Abstract
Growth hormone (GH) and insulin-like growth factor-I (IGF-I) exert powerful influences on somatic growth, metabolism, and tissue repair, and have been implicated in aging and carcinogenesis. Since the formulation of the somatomedin hypothesis over 50 years ago, GH and IGF-I have been linked intimately to one another. Recent studies have established that GH potently stimulates IGF-I gene transcription, and through this mechanism controls production of IGF-I. A key mediator of the GH - IGF-I biosynthetic pathway is the latent transcription factor Stat5b. This review summarizes the potentially complex mechanistic relationship among GH action, Stat5b, and IGF-I gene activation, and suggests that Stat5b may have a broad role in mediating IGF-I gene regulation in response to diverse physiological inputs.
The GH - IGF-I - Stat5b connection: a historical perspective
More than 50 years ago, at a time when the biochemical mechanisms of action of GH were unknown, studies assessing its acute effects on protein biosynthesis in rib cartilage explants (measured by sulfate incorporation into proteoglycans) led to the idea the GH promoted growth through a mediator molecule, a “somatomedin” [1,2]. Essential for the formulation of the somatomedin hypothesis were experiments showing that GH was inactive when added to cartilage but that “sulfation factor” activity was detected in serum within a few hours of systemic GH administration to pituitary-ablated rats [1]. Subsequent identification of the GH-dependent somatomedin as the 70-amino acid single-chain peptide, IGF-I [3], did not immediately establish how GH stimulated growth by promoting the accumulation of this protein, although it was subsequently shown in direct confirmation of studies defining the somatomedin hypothesis that GH caused a rapid increase in levels of circulating IGF-I [4]. Molecular biological experiments conducted in the mid 1980s and early 1990s then determined that GH induced IGF-I gene expression [5–7], and demonstrated that a single systemic pulse of GH to pituitary-deficient rats could stimulate IGF-I gene transcription within minutes [8], and lead to sustained IGF-I mRNA production [8]. More recent insights that were critically dependent on characterization of the GH receptor and elucidation of its intracellular signaling cascades (reviewed in [9–11]) have established that the latent transcription factor Stat5b is the essential transducer of GH-stimulated IGF-I gene activation [11,12]. These results were confirmed and extended by “experiments of nature”, in which inactivating mutations in the STAT5b gene were found in individuals with GH-resistant growth defects and low levels of circulating IGF-I [13].
The purpose of this review is to discuss the molecular and biochemical basis for IGF-I gene regulation by GH via Stat5b. A central focus will be on recent findings defining a potentially complex mechanistic relationship between GH-activated Stat5b and induction of IGF-I gene transcription, and also supporting a possibly broader role for Stat5b in controlling IGF-I gene expression via other non-GH activated regulatory pathways.
The GH receptor and GH-mediated signaling pathways
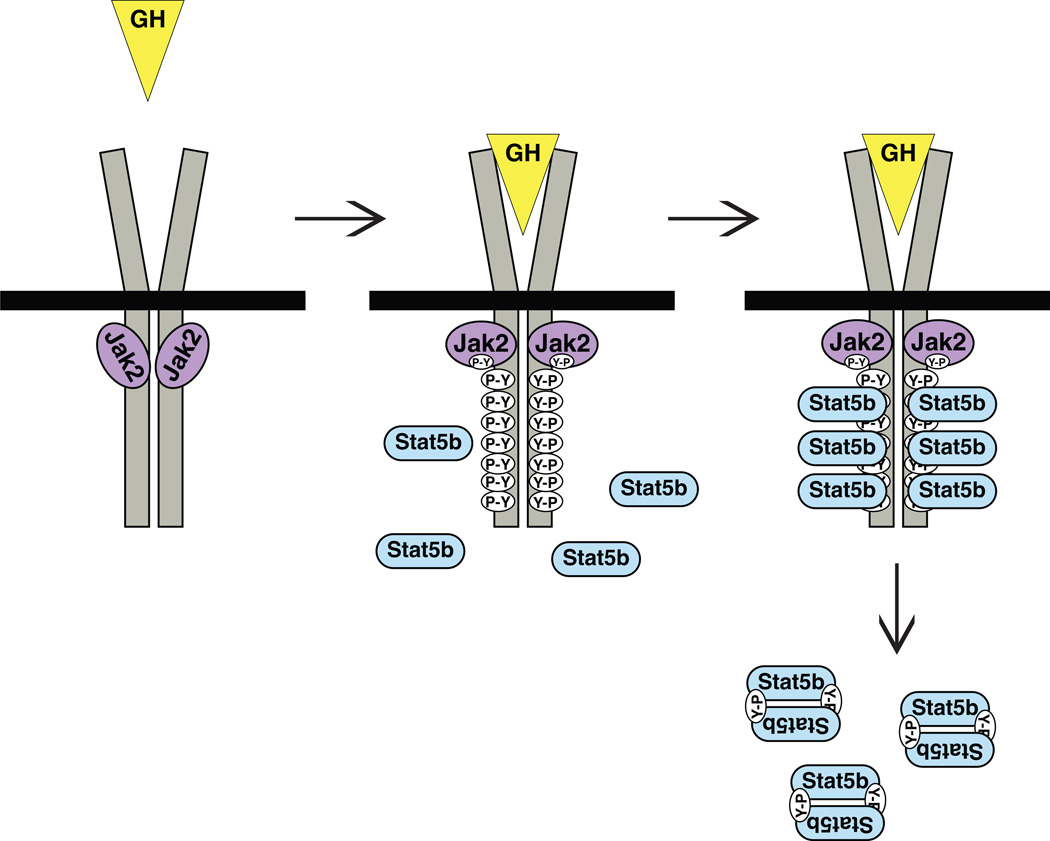
The GH receptor (GHR) was among the first members of the cytokine receptor family in which a biochemical mechanism of signaling was identified [9], and through a combination of biophysical, biochemical, genetic, molecular, and physiological studies a fairly comprehensive picture of the receptor’s mechanisms of action now can be drawn (reviewed in [10,11]) (Fig 1). In the absence of hormone, the GHR, a single-chain membrane-spanning protein, appears to exist primarily as a homodimer [10,11]. A single GH molecule binds sequentially to sites on the extracellular part of each receptor monomer, with binding to the first receptor facilitating interactions with the second [10,11]. The resultant GH-induced conformational changes to the receptor dimer promote transmission of information through the cell membrane to the intracellular part of the GHR, leading to activation of the receptor-associated tyrosine protein kinase, Jak2, and phosphorylation of multiple tyrosine residues within the intracellular region of the GHR [9–11] (Fig 1). Each of the phosphorylated tyrosines on the GHR potentially can serve as a docking site for the signaling proteins that mediate the pleiotropic biological effects of GH, with several of the sites being able to bind Stat5b (Fig 1).
