Abstract
Increased susceptibility to autoimmunity in females is often viewed as the consequence of enhanced immunoreactivity providing superior protection against infections. We paradoxically observed greater mortality in female compared to male mice during systemic viral infections with three large double-stranded DNA viruses (herpes simplex virus type I [HSV], murine cytomegalovirus [MCMV], and vaccinia virus [VV]). Indeed, female mice were 27-fold more susceptible to infection with HSV than male mice. Elimination of estrogen by ovariectomy in female mice or addition of estrogen to castrated male mice only partially eliminated the observed sex differences following HSV infection. However, the differences observed in survival between female and male mice were nearly abrogated in the absence of type I interferon receptor signaling and substantially mitigated in absence of DAP12 signaling. Interestingly, the sex-specific impact of type I interferon receptor and DAP12 signaling differentially influenced survival during systemic viral infections with type I interferon receptor signaling enhancing male survival and DAP12 signaling increasing the susceptibility of female mice. These results have potential implications for the sex disparities observed in human autoimmune disorders.
Keywords: female, IFNαβ, DAP12, HSV, MCMV, vaccinia, survival
1. Introduction
Autoimmune diseases affect approximately 5% of the population in Western countries and disproportionately impact females, as illustrated by a 9:1 female to male ratio in systemic lupus erythematosus (SLE) [1, 2]. The increased female susceptibility to autoimmunity is frequently attributed to enhanced immunoreactivity that provides more effective protection against infections that may either harm the fetus of pregnant females or limit reproductive potential [3–6].
Although a female bias in autoimmune susceptibility has been well established for decades, the mechanisms accounting for these sex differences are not well established. Hypotheses include direct effects of sex steroids [7], gene dose effects associated with the X-chromosome (e.g., varied X-chromosome inactivation [8] or microRNAs [9]), impact of non-testis determining genes on the Y-chromosome, or fetal microchimerism [10]. Most studies have focused on sex differences in immune responses secondary to the direct influence of sex steroids [3, 4, 6, 11]; however, a number of recent reports have highlighted the potential impact of dosage effects of genes associated with the X-chromosome [9, 12–14] or the influence of non-testis determining genes on the Y-chromosome [15].
Women appear to mount stronger cellular and humoral immune responses to viral infections than men (reviewed in [3, 6, 16]). Women, in general, have more robust antibody production following vaccination [17–20] or viral infection [21, 22] as well as more vigorous cytokine [23] and T cell responses [24–27]. In addition, there also appears to be sexual dimorphism in innate immune responses (reviewed in [4, 6]). For example, TLR7 ligands induce higher IFNα production in females [28], and plasmocytoid dendritic cells (pDCs) derived from women produce more INFα in response to HIV-encoded TLR7 ligands than pDCs from men [29]. Although these responses might be protective in the context of viral infections, they may predispose females to a higher incidence of autoimmune disorders.
Studies in mice have also demonstrated significant sex differences in immune responses during several viral infections. For example, female mice generate more neutralizing antibodies [30] and higher levels of many chemokines and cytokines [7] than males during infection with influenza A. Female mice also generate higher titers of neutralizing antibody and stronger peripheral T cell responses during Theiler’s murine encephalomyelitis virus (TMEV) infection [31]. Although the more robust immune response in females may be protective and accelerate viral clearance, it can in some contexts result in increased damage to the host. Indeed, the more vigorous immune response in female mice appears to protect them from TMEV-induced demyelinating disease (a murine model of multiple sclerosis) [31] while resulting in increased morbidity and mortality during influenza A infection [7, 14]. Interestingly, a number of studies have demonstrated greater morbidity and mortality in males during viral infections, including greater susceptibility to myocarditis following coxsackievirus B3 infection [14, 32] and increased paralysis following HSV-1 infection [33].
In this study, we quantitatively examined sex differences in murine survival following systemic infections with large double-stranded DNA viruses. Despite a growing body of literature documenting superior anti-pathogen immune responses in females [3–6], both male and female sex biases in survival following viral infections have been reported (Table 1). Here, we observed significantly enhanced survival of male mice compared with female mice during systemic infections with three large DNA viruses. The male survival advantage was particularly large following systemic infection with HSV.
Table 1.
Sex Differences in murine survival following viral infections
| virus (strain)a | routeb | inoculum dose |
mouse strain |
greater mortality |
survival females |
survival males |
reference |
|---|---|---|---|---|---|---|---|
| SLE virus (CDC-904) | i.v. | 107 SMLD50 | Ha/ICR | males | 50% (38/76) | 32% (24/76) | [62] |
| EMCV | i.p. | 104.3 TCID50 | Swiss | males | 52% (27/52) | 12% (6/52) | [63] |
| ectromelia (Moscow) | s.q. | 105 &107 pfu | DBA/2×(B6×D2)F1 | females | 82% (56/68) | 51% (36/70) | [64] |
| HSV-1 (strain F) | c.s. | 105–107 pfu | 129 sv/Ev | males | 92% (23/25) | 77% (115/150) | [51] |
| HSV-1 (strain 17) | c.s. | 10× LD50 | 129×(B6×129)F1 | males | 68% (89/131) | 52% (75/144) | [65] |
| HSV-1 (strain VR) | i.n. | 1.2×105 pfu | CD-1 | males | 84% (37/44) | 72% (31/43) | [52] |
| influenza A (PR8) | i.n. | 102 TCID50 | B6 | females | 0% (0/15) | 47% (7/15) | [7] |
| influenza A (PR8) | i.n. | 102 TCID50 | B6 | females | 0% (0/10) | 50% (5/10) | [14] |
| coxsackievirus (H3) | i.p. | 100 pfu | B6 | males | 100% (10/10) | 50% (5/10) | [14] |
SLE- St Louis encephalitis virus; EMCV – encephalomyocarditis virus
i.v. – intravenous injection; i.p. – intraperitoneal injection; i.n. – intranasal injection; c.s. – corneal scarification; s.q. – subcutaneous injection
Large DNA viruses, such as HSV, are recognized early during infection through pattern recognition receptors (PRR; e.g., TLR, RIG-I, MDA5, DAI, and IFI16) on the cell surface, in endosomes, and in the cytosol (reviewed in [34]). Signaling through these PRRs results in the upregulation of type I IFNs as well as other immunomodulatory cytokines. Type I IFNs induce the transcription of hundreds of interferon-stimulated genes (ISGs) that contribute to host antipathogen responses [35] and play a particularly important role in host defense against HSV [36]. TLR signaling on macrophages and dendritic cells is negatively regulated by DAP12, a highly conserved and widely expressed adaptor molecule in the innate immune response, resulting in suppressed production of type I IFNs and pro-inflammatory cytokines, such as IL-12 [37–39].
We hypothesized that sex-specific differences in early innate immune responses might contribute to the observed male survival advantage during systemic viral infections. Indeed, the sex differences observed in survival following viral infection were nearly abrogated in the absence of type I IFN receptor signaling and substantially decreased in the absence of DAP12 signaling. Interestingly, signaling through type I IFN receptors and DAP12 differentially influenced survival with type I IFN receptor signaling enhancing male survival while DAP12 signaling increased female mortality. We propose that the association of these unexpected sex differences in survival following systemic viral infections and intact type I IFN receptor and DAP12 signaling may provide novel insights into the sex disparities observed in human susceptibility to autoimmune disorders.
2. Materials and Methods
2.1. Mice
C57BL/6 (B6) mice were obtained from NCI (Charles River, MA). B6 mice that were castrated or ovariectomized at 4 weeks of age were also obtained from NCI. 129.IFNαβRdeficient mice were backcrossed onto a C57BL/6 background for 10 generations [40], and the completeness of the backcross was confirmed with speed congenic markers (>99% B6 with 136/137 B6 microsatellite markers) in the Rheumatic Diseases Core Center at Washington University. C57BL/6.DAP12 loss-of-function knock-in mice (DAP12KI; [41]) were a kind gift from Eric Vivier (CNRS-INSERM-Universite de la Mediterranee, France). BDCA2-DTR Tg mice on a C57BL/6 background [42] were a generous gift from Marco Colonna (Washington University). Plasmacytoid dendritic cells were depleted in the BDCA2-DTR Tg mice following the protocol outlined by Colonna and colleagues [42]. Mice were maintained under specific pathogen-free conditions and used between 8 and 14 weeks of age. All experiments were conducted in accordance with institutional guidelines for animal care and use based on the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Animal Studies Committee at Washington University (#20110104). Mice utilized in survival studies were observed daily after infection for 21 days, and moribund mice were euthanized per institutional guidelines.
