Abstract
The protein kinase regulated by RNA (PKR) enhances both activation of mitogen-activated protein kinases and the induction of interferon beta (IFN-β) by measles virus defective in C protein expression (Cko). Here we used complementation of human cell lines stably deficient in PKR (PKRkd) to probe the basis of these PKR-mediated responses. We found that PKRkd HeLa and amnion U cell lines were defective for virus-mediated activation of IFN induction signaling components compared to PKR-sufficient control cells. Complementation of PKRkd cells with wildtype PKR, but not with PKR mutants defective in either catalytic activity or dsRNA binding activity, restored JNK, p38 and ATF-2 phosphorylation and enhanced IFN-β induction following infection. By contrast to mammalian PKR, the Z-DNA binding domain-containing fish homologue of PKR, PKZ, lacked the capacity to enhance Cko virus-mediated IFN-β induction. Furthermore, inhibition of virus growth was observed with Cko-infected PKRkd cells complemented with PKR but not with PKZ.
Keywords: PKR, protein kinase, interferon, innate immunity
INTRODUCTION
Interferons (IFNs) are a cornerstone of the innate antiviral immune response that provides an early line of defense against viral infection. In addition to their antiviral activities, IFNs also affect the growth and development of normal and tumor cells (Borden et al., 2007; Joshi et al., 2009; Platanias, 2005; Samuel, 2001). Type I interferons (IFNs α and β) are induced by viral infection and mediate the establishment of an antiviral state in host cells (Randall and Goodbourn, 2008; Samuel, 2001); they also modulate adaptive in addition to innate immune responses (Borden et al., 2007; Le Bon and Tough, 2002). Cellular sensors involved in IFN production include RIG (retinoic acid inducible gene) I-like receptors (RLRs) and Toll-like receptors (TLRs) that recognize foreign nucleic acids generated during infection (Kawai and Akira, 2010; Yoneyama and Fujita, 2010). Both RLR and TLR signaling pathways trigger the activation of interferon regulatory factor 3 (IRF-3) and nuclear factor kappa B (NF- κB), which together with activating transcription factor 2 (ATF-2) and c-jun form an enhanceosome complex that activates IFN-β gene transcription (Panne et al., 2007).
PKR is an IFN-inducible protein kinase that mediates both antiviral and antiproliferative effects of IFNs (Garcia et al., 2006; Samuel, 2001). The PKR protein domain structure includes an N-terminal regulatory domain that binds RNA and a C-terminal kinase catalytic domain. Upon RNA binding, PKR undergoes dimerization and autophosphorylation to generate an active kinase (McCormack et al., 1992; McKenna et al., 2007; Ortega et al., 1996; Pindel and Sadler, 2011; Thomis and Samuel, 1993). The best characterized cellular substrate of PKR is the alpha subunit of translation initiation factor 2 (eIF2α), although additional substrates of PKR have been described including IκB kinase, HIV Tat protein, NFAT factor, MPP4 and B65α subunit of PP2A phosphatase (Pindel and Sadler, 2011; Toth et al., 2006). When activated, PKR catalyzes the phosphorylation of eIF2α on serine 51, a modification that leads to an inhibition of translation (Nallagatla et al., 2011; Pindel and Sadler, 2011; Samuel, 1979, 1993). In addition to altering the translation pattern within cells, PKR also modulates signal transduction responses by interacting with and activating components of signaling pathways in response to cellular stresses including virus infection (Pfaller et al., 2011; Pindel and Sadler, 2011; Samuel, 2001). Among the interacting partners described for PKR are the TRAF family of proteins (Gil et al., 2004). Stress-activated protein kinases, c-jun N-terminal kinase (JNK) and p38, also are implicated in the activation of the innate immune response and cytokine production, through phosphorylation of transcription factors c-jun and ATF2 (Liu et al., 2007; Platanias, 2005). Furthermore, PKR has been shown to modulate the activation of JNK and p38 in response to diverse stimuli including lipopolysaccharide, double-stranded RNA (dsRNA), tumor necrosis factor and viral infection (Goh et al., 2000; Iordanov et al., 2000; Sadler and Williams, 2007; Silva et al., 2004; Takada et al., 2007; Zhang et al., 2009).
Fish, like mammals, encode both IFNs and IFN-inducible gene products (Berg et al., 2009; Liu et al., 2011; Sun et al., 2011; Verrier et al., 2011). Among them, a PKR-like kinase protein identified in fish, designated PKZ, is constitutively expressed at low levels and inducible by viral infection or treatment with IFN or poly (I:C). While the C-terminal kinase domain of PKZ is closely related to the kinase domain of mammalian PKR, instead of repeated dsRNA binding domains within the N-terminal region, PKZ possesses two copies of the nucleic acid binding domain for the left-handed Z-conformation DNA (Z-DNA) similar to the Z-DNA binding domains first identified in the mammalian IFN-inducible adenosine deaminase acting on dsRNA (ADAR1) and the poxvirus E3L protein (Bergan et al., 2008; Herbert et al., 1997; Patterson and Samuel, 1995; Rothenburg et al., 2005; Su et al., 2008).
Measles virus (MV), a member of the family of Paramyxoviridae, is a nonsegmented negative-sense single-stranded RNA virus possessing 6 genes in the order 3′-N, P/V/C, M, F, H, L-5′ (Griffin, 2007). The C and V proteins are major virulence factors (Cattaneo et al., 2008; Randall and Goodbourn, 2008) that play roles in circumventing the host immune response by inhibiting IFN production and IFN signaling (Nakatsu et al., 2006; Palosaari et al., 2003; Patterson et al., 2000; Shaffer et al., 2003; Sparrer et al., 2011). Previous work showed that the PKR protein mediates a reduction in viral growth and enhancement of apoptosis in cells infected with C-deficient (Cko) measles virus (Toth et al., 2009). Furthermore, PKR plays a role in amplifying the activation of JNK and p38 signaling and the induction of IFN-β by Cko virus infection (McAllister et al., 2010). In order to gain insight into the mechanism by which PKR modulates activation of stress-activated JNK and p38 kinases and the induction of IFN-β in response to MV infection, and to test the requirement for PKR activity, we complemented PKRkd cells with either wildtype or mutant forms of PKR or PKZ. Our results reveal that the catalytic activity of PKR is required for optimal activation of the JNK and p38 kinases and maximal induction of IFN-β following infection with Cko virus. By contrast, the PKZ homolog of PKR found in fish, when expressed in PKRkd mammalian cells, does not complement the PKR-dependent phenotype as measured by increased induction of IFN-β or phosphorylation of JNK following Cko virus infection.
MATERIALS AND METHODS
Cells, viruses, and plasmids
Parental human amnion U cells and Vero cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 5% (vol/vol) fetal bovine serum (HyClone), 100 μg/ml of penicillin and 100 units/ml of streptomycin (GIBCO/Invitrogen) as previously described (McAllister et al., 2010; Zhang et al., 2009). HeLa cells and U cells made stably deficient in PKR by an integrated short hairpin silencing RNA interference strategy, designated PKRkd and UPKRkd, respectively, were as previously described as were the drug-resistant knockdown vector controls cells (CONkd) (Zhang et al., 2009; Zhang and Samuel, 2007). Knockdown cells were maintained in medium containing 1 μg/ml puromycin (Sigma).
The recombinant Moraten measles virus vaccine strain, mutated to be deficient in C protein expression (Cko) and to include the gene encoding green fluorescent protein inserted downstream of the viral H gene was generously provided by Roberto Cattaneo (Mayo Clinic, Rochester, MN) (Devaux et al., 2008).
The expression vector pcDNA6 was used for expression of the wildtype (WT) human PKR protein and mutants defective for either catalytic activity (K296R) or dsRNA binding activity (K64E) (McCormack et al., 1994; Thomis and Samuel, 1992). The PKR expression plasmid constructs were mutated at four synonymous sites (designated by underlined italics font) in the shRNA target sequence (GCAGGGAGTAGTCTTAAAGTA) located in the PKR open reading frame to circumvent knockdown by the stably expressed silencing RNA while still preserving the PKR amino acid sequence (Zhang et al., 2009). Plasmids were verified by direct sequence analysis. The expression plasmids for PKZ and the mutant deficient in catalytic activity, K199R (Rothenburg et al., 2005), were generously provided by S. Rothenberg (National Institutes of Health, Bethesda).
