Abstract
Objectives
The objectives of this study were to 1) compare the strength of associations between sleep duration and BMI in middle childhood, early and late adolescence; 2) determine whether sleep duration in middle childhood predicts BMI in early or late adolescence; 3) examine the consistency of these associations by sex.
Methods
Subjects included 313 children/adolescents aged 8–19 participating in a longitudinal cohort study on sleep and health. Participants were assessed at three time points approximately 4 years apart: ages 8–11, 12–15 and 16–19. BMI z-score (BMIz) was calculated using age and sex normative data from the Centers for Disease Control. Sleep duration was reported by the parent (ages 8–15) or the adolescent (ages 16–19).
Results
Half of the participants were male and 79% were Caucasian. Sleep duration had a negative linear association with BMIz for boys but not girls, and the magnitude of this association decreased with age. Sleep duration at age 8–11 predicted BMIz in early and late adolescence for boys but not girls, and associations were largely attenuated after adjusting for BMIz at age 8–11. The strongest predictor of adolescent BMIz was BMIz at age 8–11 for both boys and girls.
Conclusions
We conclude that the association between sleep duration and BMIz varies by sex and age, with stronger associations in boys and in middle childhood compared to adolescence.
Keywords: Adolescents, BMI, Children, Obesity, Sleep
Introduction
The prevalence of childhood and adolescent obesity in the United States has increased markedly since the 1970s (1, 2). Current estimates are that 20.1% of boys and 17.3% girls aged 6–19 in the U.S. are obese, and one in four (25.9%) Non-Hispanic black girls are obese (3). The obesity epidemic is a health concern because obese youth are at increased risk of adverse medical conditions during childhood/adolescence (4, 5) as well as chronic diseases in adulthood (6–8).
Concurrent with the childhood obesity epidemic is the increased prevalence of sleep curtailment. During the past 3 decades, children and adolescents are sleeping less, and only one-third of teens are getting the recommended 9 hours of night-time sleep (9, 10). There is growing evidence from large cross-sectional studies conducted internationally that shorter sleep duration is a risk factor for higher BMI in both children and adults, although associations may be somewhat stronger in children (see (11, 12) for literature reviews). Two recent meta-analyses of cross-sectional and longitudinal studies reported associations between short sleep duration and increased risk of overweight/obesity in children/adolescents (13, 14). Cappuccio et al. reported that short sleep duration was associated with an 89% increased odds of obesity for children/adolescents (14), while Chen et al. found that each hour of increased sleep duration was associated with a 9% decreased odds of overweight/obesity (13).
Prospective epidemiological studies provide some evidence that shorter sleep duration in early childhood is a risk factor for higher BMI in early and middle childhood (15–18). For example, Taveras et al. found that sleep duration between 6 months and 2 years of age was associated with an increased odds of obesity and higher BMI at age 3 years (15). Dieu and colleagues reported a negative linear association between sleep duration at age 4–5 years and obesity one year later (16). Similarly, two additional studies found that sleep duration in children under 6 years of age had a negative linear association with obesity at ages 7 (17) and 9.5 years (18). Moreover, Lumeng et al. reported a negative linear association between sleep duration in third grade and subsequent obesity in sixth grade that persisted after adjusting for BMI in third grade (19).
Only two prospective studies evaluated the relation between sleep duration and BMI across early/middle childhood to adolescence (20, 21). The results of these studies suggest that the association between sleep duration and BMI varies with age. Snell et al. found that after adjusting for baseline BMI, sleep duration at age 3–7.9 had a negative linear association with subsequent BMI 5 years later; however, among children age 8–12.9 at baseline, the association between baseline sleep duration and subsequent BMI was weaker and not statistically significant (20). Additionally, shorter sleep duration was associated with significantly higher odds of overweight only for the younger children (age 3–7.9 at baseline). Bell and colleagues reported that shorter nocturnal sleep duration at age 0–4 years was associated with significantly higher odds of subsequent overweight or obesity 5 years later (21). In contrast, among children aged 5–12, baseline sleep duration did not predict weight status 5 years later. However, at the 5 year follow-up (when the children were 10–18 years old), there was a significant cross-sectional association between short sleep duration and higher weight status. The results of these two studies suggest that the longitudinal association between shorter sleep duration and subsequent overweight/obesity may be attenuated as children progress from early childhood to middle childhood and pre-adolescence.
There is also evidence for sex differences in the association between sleep duration and obesity in children and adolescents (13, 22–27). Stronger associations were reported for boys compared to girls among younger children age 3–6 (22), age 6–7 (23), and age 5–10 years (24), as well as for adolescents in grades 7–12 (25). Additionally, one study reported that short sleep among 13–16 year old adolescents was associated with increased risk of overweight/obesity in males and decreased risk in females (26). Analyses from an Australian cohort of children aged 7.5–16.5 revealed that while the association between sleep duration and BMI was consistently stronger for boys than girls across all age groups, the magnitude of the difference between the sexes varied with age, with the largest difference seen at age 14–16.5 years (27).
Taken together, the extant pediatric literature suggests that the association between sleep duration and BMI varies by both sex and age. However, the prior literature has been limited by a restricted age range available for longitudinal assessments, with all studies reporting data from only two time points. The purpose of this study was to further examine variability in the relation between sleep duration and BMI by sex and age in a prospective cohort study of urban U.S. children studied at three time points over an 8 to 10 year period spanning middle childhood and late adolescence. We postulated that the cross-sectional associations between sleep duration and BMI z-score (BMIz) would be stronger in middle childhood compared to adolescence and for boys compared to girls. We also hypothesized that sleep duration in middle childhood would predict BMIz in early and late adolescence, and that the effect would be stronger in boys compared to girls. An exploratory aim of the study was to assess potential mechanisms underlying any sex differences in the association between sleep duration and BMIz by examining the relations among sleep, leptin (a satiety hormone that varies by sex), and sex hormones (testosterone and estrogen) in a subset of children with biochemical measures available.
