Abstract
Of the major vertebrate taxa, Class Aves is the most extensively studied in relation to the evolution of social systems and behavior, largely because birds exhibit an incomparable balance of tractability, diversity, and cognitive complexity. In addition, like humans, most bird species are socially monogamous, exhibit biparental care, and conduct most of their social interactions through auditory and visual modalities. These qualities make birds attractive as research subjects, and also make them valuable for comparative studies of neuroendocrine mechanisms. This value has become increasingly apparent as more and more evidence shows that social behavior circuits of the basal forebrain and midbrain are deeply conserved (from an evolutionary perspective), and particularly similar in birds and mammals. Among the strongest similarities are the basic structures and functions of avian and mammalian nonapeptide systems, which include mesotocin (MT) and arginine vasotocin (VT) systems in birds, and the homologous oxytocin (OT) and vasopressin (VP) systems, respectively, in mammals. We here summarize these basic properties, and then describe a research program that has leveraged the social diversity of estrildid finches to gain insights into the nonapeptide mechanisms of grouping, a behavioral dimension that is not experimentally tractable in most other taxa. These studies have used five monogamous, biparental finch species that exhibit group sizes ranging from territorial male-female pairs to large flocks containing hundreds or thousands of birds. The results provide novel insights into the history of nonapeptide functions in amniote vertebrates, and yield remarkable clarity on the nonapeptide biology of dinosaurs and ancient mammals.
Keywords: arginine vasotocin, mesotocin, isotocin, arginine vasopressin, oxytocin, bed nucleus of the stria terminalis, paraventricular nucleus, hypothalamus, sociality, flocking, coloniality, territoriality, pair bonding, aggression, evolution, bird, finch
The nine amino acid neuropeptides, or “nonapeptides,” evolved more than 600 million years ago (reviews: Acher, 1972; Hoyle, 1998; Donaldson and Young, 2008), and amazingly, even neurosecrotory nonapeptide neurons had evolved by the time that the major bilaterian taxa diverged, as evidenced by the fact that annelid worms possess neurosecretory nonapeptide neurons that express the same micro-RNAs and transcription factors as do the neurosecretory magnocellular neurons of vertebrates (Tessmar-Raible et al., 2007). These include the magnocellular oxytocin (OT) and vasopressin (VP) neurons of the supraoptic and paraventicular nuclei in mammals (SON and PVN, respectively). Consistent with this finding, both vertebrate and invertebrate nonapeptides regulate fluid balance and egg-laying (Oumi et al., 1996; Fujino et al., 1999), and thus, even the OT regulation of parturition in humans, which is essentially a very delayed form of egg-laying, can be traced back to the nonapeptide mechanisms of egg-laying in the ancient bilaterians that gave rise to the major vertebrate and invertebrate clades.
Although speculative, it seems likely that these basic egg-laying functions are the foundation upon which most other social functions of the nonapeptides have evolved, while the response of nonapeptide neurons to osmotic stressors likely set the stage for the evolution of nonapeptide mechanisms that modulate the pituitary-adrenal axis and the central processes of anxiety and stress response. In fact, virtually all VP cell groups of the hypothalamus are sensitive to both psychological and metabolic stressors (Sharp et al., 1995; Wotjak et al., 1996; Briski and Brandt, 2000; Ho et al., 2010).
But a large question remains – have nonapeptide systems and their socially relevant functions evolved in the same ways across the different vertebrate classes, or even in closely related species? The answer to this question is relevant not only to basic biology, but also to translation, because we need to learn how to predict mechanisms of social regulation in humans from non-human species. But this requires that we come to grips with the extraordinary amount of convergent evolution that is evident in social systems. For instance, even among amniote taxa, mother-offspring interactions and extended maternal care have probably evolved independently in crocodilians, mammals, some lepidosaurs, and neognathan birds (the largest of two avian clades that includes fowl and passerines); and in fact, paternal care appears to be the ancestral condition for birds and closely related groups of theropod dinosaurs, such as oviraptors (Clutton-Brock, 1991; e.g., Ch. 7–8; Varricchio et al., 2008). Have the nonapeptide mechanisms of maternal care evolved in similar ways across these groups? In cases where paternal care is exhbited, have males evolved mechanisms similar to females? And of the greatest relevance here – have the nonapeptide mechanisms of same-sex affiliation and grouping evolved in convergent ways across vertebrates? This dimension of behavior is highly labile, and thus the patterns of sociality that we observe across species have likely been generated through many hundreds or thousands of independent evolutionary events. The same may be said for monogamous pair bonding, which has evolved independently in a small percentage of mammals and in most avian groups (Reichard, 2003). At present, the question of whether nonapeptide systems evolve in predictable ways in relation to affiliation, grouping and monogamy is still very much open to debate, and is a question that likely cannot be answered without capitalizing on the exceptional diversity of birds. Birds alone cannot provide all of the answers, but they are an essential piece of the puzzle.
In the sections that follow, we will first describe the basic nonapeptide circuitries of amniotes, particularly with respect to mammals and birds, and then explore the ways in which nonapeptide systems have evolved in relation to affiliation, grouping and territoriality.
Shared features of nonapeptide systems in birds and other amniote vertebrates
The vertebrate nonapeptides
The earliest vertebrates likely possessed only a single nonapeptide form, arginine vasotocin (VT), although the VT gene duplicated at about the same time that jaws evolved, and thus all jawed vertebrates now exhibit two nonapeptide forms in the brain – an OT-like form and either VT or a form of VP (either arginine VP or lysine VP in mammals) (review: Hoyle, 1999). The most common OT-like forms are isotocin, which is found in bony fish, and mesotocin (MT), which is found in birds, lungfish, reptiles, amphibians, and some marsupials. Cartilaginous fish have evolved at least six OT-like forms, including OT (reviews: Acher, 1972; Acher and Chauvert, 1995; Hoyle, 1998; 1999; Donaldson and Young, 2008).
Nonapeptide cell groups
Birds produce both MT and VT in magnocellular neurons located within the preoptic area (POA), SON, and PVN, and in other small cell groups that are primarily associated with the hypothalamo-hypophyseal tract (Fig. 1) (Bons, 1980; Berk et al., 1982; Mikami and Yamada, 1984; Kiss et al., 1987; Korf et al., 1988; Robinzon et al., 1988; Voorhuis and De Kloet, 1992; Aste et al., 1996; Balthazart et al., 1997; Barth et al., 1997; Panzica et al., 1999). Although some magnocellular neurons may have small projections to central targets, they primarily innervate the neurohypophysis (Mikami et al., 1978; Grossmann et al., 1995). VT and MT are also synthesized in parvocellular PVN neurons, which project to the median eminence (Mikami et al., 1978), and likely, as in other vertebrates, give rise to intrahypothalamic projections and descending projections to the midbrain and viscerosensory areas of the hindbrain, such as the nucleus of the solitary tract (De Vries and Buijs, 1983; Goodson et al., 2003; Thompson et al., 2008; Thompson and Walton, 2009). This basic anatomy appears to be shared across all jawed vertebrates, with the exception that the major parvocellular and magnocellular populations reside within the POA in fish and amphibians, not the SON and PVN (Moore and Lowry, 1998).
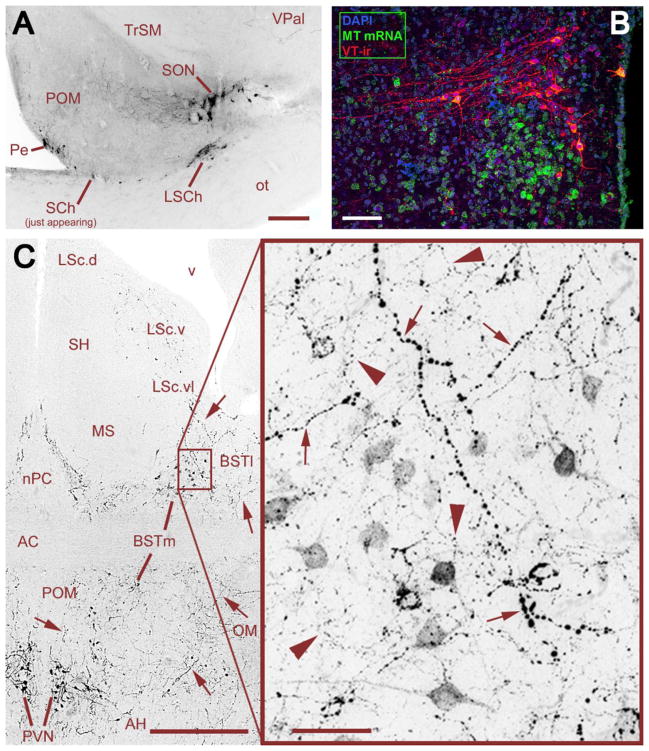
Fig. 1.
Distribution of VT-ir cell groups in estrildid finches. (A) The rostral-most cell groups in a female Angolan blue waxbill, showing VT-ir neurons in the periventricular POA (Pe), SON, suprachiasmatic nucleus (SCh), and lateral SCh (LSCh). This photo was taken rostral to the main body of cells in the SCh, thus just a few neurons are visible. Scale bar = 200 μm. (B) The PVN of a male zebra finch, showing the separation of VT and MT populations. Note that low levels of MT mRNA extend in the surrounding hypothalamus and are not restricted to the PVN. Scale bar = 50 μm. (C) VT-ir cells and fibers at the level of the anterior commissure (AC) in a male zebra finch, showing cell groups of the PVN and BSTm, and apparent overlapping projections from these cell groups in the BSTm and ventral LS. Large-caliber, heavily beaded axons (small arrows) are observed coursing from the PVN through the BSTm and directly into the ventrolateral zone of the caudal LS (LSc.vl). Relatively heavier projections are observed to the lateral BST (BSTl), but terminate immediately adjacent to the BSTm and LSc.vl. Within the BSTm (box), fine-caliber, beaded axons of local origin (large arrowheads) mix with the heavier axons of apparent PVN origin. Scale bars = 200 μm (left) and 20 μm (right). Other abbreviations: AC, anterior commissure; AH, anterior hypothalamus; LSc.d, dorsal zone of the LSc; LSc.v, ventral zone of the LSc; MS, medial septum; nPC, nucleus of the pallial commissure; OM, occipital-mesencephalic tract; POM, medial preoptic nucleus; SH, septohippocampal septum. Panel C modified from Goodson and Kabelik (2009).
Early tetrapods expanded upon this basic VT anatomy in two important ways – first, by adding a parvocellular VT cell group in the suprachiasmatic nucleus (SCN), and second, through the addition of an extrahypothalamic VT cell group in the medial bed nucleus of the stria terminalis (BSTm) (review: Moore and Lowry, 1998). Finally, with the arrival of amniotes, the major POA populations migrated into the PVN and SON of the hypothalamus, leaving a relatively smaller magnocellular population in the POA (reviews: Moore and Lowry, 1998; Goodson and Bass, 2001). In addition to these major cell groups, other small populations of VT/VP neurons may be found in any given species (Lowry et al., 1997; Moore and Lowry, 1998; Ho et al., 2010; Rood and De Vries, 2011). MT/OT anatomy has remained relatively less diversified, although low levels of MT-OT are expressed in a variety of extrahypothalamic sites (e.g., in amphibians and mice), perhaps in a species-specific manner (Chung et al., 1991; Gonzalez and Smeets, 1992). Too few data are available on this point to generalize, particularly in birds, for which extrahypothalamic MT mRNA has not yet been observed (and is absent in chickens) (Barth et al., 1997).
