Abstract
Introduction
Changes occur in muscles and nerves with aging. This study aimed to explore the relationship between unipedal stance time (UST) and frontal plane hip and ankle sensorimotor function in subjects with diabetic neuropathy.
Methods
UST, quantitative measures of frontal plane ankle proprioceptive thresholds, and ankle and hip motor function were tested in forty-one persons with a spectrum of lower limb sensorimotor function, ranging from healthy to moderately severe diabetic neuropathy.
Results
Frontal plane hip and ankle sensorimotor function demonstrated significant relationships with UST. Multivariate analysis identified only composite hip strength, composite ankle proprioceptive threshold, and age to be significant predictors of UST (R2=0.73); they explained 46%, 24% and 3% of the variance, respectively.
Discussion/Conclusions
Frontal plane hip strength was the single best predictor of UST and appeared to compensate for less precise ankle proprioceptive thresholds. This finding is clinically relevant given the possibility of strengthening the hip, even in patients with significant PN. .
Keywords: age, muscle strength, proprioception, diabetic neuropathy, balance
INTRODUCTION
Quantitative and qualitative changes occur in muscles and nerves with aging 1. These include a decrease in the number of alpha motoneurons, reduced motoneuron excitability, and loss of type II muscle fibers leading to decreased muscle mass and slower muscle response latencies 2. Such changes, which adversely affect motor control and balance in older persons, are even more marked among older persons with peripheral neuropathy (PN), a common complication of diabetes mellitus. In such patients, the neuropathy is usually length dependent and results in distal sensorimotor dysfunction of varying severity. As a result, diabetic patients have decreased balance 3–6, altered gait 7 and increased fall risk 8–9 when compared to healthy controls.
Control of frontal plane stability is particularly important given that lateral falls are associated with hip fractures in older adults 10–11. Biomechanical models and human studies suggest that control at the hip is of greater importance to equilibrium in the frontal plane than control at the ankle. For example, a whole-body inverted pendulum model of medial-lateral control during human walking, suggests that the hip exerts the primary influence, and that minor errors in hip motion are compensated by adjustments at the subtalar joint 12. Similarly, a second model demonstrated that foot placement in the frontal plane, which is regulated by hip abduction/adduction, was the most efficient method for controlling frontal plane balance while walking 13. Other studies have provided experimental support for these models and demonstrated the importance of hip frontal plane strength for balance control in elderly subjects when they negotiate obstacles 14 and for fall prevention 15–16.
However, no study has evaluated the relationship between lower limb afferent and efferent neuromuscular capacities relevant to frontal plane control in older subjects with a demonstrably significant range of peripheral neurologic function. For example, none of the above biomechanical models or experimental studies have addressed the role of distal afferent function (i.e., ankle proprioception). Similarly, evaluations of lower limb neuromuscular capacities associated with balance deficits in subjects with PN studied either ankle proprioception 5 or ankle joint motor function 17–18, but not both, and no study evaluated hip motor function in this high risk population.
Unipedal stance time (UST) is a convenient clinical measure of balance that evaluates frontal plane postural control. It is the most challenging activity within the widely used Berg Balance Scale 19. Moreover, UST is associated with frailty 20–21, PN 22,6, activity level 23, falls in older persons with 24 and without PN 25–26 and decreases markedly with age 27–28. Therefore, the objective of this study was to explore the relationships between UST and lower limb neuromuscular capacities relevant to frontal plane postural control in older subjects with a spectrum of neuromuscular function. The primary hypothesis was that hip motor function would be an independent predictor of UST. Support for this hypothesis has clinical relevance, given the fact that PN predominantly affects distal function, which leaves the potential for strengthening of hip musculature1.
MATERIALS and METHODS
Forty-one subjects (16 healthy old and 25 subjects with PN due to diabetes) were recruited under a protocol approved by the Institutional Review Board. Written informed consent was obtained from all participants. Subjects were recruited from the University of Michigan Orthotics and Prosthetics Clinic, Endocrinology Clinic and the Older Americans Independence Center Human Subjects Core. Inclusion criteria for PN subjects were:
Age 50 – 85 years
Weight < 136 kg
Known history of diabetes.
Able to walk household distances without assistance/assistive device
Strength of ankle dorsiflexors, invertors, and evertors at least anti-gravity (grade ≥3 by manual muscle testing)
Symptoms and signs consistent with PN: symmetrically altered sensation in lower extremities, Michigan Diabetes Neuropathy Score (MDNS) ≥10; 29
Electrodiagnostic evidence of a diffuse PN as evidenced by bilaterally abnormal fibular motor nerve conduction studies (absent or amplitude < 2 mV and/or latency > 6.2 msec and/or conduction velocity < 41.0 m/s) stimulating 9 centimeters from recording site over the extensor digitorum brevis distally, and distal to the fibular head proximally.
Exclusion criteria for PN subjects were:
Accidental fall one month or less prior to testing
History or evidence of any significant central nervous system dysfunction (i.e. hemiparesis, myelopathy or cerebellar ataxia)
Neuromuscular disorder other than PN (e.g. myopathy or myasthenia gravis)
Evidence of vestibular dysfunction
Angina or angina-equivalent symptoms with exercise
Plantar skin sore or joint replacement within the previous year
Symptomatic postural hypotension
Significant musculoskeletal deformity (i.e. amputation or Charcot changes)
Lower limb or spinal arthritis or pain that limits standing to less than 10 minutes, or walking to less than one block
The healthy older adults were without neuropathic symptoms, had an MDNS<10 and had normal electrodiagnostic studies. They otherwise met the same inclusion criteria as the PN subjects.
