Abstract
A coxsackievirus B4 induces acute pancreatitis with different outcomes. The study utilized a systems biology approach to identify molecular immune responses that differentiate between disease resolution and disease progression. The data establish a temporal pattern of host responses that differentiate the resolution of acute pancreatitis from the progression to chronic pancreatitis. A group of twenty-five genes exhibited characteristic expression profiles that were observed during the development of chronic pancreatitis but not during the resolution of disease. We postulate that the temporal dynamics of the twenty-five genes influence the development of pathogenic immune responses associated with chronic pancreatitis. Furthermore, a subset of eleven genes exhibited increased expression as viral titers waned. Of the eleven gene products, five are secreted molecules, TNF-α, IFN-γ, CXCL10, IL-10, and IL-22b, and represent novel potential therapeutic targets since they can be readily modulated with antibodies against the specific cytokine/chemokine or with antibodies against the corresponding receptors.
Keywords: pancreatitis, coxsackievirus, transcriptional profiling, cytokines, gene expression, therapeutic target
INTRODUCTION
The most common result of infection with the group B coxsackieviruses (CVB) is asymptomatic infection, an undifferentiated febrile illness, or mild upper respiratory symptoms (Huber & Ramsingh, 2004). However, on rare occasions, CVB infection results in chronic inflammatory disease of the pancreas, heart, or central nervous system (Tracy & Gauntt, 2008). Since CVB infections range from asymptomatic infection to severe, debilitating chronic diseases, the study of viral pathogenesis has proven challenging. The precise mechanisms by which CVB cause acute or chronic inflammatory disease remain to be determined. We have developed a mouse model of CVB4-induced pancreatitis to explore the molecular events at the virus/host interface that affect the development of acute and chronic inflammatory disease (Huber & Ramsingh, 2004; Chapman et al., 1997; Ramsingh, 2008).
Clinical pancreatitis is an inflammatory disease of the exocrine pancreas and occurs as either an acute or a chronic disease. Mild acute pancreatitis is generally self-limiting, while severe acute disease can lead to a systemic inflammatory response syndrome with respiratory and cardiovascular failure (Bhatia, 2004; Kingsnorth & O'Reilly, 2006; Whitcomb, 2006). Chronic pancreatitis, on the other hand, is a painful and debilitating disease, in which a progressive, destructive inflammatory process destroys the exocrine pancreas, resulting in exocrine pancreatic insufficiency (Mergener & Baillie, 1997; Stevens et al., 2004). Treatment is generally geared to reducing pain. Chronic pancreatitis can develop from one episode of severe acute pancreatitis, or from recurrent episodes of acute disease. Furthermore, chronic pancreatitis is a major risk factor for pancreatic cancer which has a poor prognosis.
Our experimental model utilizes a CVB4 variant designated CVB4-V which induces a severe acute pancreatitis that progresses to chronic pancreatitis (Ramsingh et al., 1989; Ramsingh, 2008). Acute pancreatitis develops during the period of viral replication while chronic pancreatitis develops after infectious virus is cleared. Chronic pancreatitis resembles the clinical disease and is characterized by exocrine pancreatic insufficiency, weight loss, and pathological changes in the pancreas (Ramsingh et al., 1999). We have shown that the severity of acute pancreatitis is determined by the viral genotype. A single amino acid residue in the DE-loop of the VP1 capsid is a major determinant of viral virulence (Caggana et al., 1993). Additional studies revealed that exogenously administered cytokines such as IL-12 or IFN-γ modulate the severity of CVB4-V-induced acute pancreatitis (Potvin et al., 2003). While the viral genotype determines the severity of acute pancreatitis, host factors govern the progression to chronic pancreatitis. We have recently shown that IL-10 plays a major role in the development of chronic pancreatitis (Gu et al., 2009). CVB4-V infection in IL-10 knockout (KO) mice or during disruption of IL-10 signaling in wild-type mice results in an acute pancreatitis that does not progress to chronic pancreatitis. Cytokines have also been shown to modulate myocarditis caused by CVB3. As was observed in the CVB4 model, IFN-γ is protective during CVB3-induced acute myocarditis (Szalay et al., 2006; Horwitz et al., 2000; Henke et al., 2001). Unlike the CVB4 model, IL-10 is beneficial during CVB3-induced acute myocarditis (Szalay et al., 2006; Henke et al., 2001). The results indicate diversity in the mechanisms underlying CVB-induced disease and caution against extrapolation from one model system to another.
Our initial studies to explore the molecular responses of the host to CVB4-V infection utilized a systems biology approach to evaluate transcriptional events during the early infectious phase of disease (Ostrowski et al., 2004). We obtained a “snapshot” of the transcriptome in the pancreas of infected mice, at an early time in acute disease. CVB4-V-induced acute pancreatitis was accompanied by increased expression of genes involved in apoptosis, acinoductular metaplasia, remodeling of the extracellular matrix, and fibrosis; consistent with the observed progression to chronic pancreatitis. We subsequently pursued a study to explore the temporal dynamics of molecular immune responses to CVB4-V infection during the early infectious phase of disease and showed that early high levels of IL-10 are associated with delayed innate and adaptive immune responses in BALB/c mice (Gu et al., 2009). The present study is an extension of this work and focuses on the temporal dynamics of molecular immune responses to CVB4-V infection at a later stage in the disease process, just prior to the development of chronic pancreatitis. The goal of the study was to identify host molecular responses that correlated with progression to chronic disease. The approach focused on comparing gene expression in two models, one in which CVB4-V infection progresses to chronic inflammatory disease (BALB/c mice) and another in which CVB4-V infection does not progress to chronic disease (IL-10 knockout mice). We identified twenty-five genes whose biphasic expression patterns correlated with disease progression. Of the twenty-five genes, five encode cytokines or chemokines and represent novel potential therapeutic targets for the treatment of chronic pancreatitis.
MATERIALS AND METHODS
Cells, viruses, and mouse strains
CVB4-V is a pathogenic variant that was selected after multiple passages of the JVB strain of CVB4 in mouse pancreas (Ramsingh et al., 1989). A large-scale stock of plaque-purified virus was grown in LLC-MK2(D) cells and viral infectivity was measured by plaque assay. Two strains of mice were used in this study. BALB/c mice, purchased from The Jackson Laboratory (Bar Harbor, ME), were housed in a Specific Pathogen Free (SPF) facility at the Wadsworth Center (Albany, NY). IL-10 KO mice on the C57BL/6 genetic background were bred onto the BALB/c background (Dr. William Lee at the Wadsworth Center) for 11 generations. After 10 generations of traditional backcrossing, the recipient genome is 99.9% (www.criver.com). Thus, after 11 generations, the IL-10 KO mice have greater than 99.9% of the BALB/c genetic background.
Five-to-six week old mice (14–20g) were used in the study. Mice were infected intraperitoneally with CVB4-V and were allowed to eat and drink ad libitum. Because male mice develop a more severe acute disease than female mice (Ramsingh et al., 1999), male mice were infected with 103 pfu of virus while female mice were given 104 pfu of virus. Mice were sacrificed at various time points after infection and organs were removed. Pancreatic tissues were fixed in Bouin’s solution (Sigma-Aldrich, St. Louis, MO), processed for routine histology, and stained with hematoxylin and eosin. All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the Wadsworth Center.
