Abstract
Introduction
HIV-infected patients commonly develop distal symmetric polyneuropathy (DSP). Age, ethnicity, and toxic exposures may influence the risk. This study examines the association between substance use, antiretrovirals, ethnicity and incident neuropathy in an HIV- infected cohort.
Methods
Data were obtained from the National NeuroAIDS Tissue Consortium (NNTC), an ongoing, prospective cohort started in 1998. Cox proportional hazards models were used to examine the association of substance use, demographics, neurotoxic antiretrovirals, and laboratory parameters with incident neuropathy in 636 participants who were neuropathy-free at baseline.
Results
The cumulative incidence of DSP was 41%. Substance use (p =.04), number of substances used (p=.04) and longer duration of HIV infection (p = .05) were associated with incident DSP, but demographic factors, use of neurotoxic antiretrovirals, and laboratory parameters were not.
Discussion
Substance use and longer duration of HIV infection are risk factors for DSP in HIV-infected patients. Use of multiple substances may be particularly risky.
Keywords: neuropathy, HIV, substance use, antiretroviral, NNTC
Introduction
Distal symmetric polyneuropathy (DSP) is a common complication of HIV infection.1, 2 Prior to the widespread use of highly active antiretroviral therapy (HAART), DSP was associated with markers of advanced HIV, such as low CD4+ count and high viral load.3 The role of toxic factors in some cases of DSP was established when a neuropathy associated with the nucleoside reverse transcriptase inhibitors stavudine (d4T), didanosine (ddI), and zalcitabine (ddC), also known as “d-drugs”, was described.4 Although DSP remains a common disorder in the HAART-era, the risk factors appear to have changed. Several HAART-era studies have shown no association of DSP with CD4+ count or viral load1, 2, 5, but rather with demographic factors such as older age6, 7, 8, male gender1, and non-Hispanic white ethnicity.2 We performed a small pilot study which suggested that a combination of toxic exposures [intravenous drug-use (IVDU) and d-drugs] and Hispanic ethnicity were associated with incident HIV-DSP.9 However it was unclear if IVDU and Hispanic ethnicity were independent risk factors, because Hispanics were overrepresented among the substance users, and the sample size was small. This study explores the association between substance use, d-drugs, and demographic factors and the risk of clinically diagnosed incident DSP in a larger HIV-infected cohort.
Materials and Methods
Study sample
The National NeuroAIDS Tissue Consortium (NNTC) is an ongoing prospective cohort study established in 1998 to provide a resource of neurologic tissues from HIV-infected donors who underwent comprehensive, longitudinal, ante-mortem evaluation. Following enrollment, participants are evaluated at three to six month intervals until the time of death. Since the endpoint of the study is autopsy and resources are limited, the interval is chosen based on a consensus among investigators at each individual site. Participants who are acutely ill, or deemed likely to become so, are followed closely with formal assessments every three months and additional monitoring of clinical status as appropriate. Participants who are stable receive formal assessments every 6 months. There are 4 sites: Manhattan HIV Brain Bank (New York), California NeuroAIDS Tissue Network (San Diego), National Neurologic AIDS Bank (Los Angeles) and Texas NeuroAIDS Research Center (Galveston). The primary enrollment criterion for the NNTC is willingness to participate in organ donation at the time of death. Other criteria were designed to target patients with advanced HIV disease (progressive multifocal leukoencephalopathy, lymphoma, disseminated mycobacteriumavium-intercellulare infection, wasting, AIDS dementia complex, cytomegalovirus end organ disease, visceral Kaposi sarcoma, congestive heart failure, serum albumin <3.2 g/dL, CD4 count ≤50 cells/mm3 for at least a 3-month period of time, or substantive risk for imminent mortality in the judgment of the participant's primary physician).10 Participants are referred from a variety of sources including health care providers, other research programs (e.g. Women's Interagency HIV Study), and other participants. As of this analysis, 1970 HIV-positive participants had enrolled in the NNTC longitudinal cohort. Of these, 705 are deceased, 579 are actively followed, and 686 are inactive (either lost to follow-up or on administrative hold). Six-hundred and thirty nine participants met the following criteria and were considered for this analysis: no DSP at baseline and at least one additional neurologic evaluation thereafter. For 29 of these participants, the first neurologic examination did not occur at their baseline visit (23 within one-year post-baseline, 3 between 1–2 years post-baseline and 3 between 4–5 years post-baseline). The 3 participants whose first neurologic examination occurred between 4–5 years post-baseline were excluded, leaving a total of 636 evaluable participants. These participants were followed for up to 10.9 years, with a mean duration of 3.4 years and a median duration of 2.6 years.