Entrance Evaluation
During the physical examination that focused on neurologic and musculoskeletal findings, inclusion and exclusion criteria were verified. Neuropathy severity was further determined using the 46 point scale MDNS 29,30, (higher score reflecting more severe neuropathy) evaluating distal sensory impairment, distal muscle strength and muscle stretch reflexes. Finally, all subjects underwent nerve conduction studies of the fibular nerve, as described above.
UST
Subjects performed three trials of UST on each foot 29,31. Subjects started with an intra-malleolar distance of approximately 15 cm, and then transferred weight to one foot. To standardize the test sequence and timing of weight transfer to the extent possible the examiner asked, “Ready?” and upon receiving assent from the subject, gave the cadence command, “one, two, up”. Subjects were required to raise their non-stance limb at the “up” command. UST maximum was set at 30 s.
Neuromuscular Capacity Testing
Hip abduction and adduction muscle strength
A custom, whole-body dynamometer (BioLogic Engineering, Inc.) was used to measure the maximum voluntary contraction (MVC) and maximum rate of torque development (RTD) in the frontal plane at the hip32. This dynamometer was found to be sensitive to the effects of age, gender and hip angle when isometric hip strength was measured in a group of 24 young and 24 older subjects. In addition, the apparatus demonstrated the ability to resolve torque with a precision of 0.5 Newton-meters. Retest reliability has not been evaluated; however, it is anticipated that reliability would be similar to that found with isometric testing in other populations (e.g., with a mean day-to-day difference of 10% and a coefficient of repeatability of 11 to 33%) 33. The dynamometer features a horizontal bench on which the subject lies fully supported, allowing all measurements to be made in a gravity-free plane. The pelvis and upper body were immobilized using adjustable harness straps at multiple points. During maximum voluntary abduction strength tests, subjects progressively increased their isometric effort from rest to their maximum over a count of three, held it for two seconds, and relaxed. Patients were encouraged verbally. To quantify rate of isometric strength development, subjects performed an abduction against the lever arm as fast and as hard as possible for three seconds 34. Three trials were performed with one minute rest between trials. Subjects performed analogous maneuvers in the opposite direction for hip adduction strength and rate of isometric strength testing.
Ankle muscle strength
During ankle rate of strength development testing, subjects stood on the test foot on a force plate (Advanced Mechanical Technology, Inc. OR-6) and moved the center of ground support reaction from the lateral margin of the foot to the medial margin as quickly as possible, then again to the lateral margin, as previously described 17. Three trials, each trial with five medial-lateral movements, were performed. Subjects were allowed to touch a horizontal railing to keep their balance.
During maximum voluntary strength testing, subjects stood on the force platform touching the hand rails on both sides as needed. Subjects were then asked to lift one leg, shift their center of gravity as far lateral under their foot as they could and lift their hands from the rails for three seconds. The test was repeated three times for the lateral, and then likewise repeated for the medial margin of the foot.
Ankle proprioception threshold
Subjects stood with the test foot in a 40 × 25 cm cradle that was rotated by an Aerotech 1000 servomotor equipped with an 8,000 line rotary encoder as described by Son et al.5. After an audible cue, a single ankle inversion or eversion rotation of 0.1 to 3° magnitude was randomly presented at 5°/s. The subject then pressed a joystick handle in the direction of the perceived foot rotation. Four blocks of 25 trials (randomly, 10 eversion, 10 inversion, and 5 dummy trials) were presented interspersed with 2 to 5 minute rest intervals. The outcome measure was the ankle proprioception threshold (TH100), defined as the smallest rotational displacement of the ankle that a subject could reliably detect with 100% accuracy 35.
Data Processing
Signals were amplified to volt levels before being acquired using a 12 bit analog-to-digital converter sampling at 100 Hz. The MVC efforts at the hip and ankle, as well as the maximal RTD, were normalized by individual body size defined as the parameter body height multiplied by weight in units of Nm. Strength data were processed using a Labview second-order least squares polynomial fit to determine the peak value. The mean peak value obtained from the three trials for each test type was used for the statistical analyses. To determine each proprioceptive threshold, the mean TH100 from the four blocks of 25 trials in each test direction was calculated. A summary measure of ankle proprioception was found from the sum of the inversion and eversion proprioception threshold.
Statistics
Statistics were conducted using SPSS (SPSS for Windows, Rel.11.0.1.2001. Chicago). Descriptive statistics were calculated for all measures, including a composite score of frontal plane ‘hip strength’, calculated as the mean of the mean peak abduction and adduction MVCs. Data were examined for normality and screened for outliers. Pearson product-moment correlation coefficients were calculated to assess relationships between neuromuscular capacities and UST.
A regression model determined independent predictors of UST. Variables were entered stepwise in the order of their strength of correlation. To reduce the number of independent variables, only the best predictor variable for ankle motor function and the best predictor variable for hip motor function were retained in the final regression model, along with the identified co-variables (age and BMI).
To determine whether hip strength might compensate for distal afferent deficiencies (less precise ankle proprioceptive thresholds), the residuals of the regression model using UST as the outcome variable and proprioceptive threshold and age as predictor variables were saved and ranked by magnitude. The hip strength of the 12 subjects with the highest residuals was then compared with the hip strength of the 12 subjects with the lowest residuals using a two-sided, student t-test. A similar analysis was performed to determine whether more precise ankle proprioceptive thresholds might compensate for decreased hip strength. The significance level for all tests was set at 0.05.