Nonapeptide fiber distributions
Both VT- and MT-immunoreactive (-ir) fibers are observed in a variety of basal forebrain and midbrain sites in birds, including the nucleus accumbens (see Husband and Shimizu, 2011, for anatomical characterization of this structure), BSTm, lateral BST (BSTl), medial amygdala (“taenial nucleus of the amygdala”), lateral septum (LS), habenula, periaqueductal gray (PAG) and ventral tegmental area. A dense fiber innervation is also observed for the medial preoptic nucleus (POM) and extensive portions of the anterior (AH), lateral and mediobasal hypothalamus. The latter includes the lateral portion of the ventromedial nucleus (VMH) and tuberomammillary areas (see references in preceding two paragraphs). These sites may receive projections from multiple VT cell groups, particularly the parvocellular PVN and the BSTm (Balthazart et al., 1997; Absil et al., 2002).
Regulation by sex steroids
Another conserved feature of vertebrate nonapeptide systems is their regulation by sex steroid hormones (reviews: Goodson and Bass, 2001; De Vries and Panzica, 2006). In the hypothalamus of birds, this regulation is fairly modest, and sex steroids appear to primarily influence VT mRNA and production within cells that are already producing VT (Seth et al., 2004). However, in numerous bird species the BSTm VT neurons completely disappear following castration or the seasonal transition to a nonreproductive state. This waxing and waning of VT cells in the BSTm is associated with similar changes in the VT-ir innervation of numerous brain areas, including the LS, POM, nucleus intercollicularis (homologous to the dorsal PAG of mammals; see Kingsbury et al., 2011), and the BSTm itself (reviews: Goodson and Bass, 2001; Panzica et al., 2001; De Vries and Panzica, 2006; also see Plumari et al., 2004). As in mammals, this circuitry is sexually dimorphic (m > f) and primarily regulated by estradiol (Panzica et al., 1998; Viglietti-Panzica et al., 2001). However, despite the similarities in the activational effects of estradiol in adults, the developmental effects of estradiol are feminizing in Japanese quail (Coturnix japonica), but masculinizing in rodents (review: De Vries and Panzica, 2006). This extreme sensitivity of VT circuitry to sex steroids means that environmental estrogens and other endocrine disruptors can have significant deleterious effects, particularly in development, as shown in a variety of studies in quail (Panzica et al., 2007; Ottinger et al., 2008).
Relatively fewer studies have focused on the BSTm cell group in species that are not strongly seasonal in their breeding, but recent experiments in the highly opportunistic zebra finch (Taeniopygia guttata) show that VT-ir cell number is not influenced by combined aromatase inhibition and androgen receptor blockade. However, hormonal manipulations do regulate constitutive Fos expression in VT-ir neurons of the BSTm, providing a potential mechanism for the rapid regulation of VT neurons by environmental stimuli (Kabelik et al., 2010b). A lack of seasonal variation in VT immunoreactivity is likewise observed for three other estrildid finch species that are highly opportunistic in their breeding, although the spice finch (Lonchura punctulata) shows strong variation in VT-ir cell number across seasons (Kabelik et al., 2010b). This may reflect the fact that, despite being highly flexible in their breeding (Immelmann, 1965), spice finches nonetheless exhibit pronounced endogenous rhythms of reproductive physiology that correlate with monsoon cyclicity, and unlike zebra finches, they become photorefractory (Goodwin, 1982; Chaturvedi and Prasad, 1991; Sikdar et al., 1992; Hahn et al., 2008).
Nonapeptide receptors
The behavioral and physiological effects of avian nonapeptides are mediated by a suite of four receptor types (VT1-VT4) that show strong sequence similarities to those of mammals and other vertebrates, although most have not been well characterized in terms of ligand sensitivities (Baeyens and Cornett, 2006; Cornett et al., 2007).
A particularly intriguing receptor is the avian VT1, which exhibits substantial sequence identity to the mammalian V2 receptor (Leung et al., 2011). However, unlike the V2, the VT1 is expressed in the brain (Tan et al., 2000; Leung et al., 2011) and has key amino acid residues that may confer V1a-like ligand sensitivities (Acharjee et al., 2004). This receptor is also expressed in the oviduct uterus (shell gland) (Tan et al., 2000). The different tissue distributions of the avian VT1 and mammalian V2 may relate to the recent discovery that ancestral jawed vertebrates expressed two V2 receptors, V2A and V2B. These are present in all fishes genomes examined, and whereas the V2A is orthologous to the mammalian V2, the V2B is orthologous to the avian VT1 (Ocampo Daza et al., 2012).
The VT2 is a clear homologue of the V1b receptor and is highly expressed in the pituitary but not the brain. The VT2 also shows osmoregulatory properties (Cornett et al., 2003; Jurkevich et al., 2005; Jurkevich et al., 2008; Sharma and Chaturvedi, 2009; Sharma et al., 2009). The VT3 is an oxytocic receptor that is expressed in the brain and also the oviduct uterus, where it is regulated by estrogens and influences oviposition (Srivastava et al., 2007; 2008; Srivastava and Chaturvedi, 2010; Srivastava et al., 2010). Like the mammalian OT receptor, VT3 is at least somewhat promiscuous (i.e., binds VT as well as MT), and as described in the next section, it mediates some of the peripheral effects of VT. Finally, the VT4 is a V1a-like receptor that is expressed in the brain (Cornett et al., 2007; Leung et al., 2011). Based on autoradiography studies, it is clear that both V1a and OT-like binding sites are present in the avian brain, and as is typical of mammals, the distributions of these binding sites are highly species-specific (Goodson et al., 2006; Goodson et al., 2009d; Leung et al., 2009).
Nonapeptide mechanisms of maternal physiology and offspring care
For many vertebrates, maternal contributions to offspring consist of little more than laying an egg in a good place. This is the case for most extant reptiles and amphibians, and appears to have been the case for most dinosaurs, as well. The extensive care that is provided by most avian and mammalian mothers is therefore the product of independent evolutionary processes (see Clutton-Brock, 1991; e.g., Ch. 7–8), although this behavior evolved against the backdrop of several pre-existing nonapeptide functions. These include 1) a deeply conserved involvement of nonapeptides in social communication and social approach behaviors, which is known for teleost fish and all tetrapod vertebrates (cartilaginous fish have not been examined), and 2) the regulation of uterine contractions by nonapeptide receptors, which results in the ejection of either eggs or live offspring (Goodson and Bass, 2001; Donaldson and Young, 2008; Goodson, 2008; Thompson et al., 2008; Thompson and Walton, 2009).
In nonmammals, VT is the primary regulator of egg-laying and associated species-specific behaviors, such as bearing down in birds (Takahashi and Kawashima, 2003) and tree climbing in reptiles (Guillette and Jones, 1982). In birds, these effects are mediated by both promiscuous oxytocic receptors (VT3) in addition to more selective VT receptors, and both receptor types show increased binding capacity at the time of oviposition (Takahashi and Kawashima, 2008). The role, if any, of MT in the egg-laying process has remained obscure until recently, when it was discovered that in White Leghorn hens, MT increases in the plasma just prior to oviposition and just prior to a rise in plasma VT, and that this spike in MT increases the receptor binding capacity for VT (Takahashi and Kawashima, 2008). Thus, egg laying is likely coordinated by complex interactions of both VT and MT.
In mammals, both VP and OT play important roles in maternal care (Pedersen et al., 1982; Bosch and Neumann, 2008), but the possibility of convergent functions in birds has only recently been addressed, with the findings that 1) brooding of poults by turkey hens is abolished by intraventricular infusions of an OT receptor antagonist, and 2) this occurs in association with elevated c-fos expression in MT neurons of the PVN and ventral SON (Thayananuphat et al., 2011). Thus, in birds as in mammals, the basic social and reproductive functions of MT/OT have likely been expanded to include the modulation of direct offspring care.
A comparative approach to avian grouping
Over the last 20 years, a great deal has been learned about the nonapeptide mechanisms of sociality broadly defined, relating to bonding, social contact (“affiliation”), and parental care, including biparental and alloparental care (reviews: Carter, 1992; Young and Wang, 2004; Donaldson and Young, 2008; Neumann, 2009; Insel, 2010). In the prairie vole (Microtus ochrogaster), nonapeptide mechanisms that influence these various aspects of sociality appear to be linked (e.g., see Lim and Young, 2006; Ross and Young, 2009). However, the social structures of vertebrate species are highly variable, such that the various components of sociality are mixed and dissociated, indicating that those behavioral dimensions often evolve independently of each other (Alexander, 1974). This suggests that there may be mechanistic trade-offs and constraints that impact the evolution of social systems, and thus without a broad sample of species, we cannot say what mechanisms commonly evolve in relation to a specific aspect of behavior.
Despite the growing literature on most aspects of sociality, mechanisms that influence large-scale variation in grouping remain poorly understood, perhaps because species differences in the grouping behavior of rodents are often difficult to examine without the confounding influences of species differences in mating system and patterns of parental care, and/or aspects of ecology that may impact relevant neuroendocrine processes (see Goodson and Kingsbury, 2011; also reviews of rodent social structures in King, 1968; Tamarin, 1985). Even so, truly large group sizes are also uncommon in rodents, and for obvious reasons, herds or troops of larger mammals are difficult to accommodate in the lab.
In contrast, birds exhibit a substantial amount of social diversity and at least a couple of avian families offer the opportunity to examine grouping while controlling for other aspects of behavior and ecology (Goodson and Kingsbury, 2011). The familiy Estrildidae (finches and waxbills) stands out in this regard, because all estrildids are biparental and exhibit long-term (typically life-long) pair bonds, but show dramatic variation in grouping (Immelmann, 1965; Goodwin, 1982). Most of the approximately 140 species (Clements, 2007) form small parties when not breeding, and loosely distribute for nesting without defense of an exclusive territory (Immelmann, 1965; Goodwin, 1982). However, a small number of species have evolved an extreme degree of gregariousness and are typically found in groups of 100–300 birds, as in the zebra finch, or even in the thousands, as in the spice finch (Lonchura punctulata). A small number of species have also evolved territoriality, such as the Melba finch (Pytilia melba) and violet-eared waxbill (Uraeginthus granatina). Independent evolution has been identified at both ends of the grouping continuum1 -- that is, convergence in territoriality and convergence in extreme gregariousness and coloniality (review: Goodson and Kingsbury, 2011). This is important, because the ability to study both divergence and convergence in grouping allows us to determine whether a given mechanism is reliably targeted by selection during social evolution, which is an essential step towards establishing predictive validity for other taxa.
The estrildid family offers several other advantages, as well, most notably the inclusion of the zebra finch. The zebra finch genome is now well known (Warren et al., 2010); zebra finch behavior is extremely robust in captivity; and virtually the full range of zebra finch social behavior can be observed and quantified in the lab. More than 20 behaviors can be quantified in captive colonies, including the establishment of pair bonds (Goodson et al., 1999; Kabelik et al., 2009), and a detailed comparison of wild-caught and domestic zebra finches revealed no differences in behavior (Morris, 1958). Zebra finches are also interesting from a translational perspective, because like humans, they communicate primarily through acoustic and visual modalities, and live in biparental nuclear families that are embedded within larger social networks.
