Abstract
As part of a festschrift issue for Philip Teitelbaum, I offer here the thesis that Teitelbaum deserves to be viewed as an important forefather to the contemporary field of affective neuroscience (which studies motivation, emotion and affect in the brain). Teitelbaum’s groundbreaking analyses of motivation deficits induced by lateral hypothalamic damage, of roles of food palatability in revealing residual function, and of recovery of ‘lost’ functions helped shape modern understanding of how motivation circuits operate within the brain. His redefinition of the minimum requirement for identifying motivation raised the conceptual bar for thinking about the topic among behavioral neuroscientists. His meticulous analyses of patterned stages induced by brain manipulations, life development and clinical disorders added new dimensions to our appreciation of how brain systems work. His steadfast highlighting of integrative functions and behavioral complexity helped provide a healthy functionalist counterbalance to reductionist trends in science of the late 20th century. In short, Philip Teitelbaum can be seen to have made remarkable contributions to several domains of psychology and neuroscience, including affective neuroscience.
Keywords: Motivation, Hypothalamus, Dopamine, Affective neuroscience, Recovery of function, Reward, Nucleus accumbens, Ventral pallidum, Behavior patterns
“It is as inappropriate to apply reductionism to psychology as it would be to apply it to mathematics.”
Teitelbaum and Pellis (page 5, 1992).
1. Introduction
This special issue of Behavioural Brain Research presents an ideal opportunity to reflect upon the contributions of a giant within the field of psychological neuroscience: Philip Teitelbaum. Especially for anyone interested in physiological psychology, behavioral neuroscience or the psychology of motivation, Teitelbaum will always be recognized as one of the most influential scientists of the past century.
Over more than 50 years, Teitelbaum has consistently contributed original conceptual analyses and classic experimental demonstrations to behavioral neuroscience. He repeatedly helped the field to think in fresh ways about how brain systems generate complex behavior. His work has also offered insights into a variety of clinical disorders. In particular, his classic studies have advanced understanding of motivational systems of the brain, and of the organization and control of behavioral patterns. A few of these outstanding themes and contributions are discussed below.
2. Affective neuroscience
I want to focus here on an aspect of Teitelbaum’s contributions that might otherwise go unrecognized: namely, his helping to set the stage for contemporary affective neuroscience. Today the field of affective neuroscience comprises brain mechanisms of pleasures and displeasures (affect), emotions and motivations. It has emerged fully as a scientific discipline only in the past decade, and I believe the term affective neuroscience was first used in the early 1990s (by Panksepp in discussing animal studies of emotion, and by Davidson and Sutton in a review of animal and human emotion [16,46]). But the conceptual and empirical seeds of affective neuroscience were planted far earlier [8,10,45,51,67,88,89,95,97], and in my view some notable seeds were planted by Teitelbaum and co-workers [75,79].
I hope to paint a brief portrait of Philip Teitelbaum’s seminal contributions to understanding brain mechanisms of motivation and affect. It is probably important to acknowledge at the outset that that Teitelbaum’s own writings only rarely make explicit mention of affective topics such as pleasure/aversion (which indeed were rather difficult to write about explicitly in the reductionist–behaviorist zeitgeist of the 1950s–1980s). But still I think that Philip Teitelbaum’s work can be seen to have consistently pushed against the restrictive boundaries of that zeitgeist, and to have made important advances relevant to affective neuroscience. He empirically explored hedonic aspects of brain mechanisms of motivation, and conceptually introduced more sophistication into motivation definitions. I will try to bring this out a bit here.
3. Structure in behavior and motivation
Again and again over his career, Philip Teitelbaum pointed out how patterns of structure could be discerned within behavior, motivation processes, and their relation to brain organization. I characterize this Teitelbaum theme as ‘whatever exists, has structure’. Behavior and motivation processes exist, and have their own patterns of structure that can be captured by scientific study. Behavioral structures exist as patterns of movement in time and space [17,21–24,58,73]. Motivation structure exists as operating rules for psychological processes that control goal-directed behavior [4,75,79,82]. Teitelbaum’s work helped to show that structure exists within these behavioral and psychological processes as vividly as within physical brain systems.
4. Stages and change over time
A major theme for Teitelbaum was how patterns of behavior change over time. This temporal change theme can be found in his work on recovery of function after brain damage, on the series of pharmacological effects of a drug on behavior at several time points, and on early life stages developmental changes in competence and behavior [73,79,81,83].
4.1. Ingestive recovery of function after hypothalamic lesions
For motivation neuroscience, perhaps the most famous example of the temporal change theme in Teitelbaum’s work was his ground-breaking analysis of the stages of recovery for eating and drinking after brain lesions in the lateral hypothalamus (LH) [31,54,78,79,81]. Lesions of LH initially abolished all intake, so that the rats needed intensive nursing and intra-gastric feeding of food and water in order not to starve to death. But if nutrition and care were artificially provided, then over weeks the abilities to eat and drink gradually and incrementally returned.