Figure 1. Activation of Stat5b by binding of GH to the GHR.
Left: Diagram of the GHR, showing that it is a constitutive dimer and that a molecule of Jak2 is bound to each monomer’s intracellular segment. Middle: Upon sequential binding of one molecule of GH to each receptor monomer, conformational changes in the dimer stimulate the kinase activity of Jak2, leading first to phosphorylation of tyrosine residues on Jak2 (Y-P), and then to phosphorylation of multiple tyrosines throughout the intracellular part of the GHR. Right: Many of the phospho-tyrosine sites can bind Stat5b, which is phosphorylated on a single tyrosine residue by Jak2, promoting its dissociation from the GHR, homo-dimerization, and activation.
Stat5b is a key mediator for GH-stimulated somatic growth
Despite convincing evidence that multiple signaling pathways are induced by the ligand-activated GHR [9–11], it has become clear that Stat5b is the critical signaling intermediate for GH-regulated growth [10,14,15]. Stat5b, one of seven members in mammals of the Stat family of latent transcription factors [12,16], has been associated with regulation of somatic growth since studies in 1997 showed that an engineered hypomorphic mutant in mice led to diminished post-natal growth [17,18]. Subsequent elegant manipulations of the GHR in mice identified a cohort of tyrosine residues within the receptor’s intracellular domain that were critical for binding Stat5b so that it could be activated by GHR-associated Jak2 [19] (Fig 1). Elimination of all Stat5b binding sites in the GHR or mutation of the binding domain for Jak2 led to loss of activation of Stat5b in response to GH and caused growth deficits in mice that were as severe as those seen with targeted loss of the GHR [19,20]. This potentially key role of Stat5b in GH-mediated growth was strengthened by the discovery of individuals with profound growth failure and short stature, and loss of responsiveness to GH, who had mutations within the STAT5b gene that either eliminated production of the Stat5b protein or rendered the mutant protein biologically inactive [13].
The molecular anatomy and physiology of the IGF-I gene
In mammals, the IGF-I gene is composed of six exons and five introns that span > 80 kb of chromosomal DNA [21] (Fig 2). Tandem promoters direct IGF-I gene transcription through unique leader exons (Fig 2). Promoter 1, which uses heterogeneous transcription initiation sites, is active in multiple animal tissues [22], while the smaller and simpler promoter 2 is primarily but not exclusively expressed in the liver [22]. Although the biochemical mechanisms responsible for different tissue-specific patterns of IGF-I promoter activity are unknown, the DNA sequences of both proximal promoters are relatively well-conserved in mammals based on analyses of available genomic databases (74% over 420 nucleotides for promoter 1, 58% over 404 nucleotides for promoter 2 between rat and human IGF-I), suggesting that functional properties of each promoter have been maintained during speciation as essential aspects of the biology of IGF-I gene regulation.
Figure 2. IGF-I gene structure and expression.
Map of a mammalian IGF-I gene, and outline of the steps leading to production of IGF-I mRNAs. Top: Transcription of the 6-exon IGF-I gene is under control of tandem promoters (P1 and P2), each using a distinct leader exon (exons 1 and 2, yellow and green, respectively). Promoter 1 recognizes heterogeneous transcription initiation sites in exon 1 (not shown). Middle: The two promoters direct transcription of two classes of nascent nuclear IGF-I RNAs, which undergo alternative splicing involving exons 5 and 6 (red and blue, respectively), and differential polyadenylation within exon 6 to yield multiple mature IGF-I mRNA species. Bottom: The IGF-I mRNAs are translated into two classes of protein precursors termed IGF-IA and IGF-IB, which differ in their COOH-terminal amino acids sequences, but are processed into the same 70-residue bioactive IGF-I protein.
In addition to transcriptional control by tandem promoters, IGF-I mRNA undergoes alternative splicing involving exons 5 and 6, and differential polyadenylation at the 3’ end of exon 6 [21,22]. Taken together, these mechanisms are responsible for the generation of over 100 distinct IGF-I mRNAs [21]. The evolutionary pressures, which have maintained multiple IGF-I transcripts in mammals, have not been elucidated, and there no compelling hypotheses to explain the existence of so many IGF-I mRNAs that encode one of two IGF-I protein precursors and the same 70-amino acid mature IGF-I peptide (Fig 2) [21,23]. It is conceivable that each class of IGF-I transcripts has unique properties, such as distinct half-lives, and that a range of mRNA turnover rates ensures sustained production of IGF-I protein after gene expression is induced, possibly through the existence of cohorts of IGF-I mRNAs that are insensitive to different micro-RNAs or other inhibitory molecules. The available data to address this idea are fragmentary, as the average half-life of IGF-I transcripts was found to exceed several hours (range 4 – 16 hr, depending on the IGF-I mRNA species [24,25]), and the population of micro-RNAs that might target IGF-I transcripts has not been elucidated. It also is possible that key evolutionary constraints act at the level of the two IGF-I protein precursors, termed IGF-IA and IGF-IB, which differ in their COOH-terminal sequences, but are processed into the same 70-amino acid bioactive IGF-I peptide (Fig 2) [21,23]. For recent reviews on the biology of IGF-IA and IGF-IB, see [23,26].
From the perspective of signaling, the activated GH receptor is able to rapidly stimulate both IGF-I promoters, and to promote accumulation of all classes of IGF-I mRNAs, at least in pituitary-deficient rats [8,27]. In addition, in the liver, GH-induced transcription of promoter 1 is several-fold higher than promoter 2 [27]. Thus, direct evidence does not support the concept of a single GH-responsive IGF-I promoter, or distinct classes of GH-regulated IGF-I mRNAs. Rather, both promoters are targets of acute GH-mediated signaling, and all IGF-I transcripts are produced as a consequence of GH action.
