Abstract
Currently available antipsychotic medications work primarily by antagonizing D2 dopamine receptors, thus raising intracellular cAMP levels. We hypothesized that intracellular stimulation of cAMP levels in the CNS would have similar effects to treatment with antipsychotic medication. To test this hypothesis, we studied the effect of an acute treatment of rolipram, an inhibitor of type 4 phosphodiesterases that degrade cAMP, on acoustic startle and prepulse inhibition (PPI) of the acoustic startle response in C57BL/6J mice known to exhibit poor PPI. PPI is disrupted in schizophrenia patients, and the ability of a drug to increase PPI in mice is predictive of antipsychotic efficacy. We show here that acute treatment with rolipram significantly increases PPI at doses that do not alter the acoustic startle response (lowest effective dose 0.66 mg/kg). In addition, rolipram (0.66 mg/kg) blocks the disruptive effects of amphetamine (10 mg/kg) on PPI. At a slightly higher dose (1.0 mg/kg), rolipram also induces catalepsy. Thus, phosphodiesterase-4 (PDE4) inhibition has many of the same behavioral effects as traditional antipsychotic medications. In contrast to traditional antipsychotics, these effects are achieved through alteration of an intracellular second messenger system rather than antagonism of neurotransmitter receptors. Given previous reports showing rolipram improves cognition, we conclude that PDE4 represents an important novel target for further antipsychotic drug development.
Keywords: prepulse inhibition, schizophrenia, animal model, psychopharmacology, novel targets, phosphodiesterase inhibitor
Antipsychotic medications are defined by their ability to improve positive symptoms of schizophrenia such as delusions, hallucinations, and disorganized thinking. There are two main classes of antipsychotics: the first-generation medications (e.g. chlorpromazine and haloperidol), and the newer, atypical antipsychotics, (e.g. clozapine, risperidone and olanzapine) (Miyamoto et al., 2005). Regardless of class, antipsychotic potency is proportional to blockade at dopamine D2 receptors (Kanes et al., 1993; Kapur and Seeman, 2001; Seeman, 2002; Kapur and Mamo, 2003), which are tonically active (Adell and Artigas, 2004). Because of D2 blockade, patients are at high risk of developing extrapyramidal side effects (EPS) and hyperprolactinemia. By varying potency at other neurotransmitter receptors including the 5-HT2a receptor, newer medications are less likely to cause EPS, but are more likely to cause cardiac conduction abnormalities, sedation, and weight gain (Serretti et al., 2004). Although not part of the formal diagnostic criteria, schizophrenia is also associated with neurocognitive and neurophysiologic dysfunctions including problems with learning and memory, executive function, sensorimotor gating, eye tracking and olfaction (Censits et al., 1997; Gur et al., 2001, 2003; Hill et al., 2002; Lowery et al., 2003; Sachs et al., 2004). Current treatments are more effective at treating the positive symptoms of schizophrenia than the negative or neurophysiologic symptoms. Although they can measurably improve some neurocognitive (Keefe et al., 2004) and neurophysiologic abnormalities (Gonul et al., 2003), others remain untouched (e.g. Sumiyoshi et al., 2003). Thus, the ability of currently available antipsychotics to improve daily functioning and productivity is questionable (Duncan et al., 2003a,b; Percudani et al., 2004), particularly when both antipsychotics and D2-antagonists have actually been shown to impair learning in several rodent models (Ploeger et al., 1992, 1994; Setlow and McGaugh, 1998; Greba et al., 2001; Rosengarten and Quartermain, 2002). Therefore, there is a need to develop novel medications to treat schizophrenia that will control all symptoms while eliminating many of the side effects associated with current medications (Meltzer, 2003).
