SUMMARY
We report that in the presence of signal 1 (NF-κB), the NLRP3 inflammasome was activated by mitochondrial apoptotic signaling that licensed production of interleukin-1β (IL-1β). NLRP3 secondary signal activators such as ATP induced mitochondrial dysfunction and apoptosis, resulting in release of oxidized mitochondrial DNA (mtDNA) into the cytosol, where it bound to and activated the NLRP3 inflammasome. The anti-apoptotic protein Bcl-2 inversely regulated mitochondrial dysfunction and NLRP3 inflammasome activation. Mitochondrial DNA directly induced NLRP3 inflammasome activation, because macrophages lacking mtDNA had severely attenuated IL-1β production, yet still underwent apoptosis. Both binding of oxidized mtDNA to the NLRP3 inflammasome and IL-1β secretion could be competitively inhibited by the oxidized nucleoside, 8-OH-dG. Thus, our data reveal that oxidized mtDNA released during programmed cell death causes activation of the NLRP3 inflammasome. These results provide a missing link between apoptosis and inflammasome activation, via binding of cytosolic oxidized mtDNA to the NLRP3 inflammasome.
INTRODUCTION
Secretion of interleukin-1β (IL-1β), a potent pyrogen that elicits a strong proinflammatory response (Dinarello, 2009), is tightly controlled by a diverse class of cytosolic complexes known as the inflammasome (Latz, 2010). The nucleotide binding domain and leucine rich repeat (NLR) pyrin domain containing 3 (NLRP3) family member forms cytosolic oligomers with apoptosis-associated speck like protein (ASC) in dendritic cells (Ghiringhelli et al., 2009) and macrophages (Franchi et al., 2009), triggering autocatalytic activation of caspase-1 (Martinon et al., 2009). Caspase-1, in turn, cleaves pro-IL-1β, producing mature IL-1β. Under normal circumstances, NLRP3 undergoes bipartite activation (Latz, 2010). The first signal (Signal 1), often NF-κB activation, induces pro-IL-1β and NLRP3 expression. The second signal, any one of a variety of unrelated entities—particulate matter, crystals, aggregated β-amyloid, extracellular ATP, or microbial toxins—activates the NLRP3 inflammasome (Gross et al., 2011). Exactly how these diverse cytosolic danger signals trigger the same inflammasome remains unclear.
In the past, three models of NLRP3 activation were proposed: reactive oxygen species (ROS) generation (Tschopp and Schroder, 2010), lysosomal damage (Hornung and Latz, 2010) and cytosolic K+ efflux (Petrilli et al., 2007). Reports on their relative contributions to NLRP3 activation have often been contradictory and have not yielded an integrated, unified model. Recently, two papers have reported an important role for mitochondria (mt) in NLRP3 inflammasome activation (Nakahira et al., 2010; Zhou et al., 2010). Specifically, Zhou et al. found that mt reactive oxygen species (ROS) is critical for NLRP3 activation, while Nakahira et al. reported that mtDNA plays an important role. However, the molecular mechanism for NLRP3 activation has not yet been defined.
Mitochondria are exquisitely complex regulators of cytosolic homeostasis, sensing and responding to changes in intracellular K+ and ROS (Tschopp, 2011). Perturbation of intracellular K+, ROS or lysosomal stability can result in mt dysfunction and apoptosis (Johansson et al., 2010; Tschopp, 2011). Therefore, mt are well-positioned to regulate NLRP3 activation. Our initial investigation focusing on NLRP3 activation by Chlamydia pneumoniae (CP) infection led us to a serendipitous result that implicated mt in NLRP3 activation (Shimada et al., 2011). We found that mt damage specifically triggered apoptotic signals and induced NLRP3-dependent IL-1β secretion in primed macrophages. This led to an innate immune defense program where primed macrophages initiated a mt-dependent apoptotic cascade in response to danger signals and cytosolic stress, necessary and sufficient for caspase-1 activation by the NLRP3 inflammasome in presence of signal 1. This apoptotic cascade activated the NLRP3 inflammasome through cytosolic release of oxidized mtDNA, which bound to and activated the NLRP3 inflammasome. Given that both mt dysfunction and apoptosis are inextricably linked to ROS, intracellular K+ and lysosomal degradation, the present work positions mt as the central hub for integration of diverse signals sensed by NLRP3.
RESULTS
Mitochondrial dysfunction leads to IL-1β secretion
During the course of investigating inflammasome activation after mouse CP infection, we found that infection-induced IL-1β secretion in macrophages required live bacteria and was NLRP3, ASC, and caspase-1 dependent (Figure 1A). On the other hand, tumor necrosis factor alpha (TNF-α) secretion was NLRP3 inflammasome-independent (Figure S1A). While investigating the effects of chloramphenicol (CAM) on CP-induced activation of NLRP3, we serendipitously discovered that TNF-α production was enhanced in high dose CAM-treated bone marrow-derived macrophages (BMDM; data not shown). Intrigued by this result, we treated lipopolysaccharide (LPS)-primed BMDM with CAM before addition of ATP. This led to an unexpected attenuation of IL-1β secretion but TNF-α production increased reciprocally in proportion to IL-1β inhibition (Figure 1B). To rule out the possibility that CAM might be cytotoxic in this scenario, we examined lactate dehydorgenase (LDH) release in CAM-treated BMDM. To our surprise, CAM caused a dose-dependent reduction in LDH release (Figure 1B), suggesting that reduced IL-1β production is proportional to BMDM survival.
Figure 1. Mitochondrial dysfunction is linked to NLRP3 inflammasome activation.
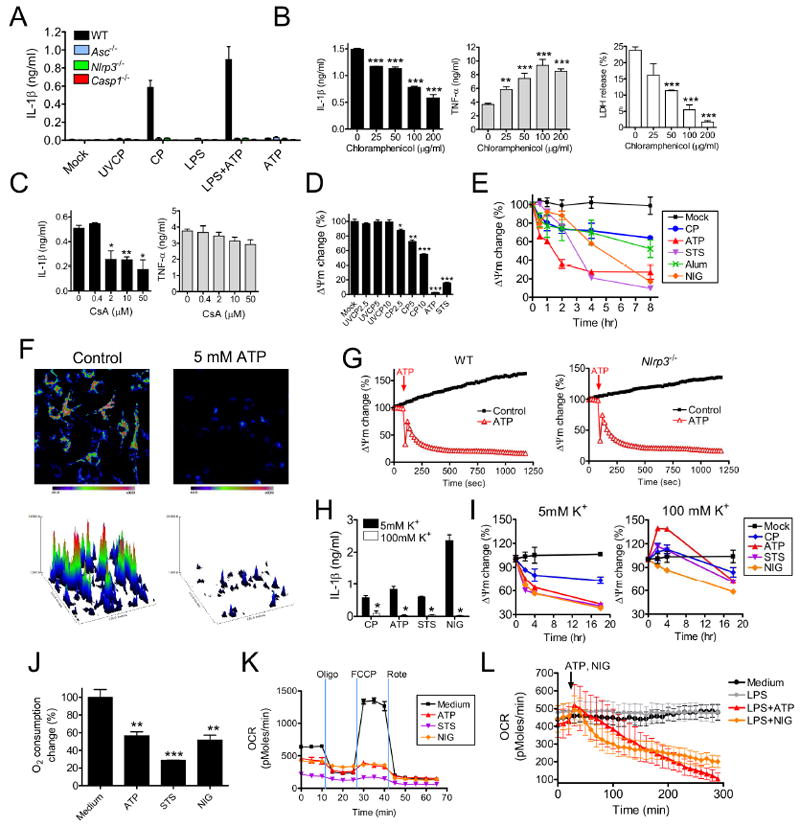
(A-C) IL-1β and TNF-α concentrations (ELISA) in BMDM culture supernatants treated with (A) NLRP3 activating stimuli or (B) LPS (1 μg/ml) plus ATP (5 mM, final 2 hr culture) in the presence of increasing doses of chloramphenicol. Also shown is an LDH release assay of the same BMDM. (C) The effect of Cyclosporin A (CsA) on LPS+ATP-induced IL-1β in WT BMDM.
(D) Mitochondrial membrane potential (ΔΨm) measurements during NLRP3 inflammasome activation. BMDM were treated with UV-killed CP (UVCP, MOI=10) or live CP (MOI=10), treated with ATP (5 mM) or staurosporine (STS, 5 μM) for 24 hr and examined for TMRM incorporation.
(E) Changes in ΔΨm were monitored over time by TMRM incorporation. BMDM were treated with live CP (MOI=10), ATP (5 mM), STS (5 μM), nigericin (NIG, 10μM), or alum (130 μg/ml).
(F and G) Changes in ΔΨm due to ATP treatment as measured by by TMRM microscopy. A representative image of mitochondrial TMRM fluorescence and associated 3D intensity map are shown in (F). The kinetic profile shown in (G) depicts the mean value +/- the SEM at each time point.
