Abstract
Objective
To determine how gestational weight gain (GWG), categorized using the 2009 Institute of Medicine recommendations, relates to changes in offspring weight-for-age (WAZ), length-for-age (LAZ), and weight-for-length z-scores (WLZ) between early infancy and 3 years.
Methods
Women with singleton infants were recruited from the third cohort of the Pregnancy, Infection, and Nutrition Study (2001-2005). Term infants with at least one weight or length measurement during the study period were included (n=476). Multivariable linear mixed effects regression models estimated longitudinal changes in WAZ, LAZ, and WLZ associated with GWG.
Results
In early infancy, compared to infants of women with adequate weight gain, those of women with excessive weight gains had higher WAZ, LAZ, and WLZ. Excessive GWG≥200% of the recommended amount was associated with faster rates of change in WAZ and LAZ and noticeably higher predicted mean WAZ and WLZ that persisted across the study period.
Conclusions
GWG represents a modifiable behavioral factor that is associated with offspring anthropometric outcomes. More longitudinal studies that utilize maternal and pediatric body composition measures are necessary to understand the nature of this association.
Keywords: gestational weight gain, obesity, longitudinal, anthropometrics, offspring
Introduction
Obesity continues to be a major health epidemic among children in the United States, and it is being documented as early as infancy. Recent studies show that 9.5% of children younger than 2 years have a weight-for-length at or above the 95th percentile (1). The prevalence of overweight/obesity (>=85th percentile) is 21.2% among children ages 2-5 years (1), and the rates among low income preschoolers are increasing (2). Treatment of pediatric obesity is an arduous task, encompassing behavioral, social, and environmental factors (3); therefore, the identification of modifiable risk factors to prevent the development of overweight and obesity during infancy and early childhood is of great importance.
Gestational weight gain (GWG) is a modifiable factor associated with offspring anthropometric outcomes (4, 5); however, relatively few studies examine these associations using longitudinal data. Li and colleagues examined the role of early life factors in overweight (>=95th percentile) trajectories in children from ages 2 to 12 years. GWG ≥20.43 kg was associated with increased odds of early onset overweight (defined as a consistent high probability of overweight status) but not late onset overweight (defined as an initial high probability of overweight status at 2 years, low probability of overweight at ages 4-6 years, and high probability of overweight after age 8 years)(6). More recently, Crozier and colleagues reported higher fat mass at birth, 4 years, and 6 years among offspring of women with excessive versus adequate GWG (based on the 2009 Institute of Medicine (IOM) recommendations) (7). These results suggest that GWG influences the development of obesity early in life.
In this study, we used data from a recent prospective pregnancy cohort and categorized GWG according to the 2009 IOM recommendations to further investigate the association of GWG and offspring anthropometrics. The main objective was to determine how GWG relates to offspring weight-for-age, length-for-age, and weight-for-length z-scores using weight and length measurements collected from physicians’ records during the first year of life and by research staff at 3 years of age. The use of longitudinal data allowed for an examination of how the associations between GWG and the selected anthropometric outcomes differed and changed over time.
Method
Study Participants
Participants were women with singleton births with no major birth defects from the third cohort of the Pregnancy Infection and Nutrition (PIN 3) study, January 1, 2001- June 30, 2005. The recruitment protocols for the study were documented previously (8). In 2003, the PIN Postpartum study recruited eligible women from PIN (n=1169) and followed the index infant at 3 months (n=689) and 12 months (n=550) postpartum (9). The PIN Kids study (beginning 2004) followed the index infant at 3 years (n=409). Mothers completed several self-administered questionnaires and two phone interviews during the prenatal period, and in-home interviews at 3 and 12 months and 3 years postpartum to provide details on health and lifestyle behaviors for themselves and their infants. Details of the PIN studies are available at the website: http://www.cpc.unc.edu/projects/pin/. The PIN study protocols were reviewed and approved by the Institutional Review Board of the School of Medicine at the University of North Carolina at Chapel Hill.
We used data from the 550 mother-child pairs who participated at 12 months postpartum. Children with physician-diagnosed illnesses related to growth (n=3) and women with missing GWG or prepregnancy BMI (n=3) were excluded. Preterm (gestational age <37 weeks) infants were also excluded (n=68) due to possible growth differences in early life. The remaining 476 mother-child pairs had at least one anthropometric measurement (weight or length) between birth and 3 years and were included in the analyses.
Distributions of selected baseline characteristics across the included (n=476) and excluded/non-participating (n=693) mother-child pairs were examined. In comparison to included mothers, those who did not participate at 12 months were more likely to be <30 years at conception, overweight/obese, black, not married, low education, low income, and smokers. They were also more likely to have infants with lower mean birth weight and gestational age. All other comparisons of characteristics were not significant.
