: CC vs. CT/TT).
Clinical and Radiation Dosimetric Covariates
Clinical covariates that may be associated with RP were retrospectively collected including, patient age, ECOG performance status (PS), smoking status, pulmonary function test data, AJCC TNM stage (6th edition), tumor size, RT technique, radiation dose, use of concurrent or consolidation chemotherapy, use of consolidation docetaxel, and use of surgery. Smoking status was categorized as: 1) never smokers; < 100 cigarettes in their lifetime; 2) former smokers; quit >1 year prior to diagnosis, and 3) current smokers; smoking at the time of diagnosis or quit < 1 year prior. Lung dosimetric variables including MLD, V5, and V20 were collected from the treatment plans. The lung volume was defined as the total volume of the two lungs minus the volume of the gross tumor volume.
Endpoints
RP events were identified retrospectively and graded using the Common Terminology Criteria for Adverse Events, (CTCAE version 4.0) by two of the investigators (B.M.A. and R.H.M). Both investigators were blinded from the genotyping results and independently reviewed all of the follow-up notes for each patient, and reviewed pertinent radiological imaging to determine a diagnosis and grade of RP. The RP scores from the two investigators were compared using the kappa statistic to estimate the concordance of grading, and any discordant results were reconciled. The RP grades were analyzed as dichotomized variables at clinically relevant cutoffs: ≥grade 2 RP (use of steroids) versus grade 0-1 RP and ≥grade 3 RP (oxygen requirement) versus grade 0-2 RP. RP was analyzed as a time dependent variable, and was calculated from the initiation of radiation therapy to the time when patients had a RP event or were censored at the time of last follow-up or death.
Statistical Analysis
The cumulative incidence of RP was estimated using the Kaplan-Meier Method. Cox proportional hazard models were performed to identify clinical variables and genotypes associated with risk of RP on univariate and multivariate analysis. For multivariate analysis, variables with a p-value < 0.10 were included in initial models as potential confounders. Stepwise selection was performed to identify variables associated with ≥grade 2 or ≥grade 3 RP. Clinically important confounders such as lung dosimetric parameters were re-introduced into the final model. Since 3 different SNPs were examined, we corrected for multiple hypothesis testing using the method of Benjamini and Hochberg in both univariate and multivariate analyses19. All statistical testing was done with a two-sided p-value < 0.05 level. All analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC, USA).
Results
Patient, Treatment and Radiation Dosimetric Characteristics
The median age of the patients was 67 (range, 37-85), 51% were female, 90% were current or former smokers, and 95% had ECOG PS of 0-1 (Table 1). In the 118 patients with pulmonary function testing, the median FEV1 was 1.98 L (range, 0.66-4.31 L). The majority of patients were treated with definitive RT without surgery (78%), and the remainder received RT preoperatively (11%) or postoperatively (11%). In total, 96% of patients received chemotherapy, including as induction prior to RT (8%), concurrently with RT (89%) and for consolidation after RT (57%). Of note, 12% of patients received consolidation docetaxel. All patients were treated with IMRT (75%) or 3D-CRT (25%). The median prescribed dose was 63 Gy (range, 41.4-72.7). Dosimetric parameters for the lungs included a median V20 of 34% (9.4-70%), V5 of 51% (14-93%) and MLD of 18.1 Gy (6.1-32.4 Gy).
Table 1.
Patient, tumor and treatment characteristics.
| All Patients (n=136) | Grade 0-1 RP (n=96) | ≥Grade 2 RP (n=40) | ≥Grade 3 RP (n=19) | |
|---|---|---|---|---|
|
| ||||
| Patient Characteristics | ||||
|
| ||||
| Median Age (range) | 66.7 (37.2-85.2) | 66.8 (37.2-85.3) | 61.4 (48.0-83.7) | 63.9 (51.2-83.4) |
|
| ||||
| Male | 67 (49.3%) | 41 (42.7%) | 26 (65.0%) | 15 (79.0%) |
| Female | 69 (50.7%) | 55 (57.3%) | 14 (35.0%) | 4 (21.0%) |
|
| ||||
| ECOG PS | ||||
| 0 | 62 (45.6%) | 45 (46.9%) | 17 (42.5%) | 7 (36.8%) |
| 1 | 67 (49.3%) | 49 (51.0%) | 18 (45.0%) | 9 (47.4%) |
| 2 | 7 (5.1%) | 2 (2.1%) | 5 (12.5%) | 3 (15.8%) |
|
| ||||
| Smoking Status | ||||
| Current | 48 (35.3%) | 37 (38.5%) | 11 (27.5%) | 6 (31.6%) |
| Former | 74 (54.4%) | 48 (50.0%) | 26 (65.0%) | 12 (63.2%) |
| Never | 14 (10.3%) | 11 (11.5%) | 3 (7.5%) | 1 (5.3%) |
|
| ||||
| Median Pack-years (range) | 49 (0-200) | 49 (0-200) | 40 (0-150) | 55 (0-120) |
|
| ||||
| Median FEV1 in Liters (range) | 1.98 (0.66-4.31; n=115) |
1.99 (0.74-4.31; n=81) | .98 (0.66-3.44; n=34) | .98 (0.92-3.44; n=18) |