(H and I) Extracellular K+ affects (H) IL-1β secretion and (I) ΔΨm as measured by TMRM incorporation. Four hours after LPS priming (1 μg/ml) or after CP infection (MOI=10), BMDM were transferred to culture media containing either 5 mM or 100 mM K+. Six h after LPS priming, BMDM were then treated with ATP (5 mM), STS (5 μM) or NIG (10 μM) and cultured for an additional 2 hr before culture supernatants were collected and IL-1β concentration was measured. CP infected BMDM were cultured for an additional 20 hr before supernatants were collected for IL-1β measurement.
(J and K) Oxygen consumption rate (OCR) was measured in LPS primed macrophages. (J) Base line OCR measurements 2 hr after ATP (5 mM), STS (5 μM) or NIG (10 μM) treatment. (K) The change of OCR was monitored after 1 μM oligomycin, 1 μM FCCP, and 1 μM Rotenone sequential additions.
(I) Kinetics of OCR were monitored in macrophages in response to NLRP3 activation.
Data shown are representative of two or more independent experiments (means ± SD). * p< 0.05, ** p<0.01, ***p<0.001.
Considering the high degree of homology between bacterial and mt ribosomes, and that CAM inhibits mt ribosomal protein synthesis (Riesbeck et al., 1990), we hypothesized that the effect of CAM on CP-induced IL-1β secretion might be due to attenuation of a mt signal that activates the NLRP3 inflammasome. To test this, we treated BMDM with LPS plus ATP in presence of cyclosporin A (CsA), a mt inhibitor that blocks cytochrome c release (Ghribi et al., 2001). CsA attenuated IL-1β secretion but had no effect on TNF-α production (Figure 1C). These results were similar to those found by others, collectively suggesting a mitochondrial role in NLRP3 inflammasome activation (Nakahira et al., 2010; Zhou et al., 2010).
To further our investigation into mt and NLRP3 inflammasome activation, we determined the effect of CP infection on inner mt membrane potential (ΔΨm), loss of which is a surrogate marker for apoptosis (Ly et al., 2003). Live CP infection, ATP, and staurosporine (STS, a pro-apoptotic compound), but not ultra-violet light-killed CP (UVCP) treatment, resulted in decreased ΔΨm (Figure 1D) as measured by tetramethyl rhodamine methyl ester (TMRM) incorporation assay, further implicating mt in NLRP3 inflammasome activation. CP infection, ATP, STS, alum, and nigericin (NIG, another pro-apoptotic compound) all led to irreversibly decreased ΔΨm (Figure 1E), with attenuation as early as 30 min post-treatment. Interestingly, incubation of BMDM with NIG also reduced ΔΨm (Figure 1E). ATP treatment of LPS-primed BMDM resulted in a similar effect on ΔΨm by TMRM fluorescence microscopy (Figure 1F and Movie S1). Of note, the kinetic differences observed between the incorporation assay (Figure 1E) and TMRM (Figure 1F) may be due to greater sensitivity of TMRM fluorescence microscopy. The addition of ATP to macrophages resulted in a permanent decrease in ΔΨm, which was independent of both NLRP3 (Figure 1G), and caspase-1 (Figure S1B). Because ATP and CP (but not UVCP) all caused reduced ΔΨm and triggerred caspase-1 activation by NLRP3, we suggest that mt dysfunction is involved in NLRP3 inflammasome activation.
Low intracellular K+ has been linked to caspase-1 activation by the NLRP3 inflammasome (Lamkanfi et al., 2009; Petrilli et al., 2007). This conclusion is supported by studies showing that cells exposed to a high concentrations (> 90 mM) of K+ or treated with K+ efflux inhibitors have attenuated NLRP3 activation in response to various NLRP3 stimuli. To determine if K+ efflux played a role in CP-induced NLRP3 activation, we infected BMDM with CP and then exposed cells to 100 mM of extracellular K+. Results showed that elevated extracellular K+ concentration almost completely blocked IL-1β secretion by CP-infected BMDM (Figure 1H). Importantly, high extracellular K+ protected BMDM from ΔΨm reduction (Figure 1I). To further characterize mt dysfunction after NLRP3 inflammasome stimulation, we analyzed oxygen consumption rate (OCR). Addition of ATP, STS, or NIG to LPS-primed macrophages all attenuated OCR and hence mt dysfunction (Figures 1J-L). LPS priming was not required for reduced OCR, as incubation with ATP or NIG alone reduced macrophage OCR (Figure S1C). Together, these results further link mt with NLRP3 activation, and also shed light on the mechanism by which cytosolic K+ efflux triggers NLRP3.
Mitochondrial apoptotic signals activate the NLRP3 inflammasome
To further their own survival, intracellular pathogens subvert, activate or otherwise manipulate host cell death signaling pathways (Arnoult et al., 2009). Since mt play a crucial role as initiators of cell death, and because our data thus far showed that mt dysfunction occurred with inflammasome activation, we next examined the putative role of mt cell death pathways in activating the NLRP3 inflammasome. We found that live CP, but not UVCP, caused LDH release from infected BMDM, albeit with reduced magnitude and slower kinetics vs. ATP, STS or alum (Figure 2A). To analyze which cell death pathway was induced by LPS+ATP or NIG, we used Annexin V flow cytometry, and found that LPS+ATP or LPS+NIG induced apoptosis in BMDM (Figure S2A). As an alternative approach, we scored DAPI-stained cells for nuclear condensation. Nuclei of apoptotic cells, unlike those of necrotic cells, exhibit highly condensed chromatin that uniformly stained with DAPI. Consistent with data from LDH release assays (Figure 2A), we observed increased apoptosis in CP infected macrophages but not in UVCP treated cells (Figure 2B). Thus, apoptosis induced by CP is linked to NLRP3 inflammasome activation. Intriguingly, the pro-apoptotic molecule STS not only led to loss of ΔΨm (Figure 1D), LDH release (Figure 2A) and nuclear condensation (Figure 2B), but also to caspase-1 activation and subsequent IL-1β maturation in LPS-primed BMDM (Figure 2C). Addition of STS 6 hr after UVCP or LPS treatment induced IL-1β secretion that was enhanced after CP infection (Figure 2D) and was predominately dependent on NLRP3/ASC/caspase-1 (Figure 2E). STS treatment did not affect TNF-α production (Figure 2D). Collectively, these results indicate that an apoptotic stimulus activates caspase-1 and induces IL-1β secretion via the NLRP3 inflammasome in primed macrophages.
Figure 2. Apoptotic stimuli activate the NLRP3 inflammasome in macrophages.
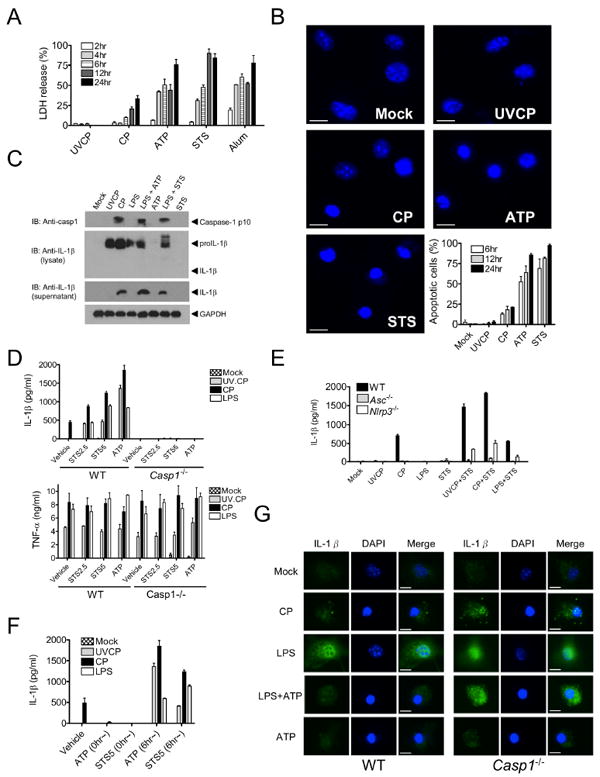
(A and B) BMDM were treated with UVCP (MOI=10), live CP (MOI=10), ATP (5 mM), STS (5 μM) or alum (130 μg/ml) and (A) LDH release was determined at the indicated time points or (B) cells were fixed, stained with DAPI, and condensed nuclei were enumerated.
(C) Immunoblotting was used to analyze mouse caspase-1, pro-IL-1β, and IL-1β in culture supernatants and lysates of BMDM treated with (left to right): UVCP (MOI of 10, 8 hr), live CP (MOI of 10, 8 hr), LPS (1 μg/ml, 8 hr), ATP alone (5 mM), LPS+ATP, STS alone (5 μM), or LPS+STS (5 μM, final 2 hr of culture).
(D and E) IL-1β or TNF-α secretion by (D) wild-type (WT) or Casp1–/– or (E) WT, Asc–/– or Nlrp3–/– BMDM was measured by ELISA after UVCP, live CP (MOI=10 and 8 hr) or LPS (1 μg/ml, for 8 hr) treatment in presence of STS (2.5 μM or 5 μM) for the final 2 hr of culture.