Defining a central role for Stat5b in GH-activated IGF-I gene transcription
Studies published in 2003 first placed Stat5b directly within a signaling pathway emanating from the activated GHR and leading to stimulation of IGF-I gene transcription [28]. In these experiments, a dominant-negative Stat5b blocked induction of IGF-I gene transcription by GH in pituitary-deficient rats, and a constitutively active Stat5b was able to promote IGF-I gene activation even in the absence of GH-mediated signaling [28]. Unlike several other genes that show similar behavior, IGF-I lacks canonical Stat5 binding sites in either promoter [29], and thus appears to belong to a different class of GH-activated genes than either murine Cish, Socs2, Igfals, or Spi2.1, where GH-induced binding of Stat5b has been identified within each proximal promoter region [29]. Other observations are in agreement with this supposition, as a combination of bioinformatics and chromatin immunoprecipitation (ChIP) experiments detected multiple Stat5b binding domains dispersed throughout human, rat, and mouse IGF-I loci, but not in either promoter [30–35] (Fig 3). DNA sequence analysis showed that 7 of the 10 elements identified contained at least two recognizable Stat5b binding sites, each with the nucleotide sequence 5’-TTCNNNGAA-3’ (top strand, where N = G, A, T, or C) [33–35], a consensus sequence found previously to preferentially bind Stat5 [36,37]. Of the three remaining elements, two encode a single site, and one contains a non-consensus sequence (Figure 3) [33–35]. These observations are not surprising, as paired binding sequences were found in the proximal promoters of other Stat5b-regulated genes, including mouse and rat Cish, Socs2, Igfals, and Spi2.1 [29]. Paired recognition sequences for Stats are thought to facilitate stronger interactions of the transcription factor with DNA, and thus potentially enhance the transmission of transcriptional information to the target gene promoters [16]. In addition, whole genome analyses performed using ChIP-seq methodology found that > 75% of chromosomal DNA binding elements for the related transcription factor, Stat1, contained consensus sequences, with half being paired sites [38]. However, since the bioinformatics strategies used to examine the role of Stat5b in IGF-I gene transcription tended to focus on conserved and paired sequences for subsequent ChIP assays [33–35], the list of putative Stat5b elements in the IGF-I gene might be considered incomplete until more comprehensive and unbiased evaluations are performed.
Figure 3. GH induces binding of Stat5b to multiple sites in the IGF-I locus.
Depicted is a map of the rat IGF-I locus illustrating the 6-exon IGF-I gene and flanking chromosomal DNA. The color-coding of individual IGF-I exons corresponds to the scheme in Locations where GH promotes binding of Stat5b in IGF-I chromatin are depicted by circles and ellipses (red circles represent canonical sequences and the pink circle non-canonical; red ellipses indicate canonical Stat5b elements possessing chromatin characteristics of transcriptional enhancers [41–43]). Illustrated below the map are the chromatin modifications found at each Stat5b sequence listed, including histone H3 lysine 4 mono-methylation (K4me1), binding of the transcriptional co-factors, p300 and Med1, and RNA polymerase II (pol II) (−, no alteration detected; ±, weak modification; +, strong modification), and their functional properties (GH-induced transcriptional activity in promoter-reporter based gene expression assays) [nd = no data]. Note that the four Stat5b elements indicated by red ellipses exhibit all of these enhancer-like characteristics.
Stat5b was found to bind directly to each consensus DNA sequence located within each putative transcriptional response element, although with variable affinities that depended upon the nucleotides found within both the central part of the 9-base pair core sequence listed above and flanking DNA [33,35]. In addition, when these putative transcriptional response elements containing Stat5b binding sites were fused to IGF-I promoters 1 or 2, and activity of chimeric promoter-reporter genes was measured after transfection into cultured cells, each chromatin segment could enhance GH-activated gene transcription [35]. However, transcriptional potency varied significantly among different response elements, yet it did not correlate closely with DNA binding affinity of individual Stat5b consensus sites [35]. These data clearly support the hypothesis that GH-induced IGF-I gene transcription is mediated by binding of Stat5b in chromatin to multiple dispersed conserved DNA elements, but leaves open for further study the elucidation of the biochemical mechanisms responsible for the transcriptional efficacy of each element. The results also raise the idea that IGF-I may be fundamentally different than other GH- and Stat5b-regulated genes, in which paired Stat5b binding domains, typically located in the proximal promoter region, mediate GH-activated transcription [29]. This paradigm also appears to be unique among all Stat-regulated genes, since to date there are no other examples in which multiple dispersed enhancers binding the same transcription factor, may exert combinatorial control over the activity of a single target gene.
GH action and the chromatin landscape of the IGF-I gene
Recent experiments have begun to characterize properties of the IGF-I gene in chromatin [27,39]. Chromatin around active genes is typically “open”, a configuration reflected by modifications of the histone-containing nucleosomes around which chromosomal DNA is wrapped [40] that usually include relatively high levels of lysine acetylation of core histones H3 and H4 and trimethylation of lysine 4 of histone H3 [40]. A consequence of this change in chromatin structure is to facilitate access of promoter DNA sequences to RNA polymerase II and associated factors. In contrast, chromatin of inactive promoters often lacks these histone modifications [40]. Surprisingly, in hepatic chromatin of young adult pituitary-deficient male rats, the two IGF-I promoters appear to reside in an open environment, even though without GH-mediated signaling IGF-I gene transcription is minimal [27] (Fig 4). The responsible biochemical mechanisms are unknown, although since rats in these experiments underwent pituitary ablative surgery as juveniles [27], it is conceivable that the presence of GH and/or other pituitary trophic hormones during earlier stages of post-natal development was responsible for these epigenetic modifications.