|
| ||||
| Tumor Characteristics | ||||
|
| ||||
| Histology: | ||||
| Squamous cell carcinoma | 29 (21.3%) | 19 (19.8%) | 10 (25.0%) | 5 (26.3%) |
| Adenocarcinoma | 64 (47.1%) | 49 (51.0%) | 15 (37.5%) | 5 (26.3%) |
| Poor differentiated carcinoma† | 28 (20.6%) | 17 (17.7%) | 11 (27.5%) | 7 (36.8%) |
| Small cell lung cancer | 15 (11.0%) | 11 (11.5%) | 4 (10.0%) | 2 (10.5%) |
|
| ||||
| T-stage | ||||
| 0 | 6 (4.4%) | 4 (4.2%) | 2 (5.0%) | 2 (10.5%) |
| 1 | 31 (22.8%) | 26 (27.1%) | 5 (12.5%) | 1 (5.3%) |
| 2 | 47 (34.6%) | 30 (31.2%) | 17 (42.5%) | 8 (42.1%) |
| 3 | 24 (17.6%) | 15 (15.6%) | 9 (22.5%) | 4 (21.0%) |
| 4 | 28 (20.6%) | 21 (21.9%) | 7 (17.5%) | 4 (21.0%) |
|
| ||||
| N-stage | ||||
| 0 | 10 (7.4%) | 10 (10.4%) | 0 (0.0%) | 0 (0.0%) |
| 1 | 11 (8.1%) | 10 (10.4%) | 1 (2.5%) | 0 (0.0%) |
| 2 | 80 (58.8%) | 53 (55.2%) | 27 (67.5%) | 14 (73.7%) |
| 3 | 35 (25.7%) | 23 (24.0%) | 12 (30.0%) | 5 (26.3%) |
|
| ||||
| Stage | ||||
| IIB | 9 (6.6%) | 9 (9.4%) | 0 (0.0%) | 0 (0.0%) |
| IIIA | 63 (46.3%) | 40 (41.7%) | 23 (57.5%) | 11 (57.9%) |
| IIIB | 52 (38.2%) | 38 (39.6%) | 14 (35.0%) | 7 (36.8%) |
| IV | 12 (8.8%) | 9 (9.4%) | 3 (7.5%) | 1 (5.3%) |
|
| ||||
| Treatment Characteristics | ||||
|
| ||||
| Median Radiation Dose (range) | 63 Gy (41.4-72.7) | 63 Gy (45-72) | 66.6 Gy (41.4-72.7) | 66.6 Gy (41.4-72.7) |
|
| ||||
| Radiation Technique: | ||||
| 3D Conformal | 34 (25%) | 26 (27.1%) | 8 (20.0%) | 2 (10.5%) |
| IMRT | 102 (75%) | 70 (72.9%) | 32 (80.0%) | 17 ( 89.5%) |
|
| ||||
| Simulation Technique: | ||||
| 3D-CT | 72 (52.9%) | 53 (55.2%) | 19 (47.5%) | 8 (42.1%) |
| 4D CT | 64 (47.1%) | 43 (44.8%) | 21 (52.5%) | 11 (57.9%) |
|
| ||||
| Radiation Sequencing: | ||||
| Pre-operative | 15 (11.0%) | 13 (13.5%) | 2 (5.0%) | 0 (0.0%) |
| Radiation alone | 106 (78.0%) | 69 (71.9%) | 37 (92.5%) | 19 (100%) |
| Post-operative | 15 (11.0%) | 14 (14.6%) | 1 (2.5%) | 0 (0.0%) |
|
| ||||
| Surgery | 30 (22.8%) | 27 (28.1%) | 3 (7.5%) | 0 (0.0%) |
|
| ||||
| Surgery Type | ||||
| Wedge | 4 (13.3%) | 4 (14.8%) | 0 | - |
| Lobectomy | 25 (83.3%) | 22 (81.5%) | 3 (100%) | - |
| Pneumonectomy | 1 (3.3%) | 1 (3.7%) | 0 | - |
|
| ||||
| Any Chemotherapy | 130 (95.6%) | 91 (94.8%) | 39 (97.5%) | 18 (94.7%) |
|
| ||||
| Induction Chemotherapy | 11 (8.1%) | 10 (10.4%) | 1 (2.5%) | 1 (5.3%) |
|
| ||||
| Concurrent Chemotherapy | 121 (89.0%) | 82 (85.4%) | 39 (97.5%) | 18 (94.7%) |
|
| ||||
| Consolidation Chemotherapy | 78 (57.4%) | 54 (56.3%) | 24 (60.0%) | 5 (26.3%) |
|
| ||||
| Consolidation Docetaxel | 16 (11.8%) | 9 (8.3%) | 8 (20.0%) | 3 (15.8%) |
|
| ||||
| Bilateral Lung Dose-Volume Histogram | ||||
|
| ||||
| Median V5 (range) | 61.7% (21.7-97.2%) | 59.4% (21.7-95.0%) | 65.3% (38.0 - 97.2%) | 63.8% (42.0-97.2%) |
|
| ||||
| Median V20 (range) | 34.0% (9.4-70.0%) | 32.4% (9.6-68.7%) | 36.3% (21.9-70.0%) | 36.4% (22.0-68.0%) |
|
| ||||
| Median MLD (range) | 18.1 Gy (6.1-32.4 Gy) | 17.6 Gy (6.1-29.3 Gy) | 19.8 Gy (11.0-32.4 Gy) | 20.5 (13.3-32.4 Gy) |
Includes carcinoma not otherwise specified (NOS) and non-small cell lung cancer NOS.
Abbreviations: CT=computed tomography; FEV1=force expiratory volume in 1 second; IMRT=intensity modulated radiation therapy; MLD=mean lung dose; PS=performance status; V5=volume receiving greater than 5 Gy; V10=volume receiving greater than 10 Gy; V20=volume receiving greater than 20 Gy; RP=radiation pneumonitis.
MTHFR and SOD2 Genotyping
The distribution of the MTHFR (rs1801131 and rs1801133) and SOD2 genotypes are shown in Table 2. All MTHFR and SOD2 polymorphisms examined were in Hardy-Weinberg equilibrium (p > 0.05, χ2 goodness of fit).
Table 2.
Genotype Frequency
| Single Nucleotide Polymorphisms | All Patients (n=136) |
Grade 0-1 RP (n=96) |
≥Grade 2 RP (n=40) |
≥Grade 3 RP (n=19) |
|---|---|---|---|---|
|
| ||||
| MTHFR (rs1801131)* | ||||
| AA | 61 (45.5%) | 38 (40.0%) | 23 (59.0%) | 13 (72.2%) |
| AC | 60 (44.8%) | 48 (50.5%) | 12 (30.8%) | 3 (11.1%) |
| CC | 13 (9.7%) | 9 (9.5%) | 4 (10.3%) | 2 (16.7%) |
| AC/CC | 73 (54.5%) | 57 (60.0%) | 16 (41.0%) | 5 (27.8%) |
|
| ||||
| MTHFR (rs1801133)* | ||||
| CC | 51 (38.1%) | 40 (42.1%) | 11 (28.2%) | 4 (22.2%) |
| CT | 59 (44.0%) | 40 (42.1%) | 19 (48.7%) | 8 (44.4%) |
| TT | 24 (17.9%) | 15 (15.8%) | 9 (23.1%) | 6 (33.3%) |
| CT/ TT | 83 (61.9%) | 55 (57.9%) | 28 (71.8%) | 14 (77.7%) |
|
| ||||
| SOD2 (rs4880) | ||||
| TT | 32 (23.5%) | 21 (21.9%) | 11 (27.5%) | 6 (31.6%) |
| TC | 76 (55.9%) | 51 (53.1%) | 25 (62.5%) | 9 (47.4%) |
| CC | 28 (20.6%) | 4 (25.0%) | 4 (10%) | 4 (21.0%) |
| TC/CC | 104 (76.5%) | 72 (78.1%) | 29 (72.5%) | 13 (68.4%) |
Data available on 134 patients
Abbreviations: RP=radiation pneumonitis.
