Abstract
Background
Childhood cancer survivors are at high risk for reduced bone mineral density (BMD). Our objective was to determine whether post-pubertal adolescent survivors of brain tumors, whose tumor or treatments placed them at risk for pituitary hormone deficiencies, have low BMD near time of peak bone mass accrual, and to assess risk factors for decreased BMD.
Procedure
Chart review of 36 post-pubertal adolescents with history of tumor or radiation therapy (RT) of the hypothalamic-pituitary area who had undergone BMD screening via dual-energy x-ray absorptiometry (DXA).
Results
Age at DXA was 16.9 ±1.9 years (mean ± SD). Time since diagnosis was 8.5 ±3.6 years. Median BMD Z-scores were −0.95 (range −2.7 to 1.7) at the femoral neck, −1.20 (−3.6 to 1.8) at the hip, and −0.90 (−3.7 to 1.8) at the spine. Bone mineral apparent density (BMAD) Z-scores were −0.23 (−2.7 to 1.9) at the femoral neck and −0.45 (−3.0 to 2.3) at the spine. Those with history of ≥1 fracture had lower BMD Z-scores of the femoral neck, total hip and spine (P<0.05). Those with treated GH deficiency had a higher BMD Z-score at the femoral neck, total hip and spine (P<0.05) than those not treated. There was no difference in BMD with respect to treatment with chemotherapy, cranial or spinal RT, or hypogonadism. Spontaneous menarche and regular periods did not correlate with BMD.
Conclusions
In post-pubertal adolescent survivors of childhood brain tumors, fracture history and untreated GH deficiency are risk factors for decreased BMD.
Keywords: Bone mineral density, brain tumors, growth hormone deficiency, fracture
Introduction
Survivors of childhood cancer are recognized as a high-risk population for the development of a reduced bone mineral density (BMD) (1–3). The malignancy itself, treatments and their sequelae, malnutrition, and limited weight-bearing exercise all likely affect BMD. Due to the complex nature of cancer and its treatment, however, the impact of each individual factor is difficult to isolate.
The effect of cancer itself on BMD and osteoporosis risk is not clear. Conflicting data exist on whether BMD is diminished at the time of cancer diagnosis. Halton et al reported “osteopenia” in 13% of children with acute lymphoblastic leukemia (ALL) prior to initiation of treatment (4). However, Arikoski et al. concluded that BMD is not compromised for age at the time of cancer diagnosis (a combined group of ALL and solid tumors), although they reported decreased markers of bone formation (5). These data suggest that some disease processes may alter bone metabolism and contribute to risk for a decreased BMD, although the changes likely vary by cancer type.
Previous research has shown that brain tumor survivors are susceptible to development of fractures and early osteoporosis (6–8). Gurney et al. found that adult survivors of childhood brain tumors were nearly 25 times more likely to report having osteoporosis or brittle bones when compared to siblings without brain tumors; 43% of these patients reported an endocrinopathy (8). More specifically, growth hormone (GH) and insulin-like growth factor I (IGF-I) are important to maintain bone mass, as both independently lead to increased bone remodeling and apposition (9). GH stimulates IGF-I synthesis in the liver, bone, and other target tissues, and GH deficiency results in low IGF-I levels. GH deficiency (GHD) may result from the location of the tumor itself, surgical resection of the hypothalamus and/or pituitary, or cranial radiation at a dose of 18 Gy or higher to the hypothalamic-pituitary region (10).
Estrogen and testosterone also affect bone mass; both have been shown to increase bone accrual (11). Primary gonadal dysfunction is a known risk factor for a decreased BMD in young adult survivors of childhood cancer (2). In addition, impairment of the hypothalamic-pituitary-gonadal axis is seen after radiation to the hypothalamic-pituitary region, most commonly after receipt of >40 Gy (10), as well as during periods of illness, undernutrition, and stress (12).
Additionally, radiation and various systemic agents targeting brain tumors appear to be directly deleterious to bone. It is thought that radiation can cause bone atrophy by damaging blood vessels and osteoblasts (13). However, Krishnamoorthy et al. reported that patients who received posterior fossa radiation alone also have diminished total body and lumbar spine BMD. Thus, the tumor itself or response to therapy may release cytokines and factors that affect bone modeling and osteoblastic and osteoclastic activity (14). Glucocorticoids promote osteoclast activity while simultaneously inhibiting osteoblast activity (15). Furthermore, they interfere with the GH–IGF-I axis (15) and suppress gonadotropins (16). Cisplatinum can cause tubular dysfunction and lead to hypomagnesemia. Alkylating agents, as well as antimetabolites and vinca alkakloids, have been linked with gonadal damage (17).