VT circuits of the BSTm-LS encode social valence and promote flocking
In order to identify brain areas that may be relevant to the species-specific processing of social stimuli in flocking and non-flocking birds, Goodson et al. (2005) exposed male and female finches of a territorial finch species (violet-eared waxbill) and three flocking finch species to a control manipulation or a same-sex conspecific through a wire barrier, and sacrificed subjects 90 min later for quantification of Fos and egr-1 response. This manipulation took place in a quiet room and elicited little overt behavior, thus neural activation should primarily reflect motivational or perceptual processes. The immediate early gene responses of territorial birds differed significantly from those in flocking birds throughout an evolutionarily conserved “social behavior network” that comprises a suite of basal forebrain and midbrain areas (network reviews: Newman, 1999; Goodson, 2005; O’Connell and Hofmann, 2011). These areas include the BSTm and LS, where VT/VP cells (BSTm only) and fibers have been linked to a variety of social behaviors (review: Goodson and Thompson, 2010). At the time of this study, however, direct functional data on the VT/VP cells of the BSTm were unavailable, beyond a single study in voles showing an increase in VP mRNA in males following overnight cohabitation with a female (Wang et al., 1994).
A subsequent experiment was therefore conducted to determine how the BSTm VT cells responded to same-sex stimuli in five estrildid finch species -- two territorial species that live in male-female pairs year-round (Melba finch and violet-eared waxbill), the modestly gregarious Angolan blue waxbill, Uraeginthus angolensis; a species that is sympatric with the two territorial species), and two highly gregarious, colonially breeding finch species (zebra finch and spice finch) (Goodson and Wang, 2006). Birds were exposed to a same-sex conspecific as just described, and were sacrificed 90 min later for quantification of VT-Fos colocalization (Fig. 2A). The 90 min time point represents two half-lives of the Fos protein (Herdegen and Leah, 1998), and both induction and suppression of Fos protein production are detectable at this time. The results of this experiment followed a striking pattern: whereas exposure to a same-sex conspecific tended to decrease VT-Fos colocalization in the territorial species, the converse was found for the flocking species (Fig. 2B–C). This produced a significant interaction effect, and a separate analysis of the two sympatric Uraeginthus species likewise yielded a significant interaction between Species and Condition. No sex differences were observed (Goodson and Wang, 2006).
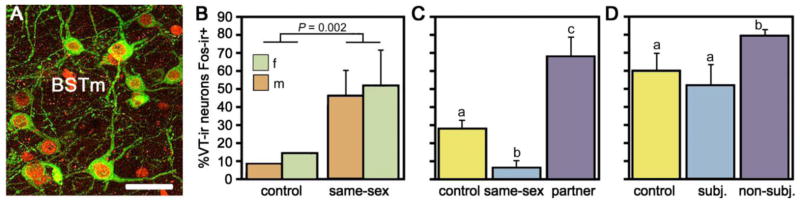
Fig. 2.
Valence sensitivity of vasotocin (VT) neurons in the bed nucleus of the stria terminalis (BSTm), as demonstrated by socially-induced changes in the immunocytochemical colocalization of VT and the proxy activity marker Fos. (A) Representative colocalization of VT (green) and Fos (red) in the BSTm of a male zebra finch following a courtship interaction. Note that most VT neurons express Fos. Scale bar = 20 μm. (B) In the zebra finch, which is a highly gregarious species, isolation in a quiet room followed by exposure to a same-sex conspecific through a wire barrier produces a robust increase in VT neuronal activity in the BSTm of both males and females. Total n = 10. (C) This same manipulation produces a significant decrease in VT-Fos colocalization in the territorial violet-eared waxbill, a species that does not naturally exhibit same-sex affiliation, but exposure to the subject’s pair bond partner (a presumably positive stimulus), produces a robust increase in neuronal activity. Sexes are shown pooled. Total n = 16. (D) VT-Fos colocalization increases in zebra finches following competition with a same-sex individual for courtship access to an opposite-sex bird, but not if the subject is paired with a highly aggressive partner and intensely subjugated. Subjugated animals were aggressively displaced or attacked 71–210 times during a 10-min interaction, demonstrating that social arousal alone does not increase VT-Fos colocalization in the BSTm. Sexes are shown pooled. Total n = 15. Panel A is modified from Goodson et al. (Goodson et al., 2009c); panels B–D are modified from Goodson and Wang (2006).
These results suggested the hypothesis that the BSTm VT cells are sensitive to the valence of social stimuli, such that they increase their Fos activity in response to positive, affiliation-related stimuli, but not to stimuli that normally elicit aggression or avoidance. This hypothesis received strong support from two additional experiments. In the first, territorial violet-eared waxbills were exposed to a control manipulation, a same-sex conspecific, or their pair bond partner (after 2 days of separation). Whereas the same-sex stimulus produced a significant decrease in VT-Fos colocalization, the partner stimulus produced an extremely robust increase (Fig. 2C). In the second experiment, zebra finches were moved to same-sex housing, which serves to increase their motivation to court, and they were subsequently exposed to a control manipulation or a mate competition interaction, in which two individuals of one sex compete for access to a single individual of the opposite sex. Aggression in this context is typically mild, and all birds have at least some opportunity to court. However, by identifying bullies in the housing cages that could be used as competitors, it was possible to pair some subjects with individuals who subjugated them intensely during the mate competition test. Non-subjugated subjects showed the expected increase in VT-Fos colocalization, but the subjugated birds exhibited a non-significant decrease (Fig. 2D). Importantly, the subjugated animals were aggressively displaced or attacked 71–205 times during a 10-min interaction, and thus it clear that social arousal alone does not induce Fos activity within BSTm VT neurons (Goodson and Wang, 2006). A later experiment further indicates that the VT neurons of the BSTm respond only to social stimuli, as providing a water bath to bath-deprived male zebra finches generated a strong behavioral response but no increase in VT-Fos colocalization, whereas exposure to a female produced a significant elevation in colocalization (Goodson et al., 2009a).
Other aspects of the BSTm-LS VT circuitry are likewise biased towards the more gregarious species: 1) Constitutive VT-Fos colocalization is significantly greater in the three flocking species than in the two territorial species (Goodson and Wang, 2006); 2) the two highly gregarious species exhibit approximately 10 times the number of VT-immunoreactive (-ir) cells in the BSTm than do the territorial and modestly gregarious species (Goodson and Wang, 2006); and 3) V1a-like binding sites in the LS are significantly more abundant in the three flocking species as compared to the territorial species (Goodson et al., 2006).
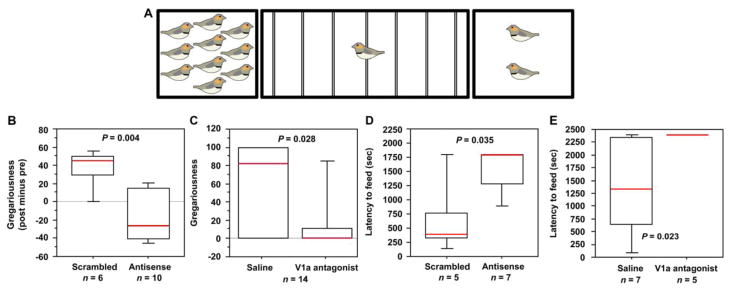
The findings described above strongly suggest the hypothesis that VT circuitry of the BSTm-LS promotes gregariousness. This hypothesis has been tested in two ways in male zebra finches -- first by knocking down VT production in the BSTm bilaterally using antisense oligonucleotides, and second by infusing a V1a antagonist into the LS (Kelly et al., 2011). Subjects were placed in a meter-wide cage containing seven perches, with the perches on the left and right sides being only a few centimeters from the cage wall. Smaller cages containing 2 and 10 same-sex individuals were placed on the sides in a counterbalanced fashion across subjects (Fig. 3A). Behavior in this apparatus yielded two primary measures -- “contact time,” which is the percent of test time that the subject spent on the two side perches combined, and “gregariousness,” which is the percent of contact time that the subject spent next to the larger group. Relative to scrambled oligonucleotide treatments, infusions of VT antisense oligonucleotides into the BSTm reduced gregariousness by 80% (Fig. 3B), although unexpectedly, a slight increase in contact time (approximately 25%) was also observed.
Fig. 3.
Antisense knockdown of VT production in the BSTm and intraseptal infusions of a VP V1a antagonist reduce gregariousness and increase anxiety-like behavior. (A) Choice apparatus design. A 1 m wide testing cage was subdivided into zones by seven perches (thin lines). Subjects were considered to be within close proximity when they were within 6 cm of a stimulus cage (i.e., on the perches closest to the sides of the testing cage). The stimulus cages contained either two or ten same-sex conspecifics. The percent of test time spent in close proximity to conspecifics yields a measure of “contact,” and the percent of contact time that is spent next to the larger group yields a measure of “gregariousness.” (B) Relative to scrambled oligonucleotide control subjects, male zebra finches infused with VT antisense oligonucleotides exhibit a median reduction in gregariousness of approximately 80% (C) Gregariousness is likewise reduced by V1a antagonist infusions into the LS, relative to vehicle. (D–E) Latency to feed in the presence of a novel object is strongly increased by both VT antisense infusions into the BSTm (D) and V1a antagonist infusions into the LS (E). Box plots show the median (red line), 75th and 25th percentile (box) and 95% confidence interval (whiskers). Modified from Kelly et al. (2011).
Remarkably, intraseptal infusions of the V1a antagonist likewise produced an 80% reduction in gregariousness relative to vehicle infusions, but there was a clear lack of effect on contact time. Both antisense and antagonist administrations also produce potent anxiogenic effects, particularly in the novelty-suppressed feeding test (Kelly et al., 2011). These results demonstrate that VT circuitry of the BSTm-LS strongly promotes preferences for larger group sizes (although the BSTm VT cells may modulate social contact elsewhere in the brain), and that this effect is associated with anxiolysis. Given that Fos induction in the BSTm VT neurons is apparently specific to positive social stimuli, the modulation of general anxiety-like processes by these cells (observed outside of a social context) may rely upon tonic VT release, an idea that is consistent with the high level of constitutive Fos activity in these neurons (Goodson and Wang, 2006; Goodson et al., 2009c).
The finding that septal VT is anxiolytic in finches is intriguing, because in rodents, septal VP tends to be anxiogenic (Liebsch et al., 1996; Bielsky et al., 2005; but see Everts and Koolhaas, 1999). The species-specific distributions of V1a-like receptors in the LS may underlie the different behavioral effects in zebra finches and rodents, and if so, then we may also expect to find that territorial and gregarious finches exhibit divergent anxiety responses to VT release in the LS. Hypothetically, such species-specific effects on anxiety could yield very different responses to social stimuli.
Whether VT circuits of the BSTm-LS evolve in finch-like ways in other taxa remains to be determined. However, recent evidence suggests that the valence sensitivity of the BSTm VT neurons may be found in distantly related taxa, as well. For instance, in male C57BL/6J mice, posterior BSTm VP neurons exhibit robust Fos responses to copulation and very modest responses to nonaggressive same-sex chemoinvestigation, but show no greater Fos response to aggressive interactions than simple chemoinvestigation (Ho et al., 2010). Similarly, VT-ir neurons in the posterior BSTm of male chickens increase their Fos activity after interactions with females, but not following agonistic interactions with other males (Xie et al., 2011).
