Abstract
The impact of the intensity of renal replacement therapy on outcomes in patients with acute kidney injury has been intensively studied over the past decade. In this review we consider the concept of dose of renal replacement therapy in acute kidney injury and summarize the recent clinical trials addressing this topic. Although several single center trials suggest that more intensive therapy is associated with improved outcomes, two large multicenter randomized trials do not find a benefit with higher doses of therapy. Based on these studies we provide recommendations for the delivered intensity of renal replacement therapy in acute kidney injury.
Acute kidney injury (AKI) is a prevalent and devastating complication in critically ill patients. It has an incidence of between 5 and 45%, depending on the specific definition used, with approximately 4% of intensive care unit (ICU) patients developing AKI severe enough to require renal replacement therapy (RRT) 1-3. The mortality associated with severe AKI remains high, ranging from 40 to 60% since the 1960's despite improvements in dialytic techniques, including the use of bio-compatible membranes, bicarbonate-buffered dialysate and integrated ultrafiltration control for intermittent hemodialysis (IHD) and the increased use of continuous renal replacement therapies since the late 1980's 4-6. Multiple factors have been posited as contributing to the persistently high mortality in patients with AKI. These have included reliance on changes in serum creatinine, which is a lagging marker of kidney function, for diagnosis of AKI; delayed initiation of renal replacement therapy; inadequate dosing of renal replacement therapy; and inability to fully replace kidney function, particularly endocrine, paracrine, metabolic and immunologic functions with current RRT modalities 7-12. Over the past decade, multiple studies have evaluated the relationship between the intensity of RRT and clinical outcomes of acute kidney injury 13-20. In this review, we provide an overview of these studies and summarize current evidence informing clinical practice and future research.
Assessment of RRT Dose
Quantification of the delivery of RRT is most commonly based on the clearance of urea as a surrogate for low-molecular weight uremic toxins 21, 22. The dose of IHD is most commonly quantified based on the urea reduction ratio (URR) or fractional urea clearance per treatment, expressed as Kt/Vurea 21, 23, 24. While urea kinetic models have been extensively validated for maintenance hemodialysis in end-stage renal disease, there are multiple limitations to their use in quantifying the dose of acute IHD as many of the fundamental assumptions underlying these models are violated in the acute setting 21, 25. Among these is the assumption that pre-dialysis volume status and nitrogen balance remain relatively stable over a repetitive cycle of dialysis treatments. Unlike end-stage renal disease patients, critically ill patients with AKI are often hypercatabolic and in negative nitrogen balance 23. In addition, alterations in regional blood flow in hemodynamically unstable patients can result in disequilibrium in urea distribution between body fluid compartments, invalidating standard single pool models 26. Finally, the volume of distribution of urea is altered in AKI, often exceeding total body water, further complicating the applications of urea kinetics to the acute setting 27, 28. Despite these limitations, and in the absence of superior metrics, URR and Kt/Vurea have been satisfactorily applied for dose quantification in critically ill patients undergoing acute dialysis 24, 26. Furthermore, a retrospective analysis of patients with AKI demonstrated that patients with intermediate severity of illness have a survival benefit with a URR >58% (Kt/Vurea >1) per treatment.29
A second consideration for the application of urea kinetics in the acute setting relates to assessing equivalence of treatments provided on different frequencies. Since urea removal during dialysis is proportional to the blood urea concentration, the absolute rate of urea removal is greatest at the start of treatment and declines over its duration. If the same weekly duration of treatment is divided among more frequent treatments (e.g. 2 hours 6 times per week as compared to 4 hours 3 times per week), the effective weekly small solute clearance is increased 30. Thus, the effective weekly dose of therapy cannot be expressed as the arithmetic sum of individual treatments. Although multiple mathematical models have been proposed for equating dialytic therapies provided on variable schedules 31-33, none have been clinically validated, particularly in the acute setting.
The dose of continuous renal replacement therapy (CRRT) is also commonly quantified on the basis of urea kinetics. CRRT solute clearance can be calculated as the ratio between the solute concentration in the effluent and the plasma multiplied by the rate of effluent flow, where the effluent is equal to the ultrafiltrate in continuous hemofiltration, the spent dialysate in continuous hemodialysis, and the sum of both in continuous hemodiafiltration. Although the mechanism of solute transfer varies with convective (hemofiltration) as opposed to diffusive (hemodialysis) modalities, under usual conditions the concentration ratio between effluent and blood for urea and other low-molecular weight solutes is close to unity 34, 35. Thus, small solute clearance is approximately equal to effluent flow allowing the dose of CRRT to be expressed as the effluent volume per unit of time, normalized to body weight. An important caveat is that pre-filter administration of replacement fluid during hemofiltration or hemodiafiltration will dilute the concentration of solutes entering the hemofilter and will decrease clearance by about 15-20% 34. In addition, equilibration between the effluent and blood may decline with time due to clotting and protein deposition fouling the hemofilter membrane 36. Thus more precise quantification of small solute clearance may be achieved by simultaneous measurement of urea in blood and effluent to monitor the decline in equilibration over time. It should be recognized, however, that this degree of monitoring was not included in the clinical trials described below 13, 15, 16, 18, 19. While the concept of URR does not have meaning in CRRT once a steady-state blood urea concentration is attained, the dose of therapy could alternatively be expressed as Kt/Vurea if there were a reliable assessment of the volume of distribution for urea. If this volume is assumed to approximate 60% of body weight, CRRT at a dose of 20 ml/kg per hour would correspond to a Kt/Vurea of approximately 0.8 per day.
