Abstract
For most people, their quality of life depends on their successful interdependence with others, which requires sophisticated social cognition, communication, and emotional bonds. Across the lifespan, new bonds must be forged and maintained, and conspecific menaces must be managed. The dynamic nature of the human social landscape suggests ongoing specific alterations in neural circuitry across several brain systems to subserve social behavior. To discover the biological mechanisms that contribute to normal social activities, animal models of social behavior have been developed. One valuable model system has been female rat sexual behavior, which is governed by cyclic variation of ovarian hormones. This behavior is modulated by the neuropeptide oxytocin (OT) through its actions in the hypothalamic ventromedial nucleus (VMH). The fluctuation of this behavior is associated with dendrite remodeling, like several other examples of behavioral plasticity. This review compares hormone-induced plasticity in the VMH with other examples of dendrite plasticity across the mammalian nervous system, namely the neurobehavioral paradigms of environmental enrichment, chronic stress, and incentive sensitization, which affect the neocortex, hippocampal formation, and ventral striatum, respectively. This comparison suggests that the effects of ovarian hormones on VMH neurons in rats, given the simple dendritic arbor and short time course for dendrite remodeling, provides an dual opportunity for mechanistic and functional studies that will shed light on i) the neural actions of OT that regulate social behavior and, ii) behaviorally relevant dendrite regulation in a variety of brain structures.
Keywords: Dendrite, estradiol, female sexual behavior, lordosis, neural plasticity, progesterone, ventromedial hypothalamus
Introduction
For humans, social interactions are an important but complex aspect of daily function, promoting prosperity and contributing to a meaningful life. Given the complexity of human social interactions, it is not surprising that a variety of mental health problems are associated with poor social function, including various personality, mood, and developmental disorders. A better understanding of the neurological underpinnings of social behavior may provide insights towards the development of interventions for diverse neurobehavioral disorders. The neuropeptide oxytocin (OT) has been implicated in social behavior in a variety of model systems, as highlighted throughout this special issue. An OT-like peptide is produced across throughout the diversity of four-limbed vertebrates (for review, see (Hoyle, 1999)). This evolutionary conservation highlights the fundamental role OT may play in sociality. Nevertheless, the mechanisms of OT action in the brain are only partially understood.
Animal models have contributed enormously to our understanding of the biological basis of social behaviors, often based on their easily quantified stereotyped social interactions. For example, sexual receptivity in female rats, characterized by the lordosis response, is a robust, tightly regulated, and evolutionarily important behavior. When a female rat engages in the lordosis response, she allows a male to make close physical contact. The lordosis response is a brief social interaction that usually occurs in the broader context of solicitous behavior. Although lordosis is admittedly not a rich social behavior, it provides several experimental advantages, including its hormonal regulation, the ease of quantification, the ability to re-test animals after changing various parameters, and a well-defined, relatively simple underlying neural circuit. Using this model system, researchers have discovered a select few peptide neuromodulators that mediate the effects of estradiol and progesterone to produce lordosis responses, including OT. A better understanding of the neural actions of OT within the lordosis circuit may alleviate the gaps in our understanding of the broad neural actions of OT.
A new social role requires that the adult mammalian brain modify itself to express adaptive behaviors. Persistent changes in behavior involve not only acute changes in neurotransmitter release, but also the enduring reconfiguration of neural circuits. One mechanism of remodeling within a neural network is neuronal replacement. This process occurs in a limited number of brain regions that can support adult neurogenesis, neural migration and circuit assimilation, such as the hippocampal formation (Deng et al., 2010). Neurogenesis allows for the broad expansion of particular nodes within a network. A second mechanism of neural plasticity occurs at the level of individual synapses. In this case, synaptic activity regulates the number of connections between two neurons, such as the ability to convert between monosynaptic and multisynaptic boutons (e.g., (Woolley et al., 1996)). A third mechanism of neural plasticity is dendritic remodeling, which involves the adjustment of dendrite length and/or branching. This form of rewiring would affect neural integration in several ways, such as providing additional distal surface for synaptic contact, altering the efficacy of other postsynaptic sites depending on their electonic distance from nascent inputs, and labeling particular inputs to distinct regions of the tree by the latency of the resulting outgoing spikes (London and Hausser, 2005). Thus, neurons that undergo changes in their dendritic arbor would experience broad modifications to their signal integration, which in turn would generate a revised pattern of action potential activity. The present review will focus on the latter form of neural plasticity.
This review first will provide an overview of the cell biology of dendrite morphology. Then we will present female mating behavior as a model system for the study of dendrite remodeling that includes a rearrangement of oxytocin (OT) synapses in the service of female reproductive behavior. Next, several other examples of dendrite remodeling in a variety of brain systems will be examined. Finally, after considering dendrite restructuring in these other systems, mechanistic hypotheses are proposed for future study regarding the role of OT in the regulation of dendrite morphology in VMH, both as a model system for social behavior and a more general model system for behaviorally relevant dendrite plasticity.
Overview of Dendrite Morphology
Dendritic elaborations exhibit a complexity and diversity recognized since the work of Ramon y Cajal (Ramon y Cajal, 1911), and Golgi impregnation continues to be employed useful method to quantify the structure of dendrites, in addition to more recent techniques. The size and shape of a dendritic tree are likely dictated by the need to sample and weight the appropriate synaptic inputs. In adult animals, the dendritic surface available for synapses is correlated with the number of synapses on a neuron (Harris and Kater, 1994). Defects in the length and branching of dendrites have been associated with diverse neurological disorders, including Alzheimer's disease, autism, Down's syndrome, epilepsy, fragile X syndrome, and Rett syndrome, and schizophrenia (Black et al., 2004; Kaufmann and Moser, 2000; Pardo and Eberhart, 2007; Teskey et al., 2006; Yamada et al., 1988). Thus, a better understanding of the regulation of dendrite morphology may provide clues to many neuropsychological disorders.
Some of the molecular mechanisms that control the structure of the dendritic tree have been identified in model organisms, such as Drosophila melanogaster and transgenic mice (Jan and Jan, 2010). The ultimate targets of regulation are the cytoskeletal proteins, namely, actin and microtubules (Schubert and Dotti, 2007). As dendrites elongate and branch, molecular motors transport Golgi outposts and endosomes along the microtubules (Satoh et al., 2008; Ye et al., 2007), which highlights the importance of an accessible depot of membrane and the appropriate membrane-associated proteins. Dendrite expansion also requires proteins that traffic messenger RNA to allow for local regulation of protein synthesis (Ye et al., 2004). Specific second messenger pathways regulate cytoskeletal elements, including small guanine triphosphate (GTP)-ases, and the mitogen-activated protein kinase (MAPK) and phosphoinositide 3-kinase-mammalian target of rapamycin (PI3K-mTOR) kinase pathways (Jaworski et al., 2005; Kumar et al., 2005). These pathways act downstream of neurotransmitters and other extrinsic factors, such as brain-derived nerve growth factor (BDNF; (McAllister et al., 1995)). Finally, dendrite remodeling is influenced in part by intrinsic factors, such as the transcription regulator neurogenic differentiation factor 1 (NeuroD1; (Gaudilliere et al., 2004)). Thus, identified mechanisms include several points in the cascades from intrinsic and extrinsic factors, their effector molecules, to their cytoskeletal targets. Although some of these mechanisms overlap in flies and mice, additional studies are needed to identify likely cell-specific and possible mammalian-specific mechanisms.