Data were obtained under protocols approved by the institutional review boards of each of the participating institutions (Mount Sinai School of Medicine; University of California, Los Angeles; University of California, San Diego; University of Texas Medical Branch). All participants gave written informed consent.
Neurologic and substance use diagnoses
The outcome of interest, DSP, was diagnosed clinically based on a standardized, comprehensive neurological examination performed by a clinician (MD, NP or RN) experienced in the care of HIV-positive patients. Participants were assigned a diagnosis of DSP if they displayed at least two of the following three signs: diminished sense of vibration at both great toes, bilateral distal decreased sensation to pin prick in both lower extremities, and ankle reflexes absent or diminished as compared to the knees. These criteria have been used previously in studies of HIV-DSP.2, 9 A DSM-IV diagnosis of past and/or current substance abuse or dependence was made using the relevant module of either the Psychiatric Research Interview for Substance and Mental Disorders or the Composite International Diagnostic Interview.11, 12 Substances or classes of substances queried by these instruments were: alcohol, cannabis, cocaine, hallucinogens, opiates, sedatives, stimulants or other substances. Other available substance use data were a self-report of intravenous drug use (IVDU) as a risk factor for HIV-infection and urine toxicology for substances of abuse (amphetamines, benzodiazepines, barbiturates, cannabinoids, cocaine, methadone, opiates, phencyclidine, propoxyphene). If detected substances were prescribed by a healthcare provider, such as opiates for pain, the urine toxicology was not considered an indicator of substance misuse.
Additional variables
Past and current antiretroviral regimens were recorded at baseline. For this analysis, 2 antiretroviral variables were considered: current d-drug use and any d-drug use. Current d-drug use was defined as use of stavudine (d4T), didanosine (ddI) or zalcitabine (ddC) upon entry into the study. Any d-drug use was defined as use of one or more of these agents either at entry to the study or any time in the past. Laboratory markers included baseline glucose, hemoglobin, CD4+ count and serum HIV viral load. Participants provided the following demographic data: date of birth, year of HIV diagnosis, gender, race (white, black/African-American, American Indian/Alaska native, Asian, Native Hawaiian or other Pacific Islander, more than one race) and ethnicity (Hispanic/Latino or not). For this analysis, ethnicity was defined as Hispanic if the participant reported Hispanic or Latino ethnicity. If the participant denied being Hispanic or Latino, the ethnicity was defined as either white, African-American or other.
Statistical Analyses
All covariate measurements were analyzed based on status at enrollment into the study. Demographic variables analyzed included age, gender, site of enrollment and race/ethnicity. D-drug use was analyzed as either current use or any use (i.e., past or current). Duration of HIV infection, CD4+ count, log serum HIV viral load, hemoglobin and glucose were analyzed as continuous variables.