MT and oxytocic (VT3) receptors modulate novel-familiar preferences and flocking
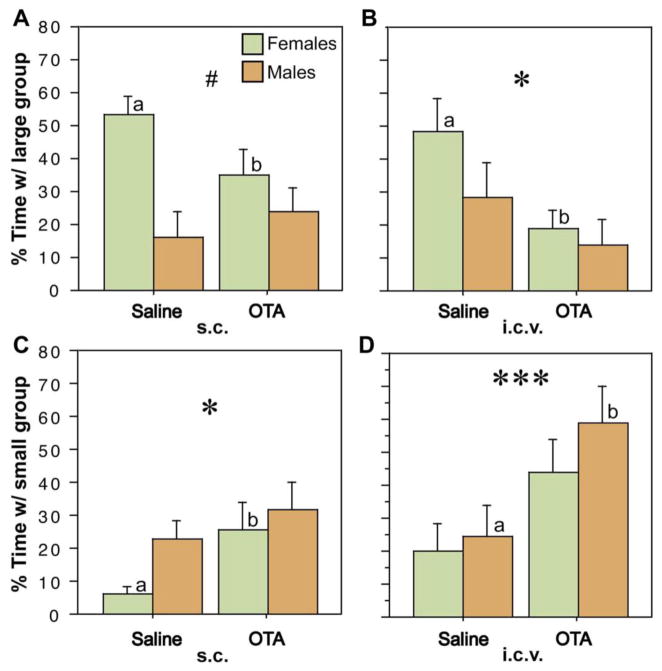
Until recently, effects of MT on avian behavior remained to be demonstrated. Using the same apparatus shown in Fig. 3A to measure the group size preferences of male and female zebra finches, Goodson et al. (2009d) showed that both peripheral and intraventricular administrations of an OT antagonist decrease the percent of test time that subjects spend in close proximity to the larger group, with a concomitant increase in the percent of time spent in close proximity to the smaller group (Fig. 4). No effect on total contact time was observed, and the effects on group size preference were reversed by central administrations of MT. Using a modification of this testing paradigm that offers a choice between novel and familiar same-sex conspecifics, it was also shown that peripheral and central administrations of the OT antagonist reduced the preference of subjects for familiar individuals. Several effects were female-specific (e.g., Fig. 4A).
Fig. 4.
Antagonism of oxytocic receptors reduces preferences for larger groups in zebra finches. Relative to vehicle treatments, subcutaneous (s.c.) or intracerebroventricular (i.c.v.) administrations of the oxytocin antagonist desGly–NH2, d(CH2)5[Tyr(Me)2, Thr4]OVT (OTA), reduce the amount of time that zebra finches spend in close proximity to the large group (B–C) and increase time in close proximity to the small group (D–E). *P < 0.05, ***P < 0.001, main effect of Treatment; #P < 0.5 Sex*Treatment; n = 12 m, 12 f. Letters above the error bars denote significant within-sex effects. Modified from Goodson et al. (2009d).
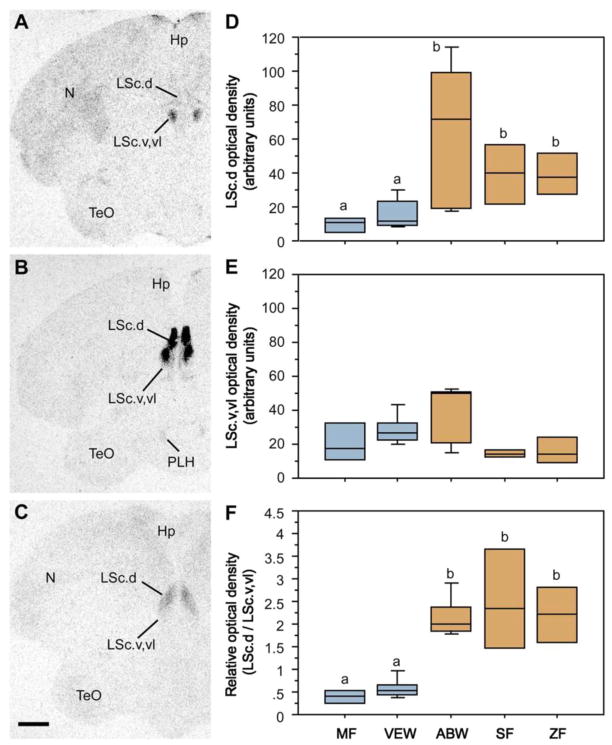
In order to determine whether the distributions of oxytocic receptors may reflect species differences in grouping, autoradiography was used to examine the oxytocic receptor densities in the brains of the five estrildid finch species introduced in the previous section. Although a variety of species differences were observed, only within the LS did the species differences in binding match the species differences in grouping (Fig. 5). Interestingly, whereas the three flocking species exhibited significantly higher binding densities in the dorsal (pallial) LS (Fig. 5D), this pattern tended to reverse in the subpallial LS (Fig. 5E), and the relative density across these divisions most strongly differentiated the territorial and flocking species (Fig. 5F). Finally, infusions of an oxytocin antagonist directly into the LS reduced preferences for the larger group in female zebra finches (Goodson et al., 2009b).
Fig. 5.
Species-specific distributions of oxytocin-like binding sites reflect evolutionary convergence and divergence in flocking and territoriality. (A–C) Representative autoradiograms of 125I-OT antagonist binding sites in the caudal LS (LSc) in two sympatric, congeneric finches – the territorial violet-eared waxbill (A) and the gregarious Angolan blue waxbill (B), plus the highly gregarious zebra finch (C). (D) Densities of binding sites in the dorsal (pallial) LSc of two territorial species (Melba finch, MF, and violet-eared waxbill, VEW), a moderately gregarious species (Angolan blue waxbill, ABW), and two highly gregarious species (spice finch, SF, and zebra finch, ZF). No sex differences are observed and sexes were pooled. Total n = 23. Different letters above the boxes denote significant species differences (Mann-Whitney P < 0.05) following significant Kruskal-Wallis. (E) Binding densities tend to reverse in the subpallial LSc (P = 0.06), suggesting that species differences in sociality are most closely associated with the relative densities of binding sites along a dorso-ventral gradient, as confirmed in the bottom panel (F) using a dorsal:ventral ratio. Abbreviations: Hp, hippocampus; LSc.d, dorsal zone of the LSc; LSc.v,vl, ventral and ventrolateral zones of the LSc; N, nidopallium; PLH, posterolateral hypothalamus; TeO, optic tectum. Modified from Goodson et al. (2009d).
Do avian nonapeptides influence monogamous pair bonding?
Perhaps no function of the nonapeptides has garnered more attention than the promotion of pair bonding. To date, however, nonapeptides have been shown to promote pair bonding only in the monogamous prairie vole, in which V1a receptors of the ventral pallidum and LS mediate VP effects on pair bonding in males, whereas OT receptors of the nucleus accumbens mediate pair bonding in females (reviews: Young and Wang, 2004; Lim and Young, 2006; Donaldson and Young, 2008). Similar nonapeptide effects have not been reported for other monogamous mammals, although to our knowledge, the necessary experimental manipulations have not been conducted (Goodson and Thompson, 2010). Outside of the Mammalia, comparable experiments have been conducted only in zebra finches. Central nonapeptide manipulations do not alter partner preferences in male or female zebra finches following a single night of cohabitation (Goodson et al., 2004), and chronic infusions of a V1-V1a antagonist cocktail do not impair natural pair bonding in males that are introduced into colony nesting cages (Kabelik et al., 2009). However, recent experiments show that chronic antagonism of central OT-like receptors severely impairs natural pair bonding of females in a colony environment, with very little effect in males (J. D. Klatt and J.L. Goodson, unpublished observations). Pair bond formation is relatively easy to observe in colony-housed zebra finches, based on the presence of distinctive behaviors such as side-by-side “clumping,” allopreening, following, and occupation of a nest cup (Zann, 1977; Adkins-Regan, 2011).
Modulation of aggression by VT is context- and phenotype-specific
Although VT and VP influence aggression across a wide range of vertebrates (Goodson and Bass, 2001), the relationship between VT/VP and aggression is highly complex and not yet fully understood. For instance, intraseptal VT infusions in male field sparrows selectively increase the use of a territorial song type during the “dawn song” period, but inhibit overt, resident-intruder aggression (Goodson, 1998a). Intraventricular infusions of VT also promote territorial singing in female white-crowned sparrows (Zonotrichia leucophrys) (Maney et al., 1997), and in rats, central VP release during aggression simultaneously increases and decreases across brain areas (Veenema et al., 2010). This complexity may reflect the involvement of many different VT/VP cell groups. For instance, at least eight distinct cell groups (mostly hypothalamic) alter their Fos activity following an aggressive encounter in male mice (Ho et al., 2010), but in virtually all cases, VP-Fos colocalization is more pronounced in subordinate animals and/or negatively correlated with aggression. Similarly, in territorial song sparrows, VT-Fos colocalization in the PVN correlates negatively with aggression (Goodson and Kabelik, 2009).
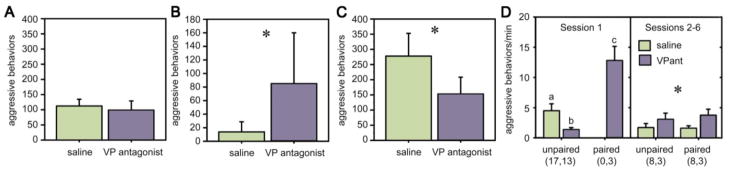
The negative correlation between aggression and VT-Fos colocalization in the PVN suggests the hypotheses that 1) endogenous VT release from PVN neurons inhibits territorial aggression, and 2) dominant males increase their aggression by reducing VT release. These hypotheses yield the prediction that antagonism of VT receptors should produce phenotype-specific effects on behavior – facilitating aggression in less aggressive males that are usually subordinate (which show higher levels of Fos activity in PVN VT neurons), while having little or no effect in aggressive, dominant males that show low levels of VT neuronal activity. These ideas receive excellent empirical support: Antagonism of V1a–like receptors has no effect on resident-intruder aggression in dominant male violet-eared waxbills (Fig. 6A), but produces a significant increase in aggression in males that are typically subordinate (Fig. 6B) (Goodson et al., 2009b). Importantly, even in very aggressive males, exogenous VT inhibits aggression (Goodson, 1998b), and thus the phenotype-specific effects just described must reflect phenotype differences in VT release, not phenotype differences in V1a-like receptor distributions. VT also reduces aggression in male Japanese quail that are paired in a neutral arena (Riters and Panksepp, 1997) and in European starlings (Sturnus vulgaris) that are subjected to crowding (Nephew et al., 2005).
Fig. 6.