RESULTS
Of 91 potential subjects, 21 did not pass the telephone screening, and 18 elected to not participate. Of the 52 remaining subjects, three had scheduling conflicts, and five failed the screen. Of the remaining 44, one was lost to follow-up, and 2 dropped out due to medical concerns. Therefore, 41 subjects were enrolled. The means and standard deviations of age, body mass index (BMI) and MDNS, together with the participants’ neuromuscular capacities and UST, are shown in Table 1.
Table 1.
Mean (SD) of Demographic and Neuromuscular Function Results
| PARAMETER | Non diabetic subjects (N=16) | Diabetic patients (N= 25) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| ALL | MEN (N=6) | WOMEN (N= 10) | ALL | MEN (N=15) | WOMEN (N=10) | |
| Age (years) | 67.81 (8.97) | 67.83 (11.02) | 67.8 (8.16) | 70.04 (8.16) | 71.53 (7.17) | 67.8 (9.39) |
|
| ||||||
| BMI (kg/m2) | 28.35 (7.18) | 26.24 (3.25) | 29.62 (8.68) | 32.41 (6.44) | 30.25 (5.36) | 35.66 (6.81) |
|
| ||||||
| Unipedal stance time (s) | 22.34 (11.1) | 21.87 (11.98) | 22.62 (11.19) | 6.9 (6.91) | 15.1 (11.03) | 9.52 (9.36) |
|
| ||||||
| MDNS (0 to 46 points) | 1.69 (3.77) | 2.5 (6.12) | 1.2 (1.48) | 13.56 (6.04) | 14.13 (6.5) | 12.7 (5.48) |
|
| ||||||
| Hip Abduction MVC (N.m/N.m) | 0.041 (0.024) | 0.051 (0.028) | 0.035 (0.02) | 0.031 (0.01) | 0.032 (0.011) | 0.03 (0.009) |
|
| ||||||
| Hip Abduction RTD (N.m/N.m.s) | 0.255 (0.188) | 0.312 (0.224) | 0.22 (0.166) | 0.154 (0.096) | 0.155 (0.104) | 0.154 (0.087) |
|
| ||||||
| Hip adduction MVC (N.m/N.m) | 0.047 (0.018) | 0.051 (0.018) | 0.045 (0.018) | 0.033 (0.012) | 0.035 (0.013) | 0.03 (0.011) |
|
| ||||||
| Hip adduction RTD (N.m/N.m.s) | 0.29 (0.226) | 0.4 (0.224) | 0.224 (0.211) | 0.199 (0.151) | 0.19 (0.176) | 0.213 (0.112) |
|
| ||||||
| Ankle eversion MVC (cm)* | 1.275 (0.502) | 1.501 (0.665) | 1.135 (0.348) | 1.017 (0.442) | 1.009 (0.541) | 1.028 (0.257) |
| Ankle inversion MVC (cm)* | 2.187 (0.501) | 2.544 (0.381) | 1.932 (0.426) | 1.596 (0.659) | 1.585 (0.729) | 1.614 (0.563) |
|
| ||||||
| Ankle inversion RTD (N.m/N.m.s)* | 0.188 (0.096) | 0.231 (0.086) | 0.161 (0.097) | 0.104 (0.064) | 0.113 (0.067) | 0.091 (0.06) |
|
| ||||||
| Ankle eversion RTD (N.m/N.m.s)* | 0.243 (0.114) | 0.326 (0.136) | 0.191 (0.059) | 0.141 (0.069) | 0.15 (0.079) | 0.128 (0.051) |
|
| ||||||
| Proprioceptive threshold (°)† | 0.986 (0.757) | 1.154 (1.093) | 0.885 (0.511) | 2.391 (1.313) | 2.208 (0.832) | 2.665 (1.839) |
N=13 valid cases for non diabetic subjects; =24 valid cases for diabetic patients and 12 for non diabetic subjects; MVC=Maximum voluntary contraction; RTD=Rate of torque development
Correlations
Correlations between UST and frontal plane lower limb neuromuscular function were strong, and many of the functions explained more than a third of the variability in UST (Table 2). This includes all of the functions measured except for ankle inversion and eversion MVC, and hip abduction and ankle eversion RTD. Age and BMI were substantially less strongly associated with UST than were the majority of neuromuscular RTD variables.
Table 2.
Bivariate Correlations between Unipedal Stance Time and Neuromuscular Function, Age and BMI
| Parameter | Correlation Coefficient with UST | p |
|---|---|---|
| Hip strength | .672 | .000 |
| Hip adduction MVC | .664 | .000 |
| Hip adduction RTD | .645 | .000 |
| Ankle inversion RTD | .644 | .000 |
| Proprioceptive threshold | −.643 | .000 |
| Hip abduction MVC | .619 | .000 |
| Age | −.492 | .001 |
| Ankle eversion RTD | .490 | .001 |
| Hip abduction RTD | .481 | .001 |
| BMI | −.392 | .009 |
| Ankle eversion MVC | .351 | .018 |
| Ankle inversion MVC | .350 | .018 |
All values have been calculated based on the 36 subjects who had valid results for all variables
Multivariate Analyses
The final regression model included UST as the outcome variable and hip strength (as defined in Methods), ankle inversion RTD, ankle proprioception and the covariates age and BMI as independent variables (Table 3). Maximum hip strength was the most important predictor of UST, explaining almost half of its variability. Ankle proprioceptive thresholds and age also contributed to the model in a significant manner. The former explained an additional 25% of the variance in UST, and age explained just 3%. Overall, the model explains nearly three quarters of the variability in UST.