Three measures of substance use behaviors were available: DSM-IV diagnoses of past or current abuse or dependence for individual substances, a self-reported history of IVDU as a risk factor for HIV infection, and urine toxicology. It was not known a priori which of these measures was best, and each provided some unique information. For example, some participants who reported IVDU or had a positive urine toxicology were not fully forthcoming about their drug use or denied distress or functional impairment. These individuals did not meet DSM-IV criteria for substance abuse or dependence. Similarly, some participants who reported significant substance use and met DSM-IV criteria had negative urine toxicology. Thus several substance use variables were defined and evaluated. These variables were based on a common-sense approach to the available data and are not previously validated measurement tools. There were 4 dichotomous “overall” substance use variables (i.e. not discriminating between particular substances): 1) the presence of one or more DSM-IV diagnoses of past or current abuse or dependence for any substance, 2) a self-reported history of IVDU as a risk factor for HIV infection, 3) urine toxicology positive at baseline, 4) any of the above three criteria met. A dichotomous variable for use of individual substances was defined as a DSM-IV abuse or dependence diagnosis or a positive illicit urine toxicology for a given substance. Finally, the number of individual substances each participant used was calculated, and participants were grouped as follows: non-user, single substance user, oligosubstance user (2–3 substances), polysubstance user (4 or more substances).
Frequencies and measures of center were calculated for baseline demographics, substance use variables and laboratory parameters. Cox proportional hazards regression models were used to initially evaluate the association between each factor and incidence of DSP in a univariate setting. Variables significant at the p ≤ .20 level in univariate models were then considered for multivariate Cox proportional hazards regression models.
To avoid multivariate models that included multiple highly-correlated substance use fields, different models were explored to examine the effect of each method of substance use categorization. Model 1 examined the overall substance use variable (DSM-IV substance use disorder OR history of IVDU as an HIV-risk factor OR positive urine toxicology); model 2 assessed each of the three individual components. Model 3 examined number of substances used. For each model, co-variates were chosen via forward selection beginning with the variables most closely associated with DSP in univariate analyses. No adjustments were made for multiple comparisons.
Initial analyses were performed using SPSS (version 18.0, SPSS Inc., Chicago IL). Additional analyses were performed using SAS PROC PHEG (version 9.2, SAS Institute, Cary, NC).
Results
Cohort characteristics at baseline
Characteristics at baseline are summarized in Table 1. The mean age of the cohort upon entry into the study was 41.6 (SD = 8.2). Seventy-seven percent of the participants were male; 36% were white, 30% Hispanic, 30% black, and 4% of another race or of more than one race. Overall the participants had fairly advanced disease with a median HIV disease duration of 10 years (IQR = 5,15), a median CD4+ count of 131 (IQR = 39, 283) and a median log VL of 3.3 (IQR = 2.6, 4.7). The mean hemoglobin was 13.2 (SD = 1.9). Ninety-four percent of participants were antiretroviral experienced. The median number of lifetime antiretrovirals per participant was 5 (IQR = 3, 8). Fifty-six percent had been exposed to non-nucleoside reverse transcriptase inhibitors (NRTIs), and 80% had been exposed to protease inhibitors. Sixty-five percent of patients had been exposed to d-drugs, and 36% were taking them on entry.
