Abstract
Objective
To investigate the effect of birth weight and early weight gain on the timing of various measures of puberty in both girls and boys.
Methods
A total of 856 newborns enrolled in the North Carolina Infant Feeding Study were followed to age 5 years, with 600 children followed up at adolescence. Birth weight was obtained from medical records and children were weighed at study visits until age 5 years; gains in standardized weights were calculated over four early age intervals: 0–6 months, 6–12 months, 1–2 years, and 2–5 years. Age at menarche in girls and age at advanced Tanner stages in both girls and boys were reported by adolescents and their parents. Survival models were used to analyze the effects of birth weight and early weight gain on these outcomes.
Results
Girls with higher birth weight and greater weight gains during the four early age intervals were younger when they reached menarche and advanced Tanner stages; boys with greater early weight gains also were younger when they reached advanced Tanner stages, but few of these effects were statistically significant.
Conclusions
Higher birth weights and greater weight gains during infancy and early childhood can lead to earlier sexual maturation in girls.
Keywords: Age at menarche, Birth weight, Puberty, Tanner stage, Weight gain
Introduction
About 40 years ago, Frisch and Revelle observed that weight at menarche was more constant than age at menarche in US girls, and hypothesized that achieving a critical weight was necessary for onset of menses (1). The relation between weight and menarche helped explain the substantial decrease in the age of menarche among white girls over the 20th century, since better nutrition had produced heavier girls during that same period (2). It also implied that the epidemic of childhood obesity that began late in the 20th century might result in many more girls achieving puberty younger. This was important because early puberty is positively associated with adult overweight status and metabolic consequences later in adulthood, even if childhood Body Mass Index (BMI) is taken into account (3).
Prospective studies so far are largely consistent in observing that girls who gain weight faster from birth to about age 2 years have earlier menarche, and higher weight gain overall from birth to school age also lowers age at menarche (4–6). Most of the studies with prospective data on menarche have relatively sparse observations on pre-school weights, and so cannot distinguish whether weight gain at particular periods in the pre-school years make independent contributions to the overall effect. Menarche is a clear and reliable pubertal event in girls. A secular trend towards early maturation has also been reported in boys, but boys have no signal event and are more difficult to study (7, 8). Unlike girls, heavier boys are generally not reported to attain puberty earlier (9–11), but studies are too sparse and inconsistent to allow any firm conclusions.
In the late 1970s, we conducted the North Carolina Infant Feeding Study, which was primarily concerned with the effects of pollutant chemicals in breast milk and their effects on children. We had relatively frequent measurements of the children from birth until school age, and we also had data on self-assessed Tanner stages during adolescence. The objective of the present study is to investigate whether birth weight or rapid weight gain in any of the pre-school periods made independent contributions to age at various pubertal measures in girls and boys.
Methods
Study participants
Between 1978 and 1982, 856 newborns from three North Carolina institutions (the East Carolina University School of Medicine at Pitt County Hospital in Greenville, the Durham Women's Clinic, and the Wake Area Health Education Center in Raleigh) were enrolled and then followed to school age; they were later asked about pubertal maturation. The mothers who volunteered were predominantly white and well educated. Detailed descriptions were published previously (12, 13). The study was approved by the review boards at our institution and at the 3 clinical sites. Written informed consent was obtained from the parents. This analysis, using de-identified data, was determined exempt from the need for further clearance.
Prenatal and infant characteristics
Data were collected by questionnaires and review of medical records for the pregnancy. The following variables were considered as covariates based on their potential associations with children’s birth weight and early weight gains: maternal education; previous pregnancies; gestational diabetes; “high risk” pregnancy (e.g., previous premature birth, spontaneous abortion, cesarean section or other illnesses); maternal smoking during pregnancy; maternal age at delivery; maternal pre-pregnancy weight; and maternal weight gain during pregnancy. The mothers also reported breastfeeding or formula feeding until the child transitioned to table food.
Birth weight and postnatal weight gain
At birth, the child’s weight was recorded by a nurse, and gestational age estimates were based on the mother’s recall of her last period and/or physical examination for the height of the uterine fundus. Subsequent visits occurred at 6 weeks, 3 months, 6 months, 1 year, 1.5 years, 2 years, 3 years, 4 years, and 5 years of age. At each visit, a nurse measured the weight of the child with light clothing but without shoes. We calculated age- and sex-specific weight z-scores at each observation time using LMSGrowth software (14) and data from the US Centers for Disease Control and Prevention 2000 growth charts (15). We used the 2000 (rather than the 2006) growth charts because the data were collected 25 or more years ago, closer to the 2000 charts. Standardized weight gains during infancy and early childhood were calculated as differences in these z-scores over four age intervals: 0–6 months, 6–12 months, 1–2 years, and 2–5 years.
Puberty
In 1992, when the children were between 10 and 15 years old, we began sending annual questionnaires to the children and their parents. A detailed description was published previously (16). We asked whether a girl had experienced her first period and, if so, the date. For stages of puberty in both sexes, we used a modification of Tanner’s method, which is based on a series of photographs of secondary sexual characteristics that appear as an ordered progression from stage 1 (pre-pubertal) to stage 5 (adult) (17). Using line drawings based on the photographs, we had the children check which of the pubertal stages was closest to their current state. Follow-up ended when a child reported Tanner stage 5; otherwise, it continued for a maximum of 5 years. In addition, children also reported their height and weight without shoes; we provided instructions and a tape measure.