Although the paradigm of urea kinetic-based dosing of renal replacement therapy has provided the basis for the majority of the clinical trials of intensity of acute RRT, assessment of adequacy of RRT solely on the basis of urea-kinetics provides an incomplete assessment of the delivered therapy. For example, the urea kinetic paradigm ignores the potential impact of clearance of higher molecular weight solutes, sodium and volume management and duration of treatment on outcomes. It should be recognized that the potential impact of these aspects on the prescription of RRT has not been assessed in the studies described below and remains an important area for future investigation.
Overview of Dosing Trials in AKI
Eight prospective clinical trials have evaluated the dosing of RRT in acute kidney injury (Tables 1 and 2). Seven of these limited their assessments to individual modalities of RRT; five evaluated modalities of CRRT 13, 15-17, 19, one evaluated IHD 14 and one evaluated slow, extended dialysis 20. The remaining study utilized a treatment strategy that allowed patients to convert between modalities of RRT as their hemodynamic status changed while maintaining dose separation 18.
Table 1. Overview of Dosing Trials in Aki.
| Study Design & Location | No. of patients | RRT Modality | Replacement Solution & Administration | Dose calculation & Prescribed dose | ||||
|---|---|---|---|---|---|---|---|---|
| Continuous renal replacement therapy trials | ||||||||
| Ronco et al13 | single center RCT; Italy | 425 | CVVH | Lactate; Post-filter | Effluent flow rate; 20mL/kg/hr vs. 35mL/kg/hr vs. 45 mL/kg/hr | |||
| Bouman et al15 | 2 center RCT; The Netherlands | 106 | CVVH | Bicarbonate; Post-filter | Effluent flow rate; 19 mL/kg/hr vs. 48mL/kg/hr | |||
| Saudan et al16 | single center RCT; Switzerland | 206 | CVVH /CVVH DF | Lactate and bicarbonate; Post-filter | Effluent flow rate; CVVH – 25mL/kg/hr CVVHDF – 42mL/kg/hr | |||
| Tolwani et al17 | single center RCT; USA | 200 | CVVHD F | Bicarbonate; Pre-filter | Effluent flow rate; 20mL/kg/hr vs. 35mL/kg/hr | |||
| Bellomo et al19 | multicenter RCT; Australia, NZ | 1,508 | CVVHD F | Bicarbonate; Post-filter | Effluent volume; 25mL/kg hr vs. 40mL/kg/hr | |||
| Intermittent hemodialysis trial | ||||||||
| Schiffl et al14 | single center Alt Assign,; Germany | 160 | IHD | NA | Kt/Vurea and frequency of IHD; Kt/Vurea of 1.2 per IHD every other day vs daily | |||
| Extended dialysis trial | ||||||||
| Faulhaber-Walter et al20 | multicenter RCT; Germany | 156 | ED | NA | Plasma urea level 120-150 mg/dl vs. <90 mg/dl | |||
| Combination modality trial | ||||||||
| Palevsky et al18 | multicenter RCT; USA | 1,124 | IHD, SLED, CVVHD F | Bicarbonate; Pre-filter | Kt/Vurea and Effluent volume; Kt/Vurea of 1.3 in IHD and SLED – 3 vs. 6 times/wk;Effluent Volume, 20mL/kg/hr vs. 35mL/kg/hr | |||
Alt Assign – alternate assignment; CVVH – continuous venovenous hemofiltration; CVVHDF – continuous venovenous hemodiafiltration; ED – extended dialysis; SLED – sustained low efficiency dialysis; IHD – intermittent hemodialysis; NA -- Not applicable; RCT – randomized controlled trial; All trials controlled for severity of illness using APACHE II or III or SOFA scoring.
Table 2. Primary Outcome Data.
| Study | Primary Outcome | Lower Dose Mortality | Higher Dose Mortality | Risk Ratio |
|---|---|---|---|---|
| Ronco et al(2000) 13 | Mortality at 15 days after stopping CVVH | 59% | 42.5% | 0.72 |
| Bouman et al (2002)15 | Mortality at 28 days after inclusion | 28.1% | 25.7% | 0.91 |
| Saudan et al (2006)16 | Mortality at 28 and 90 days | 61% | 41% | 0.67 |
| Tolwani et al (2008)17 | Mortality at 30 days or survival to ICU discharge | 44% | 51% | 1.16 |
| Bellomo et al (2009)19 | Mortality at 90 days after randomization | 44.7% | 44.7% | 1.00 |
| Schiffl et al (2002)14 | Mortality at 14 days after las hemodialysis | t 46% | 28% | 0.61 |
| Faulhaber-Walter et al (2009)20 | Mortality at 14 days after initiation of dialysis | 29.3% | 29.6% | 1.01 |
| Palevsky et al (2008)18 | Mortality at 60 days after randomization | 51.5% | 53.6% | 1.04 |
|
| ||||
| Summary | 0.89 | |||
Abbreviations: CVVH, continuous veno-venous hemofiltration; ICU, intensive care unit
CRRT
The initial study evaluating intensity of CRRT dosing was conducted by Ronco and colleagues at a single center in Vicenza, Italy 13. In this study, 425 patients undergoing continuous veno-venous hemofiltration (CVVH), with post-filter administration of lactate-buffered replacement fluid, were randomized to three doses of treatment based on prescribed effluent volumes of 20, 35 or 45 ml/kg per hour based, calculated using the patient's weight prior to admission to intensive care. All patients received at least 85% of the prescribed dose, but dosing was increased to compensate for time off of treatment. The patients randomized to receive 20 mL/kg per hour had significantly lower survival (41%), at 15 days after discontinuation of CRRT compared to the groups that received 35 mL/kg per hour (57%) or 45 mL/kg per hour (58%).