Isolation of pancreatic RNA
Pancreatic tissues were harvested at various time points after infection. Tissues were frozen immediately on dry ice and placed in TRI reagent (Molecular Research Center, Inc. Cincinnati, OH) which combines phenol and guanidine thiocyanate in a mono-phase solution to inhibit RNase activity. Tissues were homogenized in a mini-beater (Biospec Products, Bartlesville, OK) using 1 mm Zirconia beads (Biospec Products). After a clarifying spin at 10,000Xg for 10 min at 4°, the homogenate was separated into aqueous and organic phases by the addition of 1-bromo-3-chloropropane (BCP) (Molecular Research Center) and centrifugation. RNA was precipitated from the aqueous phase by the addition of isopropanol, washed with ethanol, and resuspended in water. Residual DNA was digested with DNase (Promega, Madison, WI) and purified RNA was obtained using an RNeasy column (Qiagen, Valencia, CA).
Measurement of cytokines and chemokines in pancreatic homogenates
The pancreas, harvested at various times after infection, was frozen immediately on dry ice and placed in 1 ml of lysis buffer containing protease inhibitors (50 mM Tris, 150n mM NaCl, 2 mM EDTA, 1% NP40, 30 µM PMSF, 420 µM leupeptin, 0.02% aprotinin, 3 mM NaN3) (Gu et al., 2009). Organs were homogenized in a mini-beater (Biospec Products, Bartlesville, OK) using 1 mm Zirconia beads (Biospec Products). The homogenate was centrifuged at 10,000xg for 10 min at 4°C and the supernatants were stored at −80°C. Cytokines and chemokines in the pancreatic homogenates were measured using a multiplex bead-based assay, Luminex (Invitrogen), according to the manufacturer’s protocol. Three-to-six organs were analyzed at each time point.
Gene expression in pancreatic tissues of CVB4-V-infected mice
Gene expression was quantified using the TaqMan Gene Expression Assay in a two-step RT-PCR and a TaqMan Low Density Array (LDA), a 384-well micro fluidic card, preloaded with appropriate PCR primers and probes (Applied Biosystems) (Gu et al., 2009). Total pancreatic RNA was reverse-transcribed with M-MLV RT and random hexamers in a reaction volume of 25 ul using standard conditions. Briefly, random hexamers (Invitrogen, Carlsbad, CA) were added to 3 ug total RNA which was denatured at 90°C. Samples were frozen immediately on dry ice and thawed slowly on ice. After the addition of a cocktail containing dNTPs (1mM) (Roche, Switzerland), RNasin (Promega, Madison, WI), DTT (0.1M), and M-MLV RT, the reaction was incubated at 37°C, heat-inactivated at 70°C, and cDNAs were stored at −80°C. 100 ng of cDNA were mixed with Taqman Fast Universal PCR Master Mix (Applied Biosystems) to a final 1X concentration and approximately 2 ng of the cDNA solution were applied to each of 48 wells in the LDA card. PCR amplification was carried out in an Applied Biosystems 7900HT Fast Real-Time PCR System. Eight pancreatic samples were analyzed simultaneously for the expression of 47 genes and 18S ribosomal RNA in one 384-well LDA card. The LDA card contained three possible controls, 18S RNA, Egf, and Slc2a2/Glut-2. The expression of Egf remained unchanged during CVB4-V infection while the expression of both 18S RNA and Slc2a2 varied over the course of the infection. As a result, expression of test genes was normalized to that of Egf. The expression of one additional gene, RORγt, not present on the LDA card, was quantified using the TaqMan Gene Expression Assay in a two-step RT-PCR in a single-tube format with the Applied Biosystems 7500 Real-Time PCR System. The expression of RORγt was again normalized to that of Egf.
Analysis of gene expression data
The study focused on pancreatic gene expression at multiple time points (1, 2, 4, 6, 8, 10, and 14 days post infection (dpi)) in two treatment groups, CVB4-V-infected BALB/c mice and CVB4-V-infected IL-10 KO mice. Three-to-five mice per strain were analyzed at each time point and gene expression was quantified by a comparative Ct method (Applied Biosystems). The Ct value of each test gene in each organ was normalized to that of the control gene, Egf. The resulting [delta] Ct values for samples from each time point were then averaged. Since the goal of the study was to evaluate the magnitude of gene expression over time within a single treatment group and between treatment groups, a reference group was needed. The reference group chosen for all except one test gene was CVB4-V-infected BALB/c mice, 2dpi, because gene expression was very low or undetectable prior to this time point. For the one exception, RORγt, the reference group was CVB4-V-infected BALB/c mice at 4 dpi because gene expression was undetectable prior to this time point. The change in gene expression is represented as a fold-change which was calculated by determining 2−ddCt (ddCt = averaged [delta]Ct(specific time point)- averaged [delta]Ct BALB/c 2dpi). As a result, for all genes except RORγt, the relative expression in CVB4-V-infected BALB/c mice at 2 dpi is 1. For RORγt, the relative expression in CVB4-V-infected BALB/c mice at 4 dpi is 1. By using the BALB/c reference for both the BALB/c mice and the IL-10 KO mice, we were also able to assess the relative magnitude of gene expression in the two strains.
Treatment of CVB4-V-infected mice with anti-cytokine antibodies
A pilot study was undertaken to assess whether the systems biology approach had uncovered relevant targets. We focused on two of the five potential targets, TNF-α and IFN-γ, because of their pleiotropic effects. In this initial study, we tested a combination of anti-TNF-α and anti-IFN-γ, in five CVB4-V-infected mice. Antibodies were administered intraperitoneally. Anti-TNF-α (Centocor, PA) and anti-IFN-γ (BioXCell, W. Lebanon, NH) were each administered at a dose of 0.5 mg at 8, 10, and 14 dpi. CVB4-V-infected mice in the control group were treated with an isotype control antibody. Mice were weighed daily for a 4-week follow-up period. At the end of 4 weeks, mice were sacrificed and pancreatic tissues were processed for routine histology.
RESULTS
Gene expression profiles associated with the development of chronic pancreatitis
Pathological changes in our model of CVB4-V-induced chronic pancreatitis in BALB/c mice have been extensively studied by our group and include coagulative necrosis, generalized inflammation, and acinoductular metaplasia against a background of fibrosis (Caggana et al., 1993; Ramsingh et al., 1997; Ramsingh et al., 1999; Ostrowski et al., 2004). Unlike BALB/c mice, CVB4-V infection of IL-10 KO mice results in a moderate pancreatitis that resolves without further progression. By 21 dpi, the exocrine tissues of IL-10 KO mice show almost complete recovery and contain abundant intact acini (Gu et al., 2009).
A longitudinal study of gene expression during CVB4-V infection of IL-10 KO or BALB/c mice was undertaken to identify differences in gene expression during the early infectious phase of disease and during the later phase of disease. The approach relied on monitoring the expression of inflammatory markers and T cell markers, using a PCR-based assay in a low-density microarray format. The expression of 46 test genes in pancreatic tissues was evaluated at various time points after CVB4-V infection of BALB/c and IL-10 KO mice. Genes involved in inflammatory responses included Toll-like receptors (TLRs), CC chemokines, CXC chemokines, and markers expressed by macrophages, neutrophils, and natural killer (NK) cells. Genes involved in T cell responses included T-cell associated cytokines and transcription factors for TH17, TH1, TH2, and T reg cells. Differences in gene expression during the early infectious phase of disease have already been reported (Gu et al., 2009). The present study focuses on differences in gene expression during the later phase of disease, just prior to the development of chronic pancreatitis or to the resolution of acute disease.