Table 3.
Regression Model
| 95% CI -Bound | ||||||||
|---|---|---|---|---|---|---|---|---|
| Model | R | R2 | Dependent variable | Unst. Coeff.* | Lower | Upper | t | p |
| 1 | 0.676 | 0.456 | Hip MVC* | 460.945 | 290.881 | 631.010 | 5.497 | 0.000 |
| 2 | 0.834 | 0.696 | Hip MVC* | 386.485 | 254.224 | 518.745 | 5.932 | 0.000 |
| Ankle proprioception | −4.179 | −5.794 | −2.564 | −5.254 | 0.000 | |||
| 3 | 0.856 | 0.733 | Hip MVC* | 339.517 | 205.974 | 473.060 | 5.167 | 0.000 |
| Ankle proprioception | −3.867 | −5.433 | −2.300 | −5.017 | 0.000 | |||
| Age | −0.265 | −0.515 | −0.015 | −2.156 | 0.038 | |||
Unstandardized Coefficients
UST and the Ratio of a Composite Variable of Hip Strength to Ankle Proprioception
After observing the relationship between hip strength and UST and the inverse relationship between proprioceptive threshold and UST, we formed a new variable, the ratio of hip strength to proprioceptive threshold. This variable was found to explain more than 70% of the variability of UST (Figure 1).
Figure 1.
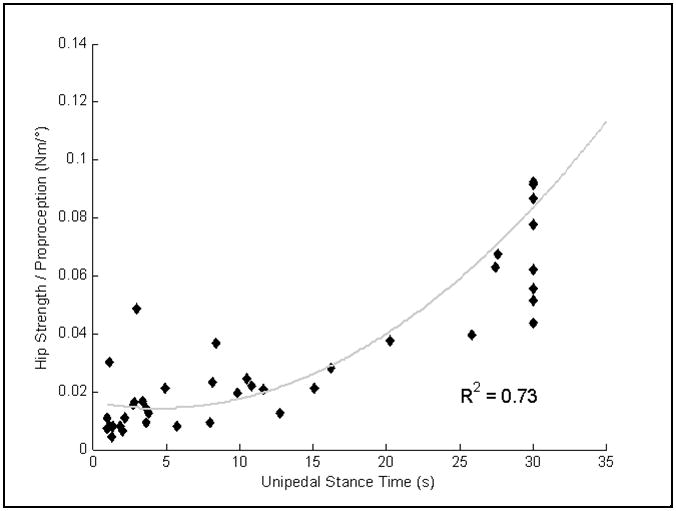
Scatterplots illustrating the relationship of the hip strength/proprioception ratio to UST. The equation for the curvilinear regression is y=0.0098e0.067x
Hip Strength Can Compensate for Imprecise Ankle Proprioception
After performing regression of ankle proprioceptive threshold and age on UST the residuals for all subjects were ranked, and the hip strength of the upper one-third (representing subjects who had longer USTs than would be expected for prioprioceptive threshold and age) was compared to that of the lower one-third. The former had significantly greater hip strength than the latter (Figure 2a), suggesting that hip strength was able to compensate for less precise ankle proprioception. When the analogous analysis was performed for ankle proprioceptive thresholds, subjects with greater UST had significantly more precise (smaller) proprioceptive thresholds (Figure 2b).
Figure 2.
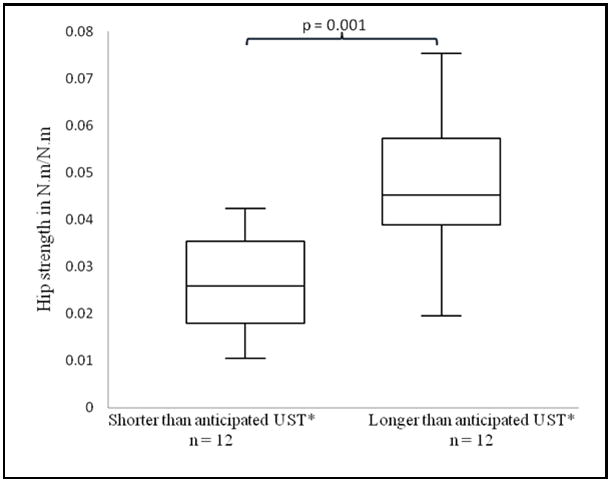
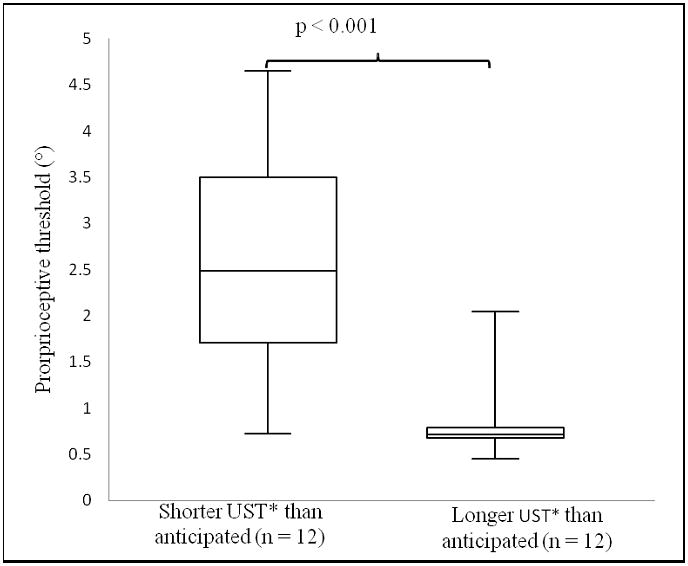
Figure 2A, B. Hip strength and ankle proprioception in patients with shorter, respectively longer UST’s than anticipated. A comparison of A) hip strength B) ankle proprioceptive thresholds in subjects who demonstrated shorter (left) and longer (right) USTs than would be anticipated based on their ankle proprioceptive threshold and age. Hip strength=calculated as the mean of the mean peak abduction and adduction maximal voluntary contractions (N.m./N.m.). UST=Unipedal Stance Time. Proprioceptive threshold=smallest rotational displacement of the ankle that a subject could reliably detect with 100% accuracy.