In another single-center study, Saudan and colleagues evaluated the impact of augmenting the clearance of small molecules by adding dialysis to the convective clearance of CVVH 16. Two hundred six patients were randomly assigned to CVVH with an effluent volume of 1 – 2.5 L/hr or continuous venovenous hemodiafiltration (CVVHDF) with an additional dialysate flow rate of 1 – 1.5 L/hr. In both groups the ultrafiltration rate was approximately 25 ml/kg per hour (rounded off to the upper 500 ml per hour within the range of 1.0 to 2.5 liters per hour) with lactate- or bicarbonate-buffered replacement fluid administered pre-filter. Patients randomized to CVVHDF received an additional 1.0 to 1.5 liters per hour of dialysate flow, dichotomized based on weight greater than or less than 70 kg. The resultant mean (±SD) ultrafiltration rates were 25±5 ml/kg per hour in the CVVH group and 24±6 ml/kg per hour in the CVVHDF group, while the mean dialysate flow rate was 18±5 ml/kg per hour. The primary endpoint of 28-day survival was significantly higher in the CVVHDF group at 28 days (59% versus 39%, p =0.03) as well as at 90 days (59% versus 34%). There was no difference in the rates of adverse events or recovery of kidney function between the two groups.
In contrast, three other studies evaluating intensity of CRRT have not demonstrated any impact of increased dose of therapy on survival. Bouman and colleagues conducted a small trial at two centers addressing the issues of both dose and timing of initiation of CVVH in critically ill patients 15. One hundred six patients with oliguria and ventilator dependency were randomized to 3 groups: early high volume CVVH (72-96 L/24 hrs); early low volume CVVH (24-36 L/24 hrs); and late low-volume CVVH (24-36 L/24hrs). In the early groups, treatment was started within 12 hours after the patient met all the inclusion criteria, while CVVH was only initiated in the late group if they met conventional criteria for initiation of RRT. Bicarbonate-buffered fluids were administered post-filter as replacement fluid. The mean (±SD) effluent volumes were 52 mL/kg per hour in the early high-volume group versus 20 and 19.0 mL/kg hour in the early and late low-volume groups, respectively. There was no difference in 28-day, ICU or hospital survival among the 3 groups (28 day survival: 74.3% in the early high-volume group; 68.6% in the early low-volume group; and 75.0% in the late low-volume group; p=0.80), and no difference in renal recovery. There was no difference in complications such as bleeding, transfusion requirements and hypothermia.
Tolwani and colleagues conducted a single-center, randomized controlled study evaluating 2 doses of CVVHDF in the United States 17. Two hundred patients were randomized to effluent rates of either 20mL/kg per hour (standard dosage) or 35mL/kg per hour (high dosage) using pre-filter bicarbonate replacement fluid. Patients with chronic kidney disease (CKD) were not excluded. Approximately 74% of patients in the standard dosage arm and 79% in the higher dosage arm received ≥ 80% of the prescribed dose, with the actual delivered dose being 17 mL/kg per hour and 29 mL/kg per hour, respectively. Thirty-day all-cause mortality was 56% in the standard dosage arm as compared to 49% in the high dosage group (p=0.32). Subgroup analyses did not reveal any differences in outcomes associated with sepsis or oliguria. Renal recovery was not statistically different between the groups. Complications of therapy including electrolyte disturbances were not reported.
The Randomized Evaluation of Normal versus Augmented Level (RENAL) Replacement Therapy Study was the largest trial of intensity of RRT in AKI. The RENAL study enrolled 1508 patients in 35 intensive care units in Australia and New Zealand between December 2005 and November 2008 19. The subjects were randomized to CVVHDF at an effluent flow of either 25 mL/kg per hour (lower-intensity) or 40 mL/kg per hour (higher-intensity). All dialysate and replacement fluids were bicarbonate-buffered; replacement fluids were infused post-filter and the ratio of dialysate to replacement fluid was set at 1:1. Study therapy was continued until kidney function recovered or the patient was discharged from the ICU; the mean (±SD) duration of study therapy was 6.3±8.7 days in the higher-intensity arm and 5.9±7.7 days in the lower-intensity arm, although overall duration of RRT including non-study therapy post-ICU discharge was 13.0±20.8 in the higher-intensity arm and 11.5±18.0 in the lower-intensity arm. The prescribed therapy was delivered more than 80% of the time. The primary study outcome of 90-day mortality was 44.7% in both treatment groups (p=0.99). Similar results were observed in pre-specified subgroups including patients with sepsis, with at least one non-renal organ failure, with a Sequential Organ Failure Assessment (SOFA) cardiovascular score of 3 or 4, or with pre-existing chronic kidney disease. The rates of renal recovery were similar in the two groups with only 6.8% of surviving patients in the higher-intensity arm and 4.4% of surviving patients in the lower-intensity arm remaining dialysis-dependent at day 90. Hypophosphatemia was more common in the higher-intensity group, but there was no difference in the incidence of other adverse events such as arrhythmias, disequilibrium and hypokalemia.
Intermittent Hemodialysis
There have been no prospective studies comparing doses of IHD (e.g., Kt/Vurea) on a fixed dialysis schedule in patients with acute kidney injury. A single study has evaluated the impact of increased frequency of IHD treatments with per-treatment dose held constant 14. In a single-center study, Schiffl and colleagues assigned 160 critically ill patients in an alternating fashion to receive IHD on either a daily or an every-other-day schedule. Dialysis was prescribed with a target Kt/Vurea of 1.2 per session; however the actual delivered Kt/Vurea was only 0.94±0.11 in the alternate-day arm and 0.92±0.16 in the daily dialysis arm. Mortality 14-days after the last dialysis session was significantly lower in patients who received daily dialysis as compared to alternate-day dialysis (28% vs. 46%, p=0.01). Recovery of kidney function, defined as dialysis independence, also occurred more rapidly with daily dialysis than with alternate-day dialysis (9±2 days vs. 16±6 days, p=0.001). Given the high rate of uremic complications, including sepsis, gastrointenstinal bleeding and alterations in mental status, observed in the alternate day arm, it has been suggested that this study demonstrated the expected hazard associated with inadequate dosing of therapy rather than a benefit to an augmented dose of therapy37.