Virus infections and plasmid transfections
Viral infections were carried out as previously described (McAllister et al., 2010; Toth et al., 2009), using a 50 percent tissue culture dose per cell multiplicity of infection (MOI) of 1. Briefly, U, UPKRkd, CONkd, and PKRkd cells were seeded into 12- or 6-well plates as indicated. After 24 h, monolayers at 80–90% confluency were washed once with OptiMEM and then infected with Cko MV. After 2 h, the monolayers were washed twice with OptiMEM, and then DMEM containing 5% FBS was added and the cultures maintained until harvest. For complementation tests, HeLa PKRkd cells were transfected with the indicated PKR or PKZ expression vector using FuGENE HD transfection reagent (Roche) according to the manufacturer’s protocol. Briefly, plasmid DNA was diluted in OptiMEM to the final concentration of 20 ng/μl, and then the FuGENE reagent was added to the diluted DNA to achieve a ratio of DNA (μg) to FuGENE (μl) of 1:2. The DNA-FuGENE complexes were incubated at room temperature for 20 min before addition to cells containing fresh DMEM with 5% FBS. The amount of DNA used was .5 μg/well (12-well plate) or 1 μg/well (6-well plate), adjusted using empty vector as necessary dependent upon the amount of expression construct utilized. The medium was changed between 5–6 h after transfection; cells were infected at 15 h after transfection as described above, or left uninfected. Virus growth assays were carried out as previously described (Toth et al., 2009). Titers of virus preparations were determined on Vero cells according to the Spearman-Kärber method (Devaux et al., 2008; Karber, 1931).
Western immunoblot analysis
At 24 h post-infection unless otherwise noted, extracts were prepared with lysis buffer [20 mM Hepes (pH 7.9), 400 mM NaCl, 1mM DTT, 1mM EDTA, and .5% NP-40] containing 50 mM NaF, 1mM Na2VO3, 1 mM phenylmethylsulfonyl fluoride (PMSF) and 1% (vol/vol) protease and phosphotase inhibitor cocktails (Sigma) as previously described (Toth et al., 2009). Protein concentration was determined by the Bradford method. Proteins were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes, and blocked in Tris-buffered saline containing 5% (wt/vol) either bovine serum albumin (for detection of phosphoproteins) or non-fat milk (for detection of total proteins) for 1 h at room temperature. Primary antibody incubation was performed overnight at 4°C. Rabbit polyclonal antibodies were used to detect PKR (Santa Cruz Biotechnology), ATF-2 (Santa Cruz Biotechnology), phospho-ATF-2 Thr71 (Cell Signaling Technology), p38 (Santa Cruz Biotechnology), Jun N-terminal kinase (JNK) (Santa Cruz Biotechnology), phospho-JNK Thr183/Tyr185 (Cell Signaling Technology), IRF-3 (Santa Cruz Biotechnology), and GFP (Santa Cruz Biotechnology). Rabbit monoclonal antibodies were used to detect phospho-PKR Thr446 (Epitomics), phospho-IRF-3 Ser396 (Cell Signaling Technology), phospho-p38 Thr180/Tyr182 (Cell Signaling Technology), and phospho-eIF2α Ser51 (Epitomics); mouse monoclonal antibodies were used to detect β-actin (Sigma) and poly (ADP-ribose) polymerase (PARP) (BD Bioscience). Anti-Myc antibody for detection of epitope tagged PKZ was from Roche, and antibody against MV viral H protein was provided by Roberto Cattaneo (Rochester, MN) as previously described (Toth et al., 2009). Western immunoblot detection was performed with IRDye 800CW-conjugated anti-rabbit immunoglobulin G or IRDye 680-conjugated anti-mouse IgG secondary antibody according to the manufacturer’s protocols. The immunoreactive bands were quantified using an Odyssey infrared imaging system (Li-COR Biosciences) and obtained values normalized to β-actin as a loading control.
Quantitative real-time PCR
IFN-β transcripts were measured by quantitative real-time PCR (qPCR) as previously described (McAllister et al., 2010). RNA was isolated from uninfected or infected cells 24 h after infection with Cko virus by the RNeasy (Qiagen) protocol. cDNA was prepared by reverse transcription carried out using 1 μg of RNA, random hexamer oligonucleotide primers, and SuperScript II (Invitrogen). qPCR analyses were performed in duplicate with each cDNA template using IQ SYBR Green Supermix (Bio-Rad) and a Bio-Rad MyIQ multicolor real-time qPCR instrument. The forward and reverse primers for IFN-β and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were as described previously (McAllister et al., 2010). For IFN-β transcripts, the qPCR program was set for 3-min hot start followed by 30 s at 95°C, 30 s at 58°C, and 30 s at 72°C, repeated 45 times. IFN-β values were normalized to GAPDH values.
Luciferase reporter assay
PKRkd Hela cells in 12-well plates were transfected with the firefly luciferase construct pGL3 control (Promega) and either an empty vector or indicated PKR or PKZ expression vector using FuGENE HD transfection reagent (Roche). To achieve comparable levels of expression, 0.5 μg of wildtype or 0.1 μg of mutant plasmid DNA construct for PKR or PKZ were co-transfected with 0.05 μg of pGL3 control plasmid. At 24 h after transfection cell extracts were prepared and analyzed for luciferase activity according to the manufacturer’s protocol (Promega) using an OPTOCOMP I luminometer. Luciferase activity was normalized to total extract protein.
RESULTS
PKR enhances the phosphorylation of MAP kinases and ATF2 following measles virus infection
Prior studies established that the attenuated Moraten vaccine strain MV Cko mutant, deficient for C protein expression, is a robust inducer of IFN-β compared to either WT or Vko virus. The enhanced IFN-inducing capacity of Cko virus is PKR-dependent and correlates with activation of MAP kinases (McAllister et al., 2010). To further define the role and generality of PKR as a mediator of IFN-β induction in MV-infected human cells, the PKR requirement for activation of signaling components following Cko virus infection was examined using two different human cell lines, HeLa and amnion U, made stably deficient in PKR expression (PKRkd) by a shRNA silencing strategy (Zhang et al., 2009; Zhang and Samuel, 2007). The HeLa PKRkd cells contain less than 5% of the PKR protein found in PKR-sufficient control HeLa cells (CONkd); the U PKR knockdown cells (UPKRkd) contain less than 10% of the PKR protein expressed in parental U cells (U) (Fig. 1).