Methods
Study Population
The sample included participants in the Cleveland Children’s Sleep and Health Study (CCSHS), an ongoing longitudinal cohort study designed to evaluate associations between sleep measures and health outcomes. As previously described (28–30), the cohort was originally assembled by stratified sampling of full-term and preterm children born between 1988–1993, and was designed to over-represent African-American and preterm children to increase the precision in estimating effects in these subgroups. At the first CCSHS examination (1998–2001), 907 children were directly studied at a dedicated clinical research unit in middle-childhood, at 8 to 11 years of age. Approximately 4 years after the first exam (2002–2006), all parents were mailed a 2-page questionnaire to obtain information about the child’s sleep duration on weekdays and weekends, BMI and health during early adolescence (exam 2: age 12 to 15), with responses obtained by mail for 572 adolescents (63.1%). Exam 2 also included direct measurements of BMI and sleep at a clinical research unit for 294 participants selected to represent the sample of children with habitual snoring and/or sleep apnea at the first exam, as well as a random sample of participants from the remaining cohort stratified by race, sex and term. All 907 subjects from the first exam were asked to participate in the third exam (TREC-Cleveland Cohort) conducted between 2006–2010, when participants were 16 to 19 years of age (late adolescence). A total of 517 participants (57.0%) were directly studied at a clinical research unit at the third exam. Data from all three time points were collected for 357 participants (39.4%).
Study Protocol
The protocols for data collection for each of the three exams have been described previously (28–30). The study protocols were approved by the University Hospitals of Cleveland Institutional Review Board. For children under age 18, written consent was obtained from the primary caregiver and assent was obtained from the participant; written consent was obtained from study participants aged 18 and older.
Measurements
Subject characteristics
Questionnaires were used to collect demographic data. Race/ethnicity was based on parent-report and coded as African-American or non-African American for analytic purposes. Birth weight was obtained via medical chart review for children born prematurely (<37 weeks gestational age) or by parent report for full-term children. Low birth weight was defined as less than 2500g. Information about socioeconomic status (SES) was obtained by matching the child’s address at the first exam to the corresponding 2000 U.S. Census Bureau tract. The lowest quartile of the CCSHS sample distribution of median income from the census tract ($40,500) was used to define low SES.
Exposure and outcome measures
For exams 1 and 2, sleep duration was based on the number of hours and minutes that the parent reported their child to be currently sleeping on weekdays and weekends. For the third exam, the adolescent directly reported how much they usually slept at night on work/school days and on days off. For these analyses, sleep duration on work/school days was considered to be weekday sleep duration while sleep duration on days off was coded as weekend sleep duration. The primary exposure in this study was sleep duration for all days, calculated as the weighted average of parent- or self-reported weekday and weekend sleep duration. Although wrist actigraphy, an objective measure of sleep duration, was performed at the second and third examinations, only 137 participants had at least 5 days of actigraphy data at both time points. For consistency across exams and to minimize biases due to missing data, we quantified sleep duration using questionnaire data in primary analyses, conducting sensitivity analyses that quantified sleep duration from actigraphy to investigate the consistency of the associations.
For the first and third exams, a rigid stadiometer (Holtain Ltd, Pembrokeshire, UK) and digital scale (Health-o-meter, Shelton, CT) were used to measure height and weight respectively. Height and weight were based on parent-report for the second exam (intraclass correlation = 0.75 for the subsample of n=130 participants with both self-report data and direct measurement). BMI was calculated by dividing weight in kilograms by height in meters squared and was then converted into sex-and age-adjusted z-scores and percentiles (http://www.cdc.gov/growthcharts/). Overweight and obesity were defined using the International Obesity Task Force reference values for children (31).
Bioassays from overnight fasting blood samples were performed in the subsample of participants who were directly studied at the second exam. These include assays for: leptin (Luminex technology multiplex ELISA using the Human Serum Adipokine Panel B LINCOplex Kit; Linco Research, Inc., St. Charles, MO); total testosterone (measured using a competitive direct chemiluminescent immunoassay on the Advia Centaur instrument; Siemens Healthcare Diagnostics, Tarrytown, NY) and estradiol–6 (measured using a sequential chemiluminescent immunoassay on the Bayer Centaur instrument; Chiron Diagnostics Corp., East Walpole, MA).
Statistical Methods
Means and standard deviations, the median, 25th and 75th percentiles, and counts and proportions were used to describe normally distributed, skewed and categorical measures, respectively. Unadjusted bivariate associations with BMIz were summarized using Pearson correlations for continuous measures and the two-sample t-test for categorical measures. For each exam, multiple linear regression analyses were used to examine cross-sectional associations between sleep duration, modeled as a linear term, and BMIz. Regression models were fitted without covariate adjustment as well as after adjusting for age, African-American race, low birth weight and low SES. Based on our hypotheses and review of the extant literature, two- and three-way interactions with sleep duration, sex and age were examined, and analyses were stratified by sex and exam. Multiple linear regression models were also used to examine if sleep duration in middle childhood predicted subsequent BMIz in early or late adolescence. Time to follow-up was included as an additional covariate in these models. Sensitivity analyses with actigraphy-based sleep duration as the exposure were used to examine the consistency of the sleep duration-BMI associations. The results of the regression models are summarized via beta coefficients (slopes for continuous measures and differences between groups for categorical measures), standard errors and p-values. Additionally, partial Spearman correlations were examined in post-hoc exploratory analyses of hormonal data obtained from a subset of participants at the early adolescent exam to further explore sex differences in the relation between sleep duration and BMIz. SAS version 9.2 was used for all analyses (SAS Institute, Inc., Cary, NC).
Results
Subject characteristics
Data from exams 1, 2 and 3 were collected for 357 children. Of these participants, 11 children were excluded due to missing BMI (n=7) or sleep duration (n=4) data, and 19 were excluded due to obstructive sleep apnea (apnea hypopnea index ≥ 5) at the first (n=10) or third exam (n=9); at the time of this report, data from the third exam had not been processed for n=14 adolescents, yielding a final sample of 313 participants. Analyses were restricted to these 313 participants so that the sample was the same when comparing the results from the middle childhood, early adolescent and late adolescent exams. Overall, half of the participants were male and 37% were low birth weight. Over three-fourths (79%) of the participants were Caucasian, 18% were African-American, and the remaining 3% were Hispanic, Asian or BiRacial. The median length of follow-up was 8.2 years (range 6.1 – 10.2 years).