Table 1.
Baseline Demographic, HIV-related and Substance Use Data
| N | 636 |
|
| |
| Length of follow-up (years), median (IQR) | 1.58 (.8, 3.4) |
|
| |
| Neuropathy Cumulative Incidence, n (%) | 261 (41%) |
|
| |
| Demographic Variables | |
|
| |
| Age (years), median (IQR) |
41.0 (36.0,46.8) |
| Male, n (%) | 489 (77%) |
|
| |
| Race/Ethnicity, n (%) | |
| White | 227 (36%) |
| Black | 189 (30%) |
| Hispanic | 193 (30%) |
| Other |
27 (4%) |
| HIV-related variables | |
|
| |
| History of d-drug use, n (%) |
413 (65%) |
| Current d-drug use, n (%) | 230 (36%) |
|
| |
| Median HIV duration (years), median (IQR) |
10 (5, 15) |
| Laboratory parameters | |
|
| |
| CD4+ count (cells/mm3), median (IQR) |
131 (39, 283) |
| HIV plasma RNA (log10(copies/ml)), median (IQR) | 3.3 (2.6, 4.7) |
|
| |
| Hemoglobin (g/dL), median (IQR) |
13.3 (12.0, 14.5) |
| Glucose mg/dL, median (IQR) | 89 (76, 102) |
|
| |
| Substance use variables | |
|
| |
| Any criteria for substance usea, n (%) | 489 (77%) |
|
| |
| Reported intravenous drug use as a risk factor for HIV-infection, n (%) |
162 (25%) |
| Urine toxicology positive, n (%) | 194 (31%) |
|
| |
| DSM-IV diagnosis of past or current substance abuse or dependence of any substance, n (%) |
427 (67%) |
| Individual substances usedb, n (%) | |
|
| |
| Alcohol |
291 (46%) |
| Cocaine | 292 (46%) |
|
| |
| Cannabis |
265 (42%) |
| Opiates | 117 (18%) |
|
| |
| Stimulants |
150 (24%) |
| Hallucinogens | 40 (6%) |
|
| |
| Sedatives |
82 (15%) |
| Other | 29 (5%) |
|
| |
| Severity of use (for any substance) |
|
| Abuse diagnosis or urine toxicology positive | 95 (15%) |
|
| |
| Dependence diagnosis |
370 (58%) |
| Number of substancesb, n (%) | |
|
| |
| 0 | 154 (24%) |
|
| |
| 1 | 124 (20%) |
|
| |
| 2–3 | 226 (36%) |
|
| |
| 4 or more | 115 (18%) |
Includes a DSM-IV diagnosis, self-report of intravenous drug use, or positive urine toxicology.
Defined as a DSM-IV diagnosis or urine toxicology positive for an individual substance.
Two-hundred and sixty-one participants (41%) developed DSP on study. The median time to incident DSP was 1.3 years. The median time of follow-up among those who did not develop DSP was 2 years. Substance use was common, with 77% of participants meeting at least one criterion for substance use. Details of substance use prevalence in the cohort are provided in table 1.
Univariate Analyses
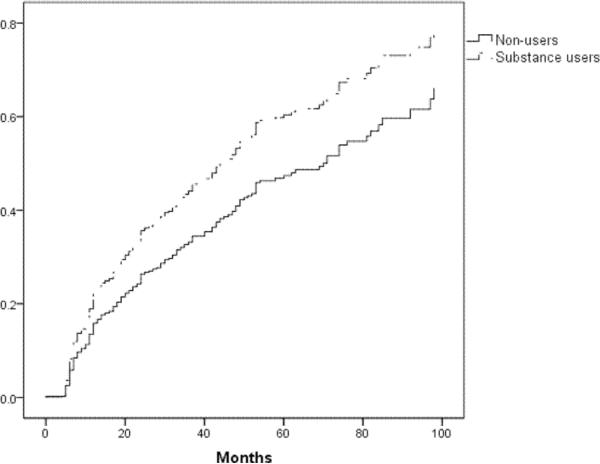
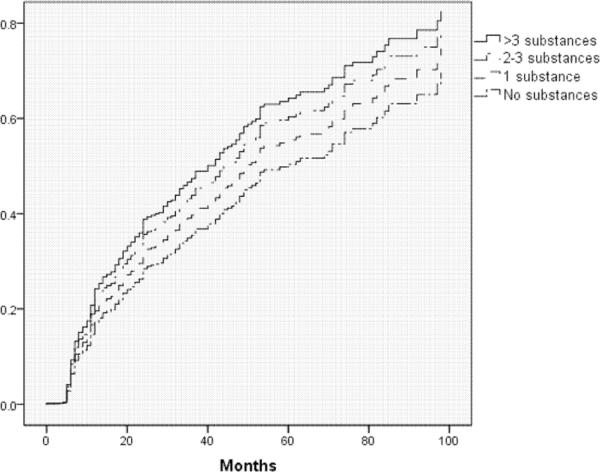
As shown in table 2, all of the overall substance use variables were found to be associated with increased hazard of DSP in univariate analyses, with a maximum hazard ratio of 1.48 (p=.02) for the most inclusive substance use variable. Using this variable, cumulative incidence of DSP in substance users was 44% compared to 33% in non-users, and median time to DSP was 44 months in substance users compared to 71 months in non-users. Among participants using 4 or more substances, the cumulative incidence of DSP was 50%. None of the individual substances stood out as more closely linked to DSP than the others in univariate analyses, with hazard ratios near 1.2 for several individual substances (all p-values >.10, see table 2). Other variables associated with increased hazard of DSP (p ≤ .20) included increasing age, longer duration of HIV infection, higher log serum viral load, and lower hemoglobin. Hispanic ethnicity and current use of d-drugs were associated with decreased hazard of DSP (p ≤ .20).