Extended dialysis
Extended dialysis or sustained low-efficiency dialysis (SLED) are hybrid modalities that generally utilize conventional intermittent hemodialysis technology to provide a slower, prolonged duration therapy 38. The Hannover dialysis outcome study randomized 156 patient to either a standard regimen of extended dialysis therapy (defined as one treatment session on the initial day followed by daily treatments to maintain the morning serum urea nitrogen level between 56 and 70 mg/dl) or to an intensified strategy of extended dialysis in which patients received two extended dialysis sessions in the first 24 hours and subsequent treatments were provided to maintain the morning serum urea nitrogen concentration less than 42 mL/dl. No other methods were used to calculate dose. Patients in the standard extended dialysis arm received a mean (±SD) of 7.7±8.1 treatments as compared to 13.3±10.2 treatments in the intensified extended dialysis arm (p<0.001), corresponding to mean (±SD) treatment times of 69.2±78.4 hours and 121.3±94.3 hours, respectively. The treatment regimens resulted in achieved serum urea nitrogen values of 53.5±19.0 mg/dL and 31.9±11.5 mg/dL 48 hours after study entry. No differences were observed in survival at day 14 (70.4% in the standard extended dialysis arm vs. 70.7% in the intensified extended dialysis arm, p= 0.97) or at day 28 (61.3% vs. 55.6%, p=0.47) or in attainment of dialysis independence in surviving patients at day 28 (63% versus 60%, p=0.77).
Combined Modalities
All of the above studies focused on single modalities of RRT. While such studies have value for evaluating the potential efficacy of higher-intensity therapy within an individual treatment modality, their study designs do not reflect typical clinical practice, in which patients switch from one modality to another as their clinical status changes over time 39. For example, in the RENAL study, dose-stratification was only maintained while patients remained in the ICU, and patients exited study therapy upon ICU discharge, even if they remained dialysis-dependent 19. Although the mean duration of study therapy in the RENAL study was approximately 6 days, the mean duration of RRT was slightly more than 12 days, with the study protocol providing no guidance regarding intensity of dialysis after ICU discharge.
In contrast, the Veterans Administration/National Institutes of Health Acute Renal Failure Trial Network (ATN) study utilized a strategy that allowed patients to switch between modalities of RRT as their hemodynamic status changed over time 18. In the ATN study 1124 critically ill patients with AKI were randomized to either an intensive strategy or less-intensive strategy. In both treatment arms, RRT was provided as IHD when patients were hemodynamically stable and as either CVVHDF or SLED when hemodynamically unstable, with the choice of modality dictated by institutional practice. In the intensive strategy, IHD and SLED were provided on a six-times per week (daily except Sunday) schedule with a target Kt/Vurea of 1.2-1.4 per treatment and CVVHDF was provided using a 1:1 ratio of bicarbonate-buffered dialysate and pre-filter replacement fluids, with a total effluent flow rate of 35 mL/kg per hour. In the less-intensive arm IHD and SLED were provided on a three-times per week (every-other day expect Sunday) schedule with the same target Kt/Vurea per treatment and CVVHDF was provided with a total effluent flow rate of 20 mL/kg per hour. Utilization of SLED during the study was minimal, accounting for fewer than 3% of study treatments. The median interval between treatments (excluding Sundays) was 1.1 days in the intensive strategy and 2.1 days in the less-intensive strategy. For treatments after the first IHD session, the mean (±SD) delivered Kt/Vurea was 1.32±0.36 per treatment across both treatment arms with the delivered Kt/Vurea exceeding 1.2 in more than two-thirds of all treatments. The mean (±SD) delivered effluent flow rate during CVVHDF was 35.8±6.4 mL/kg per hour in the intensive arm and 22.0±6.1 mL/kg per hour in the less-intensive arm. Sixty-day all-cause mortality was 53.6% in the intensive therapy arm and 51.5% in the less-intensive arm (p=0.47). There were no differences in secondary outcomes of in-hospital mortality and rate of recovery of renal function. Hypophosphatemia and hypokalemia occurred more frequently in the intensive strategy arm. Hypotension during IHD treatments occurred during a similar percentage of IHD treatments in both treatment arms but in a greater percentage of patients in the intensive-therapy arm, reflecting the greater exposure in the intensive-therapy arm. In a post hoc analysis there were no differences in the primary endpoint between treatment arms as a function of the initial modality of RRT or as a function of percentage of study therapy provided as IHD 40.
Meta-Analyses
Two recent meta-analyses have examined the issue of intensity of RRT in AKI 41, 42. Jun and colleagues included only the eight studies discussed above in their analysis 41, while Van Wert and colleagues included an additional four studies, two studies of IHD from before 1990 and two CRRT studies evaluating cytokine clearance. After pooling of data using random-effects models, both meta-analyses found no statistically significant benefit of more intensive RRT with regard to survival or dialysis dependence among survivors. Both analyses observed significant statistical heterogeneity across studies. Jun and colleagues observed that the heterogeneity was associated with year of publication and study quality, with publications prior to 2004 and with Jadad score <3 more likely to demonstrate benefit associated with more intensive therapy 41.