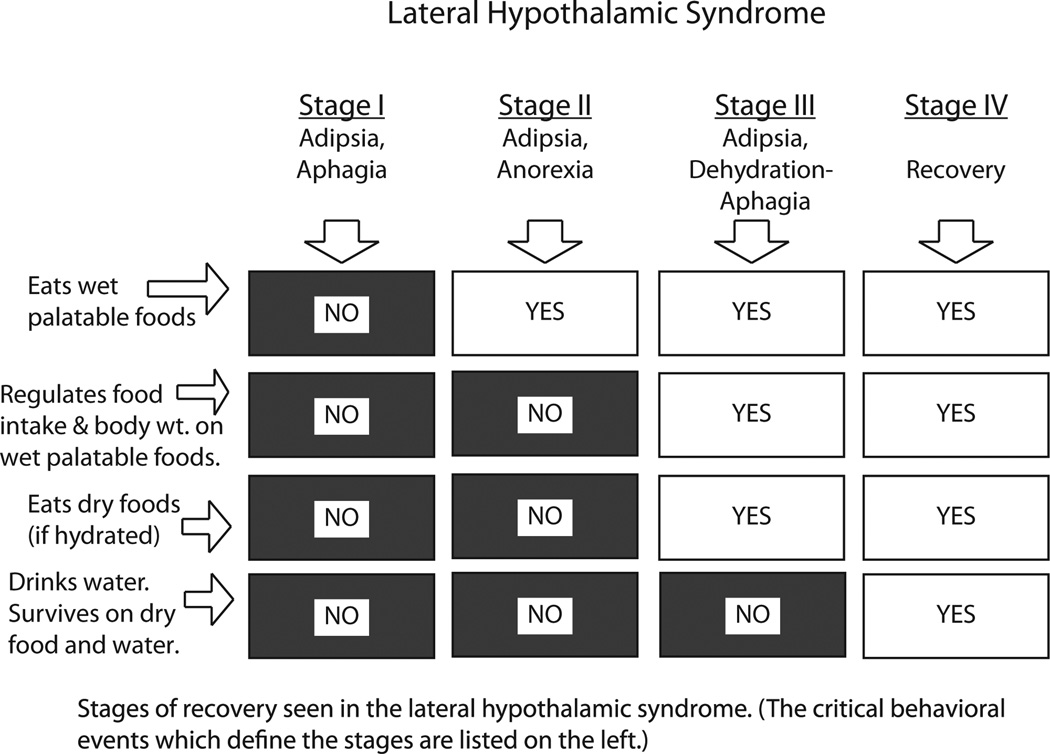
With colleagues including Eliot Stellar and Alan Epstein, Teitelbaum showed in a beautiful set of 1950s–1970s experiments how ingestive function returned in what could be viewed as a series of temporal stages (Fig. 1). Gradually, after 10 or 20 days, LH-lesion rats could be tempted by bits of chocolate to eat small amounts [79]. With further time, the rats would eat more of chocolate, milk or palatable cereal to the extent that they began to maintain their own body weight, allowing artificial feeding to be reduced. Eventually the rats became willing to eat chow pellets of mere normal palatability in sufficient quantity to remain healthy all by themselves. Soon the rats also would drink water, at least in conjunction with meals, so as not need intragastric water supplements. Yet Teitelbaum and colleagues’ careful analyses revealed that the rats were still not completely normal. Some ingestive functions remained always impaired, such as the ability to detect specific internal physiological cues for hunger or for thirst, or to learn revaluations of specific foods [42,55,56,93]. In a further intriguing extension of this analysis to normal development stages, Teitelbaum and colleagues also compared the acquisition of new behavioral competencies in early life, as rat pups proceeded from birth through infancy to weaning and adolescence, to the reacquisition of old competencies in the stages of recovery after brain lesions [77].
Fig. 1.
Stages of recovery of function after lateral hypothalamic lesions. Redrawn from Figure 3 of 1962 review article by Teitelbaum and Epstein [79].
The real issue for Teitelbaum was never merely localization of hunger in the lateral hypothalamus. To the contrary, his results along with those of others helped show that localization of function in the strongest sense can be overly simplistic [25,88]. For example, it would be wrong to think that hunger resided within the lateral hypothalamus simply because LH lesions made rats starve to death unless artificially fed. The lost functions did not need to be thought of as entirely contained within the spot where lesions disrupted them. Instead the LH site was merely an especially important node within a larger distributed circuit. The circuit became unbalanced by the lesion, producing a loss of function afterwards. But most of the brain circuit remained left behind, and the gradual recovery of some ingestive behavior showed that the residual circuit was still capable in principle of generating much of the ‘lost’ function [24,71,79]. Thus Teitelbaum and colleagues helped show both what was missing immediately after the lesion and what remained, and how what remained could be functionally pulled together again over time.
4.2. Motivation and hedonic impact mediation by hypothalamus, dopamine projection, and ventral pallidum neurons
A second major theme was separating the symptoms of hypothalamic lesions, and assignment of particular symptoms to particular substrates. For the classic LH lesion studies, this separation theme led to the eventual assignment of responsibility for particular motivational and hedonic symptoms to their respective neurobiological sources of damage.
The original LH lesions were large and neurally indiscriminate, destroying several neural systems within a radius of the coagulating electrode. Neurons that had cell bodies residing in LH were destroyed, but equally destroyed were also axon fibers that merely passed through the lateral hypothalamus on their way from another source to somewhere else, such as dopamine fibers ascending from the midbrain to forebrain targets. Anatomically, the lesions extended to damage not only a large extent of the lateral hypothalamus but often penetrated into adjacent structures that today would be recognized as important and anatomically distinct entities with their own names and functions.
First, the roles of local neuronal cell bodies versus travelling axon fibers were gradually teased apart by selective neurochemical lesions. In the 1970s, further experiments by Teitelbaum and colleagues compared recovery after nigrostriatal dopamine depletions, induced by 6-OHDA lesions of the axon pathways that run through lateral hypothalamus, and which induce a number of the same adipsia and aphagia consequences as LH lesions [41]. Additional analyses, some by others, subsequently teased apart separate symptoms for local neurons [69,87,94]. For example, symptoms of aphagia and adipsia are now known to be produced by selective neurochemical destruction of intrinsic neurons of the lateral hypothalamus that spare dopamine fibers (caused by excitotoxin microinjections: chemicals that stimulate glutamate receptors on cell bodies to overly depolarize neurons and cause them to die) [69,87,94]. By comparison, a partly overlapping but partly different range of symptoms can be produced by selective destruction of dopamine fibers from the midbrain to the neostriatum and nucleus accumbens, neurochemically targeted by a drug such as 6-hydroxydopamine, even while preserving the LH neurons [69,87,94].
The early LH lesions were also large anatomically, with diameters that extended beyond the hypothalamus itself to impinge upon bits of other structures that lay outside hypothalamic borders, including structures anterior and lateral to the hypothalamus. In the 1960s, those other structures were sometimes thought of as part of the greater lateral hypothalamus area but today the anterior-lateral region of ventral forebrain is recognized to contain several distinct structures with their own distinctive neuronal compositions, circuitry connections, and functions. These outside regions include the ventral pallidum, which is in front and immediately adjacent to the lateral hypothalamus, the basal nucleus (which is often spoken of in connection with acetylcholine projections to the neocortex) and the sublenticular extended amygdala (part of the larger extended amygdala complex that also includes the central and medial nuclei of the amygdala and the bed nucleus of the stria terminalis) [28,29,70,92].
Later studies have examined the separable roles of each of these anatomical structures. For example, a hedonic symptom of disgust and aversion is produced by the large LH lesions that damaged outside structures too [79]. In the excessive disgust syndrome, even sweet tastes elicit ‘disliking’ reactions as though they had become bitter. As Teitelbaum and Epstein wrote, the LH rat “actively resists having milk placed in its mouth by a medicine dropper, and it does not swallow the milk once it is there…”, but instead “does engage in the same paw-waving and wiping, chin-rubbing, poor grooming and rejection (as) when very bitter quinine (1% weight/volume is put in its mouth… This suggests that mouth contact with food and water is highly aversive to a rat with lateral (hypothalamic) lesions during this stage”. (pp. 75–6) [79].