Anthropometrics
Infant birthweight (n=474) and sex (n=476) were abstracted from delivery logs (birth length was not available). Gestational age was calculated using an algorithm based on the first ultrasound measurement performed prior to 22 weeks’ gestation. If no ultrasound was performed prior to the start of week 22, then the date of last menstrual period was used (n=7). Infants’ weights and lengths were recorded on study-provided doctor’s cards during well-baby visits. These visits are recommended by the American Academy of Pediatrics at 1, 2, 4, 6, 9, and 12 months of age but the frequency and times of the visits varied among infants. The difference between the recorded date of the visit and the birth date were used to calculate the exact age of the infant at each measurement. We estimated age using the 15th of the month for those infants missing the day of the visit (n=14) and we used the pediatrician recorded age when both the month and day of the visit were missing (n=64). At the 3-year home visit, children’s standing height and weight were measured by trained PIN staff using stadiometers and scales according to National Health and Nutrition Examination Surveys (NHANES) protocols (10).
Weight-for-age (WAZ), length/height-for-age (LAZ) and weight-for-length/height (WLZ) z-scores were calculated using the 2000 Centers for Disease Control and Prevention/National Center for Health Statistics growth charts (11). The use of z-scores accounts for sex differences and for variation in the actual ages of the children at the time of each measurement. Z-scores are also necessary to express relative weight (weight-for-length). They were used as the unit of measurement for all outcomes to maintain consistency and enable comparison of effect sizes across outcomes. Measurements that corresponded to z-scores <-5 or >5 were considered implausible and not included in analyses (WAZ, n=0; LAZ, n=8; WLZ, n=7). For the analyses, 476 children contributed 3050 WAZ measurements (mean 6.4 measurements per child, range 1-11 measurements); 424 children contributing 2772 LAZ measurements (mean 6.5 measurements per child, range 1-11 measurements); and 422 children contributing 2679 WLZ measurements (mean 6.3 measurements per child, range 1-11 measurements).
Maternal Exposures
Maternal prepregnancy BMI (kg/m2) and total GWG were categorized according to the 2009 IOM recommendations: underweight (BMI<18.5), 28-40 lbs; normal weight (BMI 18.5-24.9), 25-35 lbs; overweight (BMI 25.0-29.9), 15-25 lbs; and obese (BMI ≥ 30), 11-20 lbs (12). Prepregnancy BMI was calculated using self-reported prepregnancy weight and measured height. Implausible prepregnancy weights (n=15) were imputed based on the measured first trimester weights (8). GWG was defined as the difference between self-reported prepregnancy weight and the last weight measurement prior to delivery. The median difference in weeks between the gestational age at delivery and the gestational age at the last weight measurement was 0.3. There were 23 (4.8%) women with a difference between the two measurements of >2 weeks, 18 (78%) of whom were categorized as having inadequate or adequate GWG. An adequacy of GWG percentage (or ratio) was calculated by dividing the total observed GWG by the expected GWG based on the 2009 IOM recommendations (12) specific for a given prepregnancy BMI category and the trimester of gestation, using previously described methodology (8). Briefly, expected GWG was calculated based on the following formula: Expected First Trimester Total GWG + [(Gestational Age at Time of Last Weight Measurement × 13 weeks) × Rate of GWG Expected for the Second and Third Trimesters]. The use of rates of weight gain adjusts for the fact that not all women have a weight measurement at the time of delivery. Cutoffs to determine inadequate, adequate, and excessive GWG were based on ranges of adequacy percentages. Because so many women had excessive GWG, this category was further dichotomized: Excessive I GWG (n=213) was defined as excessive GWG up to 199% of the recommendations. Excessive II GWG (n=68) was defined as excessive GWG≥200% of the recommended amount.
Covariates
Maternal prenatal smoking, household income (expressed as percent of the poverty line using the 2001 U.S. Department of Health and Human Services Federal Poverty Guidelines (13)), education, marital status, age at conception, race/ethnicity, pre-existing diabetes, and parity were assessed during the prenatal period and categorized as shown in Table 1. Due to small numbers of women who self-identified as a race/ethnicity other than white or black (Hispanic, n=23; American Indian, n=2; Asian/Pacific Islander, n=15; Other, n=7), race/ethnicity was dichotomized as White/Other and Black. Household income at the 3-month interview was used if information was missing from the prenatal period (n=10). Glucose tolerance was derived from universal screens during the second trimester (mean gestational age ~27 weeks) on women without pre-existing diabetes mellitus. Women with abnormal values on the screen had a 3-hour oral glucose tolerance test (OGTT) to confirm gestational diabetes mellitus (GDM). GDM was defined as having two abnormal values (14) on the OGTT or a GDM diagnosis by a physician listed on the medical record.