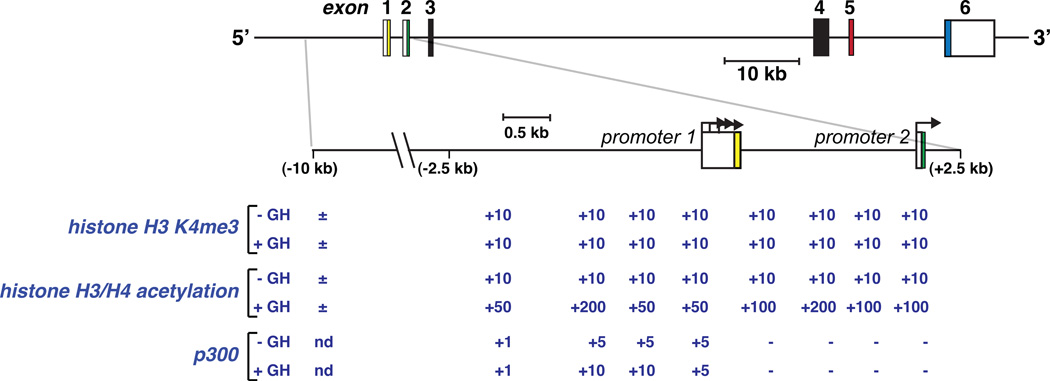
Figure 4. GH-regulated modifications within the chromatin landscape of the two IGF-I promoters.
Depicted is an outline of the rat IGF-I gene, and below is a map of the two IGF-I promoters and adjacent DNA (from −10 to +2.5 kb with respect to the beginning of exon 1). The color-coding of individual IGF-I exons corresponds to the scheme in Fig 2. Listed below the map are the relative strengths of epigenetic modifications seen in hepatic chromatin prior to and accompanying acute GH treatment to pituitary-deficient rats, and include histone H3 lysine 4 tri-methylation (K4me3), acetylation of multiple lysine residues on histones H3 and H4, and binding of the transcriptional co-factor, p300. Note the high levels of H3 K4me3 and inducible H3/H4 acetylation at both IGF-I promoters, and enhanced binding of p300 just to promoter 1 [nd = no data]. Bent arrows represent transcriptional initiation sites, and boxes depict exons.
Despite the relatively open character of IGF-I promoter chromatin in the liver in the absence of hormonal activity, GH causes a dramatic and rapid increase in the levels of core histone acetylation; a 20-fold rise is detected at the two IGF-I promoters within 60 minutes of systemic hormone administration (Fig 4) and is accompanied by induction of IGF-I gene transcription [27]. The histone acetyl-transferase and transcriptional co-activator p300 might play a role in some of these GH-mediated post-translational histone alterations as it is detected at relatively high levels at IGF-I promoter 1. However, p300 is less abundant at promoter 2 [27] (Fig 4) and at other GH- and Stat5b-activated genes, including Cish, Socs2, Igfals, and Spi2.1 [29], suggesting that other as yet unidentified histone acetyl-transferases may be responsible for these latter modifications, and indicating that potentially distinct biochemical mechanisms may regulate acute GH-mediated chromatin modifications at each IGF-I promoter and at other GH-stimulated genes.
Defining mechanisms of action of Stat5b in promoting IGF-I gene transcription
Recent studies have attempted to delineate chromosomal properties of transcriptional enhancers [41–43], and several have used activation of Stat1 by interferon γ as a test case [38,42,44]. Results have defined a series of statistical relationships, in which binding of Stat1 to chromosomal DNA often was accompanied by mono-methylation of lysine 4 on histone H3, and by binding of p300, Med1 (TRAP220, a component of the transcriptional Mediator complex [45]), and RNA polymerase II [41,42]. In addition, DNA segments possessing these chromatin properties often were able to boost activity of a neutral promoter in cell-based promoter-reporter assays [41]. Remarkably, at least four of the GH-mediated Stat5b-binding elements mapped to the IGF-I locus have a similar chromatin signature [35] (Fig 3).
Despite the correlative evidence cited above, which suggests that at least some of the Stat5b binding domains within the IGF-I locus are transcriptional enhancers for the IGF-I gene, critical studies have yet to be performed. A distal enhancer should physically interact with its cognate gene promoter in chromatin, as this is the postulated mechanism by which information is transferred to activate transcription [46,47]. An experimental approach, termed “chromatin conformation and capture” (3C) [48,49] can be used to assess these transient interactions between a distal enhancer and a promoter. The 3C assay is a molecular genetic investigative tool, which facilitates identification of a physical association between two genomic regions in chromatin that is mediated by interacting proteins that are bound to DNA at each location [48,49]. As 3C studies have not been reported for the IGF-I gene yet, it is not clear which GH-induced Stat5b-binding chromosomal elements are truly transcriptional enhancers, and which may encode other functions, for example acting as decoys that bind Stat5b and sequester it from activating sites. One implication of this latter idea would be that some Stat5b binding elements might act to modify GH-activated and Stat5b-dependent target gene transcription through titration of available Stat5b from more productive interactions. Clearly, 3C studies and other experimental approaches are needed to establish the mechanisms by which GH-induced Stat5b-binding chromosomal elements individually and collectively control IGF-I gene transcription.
Bcl6 is a transcriptional repressor whose DNA recognition sequence resembles that of Stat5b [50,51]. Recent ChIP studies have shown that Bcl6 is bound to the Stat5b element in the proximal Socs2, Cish, and Spi2.1 promoters in the absence of GH-activated signaling, and that GH treatment leads to rapid replacement of Bcl6 with Stat5b at these sites [29,52,53]. As these studies also have demonstrated that Bcl6 gene transcription is inhibited by GH via Stat5b, causing a decline in Bcl6 protein abundance [52,53], it is reasonable to postulate that GH may enhance transcription of some Stat5b-regulated target genes via Stat5b by two complementary mechanisms: one secondary to reduction in the amount of the Bcl6 repressor, and the other involving direct Stat5b-mediated transcriptional activation. Although Bcl6 is found at some of the Stat5b binding elements in the IGF-I locus, GH treatment does not promote its disappearance from several of these sites, even though IGF-I gene transcription is induced [29]. Thus, the putative role of Bcl6 in GH action may be more complicated than summarized above.
Stat5b: a potential mediator of other pathways that regulate IGF-I gene expression
The GH - IGF-I - growth pathway may be modulated by nutritional or metabolic perturbations, by a multiplicity of hormonal- and growth factor-activated signaling pathways that represent responses to different environmental inputs, and by effectors of acute and chronic disease [54–61]. Thus, it should not be surprising that Stat5b may be regulated by some of these processes and in turn modify IGF-I gene activity.