DISCUSSION
We have quantified sensory and motor lower limb neuromuscular capacities in a group of older subjects with a spectrum of peripheral neurologic health. There are three novel, clinically significant findings: 1) Maximum voluntary hip strength in the frontal plane was the single best predictor of UST, a result consistent with the primary hypothesis; 2) Maximum voluntary hip strength and ankle proprioceptive thresholds explained the majority of the variance in UST, with age playing a trivial role; 3) Increased hip strength appears to compensate for less precise ankle proprioception.
Although frontal plane hip strength is not routinely evaluated in studies of postural control, there is evidence supporting its importance. For example during bipedal stance, anterior-posterior balance is under ankle control (plantar and dorsiflexors) 36, whereas medio-lateral balance is controlled via frontal plane motion at the hip 36. Other studies have found significant correlations between hip abduction RTD and performance of reactive and voluntary frontal plane balance in older adults 37. A study of slips noted that older persons used frontal plane mechanisms for recovery, whereas young subjects did not 38. One way to interpret the importance of abductor and adductor muscles with regard to unipedal stance is to suggest that a co-contraction of these muscles allows a transient, voluntary increase in hip rotational stiffness. Given that an inverted pendulum is a commonly used model for human standing balance, this stiffness creates a longer pendulum, which requires more time to fall than a shorter pendulum. As a result, there is more time available for postural adjustments, which renders the task of one legged balance less challenging 39. However, once balance is disturbed, it is likely that the availability of a rapid rate of strength development would be more important, given that balance restoration occurs within fractions of a second 34.
The independent contribution of ankle proprioception for balancing on one leg is consistent with previous work 5 in which ankle inversion/eversion proprioceptive thresholds explained approximately half the variance in UST (R2 = 0.514) in older subjects with a range of peripheral neurologic function. More precise ankle proprioceptive thresholds may reduce the lateral distance the center of mass (COM) can travel prior to detection. Early detection of a displaced COM would then require only moderate strength that a majority of older persons likely possess. In contrast, less precise ankle proprioception would require greater intensity of motor function for appropriate repositioning of the COM. Supporting this explanation, healthy subjects demonstrate increased center of pressure velocities when the plantar aspect of the foot is anesthetized, which is consistent with the greater motor function requirement40.
Ankle motor function did not show a significant independent influence on UST, despite the fact that ankle inversion and eversion rates of torque generation explained approximately 40% and 25%, respectively, of its variance. These findings are consistent with those of Gutierrez et al. 17 who found that ankle inversion RTD explained over half of the variance in UST (R2 = 0.575). In contrast, ankle maximum isometric inversion and eversion strengths each explained only 12% of UST. When observing subjects successfully balance on one foot there are rapid postural adjustments in ankle inversion and eversion as the center of pressure is quickly manipulated to control the movements of the whole body COM. The rapid speed with which these changes occur in the subject who can reliably stand on one foot is consistent with ankle maximum RTD being an important motor function for the maintenance of unipedal stance. These findings are in line with other studies that have found that the ability of the lower limbs to create force quickly is of greater importance than the total force a muscle group can generate 41–42. Although highly correlated with UST, ankle RTD had no independent influence on UST in the presence of ankle proprioception and hip strength. This is of clinical interest, given the challenge of strengthening distal musculature in PN subjects. Given the established relationships between a diminished UST and frailty, activity level and falls, strategies to increase UST have clinical relevance. There is no clear evidence that ankle proprioceptive thresholds can be improved by therapeutic exercise 43 and recent work showed that an ankle orthosis, which had decreased the temporal and spatial variability of neuropathic gait on an irregular surface, did not improve ankle proprioceptive thresholds 35. Given these findings, frontal plane hip strengthening appears the best strategy for improving UST. This strengthening should be pursued most aggressively in persons with decreased distal afferent neurologic function, as it appears that increased frontal plane hip strength can compensate for distal sensory impairment at the ankle. Given the fact that the majority of polyneuropathies are distal, this strategy can be used in a large proportion of patients with lower limb neuromuscular disease. Conversely, persons with PN and proximal weakness that cannot be improved may be best served by an assistive device, appropriate upper limb strengthening, environmental modification and instruction 44–45. Finally, it should be noted that diminished UST need not be viewed as a natural consequence of aging, despite research which notes the inverse association between the two and even work which suggests offering age-adjusted norms for UST 27,46. Instead, a decreased UST should, in the absence of obvious musculoskeletal and/or central neurologic disorder, be considered a function of diminished lower limb neuromuscular competence.