Table 2.
Results of univariate analyses on incidence of neuropathy
| Hazard Ratio | p-value | |
|
| ||
| Overall substance use variables | ||
|
| ||
| Any substance usea | 1.48 | 0.02 |
|
| ||
| DSM-IV substance use disorder | 1.46 | 0.01 |
|
| ||
| History of intravenous drugs | 1.27 | 0.08 |
|
| ||
| Positive urine toxicology | 1.28 | 0.08 |
|
| ||
| Number of substancesb (continuous) | 1.17 | 0.009 |
| 1 | 1.25 | |
| 2–3 | 1.39 | |
| 4 or more | 1.61 | |
|
| ||
| Individual substance use variablec | ||
|
| ||
| Alcohol | 1.16 | 0.23 |
|
| ||
| Cannabis | 1.22 | 0.12 |
|
| ||
| Cocaine | 1.22 | 0.11 |
|
| ||
| Opiates | 1.23 | 0.17 |
|
| ||
| Sedatives | 1.25 | 0.20 |
|
| ||
| Demographic variables | ||
|
| ||
| Age | 1.01 | 0.13 |
|
| ||
| Gender | 1.01 | 0.95 |
|
| ||
| Ethnicityd | ||
| African-American | 1.14 | 0.38 |
| Hispanic | 0.77 | 0.10 |
| Other | 0.65 | 0.22 |
|
| ||
| HIV-related variables | ||
|
| ||
| HIV duration | 1.03 | 0.02 |
|
| ||
| Current use of d-drugs | .80 | 0.09 |
|
| ||
| Any history of d-drugs | 0.97 | 0.97 |
|
| ||
| Laboratory parameters | ||
|
| ||
| Serum glucose | 1.00 | 0.29 |
|
| ||
| CD4+ count | 1.00 | 0.76 |
|
| ||
| Hemoglobin | .93 | 0.04 |
|
| ||
| Log serum HIV viral load | 1.08 | 0.18 |
Includes DSM-IV substance use disorder, history of intravenous drug use as an HIV-risk factor, or positive urine toxicology
Defined as a DSM-IV diagnosis or urine toxicology positive for an individual substance
p-values were ≥.5 for all other individual substances
Used white as reference group.
Multivariate Analyses
The following variables were significant in univariate analyses at the p ≤ .20 level and were considered for all multivariate models: site of participation, age, ethnicity, log serum HIV viral load, hemoglobin, current use of d-drugs, and duration of HIV infection. Table 3 summarizes the results of the multivariate models. All final models included site of participation and HIV-duration. Overall substance use (defined as DSM-IV substance use disorder, history of intravenous drug use as an HIV-risk factor, or positive urine toxicology; model 1, see also figure 1) was associated with incidence of DSP (HR=1.40; 95% CI: 1.01,1.95; p=.043); when the components were assessed individually (model 2), DSM-IV substance use disorder for any substance was associated with incidence of DSP (HR=1.36; 95% CI: 1.00,1.85; p=.048). Increasing number of substances used was also associated with incidence of DSP (model 3, see also figure 2) (HR=1.14; 95% CI: 1.01,1.28).
Table 3.