Radiation Pneumonitis
With a median follow-up of 21.4 months (range, 1.6-109.5 months) after initiation of RT, the crude incidence of ≥grade 2 RP was 29% (40/136) with an 1-year Kaplan-Meier estimate of 30.5%, and the crude incidence of ≥grade 3 RP was 14% (19/136)with an 1-year Kaplan-Meier estimate of 13.7%. There was excellent concordance between the two independent measures of RP grade using CTCAE version 4.0 with a kappa statistic of 0.81 (95% confidence interval [CI]: 0.72-0.90) when examining the scores on an ordinal scale from 0 to 5. When examining the RP grading as a dichotomous variable of ≥grade 2 and ≥grade 3, the kappa statistics were 0.96 (95% CI: 0.92-1.0) and 0.94 (95% CI: 0.85-1.0), respectively. In total, two cases were discordant between the two independent measures of RP as a dichotomous variable for both ≥grade 2 and ≥grade 3.
Clinical and Dosimetric Variables Associated with RP
On univariate analysis (Table 3), clinical variables associated with ≥grade 2 RP included gender, performance status, MLD, and use of surgery. On multivariate analysis, gender, ECOG PS, surgery, concurrent chemotherapy, and consolidation docetaxel were associated with ≥grade 2 RP (Table 4). Important dosimetric predictors of RP including MLD, V20 and V5 were re-introduced into the final model. The continuous variables such as MLD, V20 and V5 were analyzed both continuously and as categorical variables (e.g. dichotomized at the median); since the models were not significantly different with either approach, the dichotomized variable analysis models are presented.
Table 3.
Univariate Cox Regression of Clinical and Dosimetric Factors for Risk of ≥ Grade 2 RP
| Total Patients (n=136) |
Patients with ≥Grade 2 RP (n=40) |
Percent of Patients with ≥Grade 2 RP |
HR | 95% CI | p-value | |
|---|---|---|---|---|---|---|
|
| ||||||
| Patient Characteristics | ||||||
|
| ||||||
| Age<66 | 64 | 17 | 26.6% | 1.0* | - | - |
| Age≥66 | 72 | 23 | 31.9% | 1.25 | 0.67-2.33 | 0.49 |
|
| ||||||
| Male | 67 | 26 | 38.8% | 2.22 | 1.16-4.26 | 0.017 |
| Female | 69 | 14 | 20.3% | 1.0* | - | - |
|
| ||||||
| ECOG PS | ||||||
| 0 | 62 | 17 | 27.4% | 1.0* | - | - |
| 1 | 67 | 18 | 26.9% | 1.01 | 0.52-1.96 | 0.98 |
| 2 | 7 | 5 | 71.4% | 3.63 | 1.34-9.87 | 0.01 |
|
| ||||||
| Smoking Status | ||||||
| Current | 48 | 11 | 22.9% | 1.0* | - | - |
| Former | 74 | 26 | 35.1% | 1.62 | 0.80-3.29 | 0.18 |
| Never | 14 | 3 | 21.4% | 0.92 | 0.26-3.31 | 0.90 |
|
| ||||||
| Pack-year<49 | 68 | 20 | 29.4% | 1.0* | - | - |
| Pack-years≥49 | 68 | 20 | 29.4% | 0.98 | 0.53-1.82 | 0.95 |
|
| ||||||
| FEV1<1.98 L | 78 | 55 | 70.5% | 1.0* | - | - |
| FEV1≥1.98 L | 58 | 41 | 70.7% | 0.99 | 0.53-1.85 | 0.97 |
|
| ||||||
| Tumor Characteristics | ||||||
|
| ||||||
| Histology: | ||||||
| SCC | 29 | 10 | 34.5% | 0.96 | 0.32-2.90 | 0.95 |
| AdenoCA | 64 | 15 | 23.5% | 1.0* | - | - |
| Carcinoma NOS | 28 | 11 | 39.3% | 1.53 | 0.69-3.40 | 0.30 |
| SCLC | 15 | 4 | 26.7% | 1.68 | 0.77-3.65 | 0.19 |
|
| ||||||
| T-stage | ||||||
| 0-1 | 37 | 7 | 18.9% | 0.62 | 0.31-1.24 | 0.17 |
| 2 | 47 | 17 | 36.2% | 1.0* | - | - |
| 3 | 24 | 9 | 37.5% | 1.19 | 0.60-2.38 | 0.62 |
| 4 | 28 | 7 | 25.0% | 0.84 | 0.39-1.84 | 0.67 |
|
| ||||||
| N-stage | ||||||
| 0-1 | 21 | 1 | 4.8% | 0.33 | 0.14-0.79 | 0.01 |
| 2 | 80 | 27 | 33.8% | 1.0* | - | - |
| 3 | 35 | 12 | 34.3% | 0.99 | 0.53-1.84 | 0.98 |
|
| ||||||
| Stage | ||||||
| IIB | 9 | 0 | 0% | 0.0 | 0.0 | 0.99 |
| IIIA | 63 | 23 | 36.5% | 1.0* | - | - |
| IIIB | 52 | 14 | 26.9% | 0.75 | 0.38-1.44 | 0.37 |
| IV | 12 | 3 | 25.0% | 0.70 | 0.21-2.33 | 0.56 |
|
| ||||||
| Treatment Characteristics | ||||||
|
| ||||||
| Radiation Dose | ||||||
| <60 Gy | 35 | 6 | 24.0% | 0.70 | 0.25-1.92 | 0.49 |
| ≥60 Gy and >66 Gy | 40 | 10 | 25.0% | 1.0* | - | - |
| ≥66 Gy and >70 Gy | 28 | 12 | 42.8% | 1.86 | 0.80-4.31 | 0.15 |
| ≥70 Gy | 33 | 12 | 36.4% | 1.63 | 0.70-3.78 | 0.25 |
|
| ||||||
| Radiation Technique: | ||||||
| 3D Conformal | 34 | 8 | 23.5% | 0.73 | 0.34-1.59 | 0.43 |
| IMRT | 102 | 32 | 31.4% | 1.0* | - | - |
|
| ||||||
| Simulation Technique: | ||||||
| 3D-CT | 72 | 19 | 26.4% | 1.0* | - | - |
| 4D CT | 64 | 21 | 32.8% | 1.28 | 0.69-2.39 | 0.43 |
|
| ||||||
| Radiation Sequencing: | ||||||
| Pre-operative | 15 | 2 | 13.3% | 0.30 | 0.07-1.24 | 0.10 |
| Radiation alone | 106 | 37 | 34.9% | 1.0* | - | - |