2.2. Viruses
A salivary gland stock of Smith strain MCMV (American Type Culture Collection [ATCC]; Manassas, VA) was prepared from young BALB/c mice that had been intra-peritoneally (i.p.) injected with 1×106 plaque-forming units (pfu) of tissue-culture propagated MCMV, and the titer of the stock was determined via standard plaque assay [43] using NIH 3T12 fibroblasts (ATCC). Vaccinia virus (ATCC) was propagated in and titered using CV-1 cells (ATCC) following standard protocols (Current Protocols in Immunology). HSV strain 17 was a kind gift from David Leib (Dartmouth Medical School, Lebanon NH). HSV was propagated in and titered using Vero cells (ATCC) following established protocols [44]. Hepatic and brainstem HSV titers were also determined using Vero cells (ATCC).
2.3. Reed Muench calculation of LD50 values
Examination of LD50 values (inoculum dose of virus at which one half of the mice die) is the most sensitive method of quantitatively comparing survival between groups of mice following infection [45]. This endpoint is less influenced by experimental variation than other assessments of survival and was calculated following the method proposed by Reed and Muench [45]. The Reed and Muench method assumes that a mouse that survived at a given inoculum dose of virus would have survived a lower inoculum dose and conversely that a mouse that died at a given inoculum dose of virus would have died a higher inoculum dose. Therefore, information from several experimental groups above and below the 50th percentile can be incorporated into the calculation, providing a more accurate estimate than simple interpolation between two arbitrary values bracketing the 50th percentile. This method allows the use of fewer animals per group in the determination of LD50 values.
2.4. Estrogen treatment
For estrogen supplementation studies, castrated mice were implanted with delayed release estrogen pellets (0.05 mg estradiol-17β/pellet, 21-day release of 2.4 µg estradiol/day; Innovative Research of America, Sarasota, FL) five days prior to HSV infection. The pellets were inserted subcutaneously in the lateral neck of sedated mice using a trochar per the manufacturer recommendations. The dose was selected based on published studies that demonstrated that 2–10 µg estradiol/day/animal resulted in normal serum estrogen levels in ovariectomized female mice [46, 47].
2.5. Cytokine measurements
Serum was collected using microtainer serum separator tubes (Becton Dickinson, Franklin Lakes, NJ) according to the manufacturer’s instructions and stored at −80°C. Fifty µl aliquots of serum were diluted 1:2 and used either in IFNα ELISAs (PBL Biomedical Laboratories, Piscataway, NJ) or Bio-Plex cytokine assays (BioRad, Hercules, CA) following the manufacturer’s protocols.
2.6. Quantitative real-time RT-PCR
Total hepatic RNA from naïve or HSV-infected mice was isolated using an RNeasy Mini kit (Qiagen, Valencia, CA), digested with RQ1 DNase (Fisher Scientific, Pittsburgh, PA), and reverse transcribed with oligo dT12–18 (Invitrogen) and SuperScript II reverse transcriptase (Invitrogen) as previously described [40]. Quantitative RTPCR was performed as described for ISG56 and IRF7 [48] and for ISG54 using forward primer 5’-CTGAAGCTTGACGCGGTACA-3’, reverse primer 5’- ACTTGGGTCTTTCTTTAAGGCTTCT-3’, and probe 5’-AAAACCAAGCAATGGCGCTGGTTG-3’. Comparisons were made by the comparative Ct method, with β-actin serving as the comparator. Data are presented as foldchange relative to samples from naïve mice, which were set to a value of one.
2.7. Statistical analysis
Unpaired, two-tailed t-tests were used to determine significant differences between experimental groups (p < 0.05). Error bars in figures represent standard deviations from the mean value. Kaplan-Meier analysis was performed with Prism software (Graph Pad, San Diego, CA).
3. Results
3.1 Increased mortality in female mice following systemic viral infections
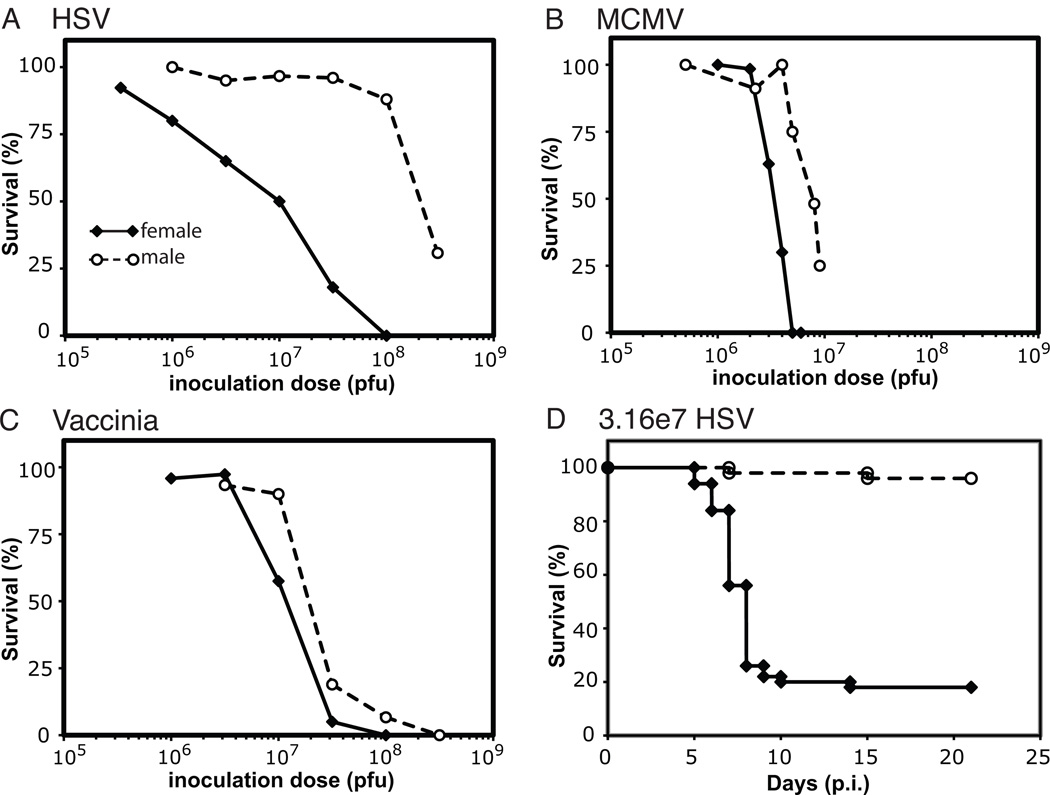
During studies of the innate immune response to HSV infection, we observed a sex bias in survival of B6 mice infected i.p. with HSV. When we systematically quantified this difference, we determined that the males were 27-fold more resistant to HSV infection than female mice with LD50 values of 2.1×108 and 7.9×106 pfu/mouse, respectively (Fig. 1A; Table 2). This large difference in survival was not accounted for by differences in weight between the male and female mice (Fig. S1). To determine if the observed sex difference was HSV-specific or generalizable to other systemic viral infections, we investigated the survival of male and female mice following i.p. inoculation with two other large DNA viruses, murine cytomegalovirus (MCMV) and vaccinia virus (VV). Similar, although substantially smaller, differences were observed in the survival of male and female mice following infection with both MCMV and VV (Figs. 1B and 1C). Males were 2.5-fold more resistant to MCMV infection than female mice (LD50 values of 6.9×105 and 2.7×105 pfu/mouse, respectively; Fig. 1B; Table 2) and nearly 2-fold more resistant to VV infection than female mice (LD50 values of 2.2×107 and 1.3×107 pfu/mouse, respectively; Fig.1C; Table 2).
Figure 1. Female mice experience reater mortality during systemic viral infections than male mice.