Currently available antipsychotics block D2 receptors (Kehne et al., 1991; Kapur and Mamo, 2003), resulting in increased cAMP levels in the brain (e.g. Kelly et al., 2006). As such, we explored the possibility that increasing cAMP levels via intracellular targets might provide a novel therapeutic avenue in the development of antipsychotics. One potential target is the phosphodiesterase-4 (PDE4) family of enzymes, one of 11 families of enzymes that degrade cyclic nucleotides (c.f., Beavo and Brunton, 2002; Lugnier, 2006). The PDE4 family has several isoforms that are widely distributed in the brain and specifically catalyze the hydrolysis of cAMP. Interestingly, a compensatory increase in cAMP PDE activity has been observed in an endophenotypic mouse model of schizophrenia (Kelly et al., 2006). Further, a physical interaction between disrupted in schizophrenia 1 (DISC1) and PDE4B, along with cytogenetic alterations in PDE4B in human patients with schizophrenia, has been observed (Millar et al., 2005). Inhibition of PDE4 is likely to result in selective physiological effects because PDE4 is compartmentalized to microdomains via binding partners, such as β-arrestins and A-kinase anchoring proteins (Houslay and Adams, 2003; Mongillo et al., 2004; Dodge-Kafka et al., 2005). As such, PDE4 regulates cAMP-stimulated crosstalk between specifically complexed proteins, such as protein kinase A and β-adrenergic receptors (Perry et al., 2002; Baillie et al., 2003). Importantly, PDE4 inhibitors are likely to be clinically available in the near future as they are currently in phase II clinical trials (Houslay et al., 2005).
Rolipram, a potent inhibitor of PDE4, increases both the intensity and duration of cAMP-mediated signaling (Scuvee-Moreau et al., 1987). Rolipram has a number of important psychoactive properties including acting like an antidepressant (Bonbon et al., 1988), reducing morphine-induced hyperlocomotion (Mori et al., 2000), and enhancing hippocampus-dependent memory and LTP (Randt et al., 1982; Barad et al., 1998; Bourtchouladze et al., 2003). In addition, rolipram blocks the development of haloperidol-induced vacuous chewing movement (Sasaki et al., 1995), a standard behavioral screen for the development and treatment of tardive dyskinesia. Because of the possibility of aberrant cAMP signaling in schizophrenia, the potential of improving learning and memory functioning, and the likelihood of preventing or treating tardive dyskinesia, we studied the effect of rolipram in several behavioral paradigms with strong predictive validity for antipsychotic activity.
To assess the potential antipsychotic activity of rolipram, we tested the effect of this drug in a paradigm of sensorimotor gating referred to as prepulse inhibition of acoustic startle (PPI). We chose this paradigm not only because patients with schizophrenia exhibit PPI deficits (Braff et al., 1978), but also because the ability of a drug to increase PPI in rodents predicts the clinical efficacy of antipsychotic medications (Swerdlow et al., 1998; Geyer et al., 2001). PPI is a behavioral paradigm in which the presentation of a non-startling stimulus (prepulse, e.g. 72 dB noise) inhibits the startle response elicited by a subsequently presented startling acoustic stimulus (e.g. 120 dB noise). Here, we tested the dose-dependent ability of rolipram to increase baseline PPI in C57BL/6J mice. C57BL/6J mice exhibit poor PPI relative to other strains (Paylor and Crawley, 1997; Dulawa and Geyer, 2000; Ouagazzal et al., 2001) and, as such, have been proposed as a predictive model for testing of antipsychotic efficacy (Ouagazzal et al., 2001). In addition, we tested the ability of rolipram to reverse amphetamine-induced disruption of PPI as well as the dose-dependent ability of rolipram to induce catalepsy, the latter of which evaluates the potential for causing EPSs. Given the ability of rolipram to improve memory, an antipsychotic-like profile in these behavioral paradigms could lead to the development of improved therapeutics that are capable of resolving the positive symptoms and the debilitating cognitive deficits experienced by patients with schizophrenia.
EXPERIMENTAL PROCEDURES
Mice
Eight to 10 week old male C57BL/6J mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA). Mice were housed in an Association for Assessment of Accreditation of Laboratory Animal Care–approved animal facility at the University of Pennsylvania. Mice were maintained on a 12-h light/dark cycle and housed in a light- and temperature-controlled facility with food and water available ad libitum. Behavioral testing was performed during the light phase. Mice were acclimated to the housing facility for 1–2 weeks prior to behavioral testing. All protocols were approved by the Institutional Animal Care and Use Committee and were conducted in accordance with and National Institutes of Health guidelines. Every effort was made to reduce suffering experienced by the subjects and the number of mice used.
