Abstract
The objective of the present study is to determine the effect of (bi)sulfite (hydrated sulfur dioxide) on human neutrophils and the ability of these immune cells to produce reactive free radicals due to (bi)sulfite oxidation. Myeloperoxidase (MPO) is an abundant heme protein in neutrophils that catalyzes the formation of cytotoxic oxidants implicated in asthma and inflammatory disorders. In the present study sulfite (•SO3−) and sulfate (SO4•−) anion radicals are characterized with the ESR spin-trapping technique using 5,5-dimethyl-1-pyrroline N-oxide (DMPO) in the reaction of (bi)sulfite oxidation by human MPO and human neutrophils via sulfite radical chain reaction chemistry. After treatment with (bi)sulfite, PMA-stimulated neutrophils produced DMPO-sulfite anion radical, -superoxide, and -hydroxyl radical adducts. The latter adduct probably resulted, in part, from the conversion of DMPO-sulfate to DMPO-hydroxyl radical adduct via a nucleophilic substitution reaction of the radical adduct. This anion radical (SO4•−) is highly reactive and, presumably, can oxidize target proteins to protein radicals, thereby initiating protein oxidation. Therefore, we propose that the potential toxicity of (bi)sulfite during pulmonary inflammation or lung-associated diseases such as asthma may be related to free radical formation.
Introduction
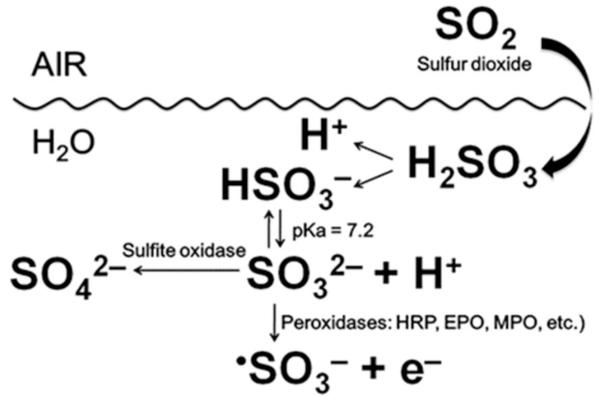
Sulfur dioxide, formed during the combustion of fossil fuels, is a major air pollutant [1]. It can be hydrated to (bi)sulfite in the lung upon contact with fluids lining the air passages (Scheme 1). Its two ionized forms in aqueous solution at physiological pH, (bi)sulfite (HSO3−) and sulfite (SO32−) [2], are widely used in the food industry – predominantly as anti-browning agents, antioxidants and preservatives [3] - and as pharmaceutical ingredients [4]. It has also been reported that oral, topical or parenteral exposure to sulfites induces a wide range of adverse reactions in sensitive individuals and bronchoconstriction in asthmatic patients [4-6]. Until recently, there were only limited restrictions on the use of approved sulfiting agents in foods. These included a prohibition against their use in meats and a limitation of their concentrations in wines and raw shrimp to 350 ppm (5.5 mM) and 100 ppm (1.6 mM) SO2 equivalents, respectively [7].
Scheme 1.
Sulfite is detoxified to sulfate by sulfite oxidase [8] (Scheme 1), present at high levels in the liver and kidney and in lower concentrations in most other tissues of the body (e.g., the lung). The enzymatic oxidation of sulfite by sulfite oxidase proceeds via a two-electron oxidation, but recent studies hypothesized that the cytotoxicity of (bi)sulfite is mediated by free radicals [9]. In fact, free radicals have been demonstrated to be produced by enzymatic initiation of the oxidation of (bi)sulfite (Scheme 1) by prostaglandin H synthase [10], horseradish peroxidase [10, 11] and human eosinophil peroxidase [12], with •SO3− anion radical formed as follows:
| (1) |
| (2) |
| (3) |
Sulfite anion radical reacts very rapidly with oxygen and gives rise to the formation of the oxygen-centered peroxymonosulfate (−O3SOO•) and sulfate (SO4•−) anion radicals through chain propagation steps:
| (4) |
| (5) |
| (6) |
Furthermore, SO4•− can react with another molecule of (bi)sulfite via Eqn. (6) but, being a very strong oxidant [14], it will oxidize almost any biomolecule. Moreover, our recently published results demonstrated that one of the mammalian peroxidases, eosinophil peroxidase (EPO), uses (bi)sulfite as a one-electron donor substrate to generate reactive intermediates that oxidize the most abundant protein present in plasma, human serum albumin (HSA), to protein radicals via Eqns (1)-(6) [12].
Neutrophils have been implicated in the pathology of many diseases. Chronic inflammation and influx of these cells into the airways in asthma results in increased generation of reactive oxygen species (ROS) in asthmatic patients [15, 16], and it is likely that ROS play a significant role in the pathophysiology of asthma [17, 18]. The oxidative or respiratory burst in neutrophils is triggered upon phagocytosis or when the pathway is activated by an appropriate synthetic stimulus in vitro. Superoxide (O2•−) is formed, initially, by the reduction of molecular oxygen by a single electron that originates from NADPH. Although O2•− may contribute to microbial killing, other more potent ROS are generated rapidly from this precursor. Hydrogen peroxide (H2O2) is formed by spontaneous dismutation of superoxide and/or catalytic action of superoxide dismutase (SOD).
Myeloperoxidase (MPO) is an abundant heme protein secreted from activated neutrophils that catalyzes the formation of cytotoxic oxidants implicated in asthma and allergic inflammatory disorders [19]. The concentration of MPO can be greater than 5% of the dry weight of these cells. In exacerbated asthma, a strong inflammation is developed and inflammatory cells become activated. Due to this activation, the respiratory burst occurs in neutrophils, eosinophils, monocytes and macrophages. ROS produced by inflammatory cells have been implicated in the pathogenesis of lung diseases such as asthma, cystic fibrosis, adult respiratory distress syndrome, and idiopathic pulmonary fibrosis [20].
We now demonstrate that MPO uses sulfite as a substrate to generate highly reactive sulfite-derived oxygen species in phorbol 12-myristate 13-acetate (PMA)-activated human neutrophils. In this report, these radicals are characterized with the electron spin resonance (ESR) technique using 5,5-dimethyl-1-pyrroline N-oxide (DMPO) as a spin trap. For the first time, we have demonstrated that sulfite anion radical is formed intracellularly in human neutrophils.
Materials and methods
Chemicals
PMA, sodium sulfite, sodium chloride, sodium bromide, sodium thiocyanate, sodium formate, sodium azide, diethylenetriaminepentaacetic acid (DTPA), Gd-DTPA complex, 4-aminobenzoic acid hydrazide (ABAH), ascorbic acid, dimethyl sulfoxide (DMSO), homovanillic acid (HVA), and hydrogen peroxide (obtained as a 30% solution) were from Sigma (St. Louis, MO). The hydrogen peroxide concentration was determined from its absorbance at 240 nm (ε = 39.4 M−1cm−1). Human MPO was purified from white blood cells (Lee Biosolutions, Inc., St. Louis, MO). The enzyme purity (≥ 95%) was assured by electrophoresis and the specific activity was 1130 units/mg protein (information provided by the company). The concentration of the enzyme was calculated from the extinction coefficient of 91 mM−1cm−1 at 430 nm [21]. Bovine kidney superoxide dismutase (SOD) was purchased from Calzyme Laboratories, Inc. (San Luis Obispo, CA). Catalase was purchased from Roche and the activity of the enzyme was 65,000 units/mL. DMPO was obtained from Dojindo Laboratories (Kumamoto, Japan) and used without further purification. The La-DTPA complex was prepared by mixing lanthanum chloride heptahydrate (Sigma) and DTPA solution in the ratio 1:2.
Optical spectroscopy
A rapid scanning diode array stopped-flow apparatus (model SF-61DX2, HiTech Scientific Ltd., UK) was used for MPO-compound I kinetic experiments. Data acquisition and analyses were performed using the Kinetic Studio software package (HiTech Scientific). Reactions were performed in 100 mM phosphate buffer (Chelex-treated with 25 μM DTPA) at pH 7.4. Sequential double-mixing experiments were performed to monitor the rate of reduction of MPO-compound I by (bi)sulfite. MPO (5 μM) was premixed with 50 μM H2O2 (conditions that allow the formation of MPO-compound I [22]) and the latter species was reacted after a 50 ms delay with 10-, 50-, 100-, 500-, and 1000-fold excess of (bi)sulfite. The slow formation of MPO-compound II, known to occur upon oxidation of H2O2 or endogenous electron donors [22, 23], was a minor side reaction and was not analyzed. Pseudo-first-order conditions were achieved by keeping the (bi)sulfite concentration in at least a tenfold excess over the enzyme.