Table 1.
Distribution of Baseline Characteristics of Women and Children in the Pregnancy, Infection, and Nutrition 3 Study (n=476)
| Variable | n | Mean(SD)/Frequency,% |
|---|---|---|
| Maternal age at conception (years) | 476 | 30.0 (5.4) |
| Maternal age at conception (years) | 476 | |
| 16-24 | 78 | 16.4 |
| 25-29 | 133 | 27.9 |
| 30-34 | 176 | 37.0 |
| 35-47 | 89 | 18.7 |
| Prepregnancy BMI (kg/m2) | 476 | 24.8 (6.3) |
| Prepregnancy BMI Category | 476 | |
| Underweight | 24 | 5.0 |
| Normal Weight | 297 | 62.4 |
| Overweight | 82 | 17.2 |
| Obese | 73 | 15.3 |
| Maternal Height (meters) | 476 | 1.7 (0.07) |
| Gestational Weight Gain (kg) | 476 | 15.7 (5.6) |
| IOM Gestational Weight Gain Category | 476 | |
| Inadequate | 57 | 12.0 |
| Adequate | 138 | 29.0 |
| Excessive I | 213 | 44.8 |
| Excessive II | 68 | 14.3 |
| Race | 476 | |
| White/Other | 423 | 88.9 |
| Black | 53 | 11.1 |
| Marital Status | 476 | |
| Married | 399 | 83.8 |
| Other | 77 | 16.2 |
| Education | 476 | |
| ≤ Grade 12 | 63 | 13.2 |
| Grades 13 -16 | 226 | 47.5 |
| ≥ Grade 17 | 187 | 39.3 |
| Household Income (% Poverty) | 475 | |
| <185% | 73 | 15.4 |
| 185-350% | 94 | 19.8 |
| >350% | 308 | 64.8 |
| GDM (excludes pre-existing diabetes mellitus) |
457 | |
| No | 437 | 95.6 |
| Yes | 20 | 4.4 |
| Pre-existing Diabetes Mellitus | 476 | |
| No | 457 | 96.0 |
| Yes | 19 | 4.0 |
| Parity | 476 | |
| Nulliparous | 232 | 48.7 |
| 1 or More Births | 244 | 51.3 |
| Smoking in Months 1-6 of Pregnancy | 460 | |
| No | 428 | 93.0 |
| Yes | 32 | 7.0 |
| Infant Sex | 476 | |
| Male | 247 | 51.9 |
| Female | 229 | 48.1 |
| Gestational Age (weeks) | 476 | 39.2 (1.2) |
Statistical Analyses
Statistical analyses were performed using STATA 11 (College Station, TX). Means, standard deviations (SD), and frequencies (%) were calculated for baseline characteristics in the population. Potential effect measure modifiers and confounders were identified a priori from a review of previous literature and causal diagrams (15, 16).
Child WAZ, LAZ, and WLZ were individually plotted between 0 and 3 years to visually inspect the longitudinal pattern. The resulting graphs suggested linear relations for LAZ and WLZ but a non-linear relation with age for WAZ. Addition of a child’s age2 term to the model was significant (p<0.01) and, therefore, included in the WAZ models. Linear mixed effects regression models (17-19) were fit using restricted maximum likelihood specified by the xtmixed function in STATA 11. These models were used to determine how GWG related to WAZ, LAZ, and WLZ between 0 and 3 years. The mixed effects model easily accommodates these data since they account for unbalanced data (unequal numbers of repeated measurements within children and irregular spacing of measurements across time and across children) and correlations of measurements within children (20). We also considered a spline approach to separately estimate changes in anthropometrics between 0 and 12 months and 12 and 36 months. The graphs produced from the mixed models and splines were similar with the exception being that graphs for mixed models that included polynomials had slight troughs (an artifact of the quadratic term) while those using splines had a straight line; therefore, we opted to present statistical analyses using the mixed models.
We included random intercepts and slopes to allow for child-specific intercepts and rates of overall growth. Interactions were examined to determine whether associations between offspring anthropometric outcomes and categories of GWG varied over time or whether the effects of gestational age and child’s age varied overtime. We also tested for an interaction between continuous and categorical measures of prepregnancy BMI and GWG. All fixed effects interaction terms were tested using Wald tests with an a priori significance p-value of <0.15. Full models for GWG were adjusted for income (185-350% used as referent), education (grades 13-16 used as referent), race (non-Black used as referent), smoking (nonsmoker used as referent), pre-existing diabetes mellitus (no diagnosis used as referent), and marital status (married used as referent) using the categories shown in Table 1 as well as prepregnancy BMI (centered at 23 kg/m2), maternal height (centered at 1.65 meters) and gestational age (centered at 40 weeks). Maternal glucose tolerance and pregnancy induced hypertension were not considered as confounders and, therefore, were not included in the full models.