Startle response and prepulse inhibition tests
Startle responses to acoustic stimuli and inhibition of startle responses due to presentation of a non-startling prepulse were initially measured, as previously described (protocol 2; Gould et al., 2004; Kelly et al., 2006). Startle responses were measured by an accelerometer in response to acoustic stimuli delivered by a white noise generator (4–19 kHz) (SR-Laboratory; San Diego, CA, USA). Testing was conducted in one of two facilities, one in which the background (bg) noise played in the chamber was set to 65 dB and the other in which the bg was set to 68 dB. There was no difference between datasets; therefore, all data collected in the two facilities were combined for subsequent analyses (see Data Analysis below). Sessions began with a 5 min block of acclimation to the bg noise, followed by a block of five 120 dB startle pulses in an effort to make subsequently measured startle responses less variable. During the next 10 min block, startle responses were measured to 40 ms pulses of 0, 90, 95, 100, 105, 110, 115 and 120 dB, presented each five times in a random order with an intertrial interval randomized from 10 to 20 s. The startle portion of the session concluded with an additional block of five 120 dB pulses to assess potential effects on habituation. Startle trials were followed by a 10 min block of PPI trials. Each prepulse trial consisted of a 20 ms prepulse 4, 8, or 16 dB above bg, followed 100 ms later by a 40 ms pulse of 120 dB. Five trials of each prepulse intensity along with 10 startle-only trials (i.e. trials with no prepulse) were presented in random order. Startle responses were collected as 60, 1 ms voltage readings, which were averaged over the collection interval to give an average measure for each trial (Gould et al., 2004).
Startle responses and inhibition of startle responses were also measured in pilot studies based on “Protocol 1” of Gould and colleagues (2004). This protocol is similar to that described above, with a 65 dB bg noise, except that the startle portion of the session also includes trials at 125 dB. Further, PPI trials consisted of 75, 80, 85, 90, and 95 dB prepulses with a 40 ms interstimulus interval between the prepulse and 120 dB startle stimulus. Finally, data were recorded as 100 1 ms voltage readings, which were averaged over the collection interval to give an average startle measure for each trial. These data are not shown, but rolipram yielded similar dose-response and amphetamine-reversal effects using this protocol.
PPI for a given prepulse intensity was calculated as percent inhibition of the startle response using the following formula: [100−(average startle response for PPI trials/average startle response for startle-only trials in PPI block)×100].
Catalepsy testing
Catalepsy testing was performed as previously described (Kanes et al., 1993, 1996). Mice were removed from their home cage to a testing cage 1 h prior to testing. Fifteen minutes after injection mice were positioned in a fixed rearing posture in the test cage. Mice were rated by two independent raters as cataleptic if they maintained this posture for 300 s or longer. Release from catalepsy was scored if one or more forepaws touched the floor of the test cage.
Drugs
Rolipram and d-amphetamine sulfate were purchased from Sigma Inc. (St. Louis, MO, USA). For behavioral testing d-amphetamine was dissolved in 0.9% sterile saline, rolipram was dissolved in 2% (v/v) DMSO/0.9% saline. Rolipram was administered 15 min prior to the behavioral session at doses of 0.1–10.0 mg/kg (as indicated), with animals remaining in a holding cage during the injection-session interim. The doses of rolipram selected (0.1–10 mg/kg) have been previously tested in paradigms of both learning and memory (Barad et al., 1998) and acoustic startle (Kehne et al., 1991) and have been shown to increase cAMP levels in the absence of neuronal stimulation (Gold et al., 2002).
d-Amphetamine was administered immediately prior to the behavioral session at a dose of 10 mg/kg, as this has previously been shown to impair PPI in C57BL/6 mice (Ralph-Williams et al., 2003). Haloperidol (Ben Venue Laboratories Inc., Bedford, OH, USA) was dissolved in saline with lactic acid, pH 3.0–3.8 (0.01 or 0.1 mg/ml) and administered at a dose of 0.1 mg/kg or 1.0 mg/kg, based on our previous work in transgenic mice (Kelly et al., 2006). All injections were given i.p. at a volume of 1.0 ml/kg body weight.