Kinetic experiments were carried out with a Cary 100 spectrophotometer (Varian, Inc., Palo Alto, CA) using a 500 μl quartz cuvette for MPO-compound II kinetics. For reduction of MPO-compound II, 400 nM myeloperoxidase was premixed with 300 nM homovanillic acid (HVA) and 50 μM hydrogen peroxide. Forty seconds after mixing, MPO-compound II was allowed to react with (bi)sulfite. Pseudo-first-order conditions were achieved by keeping the (bi)sulfite concentration in at least a fivefold excess over the enzyme. In the experiments with taurine, 1 mL samples containing 0.1 μM MPO, 100 μM H2O2, 100 mM NaCl and 50 mM taurine were incubated for 1 h at 37°C in the presence of (bi)sulfite. At the end of the incubation, 25 μg of catalase were added to the mixture before essaying for taurine chloramine formation by adding 20 mM KI. 1 mol taurine chloramine was able to oxidize 2 mol of I− to I2. Samples were performed in triplicates, and UV-spectra of solutions diluted two times were recorded over a range of 200-500 nm. The I2 concentration was determined as I3− at 355 nm (ε = 2.29 × 104 M−1cm−1)[24].
Preparation of human neutrophils
Human neutrophils from healthy donors were isolated as previously described [25] with minor changes. Briefly, venous blood was collected in sodium citrate solution (3.8%), centrifuged (SORVALL centrifuge, g-force 174, 20 min), and platelet-rich plasma was discarded. The remaining part of the blood was mixed with a solution of 6% dextran in saline (5:1, v/v); then the blood/dextran solution was diluted to a 50 ml volume with 0.9% saline in a 50 cc conical tube and fixed vertically for 30 min. After 30 minutes, supernatant containing all white blood cells and granulocytes was collected and centrifuged at 25°C (g-force 111, 6 min). The cell pellet was resuspended in platelet poor plasma (ppp), and the cell suspension was layered over 2 percoll/ppp density gradients in a 15 ml polystyrene tube and centrifuged at 25°C (g-force 121, 15 min), producing two distinct cell monolayers - one of monocytes and macrophages and a second of granulocytes. The second fraction was retrieved and centrifuged as previously mentioned. The granulocyte pellet was resuspended in MAC buffer (phosphate-balanced saline without CaCl2 or MgCl2 containing 2 mM EDTA and 0.5% bovine serum albumin) and counted. The population of neutrophils was extracted from the granulocytes by negative selection using an eosinophil isolation kit (Miltenyi Biotec, Germany). The cells were counted using the Vision Cellometer. Dual fluorescence allows for staining of both live and dead cells using acridine orange and propidium iodine for counting and viability. Total counted cells are indicated by green circles in the bright field image and dead cells stained with propidium iodine. The purity of neutrophils was >95% . Informed consent was obtained from all donors. The protocol was approved by the National Institute of Environmental Health Sciences Institutional Review Board.
Electron Spin Resonance (ESR) spectroscopy
ESR spectra were recorded using a Bruker E500 ESR spectrometer equipped with an ER4122SHQ microwave cavity and operating at 9.78 GHz and a modulation frequency of 100 kHz. The ESR spin-trapping experiments were performed by placing the samples in a 10-mm flat cell (250 μl, final volume) immediately after peroxide addition, and the spectra were recorded within 1 min after starting the reaction. The instrumental settings were as follows: field sweep, 80 G; microwave frequency, 9.78 GHz; microwave power, 20 mW, modulation amplitude, 0.5 G; conversion time, 164 ms; time constant, 164 ms; receiver gain, 5 × 104; and number of scans, 1. Computer simulations were performed using WinSim software [26]. The experiments in each figure were from a single donor, but repeated several times from different healthy patients to check the reproducibility. The variation of the ESR amplitude was not more than 10%. Each experiment was repeated at least three times with cells from different patients.
Results
Optical spectroscopy
The mechanism of peroxidase oxidation of (bi)sulfite by MPO/H2O2 proceeds in two sequential, one-electron reduction steps of MPO-compounds I and II by (bi)sulfite with the formation of sulfite anion radical (•SO3−), very similar to the enzymatic oxidation of (bi)sulfite by eosinophil peroxidase and horseradish peroxidase [12, 27].
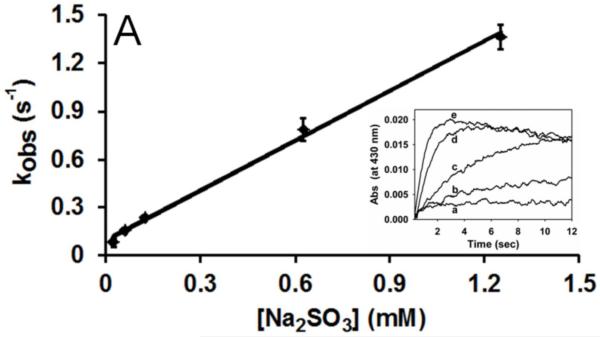
To determine the rate of reduction of the MPO-compound I intermediate, stopped-flow experiments were performed. We premixed 50 μM H2O2 with MPO (5 μM) in an aging loop for 50 ms to allow formation of MPO-compound I (Soret peak at 430 nm) [22]. After this aging time, the intermediate was mixed with various concentrations of (bi)sulfite, the final concentrations of which were at least 10-fold in excess of the enzyme to ensure pseudo-first order kinetics. The reaction was monitored at 430 nm and, under these conditions, we were able to follow monoexponential the reduction of MPO-compound I by (bi)sulfite (Figure 1A, the inset). The pseudo-first-order rate constants (kobs) were obtained from the fit of time traces for each (bi)sulfite concentration. The second-order rate constant for the reduction of MPO-compound I by (bi)sulfite was obtained from the slope of plotted kobs versus sulfite concentrations (Fig. 1A). The calculated value of 1.04 ± 0.04 × 103 M−1 s−1 at pH 7.4 is over 10-fold faster than that reported for HRP-compound I and sulfite (7.6 ± 0.8 × 10 M−1 s−1) [30].
Fig. 1.
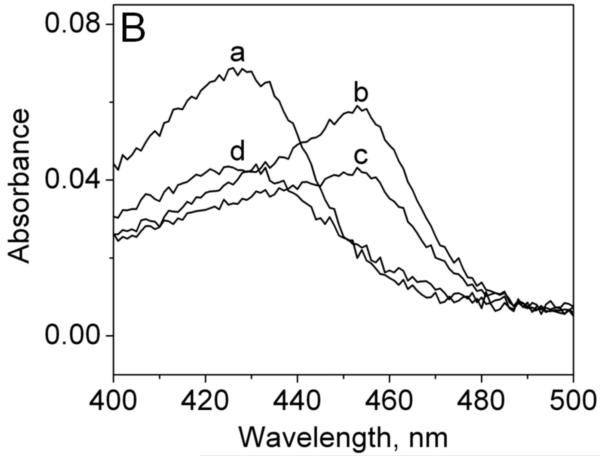
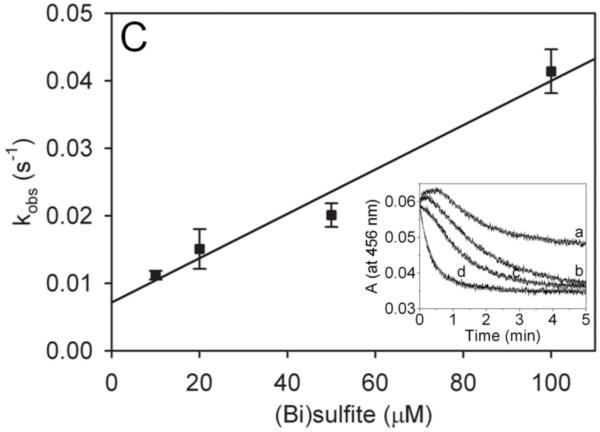
Reduction of MPO-compound II by sulfite. (A) Pseudo-first-order rate constants for reduction of MPO-compound I by (bi)sulfite. The second-order rate constant is calculated from the slope. The inset shows the time traces of the reaction followed at 430 nm using the sequential mixing mode. Final concentrations were 1.25 μM MPO and12.5 μM H2O2, and the concentration of (bi)sulfite for each time trace was (a) 12.5 μM, (b) 62.5 μM, (c) 125 μM, (d) 625 μM, and (e) 1.25 mM. (B) Spectral changes upon addition of 50 μM Na2SO3 to MPO-compound II. The resting MPO was recorded first (spectrum a). MPO-compound II was formed by mixing 400 nM ferric MPO with 300 nM homovanillic acid (HVA) and 50 μM H2O2 and waiting for 1 min (b). Spectrum c was taken 5 min after the addition of sulfite, and the resting enzyme was reformed after 30 min (spectrum d). (C) Pseudo-first-order rate constants for reduction of MPO-compound II by (bi)sulfite. The inset shows the time traces and fits of the reduction of compound II at pH 7.4 by Na2SO3. The concentration of (bi)sulfite for each time trace was (a) 10 μM, (b) 20 μM, (c) 50 μM, and (d) 100 μM.