Yet later analyses by Schallert and Whishaw, by J. Stellar and colleagues, and by Cromwell showed that the excessive disgust is produced by neither LH neuron death nor dopamine fiber death per se. Instead, hedonic disgust is produced only by larger or different lesions that extend anteriorly past the lateral hypothalamus itself [57,68] enough to project outside the hypothalamus and penetrate anteriorly-laterally into the caudal ventral pallidum [3,15,30,64]. For rats with such excitotoxin lesions that destroy neurons in ventral pallidum, even sugar water placed in the mouth is actively rejected with signs of disgust such as gapes, headshakes and forelimb flails. By contrast, equivalent damage restricted to the lateral hypothalamus does not produce the disgust syndrome, even if those rats have much greater LH damage than the rats with ventral pallidum lesions that do have exaggerated disgust [15]. The original electrolytic lesions of the lateral hypothalamus were typically large enough to damage the ventral pallidum hotspot as well as the lateral hypothalamus [57,68,79], and it may have been the ventral pallidum damage which was the real culprit in generating the hedonic reversal symptom of exaggerated disgust [15].
5. Principles of psychology and brain function
Beyond the themes above, several additional important principles emerge in Teitelbaum’s work. One principle is hierarchy and levels of function. This hierarchical principle addresses how higher levels of brain systems control the activity of lower brain levels. Hierarchy has a classical origin in neuroscience a century ago in Sherrington’s studies of complex reflexes after spinal or brainstem transections in animals, in Hughlings-Jackson’s studies of cortical lesion disruption of voluntary movements in human patients, and in Bard and Cannon’s studies on the release of aggression by removal of the forebrain in animals (based on comparisons of forebrain ablation above the thalamus [the so-called ‘thalamic preparation’] or transection below the hypothalamus [midbrain decerebration]) [2,11,32,59]. In all of those analyses, adding top layers of the brain onto lower layers gave additional psychological complexity or behavioral flexibility. A corresponding idea is that normal behavior and mind results from all the levels working together as a whole, in which the higher layers govern the lower layers but also need those lower layers in order for higher functions to be expressed. Even the highest cortical brain structures are incomplete by themselves without lower levels, just as lower levels are incomplete without the higher ones.
Teitelbaum’s work helped show that hierarchical levels of control can be discerned also in the behavioral effects of hypothalamic lesions and in recovery of functions over time after lesions. For example, Teitelbaum referred to the utter lack of motivation or movement by a rat in the early stage of a LH lesion as a ‘zerocondition’ or state of total apathy [76]. In the zero-condition, almost no function could be discerned [75,76,80,82], but as functions returned the system became more normal. The zero-condition may also be approached by the suppressive effects of drugs on behavioral patterns, and even in early life stages of development.
A related principle is the idea that the structure of organization within normal behavior could be exposed by simplifying it. Simplification could be induced via the zero-condition, or otherwise by simplifying the brain via transection, lesion or drug that reduced behavioral competency in a more graded fashion. Teitelbaum and colleagues’ innovative genius in this regard was to use the manipulations mentioned above—local hypothalamic brain lesions, circuit-wide dopamine depletion, dopamine blocking drugs or even early stages of life – combined with meticulous analyses of detailed patterns of behavior—as tools to reveal underlying structure [12,13,24,49,50,58,72]. A complex pattern of behavior could be taken apart to reveal cascades of coordinated reflexes contained inside. Clarification of how the brain worked could be achieved in this way by removing some of the higher complexity that normally obscures the simpler layers. Just as spinal or decerebrate transection simplifies a brain by stripping away higher control levels, brain lesions drugs or early life stages could achieve a conceptually similar simplification for understanding how brain systems control behavior.
A final related principle suggested by Teitelbaum and colleagues was re-synthesis: the need to try to re-build normal function back into a simplified system by adding and reconnecting the levels once again [80,82]. In a sense, successful rebuilding is the truest test of whether understanding of a mechanism has been achieved. A truly successful psychological neuroscience thus would be able to rebuild a brain, or at least ‘build a robot’ (in Teitelbaum’s phrase) to model the brain. Of course, no one can yet rebuild a brain or even a robot that has the full equivalent of a brain. But just the exercise of mentally trying to re-assemble the levels in imagination or in computational models can be very useful. Attempting a resynthesis forces one to confront the puzzle of how functional levels might work together to produce normal functions.
6. Defining motivation
Philip Teitelbaum also made a fundamental contribution to the conceptual task of identifying the nature of motivation. Traditionally in psychology and neuroscience through the 1960s, motivation was conceived simply as a homeostatic drive, triggered by internal depletion needs, or as a mere intervening variable (an intervening variable is a labeled correlation [e.g., thirst] that groups together several stimulus inputs [e.g., water deprivation, excessive heat] which cause similar outputs [e.g., drink more; work harder for a sip]). But drive as an intervening variable is only the most minimalist concept of motivation, as Teitelbaum helped point out. An intervening drive is relatively impoverished and sterile, leaving out lots of what makes motivation interesting in the psychological sense, and lots about how motivation actually works in brain systems.
Even a mere “hungry fly” has motivation in the intervening variable sense of drive. A housefly has a reflex to eat when it lands on food and a reflex to stop eating when its stomach is finally full. Its hunger drive can be viewed as the balance between the two reflexes that determines whether the fly continues to eat [18]. Yet this seems hardly satisfying as an understanding of hunger motivation, and it misses most of what hunger is in mammalian brains like ours.
Drive as intervening variable became viewed around 1970 as insufficient as a definition of motivation for many psychologists, including Philip Teitelbaum. In particular, Teitelbaum suggested that real motivation could be recognized not simply by drive as intervening variable but rather only by the capacity to motivate flexible instrumental behavior [74,75]. In practice, he argued, an animal or person must be able to learn a new operant response to gain a goal in order to prove they were motivated for that goal (for example, learning to press a bar for reinforcement). The value of an instrumental or operant response is that it can be selected arbitrarily at the whim of the experimenter, demonstrating that the response was not simply a programmed S-R reflex or an instinctive fixed action pattern such as possessed by the fly. The ability to meet the operant criterion shows the response was flexible, and that the creature was motivated in a crucial sense of the word – in the sense of being willing to do most anything to gain the goal.
Teitelbaum’s operant criterion drew conceptually on an earlier descriptive classification of motivated behavior as appetitive, by the early American ethologist, Wallace Craig (building on even earlier formulations by Charles Sherrington and others) [14,59]. Wallace Craig’s appetitive phase of motivated behavior is the flexible approach or seeking behavior that an animal or person emits before the motivational goal is found. Flexible appetitive behavior helps find the goal. Instrumental behavior or operant responses performed to gain access to a goal are a type of appetitive behavior, easily produced and measured in standard behavioral neuroscience laboratories. Teitelbaum’s criterion helped move the neuroscience of motivation away from a focus on simplistic drives and reflexes and toward incorporating more complex psychological functions that are embedded in real brains [4,47,80,82].