A recent study 47 found that improvements in trunk extension endurance, but not lower limb strength or power, were independently associated with clinically meaningful change in balance in older adults. However, the protocol measured lower limb strength while subjects performed a double leg press maneuver while seated, and so sagittal plane strength of multiple muscle groups within the lower limbs was simultaneously measured. This technique contrasts with our study which measured frontal plane sensorimotor functions discretely at the hip and ankle. Therefore, although trunk extension endurance may be more important to balance than sagittal plane lower limb strength, the relative importance of trunk endurance and lower limb frontal plane sensorimotor function with reference to balance has yet to be explored. .
The strengths of this study include the fact that sensory and motor control mechanisms were quantified simultaneously in subjects with a spectrum of neuromuscular dysfunction. The correlations and multiple regression analyses were unusually strong. Given the complexity of any human behavior it is remarkable that just two lower limb neuromuscular characteristics explain nearly 75% of UST. Limitations include the fact that UST is unlikely to perfectly reflect a variety of relevant mobility characteristics such as gait speed and the ability to recover from a perturbation while walking. The lower limb sensorimotor function(s) responsible for these deserves further attention. Additionally, only frontal plane neuromuscular functions were evaluated. It is possible that sagittal plane muscle strength also influences UST. It should also be mentioned that the ankle motor function measures assumed the ankle center of rotation to be mid-way between the malleoli. This is an estimation and therefore a study limitation, and an important one to note given that ankle motor function was not identified as an independent predictor of UST. It is possible that evaluation of ankle motor function by another means, for example an open chain technique, would have led to an alternative conclusion. However, we have used the closed chain technique in the past, and its validity is supported by the relationship between ankle strength determined in this fashion to the presence of neuropathy and unipedal stance time 17
Due to technical difficulties in the early stages of the study, a portion of the ankle motor data could not be analyzed for 5 subjects, and so the final regression model was performed on 36 subjects. Finally, UST was cut off at 30 seconds, likely creating a ceiling effect for the most able subjects.
In conclusion, increased frontal plane hip strength and/or decreased (more precise) ankle proprioceptive thresholds strongly influenced UST. Age, in contrast, had a trivial influence when these neuromuscular functions were taken into account. Frontal plane hip strength was the single best predictor of UST and appeared to compensate for less precise ankle proprioceptive thresholds. This finding is clinically relevant, given the possibility of strengthening the hip even in the setting of significant PN.
Acknowledgments
Grant support:
This work was supported by the National Institutes of Health (RO1 AG026569-01; the PHS Grants (P30AG024824) and the Swiss National Foundation (IZKOZ3_133925).
LIST OF ABREVIATIONS
- UST
unipedal stance time
- PN
peripheral neuropathy
- RTD
rate of torque development
- MVC
maximum voluntary contraction
- MDNS
Michigan Diabetes Neuropathy Score
- BMI
body mass index
- COM
center of mass
References
- 1.Narici MV, Maganaris C, Reeves N. Myotendinous alterations and effects of resistive loading in old age. Scand J Med Sci Sports. 2005;15(6):392–401. doi: 10.1111/j.1600-0838.2005.00458.x. [DOI] [PubMed] [Google Scholar]
- 2.Barry BK, Carson RG. The consequences of resistance training for movement control in older adults. J Gerontol A Biol Sci Med Sci. 2004;59(7):730–754. doi: 10.1093/gerona/59.7.m730. [DOI] [PubMed] [Google Scholar]
- 3.Kanade RV, Van Deursen RW, Harding KG, Price PE. Investigation of standing balance in patients with diabetic neuropathy at different stages of foot complications. Clin Biomech. 2008;23(9):1183–1191. doi: 10.1016/j.clinbiomech.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Turcot K, Allet L, Golay A, Hoffmeyer P, Armand S. Investigation of standing balance in diabetic patients with and without peripheral neuropathy using accelerometers. Clin Biomech. 2009;24(9):716–721. doi: 10.1016/j.clinbiomech.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Son J, Ashton-Miller JA, Richardson JK. Frontal plane ankle proprioceptive thresholds and unipedal balance. Muscle Nerve. 2009;39(2):150–157. doi: 10.1002/mus.21194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richardson JK, Ashton-Miller JA, Lee SG, Jacobs K. Moderate peripheral neuropathy impairs weight transfer and unipedal balance in the elderly. Arch Phys Med Rehabil. 1996;77(11):1152–1156. doi: 10.1016/s0003-9993(96)90139-2. [DOI] [PubMed] [Google Scholar]