Considering that dendritic morphology can undergo significant changes, both during pathological conditions and normal life experiences, as detailed below, the impact of remodeling on neuronal firing pattern also has been studied. Correlational studies based on recordings and computational modeling of hippocampal and neocortical pyramidal cells indicate that burst firing, as opposed to a steady pattern of firing, is associated with a distinct dendrite arrangement (Bilkey and Schwartzkroin, 1990; Chagnac-Amitai et al., 1990; Mainen and Sejnowski, 1996; Mason and Larkman, 1990). Burst firing, the generation of clusters of spikes with short interspike intervals, can improve signal-to-noise ratio and determine whether long-term potentiation versus long-term depression occurs. Computational models show that spatial features of the dendritic tree that promote burst firing include a specific range of total dendrite length and branching, as well as symmetry. Indeed, in computational models dendritic branching has a greater impact on burst firing than the density of ion channels or the strength of the stimulus (van Elburg and van Ooyen, 2010). Thus, dendrite remodeling does not simply quantitatively alter the number of inputs a neuron receives, but can fundamentally change the quality of its output. Of course, it would be useful to determine empirically the specific electrophysiological consequences of dendritic remodeling in a broader spectrum of neuron types involving physiologically relevant parameters.
In sum, functionally distinct neurons exhibit unique patterns of dendrite morphology, a key aspect of their information processing potential. Basic cellular mechanisms have been identified that would allow for ongoing refinements of the dendritic processes, depending on the level of synaptic input or more global transcriptional reprogramming, such as by steroid hormones. Moreover, computational studies of the effect of dendrite topology on electrophysiological output indicate that dendrite remodeling has a major impact on neuronal communication by influencing the burst versus tonic firing pattern. We now will consider dendrite remodeling in contexts associated with behavioral adaptation, beginning with female reproductive behavior.
Female sexual behavior: the role of OT and dendrite remodeling
Female sexual behavior is a robust and well-characterized behavior. The hallmark of female receptivity is the lordosis response, the rigid posture in which the female dorsiflexes her back to allow the male to mount her. A female rat displays the lordosis response at a precise time after the appropriate hormone regimen or within a specific time window in her natural estrous cycle. The ventromedial nucleus of the hypothalamus (VMH) is crucial for this behavior, and the actions of both estrogen and progesterone in the VMH are necessary for its full execution (Dörner et al., 1968; Kennedy, 1964; Pfaff and Sakuma, 1979; Rubin and Barfield, 1983). This detailed understanding of the underlying neuroendocrine mechanisms of the lordosis response makes this an exemplary model system for the study of the role of structural plasticity in behavior.
Neurons in the VMH exhibit simple dendritic arbors, with approximately three dendrites that extend directly from the soma, referred to as primary dendrites, and occasional secondary dendrites that ramify from one of the primary dendrites (Calizo and Flanagan-Cato, 2000; Griffin and Flanagan-Cato, 2008; Millhouse, 1973). A single long primary dendrite (LPD) is more than 100 μm longer than the other primary dendrites, and extends towards the surrounding neuropil. Golgi impregnation analysis revealed that estradiol treatment causes a marked shortening of these LPDs, a process that is reversed with sequential progesterone treatment (Griffin and Flanagan-Cato, 2008). Electron microscopy analysis of the surrounding fiber plexus verified that estradiol treatment caused an attrition of dendritic profiles in that region, and this effect was reversed with four hours of progesterone treatment (Griffin et al., 2010). This pattern is consistent with findings reported from intact cycling rats (Madeira et al., 2001). The striking effects of ovarian hormones on the LPD raised questions about the impact of dendrite length changes on synaptic organization in the lateral fiber plexus, the presumed source of innervation for the LPDs extending from the VMH.
Within the lateral fiber plexus, a prominent neurochemical marker is the peptide OT. The role of OT in female rat mating behavior is well documented. OT administered intracerebroventricularly significantly increases lordosis behavior in ovariectomized female rats pretreated with ovarian hormones, as well as in intact females at estrus (Arletti and Bertolini, 1985; Benelli et al., 1994; Caldwell et al., 1986; Gorzalka and Lester, 1987). Likewise, OT receptor (OTR) antagonists given centrally significantly reduce mating behavior in estradiol and progesterone-primed gonadectomized females, implicating endogenous OT in the natural expression of lordosis (Caldwell et al., 1990; Witt and Insel, 1991). Regarding the site of action, several studies have shown behavioral effects when OT action in the VMH is targeted (McCarthy et al., 1994; Schumacher et al., 1989). Additionally, OT administration increases the firing activity of ventrolateral VMH (vlVMH) neurons, and neuronal activity is further increased in animals given estradiol pretreatment (Booth et al., 2010; Kow et al., 1991). Ovarian hormones enhance OT production, the electrical activity of OT neurons, and up-regulate the level of OTRs in the VMH (see (Theodosis, 2002) for review), (Armstrong et al., 2002; Bale and Dorsa, 1995b; Bale et al., 1995c; De Kloet et al., 1986; Israel and Poulain, 2000; Quinones-Jenab et al., 1997; Schumacher et al., 1990). Thus, the effects of ovarian hormones on mating behavior are mediated in part by enhanced OT action in the VMH. Given that many LPDs from the VMH extend towards the OT-rich lateral fiber plexus, it has been proposed that OT released in the VMH lateral fiber plexus regulates female mating behavior by acting on these dendrites (Schumacher et al., 1990).
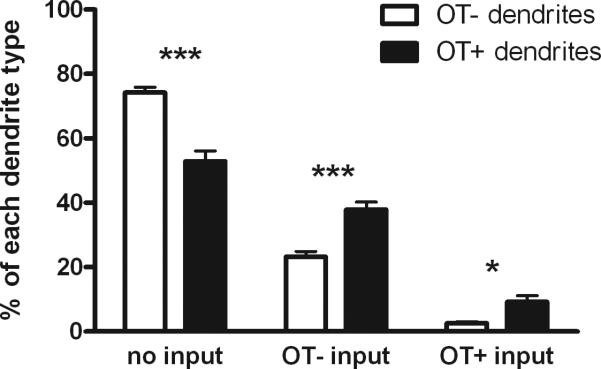
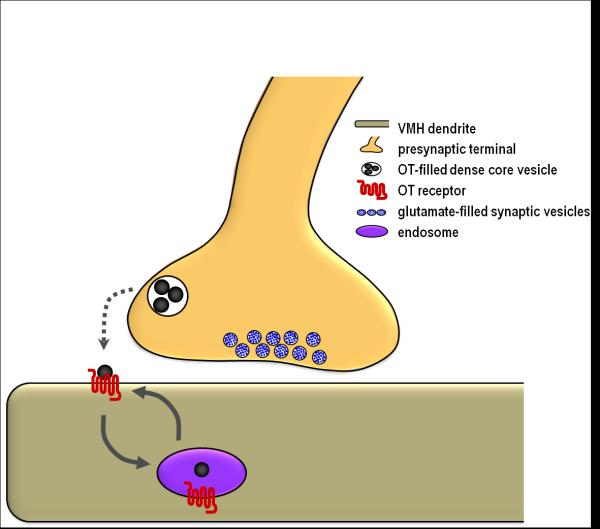
Our laboratory has recently provided ultrastructural evidence for OT-containing axodendritic, glutamatergic synapses in the VMH lateral fiber plexus. OT-labeled particles within axon terminals were segregated from vesicles and the presynaptic membrane, which is consistent with parasynaptic release, as observed for other neuromodulatory peptides (Griffin et al., 2010). In addition, a striking proportion of the dendritic profiles were immunolabeled with OT. OT-labeled synaptic terminals preferentially innervated OT-labeled dendrites, as shown in Figures 1 and 2, which suggests that OT labeling of dendrites reflects liganded receptor-mediated internalization. Interestingly, OT-labeled dendrites were protected from the aforementioned estradiol-induced dendrite retraction, increasing proportion of dendrites that were OT-labeled, and increasing the density of their synaptic inputs. Therefore, we propose that in the VMH lateral fiber plexus, OT postsynaptically modulates glutamate action, thereby preserving, and perhaps even strengthening, specific elements within this dynamic circuit (Figure 3).