Data analysis
For the rolipram experiments, data from the PPI trials were not normally distributed across trials. As such, data could not be analyzed across trials using a parametric approach (repeated measures analysis of variance (ANOVA)). These data were, however, normally distributed within trials. Thus, data from each trial were analyzed using ANOVA, with the Student-Newman-Keuls (SNK) post hoc approach used to accommodate multiple testing (Sigmastat 2.03; Sigmastat, San Jose, CA, USA). The outcomes from the startle trials were not generally normally distributed within trials. Therefore, a nonparametric alternative to the ANOVA, the Kruskal-Wallis test was used in place of the ANOVA, with post hoc testing performed using Dunn’s approach (Sigmastat 2.03). For the haloperidol experiments, the variables were normally distributed both within and across PPI trials. Thus, data for these experiments were analyzed by a repeated measures ANOVA (for effects of treatment and trial), with post hoc testing by SNK. Data for startle trials were normally distributed within trials (but not across trials), thus, each trial was analyzed separately ANOVA with post hoc by SNK. For both rolipram and haloperidol experiments, habituation of the startle response was calculated as a difference score (average startle response to the block of 120 dB trials before the randomized startle trials minus the average startle response to the block of 120 dB trials that followed the randomized startle trials). Habituation data were normally distributed and analyzed by ANOVA with post hoc by SNK. In addition, Pearson product moment correlations were conducted to determine if there was an interrelation between startle magnitude (during 120 dB pulse-only trials of the PPI section, which were normally distributed) and % PPI, as previously described (Quednow et al., 2006; Kelly et al., 2006). Catalepsy data were analyzed by χ2 analysis using Prism 4 software (GraphPad Inc., San Diego, CA, USA). Statistical significance was determined at P≤0.05.
RESULTS
Rolipram increases PPI in a dose-dependent manner