- 7.Allet L, Armand S, Golay A, Monnin D, de Bie RA, de Bruin ED. Gait characteristics of diabetic patients: a systematic review. Diabetes Metab Res Rev. 2008;24(3):173–191. doi: 10.1002/dmrr.809. [DOI] [PubMed] [Google Scholar]
- 8.Wallace C, Reiber GE, LeMaster J, Smith DG, Sullivan K, Hayes S, Vath C. Incidence of falls, risk factors for falls, and fall-related fractures in individuals with diabetes and a prior foot ulcer. Diabetes Care. 2002;25(11):1983–1986. doi: 10.2337/diacare.25.11.1983. [DOI] [PubMed] [Google Scholar]
- 9.Maurer MS, Burcham J, Cheng H. Diabetes mellitus is associated with an increased risk of falls in elderly residents of a long-term care facility. J Gerontol A Biol Sci Med Sci. 2005;60(9):1157–1162. doi: 10.1093/gerona/60.9.1157. [DOI] [PubMed] [Google Scholar]
- 10.Maki BE, Holliday PJ, Topper AK. A prospective study of postural balance and risk of falling in an ambulatory and independent elderly population. J Gerontol. 1994;49(2):M72–84. doi: 10.1093/geronj/49.2.m72. [DOI] [PubMed] [Google Scholar]
- 11.Cummings SR, Nevitt MC. Non-skeletal determinants of fractures: the potential importance of the mechanics of falls. Study of Osteoporotic Fractures Research Group. Osteoporos Int. 1994;4 (Suppl 1):67–70. doi: 10.1007/BF01623439. [DOI] [PubMed] [Google Scholar]
- 12.MacKinnon CD, Winter DA. Control of whole body balance in the frontal plane during human walking. J Biomech. 1993;26(6):633–644. doi: 10.1016/0021-9290(93)90027-c. [DOI] [PubMed] [Google Scholar]
- 13.Bauby CE, Kuo AD. Active control of lateral balance in human walking. J Biomech. 2000;33(11):1433–1440. doi: 10.1016/s0021-9290(00)00101-9. [DOI] [PubMed] [Google Scholar]
- 14.Chou L, Kaufman K, Brey R. Correlation between muscle strength and balance control when negotiating obstacles. 46th Annual Meeting, Orthopaedic Research Society; Orlando, Florida. 2000. p. 0315. [Google Scholar]
- 15.Hilliard MJ, Martinez KM, Janssen I, Edwards B, Mille ML, Zhang Y, Rogers MW. Lateral balance factors predict future falls in community-living older adults. Arch Phys Med Rehabil. 2008;89(9):1708–1713. doi: 10.1016/j.apmr.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mille ML, Johnson ME, Martinez KM, Rogers MW. Age-dependent differences in lateral balance recovery through protective stepping. Clin Biomech. 2005;20(6):607–616. doi: 10.1016/j.clinbiomech.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Gutierrez EM, Helber MD, Dealva D, Ashton-Miller JA, Richardson JK. Mild diabetic neuropathy affects ankle motor function. Clin Biomech (Bristol, Avon) 2001;16(6):522–528. doi: 10.1016/s0268-0033(01)00034-1. [DOI] [PubMed] [Google Scholar]
- 18.Giacomozzi C, D’Ambrogi E, Cesinaro S, Macellari V, Uccioli L. Muscle performance and ankle joint mobility in long-term patients with diabetes. BMC Musculoskelet Disord. 2008;9:99. doi: 10.1186/1471-2474-9-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang CY, Hsieh CL, Olson SL, Wang CH, Sheu CF, Liang CC. Psychometric properties of the Berg Balance Scale in a community-dwelling elderly resident population in Taiwan. J Formos Med Assoc. 2006;105(12):992–1000. doi: 10.1016/S0929-6646(09)60283-7. [DOI] [PubMed] [Google Scholar]
- 20.Hurvitz EA, Richardson JK, Werner RA, Ruhl AM, Dixon MR. Unipedal stance testing as an indicator of fall risk among older outpatients. Arch Phys Med Rehabil. 2000;81(5):587–591. doi: 10.1016/s0003-9993(00)90039-x. [DOI] [PubMed] [Google Scholar]
- 21.Vellas BJ, Wayne SJ, Romero L, Baumgartner RN, Rubenstein LZ, Garry PJ. One-leg balance is an important predictor of injurious falls in older persons. J Am Geriatr Soc. 1997;45(6):735–738. doi: 10.1111/j.1532-5415.1997.tb01479.x. [DOI] [PubMed] [Google Scholar]
- 22.Hurvitz EA, Richardson JK, Werner RA. Unipedal stance testing in the assessment of peripheral neuropathy. Arch Phys Med Rehabil. 2001;82(2):198–204. doi: 10.1053/apmr.2001.17830. [DOI] [PubMed] [Google Scholar]
- 23.Bulbulian R, Hargan ML. The effect of activity history and current activity on static and dynamic postural balance in older adults. Physiol Behav. 2000;70(3–4):319–325. doi: 10.1016/s0031-9384(00)00272-9. [DOI] [PubMed] [Google Scholar]
- 24.Richardson JK. Factors associated with falls in older patients with diffuse polyneuropathy. J Am Geriatr Soc. 2002;50(11):1767–1773. doi: 10.1046/j.1532-5415.2002.50503.x. [DOI] [PubMed] [Google Scholar]
- 25.Vellas BJ, Rubenstein LZ, Ousset PJ, Faisant C, Kostek V, Nourhashemi F, Allard M, Albarede JL. One-leg standing balance and functional status in a population of 512 community-living elderly persons. Aging (Milano) 1997;9(1–2):95–98. doi: 10.1007/BF03340133. [DOI] [PubMed] [Google Scholar]
- 26.Bohannon R. Single limb stance times. A descriptive meta-analysis of data from individuals at least 60 years of age. Topics in Geriatric Rehabil. 2006;22:70–77. [Google Scholar]
- 27.Bohannon RW, Larkin PA, Cook AC, Gear J, Singer J. Decrease in timed balance test scores with aging. Phys Ther. 1984;64(7):1067–1070. doi: 10.1093/ptj/64.7.1067. [DOI] [PubMed] [Google Scholar]
