Abstract
Purpose
To study the protective effects and underlying molecular mechanisms of SAMC on carbon tetrachloride (CCl4)-induced acute hepatotoxicity in the mouse model.
Methods
Mice were intraperitoneally injected with CCl4 (50 μl/kg; single dose) to induce acute hepatotoxicity with or without a 2-h pre-treatment of SAMC intraperitoneal injection (200 mg/kg; single dose). After 8 h, the blood serum and liver samples of mice were collected and subjected to measurements of histological and molecular parameters of hepatotoxicity.
Results
SAMC reduced CCl4-triggered cellular necrosis and inflammation in the liver under histological analysis. Since co-treatment of SAMC and CCl4 enhanced the expressions of antioxidant enzymes, reduced the nitric oxide (NO)-dependent oxidative stress, and inhibited lipid peroxidation induced by CCl4. SAMC played an essential antioxidative role during CCl4-induced hepatotoxicity. Administration of SAMC also ameliorated hepatic inflammation induced by CCl4 via inhibiting the activity of NF-κB subunits p50 and p65, thus reducing the expressions of pro-inflammatory cytokines, mediators, and chemokines, as well as promoting pro-regenerative factors at both transcriptional and translational levels.
Conclusions
Our results indicate that SAMC mitigates cellular damage, oxidative stress, and inflammation in CCl4-induced acute hepatotoxicity mouse model through regulation of NF-κB. Garlic or garlic derivatives may therefore be a potential food supplement in the prevention of liver damage.
Keywords: Liver injury, S-allylmercaptocysteine, Carbon tetrachloride, Oxidative stress, Necroinflammation, Nuclear factor κB
Introduction
As a potent hepatotoxin, carbon tetrachloride (CCl4) continues to serve as an important substance in medicinal research, particularly in the investigation of hepatotoxin-induced liver diseases [1]. CCl4 activates the 2E1 isoform of cytochrome P450 (CYP2E1) and then forms highly reactive species CCl3* and CCl3OO* which initiates lipid peroxidation. Those reactive oxygen species (ROS) cause oxidative stress in the liver and attack several cellular targets, including endoplasmic reticulum, mitochondrial, and plasma membranes, which contribute to further cell damage. At the molecular level, CCl4 induces the expression of pro-inflammatory cytokines of tumor necrosis factor alpha (TNF-α) and interleukin-1 beta (IL-1β), which mediate the processes of apoptosis, inflammation, and fibrosis. On the other hand, treatment of CCl4 also activates transforming growth factors (TGF-α and TGF-β) which leads to the development of liver fibrosis [2].
Daily consumption of garlic and garlic-related products in several countries, including the United States, decreased the risk of colon and stomach cancer [3]. Previous research also revealed that S-allylmercaptocysteine (SAMC), the naturally occurring water-soluble derivative of garlic, possesses anti-tumor properties in several cancers. In colon cancer cells, SAMC is shown to inhibit cell growth, arrest cell cycle, and induces apoptosis via depolymerizing microtubule, activate JNK1 and caspase-3 [4], as well as inactivating transcription factor NF-κB [5]. In addition, in human gastric cancer cells, the anti-proliferative and pro-apoptosis effects of SAMC are associated with induction of caspase-3, caspase-9, p53, and Bax, rather than Bcl-2 and p21 [6]. In vivo study of androgen-independent prostate cancer model points out that SAMC suppresses metastasis via up-regulating cell adhesion molecules E-cadherin [7]. In rat cells and animal model, SAMC is also shown to play antioxidative effects in vitro and attenuates oxidative and nitrosative stresses in vivo [8]. In an acetaminophen-induced liver injury model, SAMC exerts its protective effects against hepatotoxicity by the inhibition of CYP2E1 enzymatic activity, leading to the suppression of acetaminophen arylation of hepatic proteins [9].
Although some efforts have been made to investigate the protection of SAMC in CCl4-induced liver damage [10], there is a lack of information on the molecular mechanisms of SAMC on such protection, especially on the attenuation of hepatic oxidative stress and inflammation. In the present study, we investigated the detailed molecular mechanisms of the protective role of SAMC in CCl4-induced acute hepatotoxicity, which may shed some light on the prevention of liver damage by taking SAMC as a supplement in the daily diet.
Materials and methods
Reagents
SAMC pure powder was a generous gift from Dr. Patrick M. T. Ling. It was dissolved in solvent buffer (10% L-dextrose and 1% gum Arabic (w/v) in phosphate-buffered saline, pH 4.5). Carbon tetrachloride was purchased from Tianjin Baishi Chemical (Tianjin, China). Murine anti-nitrotyrosine monoclonal antibody was purchased from Zymed (San Francisco, CA). Rabbit anti-CYP2E1 and anti-IκBα polyclonal antibodies were purchased from Millipore (Billerica, MA) and Cell Signaling (Danvers, MA), respectively.
Animals and treatment
Eight- to ten-week healthy male and female C57BL/b6N mice were purchased from the Laboratory Animal Unit (LAU), The University of Hong Kong. Mice were kept under standard conditions 1 week before CCl4 treatment with free access to animal chow and tap water. The animals were divided into four groups (n = 8–10 in each group): (1) control with vehicle administration (PBS or olive oil); (2) CCl4 single-dose treatment (50 μl/kg in olive oil; intraperitoneal injection); (3) SAMC single-dose treatment (200 mg/kg in PBS; intraperitoneal injection); and (4) CCl4 and SAMC co-treatment. The optimum dosage of SAMC used was previously shown to be effective in protecting from oxidative stress in mice [11]. SAMC pre-treatment was performed 2 h before the CCl4 injection. After 8 h of CCl4 treatment, the mice were killed by an overdose of anesthesia according to the protocols approved by the Committee of Animal Use for Research and Teaching at The University of Hong Kong. The Laboratory Animal Unit of the University of Hong Kong is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC international). Blood and liver samples were collected for further analysis.
Tissue and blood samples processing and histopathological analysis
Serum were collected by centrifugation of whole blood sample at 1,000×g for 10 min at 4 °C and stored at −80 °C. Liver tissue samples were fixed in 10% phosphate-buffered formalin, processed for histology, and embedded in paraffin. Five-micrometer tissue sections were stained with hematoxylin and eosin for histological analysis under LEICA Qwin Image Analyser (Leica Microsystems Ltd., Milton Keynes, UK).