Survival curves are shown for female (black diamonds with solid line) and male (unfilled circles with dashed line) mice following infection over a range of inoculum doses of large double-stranded DNA viruses. A) Significantly inferior survival was observed in the female compared to the male mice following systemic infection with HSV. HSV survival curves represent cumulative results from 5 independent experiments with 5–10 mice/dose/experiment (n=198 females and n=186 males). Similar but smaller differences in male and female survival were observed following infection with MCMV (B) and vaccinia (C). MCMV survival curves are shown with cumulative results from 7 and 5 independent experiments with female (n=135) and male (n=127) mice, respectively. Vaccinia survival curves represent aggregated results from four independent experiments (n=157 females and n=106 males). D) Kaplan-Meier analysis is shown of the survival of female (black diamonds with solid line; n=50) and male (unfilled circles with dashed line; n=50) mice following i.p. infection with 3.16×107 pfu of HSV. The data are aggregated from five independent experiments.
Table 2.
Summary of LD50 values following viral infection
| Strain of mice | virus | route | female LD50 | male LD50 | fold difference |
|---|---|---|---|---|---|
| wt B6 | HSV | i.p. | 7.9×106 | 2.1×108 | 27 |
| castrated B6 | HSV | i.p. | - | 1.4×108 | - |
| ovarietomized B6 | HSV | i.p. | 3.5×107 | - | - |
| IFNαβR−/− | HSV | i.p. | 3.3×101 | 5.4×101 | 1.6 |
| IFNαβR−/− | HSV | footpad | 4.4×101 | 6.1×101 | 1.5 |
| IFNαβR+/− | HSV | i.p. | 2.6×106 | 2.1×108 | 81 |
| wt B6 | MCMV | i.p. | 2.7×105 | 6.9×105 | 2.5 |
| IFNαβR−/− | MCMV | i.p. | 1.7×104 | 2×104 | 1.2 |
| wt B6 | VV | i.p. | 1.3×107 | 2.2×107 | 1.7 |
| IFNαβR−/− | VV | i.p. | 5.1×105 | 7.4×105 | 1.45 |
Systemic infection with HSV in female mice resulted in a much broader survival curve (Fig. 1A) than observed with either MCMV or VV. However, the differences between the survival in male and female mice following HSV infection were robust and reproducible over five independent experiments. A Kaplan-Meier plot of survival following infection with 3.16×107 pfu/mouse demonstrated nearly universal survival of male mice (96%) while only 18% of the female mice survived, with most deaths occurring on days 7 and 8 post-infection (p.i.) (Fig. 1D).
3.2. Serum proinflammatory cytokine levels and viral titers during HSV infection
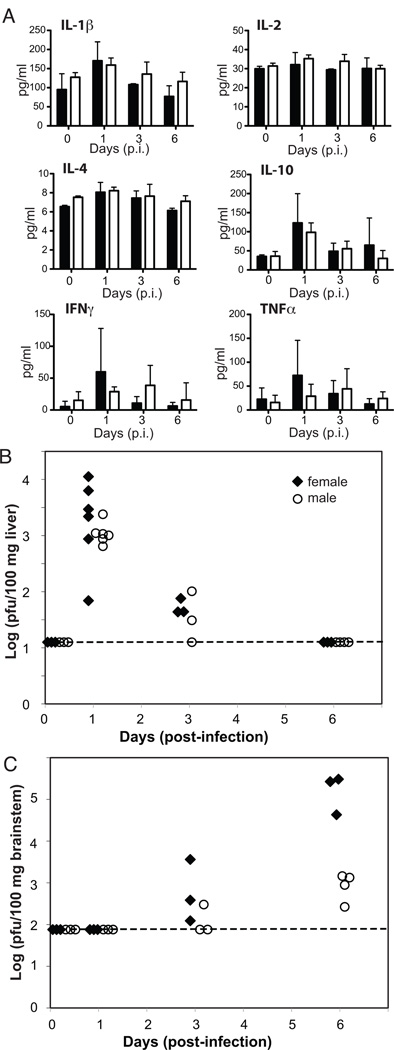
We hypothesized that the large sex differences in survival following systemic HSV infection occurred either from an overly exuberant immune response in female mice leading to host damage or from more effective host defense in male mice. To test these hypotheses, we evaluated levels of proinflammatory cytokines (e.g., IL-1β, IL-2, IFNγ, and TNFα) in the serum of female and male mice as a surrogate measure of overwhelming systemic inflammation and viral titers in the livers and brainstems of female and male mice to assess the effectiveness of host defense. No sex differences were observed in serum proinflammatory cytokines on days 0, 1, 3, or even 6 p.i. (immediately preceding the majority of deaths in females on days 7 and 8 p.i.; Fig. 1D) with 3.16×107 pfu HSV/mouse (Fig. 2A). In addition, minimal inflammatory infiltrates were observed in the organs of female mice on necropsy at day 6 p.i. with 3.16×107 pfu HSV/mouse (data not shown). Therefore, increased mortality in female mice does not appear to be due to an overwhelming systemic inflammatory response, and we focused on the second hypothesis that more effective host defense in male mice enhanced male survival.
Figure 2. Serum inflammatory cytokine levels and viral titers following i.p. infection with HSV.
A) No differences were observed in serum proinflammatory cytokine levels in female (black bars; n=3/time-point) and male (white bar; n=3/time-point) mice infected with 3.16×107 pfu of HSV. B) No differences were observed in hepatic viral titers from female (black diamonds) and male (open circles) mice following i.p. infection with 3.16×107 pfu of HSV (3–6 mice/time-point; data at day 1 p.i. are from two independent time-points). C) Significantly higher viral titers were observed in the brainstems of female (black diamonds) mice compared to male (open circles) mice on day 6 p.i. with 3.16×107 pfu of HSV (3–4 mice/time-point). Level of detection of the assay is noted in B) and C) by a dotted line.
Both female and male mice were found to have elevated hepatic titers on day 1 p.i. that decreased substantially by day 3 p.i., with no detectable hepatic titers in either female or male mice by day 6 p.i. (Fig. 2B). In contrast to the peripheral hepatic titers, brainstem titers in both female and male mice were undetectable at day 1 p.i.; however, by day 6 p.i., female mice had brainstem titers 100-fold higher than male mice (Fig. 2C). These findings support the hypothesis that male host defense is more effective, at least in preventing transit to and replication of HSV in the central nervous system.
3.3. Impact of sex steroids on sex differences in survival during HSV infection
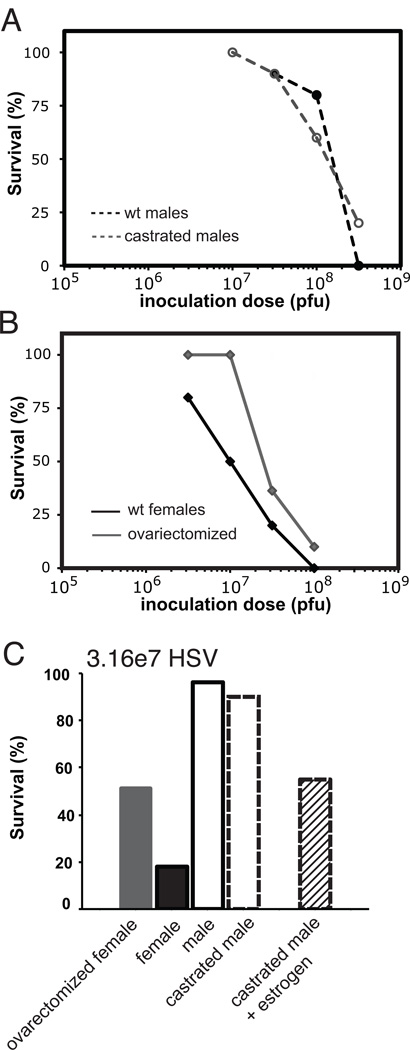
To investigate the basis for the apparent superior host defense and survival in males during systemic HSV infection, we first evaluated the impact of androgens. We found no difference in survival between castrated and wt male mice, indicating that circulating androgens do not contribute to the enhanced survival of male mice during HSV infection (Fig. 3A).