| Post-operative | 15 | 1 | 6.7% | 0.17 | 0.02-1.25 | 0.08 |
|
| ||||||
| Surgery | 30 | 3 | 10.0% | 0.23 | 0.07-0.74 | 0.014 |
|
| ||||||
| Any Chemotherapy | 130 | 39 | 30.0% | 1.66 | 0.23-12.08 | 0.62 |
|
| ||||||
| Concurrent Chemotherapy | 121 | 39 | 32.2% | 5.27 | 0.72-38.35 | 0.10 |
|
| ||||||
| Consolidation Chemotherapy | 78 | 24 | 30.8% | 1.0 | 0.84-1.18 | 0.96 |
|
| ||||||
| Consolidation Docetaxel | 16 | 8 | 50.0% | 2.11 | 0.97-4.58 | 0.06 |
|
| ||||||
|
Bilateral Lung Dose-
Volume Histogram |
||||||
|
| ||||||
| V5<60% | 62 | 13 | 21.0% | 1.0* | - | - |
|
| ||||||
| V5≥60% | 74 | 27 | 36.4% | 1.90 | 0.98-3.69 | 0.057 |
|
| ||||||
| V20<30% | 52 | 9 | 17.3% | 0.45 | 0.20-1.04 | 0.061 |
| V20≥30% and <40% | 43 | 15 | 34.9% | 1.0* | - | - |
| V20≥40% | 41 | 16 | 39.0% | 1.15 | 0.57-2.33 | 0.70 |
|
| ||||||
| MLD<18 Gy | 66 | 14 | 21.2% | 1.0* | - | - |
| MLD≥18 Gy | 70 | 26 | 37.1% | 1.95 | 1.02-3.74 | 0.044 |
Reference group for Cox regression
Abbreviations: CI=confidence interval; CT=computed tomography; FEV1=force expiratory volume in 1 second; HR=hazard ratio; IMRT=intensity modulated radiation therapy; MLD=mean lung dose; PS=performance status; RP=radiation pneumonitis; V5=volume receiving greater than 5 Gy; V10=volume receiving greater than 10 Gy; V20=volume receiving greater than 20 Gy.
Table 4.
Cox Regression Analysis of Risk of ≥Grade 2 RP
| Univariate Analysis | Multivariate Analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Total Patients (n=136) |
Number of Patients with ≥Grade 2 RP (n=40) |
Percent of Patients with ≥ Grade 2 RP |
HR | 95% CI | p-value | AHR | 95% CI | p-value | |
|
| |||||||||
| Patient Characteristics | |||||||||
|
| |||||||||
| Male | 67 | 26 | 38.8% | 2.22 | 1.16-4.26 | 0.017 | 2.69 | 1.32-5.49 | 0.006 |
| Female | 69 | 14 | 20.3% | 1.0* | - | - | 1.0* | - | - |
|
| |||||||||
| ECOG PS | |||||||||
| 0 | 62 | 17 | 27.4% | 1.0* | - | - | 1.0* | - | - |
| 1 | 67 | 18 | 26.9% | 1.01 | 0.52-1.96 | 0.98 | 1.03 | 0.51-2.08 | 0.92 |
| 2 | 7 | 5 | 71.4% | 3.63 | 1.34-9.87 | 0.01 | 2.85 | 0.90-9.00 | 0.075 |
|
| |||||||||
| Treatment Characteristics | |||||||||
|
| |||||||||
| Surgery | 30 | 3 | 10.0% | 0.23 | 0.07-0.74 | 0.014 | 0.24 | 0.06-0.91 | 0.036 |
|
| |||||||||
| Concurrent Chemotherapy | 121 | 39 | 32.2% | 5.27 | 0.72-38.35 | 0.10 | 1.27 | 1.01-1.59 | 0.04 |
|
| |||||||||
| Consolidation Docetaxel | 16 | 8 | 50.0% | 2.11 | 0.97-4.58 | 0.06 | 4.42 | 1.79-10.92 | 0.001 |
|
| |||||||||
|
Bilateral Lung Dose
Volume Histogram |
|||||||||
|
| |||||||||
| V5<60% | 62 | 13 | 21.0% | 1.0* | - | - | 1.0* | - | - |
| V5≥60% | 74 | 27 | 36.4% | 1.90 | 0.98-3.69 | 0.057 | 1.16 | 0.44-3.10 | 0.76 |
|
| |||||||||
| V20<30% | 52 | 9 | 17.3% | 0.45 | 0.20-1.04 | 0.061 | 0.77 | 0.23-2.54 | 0.66 |
| V20≥30% and < 40 | 43 | 15 | 34.9% | 1.0* | - | - | 1.0* | - | - |
| V20≥40% | 41 | 16 | 39.0% | 1.15 | 0.57-2.33 | 0.70 | 1.08 | 0.50-2.34 | 0.84 |
|
| |||||||||
| MLD<18 Gy | 66 | 14 | 21.2% | 1.0* | - | - | 1.0* | - | - |
| MLD≥18 Gy | 70 | 26 | 37.1% | 1.95 | 1.02-3.74 | 0.044 | 1.19 | 0.42-3.32 | 0.75 |
|
| |||||||||
| SNP † | |||||||||
|
| |||||||||
| MTHFR (rs1801131)□ | |||||||||
| AA | 61 | 23 | 37.7% | 1.0* | - | - | 1.0* | - | - |
| AC | 60 | 12 | 20.0% | 0.47 | 0.24-0.95 | 0.36 | 0.30 | 0.14-0.67 | 00.003 |
| CC | 13 | 4 | 30.8% | 0.68 | 0.24-1.98 | 0.48 | 0.70 | 0.24-2.07 | 0.52 |
| AC/CC | 73 | 16 | 21.9% | 0.51 | 0.27-0.97 | 0.041 | 0.37 | 0.18-0.76 | 0.006 |
|
| |||||||||
| MTHFR (rs1801133)□ | |||||||||
| CC | 51 | 11 | 21.6% | 1.0* | - | - | 1.0* | - | - |
| CT | 59 | 19 | 32.2% | 1.56 | 0.74-3.28 | 0.24 | 1.24 | 0.57-2.73 | 0.59 |
| TT | 24 | 9 | 37.5% | 2.05 | 0.85-4.94 | 0.11 | 2.29 | 0.91-5.76 | 0.078 |
| CT/TT | 83 | 28 | 33.7% | 1.69 | 0.84-3.39 | 0.14 | 1.48 | 0.71-3.08 | 0.29 |
|
| |||||||||
| SOD2 (rs4880) | |||||||||
| TT | 32 | 11 | 10.5% | 1.0* | - | - | 1.0* | - | - |
| CT | 76 | 25 | 32.9% | 0.93 | 0.46-1.88 | 0.83 | 1.24 | 0.60-2.59 | 0.56 |
| CC | 28 | 4 | 14.3% | 0.40 | 0.13-1.24 | 0.12 | 0.57 | 0.17-1.85 | 0.35 |
| CT/CC | 104 | 29 | 27.9% | 0.78 | 0.39-1.57 | 0.49 | 0.84 | 0.52-2.21 | 0.85 |
Reference group for Cox regression
Each SNP was independently entered into a multivariate model that adjusted for gender, ECOG PS, use of surgery, use of concurrent chemotherapy, use of consolidation docetaxel, MLD, V5 and V20.