Weight-bearing activity and adequate nutrition are important for increasing bone density (18). Although many brain tumor survivors ambulate, their exercise is limited due to neurologic deficits. Patients treated for craniopharyngioma have been shown to have decreased activity (19, 20). Malnutrition may contribute to poor bone health, as it leads to GH resistance and subsequent IGF-I deficiency (21). Poor diet may also lead to decreased calcium and vitamin D intake. An increase in calcium intake results in higher bone mass (18, 22) and reduced risk of fracture. Optimizing vitamin D intake is equally beneficial, as vitamin D insufficiency results in poor intestinal calcium absorption. Some studies have indicated that childhood cancer survivors have low serum calcium and are often 25-hydroxyvitamin D deficient (5, 14), while others have found survivors to have normal levels (23).
While low bone mass has been found to be common in children surviving brain tumors (6, 24, 25), studies to date have been limited by evaluation of subjects at different ages and thus different stages of bone accrual, and by the lack of longitudinal data. The objective of this study was to determine whether survivors of childhood brain tumors, whose tumor or its treatments placed them at risk for pituitary hormone deficiencies, have a low BMD as they approach the time of peak bone mass acquisition. We also sought to identify risk factors for a low BMD.
Methods
The sample included patients diagnosed with a pediatric brain tumor, at risk for pituitary hormone deficiencies and followed in the Endocrinology Program at Children’s Hospital Boston (CHB) for routine endocrine care. Data were collected both retrospectively and prospectively by review of medical records between July 2003 and May 2008. The protocol was approved by the Committee on Clinical Investigation of CHB. Patients and/or their parents or guardians signed informed consent. Two hundred thirty-seven patients were enrolled in the database, while 4 declined participation. This sub-study sample was composed of 36 patients less than 20 years of age who had completed puberty (based on physical examination), had bone age >14 years in girls and >16 years in boys, and who had been referred for a baseline dual x-ray absorptiometry (DXA) as part of their clinical care. Thirteen of these patients underwent follow-up BMD measurements by DXA. All patients were routinely monitored for endocrine dysfunction and treated accordingly.
DXA scans were performed on a Hologic 4500 scanner (Hologic, Inc., Bedford, MA) with Delphi upgrade, and BMD Z-scores were generated using recently published pediatric software (26). BMD measurements (g/cm2) of the total hip, femoral neck and L1–L4 spine were obtained and compared to age-matched, normative data (expressed as a Z-score). Height, weight, and BMI absolute values and Z-scores were collected at the time of DXA. Estimated volumetric BMD or bone mineral apparent density (BMAD) was calculated at the femoral neck and L2–L4 spine (27), and age, gender and self-reported ethnicity-matched Z-scores were generated from a database of 413 healthy Americans aged 9–25 years (28). No data were available to calculate BMAD at the total hip.
Diagnosis and treatment information for both the brain tumor and endocrinopathies were abstracted. GHD was diagnosed if either peak GH was less than 10 ng/mL after two provocative stimuli (insulin tolerance test or arginine, and glucagon), or IGF-1 level was low after pituitary stalk transection with resultant panhypopituitarism, or the patient had growth failure with low IGF-1, normal weight gain, and history of cranial RT with estimated dose to the hypothalamus and pituitary ≥23.4 Gy. Fracture history, age of pubertal onset according to Tanner staging, and menstrual history were also obtained.
Statistical analyses were performed using SPSS software (version 18.0.0, SPSS Inc., Chicago, IL). The two-tailed independent-sample t-test was used to assess differences in BMD and BMAD between treatment groups and between populations with and without relevant medical conditions. The Pearson correlation coefficient was used to assess the association between BMD Z-scores and BMAD Z-scores and height and to test for association between BMD and years since cancer diagnosis and age at cancer diagnosis. In a subsample with follow-up data, the paired t-test was used to determine whether significant change had occurred.
Results
Patient characteristics are shown in Table I. The age at initial DXA was 16.9 ±1.9 years (mean ± SD). Time since diagnosis was 8.5 ±3.6 years. Thirty of the 36 patients (83%) had received cranial RT, and 14 of those 30 patients had also received spinal RT. Brain tumor diagnoses are shown in Table II. The majority of patients were treated for astrocytoma/glioma, medulloblastoma, or craniopharyngioma.
Table I.