Results
The mean (SD) prepregnancy BMI was 24.8 (6.3) kg/m2; approximately a third of the women were overweight or obese. The mean (SD) GWG and adequacy of GWG were 15.69 (5.62) kg and 141.0 (64.0)%, respectively. Fifty-nine percent of women had excessive GWG with 14.3% having GWG>=200% of the recommendations (Table 1). The frequency (%) of inadequate, adequate, excessive I, and excessive II GWG across prepregnancy BMI categories were: 16.7, 33.3, 50.0, and 0.0% among underweight women; 14.1, 35.0, 47.8, and 3.0% among normal weight women; 2.4, 13.4, 50.0, and 34.1% among overweight women; and 12.3, 20.5, 24.7, and 42.5% among obese women. The majority of women were white, 25-34 years of age at conception, married, college educated, non-smokers, and from high income households (Table 1). The mean (SD) birth weight and gestational age were 3435.9 (434.5) g and 39.2 (1.2) weeks, respectively.
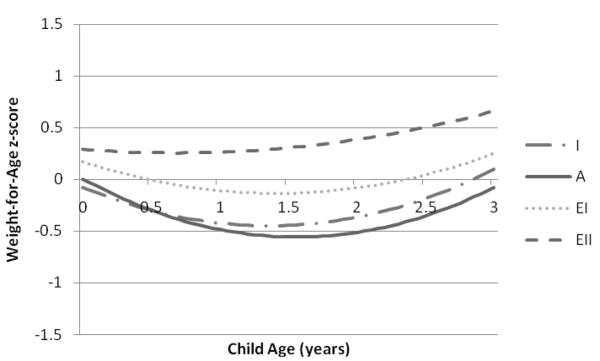
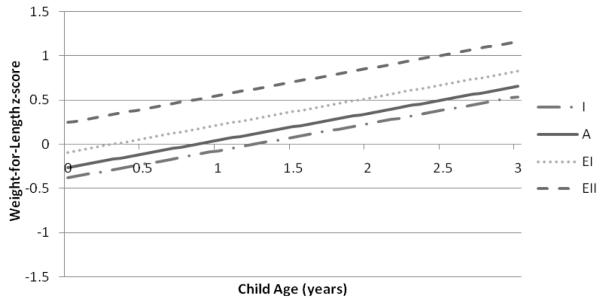
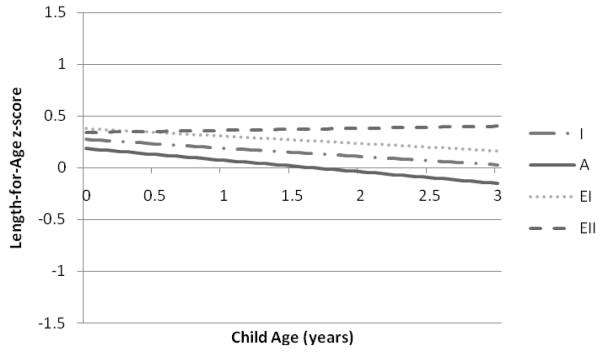
Predicted mean values of WAZ, LAZ, and WLZ were lowest among children of women with inadequate and adequate GWG and highest among children of women with excessive II GWG (Figures 1-3). Significant differences in WAZ, LAZ, and WLZ in early infancy were observed across categories of GWG (Table 2). Excessive II GWG was associated with higher WAZ at birth and a significant rate of change in WAZ across the study period (interaction of high excessive GWG and child’s age) compared to adequate GWG (Table 2). Predicted mean WAZ for children of mothers with excessive II GWG was higher and increased over time compared to children of mothers in the other GWG categories (Figure 1). Excessive I GWG was associated with higher LAZ in early infancy and excessive II GWG was associated with a significant rate of change in LAZ across the study period (Table 2). Excessive II GWG was associated with higher predicted mean WLZ in early infancy compared to adequate GWG but there were no significant interactions between GWG categories and child’s age (Table 2). Predicted mean WLZ increased between 0 and 3 years across the GWG categories with the highest values observed for children of women with excessive II GWG (Figure 3). We did not find evidence of an interaction between prepregnancy BMI and adequacy of GWG for WAZ, LAZ, or WLZ.
Figure 1.
Predicted mean child weight-for-age z-scores from birth to 3 years associated with gestational weight gain categories based on the 2009 IOM recommendations in the Pregnancy, Infection, and Nutrition 3 Study (n=459).