Figure 1. Electron photomicrographs illustrating OT-labeled dendritic profiles and axon terminals in the VMH lateral fiber plexus.
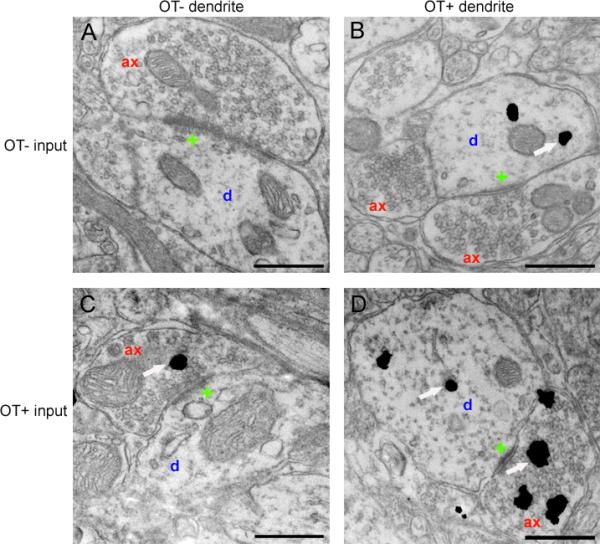
Immunoelectron micrographs were prepared and analyzed, as described in (Griffin et al., 2010). For most axodendritic synaptic contacts, both the dendrites and the axon terminals were absent of labeling for OT. For some axodendritic contacts, either the dendrites or the axon terminals were labeled for OT, but not both. In a few cases, axodendritic synaptic contacts were observed in which both the dendrites and the axon terminals were labeled for OT. ax=axon; d=dendrite; plus sign indicates an active zone of synaptic contact; white arrows indicate immunogold particles, indicative of OT. Bar=500 nm.
Figure 2. Bar graph comparing the pattern of synaptic input to dendritic profiles in the VMH lateral fiber plexus depending on whether the dendritic profile was labeled for OT.
Immunoelectron micrographs were prepared and analyzed, as described in (Griffin et al., 2010). There was no effect of hormone treatment on these measures, and therefore ovariectomized animals treated with vehicle, estradiol alone, and estradiol plus progesterone were combined (n=9 animals, including a total of 5118 dendritic profiles). OT-labeled dendrites were more likely to have synaptic inputs, including more likely to have OT-labeled synaptic inputs, compared with non-OT-labeled dendritic profiles. * indicates p<0.05; *** indicates p<0.005 based on within animal paired Student's t-test).
Figure 3.
A schematic illustrating the hypothesized parasynaptic release of OT from glutamate terminals, and its receptor binding and internalization into VMN dendrites.
OT neurons express vesicular glutamate transporters, indicating that OT is co-released with glutamate (Hrabovszky et al., 2006; Ponzio et al., 2006). In other brain systems, peptides co-localized with classical neurotransmitters act as activity-dependent neuromodulators (Eva et al., 2004). In cortex, OT modulates glutamate neurotransmission (Ninan, 2011). Likewise, we have recently shown that OT and vesicular glutamate transports are co-localized in axon terminals laterally adjacent to the VMH (Griffin et al., 2010). The co-localization of glutamate and OT is intriguing, given their reportedly opposing effects on lordosis. Whereas OT facilitates lordosis, glutamate agonists infused into the VMH attenuate female receptive behavior in estrogen plus progesterone-treated female rats (Georgescu and Pfaus, 2006; McCarthy et al., 1991). Conversely, NMDA and AMPA antagonists site-specifically facilitate sex behavior (Georgescu and Pfaus, 2006b), which indicates that endogenous glutamate release in the VMH inhibits sexual behavior. Therefore, OT may regulate lordosis behavior by dampening glutamate transmission. Future studies are needed to determine whether or not the change in dendrite organization is related to OT-glutamate modulation.
In summary, the VMH mediates the ovarian hormone regulation of female mating behavior. Among the hormone-induced changes in the VMH is the remodeling the dendritic arbor of VMH neurons. This remodeling involves an increased representation of OT-modulated connections, which may affect glutamate neurotransmission. We will now consider several other examples of dendrite remodeling in the nervous system.
Dendrite plasticity in other neurobehavioral systems
Engagement with the environment
The neocortex provides sophisticated associations across sensory systems, coding for the execution of complex motor sequences and the regulation of attention and emotion. Although seemingly minor defects in neocortical synaptic activity can be associated with severely disordered cognition (Wandinger et al., 2011), humans sometimes exhibit remarkable recovery of function after damage to discrete areas of the neocortex (Pasinetti et al., 2010). An attractive structural feature of the neocortex, at least from a research perspective, is its laminar organization, in which particular components of dendritic arbors are innervated by known afferents.
The dendritic tree of neocortical neurons is subject to regulation by experiences. For example, rats housed in complex environments have increased dendritic branching in the layer 5 pyramidal neurons of the occipital cortex compared with animals raised in unadorned cages (Greenough and Volkmar, 1973; Holloway, 1966; Volkmar and Greenough, 1972). In particular, environmental enrichment increased the number of higher order dendrites, specifically for basal, not apical, dendrites of pyramidal neurons in the occipital cortex. This enhanced complexity would likely affect input from nearby pyramidal cells, rather than input from the thalamus which innervates the apical dendrites. The enhanced branching did not result in dendrites residing beyond the normal borders of the dendritic arbor, but rather the tree was denser within its original domain. Thus, the enhanced branching did not alter the laminar or columnar context of the dendrite field. The impact of this neural plasticity is reflected by the demonstration of an overall larger cortical size (Bennett et al., 1964). The experience-induced dendrite remodeling persisted for at least 30 days after animals were removed from the enriched environment (Briones et al., 2004). Taken together, dendrite restructuring in this neurobehavioral systems is localized to a portion of the tree, and specific set of afferents, and the reconfigured arbor endures well beyond the occurrence of the stimuli that produced the enhanced connectivity.