Neuromodulation of aggression varies across contexts and phenotypes. For panels A–C, subjects were tested in a within-subjects design following injections of saline control or JNJ-17308616, a novel V1a antagonist that crossed the blood-brain barrier. Tests were 7 min. Total n = 9 for all *p = 0.015. (A) Total numbers of aggressive behaviors (means ± SEM) exhibited by aggressive, dominant male violet-eared waxbills in the context of territorial defense (resident-intruder tests). (B) Aggressive behaviors exhibited in resident-intruder tests (as in panel A) by male violet-eared waxbills that were typically subordinate. Total n = 6; *p = 0.043. (C) Aggressive behaviors exhibited by aggressive, dominant male violet-eared waxbills in the context of mate competition. Panels A–C modified from Goodson et al. (2009b). (D) Aggressive behavior per minute (when not in a nest; means ± SEM) exhibited by male zebra finches in colony cages that contained 4 males and 5 females. Subjects were administered intraventricular infusions of a V1-V1a antagonist cocktail twice daily. Focal 10-min observations were conducted in the morning and afternoon for three days (corresponding to sessions 1–6). Data are shown separately for session 1, when aggression is focused on competition for females, and during sessions 2–6 when most aggression is focused on the defense of nest cups. Data are displayed separately for unpaired and pair-bonded individuals. In session 1, paired males exhibited more aggression than unpaired males (P = 0.0002), and VP antagonist treatment resulted in a decrease in aggression relative to treatment with saline (P = 0.006). Different letters above the error bars denote significant group differences (P < 0.05). In sessions 2 to 6, the antagonist resulted in an increase in aggression levels relative to saline treatment (*P = 0.04). Data for all males are shown for session 1; analyses for sessions 2–6 are restricted to males for which unpaired and paired data are available. Modified from Kabelik et al. (2009).
As described earlier, the BSTm VT/VP neurons do not exhibit Fos responses to resident-intruder encounters in mice (Ho et al., 2010), simulated territorial intrusions in song sparrows (Goodson and Kabelik, 2009), or dominance interactions in roosters (Xie et al., 2011). However, VT-Fos colocalization increases in the BSTm during mate competition (Goodson and Wang, 2006), and endogenous VT/VP actually promotes male aggression during courtship interactions in both violet-eared waxbills (Fig. 6C) and zebra finches (Goodson and Adkins-Regan, 1999; Goodson et al., 2004; Goodson et al., 2009b).
These context-specific relationships between endogenous VT and aggression (i.e., in mate competition versus territorial contexts) have also been shown in male zebra finches that were introduced to colony nesting cages. Colonies were established with five females and four male subjects that were chronically administered either a V1 antagonist cocktail or vehicle. High levels of aggression were exhibited at the time of introduction, mostly focused on competition for mates, and in this context aggression was significantly lower in males that were treated with the antagonist. However, the antagonist effect completely reversed over subsequent days as most males paired and began to nest (Fig. 6D) (Kabelik et al., 2009). Notably, aggression in paired zebra finches is largely focused on nest defense (Zann, 1996), a context that is similar in some ways to territorial aggression.
Overall, the pharmacological and VT-Fos data for avian aggression are internally consistent to an impressive extent, particularly since they extend to multiple species of sparrows, multiple species of finches, and even chickens. The findings for VT-Fos colocalization in male birds are also virtually identical to those for VP-Fos colocalization in male mice (Ho et al., 2010). However, a couple of observations in rodents do not conform to the patterns just described, most notably evidence that VP release in the anterior hypothalamus promotes mating-induced territorial aggression in male prairie voles (Gobrogge et al., 2007; Gobrogge et al., 2009) and resident-intruder aggression in male Syrian hamsters, Mesocricetus auratus (Ferris et al., 1997) (although female hamsters show the opposite pattern; Gutzler et al., 2010). Nonetheless, the strong anatomical conservation of VT/VP circuits and the functional similarities between songbirds and mice suggest that there are generalizable frameworks to be derived if we continue to keep looking.
Nonapeptide modulation of female-directed song and sexual behavior
One of the first demonstrations that VT influences bird behavior came from male canaries (Serinus canaria) that were injected three days in a row with a VT analog. Song was measured several weeks later, and was found to increase or decrease depending upon the season, suggesting that VT may mediate seasonal transitions in song behavior (Voorhuis et al., 1991; De Kloet et al., 1993). However, immediate effects of VT on singing appear to be restricted to agonistic song types (Maney et al., 1997; Goodson, 1998a), as summarized in the previous section, and multiple experiments in male zebra finches demonstrate that directed courtship singing is not influenced by central infusions of VT, MT, or a diversity of nonapeptide receptor antagonists (Goodson and Adkins-Regan, 1999; Goodson et al., 2004; Kabelik et al., 2009). Knockdown of VT production in the BSTm by VT antisense oligonucleotides also produces no effects on directed song (Kelly et al., 2011). In contrast, intraventricular infusions of VT in male Japanese quail reduce sexual behavior and crowing (a vocalization that serves as a mate attractant (Goodson and Adkins-Regan, 1997; Castagna et al., 1998). These effects are reversed by a non-selective V1 antagonist, and thus the VT inhibition of behavior could be mediated by effects on either the brain (which expresses V1a-like receptors) or anterior pituitary (which expresses V1b-like receptors).
Correlational studies further support a role for VT in agonistic song. Male Lincoln sparrows (Melospiza lincolnii) sing more following one week of exposure to high-quality songs versus low-quality songs, and also show lower VT immunoreactivity in the BSTm and LS (Sewall et al., 2010). It remains to be determined whether this reduction in VT immunoreactivity reflects lower VT production as opposed to greater VT release, but regardless, the findings show a clear influence of agonistic stimuli on the VT circuitry of the BSTm and LS. VT correlations with behavior are somewhat different in the polymorphic white-throated sparrow (Zonotricha albicollis). The morph with white crown stripes displays more agonistic behavior than does the morph with tan crown stripes, and also exhibits greater VT immunoreactivity in the BSTm and ventrolateral LS (Maney et al., 2005).
Conclusions
Based on comparative studies of the extant vertebrate classes, it is clear that for at least 450 million years, nonapeptide systems have influenced reproductive physiology, osmoregulation, social communication, affiliation behaviors, aggression, and multiple aspects of stress response. However, there is at least some variation in these basic functions. For instance, whereas septal VP is anxiogenic in rodents, septal VT is strongly anxiolytic in male zebra finches, which may reflect species-specific needs in relation to gregariousness. Nonetheless, there is extensive conservation of function – for instance, in the differential relationships of BSTm and PVN VT/VP neurons to affiliation, aggression, and stress response. In addition, the independent evolution of multiple behavioral characters is associated with evolutionary convergence in the anatomy of nonapeptide systems and their behavioral effects. This is observed in the convergent roles of MT and OT in the extended maternal care of mammals and neognathan birds; in the independently derived effects of MT and OT on pair bonding in female prairie voles and zebra finches; and in the convergent patterns of nonapeptide receptor distributions in estrildid finch species that have independently evolved similar patterns of grouping behavior. These receptors have been experimentally shown to be strongly relevant to the expression of grouping and territorial behaviors. Most importantly, the studies reviewed here show the power of broadly comparative approaches to behavioral biology in general, and nonapeptide biology in particular. Only through broadly comparative studies can we identify common trends that yield translational insights into the most fascinating and evolutionarily labile aspects of human social behavior, such as monogamous pair bonding and grouping, and only through broadly comparative studies can we reconstruct the functional neurobiology of ancient species such as stem mammals and the fascinating theropod dinosaurs, a group that includes velociraptors and Tyrannosaurus, and gave rise to birds.
Highlights.
Avian nonapeptide systems are very similar to other amniote vertebrates, particularly mammals.
Birds and mammals exhibit convergent mechanisms of maternal care and female bonding.
Nonapeptide systems evolve convergently when territoriality evolves independently.
Nonapeptide systems evolve convergently when flocking evolves independently.
Acknowledgments
Support for much of the work reviewed here has been provided by the NIMH. We thank Richmond R. Thompson for his intellectual and technical contributions to our work, and the many students, postdocs and research associates who have contributed to our research program, most particularly Sara E. Schrock, Jacqueline M. Ho, Andrew K. Evans, Yiwei Wang, David Kabelik, James D. Klatt and Jacob Rinaldi.
Footnotes
Convergent and divergent social evolution in the Estrildidae is has been recently been described at length (Goodson and Kingsbury, 2011). Briefly, available data show that only four of the ~140 estrildid species are territorial. The first territorial species under study, Pytilia melba, is the only territorial member of the Pytilia genus (which contains five species), and the two closest outgroup genera are comprised of typical estrildids that are modestly gregarious when not breeding and that loosely distribute for nesting without territoriality. The second territorial species, Uraeginthus granatina, is virtually identical: of five species in the genus, only two are territorial and the two closest outgroup genera are typical estrildids. Another Uraeginthus species, U. angolensis, is much more social than the outgroup species. Thus U. angolensis and U. granatina have evolved in divergent ways. Although numerous estrildid species travel in small parties and breed in small colonies of 5–10 pairs, only five species breed in larger groups, and these species greatly exceed the group sizes of the other estrildids, forming colonies of 100 or more birds and flocking in even larger groups. The two Taeniopygia species are among these (including the zebra finch). The other three instances occur in Lonchura, a genus distantly related to Taeniopygia that contains 26 species, including the study species L. punctulata.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Absil P, Papello M, Viglietti-Panzica C, Balthazart J, Panzica G. The medial preoptic nucleus receives vasotocinergic inputs in male quail: a tract-tracing and immunocytochemical study. J Chem Neuroanat. 2002;24:27–39. doi: 10.1016/s0891-0618(02)00017-0. [DOI] [PubMed] [Google Scholar]