Risk for neuropathy in multivariate analyses
| Hazard Ratio | 95% CI | p-value | |
|
| |||
| Model 1: Any substance usea | 1.41 | 1.01–1.99 | 0.04 |
|
| |||
| Model 2: DSM-IV substance use disorder | 1.34 | 1.01–1.78 | 0.04 |
|
| |||
| Model 3: | |||
| Number of substances (continuous) | 1.14 | 1.01–1.28 | 0.04 |
| 1 substance | 1.15 | ||
| 2–3 substances | 1.32 | ||
| 4 or more substances | 1.47 | ||
Includes DSM-IV substance use disorder, history of intravenous drug use as an HIV-risk factor, or positive urine toxicology
Figure 1.
Incident distal symmetric polyneuropathy in substance users versus non-substance users (p=.04)
Figure 2.
Incident distal symmetric polyneuropathy by number of substances used (p=.04)
Discussion
In this study we sought to confirm the findings of a pilot study which suggested a role for substance abuse and ethnicity in the development of clinically diagnosed DSP in participants with advanced HIV. The results indicate that substance use and duration of HIV infection are risk factors for DSP in HIV-infected individuals. No one particular substance of abuse accounts for this association, however risk increases with the number of substances used, with the highest cumulative incidence of DSP (50%) among those using 4 or more substances. We did not find any role for ethnicity as a risk factor. This suggests that the apparent role of ethnicity observed in the pilot study was in fact due to a disproportionate number of substance users among Hispanics in that sample.
Although the neurotoxic effect of alcohol is well known, substance abuse in general has not been previously described as a risk factor for DSP in HIV-infected or general populations. We were unable to detect an effect of alcohol specifically, or any other individual substance. This is not particularly surprising, since the majority of substance users (73%) in this sample used more than one substance. It has been proposed that the mechanism of alcoholic neuropathy is multifactorial, including the neurotoxic effect of alcohol itself, poor nutrition, and other related toxic exposures. Similar mechanisms are likely responsible for the neuropathy observed in this study, however in our population the additional effects of HIV must also be considered. There is a substantial and rapidly developing literature on substance abuse, HIV, and neurotoxicity in the central nervous system.13 For example, cocaine and opiates have been shown to enhance HIV viral replication14 and may potentiate the neurotoxic effect of HIV proteins such as Tat and gp120 in animal and in vitro models.14–18 In many analyses, enhanced inflammatory responses and oxidative stress have been mechanistically implicated in the deleterious substance use-HIV synergy. There are no studies that examine the effect of substances of abuse alone or in combination with HIV viral proteins in models of DSP. However, the mechanisms by which HIV causes neurotoxicity in the peripheral nervous system are thought to be similar to those in the central nervous system;19, 20 thus similar harmful synergy might occur. This hypothesis is supported by our finding of HIV infection duration and substance use as predictors of DSP. In fact, the highest cumulative incidence of DSP (57%) was among the 63 polysubstance users who had been HIV positive for more than 10 years.
There are several potential limitations in this study. Although data in the NNTC are gathered prospectively, this analysis is retrospective in that it makes use of pre-existing data that was not gathered for this particular purpose. Accordingly certain variables are not ideally suited for this analysis. For example, a variable was constructed to approximate the effect of polysubstance use, but key information that would help to quantify substance use, such as duration of use, was lacking. There was variability in some results across the 4 sites included in the consortium. For example: DSP rates were higher at the Los Angeles and New York sites, which also had more minority participants; New York had greater use of cocaine and opiates; and the substance abuse effect was weaker at the San Diego site. The site of participation was included in the multivariate models in an attempt to account for this variability. No adjustment was made for multiple comparisons due to the exploratory nature of the analysis, which increases the likelihood of type 1 error. However the consistency of association between multiple substance use variables and incident DSP is reassuring. The majority of participants in this study had advanced HIV, which may restrict application of these findings to healthier HIV-infected populations. Additionally, since all participants were HIV-positive, this study does not provide information as to whether substance use is a risk factor for neuropathy in HIV-negative populations or whether the effect of substance use on risk on neuropathy in HIV is additive or multiplicative. Detailed data that characterize the nature of the DSP, including symptoms, were not available. There were no neurophysiologic data, such as nerve conduction studies, to support the clinical diagnosis of DSP. Data about co-morbid conditions and medications (other than antiretrovirals) that could cause DSP were also not available. Although serum glucose measurements were considered, there was no information about diabetes diagnoses, which is particularly important since diabetes is a common cause of DSP. The strengths of the study include the relatively large number of participants, the long period of follow up, the reproducibility of the substance use effect in different analyses and the biologic plausibility of the results.