Serum alanine aminotransferase assay
The level of hepatic injury can be detected by the increased level of alanine aminotransferase (ALT) in the serum. The ALT assay was performed by procedures described previously [12].
RNA extraction and quantitative reverse-transcription polymerase chain reaction (real-time PCR)
Total RNA was extracted from the liver samples using illustra™ RNAspin mini kit (GE healthcare, UK). The preparation of the first-strand cDNA was conducted following the instruction of the SuperScript™ First-Strand Synthesis System (Invitrogen, Calsbad, CA).
The mRNA expression levels of tumor necrosis factor alpha (TNF-α), interleukin-1 beta (IL-1β), inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), glutathione peroxidase (GPx), catalase (CAT), Cu/Zn SOD, monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-2 (MIP-2), KC (murine IL-8 ortholog), interleukin-6 (IL-6), and transforming growth factor beta1 (TGF-β1) were measured by real-time PCR. The primers used in those real-time PCR reactions are listed in Table 1. The annealing temperature of each reaction has been adjusted to 58 °C. Parallel amplification of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the internal control. Relative quantification was performed using the 2−ΔΔCt method. The relative expression of the specific gene to the internal control was obtained and then expressed as percentage of the control expression in the Figures.
Table 1.
Primer sequences for quantitative PCR
| Target gene | Direction | Sequence |
|---|---|---|
| TNF-α | Forward | 5′-ATGAGCACAGAAAGCATGATC-3′ |
| Reverse | 5′-TACAGGCTTGTCACTCGAATT-3′ | |
| IL-1β | Forward | 5′-CAGGCAGGCAGTATCACTCA-3′ |
| Reverse | 5′-AGGCCACAGGTATTTTGTCG-3′ | |
| iNOS | Forward | 5′-GTGGTGACAAGCACATTTGG-3′ |
| Reverse | 5′-GGCTGGACTTTTCACTCTGC-3′ | |
| COX-2 | Forward | 5′-GGAGAGACTATCAAGATAGTGATC3′ |
| Reverse | 5′-ATGGTCAGTAGACTTTTACAGCTC-3′ | |
| GPx | Forward | 5′-TCCACCGTGTATGCCTTCTCC-3′ |
| Reverse | 5′-CCTGCTGTATCTGCGCACTGGA-3′ | |
| CAT | Forward | 5′-GAGGCAGTGTACTGCAAGTTCC-3′ |
| Reverse | 5′-GGGACAGTTCACAGGTATCTGC-3′ | |
| Cu/Zn SOD | Forward | 5′-CGAGCAGAAGGCAAGC-3′ |
| Reverse | 5′-GCCCAGGTCTCCAACA-3′ | |
| MCP-1 | Forward | 5′-ACCAGCCAACTCTCACTGAAGC-3′ |
| Reverse | 5′-CAGAATTGCTTGAGGTGGTTGTG-3′ | |
| MIP-2 | Forward | 5′-AGTGAACTGCGCTCTCAATG-3′ |
| Reverse | 5′-CTTTGGTTCTTCCGTTGAGG-3′ | |
| KC | Forward | 5′-CTGTCAGTGCCTGCAGACCA-3′ |
| Reverse | 5′-CCAAGGGAGCTTCAGGGTCA-3′ | |
| IL-6 | Forward | 5′-CCGGAGAGGAGACTTCACAG-3′ |
| Reverse | 5′-GGAAATTGGGGTAGGAAGGA-3′ | |
| TGF-β1 | Forward | 5′-CTTCAGCTCCACAGAGAAGAACTGC-3′ |
| Reverse | 5′-CACGATCATGTTGGACAACTGCTCC-3′ | |
| GAPDH | Forward | 5′-CCTTCATTGACCTCAACTACATGGT-3′ |
| Reverse | 5′-TCATTGTCATACCAGGAAATGAGCT-3′ |
Determination of malondialdehyde level
The malondialdehyde (MDA) levels of liver samples were determined using a Bioxytech LPO-586™ kit (Oxis Research, Portland, OR). The reaction product was measured spectrophotometrically at 586 nm. Standard curves were constructed using 1,1,3,3-tetraethoxypropane as a standard. The MDA levels were normalized with corresponding protein amounts determined by Bio-Rad Protein Assay Kit (Bio-Rad, Hercules, CA) and represented as percentage against control level.
Western blotting
Protocols for cytosolic and nuclear protein extraction were previously described [12]. Protein was diluted and mixed with 2× sample buffer (0.1 M Tris–HCl, pH 6.8, 20% glycerol, 4% sodium dodecyl sulfate, 0.2% bromophenol blue, and 5.25% β-mercaptoethanol). The mixture was denatured at 95 °C for 5 min and followed by electrophoresis in a 10% polyacrylamide gel. The protein was then transferred to an Immun-Blot™ PVDF Membrane (Bio-Rad) in a TE series transfer electrophoresis unit (Hoefer Inc., Holliston, MA). The membrane was then incubated in blocking buffer (5% nonfat milk powder in TBST) for 1 h followed by incubation with primary antibodies (1:1,000 dilution for nitrotyrosine and IκBα; 1:2,000 dilution for CYP2E1) in TBST (100 mM Tris–HCl, pH 7.5, 0.9% NaCl, and 0.1% Tween 20) overnight at 4 °C with gentle agitation. On the following day, the membrane was washed with TBST and incubated with appropriate secondary antibodies (1:2,000 dilution in TBST) for 2 h at room temperature. Beta actin (Monoclonal; 1:5,000; Sigma, St. Louis, MO) was used as the internal control. After washing off the unbound antibody with TBST, the expression of the antibody-linked protein was determined by an ECL™ Western Blotting Detection Reagents (GE Healthcare). The optical density of the bands was measured and quantified by ImageJ software. The ratio of the optical density of the protein product to the internal control was obtained and was expressed as percentage of the control expression in the Figures.
Enzyme-linked immunosorbent assay measurement
Enzyme-linked immunosorbent assay (ELISA) measurements of IL-1β, KC, IL-6, and TGF-β1 protein expression levels were performed using ELISA development kits from PeproTech (PeproTech Inc., Rocky Hill, NJ). ELISA of COX-2 was conducted using kit purchased from EIAab (Wuhan EIAab Science, Wuhan, China).