Data available on 134 patients
Abbreviations: AHR=adjusted hazard ratio; CI=confidence interval; HR=hazard ratio; MLD=mean lung dose; PS=performance status; RT=radiation therapy; V5=volume receiving greater than 5 Gy; V20=volume receiving greater than 20 Gy; RP=radiation pneumonitis.
Univariate and Multivariate Analysis of SNP Association with RP
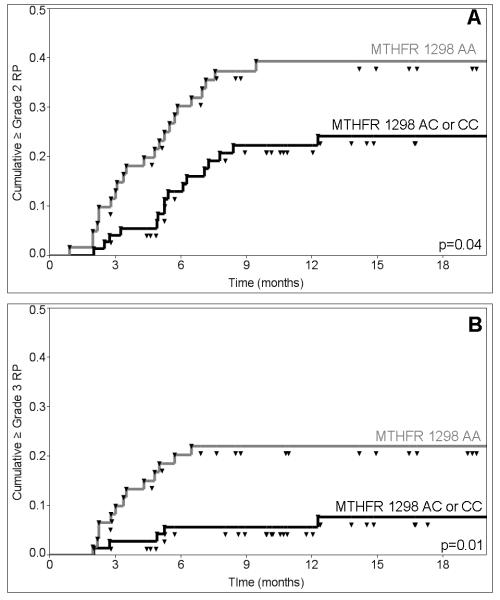
The MTHFR 1298A>C SNP (rs1801131) was associated with risk of ≥grade 2 RP on univariate analysis with a hazard ratio (HR) of 0.51 (95% CI: 0.27-0.97; p=0.04; corrected p=0.12) for the MTHFR 1298 AC/CC versus AA genotype (Table 3). Kaplan-Meier estimates of the incidence of ≥grade 2 RP at 12 months from the initiation of RT were 39.3% for patients with the MTHFR 1298 AA genotype versus 22.3% for patients with the MTHFR 1298 AC/CC genotypes (Figure 1A). Analysis of the MTHFR 1298 genotypes using a co-dominant model (AA vs. AC vs. CC) showed a significant decrease in risk of RP between 1298 AA versus AC genotype, and a non-significant decrease in risk of 1298 AA versus CC genotype on both univariate and multivariate analysis (Table 4). Genotype of SOD2 (rs4880), and the other SNP from the MTHFR gene (rs1801133; 677C>T) were not significantly associated with risk of ≥grade 2 RP on univariate analysis (Table 3).
Figure 1.
Kaplan-Meier Plots of Cumulative (A) ≥Grade 2 and (B) ≥Grade 3 Radiation Pneumonitis in Patients with MTHFR 1298 AA (gray line) versus 1298 AC/CC Genotype (black line).
After adjusting for potential clinical and dosimetric confounders, there remained an association between ≥grade 2 RP and MTHFR 1298A>C genotype (rs1801131; 1298AA vs. AC/CC) with an adjusted hazard ratio (AHR) of 0.37 (95% CI: 0.18-0.76; p=0.006; corrected p=0.018; Table 4). Neither the SOD2 nor the other MTHFR (rs1801133) SNP were associated with risk of ≥grade 2 RP on multivariate analysis (Table 4).
We also examined the relationship between the MTHFR 1298C>A SNP and risk of ≥grade 3 RP (Table 5). Kaplan-Meier estimates of the incidence of ≥grade 3 RP at 12 months from the initiation of RT were 22.0% for patients with the MTHFR 1298AA genotype versus 5.7% for patients with the MTHFR 1298AC/CC genotypes (Figure 1B). MTHFR 1298 AC/CC versus AA genotype were significantly associated with decreased risk of ≥grade 3 RP on univariate analysis (HR: 0.30; 95% CI: 0.11-0.83, p=0.02; corrected p=0.06), and multivariate analysis (AHR: 0.21; 95% CI: 0.06-0.70; p=0.01; corrected p=0.03).
Table 5.