Within the occipital cortex, a number of mechanisms have been recognized to contribute to dendrite remodeling (as reviewed in (Berardi et al., 2003)). Ionotropic receptors for glutamate and GABA have been implicated, as well as BDNF. Three kinases have been shown to integrate electrical signals and neurotropic influences, namely, protein kinase A, extracellular-signal-regulated kinase, and α Ca2+/calmodulin-dependent protein kinase II, and the regulation of extracellular matrix proteins. These kinases, in turn, regulate the activity of various signal transduction pathways, that include effects on AMPA receptors and the dendrite-specific structural protein microtubule associated protein-2. Genotropic targets of these signaling pathways include cAMP-response element binding protein (CREB). Finally, the extracellular matrix has been studied, which includes proteins that provide perineural nets, which ensheath cell bodies and dendrites, but allow synaptic contacts through fenestrations. These mechanistic studies emphasize the dendrite remodeling requires not only the necessary construction within the dendrite, but an “easement” in the surrounding tissue.
Environmental enrichment-induced dendritic morphology has been correlated with improved spatial-contextual memory (Duffy et al., 2001; Nilsson et al., 1999). In particular, animals provided with environmental enrichment display superior performance in a water maze task, swimming a shorter distance to locate the hidden platform after several days of training, compared with animals exposed to deficient sensory stimulation. Likewise, animals with environmental enrichment display enhanced context, but not cued, conditioning to an aversive stimulus. At this time, it is difficult to make a causal connection between the altered dendritic tree in the occipital cortex and these improved performances on tasks often associated with hippocampal function. Furthermore, the enriched environment affects motor activity, which may affect sensory processing indirectly, making it difficult to isolate the factor that exerts later effects on performance in memory tasks. Given the high-level, multi-system functions that are mediated by neocortical regions, it is not surprising that the behavioral significance of the enhanced dendrite morphology in the occipital cortex requires additional study.
Emotional context
The hippocampal formation is an important neural system for episodic, declarative and contextual learning, with outputs that affect cognition, mood and endocrine control. It is also vulnerable to seizure activity and damage due to stroke, head trauma, stress, recurrent depression and aging (Sapolsky, 1992). An important feature of hippocampal function, as well as a factor in its vulnerability to damage, is its high expression of adrenal steroid receptors (De Kloet et al., 1994; McEwen et al., 1968). These multi-tasking receptors 1) mediate negative feedback effects on the endocrine stress response through multisynaptic connections to the hypothalamus, 2) alter energy utilization of hippocampal neurons, and 3) promote specific modifications of dendrite morphology. Glucocorticoid action in the hippocampus represents a major crossroads for higher cognitive processing, affect and homeostasis.
Chronic treatment with glucocorticoids induces atrophy of apical dendrites on CA3 pyramidal cells, based on Golgi impregnation studies (Woolley et al., 1990b). The apical dendrites within the CA3 region of the hippocampus receive the mossy fiber projection from the granule cells of the dentate gyrus. Endogenous elevation in glucocorticoids, as occurs during chronic restraint stress, likewise produces a paring of the dendritic arbor of CA3 pyramidal neurons (Watanabe et al., 1992). In particular, the length and number of branch points of apical dendrites decreases by approximately 15 to 20 percent. The effects of adrenal steroids on dendrite arborization are mediated by neurotransmitters, peptide modulators, and calcium currents (McEwen, 1999). Dendritic remodeling in hippocampal CA3 pyramidal apical dendrites also occurs under other physiological conditions, such as during hibernation in European hamsters (Magarinos et al., 2006). Dendrite retraction during this metabolic state may represent an adaptive limit on glutamatergic activation during a prolonged period during which episodic and spatial memory formation are not needed. Thus, dendrite retraction during chronic stress may have an adaptive value, rather than being a sign of damage.
The effects of stress on dendritic arbors in the hippocampus may coordinate with similar remodeling of a broader network. For example, chronic restraint stress also reduces the length and branch points of apical dendrites in layer III of prefrontal cortex (Bloss et al., 2010). A potential outcome of stressor-induced remodeling of dendrites in prefrontal cortex in humans was shown in a study of medical students preparing for the board examination. This stressor disrupted prefrontal cortex connectivity with premotor areas and posterior parietal cortex and reduced measures of attentional control, based on functional magnetic resonance imaging and an attention shifting task (Liston et al., 2009). As with the stress-induced dendrite changes in rats, the effects of stress on cortical connectivity and attention in humans were reversed one month after the examination. The mechanisms of stress-induced dendrite remodeling in the hippocampus show some overlap with what has been shown for the neocortex (as reviewed in (McEwen, 2010)), although there has been less emphasis on the dendrite length compared with the regulation of spines. In particular, ionotropic receptors for glutamate, BDNF, CREB, and neural adhesion molecules have been implicated in dendrite restructuring in the hippocampus during chronic stress.
Behavioral changes correlate with stress-induced remodeling in the CA3 region of the hippocampus in rats. In particular, chronic restraint stress for 21 days impairs spatial memory, and antidepressant treatments prevented both the stress-induced dendrite remodeling and the memory impairment (Conrad et al., 1996; Luine et al., 1994; Wright and Conrad, 2005). Strengthening this correlation, the effects of chronic stress on both morphology and memory disappeared two weeks after the stress regimen was halted. Given that chronic stress causes plasticity in other brain regions, further experimentation is required to prove a mechanistic link between hippocampal CA3 dendrite morphology and spatial learning and memory. Furthermore, spatial memory tasks generally involve a series of mental steps, and the specific cognitive process that is dependent on the arborization of CA3 dendrites has not been well defined. Thus, although neural plasticity in this system has been associated with an important behavioral outcome, the functional specificity remains ill defined.
In sum, the dendritic arbors of hippocampal and prefrontal neocortical neurons are sensitive to chronic stressors, providing an example in which dendrites may be temporarily simplified in adulthood. As with the hormonal effects in the VMH and experiential effects in the occipital cortex, the dendrite modification occurs on a specific compartment of the dendritic tree, suggesting that the influence of a specific subset of afferents is being titrated. Whereas the elaboration of dendrites in the cortex is thought to adaptively improve sensory processing for memory formation, the pruning of dendrites in this case is thought to adaptively disrupt functional connectivity, albeit with the cost of impaired memory formation.
Value-based action
The nucleus accumbens is part of a motor loop that links the incentive properties of stimuli with the effort exerted to approach or avoid them (Berridge, 2007; Salamone et al., 2007). Studies of the dendritic structure of neurons in the nucleus accumbens have shown that they share common features with neurons in the dorsal striatum, including the phenotype of medium spiny neurons (Meredith et al., 2008). Neuroadaptations in this brain region normally optimize our motivated behaviors; however drugs of abuse can hijack this plasticity to produce addictive behaviors. Neuroimaging studies in humans have illustrated that addictions are associated with long-lasting alterations in accumbal activity (Gu et al., 2010).
The neuroadaptations in the nucleus accumbens that occur when animals become sensitized to work for specific stimuli are correlated with the remodeling of dendrites. For example, exposure to psychostimulants causes long-lasting changes in the behavioral sensitivity to these compounds, and environmental stimuli paired with these drugs exert effects on behavior in the absence of the drugs (Robinson and Becker, 1986). Animals treated chronically with psychostimulants exhibited an increased dendrite length in both the core and shell of the accumbens (Robinson and Kolb, 1997). Interestingly, a similar expansion of the dendritic arbor was observed in animals with a history of sodium depletion (Roitman et al., 2002), a treatment is known to sensitize sodium appetite (Sakai et al., 1987). Opiates, in contrast, decrease dendrite complexity (as reviewed in (Russo et al., 2010)). Thus, although the dendritic changes that are induced by drugs of abuse may be a fundamental mechanism that links environmental stimuli with natural goal-directed behaviors, there does not seem to be a simple relation between enhanced dendrite complexity and incentivized behavior. Although some signaling molecules that mediate dendrite plasticity in the nucleus accumbens have been identified that overlap with mechanisms in the neocortex and hippocampus, including ionotropic glutamate receptors, BDNF, and cell adhesion molecules, other signals, such as delta Fos B and nuclear factor κB, may be unique to dendrite plasticity in the ventral striatum (Russo et al., 2010). The possible role of CREB in accumbal dendrite remodeling is intriguing, given that opiates and psychostimulants both activate CREB while inducing opposite effects on dendrite structure.