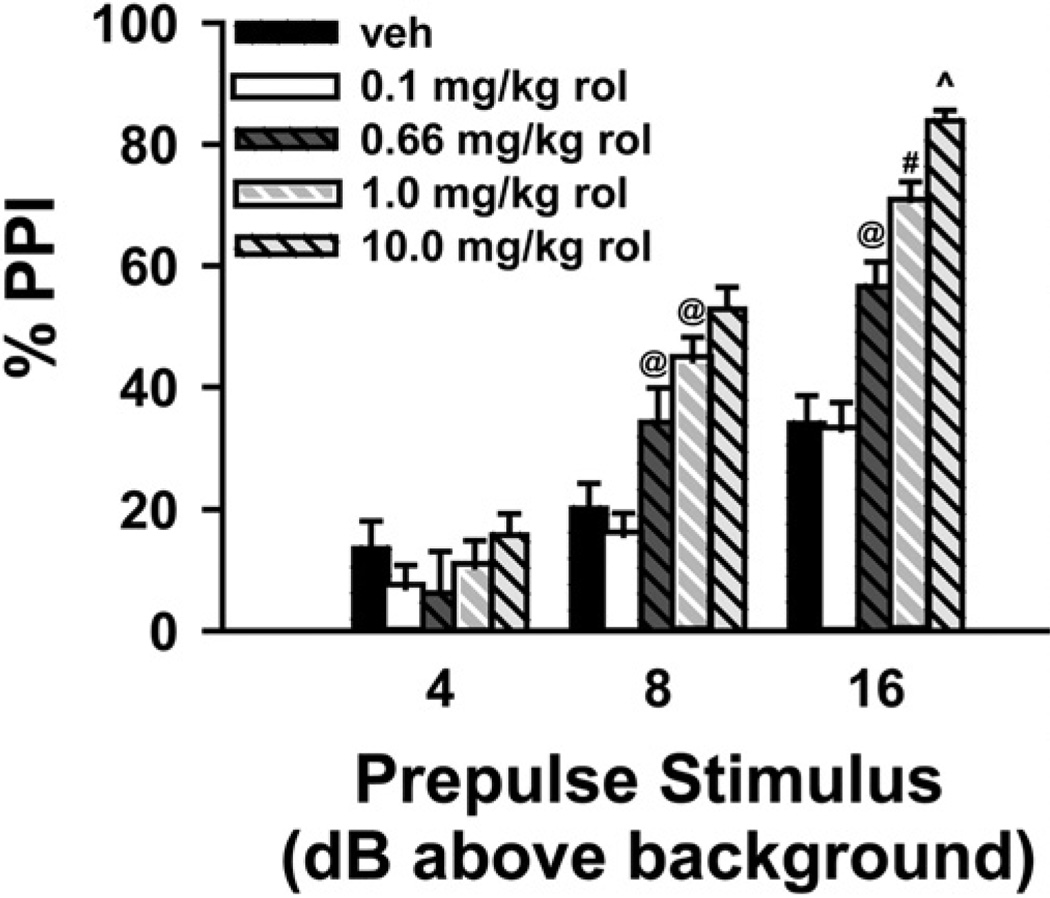
We tested directly the hypothesis that increasing cAMP levels would increase PPI (based on “Protocol 2,” Gould et al., 2004; Kelly et al., 2006). Using C57BL6/J mice, we examined the effect of vehicle vs. four doses of rolipram (0.1, 0.66, 1 and 10 mg/kg), a PDE4 inhibitor known to increase cAMP levels (n = 18 per group; Fig. 1). Rolipram increased PPI at select prepulse intensities. In trials where the prepulse was played 8 dB above bg, rolipram significantly increased PPI in a dose-dependent manner (F(4,85) = 13.67, P<0.001). Post hoc analyses revealed that 0.66 mg/kg and 1.0 mg/kg rolipram increased PPI relative to both vehicle (P = 0.022 and P<0.001, respectively) and 0.1 mg/kg rolipram (P = 0.008 and P<0.001, respectively), and 10 mg/kg rolipram increased PPI relative to vehicle (P<0.001), 0.1 mg/kg (P<0.001) and 0.66 mg/kg rolipram (P = 0.007). Rolipram also increased PPI in a dose-dependent manner when the prepulse was played 16 dB above bg (F(4,85) = 36.11, P<0.001). Post hoc analysis revealed that 0.66 mg/kg significantly increased PPI relative to vehicle and 0.1 mg/kg rolipram (P<0.001 for each), 1.0 mg/kg increased PPI relative to vehicle (P<0.001), 0.1 mg/kg (P<0.001), and 0.66 mg/kg rolipram (P = 0.009), and 10 mg/kg increased PPI relative to vehicle (P<0.001), 0.1 mg/kg (P<0.001), 0.66 mg/kg (P<0.001), and 1.0 mg/kg rolipram (P = 0.015). Importantly, rolipram did not affect the startle response itself in pulse-only trials (120 dB) during the PPI portion of the session.
Fig. 1.
Rolipram increases PPI in a dose-dependent manner. Rolipram (rol), administered 15 min before testing, produced a dose-dependent increase in PPI. Overall, 10 mg/kg rolipram had the greatest effect at all prepulse intensities, with significant increases also observed for doses of 0.66 mg/kg and 1 mg/kg. In addition, the largest effects of rolipram were seen at higher prepulse intensities. n=18 per group. Post hoc @ vs. vehicle and 0.1 mg/kg, P<0.025–0.001; # vs. vehicle, 0.1 and 0.66 mg/kg, P<0.01–0.001; ^ vs. vehicle, 0.1, 0.66, and 1 mg/kg, P<0.02–0.001.