7. Recent advances in understanding brain circuitry of hedonic ‘liking’ and incentive ‘wanting’
In the final part of this essay, I will turn away from Teitelbaum’s seminal work to subsequent developments in the past 15 years in the field of affective neuroscience, especially regarding ‘liking’ and ‘wanting’ mechanisms in motivation for rewards. This is not intended to imply that Teitelbaum would necessarily use similar language or endorse any particular conclusion below. Rather my point is that clarification of how hedonic-motivational processes relate to brain substrates began with Teitelbaum’s highlighting of hedonic features of the LH syndrome (e.g., aversion induction; loss of normal positive reaction to food; need for higher hedonic palatability to elicit residual ingestion during recovery) and with his efforts to raise the conceptual bar for defining motivational states (e.g., having an operant-style goal). Efforts by others to further clarify brain mechanisms of affect and motivation have continued along a path similar to that begun by Teitelbaum, and it may be of interest to place a few newer conclusions in that context.
As highlighted at the beginning: when motivation exists, it has structure. Beginning in the 1970s, experimental results and critical analyses began to chip away at homeostatic drive concepts of motivation structure. Drive concepts were replaced with incentive motivation concepts of structure that did a better job of describing how motivated behavior operates and relates to brain mechanisms [8,9,86] (a history of the drive-incentive transition is summarized in [4]).
Incentive motivation for rewards has in particular the structure of ‘liking’ and ‘wanting’. Rewards carry an important hedonic component: pleasure ‘liking’. Indeed, for many people, pleasure is synonymous with reward. It is a crucial goal for affective neuroscience to understand how pleasure is generated in the brain. Teitelbaum made an important early contribution toward that goal in pointing with Alan Epstein to the disgust symptoms in the early phase of the LH lesion syndrome (though actually due to ventral pallidum lesion syndrome, as discussed above), in which even sweetness becomes reacted to as though it were nasty as bitterness, and the subsequent heightened sensitivity to palatability in the later phase as recovery began to occur [79].
7.1. Affective neuroscience of pleasure and ‘liking’
The goal of understanding how pleasure is generated in the brain has become even more urgent as many of the most promising hedonic brain candidates from the 1960s to 1990s, such as so-called pleasure electrodes or mesolimbic dopamine [27,43,44,96], turn out on closer inspection not to generate much pleasure after all [5,7,36,63]. Identifying the brain mechanisms that actually generate ‘liking’ for pleasures has been a major research focus for my laboratory and colleagues in the past decades, so I would like to say a few words about how progress in understanding pleasure generation has continued to develop in recent years.
7.2. Coding versus causing pleasure
Pleasure is coded by neural activations in many brain systems (Fig. 2). Neuroimaging and neural recording studies of have found that rewards ranging from sweet taste to intravenous cocaine, winning money or a smiling human face activate many brain structures, including orbitofrontal cortex, anterior cingulate and insula, and subcortical structures such as nucleus accumbens, ventral pallidum, ventral tegmentum and mesolimbic dopamine projections, amygdala, etc. [34,38,90,91]. In human cortex, above all, a small midanterior subregion of the orbitofrontal region of prefrontal cortex may code the occurrence of pleasure [33,35,37]. Studies by Kringelbach and by Small, and their colleagues, for example have identified particular regions in human orbitofrontal cortex and in insula cortex that track pleasure decrements in the taste of tomato juice or chocolate candy as sensory-satiety sets in, helping to peel away the hedonic coding of state-dependent pleasure from coding of more stable sensory or motor functions that remain constant [34,37,90]. Deeper in the subcortical forebrain, hedonic coding of taste pleasure ‘liking’ by the firing of neurons within the ventral pallidum hedonic hotspot has been identified by Aldridge and colleagues (the same hedonic hotspot where LH lesions abolish ‘liking’ reactions and make sweetness become disgusting) [1,62,85].
Fig. 2.
Brain systems for ‘liking’ and ‘wanting’ rewards. Hedonic hotspots and hedonic circuits. Hedonic hotspots are shown in nucleus accumbens, ventral pallidum, and brainstem parabrachial nucleus where opioid or other signals cause amplification of core “liking” reactions to sweetness. Pleasure causation circuits for ‘liking’ generation are in red. Additional ‘liking’ coding circuits are in orange. ‘Wanting’ generation circuits involving mesolimbic dopamine are in green. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article).
Reprinted by permission from [63].
But as a general rule the brain contains more codes than causes for any psychological function. My colleagues and I have suggested that only a few of the brain structures that code pleasure actually also cause the pleasure that is coded [7,35,63]. Neural activation studies using electrophysiology or neuroimaging to measure coding of function are likely to implicate a relatively large number of brain structures and wide regions within structures. By contrast, causal studies that stimulate or suppress neural activation to identify psychological consequences may implicate fewer structures or smaller regions as needed for the normal function or as able to generate enhancements in function. The anatomical discrepancy between code and cause simply reflects the fact that neural activation spreads, and that some activations are secondary consequences of pleasure, which in turn are causing something else. The other pleasure-coding structures instead cause their own different functions: learning and remembering about the pleasure, thinking about the pleasure, reacting to the pleasure, introspecting about the experience of the pleasure, etc. Activations in these structures would code pleasure as well as activations in the causal mechanisms themselves.
So, which brain systems that are activated by pleasant events actually also cause the pleasure of that reward? To identify pleasure generators, my colleagues and I have searched for brain systems able to enhance objective ‘liking’ reactions to the pleasure of sweet sensations. We have assessed increases in pleasure by measuring facial ‘liking’ reactions in animals similar to the hedonic facial expressions that sweet tastes elicit from human infants (Fig. 3) [26,65,66]. Combined with painless brain manipulations to activate specific neurobiological systems, these studies reveal which brain systems can generate enhancements of a sensory pleasure.
Fig. 3.
Taste “liking” reactions and detail map of nucleus accumbens hotspot. Facial expressions of a rat, orangutan and human infant to sweet taste of sucrose and to bitter taste of quinine. Only a cubic-millimeter sized hedonic hotspot generates pleasure (red/orange). There increases in ‘liking’ for sweetness are generated by microinjections of a droplet containing drug to stimulates mu opioid receptors. By contrast, increases in ‘wanting’ to eat more food are generated by opioid microinjections throughout the entire nucleus accumbens and in related outside structures (‘wanting’ can also be stimulated by dopamine in the same structures). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article).
In hedonic-causing brain structures, stimulation of a neural system can amplify the hedonic impact of sensations, making sweetness even nicer and bitterness less bad. These causal structures are relatively tiny and specific. Only a few pleasure-generating neurochemical systems have been found so far to enhance positive ‘liking’ reactions to a sweet taste in rats. Even those neurochemicals enhance pleasure only within a few anatomically circumscribed sites. Those sites include cubic-millimeter sized ‘hedonic hotspots’ in subregions of the nucleus accumbens and of the ventral pallidum in the rat brain (Fig. 2) [7,63]. In a human brain, the corresponding hotspots should be about a cubic-centimeter in volume, if proportional to whole-brain size.