Striking behavioral changes are correlated with psychostimulant-induced remodeling in the ventral striatum. Chronic exposure to D-amphetamine for five weeks produces behavioral sensitization to amphetamine that can last a year (Robinson and Camp, 1987). Given that chronic psychostimulant treatment causes dendrite plasticity in other brain regions, such as the prefrontal cortex (Robinson and Kolb, 1999), the causal link between accumbal dendrite morphology and behavioral sensitization will require further experimentation to be established. Thus, although neural plasticity in this system has been associated with a marked behavioral change, whether this reflects a causal effect on behavior or a homeostatic compensation remains unclear. Furthermore, given that opioids have the opposite effect on dendrite morphology but can also produce sensitization, it seems likely that a broader consideration of the circuit is needed to understand the causal relations between accumbal dendritic profiles and behavior.
In summary, across diverse neurobehavioral paradigms, modifications in the dendrite arbor have been shown to be stimuli-specific, usually cell type-specific and often localized within the dendritic tree. Depending on the brain region and the nature of the stimulus, the dendritic tree can be either elaborated or simplified, adjusting the available surface for synaptic contact, altering the efficacy of other postsynaptic sites, and temporally organizing inputs based on the distance to the spike initiation zone (London and Hausser, 2005). Modeling and empiric studies indicate that the dendrite remodeling would manifest a revised pattern of action potential activity. At the level of behavior, the functional significance of dendrite modifications in these model systems are generally correlational, with a better understanding often hampered by the complexity of the broader circuit and the relevant behavior.
For most of these systems, the latency for the intervention to produce significant changes in dendrite branching seems to require many days. Experience-induced changes in the neocortex occurred within 22 days (Greenough and Volkmar, 1973); stressor-induced changes in the dentate gyrus occurred within 21 days (Watanabe et al., 1992); and psychostimulant induced remodeling in the nucleus accumbens occurred within 35 days (Robinson and Camp, 1987). Given that the effect of progesterone on dendrite length is apparent within four hours makes the VMH-lordosis model system especially valuable for revealing cellular mechanisms of dendrite modification in future studies. In addition, after considering the potential for each of these model systems to link dendrite morphology to behavior, the lordosis offers a clear advantage based on the known direct pathway from the VMH to motor outflow.
Conclusions
The goal of this review was to consider dendrite restructuring in diverse brain systems to guide future hypothesis testing with regard to the role of OT in the regulation of dendrite morphology in VMH. An examination of these other systems with the VMH reveals some specific points for future study. For example, the latency for the intervention to produce significant changes in dendrite branching is often much longer than what has been observed in the VMH. Given that the effect of progesterone on dendrite length is apparent within four hours makes the VMH-lordosis model system especially valuable for revealing cellular mechanisms of dendrite modification in future studies. A future direction would be to assess the role of OT acting as a neuromodulator to facilitate the rapidity of dendrite restructuring. The comparison of these disparate neurobehavioral systems also reveals the broader potential for dendrite regulation in the VMH to serve as a model system for understanding the links between neural plasticity and behavior.
Experimental hurdles to making the causal connection between dendrite morphology and behavior may arise from a poorly defined behavioral function, the complexity of the broader circuit, the complexity of the behavior, and the limited toolkit to selectively manipulate dendrite morphology. Nonetheless, the correlations between dendrite morphology in relevant brain regions and robust behavioral plasticity are striking enough to warrant continued active investigations. Given the plethora of neurologic and psychiatric disorders associated with defects in dendrite length and branching, basic research on the regulation of dendrite structure may offer insights into pathological mechanisms and possible interventions. It bears mentioning that for the VMH, the broader circuits are simple and well defined, and the simple social component of the behavioral output makes it relevant for mental health issues. Thus, the role of OT in VMH neural plasticity offers advantages as a model system for both mechanisms of social behavior and the behavioral significance of dendrite morphology.
Although the role of OT in dendrite remodeling has yet to be studied in these other systems, it is noteworthy that OT receptors are found in abundance certain cortical regions, especially the piriform cortex, the hippocampus (Yoshimura et al., 1993), and the nucleus accumbens in certain species (Young et al., 2001). At the behavioral level, OT promotes social perception, learning and memory, and social incentives (Ross and Young, 2009). Thus, it is possible that OT contributes to dendrite remodeling in these other neurobehavioral systems. Likewise, it is noteworthy that estradiol regulates dendrite morphology in the neocortex and hippocampus (Nuñez et al., 2000; Woolley et al., 1990a; Woolley and McEwen, 1993), dopamine release in the nucleus accumbens, and acquisition of self-administration of psychostimulants (Zhao and Becker, 2010). This suggests that gonadal steroids may have broad effects to enhance the structural plasticity that underlies learning.
As summarized in Table 1, dendrite regulation in the VMH can be contrasted with the other systems we have discussed. We propose that the unique attributes of dendrite plasticity in the VMH represent opportunities to uncover underlying mechanisms that may be similar but harder to study in other systems. By the same token, the differences already observed in dendrite regulation across these systems may reflect brain region-specific controls for dendrite remodeling. In particular, in some systems, dendrite restructuring is long lasting, such as in the accumbens after psychostimulant treatment, whereas in other cases it is reversible, such as in the hippocampus after recovery from chronic stress. In the VMH, dendrite remodeling is not only reversible, but cycles in a relatively short time frame. Insight into the mechanisms that allow for rapidly reversible dendrite restructuring could potentially provide clues for overturning the behaviorally harmful neuroadaptions of drug addition. Likewise, accelerating the reversal of stress-induced dendrite remodeling in the hippocampus may promote cognitive and emotional health, especially during aging. At the cellular level, we hypothesize that OT actions may be localized to specific zones within the dendritic tree, enhancing a particular aspect of connectivity, which involves the internalization of OT and its receptor, and an interaction with glutamate neurotransmission. Thus, continued research on the plasticity of the OT-labeled connections in the VMH, with an eye toward exploiting unique and generalized mechanisms, has intriguing potential applications to into the neurological basis of social cognition, affect, and motivation.
Table 1.
Brain regions that manifest dendrite plasticity after the relevant treatment conditions. The effects of these treatments on these diverse brain areas are compared based on the reported duration of the treatment needed to observe an effect and whether or not the treatment has been found to be reversible. The effect of ovarian hormones on dendrites in the VMH occurs in a uniquely rapid and rapidly reversible fashion compared with effects of other treatments on other brain regions. Please see the text for a fuller discussion of experimental details.
| VMH | Visual Cortex | Hippocampus | Nu. Accumbens | |
|---|---|---|---|---|
| Treatment | Ovarian hormones | Complex environment | Chronic stress | Chronic psychostimulants |
| Trt. Duration | 3 d | 22 d | 21 d | 35 d |
| Reversibility | Yes, within hours | Persists > 30 d | Yes, within 2 weeks | Persists > 24 d |
Highlights.