At least one questionnaire was returned for 600 (70%) of the 856 original newborns. We used the date at menarche reported by the girl when it was available and by the parents otherwise (the agreement between daughters and parents was > 98%). Age at menarche was categorized into three groups (18): early (more than one standard deviation (SD) below the mean), average (within one SD of the mean), and late (more than one SD above the mean). Puberty follow-up started late enough that many children had already attained an advanced Tanner stage (> 3); thus, we used the age and stage reported by the children on the first questionnaire. As surrogates for an idealized age at onset of puberty, the temporal outcomes used in our study were the ages at which children achieved menarche and advanced Tanner stages.
Statistical analysis
All statistical analyses were performed with SAS software (version 9.2, Cary, North Carolina, USA). All P values are two-sided. We used means (and SDs) or frequencies (and percentages) to describe prenatal and postnatal characteristics; Pearson correlation coefficients to summarize associations between birth weights and standardized weight gains; and univariate linear regression analysis to assess which characteristics, when considered individually, were associated with age at menarche. Our analyses excluded preterm (delivery < 37 weeks) infants.
We used parametric survival methods (LIFEREG procedure) to assess the effects of birth weight and early weight gains on the time to an event associated with puberty. Separate survival analyses were performed for event times defined as the age at menarche or the age at attaining an advanced (> 2 or > 3) Tanner stage for development of breasts (girls), genitals (boys), or pubic hair (both sexes). Age at menarche was available for all girls, so none of these event times were censored. The child’s current Tanner stage was reported, not the age at which the current stage was first achieved, so all of these event times were either left- or right-censored. For example, if Tanner stage > 2 was the endpoint of interest, ages of children who were already in stage 3 or greater at the first follow-up visit were left-censored; and ages of children who were still in stage 1 or 2 were right-censored. For each endpoint, we assumed a lognormal distribution for the event times and assessed the effects of birth weight and weight gain over the age intervals 0–6 months, 6–12 months, 1–2 years, and 2–5 years adjusted for potential confounding factors. The lognormal analysis treats the logarithm of the event time as being normally distributed with a mean that is a linear function of regression coefficients measuring the effects of explanatory variables. The anti-log of a given regression coefficient approximately equals the ratio of mean event times associated with a unit difference in the corresponding explanatory variable.
The base survival model for each temporal endpoint included the child’s birth weight and the gains in standardized weights over the four early age intervals. Other variables were included in the final model if they were statistically significant when added individually to the base model. Race was automatically included in the final model whenever possible, though the small number of black children in our study precluded this in some cases.
Results
General characteristics
Of the original 856 children, 600 (70%) returned at least one follow-up questionnaire. Of the 256 who were lost to follow-up, we had no valid address for 94 (37%), the families of 38 (15%) refused, and 124 (48%) did not respond (16). Of the remaining 600 children in the present study, we excluded 2 who returned questionnaires without any identification, 17 who were born preterm (< 37 weeks), and 6 without birth weight data. Compared to the 575 children whose data we used, children who did not participate had no significant differences (Student’s t-test) in birth weight (P = 0.43) or weight gain during the periods from 6–12 months (P = 0.76), 1–2 years (P = 0.15), and 2–5 years (P = 0.14), but had higher weight gain from 0–6 months (P = 0.03). Table 1 shows that, of the 305 girls and 270 boys analyzed, more than two-thirds were already beyond Tanner stage 2 at their first follow-up visit. For both sexes, the mean ages at first follow-up were very similar for each stage of the two Tanner endpoints – breast and pubic hair characteristics in girls, genital and pubic hair characteristics in boys. For instance, the mean age at which girls reported being in Tanner stage 3 at their first follow-up visit was 13.2 (SD=0.7) years for breast development and 13.1 (SD=0.8) years for pubic hair development. Regarding age at menarche, the mean among the 287 girls who responded was 12.7 (SD=1.2) years. Table 2 shows the category-specific frequency (and proportion) or mean (and SD) for each prenatal and postnatal characteristic, and the estimated regression coefficient (and standard error, SE) for the effect of each characteristic in a univariate linear model for age at menarche. Heavier mothers had daughters with earlier menarche (P = 0.005). Menarche occurred one year earlier in black girls than white girls (11.7 vs 12.7 years, P = 0.008). Greater weight gains from 6–12 months (P = 0.026), 1–2 years (P = 0.035), and 2–5 years (P = 0.020) were associated with earlier menarche. None of the other characteristics had a significant individual effect on age at menarche (P > 0.05). Figure 1 shows weight z-score trajectories by age for young girls who eventually experienced early, average, or late menarche; the trajectories start to diverge by 1 year of age and remain separated through 5 years of age, at least for early versus average/late.
Table 1.