Given our a priori hypotheses, we show subject characteristics by sex and exam in Table 1. BMIz increased with age for both boys (0.39 at age 8–11 and 0.60 at age 16–19) and girls (0.11 at age 8–11 and 0.46 at age 16–19). Although median weekend sleep duration was approximately 9 hours for both boys and girls at all ages, median weekday sleep duration decreased by 1.7 hours for boys and 2.5 hours for girls between age 8–11 and 16–19. Thus, overall sleep duration decreased with age, while the difference between weekday and weekend sleep duration increased with age for both boys and girls.
Table 1.
Subject Characteristics By Sex and Agea
| Middle Childhood (age 8–11) | Early Adolescence (age 12–15) | Late Adolescence (age 16–19) | |
|---|---|---|---|
| Boys (n=157) | |||
| Age | 9.5 ± 0.7 | 13.6 ± 0.8 | 17.7 ± 0.4 |
| BMIz | 0.39 ± 1.08 | 0.50 ± 1.06 | 0.60 ± 1.10 |
| BMI categoryb | |||
| Underweight/Normal | 116 (73.9%) | 106 (67.5%) | 95 (60.5%) |
| Overweight | 32 (20.4%) | 36 (22.9%) | 36 (22.9%) |
| Obese | 9 (5.7%) | 15 (9.6%) | 26 (16.6%) |
| BMI | 18.1 ± 3.3 | 21.4 ± 4.2 | 24.8 ± 5.1 |
| Sleep duration – all days (hrs) | 9.1 (8.8, 9.7) | 8.6 (8.1, 9.1) | 7.8 (7.1, 8.3) |
| Weekday sleep duration (hrs) | 9.0 (9.0, 10.0) | 8.5 (8.0, 9.0) | 7.3 (6.5, 8.0) |
| Weekend sleep duration (hrs) | 9.0 (8.5, 10.0) | 9.5 (8.5, 10.0) | 9.0 (8.3, 10.0) |
| Girls (n=156) | |||
| Age | 9.5 ± 0.6 | 13.6 ± 0.8 | 17.7 ± 0.4 |
| BMIz | 0.11 ± 1.22 | 0.32 ± 0.94 | 0.46 ± 0.91 |
| BMI categoryb | |||
| Underweight/Normal | 118 (75.6%) | 120 (76.9%) | 107 (68.6%) |
| Overweight | 27 (17.3%) | 27 (17.3%) | 33 (21.1%) |
| Obese | 11 (7.1%) | 9 (5.8%) | 16 (10.3%) |
| BMI | 17.7 ± 3.7 | 20.9 ± 4.0 | 24.0 ± 5.1 |
| Sleep duration – all days (hrs) | 9.4 (9.0, 9.8) | 8.6 (8.0, 9.0) | 7.6 (7.0, 8.3) |
| Weekday sleep duration (hrs) | 9.5 (9.0, 10.0) | 8.0 (7.5, 8.5) | 7.0 (6.2, 8.0) |
| Weekend sleep duration (hrs) | 9.0 (8.5, 10.0) | 9.5 (9.0, 10.0) | 9.0 (8.0, 10.0) |
Mean ± standard deviation shown for continuous variables; median (interquartile range) shown for non-normally distributed measures; count (proportion) shown for categorical variables.
Based on values from the International Obesity Task Force.
Cross-sectional associations of sleep duration and BMIz
Preliminary regression analyses with BMIz as the outcome were used to examine two-and three-way interactions of sex, age and sleep duration at each exam. Analyses of data from the first exam revealed significant interactions between sex and age (p=0.04), and sex and SES (p=0.03), but two- and three-way interactions with sleep duration were not statistically significant. However, at the second and third exams, p-values for the three-way interaction of sleep duration, sex and age were p=0.07 and p=0.04 respectively, suggesting that the relation between sleep duration and BMIz varied by sex and age. To facilitate the interpretation of the results, subsequent analyses were stratified by sex and exam.
Bivariate associations by sex and exam showed that for boys, sleep duration was negatively correlated with BMIz; the magnitude of the unadjusted correlation was similar in middle childhood (r = −0.17), early adolescence (r = −0.18) and late adolescence (r = −0.14). African-American race, low birth weight and low SES were not significantly associated with BMIz for boys at any age (results not shown). For girls, sleep duration was not correlated with BMIz at any age, with correlations ranging from −0.11 in middle childhood to 0.05 in late adolescence. Low SES was associated with higher BMIz for girls at all exams. Low birth weight was associated with a lower BMIz for girls in middle childhood but not at older ages, while African-American race was associated with a higher BMIz for girls at the adolescent exams but not at the middle childhood exam (results not shown).
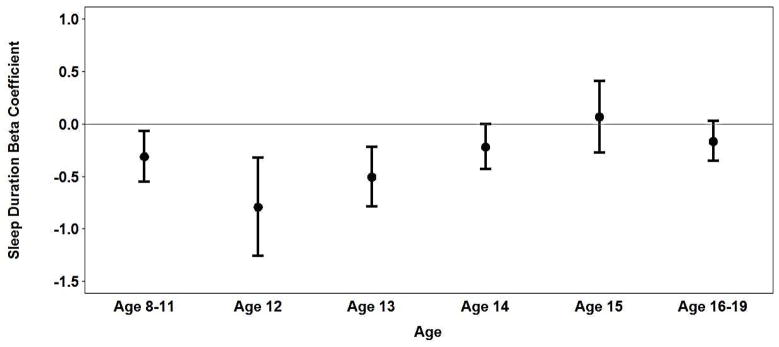
In analyses adjusted for age, race, birth weight and SES, shorter sleep duration remained associated with higher BMIz among boys in middle childhood (Table 2 & Figure 1a). Each one-hour increase in sleep duration was associated with a 0.31 decrease in BMIz for boys age 8–11. Among early adolescent boys, age modified the association between sleep duration and BMIz; as age increased from 12 to 15, the association between shorter sleep duration and BMIz decreased. At the late adolescent exam, the relation between sleep duration and BMIz was further attenuated and was not statistically significant (p=0.09).