Discussion
The current evidence does not support the hypothesis that intensification of RRT in patients with AKI improves survival. Although more intensive RRT results in greater correction of the uremic milieu, it is possible that any benefit provided by higher achieved clearances is counterbalanced by potential complications of therapy. Intensified therapy was associated with hypophosphatemia, hypokalemia and other electrolyte disturbances. Hypophosphatemia can cause respiratory muscle weakness and delay ventilator weaning, as well as rhabdomyolysis, cardiac dysfunction and impaired phagocytic function with increased risk for infection. Hypokalemia can trigger cardiac arrhythmias, although this was not evident in any of the trials. Augmented doses of therapy may exacerbate the tendency to hypothermia seen during CRRT, which in turn has been associated with a myriad of adverse outcomes. Intensified RRT may also impact nutritional parameters. Amino acid and protein losses during RRT are not insignificant, ranging from 7 to 50 gm/day. Increasing the intensity of renal support can double or triple the amount of amino acid or protein losses 43. Additionally, micronutrient losses will also be increased during more intensive RRT. Finally, increased intensity of RRT will also have the inadvertent consequence of increased drug clearances. Many of the antimicrobial agents are significantly cleared by RRT and with the exception of few drugs (vancomycin, aminoglycosides), availability of drug monitoring is often inadequate. Sub-therapeutic drug levels may adversely affect outcomes in critically ill patients, especially those in whom kidney failure is associated with sepsis.
It has been hypothesized that modulation of pro-inflammatory cytokines in patients with sepsis-associated AKI could be beneficial 44. Small studies have suggested that high volume hemofiltration is beneficial in the systemic inflammatory response syndrome as manifested by reduced vasopressor requirements 45. This benefit was not manifested in either the RENAL or ATN studies, neither of which demonstrated a benefit to intensified therapy in patients with sepsis in prespecified subgroup analyses 18, 19. These findings were confirmed in the meta-analysis by Van Wert an colleagues, in which more-intensive RRT was associated with a relative risk of death of 1.02 (95% CI 0.85-1.23) as compared to less-intensive therapy in the subgroup of patients with sepsis 42.
Volume management in AKI remains a critical issue that may be managed independently of small solute clearance. Fluid resuscitation is an essential component of early management of sepsis, however volume overload has been associated with adverse outcomes including cerebral edema, gut edema, acute lung injury and acute respiratory distress syndrome 46. A recent analysis by the Program to Improve Care in Acute Renal Disease (PICARD) group revealed that 30-day, 60-day and in-hospital mortality was significantly higher in the patients defined as fluid overloaded (>10% fluid accumulation compared to baseline weight), with an odds ratio for mortality of 2.07 (95% CI 1.27-3.37).47 The 2 larger studies, ATN and RENAL, have documented fluid balance differences in the study population. In the RENAL study, the difference in fluid balance between the 2 arms was imperceptible. In the ATN study, the net fluid balance during the first 14 days was positive (1.9L in the high dose vs. 1.7 L in the low dose. P=0.94) in both arms. Thus, it is unlikely that volume status could have had an impact on the outcomes in these trials. There is some concern that hypotension during dialytic therapy can affect renal recovery and increase mortality. Although more patients in the intensive arm of the ATN study sustained hypotensive episodes, the percent of treatments complicated by hypotension was not different in the two treatment arms and is unlikely to have masked a putative benefit in the intensive therapy arm.
An important caveat in evaluating the current literature on intensity of RRT in AKI is the recognition that all studies to date have evaluated intensity of therapy on the basis of urea kinetics. As previously discussed, this approach provides an incomplete assessment of the dose of RRT, especially in the AKI setting. The kinetic profile of small molecules such as urea is different than that of higher molecular weight solutes, whose clearance is much more dependent on treatment duration. An independent effect of time on small solute clearance due to improved clearance from slowly-exchanging compartments has also been described.48 It is plausible that duration of hemodialysis session can play a role in changing outcomes, and this needs to be systematically evaluated.
Summary
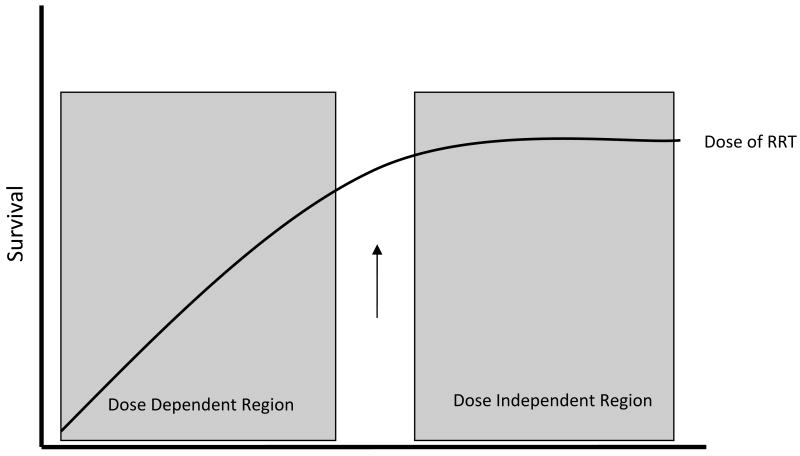
Despite the hope that more intensive RRT would improve outcomes in AKI, recent clinical trials have been unable to demonstrate this benefit (Figure 1). CRRT effluent flow rates greater than 20 to 25mL/kg per hour are not warranted for the majority of patients, although interruptions of therapy must be minimized to ensure delivery of at least 85% of the prescribed treatment. Effluent flow rates may need to be increased if there are frequent or prolonged interruptions of therapy. It must be noted that the floor of the dosing range for CRRT is not well delineated. For IHD, evidence does not support a need to routinely provide dialysis treatments more frequently than every-other day so long as a Kt/Vurea of at least 1.2 per treatment (corresponding to a URR of at least 65% to 70%) can be achieved. By inference from the ATN study experience, a target prescribed Kt/Vurea of 1.4 was required to achieve this delivered dose. The delivery of IHD should be monitored by routine measurement of Kt/Vurea or URR, and physicians should incorporate this quality metric into their daily evaluation of patients with AKI. While recognizing that URR and Kt/Vurea may not be ideal indices in AKI, in the absence of any other validated measure, it remains the best available method of monitoring the dose of intermittent hemodialysis in AKI. The use of urea-based dosing of IHD has been endorsed in clinical practice guidelines developed by the American Thoracic Society49 and in the Kidney Disease: Improving Global Outcomes (KDIGO) AKI clinical practice guidelines.50 Treatment frequency or duration may need to be increased if the target dose of therapy cannot be provided and frequent treatments may be required for volume management and in patients with extreme hypercatabolism. Firm recommendations regarding the dosing of extended dialysis or SLED modalities cannot be provided. Until further studies are performed, recommendations regarding dose must be inferred from studies of IHD and CRRT. Finally, while these dosing recommendations apply to the majority of patients, the RRT prescription must be individualized for each patient and may need to be adjusted to achieve adequate metabolic, electrolyte and fluid homeostasis. While it has been suggested that dynamic adjustment of intensity of therapy over the course of AKI may be more appropriate than the use of a fixed dose of therapy, this hypothesis has never been rigorously tested.