Determination of DNA-binding activity of nuclear factor-κB
The assay on the DNA-binding activity of nuclear factor-κB (NF-κB) was performed by electrophoretic mobility shift assay (EMSA) using the Gel Shift Assay Systems from Promega (Promega, Madison, WI). The assay was carried out following the protocol described previously [13]. Briefly, the phosphorylated and purified consensus NF-κB oligonucleotides (Promega) were mixed with 24 μg of nuclear protein extract and 10× gel shift binding buffer (200 mm Tris–HCl, pH 7.8, 1 m NaCl, 50 mm MgCl2, 10 mm EDTA, and 50 mm dithiothreitol). The mixture was incubated at room temperature for 20 min prior to electrophoresis on a 4% non-denaturing polyacrylamide gel. Signals on exposed X-ray films were quantified using laser scanning densitometry. Specificity of NF-κB binding was confirmed by competition assays and the ability of a specific antibody to supershift protein–DNA complexes. In the competition assay, the addition of 100-fold excess of unlabeled competitor consensus oligonucleotide prevented binding. Supershift experiments confirmed the presence of the p50 and p65 subunits in the binding complexes.
Statistical analysis
Data from each group were expressed as means ± SEM. Statistical comparison between groups was made using the Kruskal–Wallis test followed by Dunns post hoc test to compare all groups. A p < 0.05 was considered to be statistically significant (Prism 5.0, Graphpad software, Inc., San Diego, CA, USA).
Results
CCl4 treatment induced hepatocyte necrosis and inflammation surrounding the centrilobular veins of the liver which were obviously attenuated by the pre-treatment of SAMC (Fig. 1a–d). It should be noted that SAMC injection alone did not show any histological change in the liver (Fig. 1c). Serum ALT level of CCl4-treated mice was approximately 3.5-fold higher than that of the control group. Pre-treatment of SAMC also significantly reduced the expression of serum ALT induced by CCl4 to the control-like level. SAMC treatment alone did not change the baseline level of serum ALT (Fig. 1e; p < 0.05). Previous studies have shown that the isoform 2E1 of cytochrome P450 (CYP2E1) is transiently down-regulated in the early stage of CCl4 administration as a self-defense mechanism [14]. In the present study, protein expression of CYP2E1 was also reduced after CCl4 treatment to approximately 50% of that in the control group. Treatment of SAMC alone also led to a significant decrease in CYP2E1 expression which was around 70% of the control level. Levels were further reduced in SAMC and CCl4 co-treatment mice when compared with the control group (Fig. 2a; p < 0.05).
Fig. 1.
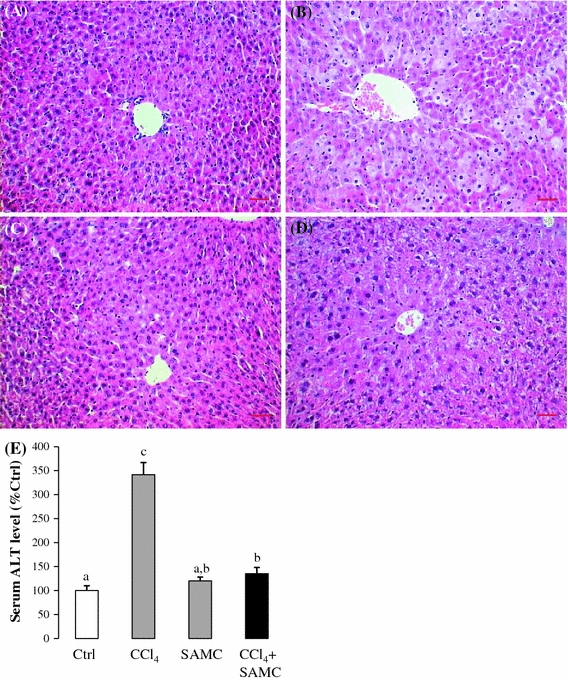
Protective effects of SAMC against CCl4-induced hepatotoxicity. H&E staining on a control, b CCl4 treatment, c SAMC treatment, and d CCl4 + SAMC groups. CCl4 treatment caused obvious centrilobular necrosis in the liver. However, no significant necrosis and inflammation are observed in control, SAMC treatment, and CCl4 + SAMC groups. e CCl4 exposure induced an increase in serum ALT level which was reversed by the pre-treatment of SAMC. Data presented are expressed as Mean ± SEM (n = 8–10) and experimental groups marked by different letters represented significant differences between groups at p < 0.05 (Kruskal–Wallis test followed by Dunns post hoc test). Bar equals 20 microns
Fig. 2.
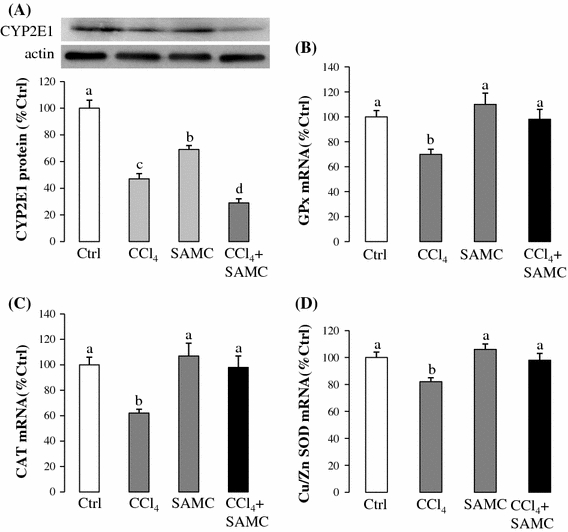
Antioxidative properties of SAMC. Expression levels of a CYP2E1 protein, b GPx mRNA, c CAT mRNA, and d Cu/Zn SOD mRNA were measured in mice liver after CCl4 with or without pre-treatment of SAMC. GAPDH and β-actin were used as internal controls for target gene’s mRNA and protein expression, respectively. Data presented are expressed as Mean ± SEM (n = 8–10) and experimental groups marked by different letters represented significant differences between groups at p < 0.05 (Kruskal–Wallis test followed by Dunns post hoc test)
The mRNA levels of antioxidant enzymes GPx, CAT, and Cu/Zn SOD were significantly reduced in CCl4 treatment group when compared with the control group. The mice pre-treated with SAMC followed by CCl4 injection exhibited similar expression levels of these antioxidant enzymes as the control group (Fig. 2b–d).