(F) IL-1β secretion was quantified by ELISA in BMDM primed with UVCP, live CP or LPS and then treated with ATP or STS at 0 h or 6 hr after priming.
(G) BMDM were treated with live CP, LPS, LPS+ATP, or ATP alone as indicated. Cells were analyzed by immunofluorescence for IL-1β and DAPI.
Data shown are representative of three or more independent experiments (means ± SD).
To further substantiate that apoptotic processes act as the second signal for NLRP3 activation, we examined the timing of NLRP3 activation with respect to LPS, UVCP and CP. If STS or ATP was added before or simultaneously with UVCP or LPS, IL-1β secretion was undetectable (Figure 2F). A similar pattern of results was observed when STS or ATP was added concomitantly with CP infection (Figure 2F). Yet, if STS or ATP was added 6 hr after LPS or UVCP priming, IL-1β secretion was increased (Figure 2F). These data further substantiate that ATP and STS rapidly induce apoptosis (as initially shown in Figures 2A and 2B), which would prevent cleavage and secretion of IL-1β because signal 1 has not had enough time to prime the cells for induction of pro-IL-1β. To determine whether apoptotic cells themselves were releasing IL-1β, we used immunohistochemistry. Indeed, secretion of IL-1β (indicated by a disappearance of cytosolic IL-1β staining) in response to CP or LPS+ATP was caspase-1-dependent and was more frequent in apoptotic cells (those with condensed nuclei, Figure 2G). This argued against the possibility that ATP released from dying cells activated the NLRP3 inflammasome of neighboring cells in a paracrine manner. Further ruling out this bystander effect, CP-infected or alum-treated macrophages given apyrase, an enzyme which rapidly hydrolyzes extracellular ATP, exhibited no difference in IL-1β secretion compared to untreated cells (data not shown). These results demonstrate that activation of the NLRP3 inflammasome by an apoptotic stimulus must occur after, but not before or concomitant with, innate immune cell priming.
Recent studies have described a caspase-1-dependent form of programmed cell death called pyroptosis (Fink and Cookson, 2005). To rule out pyroptosis as the source of mt dysfunction and cell death, we assessed ΔΨm reduction in response to CP, ATP, STS, NIG, and alum in caspase-1 deficient (Casp1–/–) BMDM. In all cases studied, mitochondrial depolarization was independent of caspase-1 (Figure S2B), suggesting that pyroptosis does not play a role. We also assessed LDH release after CP infection in BMDM and found that caspase-1 also did not play a role (Figure S2C). Additionally, LPS primed BMDM treated with necrotic stimuli did not secrete IL-1β (Figure S2D).
We also investigated the ability of another known inducer of apoptosis, A23817 (a Ca2+ ionophore), to activate the inflammasome. Importantly, A23817 did induce IL-1β under LPS activating conditions (Figure S2E). This is in agreement with our finding that A23817 resulted in loss of mitochondrial membrane potential and substantial LDH release (Figure S2E). Additionally, LPS+Fas ligand (FasL) treatment did not activate the inflammasome as determined by IL-1β secretion (Figure S2E). However, FasL did not induce copious LDH release, nor reduce the mitochondrial membrane potential, indicating that BMDM might be insensitive to FasL.
Bcl-2 expression regulates NLRP3 inflammasome activation and IL-1β secretion
To verify that macrophage apoptosis was required for NLRP3 activation, we stably transfected an immortalized macrophage cell line, MCL (Hornung et al., 2008), with a vector encoding the anti-apoptotic protein Bcl-2 (Figure 3A), and assessed IL-1β secretion in response to NLRP3 triggers. The Bcl-2 overexpressing line secreted significantly less IL-1β compared to cells with an empty vector control (or vs. cells expressing an unrelated gene, yellow fluorescent protein, YFP; Figure S2F) in response to CP, LPS+ATP, LPS+STS, or LPS+NIG (Figure 3B). TNF-α secretion was unaffected in all cases (Figure 3B). Western blot analyses revealed similar results with reduced amounts of both intracellular caspase-1 p10 or mature (secreted) IL-1β (Figure 3C). Importantly, the Bcl-2 overexpressing MCL line was resistant to both changes in ΔΨm (Figure 3D) and to apoptosis in response to numerous stimuli (Figure 3E and S2A). We also derived BMDM from Bcl-2 transgenic (Bcl-2-tg) mice and performed similar studies. Indeed, these macrophages secreted significantly less IL-1β in response to a host of NLRP3 stimuli (Figure 3F), while TNF-α secretion was unaltered. Thus, inhibition of apoptosis at the level of mt is sufficient to cause reduced IL-1β maturation and secretion triggered by NLRP3-activating stimuli. Finally, we stably transfected MCL cells with a vector encoding shRNA for Bcl-2 that efficiently reduced Bcl-2 protein abundance (Figure 3G). Attenuated Bcl-2 protein should result in increased apoptosis and, as expected, LDH release was elevated (Figure 3H). Using LPS+ATP to stimulate the NLRP3 inflammasome, these cells secreted more IL-1β but not TNF-α (Figure 3I). Based on these results, apoptotic processes blocked by Bcl-2 are necessary for NLRP3 activation.
Figure 3. Bcl-2 inversely regulates mitochondrial dysfunction and NLRP3 inflammasome activation.
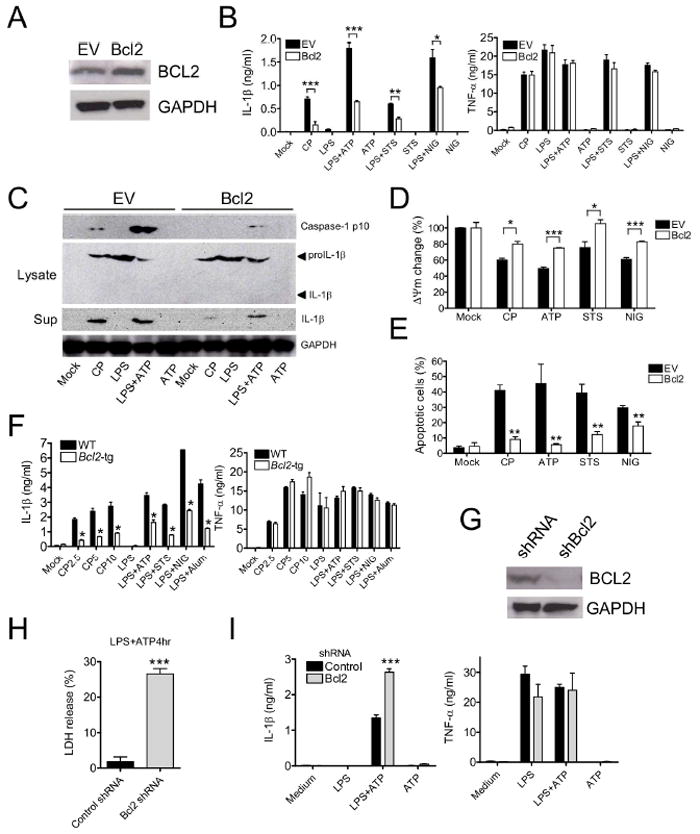
(A) Bcl-2-neor or empty vector (EV, neor) was stably expressed in MCL macrophages, and Bcl2 expression was determined by immunoblot.
(B and C) Bcl-2 overexpressing macrophages were treated with live CP (MOI=10, 24 hr), LPS (1 μg/ml, 5 hr), ATP (5 mM, final 2 hr of culture), LPS+ATP, STS (5 μM, final 2 hr of culture), LPS+STS, NIG (10 μM, final 2 hr), or LPS+NIG and then (B) IL-1β or TNF-α secretion was measured by ELISA or (C) immunoblotting was used to analyze mouse capsase-1, pro-IL-1β, or IL-1β in culture supernatants or cell lysates.
(D and E) ΔΨm (D) and apoptotic cells (E) were measured after NLRP3 inflammasome stimulation at 8 hr for CP, or at 4 hr for ATP, STS and NIG.
(F) BMDM from Bcl-2 transgenic mice were treated with live CP, LPS, LPS+ATP, LPS+STS, LPS+NIG, or LPS+alum (130 μg/ml, final 8 hr). IL-1β and TNF-α secretion were then measured using ELISA.
(G) Validation of shRNA targeting Bcl-2 in stably-expressing MCL macrophages by immunoblot.
(H and I) shBcl-2 macrophages were primed with LPS and treated with ATP (5 mM for 4 hr), and (H) LDH release, (I) IL-1β or TNF-α were then measured in supernatants. Data shown are representative of three or more independent experiments (means ± SD). * p<0.05, ** p< 0.01, *** p<0.001.