Predicted mean child WAZ from birth (age 0 years) to 3 years are based on the adjusted linear mixed model for WAZ displayed in Table 2. Categories of gestational weight gain designated as I=inadequate; A=adequate; EI=excessive I; EII=excessive II.
Figure 3.
Predicted mean child weight-for-length z-scores from birth to 3 years associated with gestational weight gain categories based on the 2009 IOM recommendations in the Pregnancy, Infection, and Nutrition 3 Study (n=412).
Predicted mean child WLZ from birth (age 0 years) to 3 years are based on the adjusted linear mixed model for WLZ displayed in Table 2. Categories of gestational weight gain designated as I=inadequate; A=adequate; EI=excessive I; EII=excessive II.
Table 2.
Adjusteda linear mixed model of the association between categories of gestational weight gain and offspring weight-for-age (WAZ), length-for-age (LAZ), and weight-for-length z-scores (WLZ) between birth and 3 years in the Pregnancy, Infection, and Nutrition 3 Study
| WAZ (n=459) bb (95% CI) |
p-value | LAZ (n=414) b (95% CI) |
p-value | WLZ (n=412) b (95% CI) |
p-value | |
|---|---|---|---|---|---|---|
| Intercept | −0.09 (−0.53, 0.36) | 0.70 | 0.32(−0.18, 0.82) | 0.21 | −0.49 (−1.09, 0.12) | 0.12 |
| Inadequate | −0.08 (−0.32, 0.16) | 0.50 | 0.09 (−0.16, 0.33) | 0.49 | −0.13 (−0.41, 0.36) | 0.38 |
| Adequate | Reference | Reference | Reference | |||
| Excessive I | 0.17 (0.00, 0.34) | 0.05 | 0.19 (0.02, 0.37) | 0.03 | 0.16 (−0.04, 0.36) | 0.11 |
| Excessive II | 0.28 (0.04, 0.53) | 0.02 | 0.16 (−0.10, 0.41) | 0.23 | 0.50 (0.20, 0.80) | 0.001 |
| Child’s Age | −0.72 (−1.00, −0.44) | <0.001 | −0.11(−0.18, −0.04) | <0.001 | 0.26 (0.21, 0.31) | <0.001 |
| Inadequate x Child’s Age | 0.17 (−0.33, 0.68) | 0.50 | 0.03 (−0.10, 0.16) | 0.64 | ¯ | |
| Adequate x Child’s Age | Reference | Reference | ¯ | |||
| Excessive I x Child’s Age | 0.29 (−0.07, 0.64) | 0.11 | 0.04 (−0.05, 0.13) | 0.37 | ¯ | |
| Excessive II x Child’s Age | 0.62 (0.14, 1.10) | 0.01 | 0.13 (0.01, 0.25) | 0.03 | ¯ |
All models are adjusted for income, education, race, smoking, marital status, prepregnancy diabetes mellitus, prepregnancy BMI (centered at 23 kg/m2), maternal height (centered at 1.65 m), and gestational age (centered at 40 weeks). WAZ model is also adjusted for: child’s age2; child’s age2 x gestational weight gain; child’s age x gestational age; and child’s age2 x gestational age. LAZ model is also adjusted for: child’s age x gestational age.
b, Coefficient from linear mixed effects model with random intercept and slope.
Discussion
In this study, we found significant differences in offspring anthropometrics in early infancy that persisted to 3 years of age across categories of GWG. Compared to infants of women with adequate GWG, those born to women with excessive GWG had higher WAZ, LAZ, and WLZ in early infancy. Excessive GWG≥200% of the recommended amount was associated with faster rates of change in WAZ and LAZ and persistently higher predicted mean WAZ and WLZ. Mothers with excessive I GWG had infants with higher WAZ and LAZ , and those with excessive II GWG had infants with higher WAZ and WLZ compared to mothers with adequate GWG.
Our study must be interpreted within the context of several limitations. First, the majority of infant measurements within the first year came from well-baby visits recorded on doctor’s cards by medical staff. These measurements, especially length (21), are subject to error because they were collected at multiple clinic sites and medical staff was not trained to use a standardized method. The number and timing of measurements also differed between children, and no measurements were available between approximately 12 and 35 months. Second, we lacked detailed information on offspring body composition, which limits our ability to determine how adequacy of GWG relate to fat and fat-free mass development. Third, though we adjusted for prenatal factors, such as prepregnancy BMI, maternal smoking, and pre-gestational diabetes, we cannot discount the possibility of residual confounding. Finally, the generalizability of the study population is limited because women in the sample had to attend the participating prenatal clinics, meet eligibility criteria, and complete several years of postpartum follow-up interviews along with their offspring. There was also a disproportionate loss-to-follow-up of women among high risk groups. However, the exposure-disease relationship is not expected to differ across many of these factors and losses from high risk groups, such as those with prepregnancy obesity and excessive II GWG.