- 28.Potvin AR, Syndulko K, Tourtellotte WW, Lemmon JA, Potvin JH. Human neurologic function and the aging process. J Am Geriatr Soc. 1980;28(1):1–9. doi: 10.1111/j.1532-5415.1980.tb00115.x. [DOI] [PubMed] [Google Scholar]
- 29.Richardson JK. The clinical identification of peripheral neuropathy among older persons. Arch Phys Med Rehabil. 2002;83(11):1553–1558. doi: 10.1053/apmr.2002.35656. [DOI] [PubMed] [Google Scholar]
- 30.Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and stagingof diabetic neuropathy. Diabetes Care. 1994;17(11):1281–1289. doi: 10.2337/diacare.17.11.1281. [DOI] [PubMed] [Google Scholar]
- 31.Richardson JK, Tang C, Nwagwu C, Nnodim J. The influence of initial bipedal stance width on the clinical measurement of unipedal balance time. PMR. 2010;2(4):254–258. doi: 10.1016/j.pmrj.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smeesters C, Ashton-Miller JA, Cole N, NBA Effect of age and gender on maximum hip flexor power. International Association of Gerontology; Vancouver, BC. 2001. [Google Scholar]
- 33.Ylinen J, Salo P, Nykanen M, Kautiainen H, Hakkinen A. Decreased isometric neck strength in women with chronic neck pain and the repeatability of neck strength measurements. Arch Phys Med Rehabil. 2004;85(8):1303–1308. doi: 10.1016/j.apmr.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 34.Thelen DG, Schultz AB, Alexander NB, Ashton-Miller JA. Effects of age on rapid ankle torque development. J Gerontol A Biol Sci Med Sci. 1996;51(5):M226–232. doi: 10.1093/gerona/51a.5.m226. [DOI] [PubMed] [Google Scholar]
- 35.Son J, Ashton-Miller JA, Richardson JK. Do ankle orthoses improve ankle proprioceptive thresholds or unipedal balance in older persons with peripheral neuropathy? Am J Phys Med Rehabil. 2010;89(5):369–375. doi: 10.1097/PHM.0b013e3181d89861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winter DA, Patla AE, Prince F, Ishac M, Gielo-Perczak K. Stiffness control of balance in quiet standing. J Neurophysiol. 1998;80(3):1211–1221. doi: 10.1152/jn.1998.80.3.1211. [DOI] [PubMed] [Google Scholar]
- 37.Chang SH, Mercer VS, Giuliani CA, Sloane PD. Relationship between hip abductor rate of force development and mediolateral stability in olderadults. Arch Phys Med Rehabil. 2005;86(9):1843–1850. doi: 10.1016/j.apmr.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 38.Liu J, Lockhart TE. Age-related joint moment characteristics during normal gait and successful reactive-recovery from unexpected slip perturbations. Gait Posture. 2009;30(3):276–281. doi: 10.1016/j.gaitpost.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reeves NP, Narendra KS, Cholewicki J. Spine stability: lessons from balancing a stick. Clin Biomech. 2011;26(4):325–330. doi: 10.1016/j.clinbiomech.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 40.Meyer PF, Oddsson LI, De Luca CJ. The role of plantar cutaneous sensation in unperturbed stance. Exp Brain Res. 2004;156(4):505–512. doi: 10.1007/s00221-003-1804-y. [DOI] [PubMed] [Google Scholar]
- 41.Bean JF, Kiely DK, Herman S, Leveille SG, Mizer K, Frontera WR, Fielding RA. The relationship between leg power and physical performance in mobility-limited older people. J Am Geriatr Soc. 2002;50(3):461–467. doi: 10.1046/j.1532-5415.2002.50111.x. [DOI] [PubMed] [Google Scholar]
- 42.Bento PC, Pereira G, Ugrinowitsch C, Rodacki AL. Peaktorque and rate of torque development in elderly with and without fall history. Clin Biomech. 2010;25(5):450–454. doi: 10.1016/j.clinbiomech.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 43.Ashton-Miller JA, Wojtys EM, Huston LJ, Fry-Welch D. Can proprioception really be improved by exercises? Knee Surg Sports Traumatol Arthrosc. 2001;9(3):128–136. doi: 10.1007/s001670100208. [DOI] [PubMed] [Google Scholar]
- 44.Ashton-Miller JA, Yeh MW, Richardson JK, Galloway T. A cane reduces loss of balance in patients with peripheral neuropathy: results from a challenging unipedal balance test. Arch Phys Med Rehabil. 1996;77(5):446–452. doi: 10.1016/s0003-9993(96)90032-5. [DOI] [PubMed] [Google Scholar]
- 45.Richardson JK, Thies SB, DeMott TK, Ashton-Miller JA. Interventions improve gait regularity in patients with peripheral neuropathy while walking on an irregular surface under low light. J Am Geriatr Soc. 2004;52(4):510–515. doi: 10.1111/j.1532-5415.2004.52155.x. [DOI] [PubMed] [Google Scholar]
- 46.Springer BA, Marin R, Cyhan T, Roberts H, Gill NW. Normative values for the unipedal stance test with eyes open and closed. J Geriatr Phys Ther. 2007;30(1):8–15. doi: 10.1519/00139143-200704000-00003. [DOI] [PubMed] [Google Scholar]
- 47.Suri P, Kiely DK, Leveille SG, Frontera WR, Bean JF. Increased trunk extension endurance is associated with meaningful improvement in balance among older adults with mobility problems. Arch Phys Med Rehabil. 2011;92(7):1038–1043. doi: 10.1016/j.apmr.2010.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]