Nutritional deficiencies in children have been associated with impaired somatic growth, as have inherited and acquired defects in metabolic pathways [58,61]. A decline in serum levels of IGF-I is seen in fasting that is reversed by re-feeding in normal adults, and similar decreases are observed in pathogenic situations in which protein-calorie malnutrition occurs [58]. In experimental animals fasting or protein restriction leads to a decrease in IGF-I mRNA levels in the liver, the major source of serum IGF-I, which also are reversed by re-feeding [62]. The biochemical mechanisms appear to be multi-factorial, with reductions seen in IGF-I gene transcription rates, and in IGF-I mRNA stability [58]. Since fasting promotes functional resistance to the actions of GH, as measured by a decline in GH-stimulated activation of Jak2 and Stat5b [62], these signaling defects could explain the negative impact on IGF-I gene activity [58,63].
FGF21, a recently described metabolic hormone, which is thought to function as a physiological mediator of severe food deprivation [64,65], may be a key agent regulating the effects of fasting or metabolic deprivation on the GH - IGF-I - growth axis. When over-expressed in mice, FGF21 is able to inhibit IGF-I gene expression and blunt somatic growth by targeting Stat5b [66], leading to a reduction in its tyrosine phosphorylation [66]. FGF21 also may participate in a GH-activated negative feedback loop [67], as its expression is induced indirectly by the acute metabolic actions of GH on adipocytes [67]. It is thus conceivable that part of the negative impact of nutritional deficiencies on somatic growth is mediated by FGF21 via inhibition of Stat5b. Further studies will be needed to strengthen this hypothesis.
Some cytokines produced in chronic inflammatory diseases impair growth through negative effects on the GH - IGF-I pathway [59,60]. For example, levels of interleukin-6 (IL-6) are elevated in active Crohn’s disease [60]. IL-6 can stimulate production of suppressor of cytokine signaling protein 3 (SOCS-3), which negatively regulates the GH receptor and Jak2, potentially explaining how IL-6 reduces IGF-I gene expression [60]. Similarly, signaling pathways induced by tumor necrosis factor-α (TNF-α), which also is elevated in chronic inflammatory diseases [60], can disrupt GH-mediated activation of Jak2 and Stat5b and also can counter the transcriptional effects of Stat5b [59,68]. Thus multiple potentially collaborative actions of different cytokines are able to perturb the GH - Stat5b - IGF-I transcriptional circuit.
Glucocorticoids when in excess, interfere with the GH - IGF-I - growth axis at many levels (for a recent review, see [61]). Among the mechanisms is resistance to the actions of GH, which appears to be secondary to a decline in GH receptor abundance [58]. Surprisingly, even though the glucocorticoid receptor (GR), a steroid-activated transcription factor and member of the nuclear receptor superfamily [69], mediates the inhibitory effect of excess glucocorticoids on the GH - IGF-I axis, its actions also appear to be required for normal GH- and IGF-I-mediated growth [70]. Targeted GR deficiency in the liver leads to growth deficits in mice that are similar to those caused by liver-specific knockout of Stat5a and Stat5b [71], and includes a comparable decline in IGF-I gene expression [71]. GR has been found to interact with Stat5b in hepatic chromatin at one of the GH-activated Stat5b-binding elements in the IGF-I locus [72], and it has been postulated that under physiological conditions GR may act as a selective transcriptional co-factor for Stat5b on a cohort of co-regulated genes, potentially including IGF-I [71]. Further studies will be needed to clearly define the relationship between levels of GR activity and modulation of components of the GH - Stat5b - IGF-I - growth pathway.
The multiple biological effects of estrogens are mediated by estrogen receptors (ER), nuclear proteins related to GR [73,74]. The actions of estrogens on uterine proliferation during the menstrual cycle appear to be mediated by IGF-I, and recent studies have shown that ERα (one of two ERs in mammals [73,74]) can stimulate production of IGF-I in epithelium and stroma of the uterus by inducing IGF-I gene transcription [75]. This involves two transcriptional mechanisms, one mediated by direct binding of ERα to several sites in IGF-I chromatin (mapped by ChIP to 5’ to IGF-I promoter 1 and to the intron between exons 3 and 4 [75]), and the other secondary to induction of Stat5a expression. In the latter pathway, Stat5a binds to at least one of the Stat5b elements in the IGF-I locus, and thus activates IGF-I gene expression by mechanisms similar to Stat5b [75]. This is not surprising, since Stat5a and Stat5b are ~96% identical in amino acid sequence [12], and recognize the same core DNA binding site [36,37]. To date it has not been established if the ERα- Stat5a pathway is unique to the uterus [75], or if it also is active in other tissues where estrogens have been proposed to promote IGF-I expression, including the mammary gland and skeleton [76–78].
Concluding remarks
The past decade has seen a remarkable increase in understanding of the mechanisms of action of GH to control biosynthesis of IGF-I, and thus to regulate somatic growth. We now know that Stat5b is the critical mediator, which is activated by Jak2 on the hormone-stimulated GH receptor. Active Stat5b interacts with multiple DNA binding sites in chromatin within the IGF-I locus, and through mechanisms not yet characterized, promotes the rapid transmission of information to the two IGF-I promoters, culminating in induction of IGF-I gene transcription and production of IGF-I mRNAs and protein. Biochemical, molecular biological, and molecular genetic studies have defined the framework for this GH - Stat5b - IGF-I transcriptional pathway, and its medical significance has been confirmed by identification of inactivating Stat5b mutations in children with profound GH-resistant growth failure [13], and by evidence that many modifiers of somatic growth target components of this signaling pathway. These recent advances now provide an impetus to address a new series of fundamental mechanistic questions that will help decipher the complexities of GH-mediated IGF-I gene regulation under both physiological and pathological conditions (see Outstanding Questions). Seeking the answers to these and other key questions also should lead to new insights about other regulatory pathways that control GH and IGF-I, bioactive molecules that play central roles not only in human growth, but also in tissue repair, aging, and neoplasia [79–82].
Outstanding Questions.
How does GH-activated Stat5b promote communication between distal hormone response elements in chromatin and the two IGF-I promoters?
What are the physiological roles of individual Stat5b-binding GH-regulated chromosomal elements in GH-stimulated IGF-I gene transcription?
Does Stat5b play a role in repressing IGF-I gene transcription in the absence of GH-activated signaling?
How does Stat5b integrate inputs from multiple physiological and pathological sources that impinge on IGF-I gene regulation?