Positive associations for GWG with infant weight, ponderal index, and length at birth (5), as well as fat mass and percent fat (7, 22) have been reported. There is some evidence to suggest that the association between GWG and infant body composition may vary by prepregnancy BMI status (23, 24). GWG is correlated with neonatal percent body fat in overweight/obese women (BMI>25 kg/m2) while it is correlated with neonatal lean body mass in lean/average women (BMI<25 kg/m2) (24). In a recent study of healthy, term neonates, those born to overweight or obese women with excessive GWG had greater percent body fat and fat mass compared to those born to normal weight women with excessive GWG(22). The greatest difference in neonatal fat mass was observed among overweight women with excessive GWG compared to overweight women with adequate GWG (22). In contrast, other studies have not reported a significant interaction (7, 25). In the current study, we did not observe an interaction between continuous or categorical measures of prepregnancy BMI and GWG (though we may have been underpowered to detect one) and cannot comment on how our findings relate to infant body composition; however, there were differences in GWG across pre-pregnancy BMI categories. Normal and overweight women were most likely to have excessive I GWG whereas obese women were most likely to have excessive II GWG. Even though analyses were adjusted for prepregnancy BMI, it is possible that some of the observed differences in early infancy may also be attributed to maternal overweight/obesity (26).
Significant interactions were observed between GWG and child’s age; children of mothers with excessive II GWG had a faster rate of increase in WAZ and a slightly faster rate of increase in LAZ between 0 and 3 years compared to children of mothers with adequate GWG. The rate of change in WAZ exceeded that in LAZ resulting in higher predicted mean WLZ from 0 to 3 years in comparison to the other GWG categories. We identified only two other studies that examined the effects of GWG on offspring anthropometric outcomes in a longitudinal nature. Li et.al.(6) demonstrated persistent effects of GWG >=20.43kg on risk of early onset child overweight (>=95th percentile) from 2 through 12 years. Using the 2009 IOM recommendations, Crozier et.al.(7) observed higher fat mass at birth and at 4 and 6 years among children of women with excessive GWG compared to those of women with adequate gain. Despite differences in methodology (Li et.al. did not base GWG on the IOM guidelines and Crozier et.al. did not adjust for prepregnancy BMI in their analyses), our results are consistent with these studies and suggest that the effects of GWG on child anthropometrics begin in infancy and persist through the early preschool years.
Whether the current observations are attributable to increased fetal growth, an in utero programming effect, an obesogenic postnatal environment, genetics, or an interaction of some or all of these factors is unknown. In comparison to children of mothers with inadequate and adequate GWG, children of mothers with excessive GWG had higher WAZ, LAZ, and WLZ in early infancy, which tended to track across the study period. This suggests that anthropometrics in early infancyplay a role in determining anthropometrics in the first years of life, such that infants who are born big tend to stay big (27, 28). We also observed faster rates of growth in weight and length in children of mothers with excessive II GWG compared to those of mothers with adequate GWG. This observation provides support for an in utero programming effect beyond fetal growth (as assessed by early infant anthropometrics). Results from animal studies show that excess maternal nutrient intakes during gestation are associated with altered programming of appetite and metabolic regulators, hyperphagia, and adiposity in the offspring (29) and in humans, GWG has been linked to early childhood weight outcomes independent of birth weight (30).
Women with excessive GWG tended to be overweight or obese. In addition to a possible genetic influence, maternal overweight/obesity is associated with factors, such as feeding behaviors (31-34) that may result in an obesogenic postnatal environment. Similarly, independent of maternal prepregnancy BMI status, women with excessive GWG may also exhibit characteristics associated with an obesogenic postnatal environment (35). Lastly, women who are genetically susceptible to weight gain may pass this susceptibility to their children (36). More sophisticated research is necessary to tease apart the various pathways that link GWG with offspring anthropometric outcomes and their contributions to the association.
Our findings provide evidence for an influence of GWG, a potentially modifiable maternal factor, on the development of offspring anthropometric outcomes and their persistence throughout early life. This study highlights the need for future research to understand the implications of fetal exposure to excessive GWG, especially GWG>=200% of the recommended amount for infant and childhood body composition, growth, and possibly later disease outcomes.
Figure 2.
Predicted mean child length-for-age z-scores from birth to 3 years associated with gestational weight gain categories based on the 2009 IOM recommendations in the Pregnancy, Infection, and Nutrition 3 Study (n=414).