Univariate and Multivariate Cox Regression analysis of Risk of Grade ≥3 RP for MTHFR
| Univariate Analysis | Multivariate Analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Total Patients (n=136) |
Number of Patients with Grade ≥ 3RP (n=19) |
Percent of Patients with ≥Grade 3 RP |
HR | 95% CI | p-value | AHR | 95% CI | p-value | |
|
| |||||||||
| Patient Characteristics | |||||||||
|
| |||||||||
| Male | 67 | 15 | 22.4% | 4.20 | 1.40-12.68 | 0.011 | 3.90 | 1.23-12.3 | 0.02 |
| Female | 69 | 4 | 5.8% | 1.0* | - | - | 1.0* | - | - |
|
| |||||||||
| ECOG PS | |||||||||
| 0 | 62 | 7 | 11.3% | 1.0* | - | - | 1.0* | - | - |
| 1 | 67 | 9 | 13.4% | 1.20 | 0.44-3.24 | 0.71 | 1.01 | 0.35-2.94 | 0.99 |
| 2 | 7 | 3 | 42.9% | 5.24 | 1.35-20.32 | 0.017 | 3.99 | 0.73-21.8 | 0.11 |
|
| |||||||||
| Treatment Characteristics | |||||||||
|
| |||||||||
| Concurrent Chemotherapy | 121 | 18 | 14.9% | 2.23 | 0.30-16.68 | 0.44 | 1.42 | 1.08-1.88 | 0.01 |
|
| |||||||||
| Consolidation Docetaxel | 16 | 3 | 18.8% | 1.39 | 0.40-4.76 | 0.60 | 2.77 | 0.71-10.9 | 0.14 |
|
| |||||||||
| Surgery | 30 | 0 | 0% | 0.0 | - | 0.99 | |||
|
| |||||||||
| Bilateral Lung DVH | |||||||||
|
| |||||||||
| V5<60% | 62 | 5 | 8.1% | 1.0* | - | - | 1.0* | - | - |
| V5≥60% | 74 | 14 | 18.9% | 2.47 | 0.89-6.87 | 0.082 | 1.86 | 0.35-9.93 | 0.47 |
|
| |||||||||
| V20<30% | 52 | 4 | 7.7% | 0.38 | 0.12-1.28 | 0.12 | 0.95 | 0.16-5.64 | 0.97 |
| V20≥30% and <40% | 43 | 8 | 18.6% | 1.0* | - | - | 1.0* | - | - |
| V20≥40% | 41 | 7 | 17.1% | 0.91 | 0.33-2.40 | 0.85 | 0.58 | 0.19-1.74 | 0.33 |
|
| |||||||||
| MLD<18 Gy | 66 | 6 | 9.1% | 1.0* | - | - | 1.0* | - | - |
| MLD≥18 Gy | 70 | 13 | 18.6% | 2.17 | 0.82-5.71 | 0.12 | 1.89 | 0.35-10.2 | 0.90 |
|
| |||||||||
| SNP | |||||||||
|
| |||||||||
| MTHFR rs1801131 † | |||||||||
| AA | 61 | 13 | 21.3% | 1.0* | - | - | 1.0* | - | - |
| AC/CC | 73 | 5 | 6.8% | 0.30 | 0.11-0.83 | 0.02 | 0.21 | 0.06-0.70 | 0.01 |
Reference group for Cox regression
Data available on 134 patients
Abbreviations: AHR=adjusted hazard ratio; CI=confidence interval; HR=hazard ratio; MLD=mean lung dose; PS=performance status; RT=radiation therapy; V5=volume receiving greater than 5 Gy; V20=volume receiving greater than 20 Gy; RP=radiation pneumonitis.
Discussion
In this cohort of patients treated with thoracic RT for lung cancer, we explored the association between RP and SNPs from candidate genes in oxidative stress pathways. We identified an association between risk of RP and the MTHFR 1298A>C genotype (rs1801131). Patients with AC/CC genotypes had a lower risk of both ≥grade 2 and of ≥grade 3 RP compared to patients with the wild-type AA genotype. This association was seen after adjusting for clinical and dosimetric variables associated with risk of RP, and correcting for multiple testing. Patients with an MTHFR 1298AC/CC genotype had an unadjusted incidence of ≥grade 2 RP of only 17%, compared to 50% in patients with an AA genotype, raising the hypothesis that AC/CC genotype may be protective. Furthermore, the adjusted hazard ratios of 0.37 (95% CI: 0.18-0.76; p=0.006; corrected p=0.018) and 0.21 (95% CI: 0.06-0.70; p=0.01; corrected p=0.03), for ≥grade 2 RP and ≥grade 3 RP, respectively, may suggest a strong underlying biological effect. While there was not a strong association between decreased risk of both 1298AC and 1298CC genotype independently versus 1298AA nor a variant allele dose effect (i.e. a decrease in risk of RP with each additional C allele), the small sample size in the 1298CC genotype subtype (n=13) likely makes this an underpowered analysis and such a dose effect cannot be excluded. Thus, further work in confirming this hypothesis-generating finding in a larger cohort of patients will be of interest.
The MTHFR 1298A>C SNP (rs1801131) results in a glutamate to alanine substitution at codon 439 in exon 7, which is located in the COOH-terminal regulatory domain of the gene, and results in a 30-40% reduction of enzymatic function in the homozygous variant genotype20. The MTHFR enzyme plays a central role in the intersection between a number of important metabolic pathways including folate metabolism, thymidine synthesis, homocysteine processing, and synthesis of both sulfhydrl- and methyl-donating species. MTHFR is a pivotal enzyme in cell metabolism by catalyzing the irreversible conversion of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate; the latter is used in methionine synthesis (a key precursor in DNA methylation), while the former is a key building block in thymidine synthesis that is catalyzed by the thymidylate synthase enzyme, and thus may play an important role in DNA repair. Of interest, Batra et al. demonstrated that after total body irradiation in mice, MTHFR activity levels decreased while thymidylate synthase activity increased suggesting that the regulation of these enzymes in diverting folate metabolism toward thymidine base synthesis may play an important role in the cellular response to ionizing radiation21. Our study identifies an increased risk of RP with the AA genotype, which is associated with higher MTHFR enzymatic function and may potentially lower both levels of thiol and thymidine synthesis. Thus, further work to study whether risk of RP is associated with genes involved in these related pathways of thiol, methyl, and pyrimidine synthesis may be of interest.
Strengths of this study include the relative genetic homogeneity of the population, which was achieved by including only white patients to reduce the influence of variation in genotype distribution by race. Secondly, by including only patients with locally advanced lung cancer, the majority received chemotherapy and were treated with similar radiation techniques. Furthermore, by including only patients that received treatment with modern 3D-CRT and IMRT techniques, we were able to control for known dosimetric predictors of RP such as MLD, V5 and V20. Of note, these previously established lung dosimetric parameters were not significantly associated with RP on multivariate analysis in our dataset. The lack of association between MLD, V5, and V20 and RP may be due to the heterogeneity of treatment techniques including IMRT and 3D-conformal RT, use of surgery and use of chemotherapy. Additionally, some of the patients in our series were treated during a time period after publications regarding V5, V20 and MLD had been published,5-8, and may have led to adjustment of clinical practice to meet these new clinical constraints, which would confound the predictive ability of these parameters. However, since the main goal of this study was to identify SNPs associated with RP, controlling for clinical and dosimetric variables as potential confounders of the primary analysis was important, but the independent predictive ability of these variables are less important.