Each small anatomical hotspot in nucleus accumbens or ventral pallidum amplifies pulses of intense sensory pleasure when its neurons are neurochemically stimulated by opioid neurotransmitters (natural brain versions of heroin), endocannabinoid neurotransmitters (natural brain versions of marijuana), and related neurochemicals to amplify the hedonic impact of a ‘liked’ sensation [40,48,60,62]. The hedonic hotspots are distributed across the brain like small islands, and are functionally linked together into an integrated hierarchical circuit, like an interconnected archipelago of islands that trade together [61,62]. Neurochemical activation of one hotspot recruits neuronal activation in other hotspots, so that the whole circuit springs into a unanimous state of activation to enhance a pleasure. The unanimity is crucial to pleasure enhancement, so that prevention of even one hotspot from joining the others in activation can completely prevent any pleasure enhancement from being generated at all [61].
7.3. ‘Wanting’ and mesolimbic dopamine systems
Usually a brain ‘likes’ the rewards that it ‘wants’. But sometimes it may just ‘want’ them [5,53]. Our research has established that ‘liking’ and ‘wanting’ rewards are dissociable psychologically and neurobiologically. ‘Wanting’ here means incentive salience, a specific process of incentive motivation that makes reward stimuli appear attractive, so that they elicit approach, and promote consumption. ‘Wanting’ is related to Teitelbaum’s early proposal that the bar for defining motivation be raised to require flexible instrumental capacity in order to gain a reward. Teitelbaum’s definition capitalized on the close interaction of incentive salience with associative learning in guiding the acquisition, direction and expression of appetitive instrumental behavior (though alternative psychological mechanisms besides incentive salience can also increase operant behavior).
Incentive salience is quite distinct as a psychological process, and is distinguishable from more cognitive forms of desire meant by the ordinary word, wanting. In ordinary wanting, you cognitively know what you want, largely mediated by cortical circuits [19]. By comparison, incentive salience is mediated by more subcortically weighted neural systems that include mesolimbic dopamine projections [5]. Incentive salience does not require elaborate cognitive expectations and is focused more directly on reward-related stimuli. Incentive salience is also distinct from pure learning processes, such as prediction errors and associative memories (one distinguishing feature is that incentive salience often changes rapidly in an unlearned fashion when appetite states change, while a learned memory remains stable). The neural generation of ‘wanting’ involves mesolimbic dopamine systems especially, and their interactions with corticolimbic glutamate signals and with other neurochemical signals in the nucleus accumbens, neostriatum, amygdala and related brain circuitry [20,29,39,62,84].
One consequence of incentive salience’s capacity to detach from cognitive desires is that it can produce excessive levels of irrational ‘wanting’: that is, an intense mesolimbic ‘want’ for what is not cognitively wanted. For example, irrational ‘wanting’ can happen in addiction: such as when a recovering addict, who is no longer in withdrawal, nonetheless relapses upon encountering drug cues despite knowing that the drug will no longer give much pleasure [6,52,53]. Neurochemical ‘wanting’ mechanisms are more numerous and robust than ‘liking’ mechanisms, which may be a reason why intense ‘wants’ occur more commonly than intense pleasures.
This account of ‘wanting’ and ‘liking’ brain mechanisms has strayed a long way from the original topics focused on Philip Teitelbaum. His writings concentrated on behavioral patterns and on integrative brain functions revealed by deficits and recovery. But the stage for contemporary ‘liking’–‘wanting’ studies was partly set by Teitelbaum’s earlier identification of hedonic mechanisms (which when destroyed by LH lesions released excessive aversion), his analysis of early-stage motivation to eat during LH recovery (which was over-sensitive to food palatability), and his concern for coming up with a better definition of motivation (which raised the conceptual bar in studies of brain function). Beyond those specifics, Philip Teitelbaum also expressed a consistent demand throughout his career that brain function and behavioral organization always be addressed at an adequate level of complexity, and that oversimplification be avoided. His insistence on recognizing integration helped raise the sights of behavioral neuroscience above those of simple reductionism, and established valuable intellectual themes that affective neuroscience still aspires to follow.
8. Conclusion
Throughout his innovative career, Philip Teitelbaum has launched several revolutions in affective neuroscience and physiological psychology. These contributions helped to re-define understanding of the recovery of function after brain damage, how behavioral patterns become constructed, and the nature of motivation. Teitelbaum’s work created a jumping-off platform for the development of modern affective neuroscience, just as for other domains of behavioral neuroscience. His accomplishments continue to serve as inspiration today. All of us in the field of affective neuroscience owe enormous respect and a debt of gratitude to Philip Teitelbaum for his remarkable contributions.
Acknowledgment
I thank Aaron Garcia for redrawing Fig. 1, and thank anonymous reviewers for helpful comments on an earlier version of this manuscript. The results from my laboratory’s research described here are from work supported by DA015188 and MH63649 grants from the NIH.