Patient characteristics, N (%) or mean ± SD.
| All | CSI + chemo |
CRT + chemo |
CRT only |
Surgery only |
|
|---|---|---|---|---|---|
| Patients | 36 | 14 | 4 | 12 | 6 |
| Male | 19 (53) | 8 (57) | 1 (25) | 9 (75) | 1 (17) |
| Female | 17 (47) | 6 (43) | 3 (75) | 3 (25) | 5 (83) |
| Age at diagnosis (years) | 8.4 ± 3.9 | 9.3 ± 3.5 | 9.3 ± 6.0 | 7.2 ± 3.7 | 8.3 ± 3.6 |
| Age at DXA (years) | 16.9 ± 1.9 | 16.8 ± 2.1 | 16.1 ± 1.9 | 17.7 ± 1.7 | 16.4 ± 1.5 |
| Height Z-score | −0.7 ± 1.3 | −1.1 ± 1.2 | −1.6 ± 1.2 | −0.1 ± 1.4 | −0.4 ± 0.7 |
| Weight Z-score | 0.7 ± 2.0 | −0.3 ± 1.6 | 0.6 ± 3.0 | 1.3 ± 2.2 | 1.7 ± 1.2 |
| BMI Z-score | 0.7 ± 1.2 | 0.2 ± 1.3 | 0.9 ± 1.6 | 0.8 ± 1.2 | 1.4 ± 0.7 |
CSI: craniospinal irradiation, CRT: cranial radiation therapy.
Table II.
Brain tumor diagnoses in 36 patients
| Brain Tumor Diagnoses | n (%) |
|---|---|
| Astrocytoma/glioma | 9 (25) |
| Craniopharyngioma | 11 (31) |
| Ependymoma | 1 (3) |
| Germ cell tumor | 2 (6) |
| Medulloblastoma | 11 (31) |
| Pineoblastoma | 2 (6) |
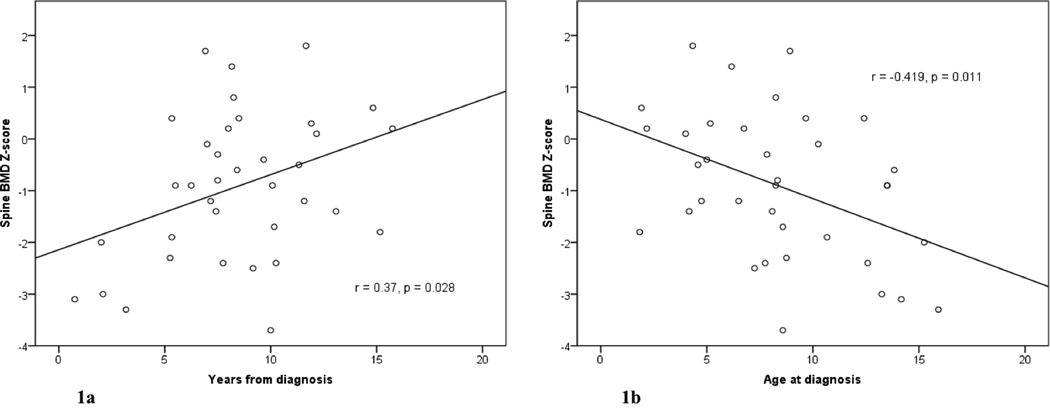
Overall, the patients had low-normal BMD and BMAD Z-scores at all sites, but wide variation was noted. The traditional method (BMD) revealed patients to be more severely affected than the volumetric method (BMAD) (Table III), though absolute BMD and BMD Z-scores were highly correlated with absolute BMAD and BMAD Z-scores at the femoral neck (r=0.774, P<0.000; r=0.884, P<0.000) and spine (r=0.846, P<0.000; r=0.938, P<0.000). Patients with fewer years since diagnosis had lower spinal BMD Z-scores than those farther from diagnosis (r=0.37, P=0.03; Figure 1a), and patients diagnosed at a younger age had higher spinal BMD Z-scores (r=−0.419, P=0.011; Figure 1b) and higher BMAD Z-score (r=−0.349, P=0.037); there were no significant correlations at the femoral neck or the hip. Those with a history of ≥1 fracture had lower BMD Z-scores of the femoral neck (mean −1.31 vs. −0.45, P=0.038), hip (−1.53 vs. −0.42, P=0.019) and spine (−1.45 vs. −0.44, P=0.042), and lower absolute hip BMD (0.830 vs. 0.944 g/cm2, P=0.042), neck BMAD (0.152 vs. 0.170 g/cm3, P=0.046) and spine BMAD (0.135 vs. 0.149 g/cm3, P=0.035) than those without. Patients with hypothyroidism (all treated) had higher spine BMD (0.922 vs. 0.773 g/cm2, P=0.003) than those without. Among patients with GHD, those treated with recombinant human GH (rhGH) for >1 year had higher BMD Z-scores and absolute BMD for the femoral neck, hip and spine. This difference was not related to differences in height, as neither absolute height nor height Z-scores were correlated with BMD Z-scores, and BMAD Z-scores and absolute BMAD for the spine were higher among the GH treated patients than among those not receiving therapy (Table IV). In the treated group, median length of treatment with rhGH was 4.6 years (1.3–12.0); one patient was treated for only 1.5 months and thus not considered among the treatment group. There was no difference in BMD or BMAD (Z-score or absolute) with respect to treatment with chemotherapy, cranial or spinal RT, or treated hypogonadism. Spontaneous menarche and regular periods did not correlate with BMD or BMAD. There were insufficient data to evaluate the relation of BMD or BMAD to calcium intake or vitamin D status.