Oxytocin is implicated in the biological basis of social behaviors, including female sexual behavior in rats.
This behavior is associated with dendrite remodeling in the hypothalamic ventromedial nucleus.
The role of dendrite remodeling in other neurobehavioral systems is considered, including the occipital cortex, hippocampus, and nucleus accumbens.
It is unclear whether or not dendrite remodeling shares mechanisms across brain regions, but glutamate has been repeatedly implicated.
Future studies should address the conspicuous gaps in our understanding of dendrite regulation and its role of behavioral plasticity.
Acknowledgements
We thank Drs. Bruce S. McEwen and John D. Salamone for helpful discussions during the preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arletti R, Bertolini A. Oxytocin stimulates lordosis behavior in female rats. Neuropeptides. 1985;6:247–253. doi: 10.1016/0143-4179(85)90095-2. [DOI] [PubMed] [Google Scholar]
- Armstrong WE, et al. Plasticity in the electrophysiological properties of oxytocin neurons. Microsc Res Tech. 2002;56:73–80. doi: 10.1002/jemt.10019. [DOI] [PubMed] [Google Scholar]
- Bale TL, Dorsa DM. Regulation of oxytocin receptor messenger ribonucleic acid in the ventromedial hypothalamus by testosterone and its metabolites. Endocrinology. 1995b;136:5135–5138. doi: 10.1210/endo.136.11.7588251. [DOI] [PubMed] [Google Scholar]
- Bale TL, et al. Oxytocin receptor mRNA expression in the ventromedial hypothalamus during the estrous cycle. J. Neurosci. 1995c;15:5058–5064. doi: 10.1523/JNEUROSCI.15-07-05058.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benelli A, et al. Oxytocin enhances, and oxytocin antagonism decreases, sexual receptivity in intact female rats. Neuropeptides. 1994;27:245–250. doi: 10.1016/0143-4179(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Bennett EL, et al. Chemical and anatomical plasticity of brain. Science. 1964;146:610–619. doi: 10.1126/science.146.3644.610. [DOI] [PubMed] [Google Scholar]
- Berardi N, et al. Molecular basis of plasticity in the visual cortex. Trends Neurosci. 2003;26:369–378. doi: 10.1016/S0166-2236(03)00168-1. [DOI] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology. 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Bilkey D, Schwartzkroin P. Variation in electrophysiology and morphology of hippocampal CA3 pyramidal cells. Brain Res. 1990;514:77–83. doi: 10.1016/0006-8993(90)90437-g. [DOI] [PubMed] [Google Scholar]
- Black JE, et al. Pathology of layer V pyramidal neurons in the prefrontal cortex of patients with schizophrenia. American Journal of Psychiatry. 2004;161:742–744. doi: 10.1176/appi.ajp.161.4.742. [DOI] [PubMed] [Google Scholar]
- Bloss EB, et al. Interactive effects of stress and aging on structural plasticity in the prefrontal cortex. J. Neurosci. 2010;30:6726–6731. doi: 10.1523/JNEUROSCI.0759-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth C, et al. An ex vivo multielectrode approach to evaluate endogenous hormones and receptor subtype pharmacology on evoked and spontaneous neural activity with the ventromedial hypothalamus: Translation from female receptivity. J. Sexual Medicine. 2010;7:2411–2423. doi: 10.1111/j.1743-6109.2010.01843.x. [DOI] [PubMed] [Google Scholar]
- Briones TL, et al. Stability of synaptic plasticity in the adult rat visual cortex induced by complex environment exposure. Brain Research. 2004;1018:130–135. doi: 10.1016/j.brainres.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Caldwell JD, et al. A uterotonic antagonist blocks the oxytocin-induced facilitation of female sexual receptivity. Brain Res. 1990;512:291–296. doi: 10.1016/0006-8993(90)90639-S. [DOI] [PubMed] [Google Scholar]
- Caldwell JD, et al. Oxytocin facilitates the sexual receptivity of estrogen-treated female rats. Neuropeptides. 1986;7:175–189. doi: 10.1016/0143-4179(86)90093-4. [DOI] [PubMed] [Google Scholar]
- Calizo LH, Flanagan-Cato LM. Estrogen selectively induces dendritic spines within the dendritic arbor of rat ventromedial hypothalamic neurons. J. Neurosci. 2000;20:1589–1596. doi: 10.1523/JNEUROSCI.20-04-01589.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagnac-Amitai Y, et al. Burst generating and regular spiking layer 5 pyramidal neurons of rat neocortex have different morphological features. J Comp Neurol. 1990;296:598–613. doi: 10.1002/cne.902960407. [DOI] [PubMed] [Google Scholar]
- Conrad CD, et al. Chronic stress impairs rat spatial memory on the Y-Maze and this effect is blocked by tianeptine pre-treatment. Behav Neurosci. 1996;110:1321–1334. doi: 10.1037//0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, et al. Brain corticosteroid receptors: studies on the mechanism, function, and neurotoxicity of corticosteroid action. Ann. New York Acad. Sci. 1994;746:1–499. [PubMed] [Google Scholar]
- De Kloet ER, et al. Estradiol modulates density of putative ‘oxytocin receptors’ in discrete rat brain regions. Neuroendocrinology. 1986;44:415–421. doi: 10.1159/000124680. [DOI] [PubMed] [Google Scholar]
- Deng W, et al. New neurons and new memories: How does adult hippocampal neurogenesis affect learning and memory? Nature reviews. Neuroscience. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörner G, et al. Differential localization of a male and a female hypothalamic mating centre. J Reprod Fertil. 1968;17:583–586. doi: 10.1530/jrf.0.0170583. [DOI] [PubMed] [Google Scholar]
- Duffy SN, et al. Environmental enrichment modifies the PKA-dependence of hippocampal LTP and improves hippocampus-dependent memory. Learn Mem. 2001;8:26–34. doi: 10.1101/lm.36301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eva C, et al. Neuroanatomical and pharmacological evidence for a functional interaction between GABAergic and NPY-Y1 transmission in the amygdala of Y 1R/LacZ transgenic mice. Critical Reviews in Neurobiology. 2004;16:33–41. doi: 10.1615/critrevneurobiol.v16.i12.30. [DOI] [PubMed] [Google Scholar]
- Gaudilliere B, et al. A CaMKII-NeuroD signaling pathway specifies dendritic morphogenesis. Neuron. 2004;41:229–241. doi: 10.1016/s0896-6273(03)00841-9. [DOI] [PubMed] [Google Scholar]
- Georgescu M, Pfaus JG. Role of glutamate receptors in the ventromedial hypothlamus in the regulation of female rat sexual behaviors. I. Behavioral effects of glutamate and its receptor agonists, AMPA, NMDA and kainate. Pharmacol. Biochem. Behav. 2006;83:322–332. doi: 10.1016/j.pbb.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Georgescu M, Pfaus JG. Role of glutamate receptors in the ventromedial hypothalamus in the regulation of female rat sexual behaviors. II. Behavioral effects of selective glutamate receptor antagonists AP-5, CNQX, and DNQX. Pharmacol Biochem Behav. 2006b;83:333–341. doi: 10.1016/j.pbb.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Gorzalka BB, Lester GLL. Oxytocin-induced facilitation of lordosis behavior in rats is progesterone-dependent. Neuropeptides. 1987;10:55–65. doi: 10.1016/0143-4179(87)90089-8. [DOI] [PubMed] [Google Scholar]
- Greenough WT, Volkmar FR. Pattern of dendritic branching in occipital cortex of rats reared in complex environments. Experimental Neurology. 1973;40:491–504. doi: 10.1016/0014-4886(73)90090-3. [DOI] [PubMed] [Google Scholar]