Mean Age (SD) at Menarche by Category in Girls and Mean Age (SD) at First Follow-up by Stage of Breast and Pubic Hair Development in Girls and by Stage of Genitalia and Pubic Hair Development in Boys
| Girls (N=305) |
Boys (N=270) |
|||
|---|---|---|---|---|
| Endpoint | N(%) | Mean (SD) |
N (%) | Mean (SD) |
| Menarche Category | ||||
| Early (< 11.5 years) | 42 (14.6) |
10.8 (0.5) |
||
| Average (11.5–13.9 years) | 201 (70.0) |
12.7 (0.6) |
||
| Late (> 13.9 years) | 44 (15.3) |
14.5 (0.6) |
||
| Tanner Stage | Breast |
Genitalia |
||
| 1 or 2 | 43 (16.0) |
12.5 (1.0) |
51 (24.3) |
12.8 (0.9) |
| 3 | 85 (31.6) |
13.2 (0.7) |
63 (30.0) |
13.4 (0.9) |
| 4 | 101 (37.5) |
13.7 (0.8) |
74 (35.2) |
13.8 (0.8) |
| 5 | 40 (14.9) |
14.0 (0.6) |
22 (10.5) |
14.3 (0.6) |
| Tanner Stage | Pubic Hair |
Pubic Hair |
||
| 1 or 2 | 48 (18.1) |
12.5 (0.9) |
62 (30.1) |
12.8 (0.9) |
| 3 | 35 (13.2) |
13.1 (0.8) |
53 (25.7) |
13.5 (0.8) |
| 4 | 92 (34.7) |
13.4 (0.7) |
68 (33.0) |
13.8 (0.8) |
| 5 | 90 (34.0) |
14.0 (0.7) |
(11.2) |
14.2 (0.6) |
Table 2.
Frequency (and Proportion) or Mean (and SD) for Prenatal and Postnatal Characteristics in Girls and Boys, and Estimated Regression Coefficient (and SE) for the Effect of Each Characteristic in a Univariate Linear Model for Age at Menarche in Girls
| Variable | Girls | Boys | |||
|---|---|---|---|---|---|
| Mother (at enrollment): | N a |
% | βb (SE) | N a |
% |
| Education (years) | |||||
| ≤12 | 3 9 |
13 | 0 | 3 6 |
13 |
| 13–15 | 9 0 |
3 0 |
0.23 (0.23) |
6 3 |
23 |
| ≥16 | 1 7 4 |
57 | 0.27 (0.21) |
1 7 1 |
63 |
| Previous Pregnancy | |||||
| No | 1 3 6 |
45 | 0 | 1 2 3 |
46 |
| Yes | 1 6 7 |
55 | 0.07 (0.14) |
1 4 7 |
54 |
| Gestational Diabetes | |||||
| No | 2 9 7 |
99 | 0 | 2 6 3 |
98 |
| Yes | 2 | 1 | −0.45 (0.84) |
5 | 2 |
| High Risk Pregnancy | |||||
| No | 2 5 4 |
84 | 0 | 2 3 4 |
87 |
| Yes | 4 7 |
16 | 0.17 (0.19) |
3 4 |
13 |
| Smoking During Pregnancy | |||||
| No | 2 7 9 |
93 | 0 | 2 4 3 |
91 |
| Yes | 2 2 |
7 | 0.04 (0.26) |
2 3 |
9 |
| N a |
Mean (SD) |
βb (SE) | N a |
Mean (SD) |
|
| Age at Delivery (years) | 3 0 3 |
27.7 (4.0) |
0.03 (0.02) |
2 7 0 |
27.8 (3.7) |
| Pre-pregnancy Weight (kg) | 3 0 3 |
58.0 (8.9) |
−0.02 (0.01)c |
2 7 0 |
59.3 (9.5) |
| Weight Gain During Pregnancy (kg) |
2 8 6 |
13.2 (4.2) |
−0.00 (0.02) |
2 6 8 |
13.5 (4.0) |
| Child: | N a |
% | βb (SE) | N a |
% |
| Race | |||||
| White | 2 9 1 |
96 | 0 | 2 5 8 |
97 |
| Black | 1 2 |
4 | −1.01 (0.37)c |
9 | 3 |
| Ever Breastfed | |||||
| No | 2 8 |
10 | 0 | 2 9 |
11 |
| 1–3 months | 6 7 |
23 | −0.10 (0.27) |
6 8 |
26 |
| 4–6 months | 1 1 9 |
41 | 0.41 (0.24) |
9 6 |
37 |
| >6 months | 7 7 |
26 | 0.14 (0.26) |
6 9 |
26 |
| N a |
Mean (SD) |
βb (SE) | N a |
Mean (SD) |
|
| Gestational Age (weeks) | 3 0 5 |
40.1 (1.0) |
−0.08 (0.07) |
2 7 0 |
40.1 (1.2) |
| Birth Weight (kg) | 3 0 5 |
3.5 (0.4) | −0.04 (0.15) |
2 6 9 |
3.6(0.5) |
| Weight Gain (z-score) | |||||
| 0–6 months | 2 8 9 |
0.1 (1.0) | −0.06 (0.07) |
2 6 1 |
0.1 (1.0) |
| 6–12 months | 2 6 6 |
−0.4 (0.6) |
−0.26 (0.12)c |
2 4 5 |
−0.4 (0.6) |
| 1–2 years | 2 5 2 |
0.1 (0.6) | −0.28 (0.13)c |
2 2 4 |
0.1 (0.5) |
| 2–5 years | 2 1 3 |
0.3 (0.6) | −0.34 (0.15)c | 2 0 3 |
0.3 (0.6) |
Totals may vary because of missing values.