Table III.
BMD and BMAD Z-scores
| Mean ± SD | Median | Range | Number (%) patients ≤ −2 |
Number (%) patients > −2 and ≤ −1 |
||
|---|---|---|---|---|---|---|
| Femoral neck | BMD | −0.83 ± 1.2 | −0.95 | −2.7 to 1.7 | 8 (22) | 10 (28) |
| BMAD | −0.38 ± 1.1 | −0.23 | −2.7 to 1.9 | 2 (6) | 8 (22) | |
| Spine | BMD | −0.91 ± 1.4 | −0.90 | −3.7 to 1.8 | 9 (25) | 7 (19) |
| BMAD | −0.43 ± 1.3 | −0.45 | −3.0 to 2.3 | 4 (11) | 9 (25) | |
| Hip | BMD | −0.91 ± 1.3 | −1.20 | −3.6 to 1.8 | 6 (17) | 15 (42) |
Figure 1.
Lumbar spine BMD by DXA relative to time from spinal radiation (a) and age at diagnosis (b).
Table IV.
Growth hormone deficiency and mean BMD and BMAD ± SEM
| Femoral neck | Hip | Spine | |||||
|---|---|---|---|---|---|---|---|
| Patients (n) |
BMD (g/cm2) | Z-score | BMD (g/cm2) | Z-score | BMD (g/cm2) | Z-score | |
| Treated | 20 | 0.878 ± 0.033 | −0.385 ± 0.273 | 0.970 ± 0.032 | −0.365 ± 0.259 | 0.978 ± 0.027 | −0.115 ± 0.255 |
| Untreated | 9 | 0.725 ± 0.034 | −1.378 ± 0.342 | 0.803 ± 0.045 | −1.533 ± 0.460 | 0.779 ± 0.036 | −2.067 ± 0.429 |
| p-value* | 0.01 | 0.043 | 0.006 | 0.025 | 0.000 | 0.000 | |
|
Patients (n) |
BMAD (g/cm3) | Z-score | BMAD (g/cm3) | Z-score | |||
| Treated | 20 | 0.168 ± 0.006 | −0.130 ± 0.25 | 0.150 ± 0.004 | 0.248 ± 0.264 | ||
| Untreated | 9 | 0.157 ± 0.009 | −0.706 ± 0.393 | 0.131 ± 0.007 | −1.368 ± 0.418 | ||
| p-value* | 0.33 | 0.22 | 0.015 | 0.002 | |||
Testing for equal mean in treated and untreated patients.
Thirteen of these patients had follow-up DXA scans a median of 2.3 years (1.7 to 3.5) after the first one. There were no significant changes in BMD or BMAD at the femoral neck, hip or spine. There was also no significant difference found in the GH deficient patients comparing those who were receiving rhGH treatment to those who were not, during the interval between DXA scans.
Discussion
This study focused on BMD in post-pubertal adolescent survivors of childhood brain tumor to determine whether they had an adequate BMD as they approached the time of peak bone mass accrual, given that the majority of bone mass is attained during the pubertal years (29). Risk factors for a decreased BMD were evaluated, in particular, age at diagnosis, time from diagnosis, tumor treatments, the development of endocrinopathies and their treatments. We found that the median BMD and BMAD in these patients was within normal limits, but some patients had a significantly low bone mass for age, gender and ethnicity. Lumbar spine BMD was significantly lower in those patients closer to diagnosis suggesting that peri-treatment factors, possibly illness, poor nutrition, and/or decreased activity, affect BMD and that there is some recovery over time. This finding was further supported by the result that patients who were diagnosed at a younger age, and therefore had longer follow-up, had higher spinal BMD and BMAD.