Figure 3. Sex differences in susceptibility to systemic HSV infection are only partially attributable to sex steroids.
A) The superior survival of male mice is not compromised in the absence of androgens. Survival curves of wt (black circles with dashed line) and castrated (gray unfilled circles with dashed line) male mice are shown following infection with HSV (10 mice/group/inoculum dose). B) The absence of estrogens only partially eliminates the increased susceptibility of female mice during systemic HSV infection. Survival curves of wt (black diamonds) and ovariectomized (gray diamonds) female mice are shown following infection with HSV (10–11 mice/group/inoculum dose; representative of two independent experiments). C) Castrated male mice treated with estrogen (stripped black bar; n=11) experience increased mortality compared to either castrated (outlined dashed bar (n=10) or wt (outlined black bar; n=50, cumulative data from five experiments) male mice following infection with 3.16×107 pfu/mouse HSV but are still substantially more resistant than female mice (filled black bar; n=50, cumulative data from five experiments). Survival data for ovarectomized female mice (gray filled bar; n=21, cumulative from two experiments) are shown for comparison.
We subsequently evaluated the impact of estrogens on the increased susceptibility of female mice by studying mice deficient in estrogen (i.e., female mice that had undergone ovariectomies) or by adding exogenous estradiol to achieve physiological levels of estrogen in castrated male mice. Ovariectomized mice had improved survival compared to wt female mice with LD50 values 4-fold higher (3.5×107 and 7.9×106 pfu/mouse, respectively); however, the ovariectomized mice were still 6-fold more susceptible to systemic HSV infection than male mice (Figs. 3B and 3C; Table 2). Adding exogenous estrogen to castrated male mice prior to HSV infection resulted in decreased survival compared to wt male mice. Fifty-five percent of castrated mice supplemented with estrogen (n=11) survived after infection with 3.16×107 pfu HSV/mouse compared with the survival of 96% male mice (n=50) and 18% of female mice (n=50) (Figs. 3C). Although estrogen appears to contribute to the enhanced susceptibility of female mice, our results demonstrate that the direct impact of estrogen at the time of infection accounts for less than half of the sex difference observed in survival after systemic HSV infection. Therefore, we conclude that the sex differences observed in survival after HSV infection were independent of androgens and only partially attributable to estrogen.
3.4. Impact of IFNαβR signaling on sex differences in survival during HSV infection
We subsequently hypothesized that more effective male host defense and survival during systemic HSV infection might result from estrogen-independent sex differences in early host immune responses. Given the critical role of type I interferons in host defense against viruses and in particular HSV, we postulated that the enhanced survival of male mice after systemic viral infections might be secondary to sex differences in type I IFN responses.
We initially evaluated serum levels of IFNα and found no significant differences in male and female mice during infection with HSV (3.16×107 pfu /mouse; Fig. 4A). We then investigated the upregulation of mRNA of a limited subset of representative interferon-stimulated genes (i.e., IRF-7, ISG54, and ISG56) that are rapidly and robustly upregulated by type I interferon stimulation [49]. These ISGs were upregulated early during HSV infection, but we found no reproducible differences in relative expression levels in the livers of male or female mice at 12 and 24 hours after HSV infection (data not shown).
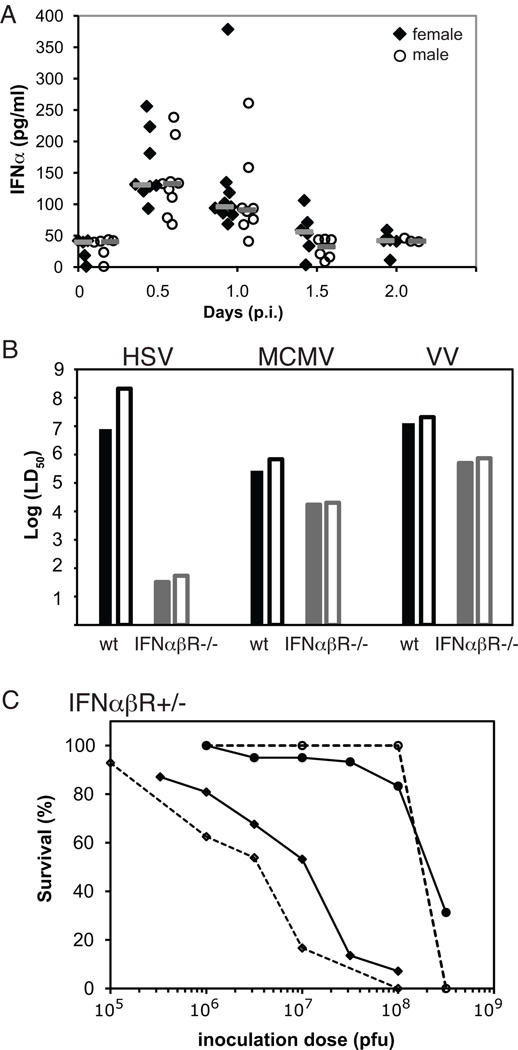
Figure 4. Sex differences in susceptibility to systemic viral infections are nearly abrogated in the absence of type I interferon signaling.
A) No differences were observed in serum IFNα levels in wt female (black diamonds; n=5–9/time-point) and male (open circles; n=6–9/time-point on days 0, 0.5, and 1 p.i. with n=3 at day 2 p.i.) mice infected with 3.16×107 pfu of HSV. Gray bars represent median values for each time-point. Data are aggregated results from two independent experiments. B) Although the absence of type I interferon signaling results in significantly increased sensitivity to HSV infection, sex differences in mortality are nearly eliminated following systemic infection with all three viruses (HSV, MCMV, and VV) in the absence of type I interferon signaling. LD50 values are shown for wt female (black bars), wt male (black outlined bars), IFNαβR-deficient female (solid grey bars), and IFNαβR-deficient male (outlined grey bars) mice following infection with HSV, MCMV, and vaccinia. C) The observed sex difference in survival following systemic HSV infection is amplified in the setting of IFNαβR-haploinsufficiency. HSV survival curves with IFNαβR+/− mice represent cumulative results from 4 independent experiments with female (diamonds with dashed line; n=61) and male (black unfilled circles with dashed line; n=43) IFNαβR+/− mice. Cumulative survival data for wt female (black diamonds with solid line) and male (black unfilled circles with solid line) mice from Fig. 1A are shown for comparison.
We then utilized IFNαβR-deficient and IFNαβR-haploinsufficient mice to investigate potential differences in type I IFN responses in mediating sex differences in survival during viral infections. Consistent with previous reports that control of HSV infection is heavily reliant on type I IFNs[50], the LD50 values in both IFNαβR-deficient male and female mice were substantially reduced (5.4×101 and 3.3×101 pfu/mouse, respectively) compared to wt mice (Table 2). However, these studies demonstrated that the 27-fold enhanced resistance of wt male compared to female mice decreased to 1.6-fold in the absence of effective IFNα/β receptor signaling (Fig. 4B).
The generalizability of IFNαβR signaling in mediating sex differences in survival was confirmed in studies of MCMV and VV infection in IFNαβR-deficient mice, which also revealed substantial decreases in sex differences in survival (without an associated large increase in susceptibility as seen with HSV; Fig. 4B). For example, the LD50 values of MCMV in IFNαβR-deficient male and female mice were 2×104 and 1.7×104 pfu MCMV/mouse, respectively, representing a decrease in the 2.5-fold enhanced resistance of wt male compared to female mice to 1.2-fold in the absence of effective IFNα/β receptor signaling (Table 2). We observed a similar decrement in sex differences in survival when using a different route of HSV infection (i.e., footpad injection), although both female and male wt mice were able to effectively suppress localized HSV infection via footpad with minimal mortality precluding the calculation of LD50 values. We saw no deaths in wt male mice following footpad infection with doses of HSV up to 1×108 pfu/mouse, while 20% and 30% of the females died after footpad injection with 1×107 or 1×108 pfu/mouse, respectively. However, no sex differences were seen following footpad injection of HSV in IFNαβ R-deficient mice with LD50 values of 6.1×101 and 4.4×101 pfu/mouse in male and female mice, respectively (Table 2). Finally, the male survival advantage was substantially more pronounced in the context of IFNαβR-haploinsufficiency (Fig. 4C), strongly implicating IFNαβreceptor signaling in mediating the observed sex bias in survival. When IFNαβRhaploinsufficient mice were infected with HSV, the survival of the males remained unchanged compared to wt males while females were more susceptible than wt females resulting in 81-fold difference in LD50 values for male and female IFNαβR+/− mice (2.1×108 and 2.6×106 pfu/mouse, respectively). Taken together, these viral studies with IFNαβR-deficient and IFNαβRhaploinsufficient mice demonstrate that disruption of IFNα/β signaling substantially reduces the survival differences between males and females during systemic herpesvirus (and to a lesser extent poxvirus) infections.