- Acharjee S, Do-Rego JL, Oh da Y, Ahn RS, Choe H, Vaudry H, Kim K, Seong JY, Kwon HB. Identification of amino acid residues that direct differential ligand selectivity of mammalian and nonmammalian V1a type receptors for arginine vasopressin and vasotocin. Insights into molecular coevolution of V1a type receptors and their ligands. J Biol Chem. 2004;279:54445–54453. doi: 10.1074/jbc.M408909200. [DOI] [PubMed] [Google Scholar]
- Acher R. Endocrinology. American Physiological Society; Washington, D.C: 1972. Chemistry of the neurohypophysial hormones: an example of molecular evolution; pp. 119–130. [Google Scholar]
- Acher R, Chauvert J. The neurohypophysial endocrine regulatory cascade: Precursors, mediators, receptors, and effectors. Front Neuroendocrinol. 1995;16:237–289. doi: 10.1006/frne.1995.1009. [DOI] [PubMed] [Google Scholar]
- Adkins-Regan E. Neuroendocrine contributions to sexual partner preference in birds. Front Neuroendocrinol. 2011;32:155–63. doi: 10.1016/j.yfrne.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Alexander RD. The evolution of social behavior. Ann Rev Ecol Systemat. 1974;5:325–383. [Google Scholar]
- Aste N, Muhlbauer E, Grossmann R. Distribution of AVT gene expressing neurons in the prosencephalon of Japanese quail and chicken. Cell Tissue Res. 1996;286:365–373. doi: 10.1007/s004410050706. [DOI] [PubMed] [Google Scholar]
- Baeyens DA, Cornett LE. The cloned avian neurohypophysial hormone receptors. Comp Biochem Physiol B Biochem Mol Biol. 2006;143:12–19. doi: 10.1016/j.cbpb.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Absil P, Viglietti-Panzica C, Panzica GC. Vasotocinergic innervation of areas containing aromatase-immunoreactive cells in the quail forebrain. J Neurobiol. 1997;33:45–60. [PubMed] [Google Scholar]
- Barth SW, Bathgate RA, Mess A, Parry LJ, Ivell R, Grossmann R. Mesotocin gene expression in the diencephalon of domestic fowl: cloning and sequencing of the MT cDNA and distribution of MT gene expressing neurons in the chicken hypothalamus. J Neuroendocrinol. 1997;9:777–787. doi: 10.1046/j.1365-2826.1997.00643.x. [DOI] [PubMed] [Google Scholar]
- Berk ML, Reaves TA, Hayward JN, Finkelstein JA. The localization of vasotocin and neurophysin neurons in the diencephalon of the pigeon, Columba livia. J Comp Neurol. 1982;204:392–406. doi: 10.1002/cne.902040410. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Hu SB, Ren X, Terwilliger EF, Young LJ. The V1a vasopressin receptor is necessary and sufficient for normal social recognition: a gene replacement study. Neuron. 2005;47:503–513. doi: 10.1016/j.neuron.2005.06.031. [DOI] [PubMed] [Google Scholar]
- Bons N. The topography of mesotocin and vasotocin systems in the brain of the domestic mallard and Japanese quail: immunocytochemical identification. Cell Tissue Res. 1980;213:37–51. doi: 10.1007/BF00236919. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Neumann ID. Brain vasopressin is an important regulator of maternal behavior independent of dams’ trait anxiety. Proc Natl Acad Sci U S A. 2008;105:17139–17144. doi: 10.1073/pnas.0807412105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briski KP, Brandt JA. Oxytocin and vasopressin neurones in principal and accessory hypothalamic magnocellular structures express fos-immunoreactivity in response to acute glucose deprivation. J Neuroendocrinol. 2000;12:409–414. doi: 10.1046/j.1365-2826.2000.00469.x. [DOI] [PubMed] [Google Scholar]
- Carter CS, Grippo AJ, Pournajafi-Nazarloo H, Ruscio MG, Porges SW. Oxytocin, vasopressin and sociality. Prog Brain Res. 2008;170:331–336. doi: 10.1016/S0079-6123(08)00427-5. [DOI] [PubMed] [Google Scholar]
- Castagna C, Absil P, Foidart A, Balthazart J. Systemic and intracerebroventricular injections of vasotocin inhibit appetitive and consummatory components of male sexual behavior in Japanese quail. Behav Neurosci. 1998;112:233–250. doi: 10.1037//0735-7044.112.1.233. [DOI] [PubMed] [Google Scholar]
- Chaturvedi CM, Prasad SK. Timed daily injections of neurotransmitter precursors alter the gonad and body weights of spotted munia, Lonchura punctulata, maintained under short daily photoperiods. J Exp Zool. 1991;260:194–201. [Google Scholar]
- Choi GB, Dong HW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Swanson LW, Anderson DJ. Lhx6 delineates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron. 2005;46:647–660. doi: 10.1016/j.neuron.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Chung SK, McCabe JT, Pfaff DW. Estrogen influences on oxytocin mRNA expression in preoptic and anterior hypothalamic regions studied by in situ hybridization. J Comp Neurol. 1991;307:281–95. doi: 10.1002/cne.903070209. [DOI] [PubMed] [Google Scholar]
- Clements JF. Birds of the world: a checklist. 6. Pica Press; Sussex: 2007. [Google Scholar]
- Clutton-Brock TH. The Evolution of Parental Care. Princeton University Press; Princeton, NJ: 1991. [Google Scholar]
- Cornett LE, Jacobi SE, Mikhailova MV. Molecular cloning of an avian vasotocin receptor with homology to the mammalian V1a-vasopressin receptor. Direct submission to Genbank. 2007 Aug 21; accession number ABV24997. [Google Scholar]
- Cornett LE, Kirby JD, Vizcarra JA, Ellison JC, Thrash J, Mayeux PR, Crew MD, Jones SM, Ali N, Baeyens DA. Molecular cloning and functional characterization of a vasotocin receptor subtype expressed in the pituitary gland of the domestic chicken (Gallus domesticus): avian homolog of the mammalian V1b-vasopressin receptor. Regul Pept. 2003;110:231–239. doi: 10.1016/s0167-0115(02)00216-1. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Elands J, Voorhuis DAM. Implication of central neurohypophyseal hormone receptor-mediated action in the timing of reproductive events: Evidence from novel observations on the effect of a vasotocin analogue on singing behaviour of the canary. Regul Pept. 1993;45:85–89. doi: 10.1016/0167-0115(93)90187-d. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Buijs RM. The origin of the vasopressinergic and oxytocinergic innervation of the rat brain with special reference to the lateral septum. Brain Res. 1983;273:307–317. doi: 10.1016/0006-8993(83)90855-7. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Panzica GC. Sexual differentiation of central vasopressin and vasotocin systems in vertebrates: Different mechanisms, similar endpoints. Neuroscience. 2006;138:947–955. doi: 10.1016/j.neuroscience.2005.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- Everts HGJ, Koolhaas JM. Differential modulation of lateral septal vasopressin receptor blockade in spatial learning, social recognition, and anxiety-related behaviors in rats. Behav Brain Res. 1999;99:7–16. doi: 10.1016/s0166-4328(98)00004-7. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Melloni RH, Koppel G, Perry KW, Fuller RW, Delville Y. Vasopressin/serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters. J Neurosci. 1997;17:4331–4340. doi: 10.1523/JNEUROSCI.17-11-04331.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino Y, Nagahama T, Oumi T, Ukena K, Morishita F, Furukawa Y, Matsushima O, Ando M, Takahama H, Satake H, Minakata H, Nomoto K. Possible functions of oxytocin/vasopressin-superfamily peptides in annelids with special reference to reproduction and osmoregulation. J Exp Zool. 1999;284:401–6. doi: 10.1002/(sici)1097-010x(19990901)284:4<401::aid-jez6>3.3.co;2-l. [DOI] [PubMed] [Google Scholar]
- Gale SD, Perkel DJ. Physiological properties of zebra finch ventral tegmental area and substantia nigra pars compacta neurons. J Neurophysiol. 2006;96:2295–2306. doi: 10.1152/jn.01040.2005. [DOI] [PubMed] [Google Scholar]
- Gobrogge KL, Liu Y, Jia X, Wang Z. Anterior hypothalamic neural activation and neurochemical associations with aggression in pair-bonded male prairie voles. J Comp Neurol. 2007;502:1109–1122. doi: 10.1002/cne.21364. [DOI] [PubMed] [Google Scholar]
- Gobrogge KL, Liu Y, Young LJ, Wang Z. Anterior hypothalamic vasopressin regulates pair-bonding and drug-induced aggression in a monogamous rodent. Proc Natl Acad Sci U S A. 2009;106:19144–19149. doi: 10.1073/pnas.0908620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez A, Smeets WJAJ. Comparative analysis of the vasotocinergic and mesotocinergic cells and fibers in the brain of two amphibians, the anuran Rana ridibunda and the urodele Pleurodeles waltlii. J Comp Neurol. 1992;315:53–73. doi: 10.1002/cne.903150105. [DOI] [PubMed] [Google Scholar]
- Goodson JL. Territorial aggression and dawn song are modulated by septal vasotocin and vasoactive intestinal polypeptide in male field sparrows (Spizella pusilla) Horm Behav. 1998a;34:67–77. doi: 10.1006/hbeh.1998.1467. [DOI] [PubMed] [Google Scholar]
- Goodson JL. Vasotocin and vasoactive intestinal polypeptide modulate aggression in a territorial songbird, the violet-eared waxbill (Estrildidae: Uraeginthus granatina) Gen Comp Endocrinol. 1998b;111:233–244. doi: 10.1006/gcen.1998.7112. [DOI] [PubMed] [Google Scholar]
- Goodson JL. The vertebrate social behavior network: Evolutionary themes and variations. Horm Behav. 2005;48:11–22. doi: 10.1016/j.yhbeh.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL. Nonapeptides and the evolutionary patterning of sociality. Prog Brain Res. 2008;170:3–15. doi: 10.1016/S0079-6123(08)00401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Adkins-Regan E. Playback of crows of male Japanese quail elicits female phonotaxis. Condor. 1997;99:990–993. [Google Scholar]
- Goodson JL, Adkins-Regan E. Effect of intraseptal vasotocin and vasoactive intestinal polypeptide infusions on courtship song and aggression in the male zebra finch (Taeniopygia guttata) J Neuroendocrinol. 1999;11:19–25. doi: 10.1046/j.1365-2826.1999.00284.x. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Social behavior functions and related anatomical characteristics of vasotocin/vasopressin systems in vertebrates. Brain Res Rev. 2001;35:246–265. doi: 10.1016/s0165-0173(01)00043-1. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Vocal-acoustic circuitry and descending vocal pathways in teleost fish: Convergence with terrestrial vertebrates reveals conserved traits. J Comp Neurol. 2002;448:298–322. doi: 10.1002/cne.10258. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Eibach R, Sakata J, Adkins-Regan E. Effect of septal lesions on male song and aggression in the colonial zebra finch (Taeniopygia guttata) and the territorial field sparrow (Spizella pusilla) Behav Brain Res. 1999;98:167–180. [PubMed] [Google Scholar]