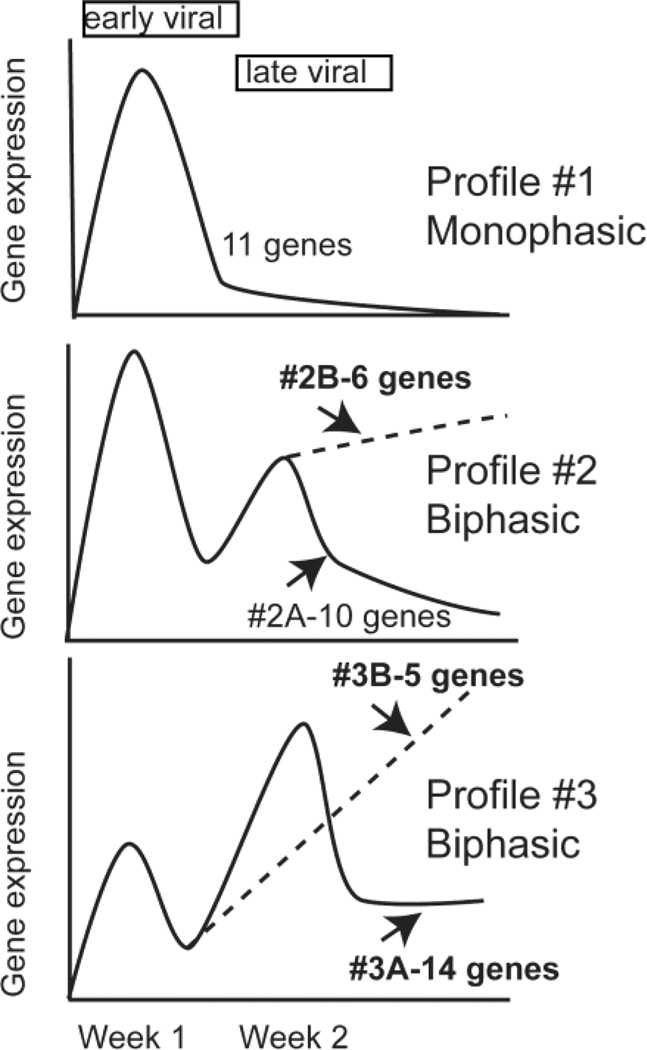
Both the magnitude and the pattern of gene expression differed between CVB4-V-infected-BALB/c and IL-10 KO mice during the later phase of disease. All 46 test genes were expressed at higher levels in BALB/c mice than in IL-10 KO mice. Profiles of the 46 genes fell into three general categories (Figure 1). Profile #1 shows a monophasic pattern with peak expression occurring during the early viral phase of disease. Profile #2 shows a biphasic pattern with higher expression during the early viral phase of disease. There were two variations of profile #2. Profile #2A shows decreasing gene expression during the late viral phase of disease (between 10 dpi and 14 dpi), while profile #2B shows increasing gene expression during the same time interval. Profile #3 also shows a biphasic pattern but with higher expression during the late viral phase of disease. Profile #3 again had two variations. Profile #3A shows decreasing gene expression between 10 dpi and 14 dpi while profile #3B shows increasing gene expression during the same time interval. Profiles #1 and #2A were common to infected BALB/c and IL-10 KO mice while profiles #2B, #3A, and #3B were unique to infected BALB/c mice. Because the latter three profiles were specific to mice that progressed to chronic inflammatory disease, we identified the 25 genes displaying these profiles as contributing to the development of chronic pancreatitis (Table 1). The profiles of two of these genes (Cxcl10 and Tnfa) have been previously reported (Gu et al., 2009).
FIG. 1.
Patterns of gene expression profiles after CVB4-V infection of BALB/c and IL-10 KO mice. Three general patterns of gene expression (#1, #2, #3) were identified in the transcriptional profiling studies. Genes displaying profiles #2B, #3A, and #3B were unique to CVB4-V-infected BALB/c mice and were identified as correlates of disease progression.
Table 1.
Genes that comprise a genetic signature of pathogenic immune responses associated with the development of CVB4-V-induced chronic pancreatitis.
| GENE | FUNCTION |
|---|---|
| Toll-like receptors | |
| Tlr2 | Cell surface expression. Binds lipopeptides |
| Tlr4 | Cell surface expression. Binds LPS |
| Tlr5 | Cell surface expression. Binds proteins |
| Tlr7 | Endosomal expression. Binds ssRNA |
| Tmed1/st2l | Modulates innate immunity by inhibiting TLR signaling |
| TH17 responses | |
| Il6ra | IL-6 receptor subunit |
| Il6st/gp130 | IL-6 receptor subunit |
| Tgfb1 | TGF-β and IL-6 promote TH17 responses |
| Il17rb | IL-17 receptor |
| Il8ra | Receptor for IL-8/CXCL8 expressed on neutrophils |
| Tnfa | Proinflammatory cytokine produced by macrophages and DCs |
| Iltifb/Il22b | TH17 cytokine |
| RORγt | Transcription factor for TH17 cells |
| TH1 responses | |
| Socs5 | Inhibits IL-4 signaling. Promotes TH1 responses |
| Ebi3/IL-27B | Subunit of IL-27. Promotes TH1 responses |
| Ccl3/Mip1a | Recruits activated T cells, NK, NKT, and macrophages. Promotes TH1 responses |
| Ccr5 | Receptor for CCL3, CCL4, and CCL5. Promotes TH1 responses |
| Ifngr1 | Receptor for IFN-γ |
| Ifng | TH1 cytokine |
| Cxcr3 | Receptor for CXCL9, CXCL10, and CXCL11. Promotes TH1 responses |
| Cxcl10/IP10 | Chemotactic for T cells and monocytes |
| Tbet/Tbx21 | Transcription factor for TH1 cells |
| Other | |
| Gata3 | Transcription factor for TH2 cells |
| Socs3 | Involved in multiple signaling pathways. Inhibits JAK-STAT pathways |
| Il10 | Regulatory cytokine. Generally considered anti-inflammatory |
Markers of TLRs, TH17, and TH1 responses are associated with the development of chronic pancreatitis
The genetic signature associated with the development of chronic pancreatitis in BALB/c mice includes 6 genes exhibiting profile #2B, 14 genes exhibiting profile #3A, and 5 genes exhibiting profile #3B. Eleven genes exhibited profile #2B or #3B which is characterized by increasing expression during the second week of infection when viral titers were diminishing (Table 2). High expression of the group of 11 genes correlates with progression to chronic pancreatitis.
Table 2.