Salmonella typhimurium infection-induced apoptosis causes IL-1β secretion
S. typhimurium (St) is recognized by both the NLRP3 and NLRC4 inflammasome pathways (Broz et al., 2010). Indeed, NLRP3 plays an important role in IL-1β secretion induced by St recognition (Figure 4A). St is also known to induce Type III secretion system (T3SS)-dependent apoptosis in macrophages (Hersh et al., 1999; Kage et al., 2008), and we investigated whether St activation of the NLRP3 inflammasome was licensed by apoptosis. St infection of BMDM for 8 hr resulted in marked LDH release (90% of control) (Figure 4B). However, T3SS-1 defective strains of St (invA, hilA, orgA, and prgH) did not induce LDH release, while a T3SS-2 defective strain (spiB), and the lipid A non-signaling strain (msbB) induced LDH release (Figure 4B). Finally, the invasin mutant (sspB) did not induce LDH release. Accordingly, LDH release inversely correlated with ΔΨm in LDH-inducing strains, while ΔΨm remained unaltered in non-LDH-releasing mutants (Figure 4C). As a control, poly(dA:dT), which utilizes absent in melanoma 2 (AIM2) but not NLRP3, did not induce changes in ΔΨm. Importantly, IL-1β secretion was only induced by St strains that caused LDH release and ΔΨm reduction (Figure 4D), and was not secreted after infection with strains that did not induce apoptosis. To further test whether IL-1β secretion due to St infection induced apoptosis, we infected the Bcl-2 stably-transfected MCL cell line with wild-type St. Bcl-2 overexpression significantly reduced IL-1β secretion after St infection with the wild-type strain (Figure 4E). As expected however, TNF-α secretion was similar between-groups (Figure 4E). It should be noted that Bcl-2 overexpression did not impact LPS+poly(dA:dT) treatment outcomes (Figure 4E), presumably since poly(dA:dT) utilizes AIM2 and not NLRP3. Together, these data further substantiate the requirement of apoptosis for NLRP3 inflammasome activation.
Figure 4. Salmonella type III secretion protein and invasion factor SipB induces mitochondrial depolarization and caspase-1 dependent IL-1β secretion.
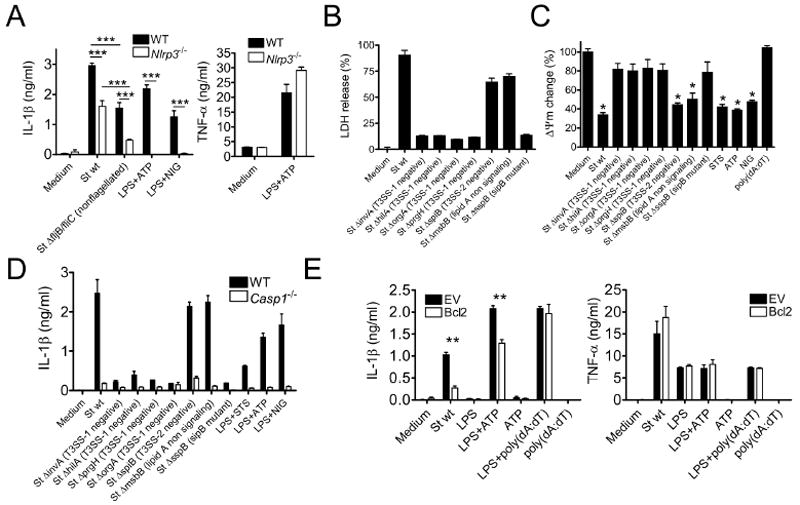
(A) Three hour-LPS-primed Nlrp3–/– BMDM were exposed to wild-type (St wt) or nonflagellated mutant St (St ΔfljB/fliC) (MOI=5) and IL-1β was quantified in culture supernatants by ELISA.
(B and C) Three hour-LPS-primed BMDM were exposed to various strains of St (MOI=5) and (B) cell viability was determined by LDH release assay or (C) ΔΨm was measured by TMRM incorporation.
(D and E) IL-1β or TNF-α were quantified in culture supernatants by ELISA in (D) WT and Casp1–/– macrophages or in (E) WT and Bcl-2 overexpressing macrophages that were exposed to St (MOI=5).
Data shown are representative of three or more independent experiments (means ± SD).* p<0.05, ** p< 0.01, *** p<0.001.
Mitochondrial DNA is required for NLRP3 inflammasome-mediated IL-1β secretion
Recently, Nakahira et al. suggested that mtDNA was involved in NLRP3 activation (Nakahira et al., 2010), but the exact mechanism for this was not elucidated. Corroborating results of Nakahira et al., we found that ρ0 cells (lacking mtDNA) were unable to secrete IL-1β in response to NLRP3-inducing stimuli, while TNF-α secretion remained unaltered (Figure S3A-D). Patrushev et al. reported that mtDNA is released into the cytosol during intrinsic apoptosis (Patrushev et al., 2006). Thus, we hypothesized that mtDNA released into the cytosol during apoptosis might trigger NLRP3 inflammasome activation. To begin testing this, we examined cytosolic DNA content following stimulation with LPS+ATP or LPS+NIG. Shortly (15-30 min) after treatment with ATP or NIG, the mitochondrial genes Cox1 and Nd3 were readily detected in the cytosol by PCR, but genomic DNA encoding Gapdh was undetectable in this cellular compartment (Figure S3E). We further hypothesized that cytosolic mtDNA might directly bind NLRP3. Thus, we co-transfected 293 cells with a NLRP3-Flag construct and purified mtDNA previously labeled with bromodeoxyuridine (BrdU). NLRP3 was subsequently immunoprecipitated (IP) using anti-Flag M2 antibody, and IP products were BrdU dot-blotted. BrdU signal was clearly detected, indicating that mtDNA directly or indirectly associated with NLRP3 (Figure 5A). To control for non-specific binding, we transfected BrdU labeled mtDNA into 293 cells previously transfected with a NOD2 expression vector. NOD2 did not co-IP with mtDNA, while NLRP3 did in the same experiment (Figure S3F), suggesting specificity amongst NLRs. Similar to Nakahira et al., IL-1β secretion induced by BMDM-transfected mtDNA was predominantly AIM2 dependent (Figure S3G) (Nakahira et al., 2010). Therefore, we also tested whether overexpressed AIM2 could bind transfected mtDNA. As expected, AIM2 interacted with the transfected DNA (Figure S3F). We probed for colocalization of BrdU-labeled mtDNA with NLRP3 by microscopy and found colocalization of BrdU-labeled mtDNA with NLRP3 (Figure 5B and C).
Figure 5. Mitochondrial DNA colocalizes with NLRP3.
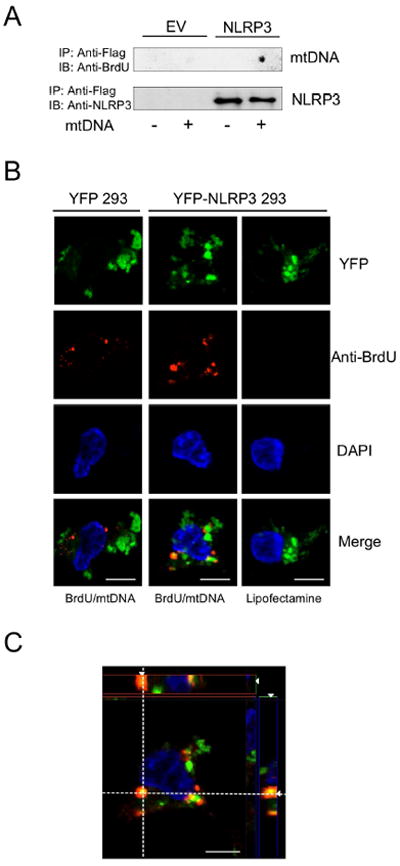
A) Flag-NLRP3 and empty vector (EV) stably-expressing 293 cells were exposed to BrdU-labeled mitochondrial DNA (mtDNA) in presence of lipofectamine. Cell lysates were collected 3 hr later and immunoprecipitated. mtDNA was detected by BrdU dot-blot, and NLRP3 Ab was used for immunoblotting as a loading control.
(B and C) YFP-tagged NLRP3 stably-expressing 293 cells were exposed to BrdU-labeled mitochondrial DNA plus lipofectamine. Three hr after exposure, cells were fixed, permeabilized, treated with DNase I (10 U/ml, 30 min), and stained with BrdU Ab. (B) Co-localization of YFP-NLRP3 and mtDNA was analyzed by structured illumination microscopy, and (C) shows an orthogonal view from a z-series.
To ensure that mtDNA-NLRP3 interaction was not owed to overexpression artifact, we investigated whether endogenous mtDNA interacted with native NLRP3 during inflammasome activation. BMDM were grown in the presence of BrdU and then treated with LPS+ATP or LPS+NIG in presence of the autophagy inhibitor 3-MA to enhance inflammasome activation. After NLRP3 IP, BrdU dot-blot was conducted. BrdU-incorporated DNA was bound to NLRP3 in cells treated with LPS+ATP or LPS+NIG, whereas LPS treatment alone did not produce NLRP3-BrdU binding (Figure 6A and S4A), indicating that secondary stimulation was necessary for this interaction. To exclude non-specific binding of BrdU-incorporated DNA with NLRP3, NLRP1 or AIM2 were subjected to IP and probed for BrdU. Importantly, BrdU-labeled DNA did not co-IP with NLRP1 or AIM2 (Figure 6B). That endogenous AIM2 did not associate with DNA under NLRP3 activating conditions, but interacted with transfected DNA (Figure S3F), suggested that cellular localization of and access to DNA are critical factors in determining whether NLRP3 or AIM2 is activated. We next investigated the origin of the DNA by performing PCR on the BrdU incorporated, NLRP3 bound DNA. Using primers specific for nuclear vs. mtDNA, we only detected mtDNA (Figure 6C) following NLRP3 IP. Thus, mtDNA released into the cytosol bound NLRP3.