Predicted mean child LAZ from birth (age 0 years) to 3 years are based on the adjusted linear mixed model for LAZ displayed in Table 2. Categories of gestational weight gain designated as I=inadequate; A=adequate; EI=excessive I; EII=excessive II.
Acknowledgments
This study was supported by the National Institute of Child Health and Human Development, National Institutes of Health (HD37584, HD39373), the National Institute of Diabetes and Digestive and Kidney Diseases (DK61981, DK56350), and the Carolina Population Center.
We would like to thank all of the PIN Study investigators: Kelly Evenson, Nancy Dole, David Savitz, June Stevens, and John Thorp for obtaining funding and designing the study. Additionally, we would like to thank Kathryn Carrier for managing the PIN study as well as the field collection staff and all of the families involved in the PIN studies.
Footnotes
Conflict of Interest Statement: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Contributor Information
Anna Maria Siega-Riz, University of North Carolina Gillings School of Global Public Health.
Amy H. Herring, University of North Carolina Gillings School of Global Public Health
Linda S. Adair, University of North Carolina Gillings School of Global Public Health
Julie L. Daniels, University of North Carolina Gillings School of Global Public Health
References
- 1.Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007-2008. JAMA. 2010;303:242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 2.Sharma AJ, Grummer-Strawn LM, Dalenius K, et al. Obesity prevalence among low-income, preschool-aged children—United States, 1998–2008. MMWR Morb Mortal Wkly Rep. 2009;58:769–773. [PubMed] [Google Scholar]
- 3.Latzer Y, Edmunds L, Fenig S, et al. Managing childhood overweight: Behavior, family, pharmacology, and bariatric surgery interventions. Obesity. 2009;17:411–423. doi: 10.1038/oby.2008.553. [DOI] [PubMed] [Google Scholar]
- 4.Catalano P, Ehrenberg H. The short-and long-term implications of maternal obesity on the mother and her offspring. BJOG. 2006;113:1126–1133. doi: 10.1111/j.1471-0528.2006.00989.x. [DOI] [PubMed] [Google Scholar]
- 5.Viswanathan M, Siega-Riz AM, Moos MK, et al. Outcomes of maternal weight gain. Evidence report/technology Assessment. 2008;168:1–223. [PMC free article] [PubMed] [Google Scholar]
- 6.Li C, Goran MI, Kaur H, Nollen N, Ahluwalia JS. Developmental trajectories of overweight during childhood: Role of early life factors. Obesity. 2007;15:760–771. doi: 10.1038/oby.2007.585. [DOI] [PubMed] [Google Scholar]
- 7.Crozier SR, Inskip HM, Godfrey KM, et al. Weight gain in pregnancy and childhood body composition: Findings from the Southampton women’s survey. Am J Clin Nutr. 2010;91:1745–1751. doi: 10.3945/ajcn.2009.29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehta UJ, Siega-Riz AM, Herring AH. Effect of body image on pregnancy weight gain. Matern Child Health J. 2011;15:324–332. doi: 10.1007/s10995-010-0578-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siega-Riz AM, Herring AH, Carrier K, Evenson KR, Dole N, Deierlein A. Sociodemographic, perinatal, behavioral, and psychosocial predictors of weight retention at 3 and 12 months postpartum. Obesity (Silver Spring) 2009;18:1996–2003. doi: 10.1038/oby.2009.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC) National Center for Health Statistics (NCHS) National Health and Nutrition Examination Protocol 1999-2000. http://www.cdc.gov.libproxy.lib.unc.edu/nchs/data/nhanes/meccomp.pdf.