Identification of putative therapeutic targets based on sustained gene expression during the late phase of disease. Genes encoding secreted molecules are in italics.
| Expression Profile |
Genes encoding |
||
|---|---|---|---|
| Secreted molecules |
Transcription factors |
Receptors/ signaling |
|
| 2B | Tnfa | Gata3 | Socs3 |
| Ifng | |||
| Cxcl10 | |||
| Il10 | |||
| 3B | Il22b | Tbet | Tlr2 |
| RORgt | Cxcr3 | ||
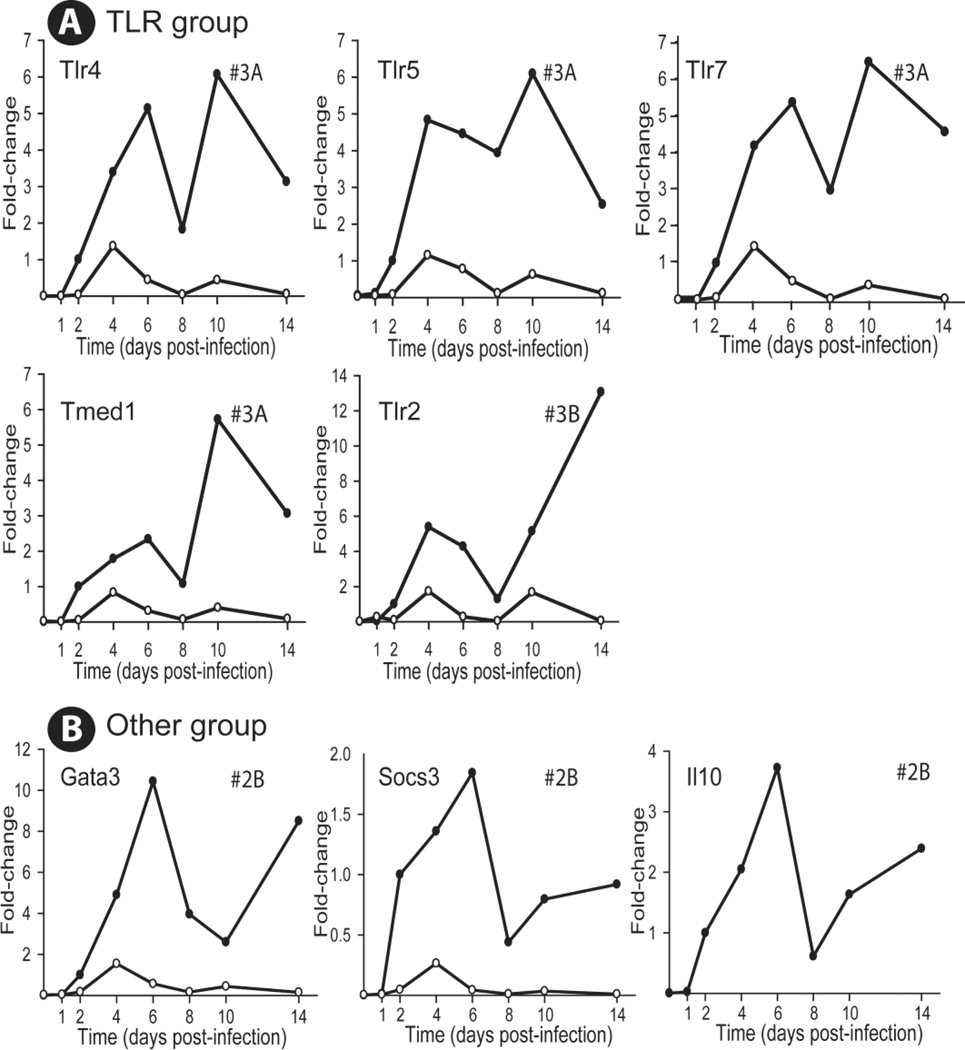
In IL-10 KO mice, all expression profiles returned to baseline 14 days after infection. The 25 genes were sorted into three functional groups; one group of genes encodes TLRs, another group encodes markers of TH17 responses while a third group encodes markers of TH1 responses. A miscellaneous group contains three genes. The TLR group contains 5 genes, Tlr2, Tlr4, Tlr5, Tlr7, and Tmed1, all of which exhibited profile #3A except Tlr2 which displayed profile #3B (Figure 2A). Three genes in the “other” category, gata3, socs3, and il10, all displayed profile #2B (Figure 2B).
FIG. 2.
Relative change in expression profiles of functional groupings of genes after CVB4-V infection of BALB/c and IL-10 KO mice. A. TLR related grouping; B. A miscellaneous grouping consisting of three genes, Gata3, Socs3, and Il10. Three-to-five mice per strain were analyzed at each time point and gene expression in the pancreas was quantified using the TaqMan Gene Expression Assay in a two-step RT-PCR and a low-density array. The Ct value of each test gene was normalized to an endogenous control, egf, and the [delta] Ct values for samples at each time point were averaged. The change in gene expression, for both BALB/c and IL-10 KO mice, is represented as a fold-change relative to the expression level of a reference group which is BALB/c mice at 2dpi. As a result, the relative expression of a gene in BALB/c mice at 2dpi is 1. Closed circles, BALB/c mice; open circles, IL-10 KO mice.
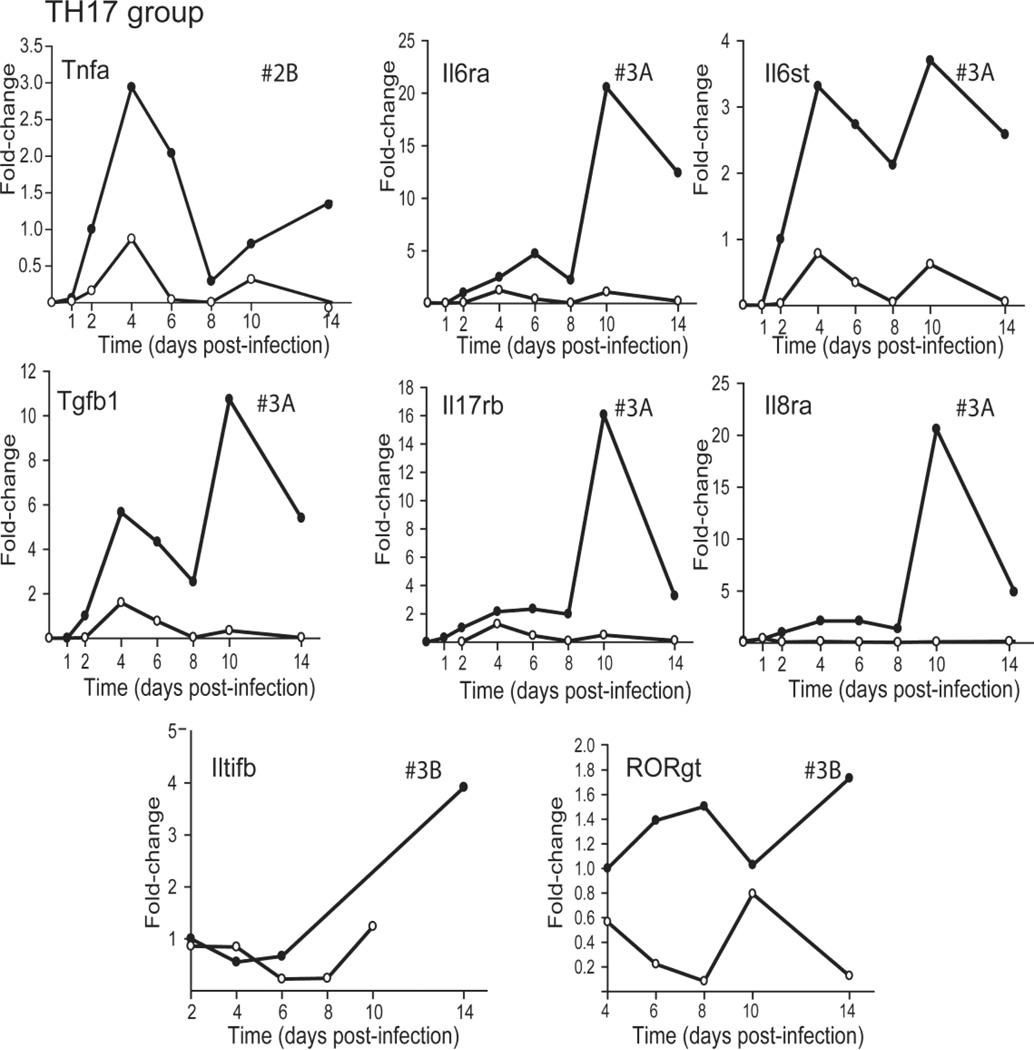
The TH17 group contains 8 genes exhibiting three different expression profiles. One gene, Tnfa, displayed profile #2B, while 5 genes, Il6ra, Il6st, Tgfb1, Il17rb, and Il8ra, exhibited profile #3A and two, Iltifb/Il22b and RORγt, showed profile #3B (Figure 3). The sustained presence of TH17 cells in the pancreatic infiltrates of BALB/c mice, as viral titers decreased, is inferred from the expression of (a) RORγt (a transcription factor for TH17 cells), the IL-6 receptor, and IL-22B; (b) an IL-17 receptor, which is expressed on a variety of cells involved in proinflammatory responses; and (c) the IL-8 receptor, which is expressed on neutrophils.