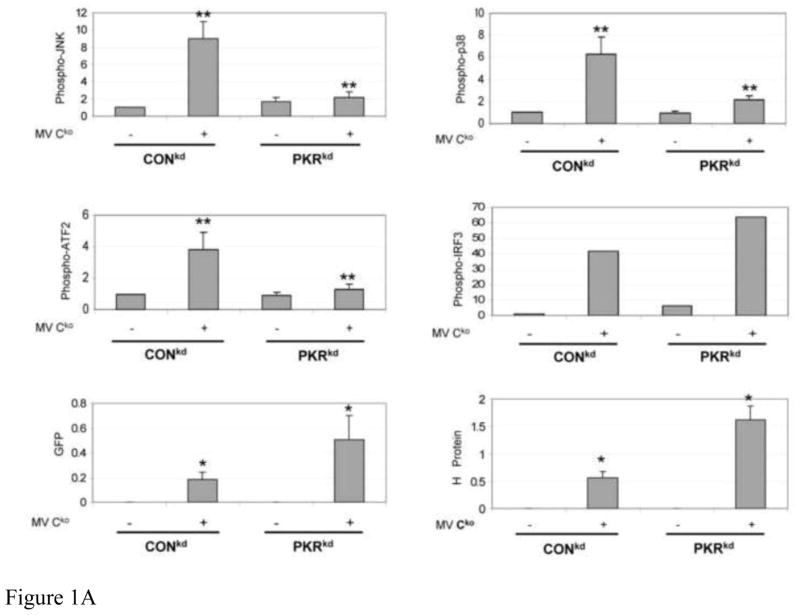
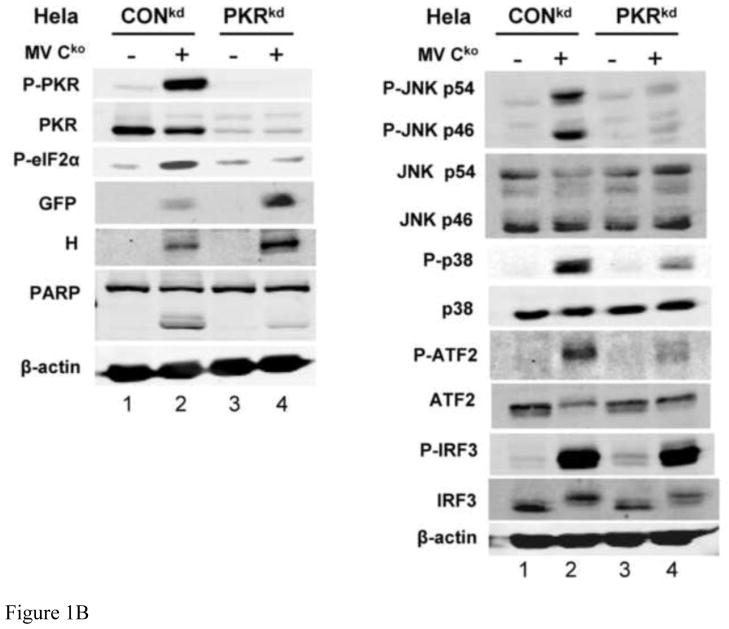
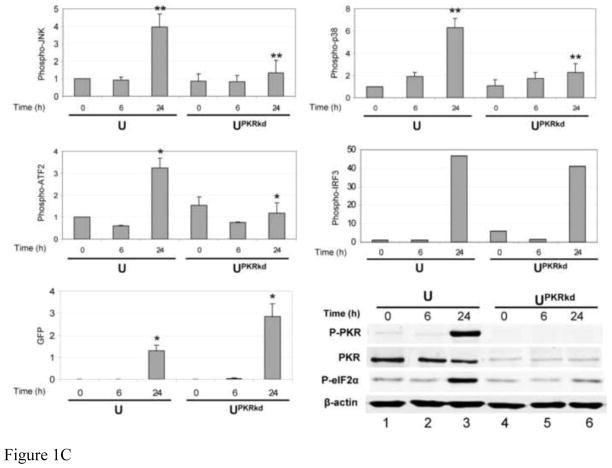
FIGURE 1. Activation of JNK, p38 and ATF2 phosphorylation is PKR dependent, but IRF3 phosphorylation is PKR independent, in Cko-infected cells.
(A) Whole-cell extracts were prepared from either uninfected (−) or infected (+) HeLa cells (CONkd or PKRkd) at 24 h after infection with Cko measles virus. Western blot analyses were performed and the blots quantified for levels of phosphorylation of JNK, p38, ATF2 and IRF3, and GFP and H protein expression, by infrared imaging as described under Materials and Methods. (B) Representative blots are also shown for PKR, phospho-PKR (P-PKR), and phospho-eIF2α (P-eIF2α), in addition to the proteins quantified in (A) above. (C) Quantitation of MAP kinase, ATF2 and IRF3 phosphorylation and GFP expression determined by immunoblot analyses of extracts prepared from amnion U cells (U) or a stable PKR knockdown clone (UPKRkd) infected for 6 or 24 h with Cko virus (6, 24), or left uninfected (0). Quantifications were performed as for Hela cells shown in (A). Representative western immunoblots for PKR, phospho-PKR (P-PKR), and phospho-eIF2α (P-eIF2α) are shown for extracts from amnion U cells, uninfected (0) or infected (6, 24 h) with Cko virus. The results shown in (A) and (C) are the means with standard deviation (n= 3). *, P< 0.05; * *, P< 0.01.
As shown by the Figure 1A quantifications of immunoblots, following Cko virus infection, the phosphorylation of JNK, p38 and ATF2 were impaired in PKRkd HeLa cells compared to control (CONkd) cells. The MAPK pathway activations seen in PKR-sufficient CONkd HeLa cells correlated with enhanced phosphorylation of both PKR and eIF2α as shown by representative immunoblots (Fig. 1B). By contrast, IRF3 phosphorylation was not impaired in PKR deficient (PKRkd) cells compared to the PKR sufficient CONkd cells following infection. Quantitation of the western blots revealed that the level of phosphorylated JNK, p38 and ATF2 was increased ~5 to 10-fold by infection of CONkd HeLa cells, and to higher levels in the PKR-sufficient CONkd compared to PKR-deficient PKRkd HeLa cells (Fig. 1A).
As a test of the generality of the results observed with PKRkd HeLa cells, we assessed the PKR dependency of Cko virus-induced activation of MAPK signaling components in a second human cell line, amnion U cells, in which PKR also had been stably knocked down using the shRNAi strategy. PKRkd U cells showed results similar to those seen with PKRkd HeLa cells. The phosphorylations of JNK, p38 and ATF2 were enhanced in Cko-infected PKR-sufficient U cells compared to PKR-deficient U PKRkd cells at 24 h after infection, and compared to uninfected cells or cells infected for only 6 h (Fig. 1C). Activation of PKR and increased phosphorylation of eIF2α likewise were increased at 24 h postinfection in PKR sufficient U cells compared to UPKRkd cells (Fig. 1C), similar to the observations with HeLa cells (Fig. 1B).
Viral protein expression was assessed by determining the levels of two proteins: GFP, a reporter engineered downstream of the viral H gene for expression by recombinant virus; and, the viral H glycoprotein. Inhibition of GFP (Fig. 1A,C) and H (Fig. 1A) protein expression were seen in CONkd and U parental cells, and this inhibition correlated with an increased activation of PKR and phosphorylation of eIF2α in these PKR-sufficient cells (Fig. 1B,C). Knockdown of PKR led to increased viral protein production. Western blot quantitation revealed that the expression levels of GFP and H were ~3-fold higher in the PKR deficient compared to PKR sufficient cell lines (Fig. 1A,C).
Catalytic and RNA binding activities of PKR are required for the PKR-mediated enhancement of MAPK signaling and increased PARP cleavage following Cko infection
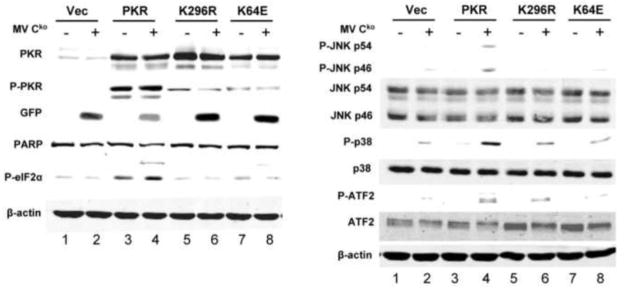
To test the requirement for PKR catalytic and RNA-binding activities in conferring the PKR dependency seen for MAP kinase and ATF2 phosphorylation in Cko-infected cells, PKRkd HeLa cells were complemented with either a catalytically inactive mutant PKR (K296R), an RNA-binding mutant (K64E), or WT PKR. To prevent knockdown of the ectopically expressed PKR by the stably expressed shRNA, the PKR cDNA sequence was mutated at the RNAi target site as described under Materials and Methods in a manner that circumvented knockdown without altering the amino acid sequence of the encoded PKR protein. Complemented cells were either left uninfected, or were infected with the Cko virus. Because K296R and K64E mutant proteins are expressed more efficiently than WT PKR protein due to PKR autoregulatory effects seen in transfected cells (Barber et al., 1993; McCormack et al., 1994; Thomis and Samuel, 1992), different amounts of plasmid DNA were used in the transfections to achieve comparable PKR expression levels of the WT, K296R and K64E proteins as measured by western blot analysis (Fig. 2, left, lanes 3–8).
FIGURE 2. Expression of wildtype PKR protein in PKRkd cells rescues JNK, p38 and ATF-2 phosphorylation and enhances PARP cleavage following MV Cko infection.