To investigate the effect of SAMC on hepatic lipid peroxidation, levels of hepatic malondialdehyde (MDA) were measured after CCl4 with or without SAMC pre-treatment. When compared with the control group, mice treated with CCl4 alone showed approximately 3.5-fold increases in the MDA level. However, pre-treatment with SAMC significantly reduced the MDA level (Fig. 3a). Although SAMC treatment alone also slightly increased the MDA level, the ameliorative effect of SAMC on hepatic lipid peroxidation is obvious.
Fig. 3.
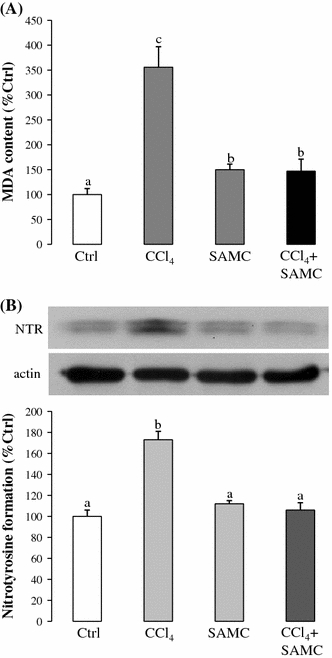
Ameliorative properties of SAMC on lipid peroxidation and NO metabolism. Formation of a MDA and b nitrotyrosine was measured in mice liver after CCl4 with or without pre-treatment of SAMC. β-actin was used as internal control for the formation of nitrotyrosine. Data presented are expressed as Mean ± SEM (n = 8–10) and experimental groups marked by different letters represented significant differences between groups at p < 0.05 (Kruskal–Wallis test followed by Dunns post hoc test)
As an indicator of cellular oxidative stress and inflammation, the formation of nitrotyrosine was highly elevated (around 1.7-fold than control level) after CCl4 treatment. However, when compared with the CCl4 group, mice pre-treated with SAMC followed by CCl4 showed a significant reduction in nitrotyrosine formation, which was comparable with the control group (Fig. 3b).
After CCl4 treatment, the mRNA expressions of pro-inflammatory factors TNF-α, iNOS, IL-1β, and COX-2 were increased by approximately 3.4, 1.6, 1.7, and 4.1-fold, respectively, when compared with the control group. In the pre-treatment with SAMC followed by CCl4 group, the mRNA expression levels of TNF-α, iNOS, and IL-1β were markedly decreased to the basal levels (Fig. 4a–c). Pre-treatment with SAMC followed by CCl4 also markedly reduced the COX-2 mRNA expression when compared with the CCl4 group, although to a level not as low as the control group (Fig. 4e). Protein expression levels of IL-1β and COX-2 confirmed the trends observed in mRNA results (Fig. 4d, f).
Fig. 4.
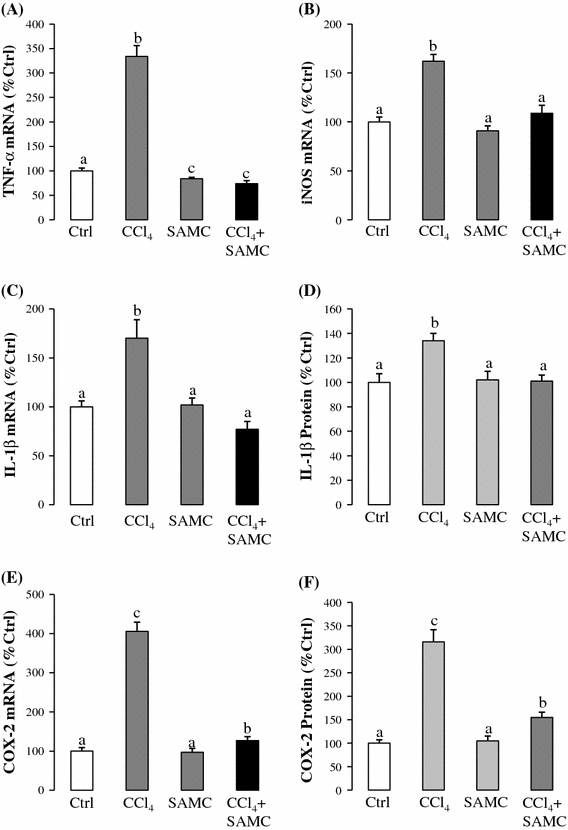
Anti-inflammatory properties of SAMC. Expression levels of a TNF-α mRNA, b iNOS mRNA, c IL-1β mRNA, d IL-1β protein, e COX-2 mRNA, and f COX-2 protein were measured in mice liver after CCl4 with or without pre-treatment of SAMC. GAPDH was used as internal controls for target gene’s mRNA expression. Data presented are expressed as Mean ± SEM (n = 8–10) and experimental groups marked by different letters represented significant differences between groups at p < 0.05 (Kruskal–Wallis test followed by Dunns post hoc test)
Since the expressions of chemokines in the liver were up-regulated by cytokines, oxidative products, and growth factors during inflammation, the change in expression level and distribution becomes an indicative parameter of hepatic inflammation and oxidation [15]. After CCl4 challenge, mRNA expressions of monocyte chemoattractant protein-1 (MCP-1, CCL2), macrophage inflammatory protein-2 (MIP-2, CXCL3), and murine KC (IL-8 ortholog, CXCL1) increased by 8, 4.5, and 1.7-fold, respectively, when compared with the control group, but significantly reduced in the SAMC and CCl4 treatment group (Fig. 5a–c). ELISA assay of KC protein expression showed similar patterns corresponding to its mRNA expression, further confirming the effect of SAMC on chemokine expressions (Fig. 5d).
Fig. 5.