β is the parameter estimate from a univariate linear regression model for age at menarche.
Parameter estimate differs significantly from 0 (P < 0.05).
Figure 1.
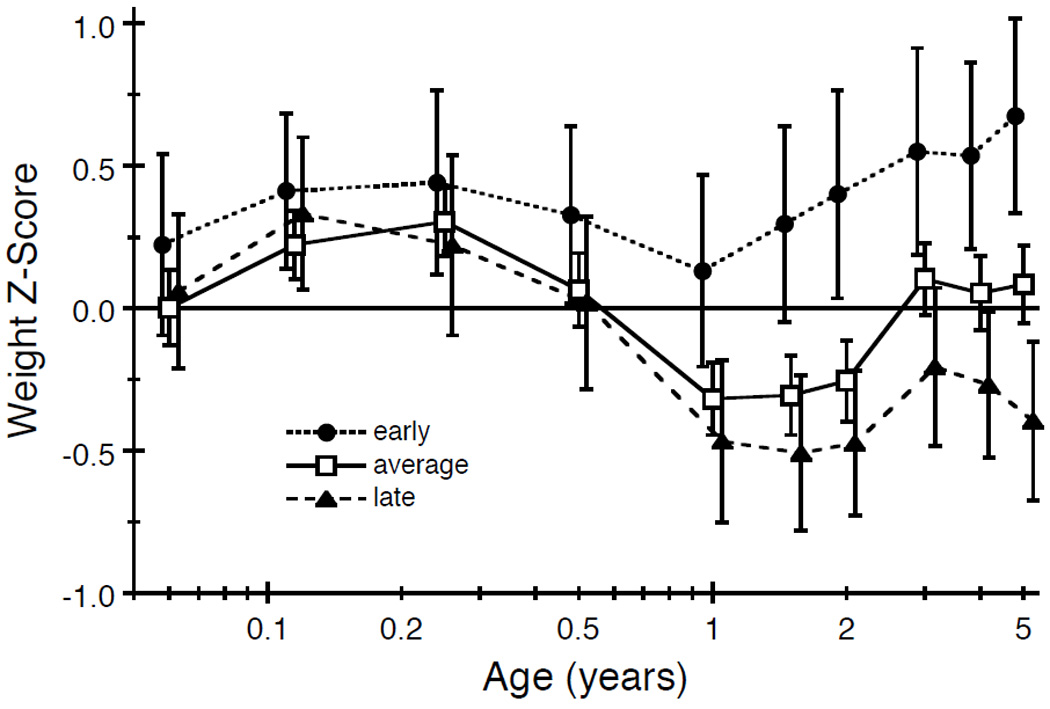
Mean Weight Z-scores and 95% Confidence Intervals by Age and Menarche Category. The means are connected by different styles of lines to better identify the menarche categories. At each age, the means and error bars are offset slightly to distinguish them from one another. The birth weight information is plotted at 0.06 because 0 is not a valid value on a log-age scale.
Association between birth weight, early weight gains and puberty
Table 3 shows that birth weight was inversely correlated with weight gain in the 0–6 month interval, but not significantly correlated with later weight gains. Weight gain in the 0–6 month interval was inversely correlated with weight gains in the other three time intervals; and weight gains from 6–12 months and 1–2 years were inversely correlated with weight gain from 2–5 years. Except for the moderate correlation between birth weight and weight gain from 0–6 months, the other correlations were relatively low (0.26 or less in magnitude).
Table 3.
Pearson Correlations for Birth Weight and Weight Gains over Four Age Intervals
| Weight Gain, 0–6 months |
Weight Gain, 6–12 months |
Weight Gain, 1–2 years |
Weight Gain, 2–5 years |
|||||
|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | |
| Birth Weight | − 0 . 54 |
< 0 . 01 |
− 0 . 0 3 |
0 . 4 4 |
− 0 . 0 3 |
0 . 4 5 |
− 0 . 0 8 |
0 . 1 2 |
| Weight Gain, 0–6 months |
− 0 . 2 6 |
< 0 . 0 1 |
− 0 . 1 0 |
0 . 0 2 |
− 0 . 1 4 |
< 0 . 0 1 |
||
| Weight Gain, 6–12 months |
− 0 . 0 3 |
0 . 4 6 |
− 0 . 1 7 |
< 0 . 0 1 |
||||
| Weight Gain, 1–2 years | − 0 . 2 4 |
< 0 . 0 1 |
||||||
We assessed the effects of birth weight and early weight gains on age at menarche in girls and age at achieving advanced Tanner stages in both girls and boys using survival models. In addition to birth weight and early weight gains, the following variables were considered for our models: maternal education, previous pregnancy, gestational diabetes, high risk pregnancy, maternal smoking during pregnancy, maternal age at delivery, maternal pre-pregnancy weight, maternal weight gain during pregnancy, breastfeeding duration, and gestational age. Based on assessments of these variables when added individually to the base survival model, the final model included maternal smoking during pregnancy for each endpoint in boys; maternal pre-pregnancy weight for each endpoint in girls; and maternal age at delivery for each Tanner stage endpoint in girls. Though not statistically significant, we also included race in the final model whenever possible; the extremely small number of black children and the distribution of the data prevented the inclusion of race in over half of the survival analyses due to numerical instability. Concurrent weight at first pubertal follow-up was also reported by children, but not only did its inclusion in the models have no effect on the magnitudes of early weight gain effects, but also it was not measured prior to pubertal maturation, so it was not included in our final models. Table 4 shows that, among girls, higher birth weight and greater weight gains over the four age intervals during infancy and early childhood were consistently associated with earlier menarche, breast development, and pubic hair development. All of the estimated regression coefficients were negative and most of the decreases were statistically significant. For example, a unit weight gain (on the z-score scale) in the 2–5 year age interval was associated with a decrease of 0.05 in the mean log age at menarche (95% confidence interval, CI: −0.08, −0.02). Thus, as exp(−0.05) ≈ 0.95, our model predicts that a white girl who gains one unit of standardized weight from 2–5 years of age and has baseline values for all other variables should experience menarche at an age 95% that of a white girl with baseline values for all variables (i.e., the mean values from Table 2); this corresponds to a decrease in mean age at menarche from 12.6 to 12.0 years.