- 11.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL. CDC growth charts: United States. Adv Data. 2000;314:1–27. [PubMed] [Google Scholar]
- 12.IOM (Institute of Medicine) NRC (National Research Council) Weight gain during pregnancy:Reexamining the guidelines. Weight gain during pregnancy:Reexamining the guidelines. The National Academies Press; Washington, DC: 2009. [Google Scholar]
- 13.Proctor BD, Dalaker J, US Census Bureau . Poverty in the United States: 2001. US Census Bureau; Washington, DC: 2002. Current Population Reports, P60-219. [Google Scholar]
- 14.Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982 Dec 1;144:768–773. doi: 10.1016/0002-9378(82)90349-0. [DOI] [PubMed] [Google Scholar]
- 15.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10:37–48. [PubMed] [Google Scholar]
- 16.Pearl J. Causal diagrams for empirical research. Biometrika. 1995;82:669–688. [Google Scholar]
- 17.Harville DA. Maximum likelihood approaches to variance component estimation and to related problems. Journal of the American Statistical Association. 1977;72:320–338. [Google Scholar]
- 18.Ware JH. Linear models for the analysis of longitudinal studies. American Statistician. 1985;39:95–101. [Google Scholar]
- 19.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 20.Fitzmaurice GM, Laird NM, Ware JH. Applied longitudinal analysis. John Wiley & Sons, Inc.; 2004. Applied longitudinal analysis. [Google Scholar]
- 21.Rifas-Shiman SL, Rich-Edwards JW, Scanlon KS, Kleinman KP, Gillman MW. Misdiagnosis of overweight and underweight children younger than 2 years of age due to length measurement bias. MedGenMed. 2005;7:56. [PMC free article] [PubMed] [Google Scholar]
- 22.Hull HR, Thornton JC, Ji Y, et al. Higher infant body fat with excessive gestational weight gain in overweight women. Am J Obstet Gynecol. 2011;205:211e.1–211e.7. doi: 10.1016/j.ajog.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ay L, Kruithof CJ, Bakker R, et al. Maternal anthropometrics are associated with fetal size in different periods of pregnancy and at birth. the generation R study. BJOG. 2009;116:953–963. doi: 10.1111/j.1471-0528.2009.02143.x. [DOI] [PubMed] [Google Scholar]
- 24.Sewell MF, Huston-Presley L, Super DM, Catalano P. Increased neonatal fat mass, not lean body mass, is associated with maternal obesity. Obstet Gynecol. 2006;195:1100–1103. doi: 10.1016/j.ajog.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 25.Jain NJ, Denk CE, Kruse LK, Dandolu V. Maternal obesity: Can pregnancy weight gain modify risk of selected adverse pregnancy outcomes? Am J Perinatol. 2007;24:291–298. doi: 10.1055/s-2007-981432. [DOI] [PubMed] [Google Scholar]
- 26.Hull HR, Dinger MK, Knehans AW, Thompson DM, Fields DA. Impact of maternal body mass index on neonate birthweight and body composition. Am J Obstet Gynecol. 2008;198:416e1–e6. doi: 10.1016/j.ajog.2007.10.796. [DOI] [PubMed] [Google Scholar]
- 27.Pietilainen KH, Kaprio J, Rasanen M, Winter T, Rissanen A, Rose RJ. Tracking of body size from birth to late adolescence: Contributions of birth length, birth weight, duration of gestation, parents’ body size, and twinship. Am J Epidemiol. 2001;154:21–29. doi: 10.1093/aje/154.1.21. [DOI] [PubMed] [Google Scholar]
- 28.Druet C, Ong KK. Early childhood predictors of adult body composition. Best Practice & Research Clinical Endocrinology & Metabolism. 2008;22:489–502. doi: 10.1016/j.beem.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 29.McMillen IC, Adam CL, Mühlhäusler BS. Early origins of obesity: Programming the appetite regulatory system. J Physiol. 2005;565:9–17. doi: 10.1113/jphysiol.2004.081992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oken E, Taveras EM, Kleinman KP, Rich-Edwards JW, Gillman MW. Gestational weight gain and child adiposity at age 3 years. Obstet Gynecol. 2007;196:322.e1–322.e8. doi: 10.1016/j.ajog.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baker JL, Michaelsen KF, Rasmussen KM, Sorensen TIA. Maternal prepregnant body mass index, duration of breastfeeding, and timing of complementary food introduction are associated with infant weight gain. Am J Clin Nutr. 2004;80:1579–1588. doi: 10.1093/ajcn/80.6.1579. [DOI] [PubMed] [Google Scholar]
- 32.Rising R, Lifshitz F. Relationship between maternal obesity and infant feeding-interactions. Nutr J. 2005 May 12;4:17. doi: 10.1186/1475-2891-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li R, Jewell S, Grummer-Strawn L. Maternal obesity and breast-feeding practices. Am J Clin Nutr. 2003;77:931–936. doi: 10.1093/ajcn/77.4.931. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen VT, Larson DE, Johnson RK, Goran MI. Fat intake and adiposity in children of lean and obese parents. Am J Clin Nutr. 1996;63:507–513. doi: 10.1093/ajcn/63.4.507. [DOI] [PubMed] [Google Scholar]
- 35.Stuebe AM, Oken E, Gillman MW. Associations of diet and physical activity during pregnancy with risk for excessive gestational weight gain. Obstet Gynecol. 2009;201:58.e1–58.e8. doi: 10.1016/j.ajog.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stuebe AM, Lyon H, Herring AH, et al. Obesity and diabetes genetic variants associated with gestational weight gain. Am J Obstet Gynecol. 2010;203:283e1–283e17. doi: 10.1016/j.ajog.2010.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]