In summary, our findings suggest that illicit substance use and longer duration of HIV infection are risk factors for DSP. Use of multiple substances may be particularly risky. Further study is needed to confirm this finding and to elucidate the mechanism by which substances of abuse may contribute to the development of DSP in the HAART-era.
Acknowledgments
Grant support: This publication was made possible from NIH funding through the NIMH and NINDS by the following grants: K23 NS066789 (PI: JRP); Manhattan HIV Brain Bank (PI: SM): U01MH083501, R24MH59724; Texas NeuroAIDS Research Center (PI: BBG) U01MH083507, R24 NS45491; National Neurological AIDS Bank (PI: ES) 5U01MH083500, NS 38841; California NeuroAIDS Tissue Network (PI: IG) U01MH083506, R24MH59745; Statistics and Data Coordinating Center U01MH083545, N01MH32002.
Abbreviations
- CI
Confidence interval
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders
- DSP
Distal symmetric polyneuropathy
- HAART
Highly active antiretroviral therapy
- HIV
Human immunodeficiency virus
- HR
Hazard ratio
- IQR
Interquartile range
- IVDU
Intravenous drug use
- MD
Medical doctor
- NNTC
National NeuroAIDS Tissue Consortium
- NP
Nurse practitioner
- RN
Registered nurse
- SD
Standard deviation
References
- 1.Morgello S, Estanislao L, Simpson D, Geraci A, DiRocco A, Gerits P, et al. HIV-associated distal sensory polyneuropathy in the era of highly active antiretroviral therapy: the Manhattan HIV Brain Bank. Arch Neurol. 2004;61:546–551. doi: 10.1001/archneur.61.4.546. [DOI] [PubMed] [Google Scholar]
- 2.Simpson DM, Kitch D, Evans SR, McArthur JC, Asmuth DM, Cohen B, et al. HIV neuropathy natural history cohort study: assessment measures and risk factors. Neurology. 2006;66:1679–1687. doi: 10.1212/01.wnl.0000218303.48113.5d. [DOI] [PubMed] [Google Scholar]
- 3.Childs EA, Lyles RH, Selnes OA, Chen B, Miller EN, Cohen BA, et al. Plasma viral load and CD4 lymphocytes predict HIV-associated dementia and sensory neuropathy. Neurology. 1999;52:607–613. doi: 10.1212/wnl.52.3.607. [DOI] [PubMed] [Google Scholar]
- 4.Simpson DM, Tagliati M. Nucleoside analogue-associated peripheral neuropathy in human immunodeficiency virus infection. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;9:153–161. [PubMed] [Google Scholar]
- 5.Schifitto G, McDermott MP, McArthur JC, Marder K, Sacktor N, McClernon DR, et al. Markers of immune activation and viral load in HIV-associated sensory neuropathy. Neurology. 2005;64:842–848. doi: 10.1212/01.WNL.0000152981.32057.BB. [DOI] [PubMed] [Google Scholar]
- 6.Cherry CL, Skolasky RL, Lal L, Creighton J, Hauer P, Raman SP, et al. Antiretroviral use and other risks for HIV-associated neuropathies in an international cohort. Neurology. 2006;66:867–873. doi: 10.1212/01.wnl.0000203336.12114.09. [DOI] [PubMed] [Google Scholar]
- 7.Ellis RJ, Rosario D, Clifford DB, McArthur JC, Simpson D, Alexander T, et al. Continued high prevalence and adverse clinical impact of human immunodeficiency virus-associated sensory neuropathy in the era of combination antiretroviral therapy: the CHARTER Study. Arch Neurol. 2010;67:552–558. doi: 10.1001/archneurol.