3.5. DAP12 signaling increases female susceptibility to systemic HSV infection
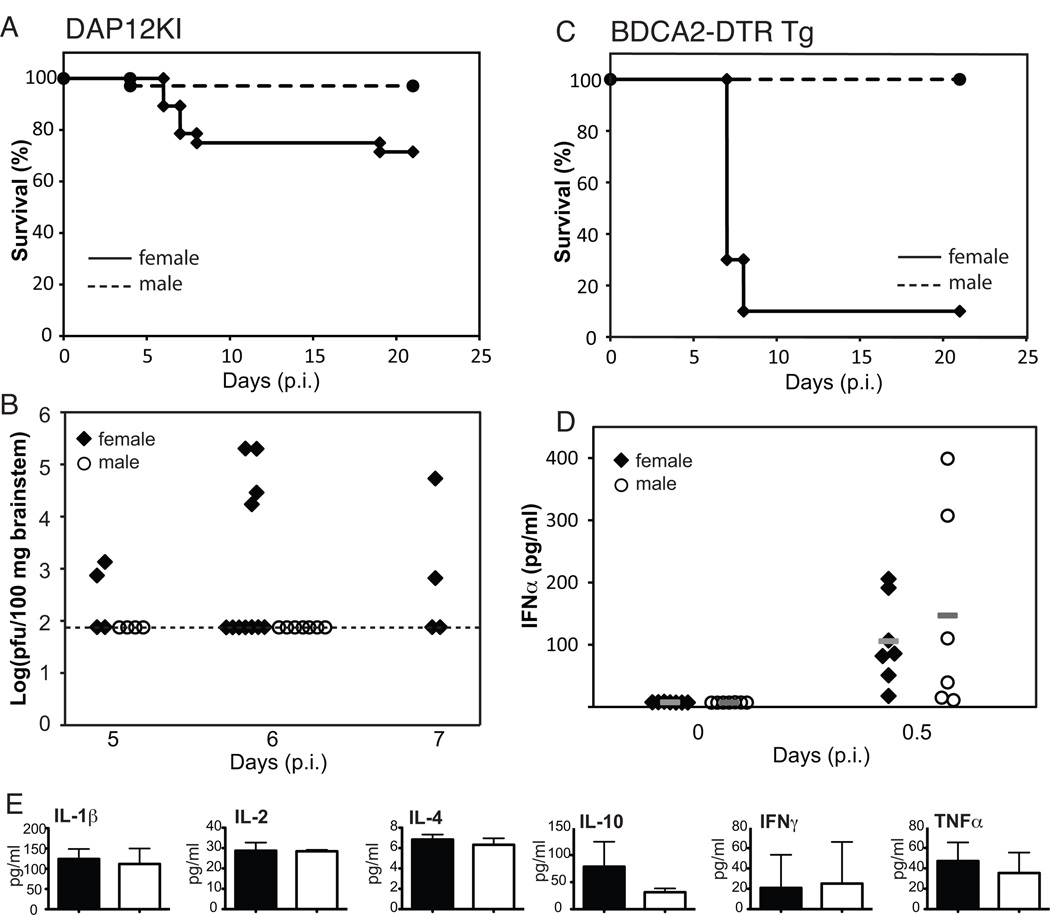
Given its broad regulatory role in the innate immune response (including negative regulation of TLRs), we hypothesized that sex differences in DAP12 signaling may also contribute to the male survival advantage during HSV infection. Using DAP12 signaling-deficient mice (DAP12KI), we observed a significant improvement in female survival (71%) compared to wt female mice (18%) after infection with 3.16×107 pfu HSV/mouse (Figs. 5A and 1D). Male DAP12KI mice had identical survival to wt male mice (97% and 96%, respectively; Figs. 1D and 5A). Interestingly, we observed dichotomous brainstem titers on day 6 p.i. in female DAP12KI mice (Fig. 5B), with 36% (4 of 11) exhibiting high titers similar to those seen in wt females (Fig. 2C) while the other 64% (7 of 11) DAP12KI females and all DAP12KI males had no detectable brainstem titers. Dichotomous brainstem titers were also observed at day 7 p.i., although the titers were lower. Therefore, in contrast to sex differences in type I interferon receptor signaling which appear to enhance male survival, intact DAP12 signaling contributes to the elevated brainstem titers and increased mortality observed in wt female mice during systemic HSV infection.
Figure 5. Sex differences in susceptibility to systemic viral infections are mitigated in the absence of DAP12 signaling.
A) Sex differences in survival following systemic infection with HSV were significantly reduced in DAP12-signaling deficient mice. Cumulative survival data with female (diamonds with solid line; n=28) and male (circles with dashed line; n=34) DAP12KI mice from four independent experiments following infection with 3.16×107 pfu of HSV demonstrate 71% survival of the female mice. Comparison with wt mice can be made by examining Fig. 1D where 18% of female wt mice survived. B) Brainstem titers are shown for female (black diamonds; n=4, 11, and 4 on days 5, 6, and 7 p.i., respectively) and male (open circles n=4 and 11 on days 5 and 6 p.i., respectively) DAP12KI mice following infection with 3.16×107 pfu of HSV/mouse. Data represent cumulative results from three independent experiments. Dotted line indicates the level of detection in the assay. C) Increased female mortality was not ameliorated by depletion of pDCs prior to infection with 3.16×107 pfu of HSV. Aggregated results from two independent experiments are shown with BDCA2-DTR Tg females (diamonds with solid line; n=10) and males (circles with dashed line; n=10) treated with DT every 2 days to selectively deplete pDCs. D) No differences were observed in serum IFNα levels in DAP12KI female (black diamonds; n=7/time-point) and male (open circles; n=6–7/time-point on days 0 and 0.5 p.i.) mice infected with 3.16×107 pfu of HSV. Gray bars represent median values for each time-point. Data was combined from two independent experiments. E) No differences were observed in serum inflammatory cytokine levels in female (black bars; n=3) and male (white bar; n=3) DAP12KI mice on day 6 p.i. with 3.16×107 pfu of HSV.
DAP12 regulates inflammatory cytokine production in dendritic cells and macrophages and appears to be particularly important in regulating IFNα and IL-12 in plasmacytoid dendritic cells (pDCs) [37, 38]. Therefore, we evaluated survival differences in female and male mice depleted of pDCs prior to HSV infection (i.e., BDCA2-DTR Tg mice injected with 100 ng diphtheria toxin (DT)/mouse i.p. every 2 days [42]) and observed no improvement in female mortality (Fig. 5C). In addition, no differences were seen in serum IFNα levels at twelve hours p.i. (Fig. 5D) or in the serum levels of pro-inflammatory cytokines on day 6 p.i. in DAP12KI female and male mice (Fig. 5E). This suggests that the influence of DAP12 signaling on female susceptibility to systemic viral infections occurs independent of type I interferons. Furthermore, these data show that intact DAP12 signaling in cells other than pDCs contributes to the increased mortality (and increased brainstem titers) observed in wt female mice during systemic HSV infection, independent of the regulation of proinflammatory cytokines in the serum at day 6 p.i.
4. Discussion
Our quantitative study of murine survival following systemic viral infection provides a robust model of sex-specific differences in innate immune responses. We observed significantly higher male than female survival following systemic infection with three large double-stranded DNA viruses, including two herpesviruses and a poxvirus. The differences were particularly striking with HSV1, where LD50 values in male mice were 27-fold higher than in female mice. The enhanced male survival was differentially mediated by sex differences in signaling through the type 1 interferon receptor and DAP12.