FIG. 3.
Relative change in expression profiles of TH17 related genes after CVB4-V infection of BALB/c and IL-10 KO mice. Three-to-five mice per strain were analyzed at each time point and gene expression in the pancreas was quantified using the TaqMan Gene Expression Assay in a two-step RT-PCR and a low-density array. The Ct value of each test gene was normalized to an endogenous control, egf, and the [delta] Ct values for samples at each time point were averaged. The change in gene expression is represented as a fold-change relative to the expression level of a reference group. For all genes except RORγt, the reference group is BALB/c at 2dpi. For RORγt, the reference group is BALB/c at 4 dpi. As a result, the relative expression of a gene, except for RORγt, in BALB/c mice at 2dpi is 1. The relative expression of RORγt in BALB/c mice at 4dpi is 1. Closed circles, BALB/c mice; open circles, IL-10 KO mice.
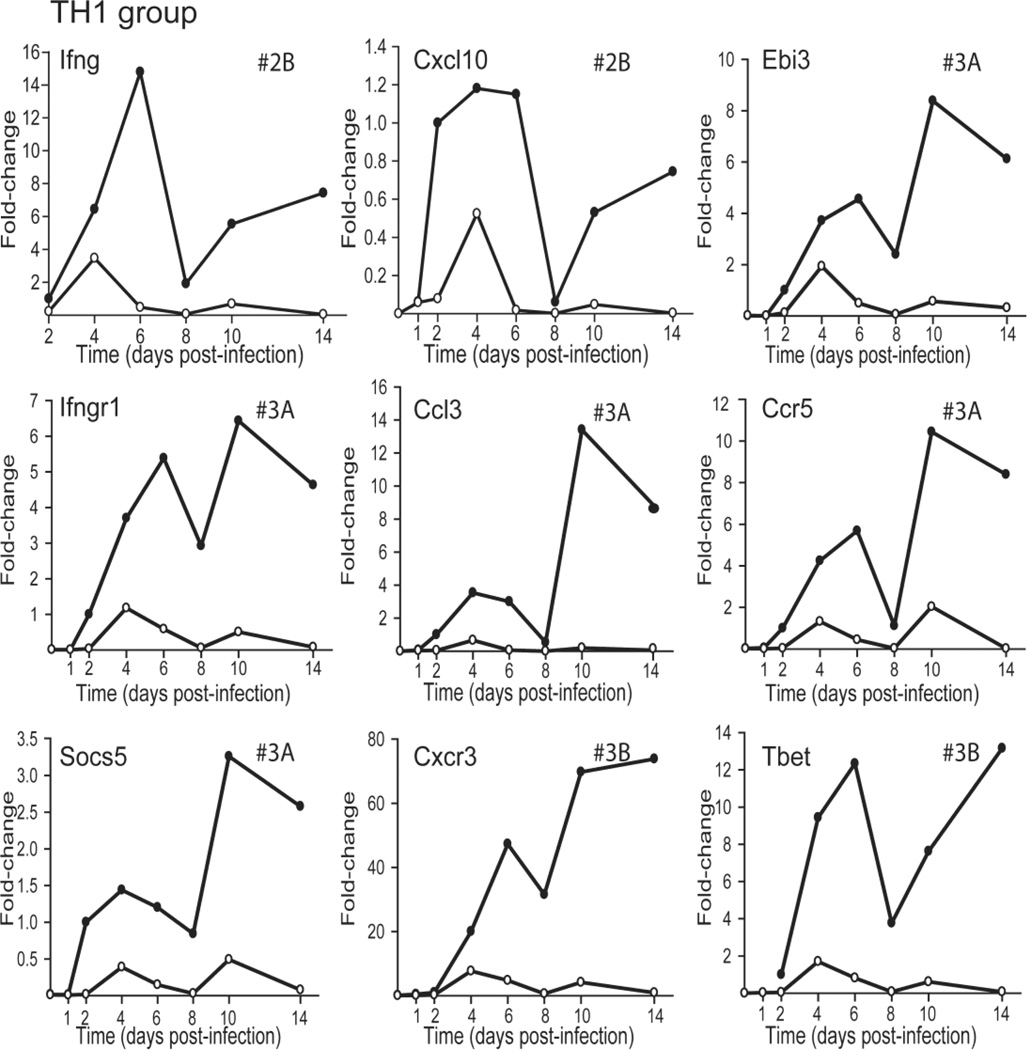
The TH1 group contains 5 genes, Socs5, Ebi3/IL-27B, Ccl3, Ccr5, and Ifngr1, which exhibited profile #3A while two, Cxcr3 and T-bet showed profile #3B (Figure 4). The sustained presence of TH1 cells in the pancreatic infiltrate as viral titers decreased is inferred from the expression of (a) T-bet (a transcription factor for TH1 cells), and the IFN-γ receptor; (b) CCL3, which recruits activated T cells and macrophages; and (c) CCR5 and CXCR3, which are expressed on activated T cells and macrophages.
FIG. 4.
Relative change in expression profiles of TH1 related genes after CVB4-V infection of BALB/c and IL-10 KO mice. Three-to-five mice per strain were analyzed at each time point and gene expression in the pancreas was quantified using the TaqMan Gene Expression Assay in a two-step RTPCR and a low-density array. The Ct value of each test gene was normalized to an endogenous control, egf, and the [delta] Ct values for samples at each time point were averaged. The change in gene expression is represented as a fold-change relative to the expression level of a reference group, CVB4-V-infected BALB/c at 2dpi. As a result, the relative expression of a gene in BALB/c mice at 2dpi is 1. Closed circles, BALB/c mice; open circles, IL-10 KO mice.