To follow the rate of reduction of the MPO-compound II intermediate, we premixed MPO (400 nM), homovanillic acid (300 nM), and hydrogen peroxide (50 μM) to generate MPO-compound II (Soret peak at 456 nm) (Fig. 1B, spectrum b). Homovanillic acid (HVA) has been shown to be a good substrate for compound I, but reacts slowly with compound II (k = 230 ± 1.9 M−1s−1 [28]). Under these conditions we were able to detect and accumulate MPO-compound II, found to be stable for at least 40 s. Fifteen seconds after generation of MPO-compound II, (bi)sulfite was added in excess to the enzyme in order to maintain pseudo first-order conditions. Then we followed the spectral changes at 456 nm, showing the transition of the intermediate back to the resting enzyme with a Soret peak at 430 nm (spectra a and d). The loss of the absorbance at 456 nm for each sulfite concentration displayed monoexponential character. The protein did not recover completely to its resting absorbance after cycling, probably due to porphyrin bleaching and irreversible inactivation, which has been observed with some other peroxidases after hydrogen peroxide treatment [29].
The pseudo-first-order rate constants (kobs) were obtained from the fit of time traces for each sulfite concentration (Fig. 1C, the inset). The second-order rate constant for the reduction of MPO-compound II by (bi)sulfite was obtained from the slope of plotted kobs versus sulfite concentrations (Fig. 1C). The calculated value of 3.1 ± 0.3 × 102 M−1s−1 at pH 7.4 is very similar to the reported rates for EPO (2.1 ± 0.6 × 102 M−1s−1 [12]) and HRP (1.8 ± 0.06 × 102 M−1s−1 [30]). Although the calculated rates show that sulfite is a relatively poor myeloperoxidase substrate, it has been demonstrated that the sulfite radical chain chemistry via Eqns (4)-(6) can be initiated by only 1.4 × 10−13 M •SO3− [31].
ESR detection of sulfite-derived radicals in a pure enzymatic system
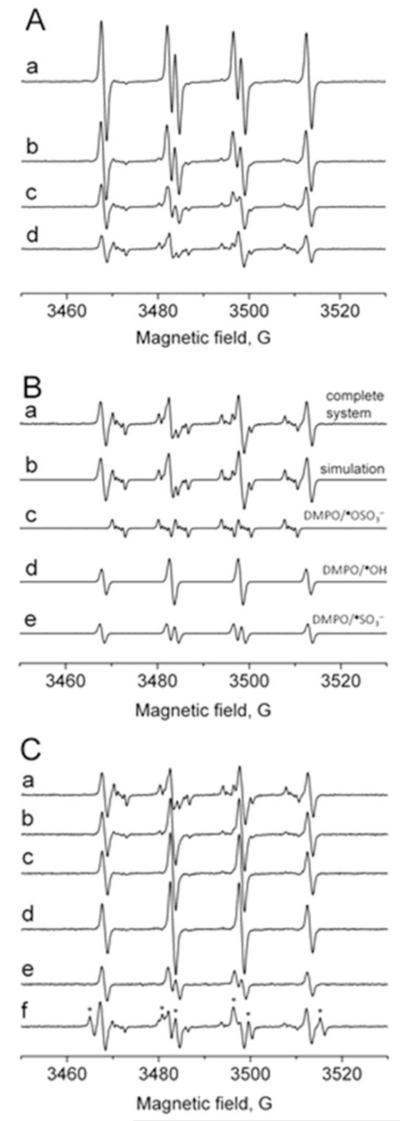
To detect the resulting •SO3−anion radical from the myeloperoxidase-initiated enzymatic oxidation of (bi)sulfite by ESR using spin trapping, we incubated MPO (1 μM) with sulfite (1 mM), which is much less than the amount used as a preservative in wines (up to 5-6 mM, [4]), plus H2O2 (100 μM) in the presence of DMPO (100 mM). The resulting ESR spectrum of the DMPO/•SO3− radical adduct is shown in Fig. 2A (spectrum a), with hyperfine coupling constants of aN = 14.7 G and aHβ = 16.0 G, consistent with previously published reports [10, 12, 32]. In the control experiments, when (bi)sulfite, MPO, H2O2, or DMPO was omitted, no radical formation was detected (data not shown). In order to trap secondary radicals formed from the sulfite chain chemistry reactions, the concentration of the spin trap was decreased in order to enhance the reaction of the primary •SO3− radical with oxygen via Eqn (4). Using DMPO concentrations of 50 and 20 mM, a secondary radical could be trapped (Fig. 2A, spectra b and c). When the DMPO concentration was reduced to 6 mM, spectrum d in Fig. 2A was detected; its computer-simulated composite is presented in Fig. 2B (spectrum b). The simulation consists of a superposition of 43% DMPO/•OSO3− (aN = 13.7 G, aHβ = 10.1 G, aHγ1 = 1.42 G, and aHγ2 = 0.75 G) [32-34], 33% DMPO/•OH (aN = 14.9 G and aHβ = 14.9 G), and 24% DMPO/•SO3−, presented in Fig. 2B as spectra c, d, and e, respectively. The SO4•− radical is oxygen-centered as indicated by DMPO/•OSO3−.
Fig. 2.
Formation of radical adducts in reaction between sulfite (Na2SO3) and myeloperoxidase (MPO)/hydrogen peroxide (H2O2) as a function of DMPO concentrations and time. (A) Reactions including Na2SO3 (1 mM) and MPO (1 μM) were initiated with H2O2 (100 μM) in 100 mM phosphate buffer (pH 7.4) in the presence of various concentrations of DMPO. After initiation with H2O2, the mixture was immediately placed into the flat cell. The concentration of the spin trap for each spectrum was (a) 100 mM, (b) 50 mM, (c) 20 mM, and (d) 6 mM. (B) Spectrum a is the same as spectrum d in Panel (A). Spectrum b is the composite simulation of 43% DMPO/•OSO3−, 33% DMPO/•OH, and 24% DMPO/•SO3−. Spectrum c is the computer simulation of DMPO/•OSO3− radical adduct (aN = 13.7 G, aHβ = 10.1 G, aHγ1 = 1.42 G, and aHγ2 = 0.75 G). Spectrum d is the simulation of DMPO/•OH (aN = 14.9 G, aHβ = 14.9 G). Spectrum e is the simulation of DMPO/•SO3− (aN = 14.6 G, aHβ = 16.2 G). (C) A reaction mixture containing Na2SO3 (1 mM), DMPO (6 mM), and MPO (1 μM) was initiated with H2O2 (100 μM) in 100 mM phosphate buffer (pH 7.4) and immediately placed into the flat cell (spectrum a). Spectra b, c, and d were detected after 3, 6, and 10 min, respectively. Spectrum e was detected immediately after the initiation, but in the presence of 100 mM DMSO. Spectrum f was detected immediately after the initiation, but in the presence of 100 mM HCOONa (the appearance of DMPO/•CO2− radical adduct is marked with asterisks; aN = 15.8 G and aHβ = 18.7 G).
Figure 2C shows the time course of the ESR spectrum representing the mixture of SO4•−, •OH, and •SO3− radical adducts in the presence of low spin trap concentrations. The ESR signals of sulfite- and sulfate-anion radicals diminished with time (spectra b and c). Ten minutes after mixing the components, only the DMPO/•OH radical adduct was detected (Fig. 2C, spectrum d) with an enhanced intensity, consistent with DMPO/•OH forming from DMPO/•OSO3−. DMPO/•OH radical adduct formation concomitant with DMPO/•OSO3− decay was also found when the sulfate anion radical was formed by the photolysis of peroxydisulfate [33, 34]. The addition of DMSO (100 mM) as a hydroxyl radical scavenger prior to the initiation with H2O2 completely eliminated the sulfate radical, but no scavenger-derived radical was trapped (Fig. 2C, spectrum e). The addition of sodium formate (100 mM) resulted in the disappearance of the DMPO/ SO4•− adduct and the appearance of a strong DMPO/•CO2− radical adduct signal (aN = 15.8 G and aHβ = 18.7 G) (lines marked with asterisks, (Fig. 2C, spectrum f) via the reaction SO4•− + HCOO− → HSO4− + •CO2− (k = 1.7 × 108 M−1s−1 [10]).