Table 2.
Adjusted cross-sectional associations between sleep duration and BMIz by sex and age
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Middle Childhood (age 8–11) | Early Adolescence (age 12–15) | Late Adolescence (age 16–19) | |||||||
| β | SE | p-val | β | SE | p-val | β | SE | p-val | |
| Boys (n=157) | |||||||||
| Sleep duration | −0.31 | 0.12 | 0.01 | −0.39 | 0.12 | 0.001 | −0.16 | 0.10 | 0.09 |
| Age | −0.22 | 0.12 | 0.08 | −0.39 | 0.17 | 0.02 | 0.13 | 0.24 | 0.59 |
| Age2 | -- | -- | -- | 0.21 | 0.10 | 0.03 | -- | -- | -- |
| Sleep duration x | |||||||||
| Agea | -- | -- | -- | 0.29 | 0.12 | 0.01 | -- | -- | -- |
| African-American | 0.41 | 0.27 | 0.14 | 0.21 | 0.27 | 0.44 | 0.40 | 0.28 | 0.17 |
| Low birth weight | −0.41 | 0.18 | 0.03 | −0.21 | 0.18 | 0.23 | 0.04 | 0.18 | 0.84 |
| Low SES | −0.38 | 0.24 | 0.11 | 0.01 | 0.23 | 0.98 | −0.35 | 0.25 | 0.16 |
| Girls (n=156) | |||||||||
| Sleep duration | −0.19 | 0.13 | 0.16 | −0.08 | 0.09 | 0.38 | 0.04 | 0.07 | 0.59 |
| Age | 0.17 | 0.15 | 0.27 | −0.01 | 0.09 | 0.88 | −0.20 | 0.17 | 0.25 |
| African-American | −0.04 | 0.31 | 0.89 | 0.30 | 0.23 | 0.20 | 0.27 | 0.23 | 0.25 |
| Low birth weight | −0.46 | 0.20 | 0.02 | −0.29 | 0.15 | 0.06 | −0.25 | 0.15 | 0.09 |
| Low SES | 0.54 | 0.28 | 0.05 | 0.56 | 0.21 | 0.008 | 0.42 | 0.21 | 0.04 |
Early adolescence model for males: age and sleep duration centered to the sample median.
Overall Model R2: Model 1 = 0.09; Model 2 = 0.10; Model 3 = 0.04.
Sleep Duration Partial R2: Model 1 = 0.04; Model 2 = 0.07; Model 3 = 0.02.
Figure 1a illustrates interaction between sleep duration and age.
Overall Model R2: Model 1 = 0.09; Model 2 = 0.14; Model 3 = 0.11.
Sleep Duration Partial R2: Model 1 = 0.01; Model 2 < 0.01; Model 3 < 0.01.
Figure 1.
Figure 1a: Association between sleep duration and BMIz in boys age 8–19.
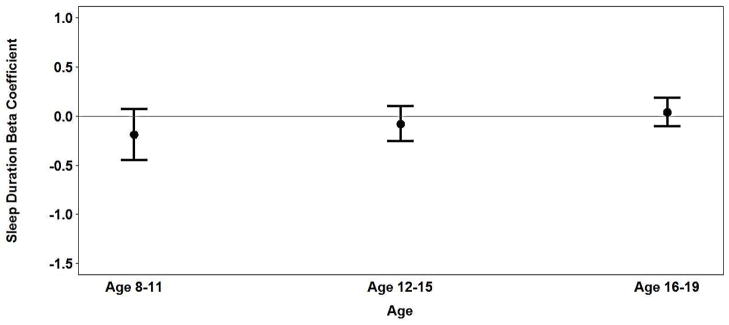
Figure 1b: Association between sleep duration and BMIz in girls age 8–19.
In contrast, among girls, sleep duration was not associated with BMIz in middle childhood or adolescence, nor did age modify the association between sleep duration and BMIz (Table 2 & Figure 1b). However, similar to the results of the bivariate analyses, low SES was associated with higher BMIz, with significant associations demonstrated at all three exams in analyses adjusted for age, race, birth weight and sleep duration. Girls who weighed less than 2500g at birth had lower BMIz on average than girls who were born weighing at least 2500g, and this difference was more pronounced at the middle childhood exam. African-American race was not significantly associated with BMIz after adjusting for SES.
Longitudinal associations of sleep duration in middle childhood and subsequent BMIz in early and late adolescence
For boys, sleep duration at age 8–11 was significantly associated with subsequent BMIz in early and late adolescence after adjusting for age, race, birth weight and SES (Table 3, models 1 and 3). On average, each hour increase in sleep duration at age 8–11 was associated with a decrease in BMIz of 0.32 in early adolescence and 0.30 in late adolescence. However, after additionally adjusting for BMIz at age 8–11, the strongest predictor of subsequent BMIz (β = 0.78 and β = 0.81 for early and late adolescent models, respectively), the association between sleep duration at age 8–11 and subsequent BMIz was largely attenuated (β = −0.08 and β = −0.06 in early and late adolescent models, respectively) and no longer statistically significant (Table 3, models 2 and 4). Additionally, after adjusting for BMIz at age 8–11, low SES in middle childhood was associated with higher BMIz in early but not late adolescence. None of the other subject characteristics were associated with BMIz in early or late adolescence for boys.
Table 3.