To our knowledge, this is the first report of a substantial male survival advantage during murine systemic HSV infection (Table 1). Two previous studies described minimally better survival of female compared to male mice following HSV infection [51, 52]. Cantin and colleagues reported that 129 Sv/Ev female mice experienced modestly better survival than male mice (92% survival in females versus 77% in males) after infection with 1×105–1×107 pfu HSV/mouse via corneal scarification [51]. However, it is difficult to interpret the significance of these results given that mice infected with inoculum doses of virus varying over 100-fold were grouped together with no information regarding the distribution of the mice within the groups. Mayer and colleagues also reported modest differences in survival between CD-1 female (84% survival) and male (72% survival) mice following intranasal inoculation of HSV [52]. Differences in the background of the mice, route of infection, and strain of HSV between these studies and our current work may contribute to the differing results. However, the magnitude of the survival difference between male and female mice observed in our study of systemic infection was substantially larger when examined either at a single inoculum dose (e.g., survival of 3% of female mice compared to 88% of male mice after i.p. infection with 1×108 pfu HSV/mouse) or more quantitatively compared with LD50 values (i.e., 27-fold higher LD50 value in male compared to female mice). Interestingly, a female sex bias in susceptibility to HSV-2 meningitis has been reported in humans, with females accounting for 75–80% of the reported cases [53–55].
We observed no evidence that the sex differences in survival occurred secondary to an inappropriately robust female immune response resulting in host damage. For example, there was no difference in serum pro-inflammatory cytokine levels in female and male mice. Rather, viral titers in the brainstem suggested that male mice were able to mount a more effective host defense, at least in preventing transit to and/or replication of HSV in the central nervous system. This observation is consistent with a previous report of higher titers in the brain and spinal cord of female mice compared to male mice on day 5.7 p.i. with HSV [56].
The male survival advantage during HSV infection was independent of androgens. Furthermore, the elimination of estrogen in female mice or the addition of estrogen in castrated male mice only partially abrogated the sex differences in survival following HSV infection. Therefore, we postulated that more effective male host defense during systemic HSV infection resulted from estrogen-independent sex differences in host innate immune responses.
Indeed, the disparities in survival during HSV infection appeared to be largely attributable to differentially regulated innate immune responses, with IFNαβreceptor signaling enhancing male survival while intact DAP12 signaling increased the susceptibility of female mice. The male advantage during systemic HSV infection was nearly abrogated in the absence of IFNαβR signaling in IFNαβR-deficient mice. The significance of this observation might be questioned given the overall sensitivity of both male and female IFNαβR-deficient mice to HSV1. However, the generalizability of IFNαβ receptor signaling in mediating sex differences in survival was confirmed with studies of MCMV and VV infection in IFNαβR-deficient mice, which revealed near eradication of sex differences in survival without substantially increasing the sensitivity of both male and female mice to either MCMV or VV. Moreover, female mice were substantially more vulnerable to HSV than male mice in the context of IFNαβR-haploinsufficiency resulting in a 81-fold difference in the LD50 values of male and female mice. Together, these observations strongly implicate sex-specific differences in IFNαβ receptor signaling in mediating superior survival of male mice during systemic HSV infection. In contrast, DAP12 signaling appears to contribute to the increased female susceptibility during systemic HSV infection. Defective DAP12-mediated signaling substantially mitigated the observed sex differences by significantly increasing female survival while male survival was unchanged. Ongoing work in our laboratory is further delineating the mechanisms by which sex differences in IFNαβR and DAP12 signaling influence survival during systemic HSV infection.
The differential impact of signaling through the IFNαβR and DAP12 on susceptibility of male and female mice to systemic viral infection may be associated with dose effects of genes on the X-or Y-chromosomes. Harley and colleagues [12] recently demonstrated a 14-fold increased prevalence of SLE in men with Klinefelter’s syndrome (47, XXY) compared with males with a normal karotype (46, XY), implicating a dose effect of an X-chromosome gene (or microRNA) in the striking sex disparity in SLE susceptibility [12]. The translocation of a portion of the Xchromosome (containing 16 genes including Tlr7) to Y-chromosome in the Yaa (y-linked autoimmune accelerating) model of murine SLE also highlights the impact of gene dosage on susceptibility to SLE [13]. In the context of the appropriate genetic background, the duplication of Tlr7 in the Yaa locus renders male mice (expressing two copies of Tlr7) susceptible to SLE while the removal of one copy of Tlr7 (e.g., eliminating the X-chromosome-associated Tlr7 in male Yaa mice by crossing the Yaa mice with TLR7-deficient mice) ameliorates the development of SLE [57–59]. We hypothesize that the survival differences in male and female mice during systemic viral infections may be at least in part due to dose effects of genes (or microRNA) associated with either the X- or Y-chromosomes [9]. Future studies with FCG (four core genotypes) mice (where Sry is deleted from the Y chromosome and reinserted as an autosomal transgene resulting in independent segregation of testis formation and the sex chromosomes [15]) will allow this hypothesis to be further investigated.
Our observations regarding sex-specific differences in IFNαβR and DAP12 signaling during systemic viral infections may yield new insights into sex disparities in human autoimmune disorders. For example, type I interferons have been implicated in SLE pathogenesis, with the upregulation of IFN-inducible genes in leukocytes from SLE patients producing a reproducible “signature” on microarrays that disappears following effective immunosuppression (reviewed in [60, 61]). Our results suggest that sex differences in IFNαβR signaling enhance male survival during HSV infection while sex differences in DAP12 signaling mediate increased female mortality. A better understanding of how sex differences in innate immune responses alter susceptibility to systemic viral infections may lead to novel therapeutic interventions in human autoimmune diseases.
Research Highlights.