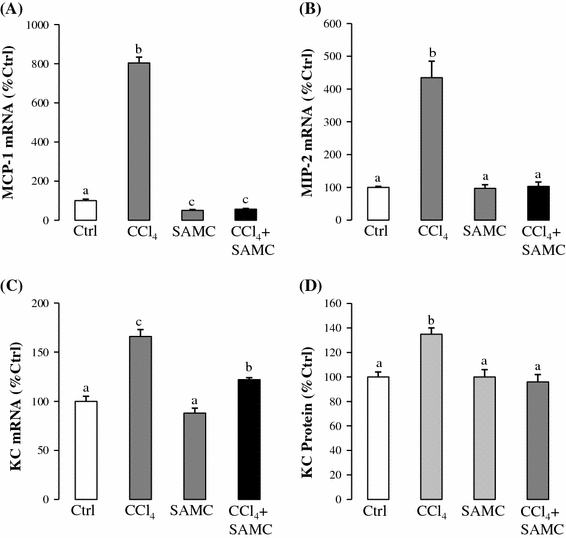
Attenuation of chemokine expression by SAMC. Expression levels of a MCP-1 mRNA, b MIP-2 mRNA, c KC mRNA, and d KC protein were measured in mice liver after CCl4 with or without pre-treatment of SAMC. GAPDH was used as internal controls for target gene’s mRNA expression. Data presented are expressed as Mean ± SEM (n = 8–10) and experimental groups marked by different letters represented significant differences between groups at p < 0.05 (Kruskal–Wallis test followed by Dunns post hoc test)
To investigate the effect of SAMC on hepatic regeneration, we measured the expressions of IL-6 and TGF-β1, which are key genes involved in the processes of regeneration, anti-inflammation, and fibrosis. CCl4 challenge significantly up-regulated the expression of these two genes, and the group treated with SAMC and CCl4 potentiated such elevated expression (Fig. 6a, c). Protein expression of IL-6 confirmed the trend of its mRNA expression (Fig. 6b).
Fig. 6.
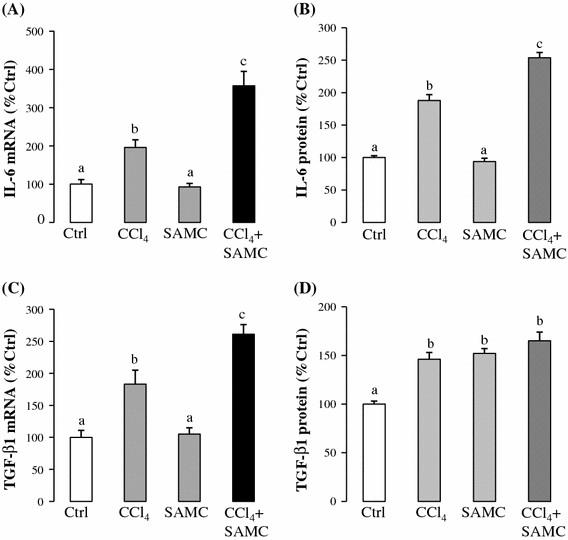
Pro-regenerative effect of SAMC. Expression levels of a IL-6 mRNA, b IL-6 protein, c TGF-β1 mRNA, and d TGF-β1 protein were measured in mice liver after CCl4 with or without co-treatment of SAMC. GAPDH was used as internal controls for target gene’s mRNA expression. Data presented are expressed as Mean ± SEM (n = 8–10) and experimental groups marked by different letters represented significant differences between groups at p < 0.05 (Kruskal–Wallis test followed by Dunns post hoc test)
Acute CCl4 treatment markedly up-regulated the activity level of NF-κB to approximately 2.5-fold of the control. Pre-treatment with SAMC followed by CCl4 showed a significant reduction in the activity of NF-κB when compared with the control group. Moreover, cytosolic content of IκBα was significantly decreased by CCl4 treatment but restored by the pre-treatment of SAMC (Fig. 7a). Supershift assay of p50 and p65 antibodies showed a lighter intensity and a slight upward retarded shift of the NF-κB band confirming the specificity of the reactions in the gel shift assay (Fig. 7b).
Fig. 7.
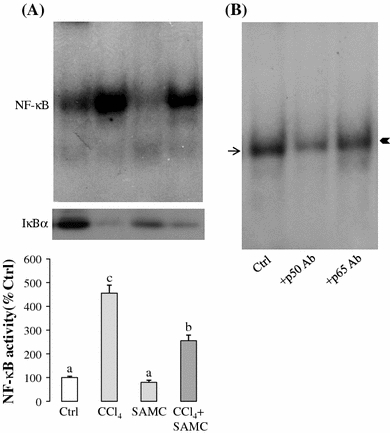
DNA-binding activity of NF-κB modulated by CCl4 and SAMC. a Gel shift assay. In CCl4-treated group, the activity level of NF-κB (p50 and p65) was significantly increased. Treatment with SAMC markedly reduced the activity level without disturbing the basal activity. In cytoplasm, CCl4 treatment stimulated the degradation of IκBα. Pre-treatment of SAMC partially restored the expression of IκBα. b Supershift assay conducted with monoclonal p50 and p65 antibodies to test the involvement of these subunits in the gel shift assay. Arrow indicates DNA–NF-κB complex. Arrowhead indicates shifted DNA–NF-κB band. Data presented are expressed as Mean ± SEM (n = 8–10) and experimental groups marked by different letters represented significant differences between groups at p < 0.05 (Kruskal–Wallis test followed by Dunns post hoc test)
Discussion
CCl4 was proven to be a useful experimental model for hepatotoxicity studies, although banned for industrial and home use as cleaner and solvent [16]. A huge number of natural products, mainly originating from traditional Asian medicine, have been tested recently for the protective effects against CCl4-induced toxicity such as vitamin E [17], green tea extracts [18], ginsan [19], coffee [20], and even mushroom [21]. Because of the similarities in the mechanism of actions, these protective agents also have the potential to be used in the treatment of AFLD (alcoholic fatty liver disease), NAFLD (non-alcoholic fatty liver disease), and cirrhosis [22, 23]. Sumioka et al. showed that in overdose acetaminophen-induced liver injury model, SAMC protected liver from drug-induced damage by inhibiting CYP2E1 and inducing a biomarker of acetaminophen arylation of liver protein [9]. In the present study, we demonstrated that administration of SAMC protected CCl4-induced acute liver injury through different mechanisms, including the inhibition of CYP2E1, down-regulation of inflammatory mediators and nuclear transcription factor, restoration of intracellular antioxidant enzymes, and promotion of liver regeneration, which added to the novel mechanistic aspects of the known beneficial properties of SAMC.
As an indicative enzyme, ALT is normally contained within liver cells. After cellular damage, the enzyme is released into the bloodstream and raising the blood ALT enzyme level, suggesting the presence of liver damage [24]. In the present study, CCl4 treatment dramatically increased the serum ALT level, induced hepatic centrilobular necrosis, and increased the number of inflammatory cells surrounding the centrilobular veins. We found that pre-treatment with SAMC significantly decreased the serum ALT biomarker as well as ameliorated hepatocellular injury against CCl4 without affecting the healthy cells (Fig. 1).