Acknowledgements
Grant support comes from the NIH (R01 DK069703). I thank current and past laboratory colleagues for their insights and efforts in developing our understanding of IGF-I gene regulation, and gratefully acknowledge Dr. William H. Daughaday, who taught me the wonders of the somatomedins. I apologize to colleagues whose work was not cited because of page limitations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Salmon WJ, Daughaday W. A hormonally controlled serum factor which stimulates sulfate incorporation by cartilage in vitro. J Lab Clin Med. 1957;49:825–836. [PubMed] [Google Scholar]
- 2.Daughaday W. A failed assay opened a new door in growth hormone research. Endocrinology. 1992;130:565–566. doi: 10.1210/endo.130.2.1733709. [DOI] [PubMed] [Google Scholar]
- 3.Blundell T, Humbel R. Hormone families: pancreatic hormones and homologous growth factors. Nature. 1980;287:781–787. doi: 10.1038/287781a0. [DOI] [PubMed] [Google Scholar]
- 4.D'ercole AJ, et al. Tissue concentrations of somatomedin C: further evidence for multiple sites of synthesis and paracrine or autocrine mechanisms of action. Proc Natl Acad Sci USA. 1984;81:935–939. doi: 10.1073/pnas.81.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murphy LJ, et al. Identification, characterization, and regulation of a rat complementary deoxyribonucleic acid which encodes insulin-like growth factor-I. Endocrinology. 1987;121:684–691. doi: 10.1210/endo-121-2-684. [DOI] [PubMed] [Google Scholar]
- 6.Roberts CJ, et al. Molecular cloning of rat insulin-like growth factor I complementary deoxyribonucleic acids: differential messenger ribonucleic acid processing and regulation by growth hormone in extrahepatic tissues. Mol Endocrinol. 1987;1:243–248. doi: 10.1210/mend-1-3-243. [DOI] [PubMed] [Google Scholar]
- 7.Norstedt G, Moller C. Growth hormone induction of insulin-like growth factor I messenger RNA in primary cultures of rat liver cells. J Endocrinol. 1987;115:135–139. doi: 10.1677/joe.0.1150135. [DOI] [PubMed] [Google Scholar]
- 8.Bichell DP, et al. Growth hormone rapidly activates insulin-like growth factor I gene transcription in vivo. Mol Endocrinol. 1992;6:1899–1908. doi: 10.1210/mend.6.11.1480177. [DOI] [PubMed] [Google Scholar]
- 9.Lanning N, Carter-Su C. Recent advances in growth hormone signaling. Rev Endocr Metab Disord. 2006;7:225–235. doi: 10.1007/s11154-007-9025-5. [DOI] [PubMed] [Google Scholar]
- 10.Waters MJ, et al. New insights into growth hormone action. J Mol Endocrinol. 2006;36:1–7. doi: 10.1677/jme.1.01933. [DOI] [PubMed] [Google Scholar]
- 11.Brooks A, Waters M. The growth hormone receptor: mechanism of activation and clinical implications. Nat Rev Endocrinol. 2010;6:515–525. doi: 10.1038/nrendo.2010.123. [DOI] [PubMed] [Google Scholar]
- 12.Hennighausen L, Robinson GW. Interpretation of cytokine signaling through the transcription factors STAT5A and STAT5B. Genes Dev. 2008;22:711–721. doi: 10.1101/gad.1643908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hwa V, et al. STAT5b deficiency: lessons from STAT5b gene mutations. Best Pract Res Clin Endocrinol Metab. 2011;25:61–75. doi: 10.1016/j.beem.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Hosui A, Hennighausen L. Genomic dissection of the cytokine-controlled STAT5 signaling network in liver. Physiol Genomics. 2008;34:135–143. doi: 10.1152/physiolgenomics.00048.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoo KH, et al. Context-Specific Growth Hormone Signaling through the Transcription Factor STAT5: Implications for the Etiology of Hepatosteatosis and Hepatocellular Carcinoma. Genes Cancer. 2011;2:3–9. doi: 10.1177/1947601911405046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levy DE, Darnell JJ. Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651–662. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- 17.Udy GB, et al. Requirement of STAT5b for sexual dimorphism of body growth rates and liver gene expression. Proc Natl Acad Sci USA. 1997;94:7239–7244. doi: 10.1073/pnas.94.14.7239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teglund S, et al. Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell. 1998;93:841–850. doi: 10.1016/s0092-8674(00)81444-0. [DOI] [PubMed] [Google Scholar]
- 19.Rowland J, et al. In vivo analysis of growth hormone receptor signaling domains and their associated transcripts. Mol Cell Biol. 2005;25:66–77. doi: 10.1128/MCB.25.1.66-77.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barclay J, et al. In vivo targeting of the growth hormone receptor (GHR) Box1 sequence demonstrates that the GHR does not signal exclusively through JAK2. Mol Endocrinol. 2010;24:204–217. doi: 10.1210/me.2009-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rotwein P. In: Molecular biology of IGF-I and IGF-II. Rosenfeld R, Roberts CJ, editors. Humana Press; 1999. pp. 19–35. [Google Scholar]
- 22.Hall LJ, et al. Functional analysis of the rat insulin-like growth factor I gene and identification of an IGF-I gene promoter. DNA Cell Biol. 1992;11:301–313. doi: 10.1089/dna.1992.11.301. [DOI] [PubMed] [Google Scholar]
- 23.Barton E. The ABCs of IGF-I isoforms: impact on muscle hypertrophy and implications for repair. Appl Physiol Nutr Metab. 2006;31:791–797. doi: 10.1139/h06-054. [DOI] [PubMed] [Google Scholar]
- 24.Hepler JE, et al. Different half-lives of insulin-like growth factor I mRNAs that differ in length of 3' untranslated sequence. Endocrinology. 1990;127:1550–1552. doi: 10.1210/endo-127-3-1550. [DOI] [PubMed] [Google Scholar]
- 25.Bichell DP, et al. Prostaglandin E2 rapidly stimulates insulin-like growth factor-I gene expression in primary rat osteoblast cultures: evidence for transcriptional control. Endocrinology. 1993;133:1020–1028. doi: 10.1210/endo.133.3.8396006. [DOI] [PubMed] [Google Scholar]