PKRkd HeLa cells were transfected with either an empty vector (Vec) or the expression construct encoding wildtype (WT), catalytic mutant (K296R) or RNA-binding mutant (K64E) PKR. At 15 h after transfection, cells were either mock infected (−) or infected with MV Cko (+) for 24 h. Western immunoblot analyses were performed on whole-cell extracts using antibodies against phospho-PKR (P-PKR), PKR, phospho-eIF2α (P-eIF2α), GFP and PARP (left panel); and, phospho-JNK (P-JNK), JNK, phospho-p38 (P-p38), p38, phospho-ATF2 (P-ATF2), and ATF2 (right panel). β–actin, loading control.
For PKRkd HeLa cells transfected with empty vector (Vec), no PKR phosphorylation was observed and only modest increases in phosphorylation of JNK, p38 and ATF2 were seen following Cko virus infection (Fig. 2, right, lanes 1,2). Complementation of PKRkd cells by expression of WT PKR protein increased the phosphorylation of JNK, p38 and ATF2 following infection (Fig. 2, right, lane 4) compared to PKRkd cells complemented with either the K296R or K64E mutant (Fig. 2, right, lanes 6,8). The PKR-mediated activation of MAP kinase and ATF2 in Cko-infected cells correlated with the phosphorylation of PKR and eIF2α (Fig. 2, left, lane 4). Furthermore, viral protein expression measured by GFP was inhibited in cells complemented with WT PKR, but in contrast, was increased in the K296R and K64E complemented cells following infection. The level of PARP cleavage, as an indicator of apoptosis, was elevated in cells expressing WT PKR but not in cells expressing either the catalytic or RNA-binding mutant protein. The increase in level of phosphorylated PKR in transfected cells observed with WT PKR in the absence of infection (Fig. 2) most likely is due to the plasmid transfection process that can give rise to activating dsRNA (Chiu et al., 2009; Wang and Samuel, 2009).
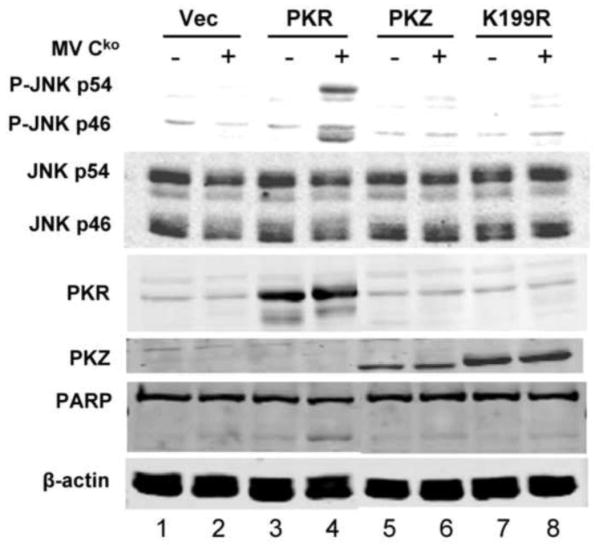
PKZ does not restore MAP kinase activation in PKRkd cells infected with Cko virus
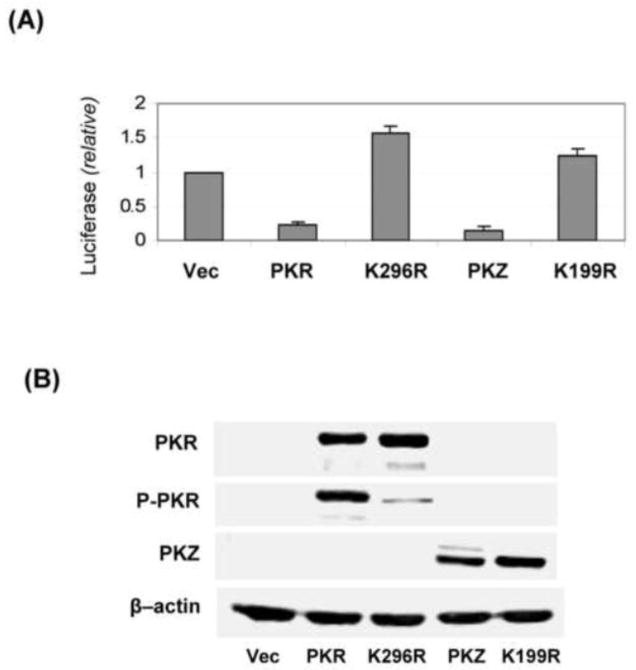
Phylogenetic analyses reveal that the kinase domain of PKZ from fish is closely related to that of mammalian PKR, but PKZ possesses two Z-DNA binding domains in the N-terminal region instead of the two RNA-binding domains found in PKR (Rothenburg et al., 2005). To test whether PKZ is able to restore MAP kinase activity in the PKR-deficient HeLa cells, WT PKZ was expressed in the PKRkd cells and activation of JNK was assessed following virus infection. Neither the expression of WT PKZ nor a catalytic mutant (K199R) form of PKZ increased JNK phosphorylation in Cko-infected cells (Fig. 3, lanes 6,8). By contrast, as a positive control, the expression of WT PKR rescued the phosphorylation of JNK relative to vector-transfected cells following Cko infection (Fig. 3, lanes 2,4). While PKZ did not restore MAP kinase activation in the PKRkd cells infected with Cko virus (Fig. 3), the WT PKZ construct like the WT PKR construct inhibited expression of the luciferase reporter when expressed in PKRkd cells, whereas neither the K199R PKZ mutant or K296R PKR mutant were inhibitory in the cotransfection reporter assay (Fig. 4). Finally, we also assessed the effect of PKZ on induction of apoptosis following infection. As shown in Figure 3, PARP cleavage was not detectably enhanced following Cko infection in cells expressing PKZ (Fig. 3, lane 6,8), but was increased in cells expressing PKR (Fig. 3, lane 4).
FIGURE 3. Expression of PKZ in PKRkd cells does not rescue JNK phosphorylation or increase PARP cleavage following MV Cko infection.
PKRkd HeLa cells were transfected with either empty vector (Vec) or the expression construct encoding wildtype PKR (PKR), wildtype PKZ (PKZ) or the PKZ catalytic mutant (K199R). After transfection for 15 h, cells then were either mock infected (−) or infected with MV Cko (+) for 24 h. Western immunoblot analyses were performed on whole-cell extracts using antibodies against phospho-JNK (P-JNK), JNK, PKR, Myc for Myc-tagged-PKZ (PKZ) and PARP. β–actin, loading control.
FIGURE 4. Expression of either PKR or PKZ in PKRkd cells inhibits luciferase reporter synthesis.
PKRkd Hela cells were co-transfected with the pGL3 control plasmid and either an empty vector (Vec) or the expression construct encoding wildtype PKR (PKR), wildtype PKZ (PKZ), PKR catalytic mutant (K296R), or the PKZ catalytic mutant (K199R). At 24 h after transfection whole-cell extracts were prepared and analyzed. (A) Luciferase activity was measured as described under Material and Methods. The means and standard deviations determined from three independent experiments. (B) Representative western immunoblots obtained with whole-cell extracts using antibodies against phospho-PKR (P-PKR), PKR, and Myc for Myc-tagged-PKZ (PKZ); β–actin, loading control.