- Goodson JL, Evans AK. Neural responses to territorial challenge and nonsocial stress in male song sparrows: segregation, integration, and modulation by a vasopressin V1 antagonist. Horm Behav. 2004;46:371–381. doi: 10.1016/j.yhbeh.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Evans AK, Bass AH. Putative isotocin distributions in sonic fish: relation to vasotocin and vocal-acoustic circuitry. J Comp Neurol. 2003;462:1–14. doi: 10.1002/cne.10679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Evans AK, Lindberg L, Allen CD. Neuro-evolutionary patterning of sociality. Proc R Soc Lond B Biol Sci. 2005;272:227–235. doi: 10.1098/rspb.2004.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Evans AK, Wang Y. Neuropeptide binding reflects convergent and divergent evolution in species-typical group sizes. Horm Behav. 2006;50:223–236. doi: 10.1016/j.yhbeh.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Kabelik D. Dynamic limbic networks and social diversity in vertebrates: From neural context to neuromodulatory patterning. Front Neuroendocrinol. 2009;30:429–441. doi: 10.1016/j.yfrne.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Kabelik D, Kelly AM, Rinaldi J, Klatt JD. Midbrain dopamine neurons reflect affiliation phenotypes in finches and are tightly coupled to courtship. Proc Natl Acad Sci U S A. 2009a;106:8737–8742. doi: 10.1073/pnas.0811821106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Kabelik D, Schrock SE. Dynamic neuromodulation of aggression by vasotocin: influence of social context and social phenotype in territorial songbirds. Biol Lett. 2009b;5:554–556. doi: 10.1098/rsbl.2009.0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Kingsbury MA. Nonapeptides and the evolution of social group sizes in birds. Front Neuroanat. 2011;5:13. doi: 10.3389/fnana.2011.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Lindberg L, Johnson P. Effects of central vasotocin and mesotocin manipulations on social behavior in male and female zebra finches. Horm Behav. 2004;45:136–143. doi: 10.1016/j.yhbeh.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Rinaldi J, Kelly AM. Vasotocin neurons in the bed nucleus of the stria terminalis preferentially process social information and exhibit properties that dichotomize courting and non-courting phenotypes. Horm Behav. 2009c;55:197–202. doi: 10.1016/j.yhbeh.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Schrock SE, Klatt JD, Kabelik D, Kingsbury MA. Mesotocin and nonapeptide receptors promote estrildid flocking behavior. Science. 2009d;325:862–866. doi: 10.1126/science.1174929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Thompson RR. Nonapeptide mechanisms of social cognition, behavior and species-specific social systems. Curr Opin Neurobiol. 2010;20:784–794. doi: 10.1016/j.conb.2010.08.020. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Wang Y. Valence-sensitive neurons exhibit divergent functional profiles in gregarious and asocial species. Proc Natl Acad Sci U S A. 2006;103:17013–17017. doi: 10.1073/pnas.0606278103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin D. Estrildid finches of the world. Cornell University Press; Ithaca, NY: 1982. [Google Scholar]
- Grossmann R, Kisliuk S, Xu B, Muhlbauer E. The hypothalamo-neurohypophyseal system in birds. Adv Exp Med Biol. 1995;395:657–66. [PubMed] [Google Scholar]
- Gubrij KI, Chaturvedi CM, Ali N, Cornett LE, Kirby JD, Wilkerson J, Mikhailova M, Turner ML, Baeyens DA. Molecular cloning of an oxytocin-like receptor expressed in the chicken shell gland. Comp Biochem Physiol B Biochem Mol Biol. 2005;142:37–45. doi: 10.1016/j.cbpc.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Guillette LJ, Jr, Jones RE. Further observations on arginine vasotocin-induced oviposition and parturition in lizards. J Herpetol. 1982;16:140–144. [Google Scholar]
- Gutzler SJ, Karom M, Erwin WD, Albers HE. Arginine-vasopressin and the regulation of aggression in female Syrian hamsters (Mesocricetus auratus) Eur J Neurosci. 2010;31:1655–1663. doi: 10.1111/j.1460-9568.2010.07190.x. [DOI] [PubMed] [Google Scholar]
- Hahn TP, Cornelius JM, Sewall KB, Kelsey TR, Hau M, Perfito N. Environmental regulation of annual schedules in opportunistically-breeding songbirds: Adaptive specializations or variations on a theme of white-crowned sparrow? Gen Comp Endocrinol. 2008;157:217–226. doi: 10.1016/j.ygcen.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Herdegen T, Leah JD. Inducible and constitutive transcription factors in the mammalian nervous system: Control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain Res Rev. 1998;28:370–490. doi: 10.1016/s0165-0173(98)00018-6. [DOI] [PubMed] [Google Scholar]
- Ho JM, Murray JH, Demas GE, Goodson JL. Vasopressin cell groups exhibit strongly divergent responses to copulation and male-male interactions in mice. Horm Behav. 2010;58:368–377. doi: 10.1016/j.yhbeh.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle CHV. Neuropeptide families: evolutionary perspectives. Regul Pept. 1998;73:1–33. doi: 10.1016/s0167-0115(97)01073-2. [DOI] [PubMed] [Google Scholar]
- Hoyle CHV. Neuropeptide families and their receptors: evolutionary perspectives. Brain Res. 1999;848:1–25. doi: 10.1016/s0006-8993(99)01975-7. [DOI] [PubMed] [Google Scholar]
- Husband SA, Shimizu T. Calcium-binding protein distributions and fiber connections of the nucleus accumbens in the pigeon (Columba livia) J Comp Neurol. 2011;519:1371–1394. doi: 10.1002/cne.22575. [DOI] [PubMed] [Google Scholar]
- Immelmann K. Australian finches in bush and aviary. Angus and Robertson; Sydney and London: 1965. [Google Scholar]
- Insel TR. The challenge of translation in social neuroscience: a review of oxytocin, vasopressin, and affiliative behavior. Neuron. 2010;65:768–779. doi: 10.1016/j.neuron.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurkevich A, Berghman L, Cornett L, Kuenzel WJ. Immunohistochemical characterization of chicken pituitary cells containing the vasotocin VT2 receptor. Cell Tissue Res. 2008;333:253–262. doi: 10.1007/s00441-008-0636-2. [DOI] [PubMed] [Google Scholar]
- Jurkevich A, Berghman LR, Cornett LE, Kuenzel WJ. Characterization and immunohistochemical visualization of the vasotocin VT2 receptor in the pituitary gland of the chicken, Gallus gallus. Gen Comp Endocrinol. 2005;143:82–91. doi: 10.1016/j.ygcen.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Kabelik D, Kelly AM, Goodson JL. Dopaminergic regulation of mate competition aggression and aromatase-Fos colocalization in vasotocin neurons. Neuropharmacology. 2010a;58:117–125. doi: 10.1016/j.neuropharm.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabelik D, Klatt JD, Kingsbury MA, Goodson JL. Endogenous vasotocin exerts context-dependent behavioral effects in a semi-naturalistic colony environment. Horm Behav. 2009;56:101–107. doi: 10.1016/j.yhbeh.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabelik D, Morrison JA, Goodson JL. Cryptic regulation of vasotocin neuronal activity but not anatomy by sex steroids and social stimuli in opportunistic desert finches. Brain Behav Evol. 2010b;75:71–84. doi: 10.1159/000297522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Kingsbury MA, Hoffbuhr K, Schrock SE, Waxman B, Kabelik D, Thompson RR, Goodson JL. Vasotocin neurons and septal V(1a)-like receptors potently modulate songbird flocking and responses to novelty. Horm Behav. 2011;60:12–21. doi: 10.1016/j.yhbeh.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JA, editor. Biology of Peromyscus (Rodentia) American Society of Mammologists; Stillwater, MN: 1968. [Google Scholar]
- Kingsbury MA, Kelly AM, Schrock SE, Goodson JL. Mammal-like organization of the avian midbrain central gray and a reappraisal of the intercollicular nucleus. PLoS ONE. 2011;6:e20720. doi: 10.1371/journal.pone.0020720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss JZ, Voorhuis TAM, Van Eekelen JAM, De Kloet ER, De Wied D. Organization of vasotocin-immunoreactive cells and fibers in the canary brain. J Comp Neurol. 1987;263:347–364. doi: 10.1002/cne.902630304. [DOI] [PubMed] [Google Scholar]
- Korf HW, Panzica GC, Viglietti-Panzica C, Oksche A. Pattern of peptidergic neurons in the avian brain: clusters--local circuitries--projections. Basic Appl Histochem. 1988;32:55–75. [PubMed] [Google Scholar]
- Leung CH, Abebe D, Earp SE, Goode CT, Grozhik AV, Minidoddi P, Maney DL. Neural distribution of vasotocin receptor mRNA in two species of songbird. Endocrinology. 2011;152:4865–4881. doi: 10.1210/en.2011-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung CH, Goode CT, Young LJ, Maney DL. Neural distribution of nonapeptide binding sites in two species of songbird. J Comp Neurol. 2009;513:197–208. doi: 10.1002/cne.21947. [DOI] [PubMed] [Google Scholar]
- Liebsch G, Wotjak CT, Landgraf R, Engelmann M. Septal vasopressin modulates anxiety-related behaviour in rats. Neurosci Lett. 1996;217:101–104. [PubMed] [Google Scholar]
- Lim MM, Young LJ. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Horm Behav. 2006;50:506–517. doi: 10.1016/j.yhbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Richardson CF, Zoeller TR, Miller LJ, Muske LE, Moore FL. Neuroanatomical distribution of vasotocin in a urodele amphibian (Taricha granulosa) revealed by immunohistochemical and in situ hybridization techniques. J Comp Neurol. 1997;385:43–70. doi: 10.1002/(sici)1096-9861(19970818)385:1<43::aid-cne3>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Maney DL, Erwin KL, Goode CT. Neuroendocrine correlates of behavioral polymorphism in white-throated sparrows. Horm Behav. 2005;48:196–206. doi: 10.1016/j.yhbeh.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Maney DL, Goode CT, Wingfield JC. Intraventricular infusion of arginine vasotocin induces singing in a female songbird. J Neuroendocrinol. 1997;9:487–491. doi: 10.1046/j.1365-2826.1997.00635.x. [DOI] [PubMed] [Google Scholar]
- Mikami S, Tokado H, Farner DS. The hypothalamic neurosecretory systems of the Japanese quail as revealed by retrograde transport of horseradish peroxidase. Cell Tissue Res. 1978;194:1–15. doi: 10.1007/BF00209230. [DOI] [PubMed] [Google Scholar]
- Mikami S, Yamada S. Immunohistochemistry of the hypothalamic neuropeptides and anterior pituitary cells in the Japanese quail. J Exp Zool. 1984;232:405–17. doi: 10.1002/jez.1402320305. [DOI] [PubMed] [Google Scholar]
- Moore FL, Lowry CA. Comparative neuroanatomy of vasotocin and vasopressin in amphibians and other vertebrates. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1998;119:251–260. doi: 10.1016/s0742-8413(98)00014-0. [DOI] [PubMed] [Google Scholar]
- Morris D. The comparative ethology of Grassfinches (Erythrurae) and Mannikins (Amadinae) Proc Zool Sci Lond. 1958;131:389–439. [Google Scholar]
- Nephew BC, Aaron RS, Romero LM. Effects of arginine vasotocin (AVT) on the behavioral, cardiovascular, and corticosterone responses of starlings (Sturnus vulgaris) to crowding. Horm Behav. 2005;47:280–289. doi: 10.1016/j.yhbeh.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Neumann ID. The advantage of social living: Brain neuropeptides mediate the beneficial consequences of sex and motherhood. Front Neuroendocrinol. 2009;30:483–496. doi: 10.1016/j.yfrne.2009.04.012. [DOI] [PubMed] [Google Scholar]
- Newman SW. The medial extended amygdala in male reproductive behavior: A node in the mammalian social behavior network. Ann N Y Acad Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- Ocampo Daza D, Lewicka M, Dan Larhammar. The oxytocin/vasopressin receptor family has at least five members in the gnathostome lineage, including two distinct V2 subtypes. Gen Comp Endocrinol. 2012;175:135–143. doi: 10.1016/j.ygcen.2011.10.011. [DOI] [PubMed] [Google Scholar]