Table 4.
Regression Coefficients (and 95% CIs) in Final Lognormal Survival Analyses of Time to Menarche, Advanced Breast Stages, and Advanced Pubic Hair Stages in Girlsa
| Menarche | Breast Stage >2 | Breast Stage >3 | Pubic Hair Stage >2 | Pubic Hair Stage >3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (n=185) | (n=174) | (n=172) | (n=172) | (n=172) | ||||||
| Variable | β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI |
| Birth Weight |
− 0 . 0 6 |
(−0.10, − 0.03) |
− 0. 0 6 |
(−0.11, − 0.01) |
− 0 . 0 5 |
(−0.10, 0.01) |
− 0. 0 4 |
(−0.10, 0.02) |
− 0. 0 6 |
(−0.11, − 0.01) |
| Weight Gain 0–6 months |
− 0 . 0 3 |
(−0.05, − 0.02) |
− 0. 0 5 |
(−0.07, − 0.02) |
− 0. 0 2 |
(−0.05, 0.01) |
− 0. 0 4 |
(−0.08, − 0.01) |
− 0. 0 4 |
(−0.07, − 0.02) |
| 6–12 months |
− 0 . 0 5 |
(−0.08, − 0.03) |
− 0. 0 5 |
(−0.09, − 0.01) |
− 0 . 0 5 |
(−0.09, − 0.01) |
− 0. 0 6 |
(−0.11, − 0.01) |
− 0. 0 5 |
(−0.08, − 0.01) |
| 1–2 years |
− 0 . 0 4 |
(−0.06, − 0.01) |
− 0. 0 5 |
(−0.09, − 0.02) |
− 0 . 0 3 |
(−0.07, 0.00) |
− 0 . 0 5 |
(−0.09, − 0.00) |
− 0. 0 7 |
(−0.10, − 0.03) |
| 2–5 years |
− 0 . 0 5 |
(−0.08, − 0.02) |
− 0. 1 0 |
(−0.15, − 0.05) |
− 0 . 0 5 |
(−0.09, − 0.00) |
− 0. 0 6 |
(−0.11, − 0.01) |
− 0. 0 6 |
(−0.10, − 0.02) |
Bold values signify coefficients that are significantly different than 0 (i.e., the variable has a significant effect on the event time). In addition to birth weight and the four early weight gains, all models were adjusted for maternal pre-pregnancy weight; the models for menarche and breast stage >3 were also adjusted for race; and all Tanner stage models were also adjusted for maternal age at delivery.
Table 5 shows the analogous results for boys. As was true with girls, higher birth weight and greater early weight gains in boys seemed to be associated with earlier genital and pubic hair development. All but two of the estimated regression coefficients were negative, but unlike the results for girls, only a few of the decreases were statistically significant.
Table 5.
Regression Coefficients (and 95% CIs) in Final Lognormal Survival Analyses of Time to Advanced Genital Stages and Advanced Pubic Hair Stages in Boysa
| Genital Stage >2 | Genital Stage >3 | Pubic Hair Stage >2 | Pubic Hair Stage >3 | |||||
|---|---|---|---|---|---|---|---|---|
| (n=151) | (n=148) | (n=148) | (n=145) | |||||
| (n=145) |
||||||||
| Variables | β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI |
| Birth Weight |
− 0.0 2 |
(−0.11, 0.06) |
− 0.0 6 |
(−0.14, 0.02) |
− 0.0 1 |
(−0.08, 0.06) |
− 0.0 8 |
(−0.16, 0.00) |
| Weight Gain |
||||||||
| 0–6 months | − 0.0 3 |
(−0.07, 0.01) |
− 0.0 3 |
(−0.07, 0.00) |
− 0.0 2 |
(−0.06, 0.01) |
− 0.0 4 |
(−0.07, − 0.01) |
| 6–12 months |
− 0.0 2 |
(−0.07, 0.04) |
0.0 2 |
(−0.03, 0.06) |
− 0.0 3 |
(−0.07, 0.02) |
0.0 0 |
(−0.04, 0.04) |
| 1–2 years | − 0.0 1 |
(−0.07, 0.05) |
− 0.0 3 |
(−0.08, 0.02) |
− 0.0 3 |
(−0.08, 0.02) |
− 0.0 4 |
(−0.08, 0.01) |
| 2–5 years | − 0.0 3 |
(−0.09, 0.03) |
− 0.0 6 |
(−0.12, − 0.00) |
− 0.0 5 |
(−0.10, 0.00) |
− 0.0 4 |
(−0.09, 0.01) |
Bold values signify coefficients that are significantly different than 0 (i.e., the variable has a significant effect on the event time). In addition to birth weight and the four early weight gains, all models were adjusted for smoking during pregnancy; the models for genital stage >3 and pubic hair stage >3 were also adjusted for race.