Through the actions of the cytochrome P450 oxygenase system within the endoplasmic reticulum, mainly the isozyme of CYP2E1, the CCl3* radical is formed by the metabolism of CCl4. In the presence of oxygen/hydrogen, CCl3* may convert to CCl3OO*/chloroform to attack many targets including DNA, amino acid, and proteins. CCl3OO* can also abstract a hydrogen to form polyunsaturated fatty acid (PUFA), initiating lipid peroxidation [2]. In a previous study, it was shown that the expression of CYP2E1 was down-regulated after CCl4 intoxication as an adaptive mechanism to limit hepatotoxicity. In addition, restoration of CYP2E1 expression or stabilization of this enzyme made CCl4-induced injury more severe [14]. This finding was further confirmed by a protective assay of ginsan, which is an antioxidant compound from plant, in CCl4-induced hepatic injury model [19]. In this study, SAMC potentiated the down-regulation of CYP2E1 expression induced by CCl4. Moreover, SAMC further demonstrated its protective properties by attenuating CCl4-induced oxidative stress through the reduction in nitrotyrosine formation and MDA, the end-products of nitric oxide (NO) and lipid peroxidation, respectively (Fig. 3). In the present study, we further investigated the expression of several hepatic antioxidant enzymes after CCl4 challenge. CCl4 treatment alone significantly decreased the mRNA expressions of GPx, CAT, and Cu/Zn SOD, whereas pre-treatment with SAMC followed by CCl4 had the expressions of those enzymes similar to baseline levels (Fig. 2). Since Cu/Zn SOD is the main antioxidant which dismutate
To further study the mechanism of the protective roles of SAMC, we measured the expressions of inflammatory mediators after CCl4 treatment. It has been reported that hepatic inflammatory responses are initiated as early as 30 min after CCl4 exposure [2]. Pro-inflammatory cytokines, TNF-α, and IL-1β are released from Kupffer cells, the resident macrophages in the liver [26]. When cellular RNA and protein synthesis are repressed by CCl4 exposure, hepatocytes becomes sensitized to TNF-α, which induces the activity of transcription factors NF-κB, AP-1, and STAT-3 [27]. Normally, TNF-α suppresses hepatocellular apoptosis or even to affect recovery through its conjugate receptor TNFR1 and another family member of interleukin, IL-6 [28]. However, when CCl4 inhibits cellular protein synthesis, this cytokine becomes a pro-apoptotic factor which potentiates the apoptotic signaling cascade from ROS-damaged mitochondria [29]. Similar to TNF-α, when associated with oxidative stress induced by CCl4, IL-1β also triggers cellular necrosis and apoptosis pathways by changing the balance of ligand and receptor [30, 31]. Moreover, elevated expression of TNF-α and IL-1β also stimulates the production of NO, a highly reactive oxidant molecule produced by both parenchymal and non-parenchymal liver cells via iNOS [32]. In the current study, SAMC greatly reduced the expression levels of pro-inflammatory mediators induced by CCl4 exposure at both transcriptional and translational level, confirming its protective role against CCl4. Since pre-treatment with SAMC also decreased the protein level of NO metabolic product nitrotyrosine, it could be suggested that the inhibition of pro-inflammatory cytokines and the restoration of antioxidant enzymes during SAMC pre-treatment followed by CCl4 intoxication not only prevented cellular necrosis and apoptosis, but also repressed the positive-feedback loop between inflammation, lipid peroxidation, and oxidative stress.
Up-regulation of chemokines in the liver is a part of the inflammation response to haloalkane intoxication, e.g., CCl4, via controlling migration and recruitment of both the inflammatory Kupffer cells and the non-inflammatory stellate cells [15]. Since MCP-1 [33], MIP-2 [34], and KC (mouse IL-8 otholog) [35] are induced by cytokines (e.g., TNF-α) in the inflammatory microenvironment, we tested the effects of SAMC pre-treatment on their expressions after CCl4 exposure. Both mRNA and protein levels of these chemokines were significantly down-regulated by the pre-treatment of SAMC followed by CCl4, which demonstrated that the anti-inflammation property of SAMC was involved in the regulation of both the inflammatory factors and chemokines.
As an acute phase response factor, IL-6 antagonizes TNFα-induced inflammation and stabilizes cell membranes after CCl4 treatment, protecting cells from oxidative stress [27]. IL-6 also binds to both membrane-bound IL-6 receptor and soluble IL-6 receptor to trigger NF-κB- and STAT3-dependent hepatic regeneration [36, 37]. TGF-β1 is mainly a pro-fibrotic factor in chronic liver disease, e.g., fatty liver disease. However, a recent study also found its role in promoting hepatic remodeling during the process of liver regeneration through increasing the expression of a cell adhesion molecule known as gicerin [38]. In the present study, SAMC potentiated the induced IL-6 and TGF-β1 expressions after CCl4 treatment, suggesting the pro-regenerative and the anti-inflammatory properties in SAMC during the early phase of CCl4 intoxication. This finding is consistent with other reported studies using different antioxidants [19].
When an intermediate amount of ROS is generated by CCl4 exposure, transcription factors (e.g., NF-κB and AP-1) are activated and translocated into the nucleus. NF-κB and AP-1 then promote the transcription of more inflammatory cytokines and chemokines which form a positive-feedback loop to potentiate the inflammatory response [39, 40]. Moreover, H2O2 and NO formed during CCl4 intoxication also directly modulate NF-κB activation via an IKK-dependent degradation of IκBα pathway, making this transcription factor a central regulatory molecule in regulating CCl4-induced oxidation and inflammation [41, 42]. In the current study, administration of SAMC prior to CCl4 challenge significantly reduced the NF-κB activity through the degradation of cytosolic IκBα. Thus, SAMC may ameliorate both oxidative stress and inflammatory signaling through regulation in the activity of the transcription factor NF-κB.
In conclusion, this study showed that the administration of garlic-derived SAMC was effective in ameliorating CCl4-induced hepatotoxicity through the regulation of oxidative stress, inflammatory signaling, and liver regeneration in association with the reduction in the activity of the transcription factor NF-κB. Since AFLD, NAFLD, and other xenobiotics share a similar mechanism in liver damage with the CCl4 intoxication model, the hepatoprotective properties of SAMC are of significant clinical application in the prevention and treatment of chronic liver diseases. Compared with other antioxidant agents reported recently, garlic is easier to purchase and in an abundant supply, so it could be used as a daily food supplement which may prevent the onset of liver injury.
Acknowledgments
We would like to thank Ms. Carman Leung for her technical help in this project. This study is partly supported by Small Project Funding, University Research Committee, The University of Hong Kong and General Research Fund, University Grant Council, Hong Kong SAR.