Figure 1.
Conceptual illustration of survival in AKI: Survival increases with increasing doses of RRT, until an inflection point (arrow), after which further increases in dose do not affect outcome. Modified with permission from Palevsky et al51.
Mortality in AKI remains unacceptably high. While initial studies suggested dramatic improvement in survival with intensification of RRT, recent large, multicenter randomized controlled trials have shown this to be a false hope. We must now look to other strategies to improve outcomes. While earlier initiation of therapy and more aggressive volume management have been suggested based on data from observational cohorts, we must insist on rigorous validation of the effectiveness of these approaches. Reliable biomarkers that will facilitate making an early diagnosis of AKI may help in guiding future trials regarding initiation, discontinuation and safety of RRT. In the interim, we should ensure that the RRT that we provide is optimized and that complications of therapy are minimized. Particular attention must be paid to ensure that the dosing of medications, particularly antibiotics, is appropriately adjusted for the dose of RRT provided. Ultimately, we must remain realistic in our expectations of what can be accomplished with dialysis and hemofiltration, and vigorously pursue other treatment strategies.
Acknowledgments
Support: None.
Financial Disclosure: Dr Vijayan is a consultant for Astute Medical and receives research support from Cytopherx, Inc and B.Braun. Dr. Palevsky is a consultant for Sanofi-Aventis and Cytopherx, Inc. and receives research support from Spectral Diagnostics, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Anitha Vijayan, Associate Professor of Medicine, Renal Division, Washington University in St. Louis School of Medicine, St. Louis, MO.
Paul M. Palevsky, Chief, Renal Section, VA Pittsburgh Healthcare System and Professor of Medicine, Renal-Electrolyte Division, University of Pittsburgh School of Medicine, Pittsburgh, PA.
References
- 1.Liano F, Junco E, Pascual J, Madero R, Verde E. The spectrum of acute renal failure in the intensive care unit compared with that seen in other settings. The Madrid Acute Renal Failure Study Group. Kidney Int Suppl. 1998 May;66:S16–24. [PubMed] [Google Scholar]
- 2.Hoste EA, Schurgers M. Epidemiology of acute kidney injury: how big is the problem? Crit Care Med. 2008 Apr;36(4 Suppl):S146–151. doi: 10.1097/CCM.0b013e318168c590. [DOI] [PubMed] [Google Scholar]
- 3.Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. Jama. 2005 Aug 17;294(7):813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 4.Ympa YP, Sakr Y, Reinhart K, Vincent JL. Has mortality from acute renal failure decreased? A systematic review of the literature. Am J Med. 2005 Aug;118(8):827–832. doi: 10.1016/j.amjmed.2005.01.069. [DOI] [PubMed] [Google Scholar]
- 5.Woodrow G, Turney JH. Cause of death in acute renal failure. Nephrol Dial Transplant. 1992;7(3):230–234. doi: 10.1093/oxfordjournals.ndt.a092111. [DOI] [PubMed] [Google Scholar]
- 6.Star RA. Treatment of acute renal failure. Kidney Int. 1998 Dec;54(6):1817–1831. doi: 10.1046/j.1523-1755.1998.00210.x. [DOI] [PubMed] [Google Scholar]
- 7.Coca SG, Parikh CR. Urinary biomarkers for acute kidney injury: perspectives on translation. Clin J Am Soc Nephrol. 2008 Mar;3(2):481–490. doi: 10.2215/CJN.03520807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bagshaw SM, Gibney RT. Ideal determinants for the initiation of renal replacement therapy: timing, metabolic threshold or fluid balance? Acta Clin Belg Suppl. 2007(2):357–361. doi: 10.1179/acb.2007.080. [DOI] [PubMed] [Google Scholar]
- 9.Bagshaw SM, Uchino S, Bellomo R, et al. Timing of renal replacement therapy and clinical outcomes in critically ill patients with severe acute kidney injury. J Crit Care. 2009 Mar;24(1):129–140. doi: 10.1016/j.jcrc.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 10.Paganini EP, Depner T, Wensley D. The Acute Dialysis Quality Initiative--part III: solute control (treatment dose) Adv Ren Replace Ther. 2002 Oct;9(4):260–264. doi: 10.1053/jarr.2002.35569. [DOI] [PubMed] [Google Scholar]
- 11.Davenport A, Bouman C, Kirpalani A, et al. Delivery of renal replacement therapy in acute kidney injury: what are the key issues? Clin J Am Soc Nephrol. 2008 May;3(3):869–875. doi: 10.2215/CJN.04821107. [DOI] [PubMed] [Google Scholar]
- 12.Palevsky PM, Baldwin I, Davenport A, Goldstein S, Paganini E. Renal replacement therapy and the kidney: minimizing the impact of renal replacement therapy on recovery of acute renal failure. Curr Opin Crit Care. 2005 Dec;11(6):548–554. doi: 10.1097/01.ccx.0000179936.21895.a3. [DOI] [PubMed] [Google Scholar]