The findings of this study must be interpreted in the context of its retrospective design. First, retrospective assessment of radiation-related toxicity is often difficult due to incomplete follow-up and reliance on physician documentation of these events, which may be biased and/or incompletely documented. To address these challenges, we utilized the CTCAE RP grading system dichotomized at clinically relevant endpoints, which allowed us to assess for RP based on the use of medical interventions (e.g. steroids) for ≥grade 2 RP or new oxygen requirement for ≥grade 3 RP. Furthermore, we used two independent retrospective reviews of the patients’ records to generate the RP grades, and had a high concordance rate in the two scores, which suggests consistency in how these events were documented in the medical records at our institution and subsequently interpreted retrospectively. A second limitation was that the study had a relatively modest number of patients and events, and thus validation in a larger data set will be required. Third, there was heterogeneity in clinical factors that could impact both the risk and/or the retrospective scoring of RP. For example, the use of surgery could certainly impact both the risk of RP and post-operative declines in pulmonary function may impact the ability to reliably score RP. However, we controlled for surgery and other clinical factors in our multivariate model, and we had stringent criteria for scoring RP with highly concordant independent measures of the endpoint as discussed above. Fourth, the rates of RP in this series are fairly high, particularly since the majority of these patients were treated with IMRT which may reflect further heterogeneity such as differences in techniques during the early adoption of IMRT, the use of concurrent chemotherapy, and the use of consolidation docetaxel in some patients, but the high event rate does increase the power of this data set to detect associations with the genotypes studied. Additionally, while the MTHFR 1298A>C (rs1801131) was associated with the risk of RP, a second MTHFR SNP (rs1801133; 677C>T) was not associated with RP in the test and validation cohorts. This inconsistency may be due to insufficient power in this study to detect an association with the other SNP, different function of these two MTHFR SNPs or greater biological effect of the 1298A>C versus the 677C>T SNP. Furthermore, there are numerous other SNPs in the SOD2 and MTHFR gene that were not analyzed in this study, and verification of the results of this study will include further study of other polymorphisms in these genes. Finally, as is the case for most candidate gene studies, the finding of an association between RP and the MTHFR SNP (rs1801131) may represent a role of the MTHFR gene in mediating the response of lung tissue to radiation injury or alternatively the SNP may simply be a marker for another gene that is in linkage disequilibrium with the SNP. Thus, to further validate these findings, the functional relationship between the MTHFR pathway and risk of RP must be determined, such as by assessment of downstream products of the pathway including homocysteine and thymidine. Nevertheless, this study is the first to our knowledge to identify a potential association between a functional SNP in the MTHFR gene and risk of radiation-related toxicity.
The results of our study build upon prior published RP and candidate gene association studies. Prior retrospective studies from MD Anderson and Beijing have identified an association between risk of RP and the pro-fibrotic cytokine, TGF-β, and DNA damage response and repair genes: ATM, p53, APEX1, and XRCC112-15. Of note, the Beijing studies were conducted in a Chinese population of 253, while the MD Anderson study was conducted in a population of 164 patients of several different races. While these studies highlighted the potential importance of circulating cytokines and DNA repair in the risk of RP, we identified a novel association between RP and the MTHFR gene, which may provide new insights into the biological mechanisms of radiation injury. However, we did not observe an association between RP and a SNP in the important oxidative stress gene SOD2 (rs4880). In all the studies to date, the effect estimate of the genotypes found to have an association with ≥grade 2 RP has been relatively large, which is likely a reflection of the relatively small sample sizes in all these studies. All of these cohorts are likely underpowered to detect SNPs with relatively minor biological effects in modulating the risk of RP. For instance, in a post-hoc power calculation for our study, with the sample size of 136 patients and a null hypothesis of a rate of RP of 29%, our study is underpowered to detect an association between RP and a SNP that would confer a less than 11% change in the rate of RP at the 80% level with an alpha level of 0.05. Furthermore, differences in the patient cohorts in each of these institutional studies including genetic variations due to race may make cross-validation and/or pooling of data sets difficult to interpret. The challenge facing the field of radiation oncology will be to improve the prospective gathering of radiation-induced toxicity events, in conjunction with collection of tissue and genetic material.
Conclusions
Our study showed an association between risk of clinically significant RP after radiation therapy for lung cancer and MTHFR genotype (1298AA vs. AC/CC; rs1801131). Further validation work including association studies with other enzymes involved in folate metabolism may identify new biomarkers for risk of RP.
Condensed abstract.
This is a candidate gene study examining the association between single nucleotide polymorphisms in oxidative stress genes and radiation pneumonitis in a retrospective cohort of patients treated with thoracic radiation therapy for lung cancer. There was an association between methylenetetrahydrofolate reductase (MTHFR) genotype and risk of clinically significant radiation pneumonitis.
Acknowledgments
Funding: Supported by the National Institutes of Health (Grant CA 74386).