Discussion
We found girls with higher birth weight had earlier ages at menarche than girls with average birth weight. In contrast, other studies found that babies who were lighter at birth had an earlier age at menarche (19–23). We excluded pre-term babies from our analyses, so it may be among them that such an association with earlier menarche would be found. In addition, our cohort was of relatively high socio-economic status and born 3 decades ago, so their postnatal experience may have modulated any effect of lower birth weight (6, 18, 24). We also found that girls with higher birth weight tended to have earlier ages at advanced breast and pubic hair development. One study reported that girls with a high birth weight (> 4 Kg) were more likely to have a Tanner stage >2 for breast development than girls of normal birth weight (25); while another study did not find any association between birth weight and timing of pubertal attainment (26).
In our analyses, greater weight gain in any of the four early age intervals was associated with earlier age at menarche, and the effect sizes for each interval were similar. Our finding was similar to those from other studies that suggested babies who gained more weight in the first year of life experienced earlier menarche (6, 26, 27). We extended these observations by our finding that girls who gained more weight in any of several periods before school age reached menarche earlier. As studies have suggested that most excess weight gained before puberty may already have been gained before 5 years of age (28, 29), it is plausible that weight gained prior to school age is critical. The assortment of growth patterns occurs during the first two years (30); then the patterns stabilize between 2 and 5 years when excess weight becomes more closely predictive of later childhood overweight and obesity (28, 29). However, we observed similar associations between events at adolescence and both weight gain before 2 years and weight gain in the 2–5 year interval, suggesting that weight gained before age 2 years may have long lasting effects.
We also found that girls with greater early weight gain reached advanced Tanner stages at earlier ages. Higher childhood BMI is associated with earlier breast development (31–33), but few studies have found prospectively that body size at younger ages predicts breast and pubic hair development. A recent British study showed that postnatal weight change during the first 20 months of life was associated with earlier menarche, breast development, and pubic hair development (26), which is consistent with our results.
Boys with higher birth weight and greater weight gain before school age generally were more likely to attain advanced Tanner stages at younger ages, but most of the estimated effect sizes were not statistically significant. This may reflect, at least in part, a difference between boys and girls in the biology of weight and puberty; boys lack a pubertal event as definitive as menarche in girls. The existing literature on boys is less consistent and less robust than on girls (9–11, 34) and, to our knowledge, there have been no studies of how weight gains in infancy and early childhood affect pubertal stages. A longitudinal study found that boys with the highest BMI z-score trajectories had the greatest relative risk of being pre-pubertal compared with boys with the lowest BMI trajectories (9), and another study found that boys reaching any given maturation stage at a younger age had lower BMI and lower adiposity (11). However, in a recent study from a British cohort, overweight and obese boys were found to have earlier transitions from Tanner stage 1 to stage 2 (34). Further prospective studies are needed to provide more evidence regarding the role of weight status on the timing of puberty in boys.
A limitation of our study is that we did not contact the families until the events of puberty were well underway, precluding us from observing associations limited to the earliest stages. In addition, Tanner stages were reported by adolescents themselves rather than being assessed by a medical professional. Although obese girls tend to report more advanced breast stages (35), our data were collected in the 1990s, when less than 3% of children were overweight; and self-assessment was used in other similar studies and was considered a practical and valid choice (36). Recall bias with respect to menarche is certainly possible, though we do not consider it a major problem (37). Another limitation is that 30% of the children did not respond to the pubertal follow-up. Although comparison of their weight status with the status of those who responded did not show much difference, we did not know their outcomes and thus misclassification bias is a possibility. Finally, we lacked information on some prenatal characteristics that could confound our results. For example, we did not collect information about maternal height, so we used maternal weight instead of maternal BMI. Despite the above limitations, we had more contemporaneous data on prenatal and postnatal periods than did prior studies; and the associations between early weight gain and multiple pubertal outcomes were robust and consistent. Overall, we found that girls who were heavier at birth or gained more weight at early ages reached menarche and advanced Tanner stages sooner. Since the girls with higher birth weight were not, in general, the same girls with greater weight gains through childhood, our findings suggest that being heavier at any pre-school age was associated with earlier maturation. To a lesser degree, our results also suggest that higher birth weight and greater early weight gains are associated with earlier ages of advanced genital and pubic hair development in boys, though the estimated effect sizes tended to be smaller than those in girls and most of the observed effects were not statistically significant. Since the present study was conducted just prior to the obesity epidemic (2), and given the recent increasing trend of obesity, more studies are needed to identify the mechanisms for the link between birth weight, early weight gain, and the onset of puberty.