- 13.Ronco C, Bellomo R, Homel P, et al. Effects of different doses in continuous veno-venous haemofiltration on outcomes of acute renal failure: a prospective randomised trial. Lancet. 2000 Jul 1;356(9223):26–30. doi: 10.1016/S0140-6736(00)02430-2. [DOI] [PubMed] [Google Scholar]
- 14.Schiffl H, Lang SM, Fischer R. Daily hemodialysis and the outcome of acute renal failure. N Engl J Med. 2002 Jan 31;346(5):305–310. doi: 10.1056/NEJMoa010877. [DOI] [PubMed] [Google Scholar]
- 15.Bouman CS, Oudemans-Van Straaten HM, Tijssen JG, Zandstra DF, Kesecioglu J. Effects of early high-volume continuous venovenous hemofiltration on survival and recovery of renal function in intensive care patients with acute renal failure: a prospective, randomized trial. Crit Care Med. 2002 Oct;30(10):2205–2211. doi: 10.1097/00003246-200210000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Saudan P, Niederberger M, De Seigneux S, et al. Adding a dialysis dose to continuous hemofiltration increases survival in patients with acute renal failure. Kidney Int. 2006 Oct;70(7):1312–1317. doi: 10.1038/sj.ki.5001705. [DOI] [PubMed] [Google Scholar]
- 17.Tolwani AJ, Campbell RC, Stofan BS, Lai KR, Oster RA, Wille KM. Standard versus high-dose CVVHDF for ICU-related acute renal failure. J Am Soc Nephrol. 2008 Jun;19(6):1233–1238. doi: 10.1681/ASN.2007111173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palevsky PM, Zhang JH, O'Connor TZ, et al. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008 Jul 3;359(1):7–20. doi: 10.1056/NEJMoa0802639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bellomo R, Cass A, Cole L, et al. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. 2009 Oct 22;361(17):1627–1638. doi: 10.1056/NEJMoa0902413. [DOI] [PubMed] [Google Scholar]
- 20.Faulhaber-Walter R, Hafer C, Jahr N, et al. The Hannover Dialysis Outcome study: comparison of standard versus intensified extended dialysis for treatment of patients with acute kidney injury in the intensive care unit. Nephrol Dial Transplant. 2009 Jul;24(7):2179–2186. doi: 10.1093/ndt/gfp035. [DOI] [PubMed] [Google Scholar]
- 21.Clark WR, Mueller BA, Kraus MA, Macias WL. Renal replacement therapy quantification in acute renal failure. Nephrol Dial Transplant. 1998;13 6:86–90. doi: 10.1093/ndt/13.suppl_6.86. [DOI] [PubMed] [Google Scholar]
- 22.Garred L, Leblanc M, Canaud B. Urea kinetic modeling for CRRT. Am J Kidney Dis. 1997 Nov;30(5 Suppl 4):S2–9. doi: 10.1016/s0272-6386(97)90535-7. [DOI] [PubMed] [Google Scholar]
- 23.Himmelfarb J, Ikizler TA. Quantitating urea removal in patients with acute renal failure: lost art or forgotten science? Semin Dial. 2000 May-Jun;13(3):147–149. doi: 10.1046/j.1525-139x.2000.00039.x. [DOI] [PubMed] [Google Scholar]
- 24.Kanagasundaram NS, Greene T, Larive AB, et al. Prescribing an equilibrated intermittent hemodialysis dose in intensive care unit acute renal failure. Kidney Int. 2003 Dec;64(6):2298–2310. doi: 10.1046/j.1523-1755.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- 25.Clark WR, Mueller BA, Kraus MA, Macias WL. Dialysis prescription and kinetics in acute renal failure. Adv Ren Replace Ther. 1997 Apr;4(2 Suppl 1):64–71. [PubMed] [Google Scholar]
- 26.Kanagasundaram NS, Greene T, Larive AB, Daugirdas JT, Depner TA, Paganini EP. Dosing intermittent haemodialysis in the intensive care unit patient with acute renal failure--estimation of urea removal and evidence for the regional blood flow model. Nephrol Dial Transplant. 2008 Jul;23(7):2286–2298. doi: 10.1093/ndt/gfm938. [DOI] [PubMed] [Google Scholar]
- 27.Himmelfarb J, Evanson J, Hakim RM, Freedman S, Shyr Y, Ikizler TA. Urea volume of distribution exceeds total body water in patients with acute renal failure. Kidney Int. 2002 Jan;61(1):317–323. doi: 10.1046/j.1523-1755.2002.00118.x. [DOI] [PubMed] [Google Scholar]
- 28.Ikizler TA, Sezer MT, Flakoll PJ, et al. Urea space and total body water measurements by stable isotopes in patients with acute renal failure. Kidney Int. 2004 Feb;65(2):725–732. doi: 10.1111/j.1523-1755.2004.00439.x. [DOI] [PubMed] [Google Scholar]
- 29.Paganini E, Tapolyai M, Goormastic M, Halstenberg W, Kozlowski L, Leblanc M, Lee JC, Moreno L, Sakai K. Establishing a dialysis therapy/patient outcome link in intensive care unit acute dialysis for patients with acute renal failure. Am J Kidney Dis. 1996;28:S81–S89. [Google Scholar]
- 30.Gotch FA, Sargent JA, Keen ML. Whither goest Kt/V? Kidney Int Suppl. 2000 Aug;76:S3–18. doi: 10.1046/j.1523-1755.2000.07602.x. [DOI] [PubMed] [Google Scholar]
- 31.Keshaviah PR, Nolph KD, Van Stone JC. The peak concentration hypothesis: a urea kinetic approach to comparing the adequacy of continuous ambulatory peritoneal dialysis (CAPD) and hemodialysis. Perit Dial Int. 1989;9(4):257–260. [PubMed] [Google Scholar]