References
- 1.Aldridge JW, Berridge KC. Neural coding of pleasure: rose-tinted glasses of the ventral pallidum. In: Kringelbach ML, Berridge KC, editors. Pleasures of the brain. Oxford: Oxford University Press; 2010. pp. 62–73. [Google Scholar]
- 2.Bard P. On emotional expression after decortication, with some remarks on certain theoretical views. Psychol Rev. 1934;41:309–329. [Google Scholar]
- 3.Berridge KC. Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev. 1996;20:1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- 4.Berridge KC. Motivation concepts in behavioral neuroscience. Physiol Behav. 2004;81:179–209. doi: 10.1016/j.physbeh.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- 6.Berridge KC, Aldridge JW. Decision utility, the brain and pursuit of hedonic goals. Social Cogn. 2008;26:621–646. doi: 10.1521/soco.2008.26.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berridge KC, Kringelbach ML. Affective neuroscience of pleasure: reward in humans and animals. Psychopharmacology (Berl) 2008;199:457–480. doi: 10.1007/s00213-008-1099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bindra D. How adaptive behavior is produced: a perceptual-motivation alternative to response reinforcement. Behav Brain Sci. 1978;1:41–91. [Google Scholar]
- 9.Bolles RC. Reinforcement, expectancy, and learning. Psychol Rev. 1972;79:394–409. [Google Scholar]
- 10.Cabanac M. Physiological role of pleasure. Science. 1971;173:1103–1107. doi: 10.1126/science.173.4002.1103. [DOI] [PubMed] [Google Scholar]
- 11.Cannon WB. The James-Lange theory of emotion: a critical examination and an alternative theory. Am J Psychol. 1927;39:10–24. [Google Scholar]
- 12.Cheng JT, Schallert T, De Ryck M, Teitelbaum P. Warm-up along dimensions of movement in the ontogeny of exploration in rats and other infant mammals. Proc Natl Acad Sci USA. 1981;78:7226–7229. doi: 10.1073/pnas.78.11.7226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chesire RM, Cheng JT, Teitelbaum P. Reversal of akinesia and release of festination by morphine or GABA applied focally to the nucleus reticularis tegmenti pontis. Behav Neurosci. 1984;98:739–742. doi: 10.1037//0735-7044.98.4.739. [DOI] [PubMed] [Google Scholar]
- 14.Craig W. Appetites and aversions as constituents of instincts. Biol Bull Woods Hole. 1918;34:91–107. [Google Scholar]
- 15.Cromwell HC, Berridge KC. Where does damage lead to enhanced food aversion: the ventral pallidum/substantia innominata or lateral hypothalamus? Brain Res. 1993;624:1–10. doi: 10.1016/0006-8993(93)90053-p. [DOI] [PubMed] [Google Scholar]
- 16.Davidson RJ, Sutton SK. Affective neuroscience: the emergence of a discipline. Cur Opin Neurobiol. 1995;5:217–224. doi: 10.1016/0959-4388(95)80029-8. [DOI] [PubMed] [Google Scholar]
- 17.De Ryck M, Schallert T, Teitelbaum P. Morphine versus haloperidol catalepsy in the rat: a behavioral analysis of postural support mechanisms. Brain Res. 1980;201:143–172. doi: 10.1016/0006-8993(80)90781-7. [DOI] [PubMed] [Google Scholar]
- 18.Dethier V. The hungry fly. Psychol Today. 1967;1:64–72. [Google Scholar]
- 19.Dickinson A, Balleine B. Hedonics: the cognitive-motivational interface. In: Kringelbach ML, Berridge KC, editors. Pleasures of the brain. Oxford, U.K: Oxford University Press; 2010. pp. 74–84. [Google Scholar]
- 20.Faure A, Richard JM, Berridge KC. Desire and dread from the nucleus accumbens: cortical glutamate and subcortical GABA differentially generate motivation and hedonic impact in the rat. PLoS One. 2010;5:e11223. doi: 10.1371/journal.pone.0011223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fentress JC. Development and patterning of movement sequences in inbred mice. In: Kiger J, editor. The biology of behavior. Corvallis: Oregon State University Press; 1972. pp. 83–132. [Google Scholar]
- 22.Fentress JC. Emergence of pattern in the development of mammalian movement sequences. J Neurobiol. 1992;23:1529–1556. doi: 10.1002/neu.480231011. [DOI] [PubMed] [Google Scholar]
- 23.Golani I, Fentress JC. Early ontogeny of face grooming in mice. Dev Psychobiol. 1985;18:529–544. doi: 10.1002/dev.420180609. [DOI] [PubMed] [Google Scholar]
- 24.Golani I, Wolgin DL, Teitelbaum P. A proposed natural geometry of recovery from akinesia in the lateral hypothalamic rat. Brain Res. 1979;164:237–267. doi: 10.1016/0006-8993(79)90019-2. [DOI] [PubMed] [Google Scholar]
- 25.Grill HJ, Kaplan JM. Caudal brainstem participates in the distributed neural control of feeding. In: Stricker EM, editor. Neurobiology of food and fluid intake. New York: Plenum Press; 1990. pp. 125–149. [Google Scholar]
- 26.Grill HJ, Norgren R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res. 1978;143:263–279. doi: 10.1016/0006-8993(78)90568-1. [DOI] [PubMed] [Google Scholar]
- 27.Heath RG. Pleasure and brain activity in man. Deep and surface electroencephalograms during orgasm. J Nerv Mental Dis. 1972;154:3–18. doi: 10.1097/00005053-197201000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Heimer L, Van Hoesen GW. The limbic lobe and its output channels: Implications for emotional functions and adaptive behavior. Neurosci Biobehav Rev. 2006;30:126–147. doi: 10.1016/j.neubiorev.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 29.Heimer L, Van Hoesen GW, Trimble M, Zahm DS. Anatomy of neuropsychiatry: the new anatomy of the basal forebrain and its implications for neuropsychiatric illness. Amsterdam: Elsevier: Academic Press; 2008. [Google Scholar]
- 30.Ho C-Y, Berridge KC. Hotspots for hedonic ‘liking’ and aversive ‘disliking’ in ventral pallidum. Soc Neurosci. 2009 Abstracts, ed2009. [Google Scholar]
- 31.Hoebel BG, Teitelbaum P. Hypothalamic control of feeding and self-stimulation. Science. 1962;135:375–377. doi: 10.1126/science.135.3501.375. [DOI] [PubMed] [Google Scholar]
- 32.Hughlings Jackson J. In: Selected writings of John Hughlings Jackson. Taylor J, editor. London: Staples Press; 1958. [Google Scholar]
- 33.Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- 34.Kringelbach ML. The hedonic brain: a functional neuroanatomy of human pleasure. In: Kringelbach ML, Berridge KC, editors. Pleasures of the brain. Oxford, U.K: Oxford University Press; 2010. pp. 202–221. [Google Scholar]
- 35.Kringelbach ML, Berridge KC. Towards a functional neuroanatomy of pleasure and happiness. Trends Cogn Sci. 2009;13:479–487. doi: 10.1016/j.tics.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kringelbach ML, Berridge KC. Pleasures of the brain. Oxford: Oxford University Press; 2010. p. 343. [Google Scholar]