Measurement of cytokines in pancreatic tissues of CVB4-V-infected mice
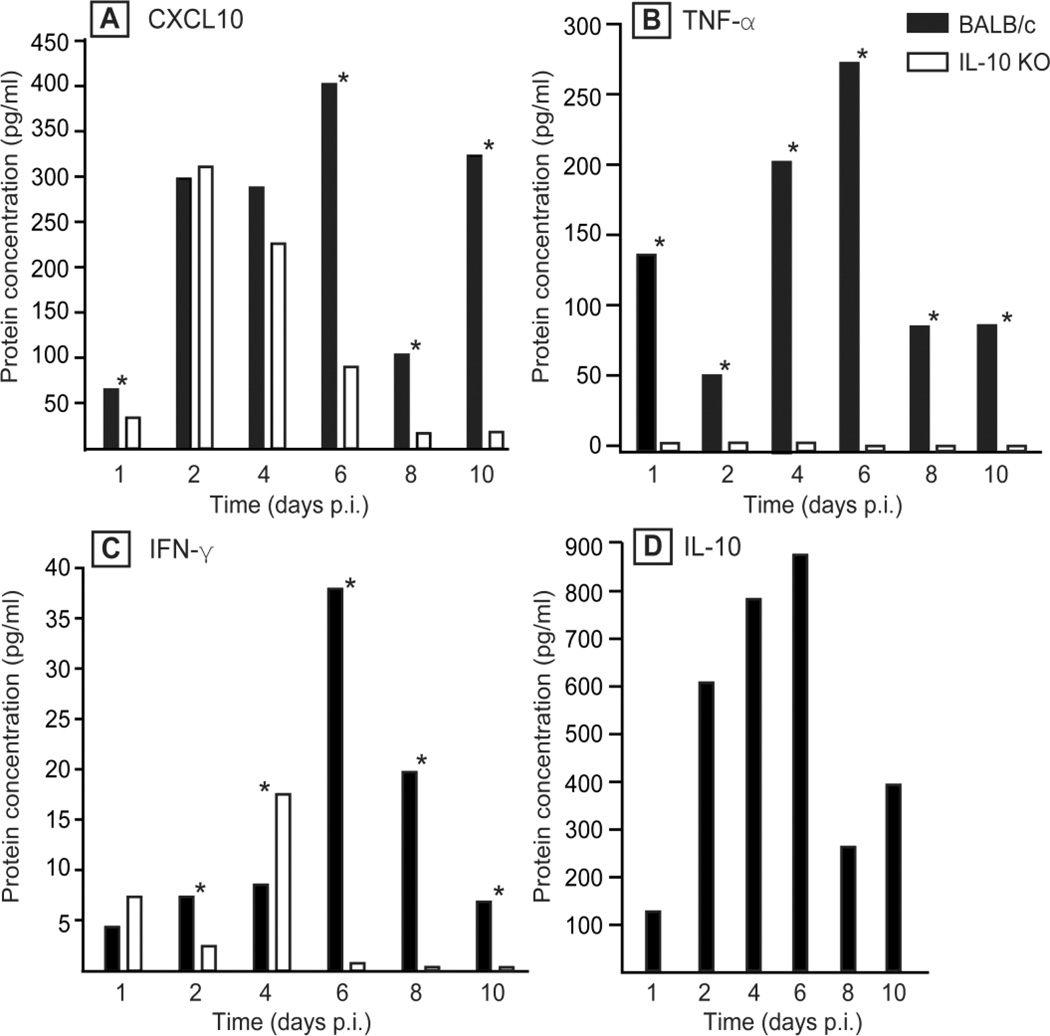
To confirm the gene array data, we measured the protein concentration of four secreted molecules (CXCL10, TNF-α, IFN-γ, and IL-10), in pancreatic tissues of CVB4-V-infected BALB/c mice or IL-10 KO mice (Fig. 5). The overall kinetics of protein expression was similar to the kinetics of RNA expression. However, while both the protein and RNA profiles for TNF-α showed higher expression in BALB/c than in IL-10 KO mice throughout the infection (Fig. 5B), protein and RNA profiles for CXCL10 and IFN-γ showed some differences. For example, the RNA data indicate that CXCL10 expression was higher in BALB/c mice throughout the infection while the protein data shows that CXCL10 expression was higher in BALB/c mice except at 2 and 4 dpi when expression levels were similar between the two strains of mice (Fig. 5A). Similarly, the RNA data indicate that IFN-γ expression was higher in BALB/c throughout the infection. The protein data shows that IFN-γ expression was higher in BALB/c mice at 2, 6, 8, and 10 dpi (Fig. 5C). However at 4 dpi, IFN-γ expression was higher in IL-10 KO mice.
FIG. 5.
Kinetics of expression of four cytokines, CXCL10, TNF-α, IFN-γ, and IL-10, in the pancreas of CVB4-V-infected BALB/c or IL-10 KO mice. Three-to-five mice per strain were analyzed at each time point and the amount of cytokines/chemokines present in pancreatic homogenates was measured using a Luminex assay. Mean values are shown. The overall kinetics of protein expression in both strains of mice is similar to that of the corresponding RNA. Statistical analysis was done using the Mann-Whitney U test. Statistically significant differences (P < 0.05) in protein expression between the two mouse strains are indicated by asterisks.
Immunomodulation using anti-cytokine antibodies
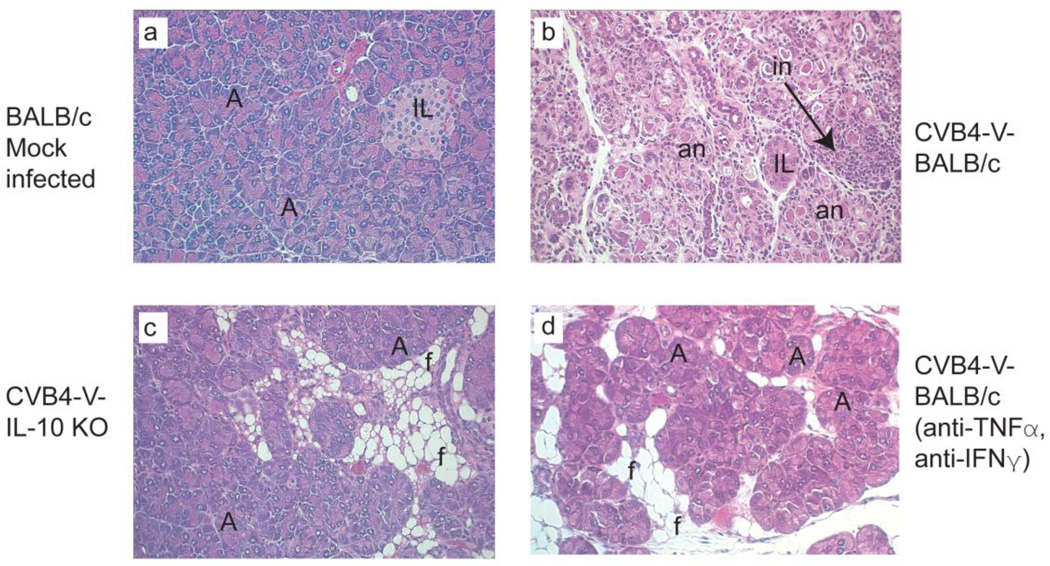
To determine whether the systems approach had uncovered relevant therapeutic targets, we undertook a pilot study to down-regulate identified molecules. We targeted secreted molecules (Table 2) because these can be down-modulated using specific antibodies. An extensive analysis of all of the identified targets was beyond the scope of the present study. Of the five secreted molecules, we targeted two cytokines, TNF-α and IFN-γ, because of their pleiotropic effects. Mice were treated, from eight to fourteen days after CVB4-V infection, with a combination of anti-TNF-α and anti-IFN-γ antibodies. Body weight and pathology were used as indicators to monitor the course of disease. At the end of the four-week follow-up period, the overall weight gain in the dual antibody treated group was 20% compared to 5% in the untreated group. The pathology results corroborate the body weight data. Pancreatic tissues from controls showed extensive destruction characterized by fat replacement of destroyed acini, acinar cell necrosis, acinoductular metaplasia, rare foci of normal acini, and inflammation (Fig. 6b). Although some exocrine tissue loss was observed in mice treated with anti-TNF-α and anti-IFN-γ, all five mice showed multiple foci of normal acini (Fig. 6d), similar to the overall histology observed in IL-10 KO mice (Fig. 6c).