ESR detection of sulfite-derived radicals in activated human neutrophils
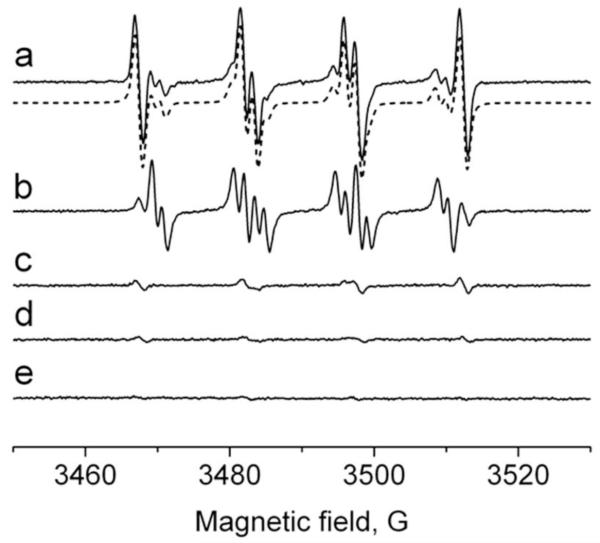
To demonstrate that sulfite oxidation is not limited to a pure enzymatic system, we investigated the formation of sulfite-derived radicals in PMA-activated human neutrophils. The cells (1 × 107 cells/ml) were premixed with Na2SO3 (1 mM) in the presence of DMPO (100 mM), stimulated with PMA (500 ng/ml), and incubated at 37°C for 3 min to initiate superoxide generation during NADPH oxidase activation of the cells. The ESR spectrum of the activated neutrophils was a composite of three radical adducts, simulated and attributed to: DMPO/superoxide, DMPO/•OOH (aN = 14.1 G, aHβ = 11.2 G, and aHγ = 1.24 G) [35]; DMPO/hydroxyl, DMPO/•OH; and DMPO/sulfur trioxide anion radical, DMPO/•SO3−, respectively (Fig. 3, spectrum a). In the absence of (bi)sulfite, a representative ESR spectrum of PMA-stimulated neutrophils shows DMPO/•OOH (~80%) and DMPO/•OH (~20%) (Fig. 3, spectrum b). The short-lived DMPO/•OSO3− signal was not detected. A very weak signal of DMPO/•SO3− was obtained without PMA stimulation possibly due to the autoxidation of (bi)sulfite by traces of transition metals or a low level of NADPH oxidase activation without added PMA (Fig. 3, spectrum c). Control experiments omitting both (bi)sulfite and PMA or omitting neutrophils resulted in no radical adduct formation (Fig. 3, spectra d and e).
Fig. 3.
Formation of radical adducts in reaction between sulfite (Na2SO3) and human neutrophils upon PMA activation in the presence of DMPO. (Spectrum a) Reaction mixture containing 1 × 107 cells/ml, Na2SO3 (1 mM), and DMPO (100 mM) in PBS, pH 7.4. After cell activation with PMA (500 ng/ml) followed by incubation at 37°C for 3 min, the mixture was placed into the flat cell. The dotted spectrum is the composite computer simulation of 26% DMPO/•OOH (aN = 14.1 G, aHβ = 11.2 G, and aHγ = 1.24 G), 42% DMPO/•OH (aN = 14.9 G and aHβ = 14.9 G), and 32% DMPO/•SO3− (aN = 14.7 G and aHβ = 16.0 G) radical adducts. (Spectrum b) Same as in spectrum a without Na2SO3. (Spectrum c) Same as in spectrum a, except PMA was not added. (Spectrum d) Same as in spectrum a without Na2SO3 and PMA. (Spectrum e) Same as inspectrum a without neutrophils.
Effect of halides and pseudohalides in their physiological concentrations on DMPO/•SO3− radical adduct formation
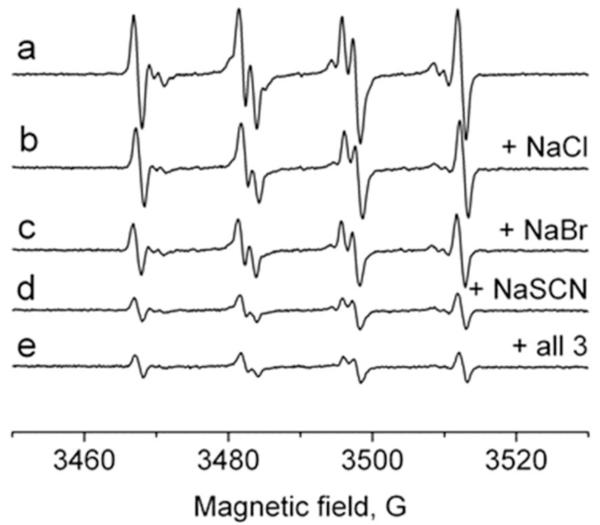
To determine the effect of bromide, chloride and thiocyanate as physiological myeloperoxidase substrates on production of sulfite-derived radicals, we recorded ESR spectra using the neutrophil-PMA-(bi)sulfite system (iodide was excluded due to its low plasma concentration, <1 μM). Neutrophil samples (1 × 107 cells/ml) containing 100 mM DMPO to trap the primary •SO3− radical, 1 mM Na2SO3, and halides or thiocyanate at plasma levels (Cl−, 100 mM; Br−, 100 μM; SCN−, 100 μM) were activated with PMA (500 ng/ml) and incubated at 37°C for 3 min. As shown in Figure 4, the ESR signal was a composite of the three radical adducts: DMPO/•SO3−, DMPO/•OOH, and DMPO/•OH (spectrum a). It was slightly inhibited in the presence of chloride (spectrum b), but significantly reduced by the addition of bromide and thiocyanate (spectra c and d). The addition of all three substrates failed to completely inhibit the radical formation (spectrum e), implying that (bi)sulfite oxidation can occur in human neutrophils even in the presence of strong competitors and preferred substrates. However, when the neutrophils were placed in human plasma instead of phosphate buffer and treated with sulfite and PMA in the presence of DMPO, there was no ESR detection of any radicals (data not shown).
Fig. 4.
Effect of halides and pseudohalides on the formation of DMPO/•SO3− radical. A reaction mixture containing 1 × 107 cells/ml, Na2SO3 (1 mM), and DMPO (100 mM) in PBS, pH 7.4. Na2SO3 (1 mM) was used for the remaining experiments. After cell activation with PMA (500 ng/ml), the mixture was placed into the flat cell. The spectrum was attenuated in the presence of 100 mM NaCl (spectrum b), 100 μM NaBr (spectrum c), 100 μM NaSCN (spectrum d), and in the presence of the mixture of NaCl, NaBr, and NaSCN with the indicated concentrations (spectrum e).
EPR experiments with cells showed a low inhibition of the •SO3− radical formation in presence of Cl−, which suggests that (bi)sulfite is a strong competitor of chloride as a substrate of MPO. So, we also studied the effect of sulfite on MPO-mediated chloride oxidation in the cell-free system by using the taurine chloramine assay. It is known that under ideal conditions, ~100% of the H2O2 is converted to HOCl when added to myeloperoxidase and Cl−. Then HOCl can be trapped as chloramine in the presence of an excess of taurine (H2N-CH2CH2SO3H). To distinguish the competition between chlorination and peroxidation, we determined taurine chloramine (ClHN-CH2CH2SO3H) generation in a system containing 0.1 μM myeloperoxidase, 50 mM taurine, and 100 mM NaCl and then added 100 μM H2O2 in the presence or absence of sulfite. The control samples without MPO, Cl−, or H2O2 inhibited the formation of taurine chloramine. The presence of taurine chloramine (78.4 ± 5.2 μM) was confirmed in the complete MPO-Cl−-H2O2 system as measured by the KI method in the absence of sulfite. However, the addition of sulfite had an inhibitory effect on the taurine chloramine formation. Indeed, the concentration of taurine chloramine was shown to be decreased by 20 % (62.7 ± 4 μM) when 1 mM sulfite was added to the sample (data not shown). A complete inhibition was observed in the presence of 3 mM sulfite since only 1 ± 0.2 μM taurine chloramine could be measured. Therefore, these results confirmed the competition between chloride and sulfite for MPO-compound I. Organic one-electron donors to MPO-compound I are already known to inhibit HOCl formation [36, 37].