Figure 6. Oxidized mitochondrial DNA binds to NLRP3 and activates the inflammasome.
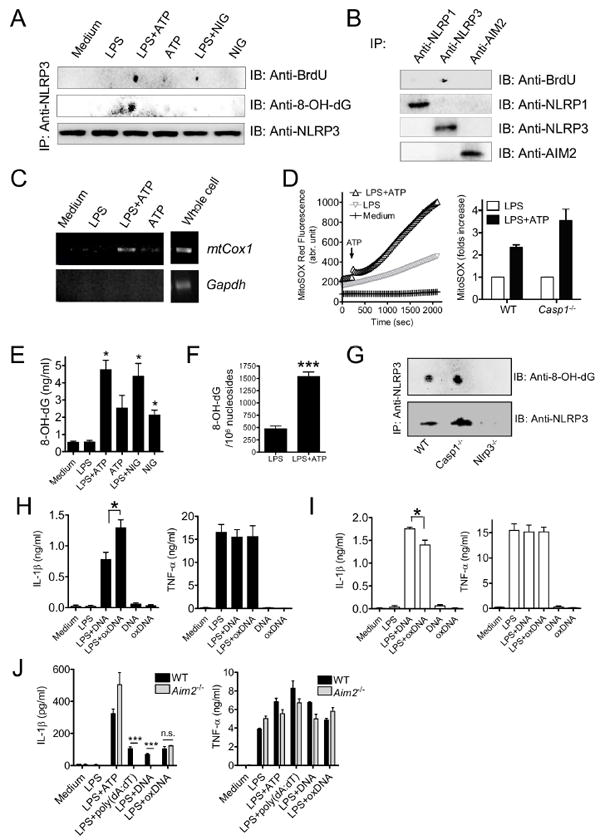
(A) BMDM were preloaded with BrdU (10 μM), and cells were treated with LPS (1 μg/ml, 3 hr), followed by 3-MA (2.5 mM, 1 hr), ATP (5 mM, 1 h) or NIG (10 μM, 1 h). Cell lysates were collected and immunoprecipitated with anti-NLRP3 Ab, then detected by dot-blots probed with anti-BrdU or anti-8OH-dG Abs. As a loading control, NLRP3 immunoblot was performed.
(B) BrdU-preloaded BMDM were treated with LPS+ATP, cell lysates were immunoprecipitated with Ab against NLRP1, NRLP3, or AIM2. Immune dot-blots with BrdU Ab, and respective control immunoblots are shown.
(C) Mitochondrial COX1 DNA associates with NLRP3 immunoprecipitates. COX1 DNA was amplified from the NLRP3 immunoprecipitates by PCR.
(D) BMDM were stimulated with or without LPS for 3 hr, then loaded with 2.5 uM MitoSOX for 20 min. Mitochondrial ROS was measured every 30 s thereafter. ATP was added 240 s after monitoring was initiated. Relative mitochondrial ROS increase at 30 min after ATP was shown.
(E and F) Mitochondrial DNA was extracted from BMDM after NLRP3 inflammasome stimulation, and amounts of 8-OH-dG were quantified by (E) ELISA and (F) LC-MS-MS (means ± SD).
(G) 8-OH-dG immune dot-blots were performed in WT, Casp1–/– and Nlrp3–/– BMDM after LPS+ATP treatment. LPS-primed BMDM were treated with 3MA for 2 hr, followed by stimulation with ATP for 30 min. Cell lysates were collected and NLRP3 Immunoprecipitation and 8-OH-dG dot-blot were performed in WT, Casp1–/– and Nlrp3–/– BMDM.
(H and I) LPS-primed (H) WT or (I) Nlrp3–/– BMDM were treated with exogenous 8-OH-dG incorporated DNA (oxDNA, 2 μg/ml) or control DNA for 8 hr. IL-1β and TNFα amounts were quantified in supernatants by ELISA (means ± SD).
(J) LPS-primed Aim2–/– BMDM were treated with exogenous oxDNA (2 μg/ml) and IL-1β and TNFα concentrations were determined in culture supernatants by ELISA (means ± SD).
Cytochrome c (Cyt-c), released from the mitochondria during apoptosis, is also liberated after ATP stimulation (Nakahira et al., 2010). We therefore investigated whether the Cyt-c released from the mitochondria during NLRP3 inflammsome activation (under LPS+ATP conditions) might also co-IP with NLRP3. However, Cyt-c was undetectable in our NLRP3 pull-downs (Figure S4B), suggesting that Cyt-c does not play a direct role in NLRP3 activation.
Previous studies have indicated that mtROS might play an important role in NLRP3 activation (Nakahira et al., 2010; Zhou et al., 2010). Indeed, upon stimulation with LPS+ATP, mtROS was substantially increased (Figure 6D and Movie S2). Importantly, generation of mtROS downstream of ATP stimulation was caspase-1 independent (Figure 6D). As ATP induced mtROS, and it is well-known that mtROS generation can result in oxidized mtDNA, we probed NLRP3 immunoprecipitates for the oxidized DNA indicator, 8-hydroxy-guanosine (8-OH-dG). We found that under LPS+ATP conditions, 8-OH-dG was detected in the pull-downs (Figure 6A and S4C). The oxidized nucleoside 8-OH-dG was also found under LPS+NIG conditions, although at a reduced amount compared with LPS+ATP (Figure 6A and S4C).
To further assess the role of oxidized mtDNA during NLRP3 activation, we investigated 8-OH-dG induction under NLRP3 activating conditions. When BMDM were exposed to either ATP or NIG, 8-OH-dG concentration increased (Figure 6E). Addition of LPS to either ATP or NIG resulted in further increased 8-OH-dG abundance (Figure 6E). This finding dovetails with a recent report showing that TLR signaling enhances mtROS production (West et al., 2011). These data were corroborated by an in-depth analysis of mtDNA by liquid chromatography-tandem mass spectrometry (LC-MS-MS) that revealed a three-fold increase in 8-OH-dG abundance in mtDNA from BMDM treated with LPS+ATP versus LPS alone (Figure 6F). We also investigated whether transfected oxidized DNA may colocalize with NLRP3 in 293 cells and found that this did indeed occur (Figure S4D and E). Finally, NLRP3 was pulled down with oxidized DNA in caspase-1 deficient macrophages, again indicating all the events take place prior to caspase-1 activation (Figure 6G and S4F), and ruling out downstream events derived from caspase-1 activity.
We next assessed whether oxidized DNA vs. normal DNA could enhance IL-1β production. Oxidized DNA was generated by PCR against the mtCOX1 template in presence of 8-OH-dGTP, and PCR products were transfected into BMDM. IL-1β production was significantly enhanced with oxidized vs. normal DNA (Figure 6H). Importantly, oxidized DNA did not impact TNF-α production. However, the same experiment performed in Nlrp3–/– BMDM resulted in significantly decreased IL-1β, but not TNF-α production (Figure 6I). Furthermore, DNA containing 8-OH-dGTP could still induce IL-1β secretion in Aim2–/– BMDM (Figure 6J), despite that these cells were refractive to mtDNA transfection-induced IL-1β release (Figure S3G). Taken together, these data indicate that oxidized DNA can induce inflammasome activation via preferential activation of NLRP3 and not AIM2.
We next investigated whether 8-OH-dG might competitively inhibit NLRP3 inflammasome activation by preventing the binding of oxidized mtDNA to NLRP3. Under NLRP3 activating conditions, we added increasing amounts of either dG or 8-OH-dG into the media concurrent with LPS treatment. While addition of dG had no effect on IL-1β production, 8-OH-dG was able to significantly reduce IL-1β secretion (Figure 7A), but did not affect TNF-α secretion (Figure S5A). As expected, 8-OH-dG did not inhibit AIM2 stimulation (Figure 7B). To ascertain the mechanism by which 8-OH-dG inhibited IL-1β secretion, we repeated the NLRP3 and BrdU-incorporated mtDNA IP experiment under LPS+ATP conditions, but in presence of either dG or 8-OH-dG. Importantly, 8-OH-dG prevented BrdU-incorporated mtDNA from co-precipitating with NLRP3 (Figure 7C and S5B). Finally, we probed 8-OH-dG in NLRP3 pull-downs and found that under 8-OH-dG conditions, instead of BrdU mtDNA, only 8-OH-dG came down with NLRP3 (Figure 7C and S5C). Thus, 8-OH-dG competitively inhibited NLRP3-mtDNA interaction and prevented IL-1β secretion. Together, these data indicate that oxidized mtDNA, generated and released into the cytosol during apoptosis, is the actual binding motif for NLRP3 and activates the NLRP3 inflammasome.