- Griffin GD, et al. Ovarian hormone-induced reorganization of oxytocin-labeled dendrites and synapses lateral to the hypothalamic ventromedial nucleus in female rats. J. Comp. Neurol. 2010;518:4531–4545. doi: 10.1002/cne.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin GD, Flanagan-Cato LM. Estradiol and progesterone differentially regulate the dendritic arbor of neurons in the hypothalamic ventromedial nucleus of the female rat (Rattus norvegicus). J. Comp. Neurol. 2008;510:631–640. doi: 10.1002/cne.21816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, et al. Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. Neuroimage. 2010;53:593–601. doi: 10.1016/j.neuroimage.2010.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, Kater SB. Dendritic spines: cellular specializations imparting both stability and flexibility to synaptic function. Annu. Rev. Neurosci. 1994;17:341–371. doi: 10.1146/annurev.ne.17.030194.002013. [DOI] [PubMed] [Google Scholar]
- Holloway RL. Dendritic branching: some preliminary results of training and complexity in the rat visual system. Brain Res. 1966;2:393–396. doi: 10.1016/0006-8993(66)90009-6. [DOI] [PubMed] [Google Scholar]
- Hoyle CHV. Neuropeptide families and their receptors: evolutionary perspectives. Brain Res. 1999;848:1–25. doi: 10.1016/s0006-8993(99)01975-7. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, et al. Localization and osmotic regulation of vesicular glutamate transporter-2 in magnocellular neurons of the rat hypothalamus. Neurochemistry International. 2006;48:753–761. doi: 10.1016/j.neuint.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Israel JM, Poulain DA. 17-Oestradiol modulates in vitro electrical properties and responses to kainate of oxytocin neurones in lactating rats. J Physiol. 2000;524:457–470. doi: 10.1111/j.1469-7793.2000.t01-2-00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan Y-N, Jan LY. Branching out: mechanisms of dendritic arborization. Nat Neurosci Rev. 2010;11:316–328. doi: 10.1038/nrn2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski J, et al. Control of dendritic arborization by the phosphoinositide-3'-kinase-Akt-mammalian target of rapamycin pathway. J. Neurosci. 2005;25:11300–11312. doi: 10.1523/JNEUROSCI.2270-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann WE, Moser HW. Dendritic anomalies in disorders associated with mental retardation. Cerebral Cortex. 2000;10:981–991. doi: 10.1093/cercor/10.10.981. [DOI] [PubMed] [Google Scholar]
- Kennedy GC. Hypothalamic control of the endocrine and behavioral changes associated with oestrus in the rat. J Physiol. 1964;172:383–392. doi: 10.1113/jphysiol.1964.sp007426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kow L-M, et al. Electrophysiological actions of oxytocin on hypothalamic neurons in vitro: Neuropharmacological characterization and effects of ovarian steroid hormones. Neuroendocrinology. 1991;54:526–535. doi: 10.1159/000125948. [DOI] [PubMed] [Google Scholar]
- Kumar V, et al. Regulation of dendrite morphogenesis by Ras-PI3K-Akt-mTOR and Ras-MAPK signaling pathways. J. Neurosci. 2005;25:11288–11299. doi: 10.1523/JNEUROSCI.2284-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, et al. Psychological stress reversibly disrupts prefrontal processing and attentional control. Proc Natl Acad Sci U S A. 2009;106:912–917. doi: 10.1073/pnas.0807041106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London M, Hausser M. Dendritic computation. Annu. Rev. Neurosci. 2005;28:503–532. doi: 10.1146/annurev.neuro.28.061604.135703. [DOI] [PubMed] [Google Scholar]
- Luine V, et al. Repeated stress causes reversible impairments of spatial memory performance. Brain Res. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- Madeira MD, et al. Influence of sex and estrus cycle on the sexual dimorphisms of the hypothalamic ventromedial nucleus: stereological evaluation and Golgi study. J. Comp. Neurol. 2001;432:329–345. doi: 10.1002/cne.1106. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, et al. Rapid and reversible changes in intrahippocampal connectivity during the course of hibernation in European hamsters. Proc Natl Acad Sci U S A. 2006;49:18775–18780. doi: 10.1073/pnas.0608785103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainen Z, Sejnowski T. Influence of dendritic structure on firing patterns in model neocortical neurons. Nature. 1996;382:363–366. doi: 10.1038/382363a0. [DOI] [PubMed] [Google Scholar]
- Mason A, Larkman A. Correlations between morphology and electrophysiology of pyramidal neurons in slices of rat visual cortex. II. Electrophysiology. J Neurosci. 1990;10:1415–1428. doi: 10.1523/JNEUROSCI.10-05-01415.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister AK, et al. Neurotrophins regulate dendritic growth in developing visual cortex. Neuron. 1995;15:791–803. doi: 10.1016/0896-6273(95)90171-x. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, et al. Excitatory amino acid modulation of lordosis in the rat. Neurosci. Lett. 1991;126:94–97. doi: 10.1016/0304-3940(91)90380-c. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, et al. Infusion of antisense oligonucleotides to the oxytocin receptor in the ventromedial hypothalamus reduces estrogen-induced sexual receptivity and oxytocin receptor binding in the female rat. Neuroendocrinology. 1994;59:432–440. doi: 10.1159/000126689. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annu. Rev. Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress, sex, and neural adaptation to a changing environment: mechanisms of neuronal remodeling. Ann. New York Acad. Sci. 2010;1204:E38–E59. doi: 10.1111/j.1749-6632.2010.05568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, et al. Selective retention of corticosterone by limbic structures in rat brain. Nature. 1968;220:911–912. doi: 10.1038/220911a0. [DOI] [PubMed] [Google Scholar]
- Meredith GE, et al. The structural basis for mapping behavior onto the ventral striatum and its subdivisions. Brain Structure & Function. 2008;213:17–27. doi: 10.1007/s00429-008-0175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millhouse OE. The organization of the ventromedial hyothalamic nucleus. Brain Res. 1973;55:71–87. [PubMed] [Google Scholar]
- Nilsson M, et al. Enriched environment increases neurogenesis in the adult rat dentate gyrus and improves spatial memory. J Neurobiol. 1999;39:569–578. doi: 10.1002/(sici)1097-4695(19990615)39:4<569::aid-neu10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Ninan I. Oxytocin suppresses basal glutamatergic transmission but facilitates activity-dependent synaptic potentiation in the medial prefrontal cortex. J Neurochemistry. 2011;119:324–331. doi: 10.1111/j.1471-4159.2011.07430.x. [DOI] [PubMed] [Google Scholar]
- Nuñez JL, et al. Androgens reduce cell death in the developing rat visual cortex. Developmental Brain Research. 2000;125:83–88. doi: 10.1016/s0165-3806(00)00126-7. [DOI] [PubMed] [Google Scholar]
- Pardo CA, Eberhart CG. The neurobiology of autism. Brain Pathology. 2007;17:434–447. doi: 10.1111/j.1750-3639.2007.00102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasinetti GM, et al. Personalized medicine in traumatic brain injury. Psychiatric Clinics of North America. 2010;33:905–913. doi: 10.1016/j.psc.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Pfaff DW, Sakuma Y. Deficit in the lordosis reflex of female rats caused by lesions in the ventromedial nucleus of the hypothalamus. J Physiol. 1979;288:203–210. [PMC free article] [PubMed] [Google Scholar]