Effect of myeloperoxidase inhibitors and antioxidants on DMPO/•SO3− radical adduct formation
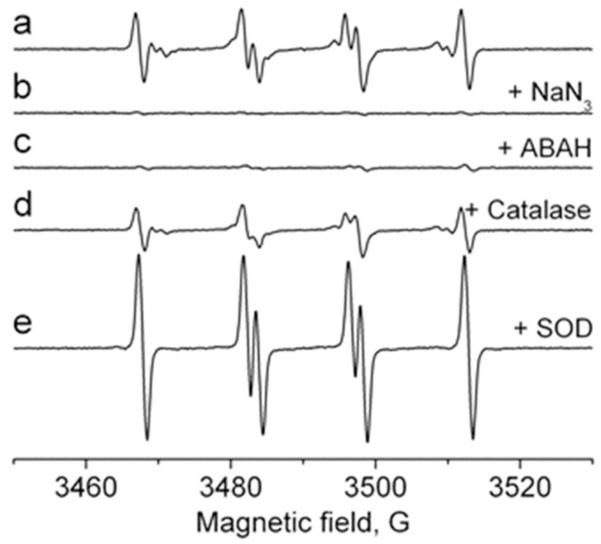
To characterize the effect of inhibitors on the generation of the primary •SO3− anion radical, we premixed neutrophils (1 × 107 cells/ml) with azide (500 μM) or 4-aminobenzoic acid hydrazide (ABAH) (500 μM) as MPO inhibitors and then added sulfite (1 mM) and PMA (500 ng/ml). The effect of each inhibitor or antioxidant was compared with a control cell sample containing no compromising agents (Fig. 5, spectrum a). With each MPO inhibitor, the signals were almost completely suppressed (Fig. 5, spectra b and c), confirming the importance of the enzyme as an initiator of (bi)sulfite oxidation. The presence of catalase (150 μg/ml), which cannot penetrate the cell membrane, caused incomplete inhibition of the ESR intensity of the DMPO radical adducts (Fig. 5, spectrum d). When SOD (50 μM) was added to the cells prior to PMA activation, neither DMPO/•OOH nor DMPO/•OH radical adduct was detected, but there was an enhanced formation of DMPO/•SO3− (Fig. 5, spectrum e). DMPO/•SO3− radical adduct formation has been found previously, showing that SOD by itself can oxidize (bi)sulfite via one-electron reduction of SOD-Cu(II) to SOD-Cu(I), leading to the formation of •SO3− [38]. Superoxide dismutase, like catalase, cannot penetrate cell membranes, so DMPO/•OOH and DMPO/•OH must be formed extracellularly with DMPO/•OH, presumably derived from DMPO/•OOH [35].
Fig. 5.
Effect of inhibitors and radical scavengers on the formation of DMPO/•SO3− radical. Reaction mixture containing 1 × 107 cells/ml, Na2SO3 (1 mM), and DMPO (100 mM) in PBS, pH 7.4. Azide and ABAH were preincubated for 15 min before the addition of DMPO. After cell activation with PMA (500 ng/ml), the mixture was placed into the flat cell. Na2SO3 (1 mM) was used for the remaining experiments. The spectrum was attenuated in the presence of NaN3 (500 μM) (spectrum b), ABAH (500 μM) (spectrum c), catalase (150 μg/ml) (spectrum d), and Cu, Zn-SOD (50 μM) (spectrum e).
Effect of line-broadening agents on DMPO/•SO3− radical adduct formation
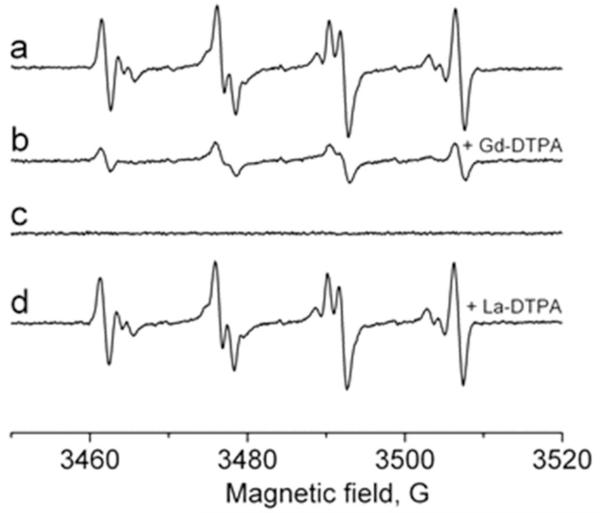
We attempted to distinguish between the intra- and extracellular fraction of sulfite- and oxygen-derived radicals. For this purpose we studied the effect of the ESR line-broadening agent Gd-DTPA, which does not enter the cells. This metal chelate does not cross the plasma membrane and should only broaden the extracellular signal [39-42]. La-DTPA, which is diamagnetic but chemically very similar to Gd-DTPA, should not have any effect on the ESR intensity and was used as a control. Figure 6 shows the effect of 20 mM Gd-DTPA (Fig. 6, spectrum b) added to neutrophils prior to their activation: the magnitude of the residual signal was approximately 25% for the cell population of 1 × 107 cells/ml compared to the signal without the metal chelate. This result suggests that this concentration of Gd-DTPA broadens a significant fraction of the ESR signal to the point of undetectability [40, 41], leaving a detectable unbroadened signal corresponding to the intracellular fraction of the primary •SO3− radical. The control experiment with La-DTPA had no effect on the ESR intensity (Fig. 6, spectrum d). Consistent with the SOD experiment, the DMPO/•OOH and DMPO/•OH signals were entirely broadened, confirming that these species are extracellular.
Fig. 6.
Effect of Gd-DTPA as a line-broadening agent on the DMPO/•SO3− radical adduct formation. The ESR spectrum of the DMPO/•SO3− generated in a system of neutrophils (1 × 107 cells/ml), Na2SO3 (1 mM), and DMPO (100 mM) in PBS, pH 7.4. After cell activation with PMA (500 ng/ml), the mixture was placed into the flat cell (spectrum a). (Spectrum b) Same as in spectrum a, but in the presence of 20 mM Gd-DTPA added prior to cell activation. (Spectrum c) Same as in spectrum b, but without neutrophils. (Spectrum d) Same as in spectrum a, but in the presence of 20 mM La-DTPA added prior to cell activation.
Discussion
The mean serum sulfite concentration in healthy individuals reported by Ji et al. is ~5 μM [43]. However, in their study, after oral metabisulfite loading with vegetable juice, there was a rapid rise of sulfite concentration in plasma (112 μM) within 30 min. The toxic potential of (bi)sulfite is most clearly indicated by the fatal loss of sulfite oxidase activity [44]. It is noteworthy that in cases of sulfite oxidase deficiency, the concentration of sulfite in plasma is abnormal (10 μM to ~2 mM) [45]. It has also been shown that the presence of sulfite oxidase is substantially reduced compared to that in normal and sulfite-sensitive asthmatic subjects [46].
Our ESR data demonstrated that in the presence of DMPO during sulfite oxidation, DMPO/•SO3−, DMPO/•OH, and DMPO/•OOH were produced in the PMA-stimulated neutrophils. Previous work on the oxidation of (bi)sulfite in polymorphonuclear (PMN) leukocytes [47, 48] was correlated with the activity of sulfite oxidase, but the formation of sulfite anion radical was attributed to the known reaction of sulfite with superoxide [49] generated from the cell activation. In another study, it was established that sulfite by itself activates the neutrophil NADPH oxidase to produce superoxide via the protein kinase C and Ca2+/calmodulin pathways [50, 51]; there, superoxide anion production of neutrophils was determined using superoxide dismutase-inhibitable, lucigenin-dependent chemiluminescence. However, there is no doubt whatsoever that lucigenin has the potential to generate superoxide radicals [52, 53]. Moreover, we did not detect any DMPO/•OOH production from sulfite-treated neutrophils without PMA activation (Fig. 3, spectrum c).
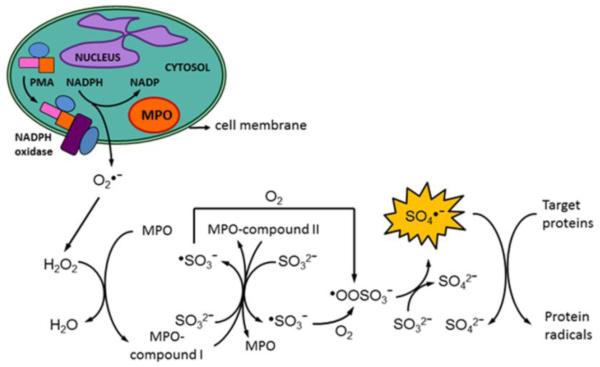
In this study, we have shown that (bi)sulfite oxidation catalyzed by myeloperoxidase resulted in the formation of highly reactive sulfite-derived radicals. To our knowledge, this is the first report of sulfite oxidation by a mammalian peroxidase-H2O2 system resulting in detection of the highly reactive SO4•− radical. Our ESR experiments in the presence of 6 mM DMPO showed the formation of •SO3−, SO4•− and •OH radical adducts, the latter building up with time (Fig. 2C). This is in agreement with previously published reports of spin trapping of SO4•− with DMPO, where nucleophilic substitution of the incipient leaving group in the initial radical adducts occurred via the reaction: DMPO/SO4•− + H2O → HSO4− + DMPO/•OH [32, 34]. We were unable to detect the DMPO/•OOSO3− radical adduct, probably because of the decomposition of DMPO/•OOSO3− to DMPO/•OH and −O3SOOH [32]. The standard redox potential of SO4•− has been determined to be between 2.4 and 3.1 V (vs. NHE) [54, 55], which makes it as strong an oxidant as hydroxyl radical (E0 = 2.31 V) [56]. Although the enzyme-catalyzed oxidation of sulfites by other peroxidases has been shown to occur in lung microsomes [10], polymorphonuclear leukocytes [48], and lymphocytes from intestinal Peyer’s patches and mesenteric lymph nodes [57], there have been no previous reports in the literature of the myeloperoxidase metabolism of (bi)sulfite in neutrophils (Scheme 2).