Rolipram alters acoustic startle response in a stimulus dependent manner
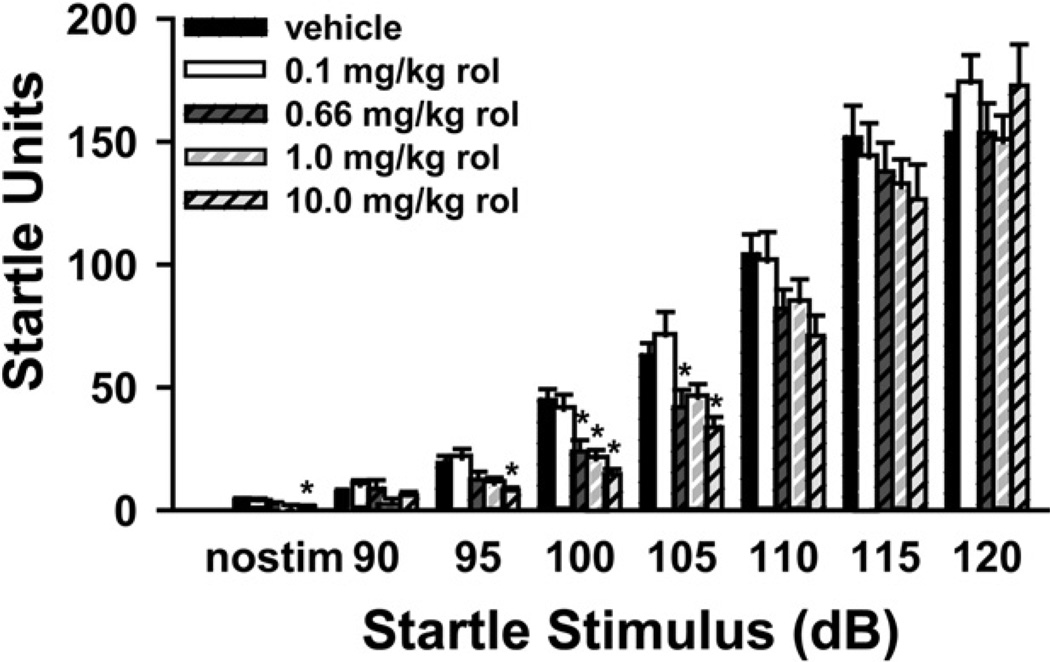
Previous studies in rats indicated that rolipram can increase acoustic startle, depending on the site of administration (Kehne et al., 1991); therefore, we tested the ability of rolipram to alter the startle response in mice. In the present study rolipram generally decreased the acoustic startle response in C57BL/6J mice (Fig. 2). In the “no stimulation” trial, there was a significant effect of rolipram (H(4) = 11.98, P = 0.017), with post hoc testing showing that 10 mg/kg reduced responses relative to vehicle. In the 90 dB trial, there was also an effect of rolipram (H(4) = 10.00, P = 0.04); however, no post hoc comparisons reached the level of significance. Similar to the “no stimulation” trial, there was a significant effect of rolipram at 95 dB (H(4) = 16.01, P<0.003), with post hoc testing showing that 10 mg/kg significantly suppressed responses relative to vehicle. In the 100 dB (H(4) = 30.20, P<0.001) and 105 dB trials (H(4) = 18.12, P = 0.001), there was even a greater effect of rolipram on startle, with post hoc testing revealing a significant suppressing effect of 0.66 mg/kg, 1.0 mg/kg, and 10 mg/kg rolipram in the former and of 0.66 mg/kg and 10 mg/kg in the latter. No significant effect of rolipram was detected in the 110 dB, 115 dB, or the 120 dB trials. Importantly, there were no significant correlations between startle magnitude in the pulse-only trials (120 dB) and % PPI for any trial in any group, suggesting that the augmentation of PPI noted above was not due to the non-significant effect of rolipram on startle. Finally, there was no significant effect of rolipram on habituation.
Fig. 2.
Rolipram decreases startle magnitude to lower intensity stimuli only. Rolipram generally decreased startle responding at doses of 0.66 mg/kg to 10 mg/kg. Importantly, there was no effect of rolipram in response to a 120 dB stimulus, the intensity used in PPI trials; n=18 mice per treatment group. Post hoc * vs. vehicle, P<0.05.