- 32.Gotch FA. The current place of urea kinetic modelling with respect to different dialysis modalities. Nephrol Dial Transplant. 1998;13 6:10–14. doi: 10.1093/ndt/13.suppl_6.10. [DOI] [PubMed] [Google Scholar]
- 33.Casino FG, Lopez T. The equivalent renal urea clearance: a new parameter to assess dialysis dose. Nephrol Dial Transplant. 1996 Aug;11(8):1574–1581. [PubMed] [Google Scholar]
- 34.Brunet S, Leblanc M, Geadah D, Parent D, Courteau S, Cardinal J. Diffusive and convective solute clearances during continuous renal replacement therapy at various dialysate and ultrafiltration flow rates. Am J Kidney Dis. 1999 Sep;34(3):486–492. doi: 10.1016/s0272-6386(99)70076-4. [DOI] [PubMed] [Google Scholar]
- 35.Troyanov S, Cardinal J, Geadah D, et al. Solute clearances during continuous venovenous haemofiltration at various ultrafiltration flow rates using Multiflow-100 and HF1000 filters. Nephrol Dial Transplant. 2003 May;18(5):961–966. doi: 10.1093/ndt/gfg055. [DOI] [PubMed] [Google Scholar]
- 36.Claure-Del Granado, Macedo E, Chertow GM, et al. Effluent Volume in Continuous Renal Replacement Therapy Overestimates the Delivered Dose of Dialysis. Clin J Am Soc Nephrol. 2011;6(3):467–75. doi: 10.2215/CJN.02500310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonventre JV. Daily hemodialysis--will treatment each day improve the outcome in patients with acute renal failure? N Engl J Med. 2002 Jan 31;346(5):362–364. doi: 10.1056/NEJM200201313460512. [DOI] [PubMed] [Google Scholar]
- 38.Marshall MR, Golper TA, Shaver MJ, Alam MG, Chatoth DK. Sustained low-efficiency dialysis for critically ill patients requiring renal replacement therapy. Kidney Int. 2001 Aug;60(2):777–785. doi: 10.1046/j.1523-1755.2001.060002777.x. [DOI] [PubMed] [Google Scholar]
- 39.Overberger P, Pesacreta M, Palevsky PM. Management of renal replacement therapy in acute kidney injury: a survey of practitioner prescribing practices. Clin J Am Soc Nephrol. 2007 Jul;2(4):623–630. doi: 10.2215/CJN.00780207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palevsky PM, O'Connor TZ, Chertow GM, Crowley ST, Zhang JH, Kellum JA. Intensity of renal replacement therapy in acute kidney injury: perspective from within the Acute Renal Failure Trial Network Study. Crit Care. 2009;13(4):310. doi: 10.1186/cc7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jun M, Heerspink HJ, Ninomiya T, et al. Intensities of renal replacement therapy in acute kidney injury: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2010 Jun;5(6):956–963. doi: 10.2215/CJN.09111209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Wert R, Friedrich JO, Scales DC, Wald R, Adhikari NK. High-dose renal replacement therapy for acute kidney injury: Systematic review and meta analysis. Crit Care Med. 2010 May;38(5):1360–1369. doi: 10.1097/CCM.0b013e3181d9d912. [DOI] [PubMed] [Google Scholar]
- 43.Btaiche IF, Mohammad RA, Alaniz C, Mueller BA. Amino Acid requirements in critically ill patients with acute kidney injury treated with continuous renal replacement therapy. Pharmacotherapy. 2008 May;28(5):600–613. doi: 10.1592/phco.28.5.600. [DOI] [PubMed] [Google Scholar]
- 44.Ronco C, Kellum JA, Bellomo R, House AA. Potential interventions in sepsis-related acute kidney injury. Clin J Am Soc Nephrol. 2008 Mar;3(2):531–544. doi: 10.2215/CJN.03830907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Piccinni P, Dan M, Barbacini S, et al. Early isovolaemic haemofiltration in oliguric patients with septic shock. Intensive Care Med. 2006 Jan;32(1):80–86. doi: 10.1007/s00134-005-2815-x. [DOI] [PubMed] [Google Scholar]
- 46.Bouchard J, Mehta RL. Volume management in continuous renal replacement therapy. Semin Dial. 2009 Mar-Apr;22(2):146–150. doi: 10.1111/j.1525-139X.2009.00561.x. [DOI] [PubMed] [Google Scholar]
- 47.Bouchard J, Soroko SB, Chertow GM, et al. Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int. 2009 Aug;76(4):422–427. doi: 10.1038/ki.2009.159. [DOI] [PubMed] [Google Scholar]
- 48.Eloot S, Van Biesen W, Dhondt A, et al. Impact of hemodialysis duration on the removal of uremic retention solutes. Kidney Int. 2008 Mar;73(6):765–770. doi: 10.1038/sj.ki.5002750. [DOI] [PubMed] [Google Scholar]
- 49.Brochard L, Abroug F, Brenner M, et al. An Official ATS/ERS/ESICM/SCCM/SRLF Statement: Prevention and Management of Acute Renal Failure in the ICU Patient: an international consensus conference in intensive care medicine. American Journal of Respiratory & Critical Care Medicine. 2010 May 15;181(10):1128–1155. doi: 10.1164/rccm.200711-1664ST. [DOI] [PubMed] [Google Scholar]
- 50.KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney International Supplements. 2012 In Press. [Google Scholar]
- 51.Palevsky PM. Intensity of continuous renal replacement therapy in acute kidney injury. Semin Dial. 2009 Mar-Apr;22(2):151–154. doi: 10.1111/j.1525-139X.2008.00543.x. [DOI] [PubMed] [Google Scholar]