Scheme 2.
Our evidence that myeloperoxidase initiates the sulfite oxidation in PMA-stimulated neutrophils is consistent with the effects of MPO substrates and inhibitors. The preferred substrates for myeloperoxidase are reported to be thiocyanate (SCN−), followed by iodide (I−), bromide (Br−), and chloride (Cl−) based on the rates of two-electron reduction of MPO-compound I with the formation of the corresponding hypohalous acid [13]. We added plasma concentrations of thiocyanate, bromide, and chloride separately and all together to the cell samples prior to PMA activation (Fig. 4). ESR data showed that the addition of the substrates significantly inhibited the formation of DMPO/•SO3− radical adduct, and this evidence supports the importance of MPO for initiation of the radical chemistry. However, incubation with a mixture of all three substrates in the presence of sulfite did not completely inhibit the enzymaticallycatalyzed oxidation of (bi)sulfite, supporting the possible physiological significance of this reaction. The lack of an ESR signal in plasma is probably due to ceruloplasmin, an acute phase protein present in plasma at 2-4 μM, which was shown to exhibit an inhibitory effect on the peroxidase acitivity of MPO [58]. In addition to ceruloplasmin, another possible reason for the absence of radical detection is the presence of unknown antioxidants, but sulfite anion radical may be present at concentrations below the detection limit of ESR. However, plasma levels of halides and (pseudo)halides can vary. For example, plasma levels for thiocyanate are from 20 to 120 μM; for Br−, from 20 to 100 μM; for I−, <1 μM; and for Cl−, 100 mM. When we added 100 μM thiocyanate or 100 μM bromide to our sulfite-mediated ESR experiments, we detected significant inhibition of radical formation, while iodide or chloride did not have such an effect. However, the concentration of the physiological substrates may be much lower in cells or tissues, in which case we can assume that the (bi)sulfite oxidation is likely to occur in vivo, particularly at sites where neutrophilic infiltration is enhanced and thiocyanate and bromide are limited. Also, the concentrations of thiocyanate and bromide are probably much lower in tissues (for example lungs) or in the intracellular space than used in the present work. For example, in the liver, the intracellular Cl− concentration was determined to be only 4.5 mM [59].
Additionally, the myeloperoxidase inhibitors azide and ABAH confirmed the strong dependence of the sulfite radical formation on myeloperoxidase (Fig. 5). Azide has been shown to be a general inhibitor for various peroxidases while 4-aminobenzoic acid hydrazide (ABAH) is a specific MPO inhibitor. The protein oxidizes the inhibitors to azidyl [60] or ABAH radicals [61]. The inactivation of MPO by azidyl radical is probably caused by covalent modification of the heme prosthetic group [62] while ABAH radical reduces the enzyme to its ferrous intermediate [61]. In addition to reacting with peroxidases, (bi)sulfite reacts with superoxide dismutase, as we recently reported [38]. For this reason, DMPO/•SO3− formation was enhanced in the presence of SOD due to the reaction: SOD-Cu(II) + SO32− → SOD-Cu(I) + •SO3−. A similar SOD effect was observed in previous work, but the authors interpreted the results as an indication that SOD-driven H2O2 participated in further enzyme-catalyzed sulfite oxidation [57].
In our ESR studies reported here, we also tried to answer the question of whether the radicals detected due to MPO-catalyzed oxidation of sulfite are formed inside or outside the cells. It has been established that cell membranes are impermeable to charged metal chelates such as Gd-DTPA and chromium(III) oxalate [K3Cr(C2O4)3 × 3H2O] [40-42, 63]. In Fig. 6, the extracellular fraction of •SO3− was broadened by 20 mM Gd-DTPA, and the remaining signal was due to the radical species present inside the cells. La-DTPA was used as a control due to its chemical similarity to Gd-DTPA, but since it is not paramagnetic, it did not broaden the DMPO/•SO3− signal. Based on the ESR intensity, we could conclude that the sulfite anion radicals generated by activated neutrophils are mainly formed outside the cells (~75%) after degranulation and MPO release, but PMA is known to cause very little degranulation [64-68]. A second possibility is that the radical formation occurred inside the cells, but the radical adducts escaped through the cell membrane. In any case, because the intracellular volume is a very, very small fraction of the total sample volume, the intracellular concentration of DMPO/•SO3− must be an order of magnitude greater than the extracellular concentration.
Our proposed mechanism of myeloperoxidase-initiated sulfite oxidation by PMA-stimulated neutrophils is shown in Scheme 2. When inflammogenic signaling is induced by PMA in human neutrophils, O2•− is produced mainly by NADPH oxidase, which disproportionates to H2O2. When MPO reacts with H2O2, it forms compound I, which contains two more oxidizing equivalents than the resting enzyme. We suggest that MPO-compound I can oxidize (bi)sulfite to sulfite anion radical (•SO3−) through the typical peroxidase cycle by a one-electron process with the formation of MPO-compound II detected in a pure enzymatic system (the same pathway as HRP). This intermediate can also oxidize another molecule of (bi)sulfite and produce another •SO3−. Once the •SO3− radical is formed, it reacts rapidly with oxygen with the formation of −O3SOO•, which undergoes self-reaction or reacts with another molecule of (bi)sulfite or other compounds to form SO4•−. The ultimately formed sulfate anion radical (SO4•−) is a very strong oxidant, and this reactive intermediate can oxidize macromolecules such as proteins, thereby propagating protein radical formation. Our neutrophil-(bi)sulfite-PMA system provides an enzymatic pathway for production of −O3SOO• and SO4•−, which may further oxidize protein and DNA macromolecules. Therefore, we propose a mechanism of MPO-dependent oxidative damage and tissue injury in (bi)sulfite (hydrated sulfur dioxide)-exacerbated inflammatory disorders (Scheme 2). Our results portend the opening of a new and exciting venue of research which we intend to explore in greater detail.
Highlights.
Sulfite (•SO3−) and sulfate (SO4•−) radicals were detected with ESR spin-trapping.
Sulfite (•SO3−) radical was detected within human neutrophils.
Adverse reactions of (bi)sulfite in asthmatics may be related to radical formation.