FIG. 6.
Simultaneous administration of anti-TNF-α and anti-IFN-γ antibodies had a beneficial effect on chronic pancreatitis. Panels a-d depict representative hematoxlyin and eosin-stained pancreatic sections from CVB4-V infected mice. a. Normal pancreatic tissue from mock-infected BALB/c mice. b. Pancreatic tissue from CVB4-V infected BALB/c mice depicting chronic pancreatitis characterized by exocrine tissue destruction and inflammation. c. Pancreatic tissue from CVB4-V infected IL-10 KO showing disease resolution. Exocrine tissues are largely intact and inflammation is absent. d. Pancreatic tissue from CVB4-V-infected BALB/c mice treated with anti-TNF-α and anti-IFN-γ antibodies. Multiple foci of normal acini are present. A, acinus; IL, islet of Langerhans; f, fat replacement; an, acinar cell necrosis; in, inflammatory infiltrates. Magnification, X250.
DISCUSSION
In this study, the data establish a temporal pattern of molecular immune responses that differentiate the resolution of acute pancreatitis from the progression to chronic pancreatitis. We identified a group of twenty-five immune-related genes (Table 1) with characteristic expression profiles that were observed during the development of chronic pancreatitis but not during the resolution of disease. We postulate that the temporal dynamics of the group of twenty-five genes contribute to the development of pathogenic immune responses associated with chronic pancreatitis. Furthermore, a subset of eleven genes (Table 2) exhibited increased expression as viral titers waned. Of the eleven gene products, five are secreted molecules, TNF-α, IFN-γ, CXCL10, IL-10, and IL-22b, and represent potential therapeutic targets since they can be readily modulated with antibodies against the specific cytokine/chemokine or with antibodies against the corresponding receptors. In a pilot study, we showed that treatment of CVB4-V-infected mice with both anti-IFN-γ and anti-TNF-α antibodies proved beneficial since treated mice gained weight and showed multiple foci of normal acini in the pancreas. The data provide support for the therapeutic potential of antibodies against the identified cytokines/chemokines.
The molecular immune responses that differentiate the resolution of acute pancreatitis from the progression to chronic pancreatitis include TLR, TH17, and TH1 responses (Table 1). TLR signaling via MyD88 can activate NF-κB, leading to a proinflammatory response due to transcription of numerous genes, including those encoding cytokines and chemokines (Liu & Malik, 2006). The genetic signature associated with the development of chronic pancreatitis contains several genes that are NF-κB dependent, including Tnfa, Cxcl10, and Ccl3/Mip1a. NF-κB activation is also inferred by the expression of cytokine and chemokine receptors, IL-6ra/st and CCR5. While TLR4 and TLR7 have been identified by others (Triantafilou et al., 2005) as initiating the inflammatory response during CVB infection, the present report also links TLR2 and TLR5 to CVB infection. Increased expression of TLR2, TLR4, and TLR5 indicates either that CVB4 virions bind to these surface receptors or else that up-regulation of the receptors is a result of the host’s response to infection. The prolonged presence of TH17 cells in the pancreas, during progression to chronic disease, is inferred from the high and sustained expression of Il22b/Iltifb and the transcription factor, RORγt. High expression of the IL-6 receptor, TGF-β, the IL-17 receptor (IL-17RB), and IL-8RA provides further evidence of a TH17 response in the pancreas. In addition, the prolonged presence of TH1 cells in the pancreas during progression to chronic disease is inferred by the high and sustained expression of Ifng, Ifngr1, Socs5, and the transcription factor, Tbet (Table 1). Further evidence of a TH1 response is provided by the high levels of expression of chemokines, Ccl3 and Cxcl10, and chemokine receptors, Ccr5 and Cxcr3 (Table 1).
In a well-regulated response to infection, innate immune responses (TLR activation, macrophage and neutrophil recruitment, NK and NKT activation) control the early stage of infection, until adaptive immune responses develop. As the infection is cleared, adaptive immune responses subside. The prolonged high expression of TLR, TH17, and TH1 markers in CVB4-V-infected BALB/c mice, during the period of viral clearance, indicates that the temporal sequence of immune responses was disrupted. The aberrant gene expression profiles were observed only in BALB/c mice which progress to chronic pancreatitis and not in IL-10 KO mice which resolve acute disease. In IL-10 KO mice, the expression of TLR, TH17, and TH1 markers returned to baseline levels during the late viral phase of disease, indicating a well-regulated immune response to CVB4-V infection. The data suggest that as virus is cleared, innate and adaptive immune responses remain in the “on” position in CVB4-V-infected BALB/c mice and are switched off in CVB4-V-infected IL-10 KO mice.
Of the five potential therapeutic targets identified in this study, IFN-γ appears to have a dual role in CVB4-V-induced pancreatitis. During the acute stage of CVB4-V-induced pancreatitis, the IFN-γ response in BALB/c mice is delayed, with peak RNA and protein expression at 6 dpi compared to a peak response at 4 dpi in IL-10 KO mice. We have previously shown that early high expression of IL-10 correlates with delays in overall innate and T cell responses (Gu et al., 2009). Early treatment with IFN-γ attenuates the severity of CVB4-V-induced acute pancreatitis in BALB/c mice (Potvin et al., 2003); presumably by overcoming the delays in ensuing immune responses. In the present work, as viral titers diminished, sustained IFN-γ correlated with progression to chronic inflammatory disease and down-modulation of IFN-γ, later in the disease, was beneficial. The combined data highlights the need for greater understanding of the pathogenesis of chronic pancreatitis and the challenges in designing immunomodulatory approaches since a cytokine that is beneficial at one stage of disease may be detrimental at a later stage.
In this study, IL-10 was also identified as a potential therapeutic target. This is a surprising result since IL-10 is generally considered to be an anti-inflammatory cytokine (Pestka et al., 2004; Couper et al., 2008). We have previously shown that early high production of IL-10 correlates with altered kinetics of regulatory T cell responses indicative of a disruption in the balance between effector and regulatory T cell responses (Gu et al., 2009). The association of high IL-10 with the progression to chronic disease may reflect the ongoing disruption between effector and regulatory T cell responses. We have previously shown that down-regulation of IL-10 early in CVB4-V infection is beneficial (Gu et al., 2009). Future studies will determine whether down-regulation of IL-10 later in infection alters the course of chronic disease.