Expression of PKR but not PKZ in PKRkd cells reduces the virus-coded GFP reporter expression
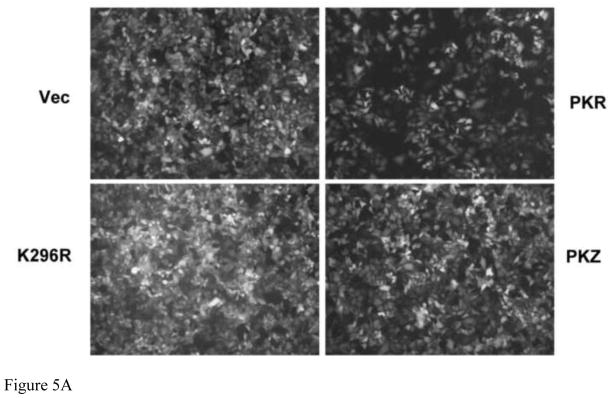
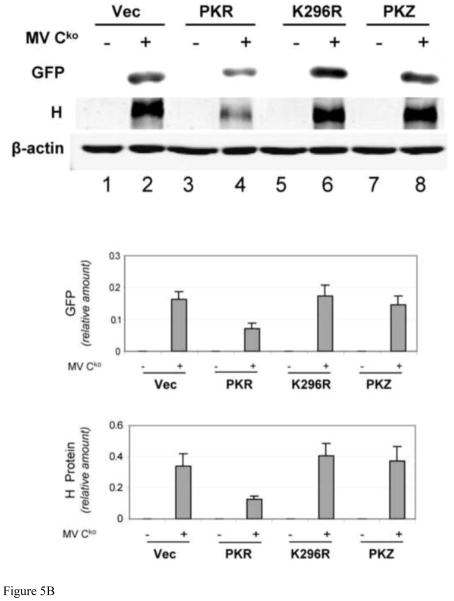
We earlier found that the knockdown of PKR rescued the growth of Cko mutant virus (Toth et al., 2009). Consistent with this finding, we observed herein that WT PKR reduced the growth of Cko virus in complemented PKRkd cells as measured by GFP expression (Fig. 2). We next examined virus growth in PKRkd HeLa cells expressing PKZ compared to PKR (Fig. 5). The GFP signal measured by fluorescence was reduced in PKRkd cells complemented with PKR relative to that of vector-transfected cells (Fig. 5A). However, the signal intensity of GFP expression was similar between vector and PKZ-transfected cells. Somewhat increased GFP expression was seen in cells expressing the K296R mutant form of PKR, an effect possibly due to a dominant negative activity of the ectopically expressed catalytic mutant on the residual endogenous WT PKR protein (Fig. 5A). Western blot analysis of GFP protein expression (Fig. 5B) was in general agreement with the results observed by fluorescence (Fig. 5A), suggesting that PKR but not PKZ was able to mediate impairment of Cko mutant virus growth. Similar to the observation for the engineered GFP reporter protein, viral H protein expression was also impaired in PKRkd cells complemented with WT PKR but not with the K296R mutant PKR or WT PKZ (Fig. 4B). As controls, western immunoblot analysis with antibody against β-actin (Fig. 5B) and phase-contrast images (data not shown) indicated comparable cell numbers for the different complemented cell cultures that were analyzed.
FIGURE 5. Expression of PKR but not PKZ impairs Cko mutant measles virus growth in PKRkd cells.
PKRkd Hela cells were transfected with an empty vector (Vec) or the expression construct encoding wildtype PKR (PKR), the PKR catalytic mutant (K296R), or wildtype PKZ as indicated. At 15 h after transfection cells were either mock infected (−) or infected with MV Cko (+). (A) Fluorescence images taken at 24 h after infection. (B) Representative western immunoblot analyses of complemented PKRkd HeLa whole-cell extracts prepared 24 h after infection, using antibodies against MV encoded proteins (GFP and H) and β-actin (upper). Quantitation of GFP and H protein expression in complemented PKRkd HeLa cells at 24 h after infection (lower).
As a direct measure of the effect of PKR or PKZ complementation on Cko virus replication in PKRkd cells, virus yields were determined at 24 h after infection at an MOI of 1 by the 50% TCID50 titration assay on Vero cells. The yields obtained for PKRkd cells complemented with vector (4.8 × 105 TCID50/ml), WT PKZ (2.7 × 105) or K296R mutant PKR (6.3 × 105) were comparable, whereas the yield for WT PKR-complemented cells (6.0 ×104) was nearly 1 log10 lower.
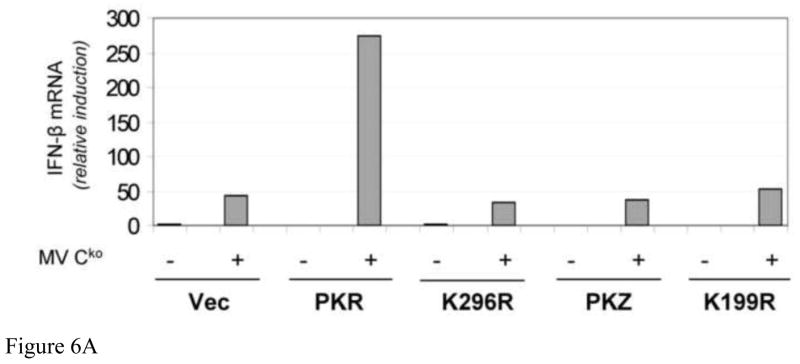
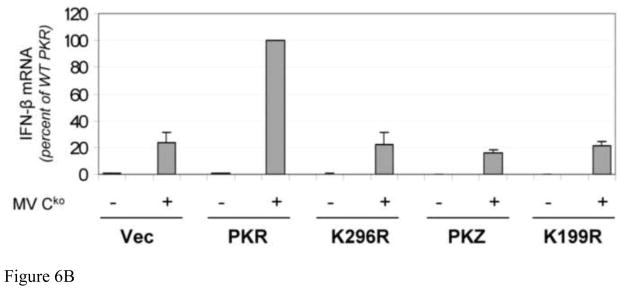
Wildtype PKR, but not PKZ, enhances IFN-β induction by Cko measles virus
Next we compared the ability of PKR and PKZ to restore the IFN-β induction phenotype in PKRkd cells following infection with Cko virus. Either WT or the catalytic mutant of PKR, or PKZ, was expressed in PKRkd HeLa cells and the level of IFN-β RNA was measured by qPCR at 24 h after Cko virus infection. As shown in Figure 6, complementation of PKRkd cells with WT PKR enhanced the induction of IFN-β ~6-fold relative to either the vector control cells or cells complemented with the K296R mutant following infection (Fig. 6A). No amplification of IFN-β expression was observed in the PKRkd cells expressing PKZ, either WT or the K199R mutant (Fig. 6A,B). These results suggest that PKR catalytic activity is required to enhance the induction of IFN-β in MV-infected cells, and that the PKR requirement cannot be fulfilled by PKZ.
FIGURE 6. Expression of wildtype PKR but not PKZ enhances IFN-β induction by measles virus.
PKRkd HeLa cells were transfected with the indicated plasmids and at 15 h after transfection were either mock infected (−) or infected (+) with Cko virus. At 24 h after infection total RNA was prepared and IFN-β transcript levels normalized to GAPDH were determined using quantitative real-time PCR. (A) Representative induction results shown for one of three independent experiments normalized to vector-transfected, uninfected cells. (B) The mean induction of IFN-β mRNA and standard deviations determined for three independent experiments. The results are normalized to Cko-infected, transfected cells expressing WT PKR taken as 100%.
DISCUSSION
The host response to viral infection includes the activation of signal transduction networks that lead to changes in gene expression in infected cells, as illustrated by the innate antiviral immune response (Kawai and Akira, 2010; Yoneyama and Fujita, 2010). Among the cellular proteins implicated in modulating virus-induced responses, including activation of stress-associated JNK and p38 MAP kinases and induction of interferon, is the RNA-dependent protein kinase PKR (Garcia et al., 2006; Pfaller et al., 2011; Pindel and Sadler, 2011; Samuel, 2001). The importance of PKR in the host response is illustrated by recent findings with measles virus. For MV, which has tropism for human cells, the virus-host interplay is affected by virulence factors encoded by the viral P/V/C gene (Cattaneo et al., 2008; Griffin, 2007; Randall and Goodbourn, 2008). Recombinant mutant virus deficient for C protein expression grows poorly relative to wildtype parental virus; a major effector of the C phenotype is the PKR kinase (Toth et al., 2009). Cko mutant virus is a robust inducer of IFN-β and a potent activator of the JNK and p38 stress-activated kinases, both of which are PKR-dependent responses (McAllister et al., 2010). What has been less clear is the mechanism by which PKR acts to affect signaling and induction of IFN-β. Does PKR act as an enzyme, or as an RNA-binding protein, or as both when mediating signaling effects (Garcia et al., 2006; Pfaller et al., 2011)?