Rolipram increases PPI at doses similar to haloperidol
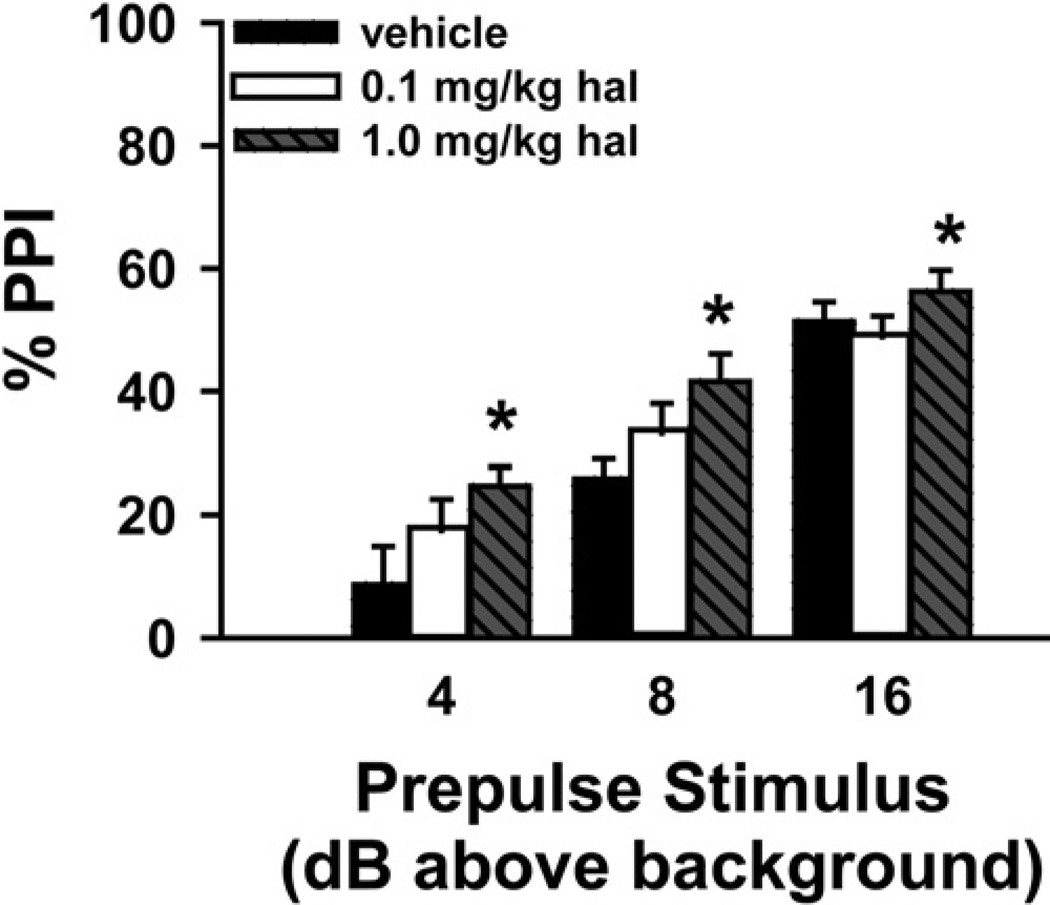
To determine the relative efficacies of rolipram vs. haloperidol in increasing PPI, we next determined at which dose haloperidol increases PPI in C57BL/6J mice using our protocol. To do so, we compared the effect of 0.1 mg/kg and 1.0 mg/kg haloperidol relative to vehicle (n = 8 per group; Fig. 3). Across prepulse intensities, there was a significant effect of treatment (F(2,42) = 3.64, P = 0.044). Post hoc tests showed that 1.0 mg/kg haloperidol significantly improved PPI across prepulse intensities relative to vehicle treatment (P = 0.037).
Fig. 3.
Haloperidol (hal) increases PPI in a dose-dependent manner. Across prepulse intensities, 1.0 mg/kg hal, but not 0.1 mg/kg hal significantly increased PPI relative to vehicle; n=8 per group. Post hoc for effect of treatment across trials, * vs. vehicle, P<0.05.
During the startle portion of the session, haloperidol significantly affected responses during the “no stimulation” trial (F(2,21) = 5.08, P = 0.016), with post hoc tests showing that both 0.1 mg/kg (3.69±0.4 units) and 1.0 mg/kg haloperidol (3.11±0.3 units) significantly reduced movement relative to vehicle (5.05±0.6 units, P = 0.041 and P = 0.014, respectively). Despite this effect, there was no effect of haloperidol in startle trials ranging from 90 dB to 120 dB. Further, there was no effect of haloperidol on habituation. Finally, mice treated with 1.0 mg/kg haloperidol showed no correlations between startle magnitude of pulse-only trials and % PPI in any trial, suggesting that the ability of haloperidol to increase PPI is not due to a non-significant change in startle.