Those patients with a history of one or more fractures had lower BMD Z-scores of the femoral neck, hip, and spine, a lower absolute BMD at the hip, and lower absolute BMAD at the femoral neck and spine. Although still limited, there are increasing data that describe the relationship between DXA measurements and fracture risk in growing children and adolescents. For example, a four-year study of children with forearm fractures demonstrated a low BMD at several skeletal sites after adjustment for bone area, height, weight and pubertal stage, confirming the premise that fractures represent a marker of skeletal fragility (30).
Many studies have shown that adults with organic GHD have low bone turnover osteoporosis with increased vertebral and non-vertebral fractures (31). Adults with childhood onset of their GHD have lower BMD than those with adult onset, suggesting that the lack of GH during late adolescence and early adulthood, a time when bone and muscle mass normally continue to increase, contributes significantly to the deterioration of these parameters in adults with childhood onset GHD (32). Likewise, it has been suggested that GHD in childhood and adolescence leads to reduced peak bone mass (33). However, size-corrective approaches yield areal (two-dimensional) BMD mean Z-scores mainly between 0 and −1 in children with this diagnosis (34), suggesting that the apparent reduced BMD may be an artifact of smaller bone size. In addition, in a study by Barr et al of 19 children, average 7 years from treatment for brain tumors, 5 of 6 subjects treated with rhGH for GHD were “osteopenic” on radiologic assessment; in children with GHD, the mean BMD Z-scores for vertebrae and femora were −1.13 and −1.45, respectively (6). In another study of survivors of childhood brain tumors, of the 3 patients who had received rhGH treatment for GHD, 2 were osteopenic (lumbar spine BMD Z-score <−1.0) and one was floridly osteoporotic (whole body BMC Z-score <−2.5) (24). We found that those with GHD treated with rhGH had higher BMAD and BMAD Z-scores at the spine than those not receiving therapy and that height was not significantly correlated with BMD Z-scores, suggesting that GHD does lead to a decreased BMD.
In survivors of childhood brain tumors, Pietilä et al. found that one-third of patients aged 3.8–28.7 years (mean 14.9, one-third >18 years) had a low total body BMD Z-score (<−2.0) with only combined craniospinal radiation significantly associated with low Z-score, although exclusive cranial radiation was of borderline statistical significance (25). However, we found no difference in BMD (Z-score or absolute) with respect to treatment with spinal and/or cranial RT.
We found no statistically significant difference in BMD between those patients who had and had not received rhGH replacement for their GHD after 1.7–3.5 years of follow-up, in keeping with the findings of Mauras et al, who showed that discontinuation of rhGH therapy for 2 years in adolescents with persistent GHD did not affect rate of bone accretion (35). However, Conway et al. showed that after 24 months, lumbar spine BMD increased more significantly in rhGH-treated young adults with childhood onset GHD than in controls (6% vs. 2%; estimated treatment difference 3.5% (95% confidence interval, 1.52–5.51) P<0.001). Treatment with rhGH also had a significant positive effect on total hip BMD (P=0.015); total body BMD was unchanged from baseline (P=0.315) (36).
We found that patients with hypothyroidism (all treated) had higher spine BMD than those without hypothyroidism. The role of the hypothalamic-pituitary-thyroid axis in bone metabolism is controversial. Hyperthyroidism may be associated with secondary osteoporosis and fractures in adults. Cross-sectional studies have demonstrated an inverse relationship between BMD and thyroxine (T4) concentrations (37). T4 is converted to triiodothyronine (T3), which regulates osteoblast proliferation, differentiation and function directly and may regulate osteoclast proliferation, differentiation and function either directly or via osteoblast-mediated effects. Since clinicians cannot use TSH for guidance regarding adequacy of therapy, our goal is to keep T4 levels mid-range. However, there is a wide normal range for T4; mid-range may be over-treatment in some patients and under-treatment in others. Thyrotropin (TSH) has been proposed as a negative regulator of bone turnover independent of T3; TSH may promote osteoblast and inhibit osteoclast progenitor commitment (37). Many of our patients have central hypothyroidism and low levels of TSH. Our analysis did not look at actual T4 and TSH levels.