Longitudinal association of weekday sleep duration at age 8–11 with BMIz in adolescence, stratified by sex
| Subject characteristics at age 8–11 | BMIz in early adolescence (age 12–15) | BMIz in late adolescence (age 16–19) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | |||||||||
| β | SE | p-val | β | SE | p-val | β | SE | p-val | β | SE | p-val | |
| Boys (n=157) | ||||||||||||
| Sleep duration | −0.32 | 0.12 | 0.01 | −0.08 | 0.08 | 0.33 | −0.30 | 0.13 | 0.02 | −0.06 | 0.08 | 0.47 |
| Age | −0.14 | 0.13 | 0.28 | 0.02 | 0.08 | 0.78 | 0.07 | 0.23 | 0.75 | 0.11 | 0.15 | 0.45 |
| Time to follow-up | 0.01 | 0.16 | 0.94 | −0.04 | 0.10 | 0.69 | 0.35 | 0.28 | 0.21 | 0.15 | 0.17 | 0.38 |
| African-American | 0.13 | 0.27 | 0.65 | −0.18 | 0.18 | 0.29 | 0.35 | 0.28 | 0.21 | 0.04 | 0.18 | 0.82 |
| Low birth weight | −0.23 | 0.18 | 0.22 | 0.09 | 0.12 | 0.45 | −0.13 | 0.19 | 0.49 | 0.20 | 0.12 | 0.09 |
| Low SES | 0.05 | 0.24 | 0.84 | 0.34 | 0.15 | 0.03 | −0.37 | 0.24 | 0.13 | −0.05 | 0.15 | 0.72 |
| BMIz | -- | -- | -- | 0.78 | 0.05 | <0.001 | -- | -- | -- | 0.81 | 0.05 | <0.001 |
| Girls (n=156) | ||||||||||||
| Sleep duration | −0.11 | 0.10 | 0.27 | −0.01 | 0.07 | 0.89 | −0.07 | 0.10 | 0.49 | 0.03 | 0.07 | 0.64 |
| Age | −0.11 | 0.17 | 0.51 | −0.14 | 0.12 | 0.24 | −0.14 | 0.17 | 0.42 | −0.16 | 0.12 | 0.17 |
| Time to follow-up | −0.29 | 0.19 | 0.14 | −0.18 | 0.13 | 0.16 | −0.27 | 0.19 | 0.15 | −0.17 | 0.13 | 0.19 |
| African-American | 0.33 | 0.23 | 0.16 | 0.34 | 0.15 | 0.03 | 0.29 | 0.23 | 0.21 | 0.31 | 0.16 | 0.05 |
| Low birth weight | −0.25 | 0.15 | 0.10 | −0.01 | 0.10 | 0.99 | −0.22 | 0.15 | 0.15 | 0.03 | 0.10 | 0.77 |
| Low SES | 0.51 | 0.21 | 0.02 | 0.22 | 0.14 | 0.13 | 0.39 | 0.21 | 0.06 | 0.10 | 0.14 | 0.46 |
| BMIz | -- | -- | -- | 0.55 | 0.04 | <0.001 | -- | -- | -- | 0.55 | 0.04 | <0.001 |
Overall Model R2: Model 1 = 0.06; Model 2 = 0.62; Model 3 = 0.07; Model 4: 0.65.
Sleep Duration Partial R2: Model 1 = 0.04; Model 2 < 0.01; Model 3 = 0.03; Model 4: <0.01.
Overall Model R2: Model 1 = 0.16; Model 2 = 0.63; Model 3 = 0.12; Model 4: 0.60.
Sleep Duration Partial R2: Model 1 = 0.01; Model 2 < 0.01; Model 3 < 0.01; Model 4: <0.01.
For girls, sleep duration in middle childhood did not predict subsequent BMIz in early or late adolescence in models adjusted for subject characteristics (Table 3, models 1 & 3) or after additionally adjusting for BMIz at age 8–11 (Table 3, models 2 & 4). BMIz in middle childhood was the strongest predictor of BMIz in early or late adolescence (β = 0.55 for both the early and late adolescent models). Moreover, the associations of birth weight and SES with BMIz in adolescence were attenuated in models that adjusted for BMIz at age 8–11. The association between race and BMIz in adolescence was more precise after adjusting for BMIz at age 8–11 (standard error decreased from 0.23 to 0.16) such that African-American race was significantly associated with higher BMIz in adolescence after adjusting for BMIz at age 8–11.
Sensitivity analyses
The cross-sectional models for the early and late adolescent exams were refitted on the subsample of adolescents for whom sleep duration from actigraphy was available (early adolescent model: n=33 females and n=44 males; late adolescent model: n=107 females; n=91 males). The pattern of results was consistent with the primary analyses. For boys age 12–15, actigraphic sleep duration was negatively associated with BMIz and the magnitude of the association decreased with age (main effect of sleep duration β = −0.60, SE = 0.22; interaction between sleep duration and age (β = 0.63, SE = 0.45). At the late adolescent exam, actigraphic sleep duration was not associated with BMIz for boys (β = −0.09, SE=0.13). For girls, actigraphic sleep duration was not associated with BMIz at the early or late adolescent exam (β = −0.16, SE=0.25 and β = −0.05, SE = 0.09, respectively). Thus, the associations observed in the adolescents are unlikely to be attributable to measurement error of sleep duration when using parent- or self-report.
Exploratory analyses
To further explore the sex differences in the association between sleep duration and BMIz, additional post-hoc exploratory analyses stratified by sex were performed. Using data from fasting venipuncture from the subset of participants that were directly studied at the early adolescent exam, associations among actigraphic sleep duration, fasting morning leptin levels (an appetite regulating hormone reduced with experimental sleep restriction (32)) and sex hormones (total testosterone level for boys, estradiol level for girls) were examined. Morning leptin level was significantly higher for girls than boys (19.2 ± 14.6 vs. 10.0 ± 9.8, p<0.001). Partial Spearman correlations adjusted for BMIz showed that for boys, sleep duration was positively correlated with morning leptin level (r = 0.19, p=0.03; n=126) and negatively, albeit insignificantly, correlated with total testosterone (r = −0.14, p=0.29; n=59), while total testosterone had a strong negative correlation with morning leptin level (r = −0.49, p<0.001; n=59). In contrast, for girls, sleep duration was not correlated with morning leptin level (r = 0.09, p=0.30; n=131) or estradiol (r = −0.01, p=0.85; n=131), although estradiol and morning leptin levels were positively correlated (r = 0.22, p=0.01; n=138).
Discussion
In this 8-year prospective cohort study examining children over critical developmental periods, we found that shorter sleep duration was associated with higher BMIz in pre- and peri-adolescent boys, and that this association was attenuated as the boys progressed through adolescence. Sleep duration in middle childhood was also significantly associated with subsequent BMIz in adolescence for boys, but the magnitude of the association diminished and was no longer statistically significant after adjusting for BMIz in middle childhood. In contrast, among girls, sleep duration was not associated with BMIz at ages 8–19 years, nor did sleep duration in middle childhood predict subsequent BMIz in adolescence. Sensitivity analyses which utilized objective actigraphy data confirmed that the findings are unlikely to be due to reporting biases. The results underscore the importance of short sleep duration during middle childhood as a risk factor for obesity in boys. Our analyses also highlight the potential complexity of the associations of sleep duration, age, sex and weight, and the need to consider issues associated with sex and development in studies of the health effects of curtailed sleep.