Acknowledgments
We would like to acknowledge Mary J. Mason, Dr. Ann Motten, and Jean Corbett for their editing of the manuscript. Also, we thank Ms. Brenda Yingling and Ms. Jamie D. Marshburn for their technical assistance. This work has been supported by the Intramural Research Program of the NIH, NIEHS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Rall DP. Review of the health effects of sulfur oxides. Environ. Health Perspect. 1974;8:97–121. doi: 10.1289/ehp.74897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hayon E, Treinin A, Wilf J. Electronic spectra, photochemistry, and autoxidation mechanism of the sulfite-bisulfite-pyrosulfite systems. The SO2−, SO3−, SO4−, and SO5− radicals. J. Amer. Chem. Soc. 1972;94:47–57. [Google Scholar]
- [3].Taylor SL, Higley NA, Bush RK. Sulfites in foods: uses, analytical methods, residues, fate, exposure assessment, metabolism, toxicity, and hypersensitivity. Adv. Food Res. 1986;30:1–76. doi: 10.1016/s0065-2628(08)60347-x. [DOI] [PubMed] [Google Scholar]
- [4].Gunnison AF. Sulphite toxicity: a critical review of in vitro and in vivo data. Food Cosmet. Toxicol. 1981;19:667–682. doi: 10.1016/0015-6264(81)90519-8. [DOI] [PubMed] [Google Scholar]
- [5].Lester MR. sulfite sensitivity - significance in human health. J. Am. Coll. Nutr. 1995;14:229–232. doi: 10.1080/07315724.1995.10718500. [DOI] [PubMed] [Google Scholar]
- [6].Vally H, Misso NLA, Madan V. Clinical effects of sulphite additives. Clin. Exp. Allergy. 2009;39:1643–1651. doi: 10.1111/j.1365-2222.2009.03362.x. [DOI] [PubMed] [Google Scholar]
- [7].Gunnison AF, Jacobsen DW. Sulfite hypersensitivity. A critical review. CRC Crit. Rev. Toxicol. 1987;17:185–214. doi: 10.3109/10408448709071208. [DOI] [PubMed] [Google Scholar]
- [8].Cohen HJ, Fridovich I. Hepatic sulfite oxidase. Purification and properties. J. Biol. Chem. 1971;246:359–366. [PubMed] [Google Scholar]
- [9].Niknahad H, O’Brien PJ. Mechanism of sulfite cytotoxicity in isolated rat hepatocytes. Chem.-Biol. Interact. 2008;174:147–154. doi: 10.1016/j.cbi.2008.05.032. [DOI] [PubMed] [Google Scholar]
- [10].Mottley C, Mason RP, Chignell CF, Sivarajah K, Eling TE. The formation of sulfur trioxide radical anion during the prostaglandin hydroperoxidase-catalyzed oxidation of bisulfite (hydrated sulfur dioxide) J. Biol. Chem. 1982;257:5050–5055. [PubMed] [Google Scholar]
- [11].Araiso T, Miyoshi K, Yamazaki I. Mechanisms of electron transfer from sulfite to horseradish peroxidase-hydroperoxide compounds. Biochemistry. 1976;15:3059–3063. doi: 10.1021/bi00659a019. [DOI] [PubMed] [Google Scholar]
- [12].Ranguelova K, Chatterjee S, Ehrenshaft M, Ramirez DC, Summers FA, Kadiiska MB, Mason RP. Protein radical formation resulting from eosinophil peroxidase-catalyzed oxidation of sulfite. J. Biol. Chem. 2010;285:24195–24205. doi: 10.1074/jbc.M109.069054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Furtmuller PG, Burner U, Obinger C. Reaction of myeloperoxidase compound I with chloride, bromide, iodide, and thiocyanate. Biochemistry. 1998;37:17923–17930. doi: 10.1021/bi9818772. [DOI] [PubMed] [Google Scholar]
- [14].Neta P, Huie RE, Ross AB. Rate constants for reactions of inorganic radicals in aqueous solution. J. Phys. Chem. Ref. Data. 1988;17:1027–1284. [Google Scholar]
- [15].Brewster CEP, Howarth PH, Djukanovic R, Wilson J, Holgate ST, Roche WR. Myofibroblasts and subepithelial fibrosis in bronchial asthma. Am. J. Respir. Cell Mol. Biol. 1990;3:507–511. doi: 10.1165/ajrcmb/3.5.507. [DOI] [PubMed] [Google Scholar]
- [16].Vachier I, Le Doucen C, Loubatiere J, Damon M, Terouanne B, Nicolas JC, Chanez P, Godard P. Imaging reactive oxygen species in asthma. J. Biolumin. Chemilumin. 1994;9:171–175. doi: 10.1002/bio.1170090311. [DOI] [PubMed] [Google Scholar]
- [17].Kanazawa H, Kurihara N, Hirata K, Takeda T. The role of free radicals in airway obstruction in asthmatic patients. Chest. 1991;100:1319–1322. doi: 10.1378/chest.100.5.1319. [DOI] [PubMed] [Google Scholar]
- [18].Barnes PJ. Reactive oxygen species and airway inflammation. Free Radic. Biol. Med. 1990;9:235–243. doi: 10.1016/0891-5849(90)90034-g. [DOI] [PubMed] [Google Scholar]
- [19].Klebanoff SJ. Myeloperoxidase: friend and foe. J. Leukoc. Biol. 2005;77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- [20].Cross CE, van der Vliet A, O’Neill CA, Eiserich JP. Reactive oxygen species and the lung. Lancet. 1994;344:930–933. doi: 10.1016/s0140-6736(94)92275-6. [DOI] [PubMed] [Google Scholar]
- [21].Furtmuller PG, Obinger C, Hsuanyu Y, Dunford HB. Mechanism of reaction of myeloperoxidase with hydrogen peroxide and chloride ion. Eur. J. Biochem. 2000;267:5858–5864. doi: 10.1046/j.1432-1327.2000.01491.x. [DOI] [PubMed] [Google Scholar]
- [22].Furtmuller PG, Burner U, Obinger C. Reaction of myeloperoxidase compound I with chloride, bromide, iodide, and thiocyanate. Biochemistry. 1998;37:17923–17930. doi: 10.1021/bi9818772. [DOI] [PubMed] [Google Scholar]
- [23].Marquez LA, Huang JT, Dunford HB. Spectral and kinetic studies on the formation of myeloperoxidase compounds I and II: roles of hydrogen peroxide and superoxide. Biochemistry. 1994;33:1447–1454. doi: 10.1021/bi00172a022. [DOI] [PubMed] [Google Scholar]
- [24].Weiss SJ, Klein R, Slivka A, Wei M. Chlorination of taurine by human neutrophils. Evidence for hypochlorous acid generation. J. Clin. Invest. 1982;70:598–607. doi: 10.1172/JCI110652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand. J. Clin. Lab. Invest. Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- [26].Duling DR. Simulation of multiple isotropic spin-trap EPR spectra. J. Magn. Reson. B. 1994;104:105–110. doi: 10.1006/jmrb.1994.1062. [DOI] [PubMed] [Google Scholar]
- [27].Mottley C, Trice TB, Mason RP. Direct detection of the sulfur trioxide radical anion during the horseradish peroxidase-hydrogen peroxide oxidation of sulfite (aqueous sulfur dioxide) Mol. Pharmacol. 1982;22:732–737. [PubMed] [Google Scholar]
- [28].Burner U, Obinger C, Paumann M, Furtmuller PG, Kettle AJ. Transient and steady-state kinetics of the oxidation of substituted benzoic acid hydrazides by myeloperoxidase. J. Biol. Chem. 1999;274:9494–9502. doi: 10.1074/jbc.274.14.9494. [DOI] [PubMed] [Google Scholar]
- [29].Valderrama B, Ayala M, Vazquez-Duhalt R. Suicide inactivation of peroxidases and the challenge of engineering more robust enzymes. Chem. Biol. 2002;9:555–565. doi: 10.1016/s1074-5521(02)00149-7. [DOI] [PubMed] [Google Scholar]
- [30].Roman R, Dunford HB. Studies on horseradish peroxidase. XII. A kinetic study of the oxidation of sulfite and nitrite by compounds I and II. Can. J. Chem. 1973;51:588–596. [Google Scholar]
- [31].Ranguelova K, Mason RP. New insights into the detection of sulfur trioxide anion radical by spin trapping: radical trapping versus nucleophilic addition. Free Radic. Biol. Med. 2009;47:128–134. doi: 10.1016/j.freeradbiomed.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Mottley C, Mason RP. Sulfate anion free radical formation by the peroxidation of (bi)sulfite and its reaction with hydroxyl radical scavengers. Arch. Biochem. Biophys. 1988;267:681–689. doi: 10.1016/0003-9861(88)90077-x. [DOI] [PubMed] [Google Scholar]
- [33].Harbour JR, Hair ML. Spin trapping of the .CO − 2radical in aqueous medium. Can. J. Chem. 1979;57:1150–1152. [Google Scholar]
- [34].Davies MJ, Gilbert BC, Stell JK, Whitwood AC. Nucleophilic substitution reactions of spin adducts. Implications for the correct identification of reaction intermediates by EPR/spin trapping. J. Chem. Soc., Perkin Trans. 2. 1992:333–335. [Google Scholar]
- [35].Buettner GR, Mason RP. Spin-trapping methods for detecting superoxide and hydroxyl free radicals in vitro and in vivo. Methods Enzymol. 1990;186:127–133. doi: 10.1016/0076-6879(90)86101-z. [DOI] [PubMed] [Google Scholar]