Here we present evidence obtained through complementation analyses that PKR plays a key role in triggering activation of JNK and p38 and amplifying the induction of IFN-β in measles virus infected cells in a manner that is dependent upon PKR catalytic activity. Ectopic expression of wildtype PKR restored MAPK activation and IFN-β induction in PKR-deficient human cells. By contrast, neither catalytically deficient PKR nor RNA binding deficient PKR nor the related PKZ kinase was able to complement PKR-deficient human cells to rescue PKR-dependent signaling phenotypes. Several important points emerge from these findings.
First, we demonstrate using two types of human cell lines, HeLa and amnion U, the generality of the finding that the stable knockdown of PKR leads to an impaired phosphorylation of JNK and p38 as well as ATF2 following infection with Cko mutant measles virus. While the Cko virus also was a robust inducer of IRF-3 phosphorylation, this IRF3 response was independent of PKR both in HeLa and U cells infected with MV. Our results obtained with the two human cell lines demonstrating PKR dependency for MAPK activation in Cko virus-infected cells are consistent with and extend earlier findings obtained with the HeLa PKRkd cells infected with Cko measles virus (McAllister et al., 2010) or ΔE3L mutant vaccinia virus (Zhang et al., 2009).
What is the mechanism by which PKR mediates activation of JNK and p38 MAP kinases? Likewise, what is the mechanistic basis of the PKR-mediated amplification of IFN-β induction observed with Cko measles virus (McAllister et al., 2010)? Among the ways by which PKR might enhance JNK and p38 phosphorylation and amplify IFN production are those that would include a catalytic-dependent enzymatic function for PKR, or alternatively, those that might require the PKR protein only in a catalytic-independent role perhaps as an adaptor or scaffold protein rather than or in addition to a role as an enzyme. Our results argue against simply an adaptor or scaffold role for the PKR protein that does not also involve PKR as an enzyme. The K296R mutant of PKR, that lacks catalytic activity but binds dsRNA normally (McCormack and Samuel, 1995; Thomis and Samuel, 1992; Toth et al., 2006), was not able to complement the PKRkd cells to restore either the activation of MAP kinases or the induction of IFN-β following Cko infection. While the observed phenotype of C-protein deficient virus characterized by enhanced activation of PKR and subsequent PKR-dependent signaling responses might implicate C protein as an antagonist of PKR, the inability of Sendai virus C protein to impair PKR activation by Newcastle disease virus (Takeuchi et al., 2008) and the inability of MV C protein to impair PKR activation by vaccinia virus deficient for E3L protein expression (Toth et al., 2009), together with the inability to demonstrate interaction between PKR and MV C proteins (Toth et al., 2009), suggest that C protein does not inhibit PKR activation directly. The alternative possibility, that paramyxovirus C proteins are indirect inhibitors of PKR activation by limiting production of aberrant dsRNAs that activate PKR, seems more likely (Boonyaratanakornkit et al., 2011; Takeuchi et al., 2008; Toth et al., 2009). Interestingly, wild-type MV displays a PKR activation phenotype similar to Cko MV in cells deficient for adenosine deaminase acting on RNA (ADAR1), an editing enzyme that destabilizes dsRNA structure through deamination (Li et al., 2012).
The finding that PKR is required for maximal induction of IFN-β is not limited to measles virus-infected (McAllister et al., 2010) or dsRNA-transfected (McAllister and Samuel, 2009) cells. PKR also is necessary for maximal production of IFN-β in cells infected with other RNA viruses, both positive-stranded and double-stranded, including West Nile virus, encephalomyocarditis virus, Semliki Forest virus and rotavirus (Schulz et al., 2010; Sen et al., 2011). However, the basis of the PKR effect in the different virus-cell systems is not sufficiently well defined to conclude whether the biochemical mechanism is possibly qualitatively similar for the different viruses, or whether PKR is affecting the host response to viral infection differently for different viruses (Pfaller et al., 2011).
Given the requirement for catalytic activity, it is tempting to speculate that the amplification of IFN-β expression mediated by PKR is in part a translational effect through eIF-2α phosphorylation or phosphorylation of additional substrates in manner whereby the functional consequence is displayed at the level of translation, for example phosphorylation of IκB kinase by PKR which has been described (Zamanian-Daryoush et al., 2000). We find that PKR catalytic activity is required for rescue of IFN-β induction and MAPK phosphorylation in the HeLa PKRkd cells following Cko infection, which would be consistent with this notion. Furthermore, activation of JNK, p38 and ATF2 phosphorylation in a PKR-dependent manner in the HeLa and U cell lines correlated with PKR autophosphorylation on Thr446, an increase in eIF-2α phosphorylation and the inhibition of viral protein expression.
PKZ, a PKR-like kinase present in fish, was unable to rescue the phosphorylation of JNK or restore IFN-β induction as was seen for PKR when expressed in the HeLa PKRkd cells. PKZ has been reported to phosphorylate eIF2α in vitro and to inhibit reporter expression in transfected mammalian cells (Bergan et al., 2008; Rothenburg et al., 2005, 2008). We confirmed that PKZ inhibits reporter gene expression in transfected cells, however the mechanism of activation of PKZ, unlike PKR, is not defined. Also, it is unknown whether PKR and PKZ display similar substrate specificity beyond eIF2α. The fact that both PKR and PKZ affect translation by a mechanism that reportedly involves eIF2α phosphorylation (Bergan et al., 2008; Rothenburg et al., 2008), but that only PKR and not PKZ is able to complement the signaling defect in the PKRkd cells, is consistent with a dual function for PKR, acting both as an enzyme and as a scaffold adaptor protein. Activation of NFκB and IFN-β induction by PKR is dependent upon the IPS-1 adaptor (McAllister et al., 2010) and TRAFs (Gil et al., 2004). Colocalization and physical interaction between PKR and TRAF family proteins has been described, in which the K296R mutant form of PKR or the C-terminal PKR kinase alone, is sufficient for interaction at least with TRAF5 (Gil et al., 2004). However, others have concluded that the ability of PKR to mediate NFκB activation resides in the N-terminal region of PKR and requires both dsRNA binding domains (Bonnet et al., 2006).
Taken together, our results firmly establish that PKR catalytic activity is required for amplification of IFN-β induction and enhancement of MAP kinase activation in Cko measles virus infected cells, and that the PKR requirement can not be fulfilled by PKZ. Our results furthermore suggest the intriguing possibility that PKR functions both as an enzyme and a scaffold protein in modulating signaling events in measles virus Cko infected cells. Although PKZ is related to PKR, PKZ seemingly is unable to complement the functional roles of PKR, as an enzyme or as an interacting protein per se.