A systems biology approach allowed us to identify a genetic signature of immune responses that correlated with disease progression and to uncover five potential therapeutic cytokine targets for the treatment of chronic pancreatitis. The data also highlight the complexity of the disease process that ultimately results in chronic pancreatitis and the need for caution in designing immunomodulatory approaches for the treatment of chronic pancreatitis.
Highlights.
Expression profiles of 25 genes correlate with progression to chronic pancreatitis
The 25 genes encode TLRs and markers of TH17 and TH1 responses
Eleven genes exhibited increased expression as viral titers waned
Five gene products were identified as novel potential therapeutic targets
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant AI066938 from the NIH/NIAID and by research funding from Centocor Inc. (Malvern, PA). The technical assistance of Xiaoyan Huang was greatly appreciated. We thank Helen Johnson (Zoonotic Disease and Clinical Virology, Wadsworth Center) for processing tissue samples for histology. We also acknowledge the secretarial assistance provided by Sandra Lionarons and the administrative assistance provided by Elizabeth Cavosie.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bhatia M. Apoptosis of pancreatic acinar cells in acute pancreatitis: is it good or bad? J.Cell Mol.Med. 2004;8:402–409. doi: 10.1111/j.1582-4934.2004.tb00330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggana M, Chan P, Ramsingh A. Identification of a single amino acid residue in the capsid protein VP1 of coxsackievirus B4 that determines the virulent phenotype. J.Virol. 1993;67:4797–4803. doi: 10.1128/jvi.67.8.4797-4803.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman NM, Ramsingh AI, Tracy S. Genetics of coxsackievirus virulence. Curr.Top.Microbiol.Immunol. 1997;223:227–258. doi: 10.1007/978-3-642-60687-8_11. [DOI] [PubMed] [Google Scholar]
- Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J.Immunol. 2008;180:5771–5777. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- Gu R, Shampang A, Reilly A, Fisher D, Glass W, Ramsingh AI. IL-10 is pathogenic during the development of coxsackievirus B4-induced chronic pancreatitis. Virology. 2009;395:77–86. doi: 10.1016/j.virol.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke A, Zell R, Ehrlich G, Stelzner A. Expression of immunoregulatory cytokines by recombinant coxsackievirus B3 variants confers protection against virus-caused myocarditis. J.Virol. 2001;75:8187–8194. doi: 10.1128/JVI.75.17.8187-8194.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz MS, La CA, Fine C, Rodriguez E, Ilic A, Sarvetnick N. Pancreatic expression of interferon-gamma protects mice from lethal coxsackievirus B3 infection and subsequent myocarditis. Nat.Med. 2000;6:693–697. doi: 10.1038/76277. [DOI] [PubMed] [Google Scholar]
- Huber S, Ramsingh AI. Coxsackievirus-induced Pancreatitis. Viral Immunology. 2004;17:358–369. doi: 10.1089/vim.2004.17.358. [DOI] [PubMed] [Google Scholar]
- Kingsnorth A, O'Reilly D. Acute pancreatitis. BMJ. 2006;332:1072–1076. doi: 10.1136/bmj.332.7549.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SF, Malik AB. NF-kappa B activation as a pathological mechanism of septic shock and inflammation. Am.J.Physiol Lung Cell Mol.Physiol. 2006;290:L622–L645. doi: 10.1152/ajplung.00477.2005. [DOI] [PubMed] [Google Scholar]
- Mergener K, Baillie J. Chronic pancreatitis. Lancet. 1997;350:1379–1385. doi: 10.1016/S0140-6736(97)07332-7. [DOI] [PubMed] [Google Scholar]
- Ostrowski SE, Reilly AA, Collins DN, Ramsingh AI. Progression or Resolution of Coxsackievirus B4-Induced Pancreatitis - A Genomic Analysis. J.Virol. 2004;78:8229–8237. doi: 10.1128/JVI.78.15.8229-8237.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka S, Krause CD, Sarkar D, Walter MR, Shi Y, Fisher PB. Interleukin-10 and related cytokines and receptors. Annu.Rev.Immunol. 2004;22:929–979. doi: 10.1146/annurev.immunol.22.012703.104622. [DOI] [PubMed] [Google Scholar]
- Potvin DM, Metzger DW, Lee WT, Collins DN, Ramsingh AI. Exogenous interleukin- 12 protects against lethal infection with coxsackievirus B4. Journal of Virology. 2003;77:8272–8279. doi: 10.1128/JVI.77.15.8272-8279.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsingh A, Slack J, Silkworth J, Hixson A. Severity of disease induced by a pancreatropic Coxsackie B4 virus correlates with the H-2Kq locus of the major histocompatibility complex. Virus Res. 1989;14:347–358. doi: 10.1016/0168-1702(89)90027-0. [DOI] [PubMed] [Google Scholar]
- Ramsingh AI. CVB-induced pancreatitis and alterations in gene expression. Curr.Top.Microbiol.Immunol. 2008;323:241–258. doi: 10.1007/978-3-540-75546-3_11. [DOI] [PubMed] [Google Scholar]
- Ramsingh AI, Lee WT, Collins DN, Armstrong LE. Differential recruitment of B and T cells in coxsackievirus B4-induced pancreatitis is influenced by a capsid protein. J.Virol. 1997;71:8690–8697. doi: 10.1128/jvi.71.11.8690-8697.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsingh AI, Lee WT, Collins DN, Armstrong LE. T cells contribute to disease severity during coxsackievirus B4 infection. J.Virol. 1999;73:3080–3086. doi: 10.1128/jvi.73.4.3080-3086.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens T, Conwell DL, Zuccaro G. Pathogenesis of chronic pancreatitis: an evidencebased review of past theories and recent developments. Am.J.Gastroenterol. 2004;99:2256–2270. doi: 10.1111/j.1572-0241.2004.40694.x. [DOI] [PubMed] [Google Scholar]
- Szalay G, Sauter M, Hald J, Weinzierl A, Kandolf R, Klingel K. Sustained nitric oxide synthesis contributes to immunopathology in ongoing myocarditis attributable to interleukin-10 disorders. Am.J.Pathol. 2006;169:2085–2093. doi: 10.2353/ajpath.2006.060350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy S, Gauntt C. Group B coxsackievirus virulence. Curr.Top.Microbiol.Immunol. 2008;323:49–63. doi: 10.1007/978-3-540-75546-3_3. [DOI] [PubMed] [Google Scholar]
- Triantafilou K, Orthopoulos G, Vakakis E, Ahmed MA, Golenbock DT, Lepper PM, Triantafilou M. Human cardiac inflammatory responses triggered by Coxsackie B viruses are mainly Toll-like receptor (TLR) 8-dependent. Cell Microbiol. 2005;7:1117–1126. doi: 10.1111/j.1462-5822.2005.00537.x. [DOI] [PubMed] [Google Scholar]
- Whitcomb DC. Clinical practice. Acute pancreatitis. N.Engl.J.Med. 2006;354:2142–2150. doi: 10.1056/NEJMcp054958. [DOI] [PubMed] [Google Scholar]