Figure 7. 8-OH-dG inhibits mtDNA from binding to and activating the NLRP3 inflammasome.
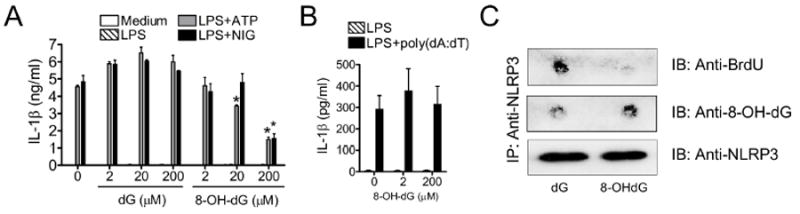
(A and B) BMDM were stimulated with LPS (1 μg/ml) in the presence of deoxyguanosine (dG) and 8-OH-dG, then treated with (A) ATP ± NIG or (B) poly(dA:dT) (2 μg/ml), and IL-1β release was quantified by ELISA (means ± SD).
(C) BMDM were primed with LPS (1 μg/ml, 3 hr) in the presence of dG or 8-OH-dG (200 μM), pretreated with 3-MA (2.5 mM for 1 hr), and then treated with ATP (5 mM, 1 hr). IP with anti-NLRP3 Ab was performed, and BrdU or 8-OH-dG dot-blotting was carried out by immunoblotting. NLRP3 is shown as a loading control.
Data shown are representative of three or more independent experiments.
*p<0.05.
DISCUSSION
The NLRP3 inflammasome is a critical nexus mediating IL-1β and IL-18 responses to pathogens and innate immune stimuli. Because inflammasome stimuli are diverse and often unrelated, discerning the mechanism of NLRP3 activation has been elusive. Recent evidence has begun to indicate mt as key players in NLRP3 inflammasome signaling (Nakahira et al., 2010; Zhou et al., 2010). During our analysis of CP infection, we serendipitously uncovered evidence that mt might play an important role in activating the NLRP3 inflammasome. In this report, we have provided a mechanistic explanation for NLRP3 activation, where mt sense cellular danger that results in apoptosis, during which oxidized mtDNA is released into the cytosol and binds to NLRP3. This cascade of events resulted in activation of the NLRP3 inflammasome and caspase-1 maturation.
The identification of mt as key players in NLRP3 inflammasome induction places apoptosis at the epicenter of this important process. As both sensors and robust sources of ROS, mt rapidly respond by releasing Cyt-c and inducing apoptosis (Ott et al., 2007). Moreover, cathepsins and ROS released during lysosomal rupture can also profoundly impact mt membrane integrity, causing membrane permeabilization and subsequent initiation of apoptosis (Boya et al., 2003; Ferri and Kroemer, 2001). The mt K+ cycle, inexorably linked to intracellular K+ concentration, is obligatorily central to maintaining mt volume, controlling metabolic ROS (Garlid and Paucek, 2003) and inducing apoptosis (Park and Kim, 2002). We have shown that, while capable of blocking IL-1β secretion in response to NLRP3 triggers, inhibiting K+ efflux using high concentrations of extracellular K+ exerted profound effects on mt, and mitigated loss of ΔΨm in response to NLRP3 activators. Indeed, it has often been overlooked that modulating cell surface K+ channels inevitably results in altered activation of mitochondrial K+ channels. But how do mt activate the NLRP3 inflammasome?
Our data demonstrated that NLRP3 triggers, such as alum, ATP, NIG, and live CP cause mt dysfunction and cell death in macrophages. We have also shown that STS, a pro-apoptotic compound, was sufficient to act as a second signal for NLRP3 activation. Moreover, LPS-primed macrophages treated with NLRP3 activators secreted less IL-1β, but similar amounts of TNF-α in presence of CsA. Apoptosis was critical for NLRP3 inflammasome induction, because overexpression of anti-apoptotic Bcl2 attenuated IL-1β secretion by LPS-primed macrophages, and Bcl2 silencing resulted in the converse. St type III secretion mutants unable to induce apoptosis did not promote IL-1β secretion, and Bcl2 overexpression inhibited wild-type St infection-induced IL-1β secretion. Given that the physiological triggers of NLRP3 are inextricably linked to mt and apoptosis, and that a causal relationship exists between pro-apoptotic signals and NLRP3, it seems that apoptosis is an indispensable step in NLRP3 inflammasome activation. While apoptosis is often portrayed as ‘silent’ cell death, our data suggest that in the presence of proinflammatory signal 1, the apoptotic machinery activates the NLRP3 inflammasome. Thus, apoptosis can activate the NLRP3 inflammasome, but remains a silent death unless in the context of signal 1.
While the role of Bcl-2 in apoptosis is well-appreciated, it has been suggested that Bcl-2 may more directly activate the inflammasome. For example, Bcl-2 can directly bind NLRP1 and inhibit NLRP1 activation (Bruey et al., 2007; Faustin et al., 2009). These data raise the possibility of alternative pathways by which Bcl-2 might inhibit NLRP3 activation. However, these investigators did find that Bcl-2 does not bind NLRP3. Additionally, whether inhibition of NLRP1 activation occurs in vivo is unclear as they stimulated Bcl2-/- and Bcl2-overexpressing macrophages with MDP+ATP, and ATP is a well-known NLRP3 activator (Mariathasan et al., 2006b; Sutterwala et al., 2006). Given these data, plus the known effect of Bcl-2 on the apoptotic machinery, it is unlikely that Bcl-2 is directly involved in NLRP3 inflammasome activation.
At this point, it is not known whether both intrinsic and extrinsic apoptosis can induce the NLRP3 inflammasome together with signal 1, as all of the apoptosis inducers we tested were intrinsic. Therefore, we also investigated whether Fas ligand (FasL) could induce IL-1β secretion in LPS-primed BMDM, but did not detect IL-1β release or any reduction in mt membrane potential. However, exposure of BMDM to FasL did not induce a robust apoptotic response, suggesting that BMDM are partially insensitive to the apoptotic effects of FasL. We therefore cannot exclude a potential role for extrinsic apoptosis in NLRP3 inflammasome activation.
It is not clear how oligomeric NLRP3 inflammasome complexes sense such a wide range of cytosolic danger signals, including ATP, K+ efflux, alum, uric acid crystals, β-amyloid, and various microbial infections (Jin and Flavell, 2010; Schroder and Tschopp, 2010). As such, in a mechanism distinct from pattern recognition receptors, NLRP3 does not seem to sense each of these diverse ligands directly with its leucine-rich repeat (LRR) domain. Instead, it is believed that three broad physiological changes—ROS generation, K+ efflux, or lysosomal leakage—activate the NLRP3 inflammasome (Stutz et al., 2009). Yet, it did not seem possible to reconcile these three NLRP3 activation models with one another, and so the mechanism by which the NLRP3 inflammasome was activated by diverse signals remained perplexing. Our model of oxidized mtDNA binding to NLRP3 as the activation step neatly assembles and unifies previous models of NLRP3 activation. While we found that oxidized mtDNA associated with the NLRP3 inflammasome after stimulation, our data utilizing 293 cells transfected with mtDNA suggest that mtDNA can directly bind NLRP3. However, these results do not rule out the association of the mtDNA with other members of the NLRP3 inflammasome complex.
Two recent investigations have linked autophagy and inflammasome activation with mt activity. The first report found that blocking autophagy results in accumulation of mt-driven ROS formation, which in turn activates the NLRP3 inflammasome (Zhou et al., 2010). Our results presented here generally agree with the basic findings of the Zhou et al. report. Specifically, those authors concluded that apoptosis was not involved in their system based on lack of LDH release after various stimuli. Moreover they were unable to detect LDH release after exposure to NIG; a proton ionophore that causes cytosolic acidification and has been shown to decrease intracellular pH and induce apoptosis in other models (Yamagata and Tannock, 1996; Yang et al., 2008). Indeed, we found that NIG induced nuclear condensation, and another group reported LDH release after NIG exposure (Shimada et al., 2010). Interestingly, while Zhou et al. did not detect LDH release after monosodium urate treatment, this compound may have off-target effects as it has been shown to inhibit neutrophil apoptosis at low concentrations, but causes LDH release at higher levels (Akahoshi et al., 1997). Nonetheless, when considering the preponderance of data we present in this study linking apoptosis and NLRP3 activation, it seems that initiation of apoptotic events is required for proper NLRP3 activation.
Both Zhou et al. and Nakahira et al. found that mt-derived ROS are required for NLRP3 activation (Nakahira et al., 2010; Zhou et al., 2010). However, Nakahira et al. also found that mtDNA release is critical for NLRP3 activation, and this is dependent on ROS generation. We also observed that mtDNA was released into the cytosol and that its presence was absolutely required for NLRP3 activation. However, Nakahira et al. concluded that NLRP3 itself is required for mtDNA release, as they did not detect mtDNA in the cytosol of NLRP3 deficient macrophages. Importantly, our results suggest that transfected mtDNA can bind to NLRP3 overexpressed in 293 cells and that DNA released from mt during apoptosis binds to the NLRP3 inflammasome. Therefore, a likely explanation for the lack of cytosolic mtDNA in NLRP3 deficient macrophages in the study by Nakahira et al. is that NLRP3 itself stabilizes mtDNA in the cytosol by binding to it. The direct binding of mtDNA to NLRP3 could therefore be the triggering mechanism for NLRP3 activation.