Acknowledgments
This work was supported in part by research grants AI-12520 and AI-20611 to CES from the National Institute of Allergy and Infectious Diseases, NIH, U.S. Public Health Service.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barber GN, Wambach M, Wong ML, Dever TE, Hinnebusch AG, Katze MG. Translational regulation by the interferon-induced double-stranded-RNA-activated 68-kDa protein kinase. Proc Natl Acad Sci U S A. 1993;90:4621–4625. doi: 10.1073/pnas.90.10.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg K, Svingerud T, Sun B, Robertsen B. An antiserum against Atlantic salmon IFNa1 detects IFN and neutralizes antiviral activity produced by poly I:C stimulated cells. Dev Comp Immunol. 2009;33:638–645. doi: 10.1016/j.dci.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Bergan V, Jagus R, Lauksund S, Kileng O, Robertsen B. The Atlantic salmon Z-DNA binding protein kinase phosphorylates translation initiation factor 2 alpha and constitutes a unique orthologue to the mammalian dsRNA-activated protein kinase R. Febs J. 2008;275:184–197. doi: 10.1111/j.1742-4658.2007.06188.x. [DOI] [PubMed] [Google Scholar]
- Bonnet MC, Daurat C, Ottone C, Meurs EF. The N-terminus of PKR is responsible for the activation of the NF-kappaB signaling pathway by interacting with the IKK complex. Cell Signal. 2006;18:1865–1875. doi: 10.1016/j.cellsig.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Boonyaratanakornkit J, Bartlett E, Schomacker H, Surman S, Akira S, Bae YS, Collins P, Murphy B, Schmidt A. The C proteins of human parainfluenza virus tye I limit double-stranded RNA accumulation that would otherwise trigger activation of MDA5 and protein kinase R. J Virol. 2011;85:1495–1506. doi: 10.1128/JVI.01297-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borden EC, Sen GC, Uze G, Silverman RH, Ransohoff RM, Foster GR, Stark GR. Interferons at age 50: past, current and future impact on biomedicine. Nat Rev Drug Discov. 2007;6:975–990. doi: 10.1038/nrd2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R, Miest T, Shashkova EV, Barry MA. Reprogrammed viruses as cancer therapeutics: targeted, armed and shielded. Nat Rev Microbiol. 2008;6:529–540. doi: 10.1038/nrmicro1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YH, Macmillan JB, Chen ZJ. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell. 2009;138:576–591. doi: 10.1016/j.cell.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux P, Hodge G, McChesney MB, Cattaneo R. Attenuation of V- or C-defective measles viruses: infection control by the inflammatory and interferon responses of rhesus monkeys. J Virol. 2008;82:5359–5367. doi: 10.1128/JVI.00169-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia MA, Gil J, Ventoso I, Guerra S, Domingo E, Rivas C, Esteban M. Impact of protein kinase PKR in cell biology: from antiviral to antiproliferative action. Microbiol Mol Biol Rev. 2006;70:1032–1060. doi: 10.1128/MMBR.00027-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil J, Garcia MA, Gomez-Puertas P, Guerra S, Rullas J, Nakano H, Alcami J, Esteban M. TRAF family proteins link PKR with NF-kappa B activation. Mol Cell Biol. 2004;24:4502–4512. doi: 10.1128/MCB.24.10.4502-4512.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh KC, deVeer MJ, Williams BR. The protein kinase PKR is required for p38 MAPK activation and the innate immune response to bacterial endotoxin. EMBO J. 2000;19:4292–4297. doi: 10.1093/emboj/19.16.4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin DE. Measles virus. In: Griffin DE, Howley PM, Lamb RA, Martin MA, Roizman B, Straus SE, Knipe DM, editors. Fields virology. 5. Lippincott Williams; Philadelphia, PA: 2007. pp. 1551–1585. [Google Scholar]
- Herbert A, Alfken J, Kim YG, Mian IS, Nishikura K, Rich A. A Z-DNA binding domain present in the human editing enzyme, double-stranded RNA adenosine deaminase. Proc Natl Acad Sci U S A. 1997;94:8421–8426. doi: 10.1073/pnas.94.16.8421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordanov MS, Paranjape JM, Zhou A, Wong J, Williams BR, Meurs EF, Silverman RH, Magun BE. Activation of p38 mitogen-activated protein kinase and c-Jun NH(2)-terminal kinase by double-stranded RNA and encephalomyocarditis virus: involvement of RNase L, protein kinase R, and alternative pathways. Mol Cell Biol. 2000;20:617–627. doi: 10.1128/mcb.20.2.617-627.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S, Kaur S, Redig AJ, Goldsborough K, David K, Ueda T, Watanabe-Fukunaga R, Baker DP, Fish EN, Fukunaga R, Platanias LC. Type I interferon (IFN)-dependent activation of Mnk1 and its role in the generation of growth inhibitory responses. Proc Natl Acad Sci U S A. 2009;106:12097–12102. doi: 10.1073/pnas.0900562106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karber G. Beitrag zur kollektiven behandlung pharmakologischer reihenversuche. Naunyn-Schmiedeberg’s Arch Pharmacol. 1931;162:480–483. [Google Scholar]
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- Le Bon A, Tough DF. Links between innate and adaptive immunity via type I interferon. Curr Opin Immunol. 2002;14:432–436. doi: 10.1016/s0952-7915(02)00354-0. [DOI] [PubMed] [Google Scholar]
- Li Z, Okonski KM, Samuel CE. Adenosine deaminase acting on RNA (ADAR1) suppresses the induction of interferon by measles virus. J Virol. 2012:86. doi: 10.1128/JVI.06307-11. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TK, Zhang YB, Liu Y, Sun F, Gui JF. Cooperative roles of fish PKZ and PKR in IFN-mediated antiviral response. J Virol. 2011;85:12769–12780. doi: 10.1128/JVI.05849-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Shepherd EG, Nelin LD. MAPK phosphatases--regulating the immune response. Nat Rev Immunol. 2007;7:202–212. doi: 10.1038/nri2035. [DOI] [PubMed] [Google Scholar]
- McAllister CS, Samuel CE. The RNA-activated protein kinase enhances the induction of interferon-beta and apoptosis mediated by cytoplasmic RNA sensors. J Biol Chem. 2009;284:1644–1651. doi: 10.1074/jbc.M807888200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister CS, Toth AM, Zhang P, Devaux P, Cattaneo R, Samuel CE. Mechanisms of protein kinase PKR-mediated amplification of beta interferon induction by C protein-deficient measles virus. J Virol. 2010;84:380–386. doi: 10.1128/JVI.02630-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack SJ, Ortega LG, Doohan JP, Samuel CE. Mechanism of interferon action motif I of the interferon-induced, RNA-dependent protein kinase (PKR) is sufficient to mediate RNA-binding activity. Virology. 1994;198:92–99. doi: 10.1006/viro.1994.1011. [DOI] [PubMed] [Google Scholar]
- McCormack SJ, Samuel CE. Mechanism of interferon action: RNA-binding activity of full-length and R-domain forms of the RNA-dependent protein kinase PKR--determination of KD values for VAI and TAR RNAs. Virology. 1995;206:511–519. doi: 10.1016/s0042-6822(95)80067-0. [DOI] [PubMed] [Google Scholar]
- McCormack SJ, Thomis DC, Samuel CE. Mechanism of interferon action: identification of a RNA binding domain within the N-terminal region of the human RNA-dependent P1/eIF-2 alpha protein kinase. Virology. 1992;188:47–56. doi: 10.1016/0042-6822(92)90733-6. [DOI] [PubMed] [Google Scholar]
- McKenna SA, Lindhout DA, Kim I, Liu CW, Gelev VM, Wagner G, Puglisi JD. Molecular framework for the activation of RNA-dependent protein kinase. J Biol Chem. 2007;282:11474–11486. doi: 10.1074/jbc.M700301200. [DOI] [PubMed] [Google Scholar]
- Nakatsu Y, Takeda M, Ohno S, Koga R, Yanagi Y. Translational inhibition and increased interferon induction in cells infected with C protein-deficient measles virus. J Virol. 2006;80:11861–11867. doi: 10.1128/JVI.00751-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nallagatla SR, Toroney R, Bevilacqua PC. Regulation of innate immunity through RNA structure and the protein kinase PKR. Curr Opin Struct Biol. 2011;21:119–127. doi: 10.1016/j.sbi.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega LG, McCotter MD, Henry GL, McCormack SJ, Thomis DC, Samuel CE. Mechanism of interferon action. Biochemical and genetic evidence for the intermolecular association of the RNA-dependent protein kinase PKR from human cells. Virology. 1996;215:31–39. doi: 10.1006/viro.1996.0004. [DOI] [PubMed] [Google Scholar]