- Ponzio TA, et al. Vesicular glutamate transporter expression in supraoptic neurones suggests a glutamatergic phenotype. Journal of Neuroendocrinology. 2006;18:253–265. doi: 10.1111/j.1365-2826.2006.01410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinones-Jenab V, et al. Effects of estrogen on oxytocin receptor messenger ribonucleic acid expression in the uterus, pituitary, and forebrain of the female rat. Neuroendocrinology. 1997;65:9–17. doi: 10.1159/000127160. [DOI] [PubMed] [Google Scholar]
- Ramon y Cajal S. Histologie due Systems Nerveux de l'homme et des Vertebres. Maloine; Paris: 1911. [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res Rev. 1986;11:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Camp DM. Long lasting effects of escalating doses of D-amphetamine on brain monoamines, amphetamine-induced stereotyped behavior, and spontaneous nocturnal locomotion. Pharmacol Biochem Behav. 1987;26:821–827. doi: 10.1016/0091-3057(87)90616-2. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J. Neurosci. 1997;17:8491–8497. doi: 10.1523/JNEUROSCI.17-21-08491.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur J Neurosci. 1999;11:1598–1604. doi: 10.1046/j.1460-9568.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- Roitman MF, et al. Induction of a salt appetite alters dendritic morphology in nucleus accumbens and sensitizes rats to amphetamine. J. Neurosci. 2002;22:RC225, 1–5. doi: 10.1523/JNEUROSCI.22-11-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Frontiers in Neuroendocrinology. 2009;30:534–547. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin BS, Barfield RJ. Induction of estrous behavior in ovariectomized rats by sequential replacement of estrogen and progesterone to the ventromedial hypothalamus. Neuroendocrin. 1983;37:218–224. doi: 10.1159/000123546. [DOI] [PubMed] [Google Scholar]
- Russo SJ, et al. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci. 2010;33:267–276. doi: 10.1016/j.tins.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai R, et al. Salt appetite is enhanced by one prior episode of sodium depletion in the rat. Behav Neurosci. 1987;101:724–731. doi: 10.1037//0735-7044.101.5.724. [DOI] [PubMed] [Google Scholar]
- Salamone JD, et al. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology. 2007;191:461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- Sapolsky R. Stress, the Aging Brain and the Mechanisms of Neuronal Death. MIT Press; Cambridge: 1992. p. 423. [Google Scholar]
- Satoh D, et al. Spatial control of branching within dendritic arbors by dynein-dependent transport of Rab5-endosomes. Nature Cell Biology. 2008;10:1164–1171. doi: 10.1038/ncb1776. [DOI] [PubMed] [Google Scholar]
- Schubert V, Dotti CG. Transmitting on actin: synaptic control of dendritic architecture. J. Cell Sci. 2007;120:205–212. doi: 10.1242/jcs.03337. [DOI] [PubMed] [Google Scholar]
- Schumacher M, et al. Localized actions of progesterone in hypothalamus involve oxytocin. Proc Natl Acad Sci USA. 1989;86:6798–6801. doi: 10.1073/pnas.86.17.6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher M, et al. Behavioral effects of progesterone associated with rapid modulation of oxytocin receptors. Science. 1990;250:691–694. doi: 10.1126/science.2173139. [DOI] [PubMed] [Google Scholar]
- Teskey GC, et al. Neocortical kindling is associated with opposing alterations in dendritic morphology in neocortical layer V and striatum from neocortical layer III. Synapse. 2006;59:1–9. doi: 10.1002/syn.20215. [DOI] [PubMed] [Google Scholar]
- Theodosis DT. Oxytocin-secreting neurons: a physiological model of morphological neuronal and glial plasticity in the adult hypothalamus. Front Neuroendocrinol. 2002;23:101–135. doi: 10.1006/frne.2001.0226. [DOI] [PubMed] [Google Scholar]
- van Elburg RAJ, van Ooyen A. Impact of Dendritic Size and Dendritic Topology on Burst Firing in Pyramidal Cells. PLoS Comput Biol. 2010;6:e1000781. doi: 10.1371/journal.pcbi.1000781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmar FR, Greenough WT. Rearing complexity affects branching of dendrites in the visual cortex of the rat. Science. 1972;176:1445–1447. doi: 10.1126/science.176.4042.1445. [DOI] [PubMed] [Google Scholar]
- Wandinger KP, et al. Anti-NMDA-receptor encephalitis: a severe, multistage, treatable disorder presenting with psychosis. Journal of Neuroimmunology. 2011:86–91. doi: 10.1016/j.jneuroim.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, et al. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brian Research. 1992;588:341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- Witt DM, Insel TR. A selective oxytocin antagonist attenuates progesterone facilitation of female sexual behavior. Endocrinology. 1991;128:3269–3276. doi: 10.1210/endo-128-6-3269. [DOI] [PubMed] [Google Scholar]
- Woolley CS, et al. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J. Neurosci. 1990a;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, et al. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Research. 1990b;531:225–231. doi: 10.1016/0006-8993(90)90778-a. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J. Comp. Neurol. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- Woolley CS, et al. Estradiol increases the frequency of multiple synapse boutons in the hippocampal CA1 region of the adult female rat. J. Comp. Neurol. 1996;373:108–117. doi: 10.1002/(SICI)1096-9861(19960909)373:1<108::AID-CNE9>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Wright RL, Conrad CD. Chronic stress leaves novelty-seeking behavior intact while impairing spatial recognition memory in the Y-maze. Stress. 2005;8:151–154. doi: 10.1080/10253890500156663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, et al. A quantitative Golgi study of basal dendrites of hippocampal CA1 pyramidal cells in senile dementia of Alzheimer type. J Neurol Neurosurg Psyhiatry. 1988;51:1088–1090. doi: 10.1136/jnnp.51.8.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye B, et al. Nanos and pumilio are essential for dendrite morphogenesis in Drosophila peripheral neurons. Current Biology. 2004;14:314–321. doi: 10.1016/j.cub.2004.01.052. [DOI] [PubMed] [Google Scholar]
- Ye B, et al. Growing Dendrites and Axons Differ in Their Reliance on the Secretory Pathway. Cell. 2007;130:717–729. doi: 10.1016/j.cell.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura R, et al. Localization of oxytocin receptor messenger ribonucleic acid in the rat brain. Endocrinology. 1993;133:1239–1246. doi: 10.1210/endo.133.3.8396014. [DOI] [PubMed] [Google Scholar]
- Young LJ, et al. Cellular mechanisms of social attachment. Horm Behav. 2001;40:133–138. doi: 10.1006/hbeh.2001.1691. [DOI] [PubMed] [Google Scholar]
- Zhao W, Becker JB. Sensitization enhances acquisition of cocaine self-administration in female rats: Estradiol further enhances cocaine intake after acquisition. Hormones and Behavior. 2010;58:8–12. doi: 10.1016/j.yhbeh.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]