Conflict of interest
None.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
J. Xiao, E. C. Liong, G. L. Tipoe contributed equally to this work.
References
- 1.Muriel P. Nitric oxide protection of rat liver from lipid peroxidation, collagen accumulation, and liver damage induced by carbon tetrachloride. Biochem Pharmacol. 1998;56:773–779. doi: 10.1016/S0006-2952(98)00220-2. [DOI] [PubMed] [Google Scholar]
- 2.Weber LW, Boll M, Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol. 2003;33:105–136. doi: 10.1080/713611034. [DOI] [PubMed] [Google Scholar]
- 3.Fleischauer AT, Arab L. Garlic and cancer: a critical review of the epidemiologic literature. J Nutr. 2001;131:1032S–1040S. doi: 10.1093/jn/131.3.1032S. [DOI] [PubMed] [Google Scholar]
- 4.Xiao D, Pinto JT, Soh JW, Deguchi A, Gundersen GG, Palazzo AF, Yoon JT, Shirin H, Weinstein IB. Induction of apoptosis by the garlic-derived compound S-allylmercaptocysteine (SAMC) is associated with microtubule depolymerization and c-Jun NH(2)-terminal kinase 1 activation. Cancer Res. 2003;63:6825–6837. [PubMed] [Google Scholar]
- 5.Ban JO, Yuk DY, Woo KS, Kim TM, Lee US, Jeong HS, Kim DJ, Chung YB, Hwang BY, Oh KW, Hong JT. Inhibition of cell growth and induction of apoptosis via inactivation of NF-kappaB by a sulfur compound isolated from garlic in human colon cancer cells. J Pharmacol Sci. 2007;104:374–383. doi: 10.1254/jphs.FP0070789. [DOI] [PubMed] [Google Scholar]
- 6.Lee Y. Induction of apoptosis by S-allylmercapto-l-cysteine, a biotransformed garlic derivative, on a human gastric cancer cell line. Int J Mol Med. 2008;21:765–770. [PubMed] [Google Scholar]
- 7.Howard EW, Ling MT, Chua CW, Cheung HW, Wang X, Wong YC. Garlic-derived S-allylmercaptocysteine is a novel in vivo antimetastatic agent for androgen-independent prostate cancer. Clin Cancer Res. 2007;13:1847–1856. doi: 10.1158/1078-0432.CCR-06-2074. [DOI] [PubMed] [Google Scholar]
- 8.Pedraza-Chaverri J, Barrera D, Maldonado PD, Chirino YI, Macias-Ruvalcaba NA, Medina-Campos ON, Castro L, Salcedo MI, Hernandez-Pando R. S-allylmercaptocysteine scavenges hydroxyl radical and singlet oxygen in vitro and attenuates gentamicin-induced oxidative and nitrosative stress and renal damage in vivo. BMC Clin Pharmacol. 2004;4:5. doi: 10.1186/1472-6904-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sumioka I, Matsura T, Yamada K. Therapeutic effect of S-allylmercaptocysteine on acetaminophen-induced liver injury in mice. Eur J Pharmacol. 2001;433:177–185. doi: 10.1016/S0014-2999(01)01503-5. [DOI] [PubMed] [Google Scholar]
- 10.Nakagawat S, Kasuga S, Matsuura H. Prevention of liver damage by aged garlic extract and its components in mice. Phytother Res. 1989;3:50–53. doi: 10.1002/ptr.2650030203. [DOI] [Google Scholar]
- 11.Sumioka I, Matsura T, Kasuga S, Itakura Y, Yamada K. Mechanisms of protection by S-allylmercaptocysteine against acetaminophen-induced liver injury in mice. Jpn J Pharmacol. 1998;78:199–207. doi: 10.1254/jjp.78.199. [DOI] [PubMed] [Google Scholar]
- 12.Tipoe GL, Leung TM, Hung MW, Fung ML. Green tea polyphenols as an anti-oxidant and anti-inflammatory agent for cardiovascular protection. Cardiovasc Hematol Disord Drug Target. 2007;7:135–144. doi: 10.2174/187152907780830905. [DOI] [PubMed] [Google Scholar]
- 13.Leung TM, Tipoe GL, Liong EC, Lau TY, Fung ML, Nanji AA. Endothelial nitric oxide synthase is a critical factor in experimental liver fibrosis. Int J Exp Pathol. 2008;89:241–250. doi: 10.1111/j.1365-2613.2008.00590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taieb D, Malicet C, Garcia S, Rocchi P, Arnaud C, Dagorn JC, Iovanna JL, Vasseur S. Inactivation of stress protein p8 increases murine carbon tetrachloride hepatotoxicity via preserved CYP2E1 activity. Hepatology. 2005;42:176–182. doi: 10.1002/hep.20759. [DOI] [PubMed] [Google Scholar]
- 15.Marra F. Chemokines in liver inflammation and fibrosis. Front Biosci. 2002;7:d1899–d1914. doi: 10.2741/marra. [DOI] [PubMed] [Google Scholar]
- 16.Slater TF, Cheeseman KH, Ingold KU. Carbon tetrachloride toxicity as a model for studying free-radical mediated liver injury. Philos Trans R Soc Lond B Biol Sci. 1985;311:633–645. doi: 10.1098/rstb.1985.0169. [DOI] [PubMed] [Google Scholar]
- 17.Shaker ME, Houssen ME, Abo-Hashem EM, Ibrahim TM. Comparison of vitamin E, L-carnitine and melatonin in ameliorating carbon tetrachloride and diabetes induced hepatic oxidative stress. J Physiol Biochem. 2009;65:225–233. doi: 10.1007/BF03180575. [DOI] [PubMed] [Google Scholar]
- 18.Tipoe GL, Leung TM, Liong EC, Lau TY, Fung ML, Nanji AA. Epigallocatechin-3-gallate (EGCG) reduces liver inflammation, oxidative stress and fibrosis in carbon tetrachloride (CCl4)-induced liver injury in mice. Toxicology. 2010;273:45–52. doi: 10.1016/j.tox.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 19.Shim JY, Kim MH, Kim HD, Ahn JY, Yun YS, Song JY. Protective action of the immunomodulator ginsan against carbon tetrachloride-induced liver injury via control of oxidative stress and the inflammatory response. Toxicol Appl Pharmacol. 2010;242:318–325. doi: 10.1016/j.taap.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 20.Shi H, Dong L, Zhang Y, Bai Y, Zhao J, Zhang L. Protective effect of a coffee preparation (Nescafe pure) against carbon tetrachloride-induced liver fibrosis in rats. Clin Nutr. 2010;29:399–405. doi: 10.1016/j.clnu.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Popovic M, Vukmirovic S, Stilinovic N, Capo I, Jakovljevic V. Anti-oxidative activity of an aqueous suspension of commercial preparation of the mushroom Coprinus comatus. Molecules. 2010;15:4564–4571. doi: 10.3390/molecules15074564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larrey D. Drug-induced liver diseases. J Hepatol. 2000;32:77–88. doi: 10.1016/S0168-8278(00)80417-1. [DOI] [PubMed] [Google Scholar]