-
➢
We observed substantial sex differences in survival following viral infections
-
➢
The differences were particularly striking with herpes simplex virus 1, HSV1
-
➢
Sex differences were independent of androgens and only partially attributable to estrogens
-
➢
Enhanced male survival was mediated by sex differences in signaling through IFNabR and DAP12
-
➢
This provides a robust model of sex-specific differences in innate immune responses
Supplementary Material
Acknowledgements
This research was supported by NIAID R01 AI078994 and AI073552 grants, a pilot grant from the Center for Women’s Infectious Disease Research (cWIDR) at Washington University, and the Washington University Child Health Research Center K12-HD01487 grant. We appreciate the experimental help provided by L. Muglia with the delayed release estradiol experiments and the guidance provided by S. Gilfillan and M. Swiecki in the BDCA2-DTR Tg experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors have no financial conflicts of interest
References
- 1.Davidson A, Diamond B. Autoimmune diseases. N Engl J Med. 2001;345:340–350. doi: 10.1056/NEJM200108023450506. [DOI] [PubMed] [Google Scholar]
- 2.Lleo A, Battezzati PM, Selmi C, Gershwin ME, Podda M. Is autoimmunity a matter of sex? Autoimmun Rev. 2008;7:626–630. doi: 10.1016/j.autrev.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 3.Bouman A, Heineman MJ, Faas MM. Sex hormones and the immune response in humans. Hum Reprod Update. 2005;11:411–423. doi: 10.1093/humupd/dmi008. [DOI] [PubMed] [Google Scholar]
- 4.Marriott I, Huet-Hudson YM. Sexual dimorphism in innate immune responses to infectious organisms. Immunol Res. 2006;34:177–192. doi: 10.1385/IR:34:3:177. [DOI] [PubMed] [Google Scholar]
- 5.Zandman-Goddard G, Peeva E, Shoenfeld Y. Gender and autoimmunity. Autoimmun Rev. 2007;6:366–372. doi: 10.1016/j.autrev.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Beagley KW, Gockel CM. Regulation of innate and adaptive immunity by the female sex hormones oestradiol and progesterone. FEMS Immunol Med Microbiol. 2003;38:13–22. doi: 10.1016/S0928-8244(03)00202-5. [DOI] [PubMed] [Google Scholar]
- 7.Robinson DP, Lorenzo ME, Jian W, Klein SL. Elevated 17beta-estradiol protects females from influenza A virus pathogenesis by suppressing inflammatory responses. PLoS Pathog. 2011;7:e1002149. doi: 10.1371/journal.ppat.1002149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carrel L, Willard HF. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature. 2005;434:400–404. doi: 10.1038/nature03479. [DOI] [PubMed] [Google Scholar]
- 9.Pinheiro I, Dejager L, Libert C. X-chromosome-located microRNAs in immunity: Might they explain male/female differences?: The X chromosome-genomic context may affect X-located miRNAs and downstream signaling, thereby contributing to the enhanced immune response of females. Bioessays. 2011;33:791–802. doi: 10.1002/bies.201100047. [DOI] [PubMed] [Google Scholar]
- 10.Gleicher N, Barad DH. Gender as risk factor for autoimmune diseases. J Autoimmun. 2007;28:1–6. doi: 10.1016/j.jaut.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Hughes GC, Clark EA. Regulation of dendritic cells by female sex steroids: relevance to immunity and autoimmunity. Autoimmunity. 2007;40:470–481. doi: 10.1080/08916930701464764. [DOI] [PubMed] [Google Scholar]
- 12.Scofield RH, Bruner GR, Namjou B, Kimberly RP, Ramsey-Goldman R, Petri M, et al. Klinefelter's syndrome (47,XXY) in male systemic lupus erythematosus patients: support for the notion of a gene-dose effect from the X chromosome. Arthritis Rheum. 2008;58:2511–2517. doi: 10.1002/art.23701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy ED, Roths JB. A Y chromosome associated factor in strain BXSB producing accelerated autoimmunity and lymphoproliferation. Arthritis Rheum. 1979;22:1188–1194. doi: 10.1002/art.1780221105. [DOI] [PubMed] [Google Scholar]
- 14.Robinson DP, Huber SA, Moussawi M, Roberts B, Teuscher C, Watkins R, et al. Sex chromosome complement contributes to sex differences in coxsackievirus B3 but not influenza A virus pathogenesis. Biol Sex Differ. 2011;2:8. doi: 10.1186/2042-6410-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arnold AP, Chen X. What does the "four core genotypes" mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol. 2009;30:1–9. doi: 10.1016/j.yfrne.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McClelland EE, Smith JM. Gender specific differences in the immune response to infection. Arch Immunol Ther Exp (Warsz) 2011;59:203–213. doi: 10.1007/s00005-011-0124-3. [DOI] [PubMed] [Google Scholar]
- 17.Cook IF. Sexual dimorphism of humoral immunity with human vaccines. Vaccine. 2008;26:3551–3555. doi: 10.1016/j.vaccine.2008.04.054. [DOI] [PubMed] [Google Scholar]
- 18.Kennedy RB, Ovsyannikova IG, Pankratz VS, Vierkant RA, Jacobson RM, Ryan MA, et al. Gender effects on humoral immune responses to smallpox vaccine. Vaccine. 2009;27:3319–3323. doi: 10.1016/j.vaccine.2009.01.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rusnak JM, Gibbs P, Boudreau E, Clizbe DP, Pittman P. Immunogenicity and safety of an inactivated Rift Valley fever vaccine in a 19-year study. Vaccine. 2011;29:3222–3229. doi: 10.1016/j.vaccine.2011.02.037. [DOI] [PubMed] [Google Scholar]
- 20.Klein SL, Hodgson A, Robinson DP. Mechanisms of sex disparities in influenza pathogenesis. J Leukoc Biol. 2011 doi: 10.1189/jlb.0811427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell LA, Zhang T, Tingle AJ. Differential antibody responses to rubella virus infection in males and females. J Infect Dis. 1992;166:1258–1265. doi: 10.1093/infdis/166.6.1258. [DOI] [PubMed] [Google Scholar]
- 22.Nielsen TR, Pedersen M, Rostgaard K, Frisch M, Hjalgrim H. Correlations between Epstein-Barr virus antibody levels and risk factors for multiple sclerosis in healthy individuals. Mult Scler. 2007;13:420–423. doi: 10.1177/1352458506071470. [DOI] [PubMed] [Google Scholar]
- 23.Klingstrom J, Lindgren T, Ahlm C. Sex-dependent differences in plasma cytokine responses to hantavirus infection. Clin Vaccine Immunol. 2008;15:885–887. doi: 10.1128/CVI.00035-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Villacres MC, Longmate J, Auge C, Diamond DJ. Predominant type 1 CMV-specific memory T-helper response in humans: evidence for gender differences in cytokine secretion. Hum Immunol. 2004;65:476–485. doi: 10.1016/j.humimm.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 25.Sterling TR, Pisell-Noland T, Perez JL, Astemborski J, McGriff JR, Nutting L, et al. Sex-based differences in T lymphocyte responses in HIV-1-seropositive individuals. J Infect Dis. 2005;191:881–885. doi: 10.1086/427827. [DOI] [PubMed] [Google Scholar]
- 26.Hewagama A, Patel D, Yarlagadda S, Strickland FM, Richardson BC. Stronger inflammatory/cytotoxic T-cell response in women identified by microarray analysis. Genes Immun. 2009;10:509–516. doi: 10.1038/gene.2009.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Camara M, Dieye TN, Seydi M, Diallo AA, Fall M, Diaw PA, et al. Low-level CD4+ T cell activation in HIV-exposed seronegative subjects: influence of gender and condom use. J Infect Dis. 2010;201:835–842. doi: 10.1086/651000. [DOI] [PubMed] [Google Scholar]
- 28.Berghofer B, Frommer T, Haley G, Fink L, Bein G, Hackstein H. TLR7 ligands induce higher IFN-alpha production in females. J Immunol. 2006;177:2088–2096. doi: 10.4049/jimmunol.177.4.2088. [DOI] [PubMed] [Google Scholar]
- 29.Meier A, Chang JJ, Chan ES, Pollard RB, Sidhu HK, Kulkarni S, et al. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat Med. 2009;15:955–959. doi: 10.1038/nm.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lorenzo ME, Hodgson A, Robinson DP, Kaplan JB, Pekosz A, Klein SL. Antibody responses and cross protection against lethal influenza A viruses differ between the sexes in C57BL/6 mice. Vaccine. 2011;29:9246–9255. doi: 10.1016/j.vaccine.2011.09.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fuller AC, Kang B, Kang HK, Yahikozowa H, Dal Canto MC, Kim BS. Gender bias in Theiler's virus-induced demyelinating disease correlates with the level of antiviral immune responses. J Immunol. 2005;175:3955–3963. doi: 10.4049/jimmunol.175.6.3955. [DOI] [PubMed] [Google Scholar]
- 32.Huber SA. Increased susceptibility of male BALB/c mice to coxsackievirus B3-induced myocarditis: role for CD1d. Med Microbiol Immunol. 2005;194:121–127. doi: 10.1007/s00430-004-0221-6. [DOI] [PubMed] [Google Scholar]