- 26.Matheny RJ, et al. Minireview: Mechano-growth factor: a putative product of IGF-I gene expression involved in tissue repair and regeneration. Endocrinology. 2010;151:865–875. doi: 10.1210/en.2009-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chia D, et al. Distinct alterations in chromatin organization of the two IGF-I promoters precede growth hormone-induced activation of IGF-I gene transcription. Mol Endocrinol. 2010;24:779–789. doi: 10.1210/me.2009-0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woelfle J, et al. Acute control of insulin-like growth factor-I gene transcription by growth hormone through Stat5b. J Biol Chem. 2003;278:22696–22702. doi: 10.1074/jbc.M301362200. [DOI] [PubMed] [Google Scholar]
- 29.Chia DJ, Rotwein P. Defining the epigenetic actions of growth hormone: acute chromatin changes accompany GH-activated gene transcription. Mol Endocrinol. 2010;24:2038–2049. doi: 10.1210/me.2010-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woelfle J, et al. Mechanisms of growth hormone (GH) action. Identification of conserved Stat5 binding sites that mediate GH-induced insulin-like growth factor-I gene activation. J Biol Chem. 2003;278:51261–51266. doi: 10.1074/jbc.M309486200. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Jiang H. Identification of a distal STAT5-binding DNA region that may mediate growth hormone regulation of insulin-like growth factor-I gene expression. J Biol Chem. 2005;280:10955–10963. doi: 10.1074/jbc.M412808200. [DOI] [PubMed] [Google Scholar]
- 32.Chia DJ, et al. Characterization of distinct Stat5b binding sites that mediate growth hormone-stimulated IGF-I gene transcription. J Biol Chem. 2006;281:3190–3197. doi: 10.1074/jbc.M510204200. [DOI] [PubMed] [Google Scholar]
- 33.Eleswarapu S, et al. Growth hormone regulation of insulin-like growth factor-I gene expression may be mediated by multiple distal signal transducer and activator of transcription 5 binding sites. Endocrinology. 2008;149:2230–2240. doi: 10.1210/en.2007-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laz EV, et al. Dynamic in vivo binding of STAT5 to growth hormone-regulated genes in intact rat liver. Sex-specific binding at low- but not high-affinity STAT5 sites. Mol Endocrinol. 2009;23:1242–1254. doi: 10.1210/me.2008-0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chia D, et al. Dispersed chromosomal Stat5b-binding elements mediate growth hormone-activated insulin-like growth factor-I gene transcription. J Biol Chem. 2010;285:17636–17647. doi: 10.1074/jbc.M110.117697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ehret GB, et al. DNA binding specificity of different STAT proteins. Comparison of in vitro specificity with natural target sites. J Biol Chem. 2001;276:6675–6688. doi: 10.1074/jbc.M001748200. [DOI] [PubMed] [Google Scholar]
- 37.Soldaini E, et al. DNA binding site selection of dimeric and tetrameric Stat5 proteins reveals a large repertoire of divergent tetrameric Stat5a binding sites. Mol Cell Biol. 2000;20:389–401. doi: 10.1128/mcb.20.1.389-401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jothi R, et al. Genome-wide identification of in vivo protein-DNA binding sites from ChIP-Seq data. Nucleic Acids Res. 2008;36:5221–5231. doi: 10.1093/nar/gkn488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ling G, et al. Unbiased, genome-wide in vivo mapping of transcriptional regulatory elements reveals sex differences in chromatin structure associated with sex-specific liver gene expression. Mol Cell Biol. 2010;30:5531–5544. doi: 10.1128/MCB.00601-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rando OJ, Chang HY. Genome-wide views of chromatin structure. Annu Rev Biochem. 2009;78:245–271. doi: 10.1146/annurev.biochem.78.071107.134639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heintzman ND, et al. Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat Genet. 2007;39:311–318. doi: 10.1038/ng1966. [DOI] [PubMed] [Google Scholar]
- 42.Heintzman N, et al. Histone modifications at human enhancers reflect global cell-type-specific gene expression. Nature. 2009;459:108–112. doi: 10.1038/nature07829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robertson A, et al. Genome-wide relationship between histone H3 lysine 4 mono- and tri-methylation and transcription factor binding. Genome Res. 2008;18:1906–1917. doi: 10.1101/gr.078519.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robertson G, et al. Genome-wide profiles of STAT1 DNA association using chromatin immunoprecipitation and massively parallel sequencing. Nat Methods. 2007;4:651–657. doi: 10.1038/nmeth1068. [DOI] [PubMed] [Google Scholar]
- 45.Conaway RC, et al. The mammalian Mediator complex and its role in transcriptional regulation. Trends Biochem Sci. 2005;30:250–255. doi: 10.1016/j.tibs.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 46.Levine M. Transcriptional enhancers in animal development and evolution. Curr Biol. 2010;20:R754–R763. doi: 10.1016/j.cub.2010.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bulger M, Groudine M. Functional and mechanistic diversity of distal transcription enhancers. Cell. 2011;144:327–339. doi: 10.1016/j.cell.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dekker J, et al. Capturing chromosome conformation. Science. 2002;295:1306–1311. doi: 10.1126/science.1067799. [DOI] [PubMed] [Google Scholar]
- 49.Fullwood M, Ruan Y. ChIP-based methods for the identification of long-range chromatin interactions. J Cell Biochem. 2009;107:30–39. doi: 10.1002/jcb.22116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang CC, et al. BCL-6, a POZ/zinc-finger protein, is a sequence-specific transcriptional repressor. Proc Natl Acad Sci USA. 1996;93:6947–6952. doi: 10.1073/pnas.93.14.6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seyfert VL, et al. Transcriptional repression by the proto-oncogene BCL-6. Oncogene. 1996;12:2331–2342. [PubMed] [Google Scholar]