- O’Connell LA, Hofmann HA. The Vertebrate mesolimbic reward system and social behavior network: A comparative synthesis. J Comp Neurol. 2011;519:3599–3639. doi: 10.1002/cne.22735. [DOI] [PubMed] [Google Scholar]
- Ottinger MA, Lavoie E, Thompson N, Barton A, Whitehouse K, Barton M, Abdelnabi M, Quinn M, Jr, Panzica G, Viglietti-Panzica C. Neuroendocrine and behavioral effects of embryonic exposure to endocrine disrupting chemicals in birds. Brain Res Rev. 2008;57:376–85. doi: 10.1016/j.brainresrev.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Oumi T, Ukena K, Matsushima O, Ikeda T, Fujita T, Minakata H, Nomoto K. Annetocin, an annelid oxytocin-related peptide, induces egg-laying behavior in the earthworm, Eisenia foetida. J Exp Zool. 1996;276:151–6. doi: 10.1002/(SICI)1097-010X(19961001)276:2<151::AID-JEZ8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Panzica GC, Aste N, Castagna C, Viglietti-Panzica C, Balthazart J. Steroid-induced plasticity in the sexually dimorphic vasotocinergic innervation of the avian brain: Behavioral implications. Brain Res Rev. 2001;37:178–200. doi: 10.1016/s0165-0173(01)00118-7. [DOI] [PubMed] [Google Scholar]
- Panzica GC, Castagna C, Viglietti-Panzica C, Russo C, Tlemcani O, Balthazart J. Organizational effects of estrogens on brain vasotocin and sexual behavior in quail. J Neurobiol. 1998;37:684–699. doi: 10.1002/(sici)1097-4695(199812)37:4<684::aid-neu15>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Panzica GC, Plumari L, Garcia-Ojeda E, Deviche P. Central vasotocin-immunoreactive system in a male passerine bird (Junco hyemalis) J Comp Neurol. 1999;409:105–117. [PubMed] [Google Scholar]
- Panzica GC, Viglietti-Panzica C, Mura E, Quinn MJ, Jr, Lavoie E, Palanza P, Ottinger MA. Effects of xenoestrogens on the differentiation of behaviorally-relevant neural circuits. Front Neuroendocrinol. 2007;28:179–200. doi: 10.1016/j.yfrne.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Ascher JA, Monroe YL, Prange AJJ. Oxytocin induces maternal behavior in virgin female rats. Science. 1982;216:648–650. doi: 10.1126/science.7071605. [DOI] [PubMed] [Google Scholar]
- Plumari L, Plateroti S, Deviche P, Panzica GC. Region-specific testosterone modulation of the vasotocin-immunoreactive system in male dark-eyed juncos, Junco hyemalis. Brain Res. 2004;999:1–8. doi: 10.1016/j.brainres.2003.10.037. [DOI] [PubMed] [Google Scholar]
- Reichard UH. Monogamy: past and present. In: Reichard UH, Boesch C, editors. Monogamy Mating Strategies and Partnerships in Birds, Humans and Other Mammals. Cambridge University Press; Cambridge: 2003. pp. 3–25. [Google Scholar]
- Riters LV, Panksepp J. Effects of vasotocin on aggressive behavior in male Japanese quail. Ann N Y Acad Sci. 1997;807:478–80. doi: 10.1111/j.1749-6632.1997.tb51943.x. [DOI] [PubMed] [Google Scholar]
- Robinzon B, Koike TI, Neldon HL, Kinzler SL. Distribution of immunoreactive mesotocin and vasotocin in the brain and pituitary of chickens. Peptides. 1988;9:829–33. doi: 10.1016/0196-9781(88)90129-5. [DOI] [PubMed] [Google Scholar]
- Rood BD, De Vries GJ. Vasopressin innervation of the mouse (Mus musculus) brain and spinal cord. J Comp Neurol. 2011;519:2434–2474. doi: 10.1002/cne.22635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol. 2009;30:534–547. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth R, Kohler A, Grossmann R, Chaturvedi CM. Expression of hypothalamic arginine vasotocin gene in response to water deprivation and sex steroid administration in female Japanese quail. J Exp Biol. 2004;207:3025–33. doi: 10.1242/jeb.01118. [DOI] [PubMed] [Google Scholar]
- Sewall KB, Dankoski EC, Sockman KW. Song environment affects singing effort and vasotocin immunoreactivity in the forebrain of male Lincoln’s sparrows. Horm Behav. 2010;58:544–553. doi: 10.1016/j.yhbeh.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma D, Chaturvedi C. Effects of thyroid status on arginine vasotocin receptor VT2R expression and adrenal function in osmotically stimulated domestic fowl. J Comp Physiol B Biochem System Environ Physiol. 2009;179:811–819. doi: 10.1007/s00360-009-0362-4. [DOI] [PubMed] [Google Scholar]
- Sharma D, Cornett LE, Chaturvedi CM. Osmotic stress induced alteration in the expression of arginine vasotocin receptor VT2 in the pituitary gland and adrenal function of domestic fowl. Gen Comp Endocrinol. 2009;160:216–222. doi: 10.1016/j.ygcen.2008.11.025. [DOI] [PubMed] [Google Scholar]
- Sharp PJ, Li Q, Talbot RT, Barker P, Huskisson N, Lea RW. Identification of hypothalamic nuclei involved in osmoregulation using fos immunocytochemistry in the domestic hen (Gallus domesticus), ring dove (Streptopelia risoria), Japanese quail (Coturnix japonica) and zebra finch (Taeniopygia guttata) Cell Tissue Res. 1995;282:351–361. [Google Scholar]
- Sikdar M, Kar A, Prakash P. Role of humidity in the seasonal reproduction of male spotted munia, Lonchura punctulata. J Exp Zool. 1992;264:82–84. [Google Scholar]
- Srivastava R, Chaturvedi CM. Effect of estrogen and tamoxifen on the shell gland AVT and VT3R of scotosensitive and scotorefractory Japanese quail. Gen Comp Endocrinol. 2010;167:104–112. doi: 10.1016/j.ygcen.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Srivastava R, Cornett LE, Chaturvedi CM. Effect of photoperiod and estrogen on expression of arginine vasotocin and its oxytocic-like receptor in the shell gland of the Japanese quail. Comp Biochem Physiol A. 2007;148:451–457. doi: 10.1016/j.cbpa.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Srivastava R, Cornett LE, Chaturvedi CM. Effect of estrogen and its antagonist on the expression of arginine vasotocin (AVT) and its oxytocic-like receptor VT3 in the shell gland of Japanese quail, Coturnix coturnix japonica. Comp Biochem Physiol A. 2008;151:551–559. doi: 10.1016/j.cbpa.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Srivastava R, Cornett LE, Chaturvedi CM. Age-dependent expression of AVT and its oxytocic-like receptor VT3 in the shell gland of Japanese quail, Coturnix coturnix japonica. Gen Comp Endocrinol. 2010;165:47–52. doi: 10.1016/j.ygcen.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Kawashima M. Arginine vasotocin induces bearing down for oviposition in the hen. Poult Sci. 2003;82:345–346. doi: 10.1093/ps/82.2.345. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Kawashima M. Mesotocin increases the sensitivity of the hen oviduct uterus to arginine vasotocin. Poult Sci. 2008;87:2107–2111. doi: 10.3382/ps.2008-00076. [DOI] [PubMed] [Google Scholar]
- Tamarin RH, editor. Biology of new world Microtus. American Society of Mammologists; Stillwater, OK: 1985. [Google Scholar]
- Tan F, Lolait SJ, Brownstein MJ, Saito N, MacLeod V, Baeyens DA, Mayeux PR, Jones SM, Cornett LE. Molecular cloning and functional characterization of a vasotocin receptor subtype that is expressed in the shell gland and brain of the domestic chicken. Biol Reprod. 2000;62:8–15. doi: 10.1095/biolreprod62.1.8. [DOI] [PubMed] [Google Scholar]
- Tessmar-Raible K, Raible F, Christodoulou F, Guy K, Rembold M, Hausen H, Arendt D. Conserved sensory-neurosecretory cell types in annelid and fish forebrain: Insights into hypothalamus evolution. Cell. 2007;129:1389–1400. doi: 10.1016/j.cell.2007.04.041. [DOI] [PubMed] [Google Scholar]
- Thayananuphat A, Youngren OM, Kang SW, Bakken T, Kosonsiriluk S, Chaiseha Y, El Halawani ME. Dopamine and mesotocin neurotransmission during the transition from incubation to brooding in the turkey. Horm Behav. 2011 doi: 10.1016/j.yhbeh.2011.06.009. e-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- Thompson RR, Walton JC. Vasotocin immunoreactivity in goldfish brains: characterizing primitive circuits associated with social regulation. Brain Behav Evol. 2009;73:153–164. doi: 10.1159/000219485. [DOI] [PubMed] [Google Scholar]
- Thompson RR, Walton JC, Bhalla R, George KC, Beth EH. A primitive social circuit: vasotocin-substance P interactions modulate social behavior through a peripheral feedback mechanism in goldfish. Eur J Neurosci. 2008;27:2285–2293. doi: 10.1111/j.1460-9568.2008.06210.x. [DOI] [PubMed] [Google Scholar]
- Varricchio DJ, Moore JR, Erickson GM, Norell MA, Jackson FD, Borkowski JJ. Avian paternal care had dinosaur origin. Science. 2008;322:1826–8. doi: 10.1126/science.1163245. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Beiderbeck DI, Lukas M, Neumann ID. Distinct correlations of vasopressin release within the lateral septum and the bed nucleus of the stria terminalis with the display of intermale aggression. Horm Behav. 2010;58:273–281. doi: 10.1016/j.yhbeh.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Neumann ID. Central vasopressin and oxytocin release: regulation of complex social behaviours. Prog Brain Res. 2008;170:261–276. doi: 10.1016/S0079-6123(08)00422-6. [DOI] [PubMed] [Google Scholar]
- Viglietti-Panzica C, Balthazart J, Plumari L, Fratesi S, Absil P, Panzica GC. Estradiol mediates effects of testosterone on vasotocin immunoreactivity in the adult quail brain. Horm Behav. 2001;40:445–461. doi: 10.1006/hbeh.2001.1710. [DOI] [PubMed] [Google Scholar]
- Voorhuis TAM, De Kloet ER. Immunoreactive vasotocin in the zebra finch brain Taeniopygia guttata. Develop Brain Res. 1992;69:1–10. doi: 10.1016/0165-3806(92)90116-e. [DOI] [PubMed] [Google Scholar]
- Voorhuis TAM, De Kloet ER, De Wied D. Effect of a vasotocin analog on singing behavior in the canary. Horm Behav. 1991;25:549–559. doi: 10.1016/0018-506x(91)90020-i. [DOI] [PubMed] [Google Scholar]
- Wang Z, Smith W, Major DE, De Vries GJ. Sex and species differences in the effects of cohabitation on vasopressin messenger RNA expression in the bed nucleus of the stria terminalis in prairie voles (Microtus ochrogaster) and meadow voles (Microtus pennsylvanicus) Brain Res. 1994;650:212–218. doi: 10.1016/0006-8993(94)91784-1. [DOI] [PubMed] [Google Scholar]
- Warren WC, Clayton DF, Ellegren H, et al. The genome of a songbird. Nature. 2010;464:757–62. doi: 10.1038/nature08819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wotjak CT, Kubota M, Liebsch G, Montkowski A, Holsboer F, Neumann I, Landgraf R. Release of vasopressin within the rat paraventricular nucleus in response to emotional stress: A novel mechanism of regulating adrenocorticotropic hormone secretion? J Neurosci. 1996;16:7725–7732. doi: 10.1523/JNEUROSCI.16-23-07725.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Kuenzel WJ, Sharp PJ, Jurkevich A. Appetitive and consummatory sexual and agonistic behaviour elicits FOS expression in aromatase and vasotocin neurones within the preoptic area and bed nucleus of the stria terminalis of male domestic chickens. J Neuroendocrinol. 2011;23:232–43. doi: 10.1111/j.1365-2826.2011.02108.x. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- Zann R. Pair-bond and bonding behavior in three species of grassfinches of the genus Poephila (Gould) Emu. 1977;77:97–106. [Google Scholar]
- Zann RA. The zebra finch: a synthesis of field and laboratory studies. Oxford University Press; Oxford: 1996. [Google Scholar]