- 23.Lieber CS. Alcoholic liver disease: new insights in pathogenesis lead to new treatments. J Hepatol. 2000;32:113–128. doi: 10.1016/S0168-8278(00)80420-1. [DOI] [PubMed] [Google Scholar]
- 24.Ozer J, Ratner M, Shaw M, Bailey W, Schomaker S. The current state of serum biomarkers of hepatotoxicity. Toxicology. 2008;245:194–205. doi: 10.1016/j.tox.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 25.Rhee SG, Chang TS, Bae YS, Lee SR, Kang SW. Cellular regulation by hydrogen peroxide. J Am Soc Nephrol. 2003;14:S211–S215. doi: 10.1097/01.ASN.0000077404.45564.7E. [DOI] [PubMed] [Google Scholar]
- 26.Edwards MJ, Keller BJ, Kauffman FC, Thurman RG. The involvement of Kupffer cells in carbon tetrachloride toxicity. Toxicol Appl Pharmacol. 1993;119:275–279. doi: 10.1006/taap.1993.1069. [DOI] [PubMed] [Google Scholar]
- 27.Streetz KL, Wustefeld T, Klein C, Manns MP, Trautwein C. Mediators of inflammation and acute phase response in the liver. Cell Mol Biol (Noisy-le-grand) 2001;47:661–673. [PubMed] [Google Scholar]
- 28.Smart DE, Vincent KJ, Arthur MJ, Eickelberg O, Castellazzi M, Mann J, Mann DA. JunD regulates transcription of the tissue inhibitor of metalloproteinases-1 and interleukin-6 genes in activated hepatic stellate cells. J Biol Chem. 2001;276:24414–24421. doi: 10.1074/jbc.M101840200. [DOI] [PubMed] [Google Scholar]
- 29.Roberts RA, James NH, Cosulich S, Hasmall SC, Orphanides G. Role of cytokines in non-genotoxic hepatocarcinogenesis: cause or effect? Toxicol Lett. 2001;120:301–306. doi: 10.1016/S0378-4274(01)00282-X. [DOI] [PubMed] [Google Scholar]
- 30.Zhu RZ, Xiang D, Xie C, Li JJ, Hu JJ, He HL, Yuan YS, Gao J, Han W, Yu Y. Protective effect of recombinant human IL-1Ra on CCl4-induced acute liver injury in mice. World J Gastroenterol. 2010;16:2771–2779. doi: 10.3748/wjg.v16.i22.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoek JB, Pastorino JG. Ethanol, oxidative stress, and cytokine-induced liver cell injury. Alcohol. 2002;27:63–68. doi: 10.1016/S0741-8329(02)00215-X. [DOI] [PubMed] [Google Scholar]
- 32.Wahl SM, McCartney-Francis N, Chan J, Dionne R, Ta L, Orenstein JM (2003) Nitric oxide in experimental joint inflammation. Benefit or detriment? Cells Tissues Organs 174:26–33. doi:10.1159/000070572 [DOI] [PubMed]
- 33.Tsuchiyama T, Nakamoto Y, Sakai Y, Mukaida N, Kaneko S. Optimal amount of monocyte chemoattractant protein-1 enhances antitumor effects of suicide gene therapy against hepatocellular carcinoma by M1 macrophage activation. Cancer Sci. 2008;99:2075–2082. doi: 10.1111/j.1349-7006.2008.00951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bautista AP. Chronic alcohol intoxication induces hepatic injury through enhanced macrophage inflammatory protein-2 production and intercellular adhesion molecule-1 expression in the liver. Hepatology. 1997;25:335–342. doi: 10.1002/hep.510250214. [DOI] [PubMed] [Google Scholar]
- 35.Chen X. Protective effects of quercetin on liver injury induced by ethanol. Pharmacogn Mag. 2010;6:135–141. doi: 10.4103/0973-1296.62900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gewiese-Rabsch J, Drucker C, Malchow S, Scheller J, Rose-John S. Role of IL-6 trans-signaling in CCl(4) induced liver damage. Biochim Biophys Acta. 2010;1802:1054–1061. doi: 10.1016/j.bbadis.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 37.Zuinen R, Yamaji K, Aoki M, Chikuma T, Hojo H. Early induced, high-level interleukin-6 expression in the rat peritoneal cavity into which a hepatotoxicant carbon tetrachloride was administered. Toxicol Lett. 2007;170:42–48. doi: 10.1016/j.toxlet.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 38.Tsuchiya S, Tsukamoto Y, Taira E, LaMarre J. Involvement of transforming growth factor-beta in the expression of gicerin, a cell adhesion molecule, in the regeneration of hepatocytes. Int J Mol Med. 2007;19:381–386. [PubMed] [Google Scholar]
- 39.Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–288. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 40.Gloire G, Legrand-Poels S, Piette J. NF-kappaB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol. 2006;72:1493–1505. doi: 10.1016/j.bcp.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 41.Liaudet L, Soriano FG, Szabo C. Biology of nitric oxide signaling. Crit Care Med. 2000;28:N37–N52. doi: 10.1097/00003246-200004001-00005. [DOI] [PubMed] [Google Scholar]
- 42.Takada Y, Mukhopadhyay A, Kundu GC, Mahabeleshwar GH, Singh S, Aggarwal BB. Hydrogen peroxide activates NF-kappa B through tyrosine phosphorylation of I kappa B alpha and serine phosphorylation of p65: evidence for the involvement of I kappa B alpha kinase and Syk protein-tyrosine kinase. J Biol Chem. 2003;278:24233–24241. doi: 10.1074/jbc.M212389200. [DOI] [PubMed] [Google Scholar]