The stronger associations in boys compared to girls and in younger children compared to adolescents are consistent with prior reports (13, 20, 22–25, 27). A meta-analysis by Chen and colleagues reported that the association between sleep duration and overweight/obesity was stronger in boys than girls, and there was a significant linear dose-response association in younger children (age < 10) (13). Our data also showed a linear exposure-response association between BMIz and sleep duration for pre- and peri-adolescent boys but not among girls or adolescent boys.
Our analysis of data from children ranging from 8 to 19 years identified a stronger association between short sleep and BMIz in younger compared to older boys. This finding is somewhat consistent with a prior study Biggs & Dollman that reported a stronger association between shorter sleep duration and larger waist girth among boys age 9–11.9 compared to boys age 13–16; however, in that study, the magnitude of the associations between sleep duration and the odds of overweight/obesity were the same for boys in both age groups (26). Although Biggs & Dollman and the present study compared associations for groups defined by relatively narrow age ranges, our finding of an interaction between age and sleep duration within the group of boys aged 12–15 in the current study highlights the importance of even incremental age effects during critical developmental periods. Perhaps the stronger association of sleep duration with BMIz in younger boys compared to adolescent boys is partially due to pubertal development; puberty is accompanied by a higher basal metabolic rate and increased muscle mass in boys (25) which might mitigate any effects of short sleep on BMI.
Consistent with our cross-sectional findings, our longitudinal analyses showed that sleep duration at age 8–11 predicted BMIz in both early and late adolescence for boys but not girls. However, this association was largely attenuated after adjusting for BMIz in middle childhood, suggesting that the effects of short sleep duration on BMI (if causal) likely manifest early in childhood at a time when weight trajectories are being established. It is important to note that among both boys and girls, the strongest predictor of BMIz in adolescence was BMIz at age 8–11. This is consistent with prior literature that obese children and adolescents are at a markedly increased risk of obesity in adulthood (33, 34) and further underscores the importance of prevention efforts that target young children. Given the results of the current study in conjunction with the extant literature, an area of future research is to examine the impact of intervention studies of sleep duration on obesity outcomes in younger children.
Several mechanisms have been postulated to explain the associations between short sleep and altered energy balance (35). Experimental and epidemiological evidence indicate that short sleep is associated with decreased secretion of leptin (35), a hormone produced by adipocytes that plays a key role in energy balance and correlates with fat mass. Since experimental sleep restriction has been shown to decrease leptin levels, it has been suggested that a low leptin level may be a factor driving increased energy intake in shorter sleepers (32). Females have higher leptin levels than males (36), and with puberty, leptin levels increase in girls but decrease in boys (37). We therefore evaluated the associations among leptin levels, sex hormones (which may modulate leptin levels) and sleep duration in sex-stratified analyses to explore mechanisms for the apparent differences in the associations between sleep duration and BMI levels in boys and girls. These exploratory analyses showed that leptin levels, as expected, were lower in boys than girls, varied inversely with testosterone levels in boys and were positively correlated with estradiol levels in girls. Notably, sleep duration was positively correlated with BMIz-adjusted leptin levels only in boys. Thus, if leptin is a key mediator between sleep deprivation and obesity, then sex differences in background hormone levels that modulate appetite regulation may partially explain differences in the apparent effects of sleep duration on BMI levels in boys and girls. It may be speculated that boys may have an increased susceptibility to the effects of sleep deprivation due to the effects of both high testosterone levels and short sleep duration on lowering leptin levels and thus increasing appetite, while peri-pubertal girls, who have increasing and relatively high circulating leptin levels, may be less sensitive to the hormonal effects of sleep deprivation on appetite.
It is also possible that age and sex differences in sleep architecture could influence the findings. Males tend to have less slow-wave sleep than females (25). Experimentally reduced slow-wave sleep has been shown to adversely influence insulin sensitivity in young adults (38), and reduced slow wave sleep has been linked to obesity in older adults (39). Thus, females may be less vulnerable to some of the negative effects of modest reductions in sleep duration due to a relatively high duration of slow wave sleep. Differences in the timing and patterns of sleeping, which vary across childhood as sleep duration decreases and circadian rhythms become more advanced, may also be important. In this sample, boys, compared to girls, had significantly shorter weekday, but not weekend, sleep duration at 8–11 years of age, and significantly longer weekday sleep duration at age 12–15 years. Further research is needed to examine how sex differences in sleep architecture and sleep patterns during childhood and adolescence may influence energy balance and weight status. This area may be especially important given the recognition of the importance of appropriate circadian alignment of sleep and eating behaviors with metabolism (40).
An area of future research is to elucidate the interactions among various physical and sedentary behaviors, sleep, development and obesity in girls and boys. While decreased sleep duration is associated with increased opportunities to eat (41), it is possible that among girls and boys who are relatively short sleepers, wake time is spent differently in regards to eating, physical activity and sedentary behaviors. For example, during adolescence, levels of physical activity decline most markedly among girls, particularly for African-American girls (42). Furthermore, sociocultural factors that influence eating and exercise behaviors, such as the desire to be thin and body dissatisfaction, are stronger in girls than boys (43, 44). It is possible that these sociocultural factors mitigate the impact of short sleep on BMI for girls.