- 52.Meyer RD, et al. Male-specific hepatic Bcl6: growth hormone-induced block of transcription elongation in females and binding to target genes inversely coordinated with STAT5. Mol Endocrinol. 2009;11:1914–1926. doi: 10.1210/me.2009-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen Y, et al. Computational and functional analysis of growth hormone (GH)- regulated genes identifies the transcriptional repressor B-cell lymphoma 6 (Bc16) as a participant in GH-regulated transcription. Endocrinology. 2009;150:3645–3654. doi: 10.1210/en.2009-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jorgensen J, et al. Effects of GH in human muscle and fat. Pediatr Nephrol. 2010;25:705–709. doi: 10.1007/s00467-009-1334-3. [DOI] [PubMed] [Google Scholar]
- 55.Vijayakumar A, et al. Biological effects of growth hormone on carbohydrate and lipid metabolism. Growth Horm IGF Res. 2010;20:1–7. doi: 10.1016/j.ghir.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baik M, et al. Growth hormone-STAT5 regulation of growth, hepatocellular carcinoma, and liver metabolism. Ann N Y Acad Sci. 2011;1229:29–37. doi: 10.1111/j.1749-6632.2011.06100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berryman D, et al. Growth hormone and adipose tissue: beyond the adipocyte. Growth Horm IGF Res. 2011;21:113–123. doi: 10.1016/j.ghir.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thissen J, et al. Regulation of insulin-like growth factor-I in starvation and injury. Nutr Rev. 1999;57:167–176. doi: 10.1111/j.1753-4887.1999.tb06939.x. [DOI] [PubMed] [Google Scholar]
- 59.Lang C, et al. Cytokine inhibition of JAK-STAT signaling: a new mechanism of growth hormone resistance. Pediatr Nephrol. 2005;20:306–312. doi: 10.1007/s00467-004-1607-9. [DOI] [PubMed] [Google Scholar]
- 60.Walters TD, Griffiths A. Mechanisms of growth impairment in pediatric Crohn's disease. Nat Rev Gastroenterol Hepatol. 2009;6:513–523. doi: 10.1038/nrgastro.2009.124. [DOI] [PubMed] [Google Scholar]
- 61.Mauras N. Can growth hormone counteract the catabolic effects of steroids? Horm Res. 2009;72 Suppl 1:48–54. doi: 10.1159/000229764. [DOI] [PubMed] [Google Scholar]
- 62.Beauloye V, et al. Impairment of liver GH receptor signaling by fasting. Endocrinology. 2002;143:792–800. doi: 10.1210/endo.143.3.8692. [DOI] [PubMed] [Google Scholar]
- 63.Straus DS. Nutritional regulation of hormones and growth factors that control mammalian growth. FASEB J. 1994;8:6–12. doi: 10.1096/fasebj.8.1.8299891. [DOI] [PubMed] [Google Scholar]
- 64.Kliewer S, Mangelsdorf D. Fibroblast growth factor 21: from pharmacology to physiology. Am J Clin Nutr. 2010;91:254S–257S. doi: 10.3945/ajcn.2009.28449B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Itoh N. Hormone-like (endocrine) Fgfs: their evolutionary history and roles in development, metabolism, and disease. Cell Tissue Res. 2010;342:1–11. doi: 10.1007/s00441-010-1024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Inagaki T, et al. Inhibition of growth hormone signaling by the fasting-induced hormone FGF21. Cell Metab. 2008;8:77–83. doi: 10.1016/j.cmet.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen W, et al. Growth hormone induces hepatic production of fibroblast growth factor 21 through a mechanism dependent on lipolysis in adipocytes. J Biol Chem. 2011;286:34559–34566. doi: 10.1074/jbc.M111.285965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ahmed T, et al. Tumor necrosis factor inhibits growth hormone-mediated gene expression in hepatocytes. Am J Physiol Gastrointest Liver Physiol. 2006;291:G35–G44. doi: 10.1152/ajpgi.00550.2005. [DOI] [PubMed] [Google Scholar]
- 69.Mcewan IJ. Nuclear receptors: one big family. Methods Mol Biol. 2009;505:3–18. doi: 10.1007/978-1-60327-575-0_1. [DOI] [PubMed] [Google Scholar]
- 70.Heitzer M, et al. Glucocorticoid receptor physiology. Rev Endocr Metab Disord. 2007;8:321–330. doi: 10.1007/s11154-007-9059-8. [DOI] [PubMed] [Google Scholar]
- 71.Engblom D, et al. Direct glucocorticoid receptor-Stat5 interaction in hepatocytes controls body size and maturation-related gene expression. Genes Dev. 2007;21:1157–1162. doi: 10.1101/gad.426007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tronche F, et al. Glucocorticoid receptor function in hepatocytes is essential to promote postnatal body growth. Genes Dev. 2004;18:492–497. doi: 10.1101/gad.284704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thomas C, Gustafsson J. The different roles of ER subtypes in cancer biology and therapy. Nat Rev Cancer. 2011;11:597–608. doi: 10.1038/nrc3093. [DOI] [PubMed] [Google Scholar]
- 74.Nilsson S, Gustafsson J. Estrogen receptors: therapies targeted to receptor subtypes. Clin Pharmacol Ther. 2011;89:44–55. doi: 10.1038/clpt.2010.226. [DOI] [PubMed] [Google Scholar]
- 75.Hewitt S, et al. Estrogen-mediated regulation of Igf1 transcription and uterine growth involves direct binding of estrogen receptor alpha to estrogen-responsive elements. J Biol Chem. 2010;285:2676–2685. doi: 10.1074/jbc.M109.043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Feng X, Mcdonald J. Disorders of bone remodeling. Annu Rev Pathol. 2011;6:121–145. doi: 10.1146/annurev-pathol-011110-130203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zaidi M. Skeletal remodeling in health and disease. Nat Med. 2007;13:791–801. doi: 10.1038/nm1593. [DOI] [PubMed] [Google Scholar]
- 78.Kleinberg D, Barcellos-Hoff MH. The pivotal role of insulin-like growth factor I in normal mammary development. Endocrinol Metab Clin North Am. 2011;40:461–471. doi: 10.1016/j.ecl.2011.06.001. vii. [DOI] [PubMed] [Google Scholar]
- 79.Rosenfeld R, Hwa V. The growth hormone cascade and its role in mammalian growth. Horm Res. 2009;71 Suppl 2:36–40. doi: 10.1159/000192434. [DOI] [PubMed] [Google Scholar]
- 80.Berryman D, et al. Role of the GH/IGF-1 axis in lifespan and healthspan: lessons from animal models. Growth Horm IGF Res. 2008;18:455–471. doi: 10.1016/j.ghir.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fontana L, et al. Extending healthy life span--from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]