Footnotes
Financial Disclosures: None
References
- 1.Yom SS, Liao Z, Liu HH, Tucker SL, Hu CS, Wei X, Wang X, Wang S, Mohan R, Cox JD, Komaki R. Initial evaluation of treatment-related pneumonitis in advanced-stage non-small-cell lung cancer patients treated with concurrent chemotherapy and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2007;68(1):94–102. doi: 10.1016/j.ijrobp.2006.12.031. Available from http://www.ncbi.nlm.nih.gov/pubmed/17321067. [DOI] [PubMed] [Google Scholar]
- 2.Curran W, Scott C, Langer C, et al. Long-term benefit is observed in a phase III comparison of sequential vs concurrent chemo-radiation for patients with unresected stage III non small cell lung cancer: RTOG 9410 (abstract) Proc Am Soc Clin Oncol. 2003;22 [Google Scholar]
- 3.Furuse K, Fukuoka M, Kawahara M, Nishikawa H, Takada Y, Kudoh S, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol. 1999;17(9):2692–9. doi: 10.1200/JCO.1999.17.9.2692. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10561343. [DOI] [PubMed] [Google Scholar]
- 4.Hanna N, Neubauer M, Yiannoutsos C, McGarry R, Arseneau J, Ansari R, et al. Phase III study of cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel in patients with inoperable stage III non-small-cell lung cancer: the Hoosier Oncology Group and U.S. Oncology. J Clin Oncol. 2008;26(35):5755–60. doi: 10.1200/JCO.2008.17.7840. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19001323. [DOI] [PubMed] [Google Scholar]
- 5.Graham MV, Purdy JA, Emami B, Harms W, Bosch W, Lockett MA, et al. Clinical dose-volume histogram analysis for pneumonitis after 3D treatment for non-small cell lung cancer (NSCLC) Int J Radiat Oncol Biol Phys. 1999;45(2):323–9. doi: 10.1016/s0360-3016(99)00183-2. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10487552. [DOI] [PubMed] [Google Scholar]
- 6.Hernando ML, Marks LB, Bentel GC, Zhou SM, Hollis D, Das SK, et al. Radiation-induced pulmonary toxicity: a dose-volume histogram analysis in 201 patients with lung cancer. Int J Radiat Oncol Biol Phys. 2001;51(3):650–9. doi: 10.1016/s0360-3016(01)01685-6. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11597805. [DOI] [PubMed] [Google Scholar]
- 7.Kim TH, Cho KH, Pyo HR, Lee JS, Zo JI, Lee DH, et al. Dose-volumetric parameters for predicting severe radiation pneumonitis after three-dimensional conformal radiation therapy for lung cancer. Radiology. 2005;235(1):208–15. doi: 10.1148/radiol.2351040248. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15703313. [DOI] [PubMed] [Google Scholar]
- 8.Kwa SL, Lebesque JV, Theuws JC, Marks LB, Munley MT, Bentel G, et al. Radiation pneumonitis as a function of mean lung dose: an analysis of pooled data of 540 patients. Int J Radiat Oncol Biol Phys. 1998;42(1):1–9. doi: 10.1016/s0360-3016(98)00196-5. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9747813. [DOI] [PubMed] [Google Scholar]
- 9.Wang S, Liao Z, Wei X, Liu HH, Tucker SL, Hu CS, et al. Analysis of clinical and dosimetric factors associated with treatment-related pneumonitis (TRP) in patients with non-small-cell lung cancer (NSCLC) treated with concurrent chemotherapy and three-dimensional conformal radiotherapy (3D-CRT) Int J Radiat Oncol Biol Phys. 2006;66(5):1399–407. doi: 10.1016/j.ijrobp.2006.07.1337. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16997503. [DOI] [PubMed] [Google Scholar]
- 10.Barriger RB, Fakiris AJ, Hanna N, Yu M, Mantravadi P, McGarry RC. Dose-volume analysis of radiation pneumonitis in non-small-cell lung cancer patients treated with concurrent cisplatinum and etoposide with or without consolidation docetaxel. Int J Radiat Oncol Biol Phys. 2010;78(5):1381–6. doi: 10.1016/j.ijrobp.2009.09.030. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20231061. [DOI] [PubMed] [Google Scholar]
- 11.Marks LB, Yu X, Vujaskovic Z, Small W, Jr., Folz R, Anscher MS. Radiation-induced lung injury. Semin Radiat Oncol. 2003;13(3):333–45. doi: 10.1016/S1053-4296(03)00034-1. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12903021. [DOI] [PubMed] [Google Scholar]
- 12.Zhang L, Yang M, Bi N, Fang M, Sun T, Ji W, et al. ATM polymorphisms are associated with risk of radiation-induced pneumonitis. Int J Radiat Oncol Biol Phys. 2010;77(5):1360–8. doi: 10.1016/j.ijrobp.2009.07.1675. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20171797. [DOI] [PubMed] [Google Scholar]
- 13.Yin M, Liao Z, Liu Z, Wang LE, Gomez D, Komaki R, et al. Functional Polymorphisms of Base Excision Repair Genes XRCC1 and APEX1 Predict Risk of Radiation Pneumonitis in Patients with Non-Small Cell Lung Cancer Treated with Definitive Radiation Therapy. Int J Radiat Oncol Biol Phys. 2011 doi: 10.1016/j.ijrobp.2010.11.079. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=21420246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang M, Zhang L, Bi N, Ji W, Tan W, Zhao L, et al. Association of P53 and ATM Polymorphisms With Risk of Radiation-Induced Pneumonitis in Lung Cancer Patients Treated With Radiotherapy. Int J Radiat Oncol Biol Phys. 2011;79(5):1402–7. doi: 10.1016/j.ijrobp.2009.12.042. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20729006. [DOI] [PubMed] [Google Scholar]
- 15.Yuan X, Liao Z, Liu Z, Wang LE, Tucker SL, Mao L, et al. Single nucleotide polymorphism at rs1982073:T869C of the TGFbeta 1 gene is associated with the risk of radiation pneumonitis in patients with non-small-cell lung cancer treated with definitive radiotherapy. J Clin Oncol. 2009;27(20):3370–8. doi: 10.1200/JCO.2008.20.6763. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19380441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee MS, Su L, Mark EJ, Wain JC, Christiani DC. Genetic modifiers of carcinogen DNA adducts in target lung and peripheral blood mononuclear cells. Carcinogenesis. 2010;31(12):2091–6. doi: 10.1093/carcin/bgq208. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20935060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu G, Zhou W, Wang LI, Park S, Miller DP, Xu LL, et al. MPO and SOD2 polymorphisms, gender, and the risk of non-small cell lung carcinoma. Cancer Lett. 2004;214(1):69–79. doi: 10.1016/j.canlet.2004.06.027. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15331175. [DOI] [PubMed] [Google Scholar]
- 18.Wang LI, Miller DP, Sai Y, Liu G, Su L, Wain JC, et al. Manganese superoxide dismutase alanine-to-valine polymorphism at codon 16 and lung cancer risk. J Natl Cancer Inst. 2001;93(23):1818–21. doi: 10.1093/jnci/93.23.1818. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11734599. [DOI] [PubMed] [Google Scholar]
- 19.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B (Methodological) 1995;57(1):289–300. [Google Scholar]
- 20.Weisberg I, Tran P, Christensen B, Sibani S, Rozen R. A second genetic polymorphism in methylenetetrahydrofolate reductase (MTHFR) associated with decreased enzyme activity. Mol Genet Metab. 1998;64(3):169–72. doi: 10.1006/mgme.1998.2714. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9719624. [DOI] [PubMed] [Google Scholar]
- 21.Batra V, Kesavan V, Mishra KP. Modulation of enzymes involved in folate dependent one-carbon metabolism by gamma-radiation stress in mice. J Radiat Res (Tokyo) 2004;45(4):527–33. doi: 10.1269/jrr.45.527. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15635262. [DOI] [PubMed] [Google Scholar]