Strengths of this prospective cohort study include a longer follow-up duration (median = 8.2 years) than previous pediatric cohort studies and inclusion of a diverse SES sample. Moreover, this study obtained measurements of sleep duration and BMI at three time points rather than two. Polysomnography data obtained at the first and third examinations enabled us to exclude children/adolescents with obstructive sleep apnea, a potentially important confounder of the results. By including the same subjects at all three time points and stratifying by sex and exam, we were also able to compare the strength of these cross-sectional associations by sex and age. There are also several limitations to this study. As with many other longitudinal studies, attrition occurred over time, resulting in data from all three time points only being available for 357 (39.4%) of the original 907 participants. Participants with data from all three exams included a lower proportion of African-American and low birthweight children than the parent cohort. While this loss to follow up can potentially lead to biases, both the average sleep duration and BMIz of the subset with complete follow-up data were similar to those excluded from the analyses due to missing follow-up data. While participants were recruited from the community, the majority of the participants were either Caucasian or African-American; there were few Hispanic or Asian participants, limiting the generalizability of the results to these populations. Additionally, although models were adjusted for potential confounders including age, race, low birth weight, and low SES, we did not adjust for other potential confounders such as TV watching, dietary patterns or physical activity, which were not consistently measured across exams. It is likely that using the parent report of the child’s height and weight at the early adolescent exam resulted in measurement error for BMI at the second exam; parents may have under-reported the weight of heavier adolescents and over-reported the weight of underweight adolescents as found in prior studies (45, 46). Although objective measurements of sleep duration were available on a sample of participants in Exams 2 and 3, we elected to use questionnaire-based data to improve the consistency of the exposure measurements across exams and to minimize biases due to missing data. While parent- or self-reported sleep duration may increase measurement error, such data have been used in the majority of epidemiological studies and have yielded generally consistent results across populations. Additionally, cross-sectional analyses at the early and late adolescent exams using actigraphy-based sleep duration showed comparable results, suggesting that measurement error was an unlikely cause of the age-specific findings. Finally, we cannot exclude the possibility of reverse causality whereby BMI influences subsequent sleep duration.
The results of this study support a growing body of literature suggesting that shorter sleep duration is a risk factor for increased BMI among pre- and peri-adolescent children. These findings suggest that an important area for future research is to examine the impact of improving children’s sleep on obesity outcomes, especially at ages when weight trajectories are being established. The association between sleep duration and BMI varies by sex and child age and appears stronger in boys compared to girls and in younger children compared to adolescents. Research that addresses sex-specific responses to sleep duration, including factors associated with energy balance, other behaviors, and hormonal modulation, is needed to further elucidate the mechanisms underlying associations between short sleep and obesity.
Acknowledgments
This work was supported by NIH grants: NIH HL07567, HL60957, UL1-RR024989 and 1U54CA116867, as well as UL1-RR024989 and 1U54CA116867.
We are grateful for the ongoing participation and support of the children and families in the Cleveland Children’s Sleep and Health Cohort. We also are indebted to the expert assistance of our research assistants: Joan Aylor, Kathryn Clark, Jennifer Frame, and Heather Rogers.
Footnotes
The authors declare no conflicts of interest with the material presented in this paper.
This research was carried out while all of the authors were at Center for Clinical Investigation, Case Western Reserve University School of Medicine
References
- 1.Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA. 2002;288:1728–32. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- 2.Freedman DS, Khan LK, Serdula MK, Ogden CL, Dietz WH. Racial and ethnic differences in secular trends for childhood BMI, weight, and height. Obesity (Silver Spring) 2006;14:301–8. doi: 10.1038/oby.2006.39. [DOI] [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA. 2010;303:242–9. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 4.Kiess W, Galler A, Reich A, et al. Clinical aspects of obesity in childhood and adolescence. Obes Rev. 2001;2:29–36. doi: 10.1046/j.1467-789x.2001.00017.x. [DOI] [PubMed] [Google Scholar]
- 5.Gunturu SD, Ten S. Complications of obesity in childhood. Pediatr Ann. 2007;36:96–101. doi: 10.3928/0090-4481-20070201-08. [DOI] [PubMed] [Google Scholar]
- 6.Must A. Does overweight in childhood have an impact on adult health? Nutr Rev. 2003;61:139–42. doi: 10.1301/nr.2003.apr.139-142. [DOI] [PubMed] [Google Scholar]
- 7.Dietz WH. Health consequences of obesity in youth: childhood predictors of adult disease. Pediatrics. 1998;101:518–25. [PubMed] [Google Scholar]
- 8.Cheung YB, Machin D, Karlberg J, Khoo KS. A longitudinal study of pediatric body mass index values predicted health in middle age. J Clin Epidemiol. 2004;57:1316–22. doi: 10.1016/j.jclinepi.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Bonnet MH, Arand DL. We are chronically sleep deprived. Sleep. 1995;18:908–11. doi: 10.1093/sleep/18.10.908. [DOI] [PubMed] [Google Scholar]
- 10.Carskadon MA, Acebo C. Regulation of sleepiness in adolescents: update, insights, and speculation. Sleep. 2002;25:606–14. doi: 10.1093/sleep/25.6.606. [DOI] [PubMed] [Google Scholar]
- 11.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring) 2008;16:643–53. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marshall NS, Glozier N, Grunstein RR. Is sleep duration related to obesity? A critical review of the epidemiological evidence. Sleep Med Rev. 2008;12:289–98. doi: 10.1016/j.smrv.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Chen X, Beydoun MA, Wang Y. Is sleep duration associated with childhood obesity? A systematic review and meta-analysis. Obesity (Silver Spring) 2008;16:265–74. doi: 10.1038/oby.2007.63. [DOI] [PubMed] [Google Scholar]