- Palosaari H, Parisien JP, Rodriguez JJ, Ulane CM, Horvath CM. STAT protein interference and suppression of cytokine signal transduction by measles virus V protein. J Virol. 2003;77:7635–7644. doi: 10.1128/JVI.77.13.7635-7644.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panne D, Maniatis T, Harrison SC. An atomic model of the interferon-beta enhanceosome. Cell. 2007;129:1111–1123. doi: 10.1016/j.cell.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson JB, Samuel CE. Expression and regulation by interferon of a double-stranded-RNA-specific adenosine deaminase from human cells: evidence for two forms of the deaminase. Mol Cell Biol. 1995;15:5376–5388. doi: 10.1128/mcb.15.10.5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson JB, Thomas D, Lewicki H, Billeter MA, Oldstone MB. V and C proteins of measles virus function as virulence factors in vivo. Virology. 2000;267:80–89. doi: 10.1006/viro.1999.0118. [DOI] [PubMed] [Google Scholar]
- Pfaller CK, Li Z, George CX, Samuel CE. Protein Kinase PKR and RNA Adenosine Deaminase ADAR1: New Roles for Old Players as Modulators of the Interferon Response. Curr Opin Immunol. 2011;23:573–582. doi: 10.1016/j.coi.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pindel A, Sadler A. The role of protein kinase R in the interferon response. J Interferon Cytokine Res. 2011;31:59–70. doi: 10.1089/jir.2010.0099. [DOI] [PubMed] [Google Scholar]
- Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005;5:375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- Randall RE, Goodbourn S. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J Gen Virol. 2008;89:1–47. doi: 10.1099/vir.0.83391-0. [DOI] [PubMed] [Google Scholar]
- Rothenburg S, Deigendesch N, Dey M, Dever TE, Tazi L. Double-stranded RNA-activated protein kinase PKR of fishes and amphibians: varying the number of double-stranded RNA binding domains and lineage-specific duplications. BMC Biol. 2008;6:12. doi: 10.1186/1741-7007-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenburg S, Deigendesch N, Dittmar K, Koch-Nolte F, Haag F, Lowenhaupt K, Rich A. A PKR-like eukaryotic initiation factor 2alpha kinase from zebrafish contains Z-DNA binding domains instead of dsRNA binding domains. Proc Natl Acad Sci U S A. 2005;102:1602–1607. doi: 10.1073/pnas.0408714102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler AJ, Williams BR. Structure and function of the protein kinase R. Curr Top Microbiol Immunol. 2007;316:253–292. doi: 10.1007/978-3-540-71329-6_13. [DOI] [PubMed] [Google Scholar]
- Samuel CE. Mechanism of interferon action: phosphorylation of protein synthesis initiation factor eIF-2 in interferon-treated human cells by a ribosome-associated kinase processing site specificity similar to hemin-regulated rabbit reticulocyte kinase. Proc Natl Acad Sci U S A. 1979;76:600–604. doi: 10.1073/pnas.76.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel CE. The eIF-2 alpha protein kinases, regulators of translation in eukaryotes from yeasts to humans. J Biol Chem. 1993;268:7603–7606. [PubMed] [Google Scholar]
- Samuel CE. Antiviral actions of interferons. Clin Microbiol Rev. 2001;14:778–809. doi: 10.1128/CMR.14.4.778-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz O, Pichlmair A, Rehwinkel J, Rogers NC, Scheuner D, Kato H, Takeuchi O, Akira S, Kaufman RJ, Reis e Sousa C. Protein kinase R contributes to immunity against specific viruses by regulating interferon mRNA integrity. Cell Host Microbe. 2010;7:354–361. doi: 10.1016/j.chom.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen A, Pruijssers AJ, Dermody TS, Garcia-Sastre A, Greenberg HB. The early interferon response to rotavirus is regulated by PKR and depends on MAVS/IPS-1, RIG-I, MDA-5, and IRF3. J Virol. 2011;85:3717–3732. doi: 10.1128/JVI.02634-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer JA, Bellini WJ, Rota PA. The C protein of measles virus inhibits the type I interferon response. Virology. 2003;315:389–397. doi: 10.1016/s0042-6822(03)00537-3. [DOI] [PubMed] [Google Scholar]
- Silva AM, Whitmore M, Xu Z, Jiang Z, Li X, Williams BR. Protein kinase R (PKR) interacts with and activates mitogen-activated protein kinase kinase 6 (MKK6) in response to double-stranded RNA stimulation. J Biol Chem. 2004;279:37670–37676. doi: 10.1074/jbc.M406554200. [DOI] [PubMed] [Google Scholar]
- Sparrer KMJ, Pfaller CK, Conzelmann KK. Measles virus C protein interferes with interferon beta transcription in the nucleus. J Virol. 2011 doi: 10.1128/JVI.05899–05811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J, Zhu Z, Wang Y. Molecular cloning, characterization and expression analysis of the PKZ gene in rare minnow Gobiocypris rarus. Fish Shellfish Immunol. 2008;25:106–113. doi: 10.1016/j.fsi.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Sun B, Skjaeveland I, Svingerud T, Zou J, Jorgensen J, Robertsen B. Antiviral activity of salmonid gamma interferon against infectious pancreatic necrosis virus and salmonid alphavirus and its dependency on type I interferon. J Virol. 2011;85:9188–9198. doi: 10.1128/JVI.00319-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada Y, Ichikawa H, Pataer A, Swisher S, Aggarwal BB. Genetic deletion of PKR abrogates TNF-induced activation of IkappaBalpha kinase, JNK, Akt and cell proliferation but potentiates p44/p42 MAPK and p38 MAPK activation. Oncogene. 2007;26:1201–1212. doi: 10.1038/sj.onc.1209906. [DOI] [PubMed] [Google Scholar]
- Takeuchi K, Komatsu T, Kitagawa Y, Sada K, Gotoh B. Sendai virus C protein plays a role in restricting PKR activation by limiting the generation of intracellular double-stranded RNA. J Virol. 2008;82:10102–10110. doi: 10.1128/JVI.00599-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomis DC, Samuel CE. Mechanism of interferon action: autoregulation of RNA-dependent P1/eIF-2 alpha protein kinase (PKR) expression in transfected mammalian cells. Proc Natl Acad Sci U S A. 1992;89:10837–10841. doi: 10.1073/pnas.89.22.10837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomis DC, Samuel CE. Mechanism of interferon action: evidence for intermolecular autophosphorylation and autoactivation of the interferon-induced, RNA-dependent protein kinase PKR. J Virol. 1993;67:7695–7700. doi: 10.1128/jvi.67.12.7695-7700.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth AM, Devaux P, Cattaneo R, Samuel CE. Protein kinase PKR mediates the apoptosis induction and growth restriction phenotypes of C protein-deficient measles virus. J Virol. 2009;83:961–968. doi: 10.1128/JVI.01669-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth AM, Zhang P, Das S, George CX, Samuel CE. Interferon action and the double-stranded RNA-dependent enzymes ADAR1 adenosine deaminase and PKR protein kinase. Prog Nucleic Acid Res Mol Biol. 2006;81:369–434. doi: 10.1016/S0079-6603(06)81010-X. [DOI] [PubMed] [Google Scholar]
- Verrier ER, Langevin C, Benmansour A, Boudinot P. Early antiviral response and virus-induced genes in fish. Dev Comp Immunol. 2011 doi: 10.1016/j.dci.2011.1003.1012. [DOI] [PubMed] [Google Scholar]
- Wang Y, Samuel CE. Adenosine deaminase ADAR1 increases gene expression at the translational level by decreasing protein kinase PKR-dependent eIF-2alpha phosphorylation. J Mol Biol. 2009;393:777–787. doi: 10.1016/j.jmb.2009.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama M, Fujita T. Recognition of viral nucleic acids in innate immunity. Rev Med Virol. 2010;20:4–22. doi: 10.1002/rmv.633. [DOI] [PubMed] [Google Scholar]
- Zamanian-Daryoush M, Mogensen TH, DiDonato JA, Williams BR. NF-kappaB activation by double-stranded-RNA-activated protein kinase (PKR) is mediated through NF-kappaB-inducing kinase and IkappaB kinase. Mol Cell Biol. 2000;20:1278–1290. doi: 10.1128/mcb.20.4.1278-1290.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Langland JO, Jacobs BL, Samuel CE. Protein kinase PKR-dependent activation of mitogen-activated protein kinases occurs through mitochondrial adapter IPS-1 and is antagonized by vaccinia virus E3L. J Virol. 2009;83:5718–5725. doi: 10.1128/JVI.00224-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Samuel CE. Protein kinase PKR plays a stimulus- and virus-dependent role in apoptotic death and virus multiplication in human cells. J Virol. 2007;81:8192–8200. doi: 10.1128/JVI.00426-07. [DOI] [PMC free article] [PubMed] [Google Scholar]