The strength of this study is that it is the only report to date examining a cohort of childhood survivors of brain tumor who are of similar age and pubertal status, removing the potential confounding of Tanner staging. There are also no published data on BMD changes over time in the same patient in childhood cancer survivors. There are several limitations of this study. DXA scans were performed as part of a routine clinical evaluation, not as part of a formal research study. DXA is a 2-dimensional study and bone depth can affect measured BMD. To address this issue, we calculated BMAD to estimate 3-dimensional density but did not directly measure it. Data on potential risk factors of calcium and vitamin D intake, vitamin D levels, and type of fracture (traumatic or fragility) were not collected or were missing. Future prospective studies with longer follow up and that use a larger sample size and modalities that evaluate true volumetric BMD (e.g., pQCT and/or MRI) are needed.
In childhood cancer survivors, the disease, its treatments, poor nutrition, lack of exercise, and the development of certain endocrinopathies are all likely to interact, resulting in a compromised BMD during a crucial period for bone growth and skeletal accretion. Bone mass should normally increase steadily throughout childhood and reach a maximum following 20 years of age (9). Normal increases in circulating GH and sex hormones during puberty increase rate of bone mass accrual during this time (9). It is not known whether a failure to acquire cortical and trabecular bone mass during childhood and adolescence is reversible in the long-term. The magnitude of the deficit and the potential for recovery likely depend upon the timing and duration of the insults relative to periods of rapid growth and maturation. As there are no prospective studies relating bone mass to fracture risk in these patients, the significance of BMD deficits in children and young adult cancer survivors is unknown (38, 39). However, since decreased DXA-derived areal BMD values have been associated with increased fracture rates in peri- and postmenopausal women (40), there is concern that if BMD remains low after achievement of peak mass, these survivors could be at increased risk for osteoporotic fractures later in life. Providers caring for survivors of childhood brain tumors should assess their patients for risk factors for a low BMD, aim to maximize nutrition, exercise, and calcium and vitamin D intake, and refer to an endocrinologist for a potential bone health assessment and treatment of any hormonal deficiencies.
Acknowledgments
Supported in part by the Harvard Clinical and Translational Science Center under NIH UL1 RR-025758.
References
- 1.Vassilopoulou-Sellin R, Brosnan P, Delpassand A, et al. Osteopenia in young adult survivors of childhood cancer. Med Pediatr Oncol. 1999;32:272–278. doi: 10.1002/(sici)1096-911x(199904)32:4<272::aid-mpo6>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 2.Aisenberg J, Hsieh K, Kalaitzoglou G, et al. Bone mineral density in young adult survivors of childhood cancer. J Pediatr Hematol Oncol. 1998;20:241–245. doi: 10.1097/00043426-199805000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Tillman V, Darlington ASE, Eiser C, et al. Male sex and low physical activity are associated with reduced spine bone mineral density in survivors of childhood lymphoblastic leukemia. J Bone Miner Res. 2002;17:1073–1080. doi: 10.1359/jbmr.2002.17.6.1073. [DOI] [PubMed] [Google Scholar]
- 4.Halton JM, Atkinson SA, Fraher L, et al. Mineral homeostasis and bone mass at diagnosis in children with acute lymphoblastic leukemia. J Pediatr. 1995;126:557–564. doi: 10.1016/s0022-3476(95)70349-7. [DOI] [PubMed] [Google Scholar]
- 5.Arikoski P, Komulainen J, Riikonen P, et al. Alterations in bone turnover and impaired development of bone mineral density in newly diagnosed children with cancer: A 1-year prospective study. J Clin Endocrinol Metab. 1999;84:3174–3181. doi: 10.1210/jcem.84.9.5968. [DOI] [PubMed] [Google Scholar]