During apoptosis, ROS and oxidized mtDNA are generated (Esteve et al., 1999). Our data indicate that it is this oxidized form of mtDNA that binds to and activates the NLRP3 inflammasome and that this interaction can be competitively inhibited by oxidized dG. One possibility for the inhibition by 8-OH-dG could be through the prevention of ATP binding on NLRP3 (Duncan et al., 2007) instead of directly competing with oxidized mtDNA binding. Perhaps ATP binding and hydrolysis is required for proper mtDNA binding. However, while our data does not rule out this possibility, the end result is a lack of mtDNA binding, which is required for NLRP3 activation. Therefore, our data are in agreement with previous studies regarding the importance of mtROS, and now provide the putative mechanism for NLRP3 inflammasome activation. Additionally, while it appears that transfected oxidized mtDNA can activate both NLRP3 and AIM2, AIM2 is preferentially activated by normal DNA, and NLRP3, by oxidized DNA.
Our data clearly show that induction of apoptosis is required for NLRP3 inflammasome activation; however, we do not know at what point in the signaling cascade the two events diverge. According to our model, both the apoptosome and the NLRP3 inflammasome share common upstream activating factors derived from mt. However, it is a byproduct of apoptosis, oxidized mtDNA released into the cytosol, which seems to be the activating factor for the NLRP3 inflammasome. The divergence of the two pathways after initiation of apoptosis is evidenced by the fact that mice deficient in Apaf-1, caspase-9 or caspase-3 exhibit infertility, abnormal brain development, and lethality (Honarpour et al., 2000; Kuida et al., 1998; Kuida et al., 1996) whereas no such phenotypes have been found for caspase-1, NLRP3- or ASC deficient mice. Thus, while the two pathways share a common origin, their downstream effects are different.
In toto, we have shown that mt dysfunction leading to apoptosis occurs with, and is necessary and sufficient for, NLRP3 activation in the presence of signal 1. The generation of oxidized mtDNA in this process of apoptosis and NLRP3 inflammasome activation presents some intriguing possibilities regarding diseases such as diabetes. NLRP3 has been shown to play an important role in type II diabetes (Masters et al., 2010) and interestingly, high concentrations of 8-OH-dG (representative of damaged DNA) have been associated with both type I and type II diabetes (Hinokio et al., 1999; Simone et al., 2008). One could speculate that the increased amounts of oxidized DNA generated in diabetes could be derived from the processes that induce NLRP3 activation.
A key implication of the present study is that inhibition of apoptosis by intracellular microbes serves a dual role: attenuation of IL-1β secretion and maintenance of a viable host cell for intracellular growth. Moreover, our results suggest that evolution has developed an innate immune strategy that relies on mt to determine the right time to sacrifice a jeopardized host cell for the sake of initiating a strong inflammatory cascade via IL-1β. Therefore, apoptosis is not always ‘silent’; rather, it can be a powerful voice to instruct nearby cells of imminent danger in the presence of NF-κB-activating signal 1.
EXPERIMENTAL PROCEDURES
Mice
Casp1–/– mice (Kuida et al., 1995) were kindly provided by R. A. Flavell (Yale Univ., New Haven, CT). Nlrp3–/–, Asc–/– (Mariathasan et al., 2006a), and Aim2–/– mice were supplied by K. A. Fitzgerald (Univ. of Massachusetts Medical School, Worcester, MA). C57BL/6 mice were obtained from the Jackson Laboratories (Bar Harbor, ME) and all animal experiments were conducted according to Cedars-Sinai Medical Center Institutional Animal Care and Use Committee guidelines. All mice were used at 8-12 weeks of age and were matched for gender.
Measurement of mitochondrial membrane potential and ROS
Cells were stained with the cationic dye TMRM (AnaSpec, Fremont, CA, USA) or mitochondrial superoxide indicator MitoSOX (Invitrogen) as described in the manufacturer’s protocol. Cells were loaded with 200 nM of TMRM for 30 min or 2.5 μM of MitoSOX for 20 min, washed three times with PBS. TMRM fluorescence was measured using a SpectraMaX M2 microplate reader (Molecular Devices Corp., Sunnyvale, CA, USA) or by fluorescence microscopy (Nikon Eclipse T2000). MitoSOX fluorescence was measured by an epifluorescence microscope (Olympus IX70; 20X Objective, Texas Red filter cube). Time-lapse images of live-cell fluorescence with and without ATP stimulus were obtained by CellSens software and quantitative analysis was performed using ImageJ software. Care was taken to optimize the fluorescence excitation conditions so as to minimize photobleaching of the fluoropores during data acquisition.
Immunoblot
BMDM were stimulated for various times, supernatants were collected, proteins were precipitated by methanol-chloroform extraction, and cell lysates were collected. Immunoblot analysis was done using various primary antibodies: anti mouse caspase-1 p10 (sc-514; Santa Cruz Biotechnology), anti-mouse IL-1β (AF-401-NA; R&D Systems), or anti-GAPDH (6C5; Santa Cruz Biotechnology).
Immunoprecipitation
BMDM were preloaded with BrdU (10 μM) for 48 hr and treated as indicated. For IPs, the rabbit anti-NLRP3 polyclonal Ab (LifeSpan BioSciences Inc., Seatle, WA) was incubated with the cell lysates for 2 hr or overnight at 4°C. Subsequently, Trueblot IgG beads (eBioscience, San Diego, CA) were added and the samples were incubated at 4°C for 1 hr. For IPs from Flag-NLRP3 stably expres sing 293 cells, mouse anti-Flag mAb (M2, Sigma) was used. The immune complexes were then washed and the associated proteins were eluted from the beads by boiling and then the supernatant was dot-blotted and UV cross-linked to a nitrocellulose membrane. Immunoblotting was performed using anti-BrdU mAb (BU33; Sigma) or mouse anti-8OH-dG mAb (15A3; Rockland Immunochemicals Inc., Gilbertsville, PA).
Supplementary Material
Acknowledgments
We thank P. Sun, W. Zhang, and G. Huang for expert technical assistance. We are grateful to G. Nuñez (Univ. of Michigan) for kindly providing the pSFFV-neo plasmid, to R.A. Flavell (Yale Univ.) for supplying Casp1–/– mice, and to E. Latz (Univ. of Bonn) for kindly providing the NLRP3 plasmid. This work was supported by National Institutes of Health (NIH) Grants HL-66436 and AI-067995 (to M.A.). T.T. is supported by NIH Grants AG-029726 and NS-076794, and is the inaugural holder of the Ben Winters Endowed Chair in Regenerative Medicine at CSMC. D.M. Underhill is supported a NIH Grant GM-085796 and holds the Janis and William Wetsman Family Chair in Inflammatory Bowel Disease at CSMC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akahoshi T, Nagaoka T, Namai R, Sekiyama N, Kondo H. Prevention of neutrophil apoptosis by monosodium urate crystals. Rheumatol Int. 1997;16:231–235. doi: 10.1007/BF01375654. [DOI] [PubMed] [Google Scholar]
- Arnoult D, Carneiro L, Tattoli I, Girardin SE. The role of mitochondria in cellular defense against microbial infection. Semin Immunol. 2009;21:223–232. doi: 10.1016/j.smim.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Boya P, Gonzalez-Polo RA, Poncet D, Andreau K, Vieira HL, Roumier T, Perfettini JL, Kroemer G. Mitochondrial membrane permeabilization is a critical step of lysosome-initiated apoptosis induced by hydroxychloroquine. Oncogene. 2003;22:3927–3936. doi: 10.1038/sj.onc.1206622. [DOI] [PubMed] [Google Scholar]
- Broz P, Newton K, Lamkanfi M, Mariathasan S, Dixit VM, Monack DM. Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against Salmonella. J Exp Med. 2010;207:1745–1755. doi: 10.1084/jem.20100257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruey JM, Bruey-Sedano N, Luciano F, Zhai D, Balpai R, Xu C, Kress CL, Bailly-Maitre B, Li X, Osterman A, et al. Bcl-2 and Bcl-XL regulate proinflammatory caspase-1 activation by interaction with NALP1. Cell. 2007;129:45–56. doi: 10.1016/j.cell.2007.01.045. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- Duncan JA, Bergstralh DT, Wang Y, Willingham SB, Ye Z, Zimmermann AG, Ting JP-Y. Cryopyrin/NALP3 binds ATP/dATP, is an ATPase, and requires ATP binding to mediate inflammatory signaling. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:8041–8046. doi: 10.1073/pnas.0611496104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteve JM, Mompo J, Garcia de la Asuncion J, Sastre J, Asensi M, Boix J, Vina JR, Vina J, Pallardo FV. Oxidative damage to mitochondrial DNA and glutathione oxidation in apoptosis: studies in vivo and in vitro. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1999;13:1055–1064. doi: 10.1096/fasebj.13.9.1055. [DOI] [PubMed] [Google Scholar]