- 37.Kringelbach ML, O’Doherty J, Rolls ET, Andrews C. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cereb Cortex. 2003;13:1064–1071. doi: 10.1093/cercor/13.10.1064. [DOI] [PubMed] [Google Scholar]
- 38.Leknes S, Tracey I. Pleasure and pain: masters of mankind. In: Kringelbach ML, Berridge KC, editors. Pleasures of the brain. Oxford, U.K: Oxford University Press; 2010. pp. 320–336. [Google Scholar]
- 39.Mahler SV, Berridge KC. Which cue to want? Central amygdala opioid activation enhances and focuses incentive salience on a prepotent reward cue. J Neurosci. 2009;29:6500–6513. doi: 10.1523/JNEUROSCI.3875-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mahler SV, Smith KS, Berridge KC. Endocannabinoid hedonic hotspot for sensory pleasure: anandamide in nucleus accumbens shell enhances ‘liking’ of a sweet reward. Neuropsychopharmacology. 2007;32:2267–2278. doi: 10.1038/sj.npp.1301376. [DOI] [PubMed] [Google Scholar]
- 41.Marshall JF, Richardson JS, Teitelbaum P. Nigrostriatal bundle damage and the lateral hypothalamic syndrome. J Comp Physiol Psychol. 1974;87:808–830. doi: 10.1037/h0037223. [DOI] [PubMed] [Google Scholar]
- 42.Marshall JF, Teitelbaum P. A comparison of the eating in response to hypothermic and glucoprivic challenges after nigral 6-hydroxydopamine and lateral hypothalamic electrolytic lesions in rats. Brain Res. 1973;55:229–233. doi: 10.1016/0006-8993(73)90507-6. [DOI] [PubMed] [Google Scholar]
- 43.Olds J. Pleasure centers in the brain. Sci Am. 1956;195:105–116. [Google Scholar]
- 44.Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol. 1954;47:419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- 45.Panksepp J. The anatomy of emotions. In: Plutchik R, Kellerman H, editors. Emotion: theory, research, and experience. New York: Academic press; 1986. pp. 91–124. [Google Scholar]
- 46.Panksepp J. Affective neuroscience: a conceptual framework for the study of emotions. In: Strongman K, editor. International reviews of studies in emotions. Chichester: Wiley; 1991. pp. 59–99. [Google Scholar]
- 47.Panksepp J. Affective neuroscience: the foundations of human and animal emotions. Oxford U.K.: Oxford University Press; 1998. [Google Scholar]
- 48.Peciña S, Berridge KC. Hedonic hot spot in nucleus accumbens shell: where do mu-opioids cause increased hedonic impact of sweetness? J Neurosci. 2005;25:11777–11786. doi: 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pellis SM, O’Brien DP, Pellis VC, Teitelbaum P, Wolgin DL, Kennedy S. Escalation of feline predation along a gradient from avoidance through play to killing. Behav Neurosci. 1988;102:760–777. doi: 10.1037//0735-7044.102.5.760. [DOI] [PubMed] [Google Scholar]
- 50.Pellis SM, Pellis VC, O’Brien DP, de la Cruz F, Teitelbaum P. Pharmacological subtraction of the sensory controls over grasping in rats. Physiol Behav. 1987;39:127–133. doi: 10.1016/0031-9384(87)90409-4. [DOI] [PubMed] [Google Scholar]
- 51.Pfaffmann C. The pleasures of sensation. Psychol Rev. 1960;67:253–268. doi: 10.1037/h0045838. [DOI] [PubMed] [Google Scholar]
- 52.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 53.Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rodgers WL, Epstein AN, Teitelbaum P. Lateral hypothalamic aphagia: motor failure or motivational deficit? Am J Physiol. 1965;208:334–342. doi: 10.1152/ajplegacy.1965.208.2.334. [DOI] [PubMed] [Google Scholar]
- 55.Roth SR, Schwartz M, Teitelbaum P. Failure of recovered lateral hypothalamic rats to learn specific food aversions. J Comp Physiol Psychol. 1973;83:184–197. doi: 10.1037/h0034415. [DOI] [PubMed] [Google Scholar]
- 56.Satinoff E, Valentino D, Teitelbaum P. A comparison of the eating in response to hypothermic and glucoprivic challenges after nigral 6-hydroxydopamine and lateral hypothalamic electrolytic lesions in rats. Brain Res. 1973;55:229–233. doi: 10.1016/0006-8993(73)90507-6. [DOI] [PubMed] [Google Scholar]
- 57.Schallert T, Whishaw IQ. Two types of aphagia and two types of sensorimotor impairment after lateral hypothalamic lesions: observations in normal weight, dieted, and fattened rats. J Comp Physiol Psychol. 1978;92:720–741. doi: 10.1037/h0077504. [DOI] [PubMed] [Google Scholar]
- 58.Schallert T, Whishaw IQ, De Ryck M, Teitelbaum P. The postures of catecholamine-depletion catalepsy: their possible adaptive value in thermoregulation. Physiol Behav. 1978;21:817–820. doi: 10.1016/0031-9384(78)90023-9. [DOI] [PubMed] [Google Scholar]
- 59.Sherrington CS. The integrative action of the nervous system. New York: C Scribner’s sons; 1906. [Google Scholar]
- 60.Smith KS, Berridge KC. The ventral pallidum and hedonic reward: neurochemical maps of sucrose liking and food intake. J Neurosci. 2005;25:8637–8649. doi: 10.1523/JNEUROSCI.1902-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith KS, Berridge KC. Opioid limbic circuit for reward: interaction between hedonic hotspots of nucleus accumbens and ventral pallidum. J Neurosci. 2007;27:1594–1605. doi: 10.1523/JNEUROSCI.4205-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith KS, Berridge KC, Aldridge JW. Disentangling pleasure from incentive salience and learning signals in brain reward circuitry. Proc Natl Acad Sci USA. 2011;108:E255–E264. doi: 10.1073/pnas.1101920108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smith KS, Mahler SV, Pecina S, Berridge KC. Kringelbach ML, Berridge KC. Pleasures of the brain. Oxford U.K.: Oxford University Press; 2010. Hedonic hotspots: generating sensory pleasure in the brain; pp. 27–49. [Google Scholar]
- 64.Smith KS, Tindell AJ, Aldridge JW, Berridge KC. Ventral pallidum roles in reward and motivation. Behav Brain Res. 2009;196:155–167. doi: 10.1016/j.bbr.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Steiner JE. The gustofacial response: observation on normal and anencephalic newborn infants. Symp Oral Sens Percept. 1973;4:254–278. [PubMed] [Google Scholar]
- 66.Steiner JE, Glaser D, Hawilo ME, Berridge KC. Comparative expression of hedonic impact: affective reactions to taste by human infants and other primates. Neurosci Biobehav Rev. 2001;25:53–74. doi: 10.1016/s0149-7634(00)00051-8. [DOI] [PubMed] [Google Scholar]
- 67.Stellar E. Brain mechanisms in hedonic processes. In: Pfaff DW, editor. The physiological mechanisms of motivation. New York: Springer-Verlag; 1982. pp. 377–408. [Google Scholar]