- 6.Barr RD, Simpson T, Webber CE, et al. Osteopenia in children surviving brain tumours. Eur J Cancer. 1998;34:873–877. doi: 10.1016/s0959-8049(98)00011-2. [DOI] [PubMed] [Google Scholar]
- 7.Petraroli M, D'Alessio E, Ausili E, et al. Bone mineral density in survivors of childhood brain tumours. Childs Nerv Syst. 2007;23:59–65. doi: 10.1007/s00381-006-0175-7. [DOI] [PubMed] [Google Scholar]
- 8.Gurney JG, Kadan-Lottick NS, Packer RJ, et al. Endocrine and cardiovascular late effects among adult survivors of childhood brain tumors: Childhood cancer survivor study. Cancer. 2003;97:663–673. doi: 10.1002/cncr.11095. [DOI] [PubMed] [Google Scholar]
- 9.Olney RC. Regulation of bone mass by growth hormone. Med Pediatr Oncol. 2003;41:228–234. doi: 10.1002/mpo.10342. [DOI] [PubMed] [Google Scholar]
- 10.Sklar CA, Constine LS. Chronic neuroendocrinological sequelae of radiation therapy. Int J Radiat Oncol Biol Phys. 1995;31:1113–1121. doi: 10.1016/0360-3016(94)00427-M. [DOI] [PubMed] [Google Scholar]
- 11.Frank GR. Role of estrogen and androgen in pubertal skeletal physiology. Med Pediatr Oncol. 2003;41:217–221. doi: 10.1002/mpo.10340. [DOI] [PubMed] [Google Scholar]
- 12.Layman LC. Hypogonadotropic hypogonadism. Endocrinol Metab Clin North Am. 2007;36:283–296. doi: 10.1016/j.ecl.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 13.Hopewell JW. Radiation-therapy effects on bone density. Med Pediatr Oncol. 2003;41:208–211. doi: 10.1002/mpo.10338. [DOI] [PubMed] [Google Scholar]
- 14.Krishnamoorthy P, Freeman C, Bernstein ML, et al. Osteopenia in children who have undergone posterior fossa or craniospinal irradiation for brain tumors. Arch Pediatr Adolesc Med. 2004;158:491–496. doi: 10.1001/archpedi.158.5.491. [DOI] [PubMed] [Google Scholar]
- 15.Hochberg Z. Mechanisms of steroid impairment of growth. Horm Res. 2002;58:33–38. doi: 10.1159/000064764. [DOI] [PubMed] [Google Scholar]
- 16.Rehman Q, Lane NE. Effect of glucocorticoids on bone density. Med Pediatr Oncol. 2003;41:212–216. doi: 10.1002/mpo.10339. [DOI] [PubMed] [Google Scholar]
- 17.Muller J. Impact of cancer therapy on the reproductive axis. Horm Res. 2003;59(Suppl 1):12–20. doi: 10.1159/000067835. [DOI] [PubMed] [Google Scholar]
- 18.French SA, Fulkerson JA, Story M. Increasing weight-bearing physical activity and calcium intake for bone mass growth in children and adolescents: A review of intervention trials. Prev Med. 2000;31:722–731. doi: 10.1006/pmed.2000.0758. [DOI] [PubMed] [Google Scholar]
- 19.Harz KJ, Muller HL, Waldeck E, et al. Obesity in patients with craniopharyngioma: Assessment of food intake and movement counts indicating physical activity. J Clin Endocrinol Metab. 2003;88:5227–5231. doi: 10.1210/jc.2002-021797. [DOI] [PubMed] [Google Scholar]
- 20.Dekkers OM, Biermasz NR, Smit JW, et al. Quality of life in treated adult craniopharyngioma patients. Eur J Endocrinol. 2006;154:483–489. doi: 10.1530/eje.1.02114. [DOI] [PubMed] [Google Scholar]
- 21.Brabant G, Wallaschofski H. Normal levels of serum igf-i: Determinants and validity of current reference ranges. Pituitary. 2007;10:129–133. doi: 10.1007/s11102-007-0035-9. [DOI] [PubMed] [Google Scholar]
- 22.Matkovic V, Landoll JD, Badenhop-Stevens NE, et al. Nutrition influences skeletal development from childhood to adulthood: A study of hip, spine, and forearm in adolescent females. J Nutr. 2004;134:701S–705S. doi: 10.1093/jn/134.3.701S. [DOI] [PubMed] [Google Scholar]
- 23.Henderson RC, Madsen CD, Davis C, et al. Bone density in survivors of childhood malignancies. J Pediatr Hematol Oncol. 1996;18:367–371. doi: 10.1097/00043426-199611000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Odame I, Duckworth J, Talsma D, et al. Osteopenia, physical activity and health-related quality of life in survivors of brain tumors treated in childhood. Pediatr Blood Cancer. 2006;46:357–362. doi: 10.1002/pbc.20512. [DOI] [PubMed] [Google Scholar]