- Faustin B, Chen Y, Zhai D, Le Negrate G, Lartigue L, Satterthwait A, Reed JC. Mechanism of Bcl-2 and Bcl-X(L) inhibition of NLRP1 inflammasome: loop domain-dependent suppression of ATP binding and oligomerization. Proc Natl Acad Sci U S A. 2009;106:3935–3940. doi: 10.1073/pnas.0809414106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri KF, Kroemer G. Organelle-specific initiation of cell death pathways. Nat Cell Biol. 2001;3:E255–263. doi: 10.1038/ncb1101-e255. [DOI] [PubMed] [Google Scholar]
- Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005;73:1907–1916. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009;10:241–247. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlid KD, Paucek P. Mitochondrial potassium transport: the K(+) cycle. Biochim Biophys Acta. 2003;1606:23–41. doi: 10.1016/s0005-2728(03)00108-7. [DOI] [PubMed] [Google Scholar]
- Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G, Ullrich E, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15:1170–1178. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- Ghribi O, DeWitt DA, Forbes MS, Arad A, Herman MM, Savory J. Cyclosporin A inhibits Al-induced cytochrome c release from mitochondria in aged rabbits. J Alzheimers Dis. 2001;3:387–391. doi: 10.3233/jad-2001-3404. [DOI] [PubMed] [Google Scholar]
- Gross O, Thomas CJ, Guarda G, Tschopp J. The inflammasome: an integrated view. Immunol Rev. 2011;243:136–151. doi: 10.1111/j.1600-065X.2011.01046.x. [DOI] [PubMed] [Google Scholar]
- Hersh D, Monack DM, Smith MR, Ghori N, Falkow S, Zychlinsky A. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proceedings of the National Academy of Sciences. 1999;96:2396–2401. doi: 10.1073/pnas.96.5.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinokio Y, Suzuki S, Hirai M, Chiba M, Hirai A, Toyota T. Oxidative DNA damage in diabetes mellitus: its association with diabetic complications. Diabetologia. 1999;42:995–998. doi: 10.1007/s001250051258. [DOI] [PubMed] [Google Scholar]
- Hogquist KA, Nett MA, Unanue ER, Chaplin DD. Interleukin 1 is processed and released during apoptosis. Proc Natl Acad Sci U S A. 1991;88:8485–8489. doi: 10.1073/pnas.88.19.8485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honarpour N, Du C, Richardson JA, Hammer RE, Wang X, Herz J. Adult Apaf-1-deficient mice exhibit male infertility. Developmental biology. 2000;218:248–258. doi: 10.1006/dbio.1999.9585. [DOI] [PubMed] [Google Scholar]
- Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V, Latz E. Critical functions of priming and lysosomal damage for NLRP3 activation. Eur J Immunol. 2010;40:620–623. doi: 10.1002/eji.200940185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C, Flavell RA. Molecular Mechanism of NLRP3 Inflammasome Activation. Journal of Clinical Immunology. 2010 doi: 10.1007/s10875-010-9440-3. [DOI] [PubMed] [Google Scholar]
- Johansson AC, Appelqvist H, Nilsson C, Kagedal K, Roberg K, Ollinger K. Regulation of apoptosis-associated lysosomal membrane permeabilization. Apoptosis. 2010;15:527–540. doi: 10.1007/s10495-009-0452-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kage H, Takaya A, Ohya M, Yamamoto T. Coordinated Regulation of Expression of Salmonella Pathogenicity Island 1 and Flagellar Type III Secretion Systems by ATP-Dependent ClpXP Protease. J Bacteriol. 2008;190:2470–2478. doi: 10.1128/JB.01385-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuida K, Haydar TF, Kuan CY, Gu Y, Taya C, Karasuyama H, Su MS, Rakic P, Flavell RA. Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell. 1998;94:325–337. doi: 10.1016/s0092-8674(00)81476-2. [DOI] [PubMed] [Google Scholar]
- Kuida K, Lippke JA, Ku G, Harding MW, Livingston DJ, Su MS, Flavell RA. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- Kuida K, Zheng TS, Na S, Kuan C, Yang D, Karasuyama H, Rakic P, Flavell RA. Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature. 1996;384:368–372. doi: 10.1038/384368a0. [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Mueller JL, Vitari AC, Misaghi S, Fedorova A, Deshayes K, Lee WP, Hoffman HM, Dixit VM. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J Cell Biol. 2009;187:61–70. doi: 10.1083/jcb.200903124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latz E. The inflammasomes: mechanisms of activation and function. Curr Opin Immunol. 2010 doi: 10.1016/j.coi.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly JD, Grubb DR, Lawen A. The mitochondrial membrane potential (deltapsi(m)) in apoptosis; an update. Apoptosis. 2003;8:115–128. doi: 10.1023/a:1022945107762. [DOI] [PubMed] [Google Scholar]
- Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006a;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006b;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- Masters SL, Dunne A, Subramanian SL, Hull RL, Tannahill GM, Sharp FA, Becker C, Franchi L, Yoshihara E, Chen Z, et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat Immunol. 2010;11:897–904. doi: 10.1038/ni.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahira K, Haspel JA, Rathinam VAK, Lee S-J, Dolinay T, Lam HC, Englert JA, Rabinovitch M, Cernadas M, Kim HP, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nature immunology. 2010 doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott M, Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria, oxidative stress and cell death. Apoptosis. 2007;12:913–922. doi: 10.1007/s10495-007-0756-2. [DOI] [PubMed] [Google Scholar]
- Park IS, Kim JE. Potassium efflux during apoptosis. J Biochem Mol Biol. 2002;35:41–46. doi: 10.5483/bmbrep.2002.35.1.041. [DOI] [PubMed] [Google Scholar]
- Patrushev M, Kasymov V, Patrusheva V, Ushakova T, Gogvadze V, Gaziev AI. Release of mitochondrial DNA fragments from brain mitochondria of irradiated mice. Mitochondrion. 2006;6:43–47. doi: 10.1016/j.mito.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Petrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007;14:1583–1589. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- Riesbeck K, Bredberg A, Forsgren A. Ciprofloxacin does not inhibit mitochondrial functions but other antibiotics do. Antimicrob Agents Chemother. 1990;34:167–169. doi: 10.1128/aac.34.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- Shimada K, Crother TR, Karlin J, Chen S, Chiba N, Ramanujan K, Vergnes L, Ojcius D, Arditi M. Caspase-1 dependent IL-1β Secretion is Critical for Host Defense in a Mouse Model of Chlamydia pneumoniae Lung Infection. PLoS ONE. 2011 doi: 10.1371/journal.pone.0021477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, Park BG, Wolf AJ, Brikos C, Goodridge HS, Becker CA, Reyes CN, Miao EA, Aderem A, Götz F, et al. Staphylococcus aureus Evades Lysozyme-Based Peptidoglycan Digestion that Links Phagocytosis, Inflammasome Activation, and IL-1beta Secretion. Cell host & microbe. 2010;7:38–49. doi: 10.1016/j.chom.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone S, Gorin Y, Velagapudi C, Abboud HE, Habib SL. Mechanism of oxidative DNA damage in diabetes: tuberin inactivation and downregulation of DNA repair enzyme 8-oxo-7,8-dihydro-2’-deoxyguanosine-DNA glycosylase. Diabetes. 2008;57:2626–2636. doi: 10.2337/db07-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutz A, Golenbock DT, Latz E. Inflammasomes: too big to miss. J Clin Invest. 2009;119:3502–3511. doi: 10.1172/JCI40599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutterwala FS, Ogura Y, Szczepanik M, Lara-Tejero M, Lichtenberger GS, Grant EP, Bertin J, Coyle AJ, Galán JE, Askenase PW, Flavell RA. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24:317–327. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Tschopp J. Mitochondria: Sovereign of inflammation? Eur J Immunol. 2011;41:1196–1202. doi: 10.1002/eji.201141436. [DOI] [PubMed] [Google Scholar]
- Tschopp J, Schroder K. NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nature Reviews Immunology. 2010 doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- West AP, Brodsky IE, Rahner C, Woo DK, Erdjument-Bromage H, Tempst P, Walsh MC, Choi Y, Shadel GS, Ghosh S. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature. 2011;472:476–480. doi: 10.1038/nature09973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata M, Tannock IF. The chronic administration of drugs that inhibit the regulation of intracellular pH: in vitro and anti-tumour effects. Br J Cancer. 1996;73:1328–1334. doi: 10.1038/bjc.1996.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Mei Y, Xie Q, Han X, Zhang F, Gu L, Zhang Y, Chen Y, Li G, Gao Z. Acidification induces Bax translocation to the mitochondria and promotes ultraviolet light-induced apoptosis. Cell Mol Biol Lett. 2008;13:119–129. doi: 10.2478/s11658-007-0042-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2010 doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