- 14.Cappuccio FP, Taggart FM, Kandala NB, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31:619–26. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taveras EM, Rifas-Shiman SL, Oken E, Gunderson EP, Gillman MW. Short sleep duration in infancy and risk of childhood overweight. Arch Pediatr Adolesc Med. 2008;162:305–11. doi: 10.1001/archpedi.162.4.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dieu HT, Dibley MJ, Sibbritt D, Hanh TT. Prevalence of overweight and obesity in preschool children and associated socio-demographic factors in Ho Chi Minh City, Vietnam. Int J Pediatr Obes. 2007;2:40–50. doi: 10.1080/17477160601103922. [DOI] [PubMed] [Google Scholar]
- 17.Reilly JJ, Armstrong J, Dorosty AR, et al. Early life risk factors for obesity in childhood: cohort study. BMJ. 2005;330:1357. doi: 10.1136/bmj.38470.670903.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agras WS, Hammer LD, McNicholas F, Kraemer HC. Risk factors for childhood overweight: a prospective study from birth to 9.5 years. J Pediatr. 2004;145:20–5. doi: 10.1016/j.jpeds.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 19.Lumeng JC, Somashekar D, Appugliese D, Kaciroti N, Corwyn RF, Bradley RH. Shorter sleep duration is associated with increased risk for being overweight at ages 9 to 12 years. Pediatrics. 2007;120:1020–9. doi: 10.1542/peds.2006-3295. [DOI] [PubMed] [Google Scholar]
- 20.Snell EK, Adam EK, Duncan GJ. Sleep and the body mass index and overweight status of children and adolescents. Child Dev. 2007;78:309–23. doi: 10.1111/j.1467-8624.2007.00999.x. [DOI] [PubMed] [Google Scholar]
- 21.Bell JF, Zimmerman FJ. Shortened Nighttime Sleep Duration in Early Life and Subsequent Childhood Obesity. Arch Pediatr Adolesc Med. 2010;164:840–5. doi: 10.1001/archpediatrics.2010.143. [DOI] [PubMed] [Google Scholar]
- 22.Sugimori H, Yoshida K, Izuno T, et al. Analysis of factors that influence body mass index from ages 3 to 6 years: A study based on the Toyama cohort study. Pediatr Int. 2004;46:302–10. doi: 10.1111/j.1442-200x.2004.01895.x. [DOI] [PubMed] [Google Scholar]
- 23.Sekine M, Yamagami T, Handa K, et al. A dose-response relationship between short sleeping hours and childhood obesity: results of the Toyama Birth Cohort Study. Child Care Health Dev. 2002;28:163–70. doi: 10.1046/j.1365-2214.2002.00260.x. [DOI] [PubMed] [Google Scholar]
- 24.Chaput JP, Brunet M, Tremblay A. Relationship between short sleeping hours and childhood overweight/obesity: results from the ‘Quebec en Forme’ Project. Int J Obes (Lond) 2006;30:1080–5. doi: 10.1038/sj.ijo.0803291. [DOI] [PubMed] [Google Scholar]
- 25.Knutson KL. Sex differences in the association between sleep and body mass index in adolescents. J Pediatr. 2005;147:830–4. doi: 10.1016/j.jpeds.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 26.Biggs SN, Dollman J. Association between sleep, BMI and waist girth in children and adolescents: a retrospective analysis. Acta Paediatr. 2007;96:1839–40. doi: 10.1111/j.1651-2227.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- 27.Eisenmann JC, Ekkekakis P, Holmes M. Sleep duration and overweight among Australian children and adolescents. Acta Paediatr. 2006;95:956–63. doi: 10.1080/08035250600731965. [DOI] [PubMed] [Google Scholar]
- 28.Rosen CL, Larkin EK, Kirchner HL, et al. Prevalence and risk factors for sleep-disordered breathing in 8- to 11-year-old children: association with race and prematurity. J Pediatr. 2003;142:383–9. doi: 10.1067/mpd.2003.28. [DOI] [PubMed] [Google Scholar]
- 29.Weiss A, Xu F, Storfer-Isser A, Thomas A, Ievers-Landis C, Redline S. The Association of Sleep Duration to Adolescents’ Fat and Carbohydrate Consumption. Sleep. 2010;33:1201–9. doi: 10.1093/sleep/33.9.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Javaheri S, Storfer-Isser A, Rosen CL, Redline S. Sleep quality and elevated blood pressure in adolescents. Circulation. 2008;118:1034–40. doi: 10.1161/CIRCULATIONAHA.108.766410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240–43. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–50. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 33.Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med. 1997;337:869–73. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
- 34.Serdula MK, Ivery D, Coates RJ, Freedman DS, Williamson DF, Byers T. Do obese children become obese adults? A review of the literature. Prev Med. 1993;22:167–77. doi: 10.1006/pmed.1993.1014. [DOI] [PubMed] [Google Scholar]
- 35.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pratt M, Macera CA, Blanton C. Levels of physical activity and inactivity in children and adults in the United States: current evidence and research issues. Med Sci Sports Exerc. 1999;31:S526–33. doi: 10.1097/00005768-199911001-00007. [DOI] [PubMed] [Google Scholar]
- 37.Ahmed ML, Ong KK, Morrell DJ, et al. Longitudinal study of leptin concentrations during puberty: sex differences and relationship to changes in body composition. J Clin Endocrinol Metab. 1999;84:899–905. doi: 10.1210/jcem.84.3.5559. [DOI] [PubMed] [Google Scholar]
- 38.Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A. 2008;105:1044–9. doi: 10.1073/pnas.0706446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rao MN, Blackwell T, Redline S, Stefanick ML, Ancoli-Israel S, Stone KL. Association between sleep architecture and measures of body composition. Sleep. 2009;32:483–90. doi: 10.1093/sleep/32.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106:4453–8. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr. 2009;89:126–33. doi: 10.3945/ajcn.2008.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kimm SY, Glynn NW, Kriska AM, et al. Decline in physical activity in black girls and white girls during adolescence. N Engl J Med. 2002;347:709–15. doi: 10.1056/NEJMoa003277. [DOI] [PubMed] [Google Scholar]
- 43.Wood KC, Becker JA, Thompson JK. Body image dissatisfaction in preadolescent children. J Appl Dev Psychol. 1996;17:85–100. [Google Scholar]
- 44.Hargreaves DA, Tiggemann M. Idealized media images and adolescent body image: “comparing” boys and girls. Body Image. 2004;1:351–61. doi: 10.1016/j.bodyim.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 45.Akerman A, Williams ME, Meunier J. Perception versus reality: an exploration of children’s measured body mass in relation to caregivers’ estimates. J Health Psychol. 2007;12:871–82. doi: 10.1177/1359105307082449. [DOI] [PubMed] [Google Scholar]
- 46.Boutelle K, Fulkerson JA, Neumark-Sztainer D, Story M. Mothers’ perceptions of their adolescents’ weight status: are they accurate? Obes Res. 2004;12:1754–7. doi: 10.1038/oby.2004.217. [DOI] [PubMed] [Google Scholar]