- 25.Pietila S, Sievanen H, Ala-Houhala M, et al. Bone mineral density is reduced in brain tumour patients treated in childhood. Acta Paediatr. 2006;95:1291–1297. doi: 10.1080/08035250600586484. [DOI] [PubMed] [Google Scholar]
- 26.Kalkwarf HJ, Zemel BS, Gilsanz V, et al. The bone mineral density in childhood study: Bone mineral content and density according to age, sex, and race. J Clin Endocrinol Metab. 2007;92:2087–2099. doi: 10.1210/jc.2006-2553. [DOI] [PubMed] [Google Scholar]
- 27.Katzman DK, Bachrach LK, Carter DR, et al. Clinical and anthropometric correlates of bone mineral acquisition in healthy adolescent girls. J Clin Endocrinol Metab. 1991;73:1332–1339. doi: 10.1210/jcem-73-6-1332. [DOI] [PubMed] [Google Scholar]
- 28.Bhudhikanok GS, Wang MC, Eckert K, et al. Differences in bone mineral in young asian and caucasian americans may reflect differences in bone size. J Bone Miner Res. 1996;11:1545–1556. doi: 10.1002/jbmr.5650111023. [DOI] [PubMed] [Google Scholar]
- 29.Theintz G, Buchs B, Rizzoli R, et al. Longitudinal monitoring of bone mass accumulation in healthy adolescents: Evidence for a marked reduction after 16 years of age at the levels of lumbar spine and femoral neck in female subjects. J Clin Endocrinol Metab. 1992;75:1060–1065. doi: 10.1210/jcem.75.4.1400871. [DOI] [PubMed] [Google Scholar]
- 30.Jones IE, Taylor RW, Williams SM, et al. Four-year gain in bone mineral in girls with and without past forearm fractures: A dxa study. Dual energy x-ray absorptiometry. J Bone Miner Res. 2002;17:1065–1072. doi: 10.1359/jbmr.2002.17.6.1065. [DOI] [PubMed] [Google Scholar]
- 31.Giustina A, Mazziotti G, Canalis E. Growth hormone, insulin-like growth factors, and the skeleton. Endocr Rev. 2008;29:535–559. doi: 10.1210/er.2007-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Radovick S, DiVall S. Approach to the growth hormone-deficient child during transition to adulthood. J Clin Endocrinol Metab. 2007;92:1195–1200. doi: 10.1210/jc.2007-0167. [DOI] [PubMed] [Google Scholar]
- 33.Soyka LA, Fairfield WP, Klibanski A. Clinical review 117: Hormonal determinants and disorders of peak bone mass in children. J Clin Endocrinol Metab. 2000;85:3951–3963. doi: 10.1210/jcem.85.11.6994. [DOI] [PubMed] [Google Scholar]
- 34.Hogler W, Shaw N. Childhood growth hormone deficiency, bone density, structures and fractures: Scrutinizing the evidence. Clin Endocrinol (Oxf) 2010;72:281–289. doi: 10.1111/j.1365-2265.2009.03686.x. [DOI] [PubMed] [Google Scholar]
- 35.Mauras N, Pescovitz OH, Allada V, et al. Limited efficacy of growth hormone (gh) during transition of gh-deficient patients from adolescence to adulthood: A phase iii multicenter, double-blind, randomized two-year trial. J Clin Endocrinol Metab. 2005;90:3946–3955. doi: 10.1210/jc.2005-0208. [DOI] [PubMed] [Google Scholar]
- 36.Conway GS, Szarras-Czapnik M, Racz K, et al. Treatment for 24 months with recombinant human gh has a beneficial effect on bone mineral density in young adults with childhood-onset gh deficiency. Eur J Endocrinol. 2009;160:899–907. doi: 10.1530/EJE-08-0436. [DOI] [PubMed] [Google Scholar]
- 37.Bassett JH, Williams GR. Critical role of the hypothalamic-pituitary-thyroid axis in bone. Bone. 2008;43:418–426. doi: 10.1016/j.bone.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 38.Leonard MB. Assessment of bone health in children and adolescents with cancer: Promises and pitfalls of current techniques. Med Pediatr Oncol. 2003;41:198–207. doi: 10.1002/mpo.10337. [DOI] [PubMed] [Google Scholar]
- 39.Kaste SC. Bone-mineral density deficits from childhood cancer and its therapy. A review of at-risk patient cohorts and available imaging methods. Pediatr Radiol. 2004;34:373–378. doi: 10.1007/s00247-003-1132-1. quiz 443-374. [DOI] [PubMed] [Google Scholar]
- 40.Arikoski P, Voutilainen R, Kroger H. Bone mineral density in long-term survivors of childhood cancer. J Pediatr Endocrinol Metab. 2003;16(Suppl 2):343–353. [PubMed] [Google Scholar]