- 33.Yirrell DL, Blyth WA, Hill TJ. The influence of androgens on paralysis in mice following intravenous inoculation of herpes simplex virus. J Gen Virol. 1987;68(Pt 9):2461–2464. doi: 10.1099/0022-1317-68-9-2461. [DOI] [PubMed] [Google Scholar]
- 34.Paludan SR, Bowie AG, Horan KA, Fitzgerald KA. Recognition of herpesviruses by the innate immune system. Nat Rev Immunol. 11:143–154. doi: 10.1038/nri2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Veer MJ, Holko M, Frevel M, Walker E, Der S, Paranjape JM, et al. Functional classification of interferon-stimulated genes identified using microarrays. J Leukoc Biol. 2001;69:912–920. [PubMed] [Google Scholar]
- 36.Zawatzky R, Gresser I, DeMaeyer E, Kirchner H. The role of interferon in the resistance of C57BL/6 mice to various doses of herpes simplex virus type 1. J Infect Dis. 1982;146:405–410. doi: 10.1093/infdis/146.3.405. [DOI] [PubMed] [Google Scholar]
- 37.Sjolin H, Robbins SH, Bessou G, Hidmark A, Tomasello E, Johansson M, et al. DAP12 signaling regulates plasmacytoid dendritic cell homeostasis and down-modulates their function during viral infection. J Immunol. 2006;177:2908–2916. doi: 10.4049/jimmunol.177.5.2908. [DOI] [PubMed] [Google Scholar]
- 38.Blasius AL, Cella M, Maldonado J, Takai T, Colonna M. Siglec-H is an IPC-specific receptor that modulates type I IFN secretion through DAP12. Blood. 2006;107:2474–2476. doi: 10.1182/blood-2005-09-3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamerman JA, Ni M, Killebrew JR, Chu CL, Lowell CA. The expanding roles of ITAM adapters FcRgamma and DAP12 in myeloid cells. Immunol Rev. 2009;232:42–58. doi: 10.1111/j.1600-065X.2009.00841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geurs TL, Zhao YM, Hill EB, French AR. Ly49H engagement compensates for the absence of type I interferon signaling in stimulating NK cell proliferation during murine cytomegalovirus infection. J Immunol. 2009;183:5830–5836. doi: 10.4049/jimmunol.0901520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tomasello E, Desmoulins P-O, Chemin K, Guia S, Cremer H, Ortaldo J, et al. Combined natural killer cell and dendritic cell functional deficiency in KARAP/DAP12 loss-of-function mutant mice. Immunity. 2000;13:355–364. doi: 10.1016/s1074-7613(00)00035-2. [DOI] [PubMed] [Google Scholar]
- 42.Swiecki M, Gilfillan S, Vermi W, Wang Y, Colonna M. Plasmacytoid dendritic cell ablation impacts early interferon responses and antiviral NK and CD8(+) T cell accrual. Immunity. 2010;33:955–966. doi: 10.1016/j.immuni.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown MG, Dokun AO, Heusel JW, Smith HR, Beckman DL, Blattenberger EA, et al. Vital involvement of a natural killer cell activation receptor in resistance to viral infection. Science. 2001;292:934–937. doi: 10.1126/science.1060042. [DOI] [PubMed] [Google Scholar]
- 44.Rader KA, Ackland-Berglund CE, Miller JK, Pepose JS, Leib DA. In vivo characterization of site-directed mutations in the promoter of the herpes simplex virus type 1 latency-associated transcripts. J Gen Virol. 1993;74(Pt 9):1859–1869. doi: 10.1099/0022-1317-74-9-1859. [DOI] [PubMed] [Google Scholar]
- 45.Reed LJ, Muench H. A Simple Method of Estimating Fifty Percent Endpoints. Am J Hygiene. 1938;27:493–497. [Google Scholar]
- 46.Brown AS, Davis JM, Murphy EA, Carmichael MD, Carson JA, Ghaffar A, et al. Susceptibility to HSV-1 infection and exercise stress in female mice: role of estrogen. J Appl Physiol. 2007;103:1592–1597. doi: 10.1152/japplphysiol.00677.2007. [DOI] [PubMed] [Google Scholar]
- 47.Pung OJ, Luster MI. Toxoplasma gondii: decreased resistance to infection in mice due to estrogen. Exp Parasitol. 1986;61:48–56. doi: 10.1016/0014-4894(86)90134-7. [DOI] [PubMed] [Google Scholar]
- 48.Daffis S, Samuel MA, Keller BC, Gale M, Jr, Diamond MS. Cell-specific IRF-3 responses protect against West Nile virus infection by interferon-dependent and - independent mechanisms. PLoS Pathog. 2007;3:e106. doi: 10.1371/journal.ppat.0030106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Der SD, Zhou A, Williams BR, Silverman RH. Identification of genes differentially regulated by interferon alpha, beta, or gamma using oligonucleotide arrays. Proc Natl Acad Sci U S A. 1998;95:15623–15628. doi: 10.1073/pnas.95.26.15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leib DA, Harrison TE, Laslo KM, Machalek MA, Moorman NJ, Virgin HW. Interferons regulate the phenotype of wild-type and mutant herpes simplex viruses in vivo. J Exp Med. 1999;189:663–672. doi: 10.1084/jem.189.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Han X, Lundberg P, Tanamachi B, Openshaw H, Longmate J, Cantin E. Gender influences herpes simplex virus type 1 infection in normal and gamma interferon-mutant mice. J Virol. 2001;75:3048–3052. doi: 10.1128/JVI.75.6.3048-3052.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brown AS, Davis JM, Murphy EA, Carmichael MD, Ghaffar A, Mayer EP. Gender differences in viral infection after repeated exercise stress. Med Sci Sports Exerc. 2004;36:1290–1295. doi: 10.1249/01.mss.0000135798.72735.b3. [DOI] [PubMed] [Google Scholar]
- 53.Landry ML, Greenwold J, Vikram HR. Herpes simplex type-2 meningitis: presentation and lack of standardized therapy. Am J Med. 2009;122:688–691. doi: 10.1016/j.amjmed.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 54.Omland LH, Vestergaard BF, Wandall JH. Herpes simplex virus type 2 infections of the central nervous system: A retrospective study of 49 patients. Scand J Infect Dis. 2008;40:59–62. doi: 10.1080/00365540701509881. [DOI] [PubMed] [Google Scholar]
- 55.Glynn JR, Crampin AC, Ngwira BM, Ndhlovu R, Mwanyongo O, Fine PE. Herpes simplex virus type 2 trends in relation to the HIV epidemic in northern Malawi. Sex Transm Infect. 2008;84:356–360. doi: 10.1136/sti.2008.030056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burgos JS, Ramirez C, Sastre I, Alfaro JM, Valdivieso F. Herpes simplex virus type 1 infection via the bloodstream with apolipoprotein E dependence in the gonads is influenced by gender. J Virol. 2005;79:1605–1612. doi: 10.1128/JVI.79.3.1605-1612.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pisitkun P, Deane JA, Difilippantonio MJ, Tarasenko T, Satterthwaite AB, Bolland S. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science. 2006;312:1669–1672. doi: 10.1126/science.1124978. [DOI] [PubMed] [Google Scholar]
- 58.Subramanian S, Tus K, Li QZ, Wang A, Tian XH, Zhou J, et al. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc Natl Acad Sci U S A. 2006;103:9970–9975. doi: 10.1073/pnas.0603912103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fairhurst AM, Hwang SH, Wang A, Tian XH, Boudreaux C, Zhou XJ, et al. Yaa autoimmune phenotypes are conferred by overexpression of TLR7. Eur J Immunol. 2008;38:1971–1978. doi: 10.1002/eji.200838138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pascual V, Allantaz F, Patel P, Palucka AK, Chaussabel D, Banchereau J. How the study of children with rheumatic diseases identified interferon-alpha and interleukin-1 as novel therapeutic targets. Immunol Rev. 2008;223:39–59. doi: 10.1111/j.1600-065X.2008.00643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Crow MK, Kirou KA, Wohlgemuth J. Microarray analysis of interferon-regulated genes in SLE. Autoimmunity. 2003;36:481–490. doi: 10.1080/08916930310001625952. [DOI] [PubMed] [Google Scholar]
- 62.Andersen AA, Hanson RP. Influence of sex and age on natural resistance to St. Louis encephalitis virus infection in mice. Infect Immun. 1974;9:1123–1125. doi: 10.1128/iai.9.6.1123-1125.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pozzetto B, Gresser I. Role of sex and early interferon production in the susceptibility of mice to encephalomyocarditis virus. J Gen Virol. 1985;66(Pt 4):701–709. doi: 10.1099/0022-1317-66-4-701. [DOI] [PubMed] [Google Scholar]
- 64.Brownstein D, Bhatt PN, Jacoby RO. Mousepox in inbred mice innately resistant or susceptible to lethal infection with ectromelia virus. V. Genetics of resistance to the Moscow strain. Arch Virol. 1989;107:35–41. doi: 10.1007/BF01313876. [DOI] [PubMed] [Google Scholar]
- 65.Lundberg P, Welander P, Openshaw H, Nalbandian C, Edwards C, Moldawer L, et al. A locus on mouse chromosome 6 that determines resistance to herpes simplex virus also influences reactivation, while an unlinked locus augments resistance of female mice. J Virol. 2003;77:11661–11673. doi: 10.1128/JVI.77.21.11661-11673.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.