- 68.Stellar JR, Brooks FH, Mills LE. Approach and withdrawal analysis of the effects of hypothalamic stimulation and lesions in rats. J Comp Physiol Psychol. 1979;93:446–466. doi: 10.1037/h0077590. [DOI] [PubMed] [Google Scholar]
- 69.Stricker EM, Zigmond MJ. Brain catecholamines and the lateral hypothalamic syndrome. In: Novin D, Wyrwicka W, Bray G, editors. Hunger: basic mechanisms and clinical implications. New York: Raven Press; 1976. pp. 19–32. [Google Scholar]
- 70.Swanson LW. Anatomy of the soul as reflected in the cerebral hemispheres: neural circuits underlying voluntary control of basic motivated behaviors. J Comp Neurol. 2005;493:122–131. doi: 10.1002/cne.20733. [DOI] [PubMed] [Google Scholar]
- 71.Szechtman H, Ornstein K, Teitelbaum P, Golani I. Functional recovery after lesions of the nervous system. V. Neural plasticity and behavioral recovery in the central nervous system. The use of recovery of function to analyze the organization of motivated behavior in the nervous system. Neurosci Res Program Bull. 1974;12:255–260. [PubMed] [Google Scholar]
- 72.Szechtman H, Ornstein K, Teitelbaum P, Golani I. The morphogenesis of stereotyped behavior induced by the dopamine receptor agonist apomorphine in the laboratory rat. Neuroscience. 1985;14:783–798. doi: 10.1016/0306-4522(85)90143-5. [DOI] [PubMed] [Google Scholar]
- 73.Teitelbaum O, Benton T, Shah PK, Prince A, Kelly JL, Teitelbaum P. Eshkol-Wachman movement notation in diagnosis: the early detection of Asperger’s syndrome. Proc Natl Acad Sci. 2004;101:11909–11914. doi: 10.1073/pnas.0403919101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Teitelbaum P. The use of operant methods in the assessment and control of motivational states. In: Honig WK, editor. Operant Behavior: Areas of research and application. New York: Appleton-Century-Crofts; 1966. pp. 565–608. [Google Scholar]
- 75.Teitelbaum P. Levels of integration of the operant. In: Honig WK, Staddon JER, editors. Handbook of operant behavior. Englewood Cliffs, N.J: Prentice-Hall Inc; 1977. pp. 7–27. [Google Scholar]
- 76.Teitelbaum P. What is the ‘zero condition’ for motivated behavior? In: Hoebel BG, Novin D, editors. The neural basis of feeding and reward. Brunswick, ME: Haer Institute; 1982. pp. 8–23. [Google Scholar]
- 77.Teitelbaum P, Cheng MF, Rozin P. Development of feeding parallels its recovery after hypothalamic damage. J Comp Physiol Psychol. 1969;67:430–441. doi: 10.1037/h0027288. [DOI] [PubMed] [Google Scholar]
- 78.Teitelbaum P, Cheng MF, Rozin P. Stages of recovery and development of lateral hypothalamic control of food and water intake. Ann N Y Acad Sci. 1969;157:849–860. doi: 10.1111/j.1749-6632.1969.tb12923.x. [DOI] [PubMed] [Google Scholar]
- 79.Teitelbaum P, Epstein AN. The lateral hypothalamic syndrome: recovery of feeding and drinking after lateral hypothalamic lesions. Psychol Rev. 1962;69:74–90. doi: 10.1037/h0039285. [DOI] [PubMed] [Google Scholar]
- 80.Teitelbaum P, Pellis SM. Toward a synthetic physiological-psychology. Psychol Sci. 1992;3:4–20. [Google Scholar]
- 81.Teitelbaum P, Stellar E. Recovery from the failure to eat produced by hypothalamic lesions. Science. 1954;120:894–895. doi: 10.1126/science.120.3126.894. [DOI] [PubMed] [Google Scholar]
- 82.Teitelbaum P, Stricker EM. Compound complementarities in the study of motivated behavior. Psychol Rev. 1994;101:312–317. doi: 10.1037/0033-295x.101.2.312. [DOI] [PubMed] [Google Scholar]
- 83.Teitelbaum P, Teitelbaum O, Nye J, Fryman J, Maurer RG. Movement analysis in infancy may be useful for early diagnosis of autism. Proc Natl Acad Sci. 1998;95:13982–13987. doi: 10.1073/pnas.95.23.13982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thompson RH, Swanson LW. Hypothesis-driven structural connectivity analysis supports network over hierarchical model of brain architecture. Proc Natl Acad Sci USA. 2010;107:15235–15239. doi: 10.1073/pnas.1009112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tindell AJ, Smith KS, Pecina S, Berridge KC, Aldridge JW. Ventral pallidum firing codes hedonic reward: when a bad taste turns good. J Neurophysiol. 2006;96:2399–2409. doi: 10.1152/jn.00576.2006. [DOI] [PubMed] [Google Scholar]
- 86.Toates F. Motivational systems. Cambridge: Cambridge University Press; 1986. [Google Scholar]
- 87.Ungerstedt U. Adipsia and aphagia after 6-hydroxydopamine induced degeneration of the nigro-striatal dopamine system. Acta Physiol Scand Suppl. 1971;367:95–122. doi: 10.1111/j.1365-201x.1971.tb11001.x. [DOI] [PubMed] [Google Scholar]
- 88.Valenstein ES, Cox VC, Kakolewski JW. Reexamination of the role of the hypothalamus in motivation. Psychol Rev. 1970;77:16–31. doi: 10.1037/h0028581. [DOI] [PubMed] [Google Scholar]
- 89.Valenstein ES, Valenstein T. Interaction of positive and negative reinforcing neural systems. Science. 1964;145:1456–1458. doi: 10.1126/science.145.3639.1456. [DOI] [PubMed] [Google Scholar]
- 90.Veldhuizen MG, Rudenga KJ, Small D. The pleasure of taste flavor and food. In: Kringelbach ML, Berridge KC, editors. Pleasures of the brain. Oxford, U.K: Oxford University Press; 2010. pp. 146–168. [Google Scholar]
- 91.Vuust P, Kringelbach ML. The pleasure of music. In: Kringelbach ML, Berridge KC, editors. Pleasures of the brain. Oxford, U.K: Oxford University Press; 2010. pp. 255–269. [Google Scholar]
- 92.Watts AG, Swanson LW. Anatomy of motivation. In: Gallistel CR, editor. Steven’s handbook of experimental psychology: learning, motivation, and emotion. New York: John Wiley & Sons, Inc; 2002. pp. 563–632. [Google Scholar]
- 93.Winn P. The lateral hypothalamus and motivated behavior: an old syndrome reassessed and a new perspective gained. Curr Dir Psychol. 1995;4:182–187. [Google Scholar]
- 94.Winn P, Tarbuck A, Dunnett SB. Ibotenic acid lesions of the lateral hypothalamus: comparison with the electrolytic lesion syndrome. Neuroscience. 1984;12:225–240. doi: 10.1016/0306-4522(84)90149-0. [DOI] [PubMed] [Google Scholar]
- 95.Wise RA. The dopamine synapse and the notion of ‘pleasure centers’ in the brain. Trends Neurosci. 1980;3:91–95. [Google Scholar]
- 96.Wise RA. The anhedonia hypothesis: Mark III. Behav Brain Sci. 1985;8:178–186. [Google Scholar]
- 97.Young PT. Hedonic organization and regulation of behavior. Psychol Rev. 1966;73:59–86. doi: 10.1037/h0022630. [DOI] [PubMed] [Google Scholar]