Acknowledgement
This research was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
Footnotes
There is no conflict of interest.
References
- 1.Frisch RE, Revelle R. Height and weight at menarche and a hypothesis of menarche. Arch Dis Child. 1971;46(249):695–701. doi: 10.1136/adc.46.249.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA. 2002;288(14):1728–1732. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- 3.He C, Zhang C, Hunter DJ, et al. Age at menarche and risk of type 2 diabetes: results from 2 large prospective cohort studies. Am J Epidemiol. 2010;171(3):334–344. doi: 10.1093/aje/kwp372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karaolis-Danckert N, Buyken AE, Sonntag A, Kroke A. Birth and early life influences on the timing of puberty onset: results from the DONALD (DOrtmund Nutritional and Anthropometric Longitudinally Designed) Study. Am J Clin Nutr. 2009;90(6):1559–1565. doi: 10.3945/ajcn.2009.28259. [DOI] [PubMed] [Google Scholar]
- 5.Ong KK, Emmett P, Northstone K, et al. Infancy weight gain predicts childhood body fat and age at menarche in girls. J Clin Endocrinol Metab. 2009;94(5):1527–1532. doi: 10.1210/jc.2008-2489. [DOI] [PubMed] [Google Scholar]
- 6.Adair LS. Size at birth predicts age at menarche. Pediatrics. 2001;107(4):E59. doi: 10.1542/peds.107.4.e59. [DOI] [PubMed] [Google Scholar]
- 7.Sun SS, Schubert CM, Chumlea WC, et al. National estimates of the timing of sexual maturation and racial differences among US children. Pediatrics. 2002;110(5):911–919. doi: 10.1542/peds.110.5.911. [DOI] [PubMed] [Google Scholar]
- 8.Herman-Giddens ME, Wang L, Koch G. Secondary sexual characteristics in boys: estimates from the national health and nutrition examination survey III, 1988-1994. Arch Pediatr Adolesc Med. 2001;155(9):1022–1028. doi: 10.1001/archpedi.155.9.1022. [DOI] [PubMed] [Google Scholar]
- 9.Lee JM, Kaciroti N, Appugliese D, Corwyn RF, Bradley RH, Lumeng JC. Body mass index and timing of pubertal initiation in boys. Arch Pediatr Adolesc Med. 2010;164(2):139–144. doi: 10.1001/archpediatrics.2009.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y. Is obesity associated with early sexual maturation? A comparison of the association in American boys versus girls. Pediatrics. 2002;110(5):903–910. doi: 10.1542/peds.110.5.903. [DOI] [PubMed] [Google Scholar]
- 11.Biro FM, Lucky AW, Huster GA, Morrison JA. Pubertal staging in boys. J Pediatr. 1995;127(1):100–102. doi: 10.1016/s0022-3476(95)70265-2. [DOI] [PubMed] [Google Scholar]
- 12.Rogan WJ, Gladen BC, McKinney JD, et al. Polychlorinated biphenyls (PCBs) and dichlorodiphenyl dichloroethene (DDE) in human milk: effects of maternal factors and previous lactation. Am J Public Health. 1986;76(2):172–177. doi: 10.2105/ajph.76.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rogan WJ, Gladen BC, McKinney JD, et al. Neonatal effects of transplacental exposure to PCBs and DDE. J Pediatr. 1986;109(2):335–341. doi: 10.1016/s0022-3476(86)80397-3. [DOI] [PubMed] [Google Scholar]
- 14.TJ C. Software for LMS method: LMSGrowth PC. http://wwwhealthforallchildren.co.uk/pro.epl?DO=PRODUCT&WAY=INFO&ID=185.
- 15.National Center for Health Statistics at Center for Chronic Disease Prevention and Health. 2000 CDC Growth Charts. http://www.cdc.gov/growthcharts.