- [36].Kettle AJ, Winterbourn CC. Mechanism of inhibition of myeloperoxidase by anti-inflammatory drugs. Biochem. Pharmacol. 1991;41:1485–1492. doi: 10.1016/0006-2952(91)90565-m. [DOI] [PubMed] [Google Scholar]
- [37].Nève J, Parij N, Moguilevsky N. Inhibition of the myeloperoxidase chlorinating activity by non-steroidal anti-inflammatory drugs investigated with a human recombinant enzyme. Eur. J. Pharmacol. 2001;417:37–43. doi: 10.1016/s0014-2999(01)00895-0. [DOI] [PubMed] [Google Scholar]
- [38].Ranguelova K, Bonini MG, Mason RP. (Bi)sulfite oxidation by copper, zinc-superoxide dismutase: sulfite-derived, radical-initiated protein radical formation. Environ. Health. Perspect. 2010;118:970–975. doi: 10.1289/ehp.0901533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Morse PD., 2nd Use of the spin label tempamine for measuring the internal viscosity of red blood cells. Biochem. Biophys. Res. Commun. 1977;77:1486–1491. doi: 10.1016/s0006-291x(77)80146-0. [DOI] [PubMed] [Google Scholar]
- [40].Berg SP, Nesbitt DM. Chromium oxalate: a new spin label broadening agent for use with thylakoids. Biochim. Biophys. Acta. 1979;548:608–615. doi: 10.1016/0005-2728(79)90068-9. [DOI] [PubMed] [Google Scholar]
- [41].Samuni A, Carmichael AJ, Russo A, Mitchell JB, Riesz P. On the spin trapping and ESR detection of oxygen-derived radicals generated inside cells. Proc. Natl. Acad. Sci. USA. 1986;83:7593–7597. doi: 10.1073/pnas.83.20.7593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].DeGray JA, Rao DNR, Mason RP. Reduction of paraquat and related bipyridylium compounds to free radical metabolites by rat hepatocytes. Arch. Biochem. Biophys. 1991;289:145–152. doi: 10.1016/0003-9861(91)90454-q. [DOI] [PubMed] [Google Scholar]
- [43].Ji AJ, Savon SR, Jacobsen DW. Determination of total serum sulfite by HPLC with fluorescence detection. Clin. Chem. 1995;41:897–903. [PubMed] [Google Scholar]
- [44].Johnson JL, Waud WR, Rajagopalan KV, Duran M, Beemer FA, Wadman SK. Inborn errors of molybdenum metabolism: combined deficiencies of sulfite oxidase and xanthine dehydrogenase in a patient lacking the molybdenum cofactor. Proc. Natl. Acad. Sci. USA. 1980;77:3715–3719. doi: 10.1073/pnas.77.6.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Acosta R, Granados J, Mourelle M, Perez-Alvarez V, Quezada E. Sulfite sensitivity: relationship between sulfite plasma levels and bronchospasm: case report. Ann. Allergy. 1989;62:402–405. [PubMed] [Google Scholar]
- [46].Stevenson DD, Simon RA. Sulfites and asthma. J. Allergy Clin. Immunol. 1984;74:469–472. doi: 10.1016/0091-6749(84)90380-4. [DOI] [PubMed] [Google Scholar]
- [47].Constantin D, Mehrotra K, Jernstrom B, Tomasi A, Moldeus P. Alternative pathways of sulfite oxidation in human polymorphonuclear leukocytes. Pharmacol. Toxicol. 1994;74:136–140. doi: 10.1111/j.1600-0773.1994.tb01088.x. [DOI] [PubMed] [Google Scholar]
- [48].Constantin D, Bini A, Meletti E, Moldeus P, Monti D, Tomasi A. Age-related differences in the metabolism of sulphite to sulphate and in the identification of sulphur trioxide radical in human polymorphonuclear leukocytes. Mech. Ageing Dev. 1996;88:95–109. doi: 10.1016/0047-6374(96)01728-9. [DOI] [PubMed] [Google Scholar]
- [49].Fridovich I, Handler P. Detection of free radicals generated during enzymic oxidations by the initiation of sulfite oxidation. J. Biol. Chem. 1961;236:1836–1840. [PubMed] [Google Scholar]
- [50].Beck-Speier I, Liese JG, Belohradsky BH, Godleski JJ. Sulfite stimulates NADPH oxidase of human neutrophils to produce active oxygen radicals via protein kinase C and Ca-/calmodulin pathways. Free Radic. Biol. Med. 1993;14:661–668. doi: 10.1016/0891-5849(93)90148-n. [DOI] [PubMed] [Google Scholar]
- [51].Beck-Speier I, Lenz AG, Godleski JJ. Responses of human neutrophils to sulfite. J. Toxicol. Environ. Health. 1994;41:285–297. doi: 10.1080/15287399409531844. [DOI] [PubMed] [Google Scholar]
- [52].Vasquez-Vivar J, Hogg N, Pritchard KA, Jr., Martasek P, Kalyanaraman B. Superoxide anion formation from lucigenin: an electron spin resonance spin-trapping study. FEBS Lett. 1997;403:127–130. doi: 10.1016/s0014-5793(97)00036-7. [DOI] [PubMed] [Google Scholar]
- [53].Wardman P. Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: progress, pitfalls, and prospects. Free Radic. Biol. Med. 2007;43:995–1022. doi: 10.1016/j.freeradbiomed.2007.06.026. [DOI] [PubMed] [Google Scholar]
- [54].Huie RE, Neta P. Chemical behavior of SO3− and SO SO5− radicals in aqueous solutions. J. Phys. Chem. 1984;88:5665–5669. [Google Scholar]
- [55].Yeber MC, Diaz L, Fernandez J. Catalytic activity of the SO4•− radical for photodegradation of the azo dye Cibacron Brilliant Yellow 3 and 3,4-dichlorophenol: optimization by application of response surface methodology. J. Photochem. Photobiol. 2010;215:90–95. [Google Scholar]
- [56].Koppenol WH, Stanbury DM, Bounds PL. Electrode potentials of partially reduced oxygen species, from dioxygen to water. Free Radic. Biol. Med. 2010;49:317–322. doi: 10.1016/j.freeradbiomed.2010.04.011. [DOI] [PubMed] [Google Scholar]
- [57].Chamulitrat W. Activation of the superoxide-generating NADPH oxidase of intestinal lymphocytes produces highly reactive free radicals from sulfite. Free Radic. Biol. Med. 1999;27:411–421. doi: 10.1016/s0891-5849(99)00088-x. [DOI] [PubMed] [Google Scholar]
- [58].Segelmark M, Persson B, Hellmark T, Wieslander J. Binding and inhibition of myeloperoxidase (MPO): a major function of ceruloplasmin? Clin. Exp. Immunol. 1997;108:167–174. doi: 10.1046/j.1365-2249.1997.d01-992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Krogh A. Extracellular and intracellular fluid. Acta Medica Scandinavica. 1938;95:9–18. [Google Scholar]
- [60].Kalyanaraman B, Janzen EG, Mason RP. Spin trapping of the azidyl radical in azide/catalase/H2O2 and various azide/peroxidase/H2O2 peroxidizing systems. J. Biol. Chem. 1985;260:4003–4006. [PubMed] [Google Scholar]
- [61].Kettle AJ, Gedye CA, Winterbourn CC. Mechanism of inactivation of myeloperoxidase by 4-aminobenzoic acid hydrazide. Biochem. J. 1997;321:503–508. doi: 10.1042/bj3210503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Tatarko M, Bumpus JA. Further studies on the inactivation by sodium azide of lignin peroxidase from Phanerochaete chrysosporium. Arch. Biochem. Biophys. 1997;339:200–209. doi: 10.1006/abbi.1996.9839. [DOI] [PubMed] [Google Scholar]
- [63].Rao DNR, Jordan S, Mason RP. Generation of nitro radical anions of some 5-nitrofurans, and 2- and 5-nitroimidazoles by rat hepatocytes. Biochem. Pharmacol. 1988;37:2907–2913. doi: 10.1016/0006-2952(88)90275-4. [DOI] [PubMed] [Google Scholar]
- [64].White JG, Estensen RD. Selective labilization of specific granules in polymorphonuclear leukocytes by phorbol myristate acetate. Am. J. Pathol. 1974;75:45–60. [PMC free article] [PubMed] [Google Scholar]
- [65].Fletcher MP, Seligmann BE. Monitoring human neutrophil granule secretion by flow cytometry: secretion and membrane potential changes assessed by light scatter and a fluorescent probe of membrane potential. J. Leuk. Biol. 1985;37:431–447. doi: 10.1002/jlb.37.4.431. [DOI] [PubMed] [Google Scholar]
- [66].Abdel-Latif D, Steward M, Lacy P. Neutrophil primary granule release and maximal superoxide generation depend on Rac2 in a common signalling pathway. Can. J. Physiol. Pharmacol. 2005;83:69–75. doi: 10.1139/y04-123. [DOI] [PubMed] [Google Scholar]
- [67].Xu S, Zhao L, Larsson A, Smeds E, Kusche-Gullberg M, Venge P. Purification of a 75 kDa protein from the organelle matrix of human neutrophils and identification as N-acetylglucosamine-6-sulphatase. Biochem. J. 2005;387:841–847. doi: 10.1042/BJ20041811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].White MR, Tecle T, Crouch EC, Hartshorn KL. Impact of neutrophils on antiviral activity of human bronchoalveolar lavage fluid. Am. J. Physiol. Lung Cell Mol. Physiol. 2007;293:L1293–L1299. doi: 10.1152/ajplung.00266.2007. [DOI] [PubMed] [Google Scholar]