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans SR, Ellis RJ, Chen H, Yeh TM, Lee AJ, Schifitto G, et al. Peripheral Neuropathy in HIV: prevalence and risk factors. AIDS. 2011;25(7):919–28. doi: 10.1097/QAD.0b013e328345889d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robinson-Papp J, Gonzalez-Duarte A, Simpson DM, Rivera-Mindt M, Morgello S, Manhattan HIV Brain Bank The roles of ethnicity and antiretrovirals in HIV-associated polyneuropathy: a pilot study. J Acquir Immune Defic Syndr. 2009;51:569–573. doi: 10.1097/QAI.0b013e3181adcefa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryan EL, Morgello S, Isaacs K, Naseer M, Gerits P, Manhattan HIV Brain Bank Neuropsychiatric impact of hepatitis C on advanced HIV. Neurology. 2004;62:957–962. doi: 10.1212/01.wnl.0000115177.74976.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wittchen HU. Reliability and validity studies of the WHO--Composite International Diagnostic Interview (CIDI): a critical review. J Psychiatr Res. 1994;28:57–84. doi: 10.1016/0022-3956(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 12.Hasin DS, Trautman KD, Miele GM, Samet S, Smith M, Endicott J. Psychiatric Research Interview for Substance and Mental Disorders (PRISM): reliability for substance abusers. Am J Psychiatry. 1996;153:1195–1201. doi: 10.1176/ajp.153.9.1195. [DOI] [PubMed] [Google Scholar]
- 13.Nath A. Human immunodeficiency virus-associated neurocognitive disorder: pathophysiology in relation to drug addiction. Ann N Y Acad Sci. 2010;1187:122–128. doi: 10.1111/j.1749-6632.2009.05277.x. [DOI] [PubMed] [Google Scholar]
- 14.Peterson PK, Gekker G, Schut R, Hu S, Balfour HH, Jr, Chao CC. Enhancement of HIV-1 replication by opiates and cocaine: the cytokine connection. Adv Exp Med Biol. 1993;335:181–188. doi: 10.1007/978-1-4615-2980-4_26. [DOI] [PubMed] [Google Scholar]
- 15.Aksenov MY, Aksenova MV, Nath A, Ray PD, Mactutus CF, Booze RM. Cocaine-mediated enhancement of Tat toxicity in rat hippocampal cell cultures: the role of oxidative stress and D1 dopamine receptor. Neurotoxicology. 2006;27:217–228. doi: 10.1016/j.neuro.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Bruce-Keller AJ, Turchan-Cholewo J, Smart EJ, Geurin T, Chauhan A, Reid R, et al. Morphine causes rapid increases in glial activation and neuronal injury in the striatum of inducible HIV-1 Tat transgenic mice. Glia. 2008;56:1414–1427. doi: 10.1002/glia.20708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gurwell JA, Nath A, Sun Q, Zhang J, Martin KM, Chen Y, et al. Synergistic neurotoxicity of opioids and human immunodeficiency virus-1 Tat protein in striatal neurons in vitro. Neuroscience. 2001;102:555–563. doi: 10.1016/s0306-4522(00)00461-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yao H, Allen JE, Zhu X, Callen S, Buch S. Cocaine and human immunodeficiency virus type 1 gp120 mediate neurotoxicity through overlapping signaling pathways. J Neurovirol. 2009;15:164–175. doi: 10.1080/13550280902755375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wallace VC, Blackbeard J, Segerdahl AR, Hasnie F, Pheby T, McMahon SB, et al. Characterization of rodent models of HIV-gp120 and anti-retroviral-associated neuropathic pain. Brain. 2007;130:2688–2702. doi: 10.1093/brain/awm195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melli G, Keswani SC, Fischer A, Chen W, Hoke A. Spatially distinct and functionally independent mechanisms of axonal degeneration in a model of HIV-associated sensory neuropathy. Brain. 2006;129:1330–1338. doi: 10.1093/brain/awl058. [DOI] [PubMed] [Google Scholar]