- 16.Gladen BC, Ragan NB, Rogan WJ. Pubertal growth and development and prenatal and lactational exposure to polychlorinated biphenyls and dichlorodiphenyl dichloroethene. J Pediatr. 2000;136(4):490–496. doi: 10.1016/s0022-3476(00)90012-x. [DOI] [PubMed] [Google Scholar]
- 17.Morris NM, Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolesc. 1980;9:271–280. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- 18.dos Santos Silva I, De Stavola BL, Mann V, Kuh D, Hardy R, Wadsworth ME. Prenatal factors, childhood growth trajectories and age at menarche. Int J Epidemiol. 2002;31(2):405–412. doi: 10.1093/ije/31.2.405. [DOI] [PubMed] [Google Scholar]
- 19.Koziel S, Jankowska EA. Effect of low versus normal birthweight on menarche in 14-year-old Polish girls. J Paediatr Child Health. 2002;38(3):268–271. doi: 10.1046/j.1440-1754.2002.00793.x. [DOI] [PubMed] [Google Scholar]
- 20.Sloboda DM, Hart R, Doherty DA, Pennell CE, Hickey M. Age at menarche: Influences of prenatal and postnatal growth. J Clin Endocrinol Metab. 2007;92(1):46–50. doi: 10.1210/jc.2006-1378. [DOI] [PubMed] [Google Scholar]
- 21.Romundstad PR, Vatten LJ, Nilsen TI, et al. Birth size in relation to age at menarche and adolescent body size: implications for breast cancer risk. Int J Cancer. 2003;105(3):400–403. doi: 10.1002/ijc.11103. [DOI] [PubMed] [Google Scholar]
- 22.Tam CS, de Zegher F, Garnett SP, Baur LA, Cowell CT. Opposing influences of prenatal and postnatal growth on the timing of menarche. J Clin Endocrinol Metab. 2006;91(11):4369–4373. doi: 10.1210/jc.2006-0953. [DOI] [PubMed] [Google Scholar]
- 23.Cooper C, Kuh D, Egger P, Wadsworth M, Barker D. Childhood growth and age at menarche. Br J Obstet Gynaecol. 1996;103(8):814–817. doi: 10.1111/j.1471-0528.1996.tb09879.x. [DOI] [PubMed] [Google Scholar]
- 24.Persson I, Ahlsson F, Ewald U, et al. Influence of perinatal factors on the onset of puberty in boys and girls: implications for interpretation of link with risk of long term diseases. Am J Epidemiol. 1999;150(7):747–755. doi: 10.1093/oxfordjournals.aje.a010077. [DOI] [PubMed] [Google Scholar]
- 25.Olivo-Marston S, Graubard BI, Visvanathan K, Forman MR. Gender-specific differences in birthweight and the odds of puberty: NHANES III, 1988-94. Paediatr Perinat Epidemiol. 2010;24(3):222–231. doi: 10.1111/j.1365-3016.2010.01097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maisonet M, Christensen KY, Rubin C, et al. Role of prenatal characteristics and early growth on pubertal attainment of British girls. Pediatrics. 2010;126(3):e591–e600. doi: 10.1542/peds.2009-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Terry MB, Ferris JS, Tehranifar P, Wei Y, Flom JD. Birth weight, postnatal growth, and age at menarche. Am J Epidemiol. 2009;170(1):72–79. doi: 10.1093/aje/kwp095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gardner DS, Hosking J, Metcalf BS, Jeffery AN, Voss LD, Wilkin TJ. Contribution of early weight gain to childhood overweight and metabolic health: a longitudinal study (EarlyBird 36) Pediatrics. 2009;123(1):e67–e73. doi: 10.1542/peds.2008-1292. [DOI] [PubMed] [Google Scholar]
- 29.Nader PR, O'Brien M, Houts R, et al. Identifying risk for obesity in early childhood. Pediatrics. 2006;118(3):e594–e601. doi: 10.1542/peds.2005-2801. [DOI] [PubMed] [Google Scholar]
- 30.Ong KK, Ahmed ML, Emmett PM, Preece MA, Dunger DB. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. BMJ. 2000;320(7240):967–971. doi: 10.1136/bmj.320.7240.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mamun AA, Hayatbakhsh MR, O'Callaghan M, Williams G, Najman J. Early overweight and pubertal maturation--pathways of association with young adults' overweight: a longitudinal study. Int J Obes (Lond.) 2009;33(1):14–20. doi: 10.1038/ijo.2008.220. [DOI] [PubMed] [Google Scholar]
- 32.Zukauskaite S, Lasiene D, Lasas L, Urbonaite B, Hindmarsh P. Onset of breast and pubic hair development in 1231 preadolescent Lithuanian schoolgirls. Arch Dis Child. 2005;90(9):932–936. doi: 10.1136/adc.2004.057612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davison KK, Susman EJ, Birch LL. Percent body fat at age 5 predicts earlier pubertal development among girls at age 9. Pediatrics. 2003;111(4 Pt 1):815–821. doi: 10.1542/peds.111.4.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monteilh C, Kieszak S, Flanders WD, et al. Timing of maturation and predictors of Tanner stage transitions in boys enrolled in a contemporary British cohort. Paediatr Perinat Epidemiol. 2011;25(1):75–87. doi: 10.1111/j.1365-3016.2010.01168.x. [DOI] [PubMed] [Google Scholar]
- 35.Desmangles JC, Lappe JM, Lipaczewski G, Haynatzki G. Accuracy of pubertal Tanner staging self-reporting. J Pediatr Endocrinol Metab. 2006;19(3):213–221. doi: 10.1515/jpem.2006.19.3.213. [DOI] [PubMed] [Google Scholar]
- 36.Rockett JC, Lynch CD, Buck GM. Biomarkers for assessing reproductive development and health: Part 1--Pubertal development. Environ Health Perspect. 2004;112(1):105–112. doi: 10.1289/ehp.6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Must A, Phillips SM, Naumova EN, et al. Recall of early menstrual history and menarcheal body size: after 30 years, how well do women remember? Am J Epidemiol. 2002;155(7):672–679. doi: 10.1093/aje/155.7.672. [DOI] [PubMed] [Google Scholar]