Rolipram ameliorates disruption of PPI by d-amphetamine
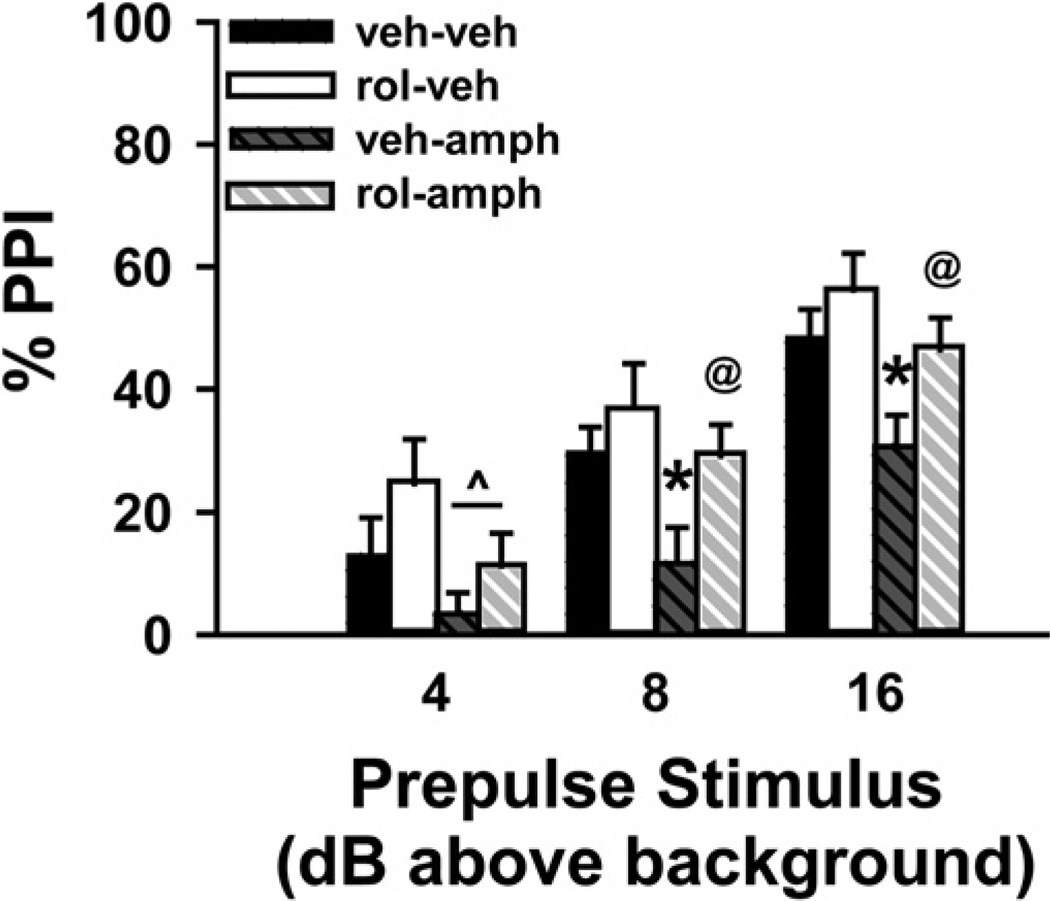
Given that rolipram was able to increase baseline PPI, we next tested the ability of 0.66 mg/kg rolipram to block disruption of PPI by d-amphetamine (n = 14 for each group; Fig. 4). d-Amphetamine is a psychotomimetic known to disrupt PPI, and the ability of an antipsychotic to reverse this disruption strongly predicts clinical efficacy (Swerdlow et al., 1998; Geyer et al., 2001). As previously shown in C57BL/6J mice, d-amphetamine impaired PPI at all prepulse intensities (4 above bg, F(1,52) = 4.04, P = 0.05; 8 above bg, F(1,52) = 5.01, P = 0.03; 16 above bg, F(1,52) = 6.78, P = 0.012). Despite a disruptive effect of d-amphetamine, pretreatment with 0.66 mg/kg rolipram significantly increased PPI in trials with prepulses 8 dB (F(1,52) = 4.65, P = 0.036) and 16 dB above bg (F(1,52) = 5.08, P = 0.028) and showed a trend toward increasing PPI in trials with prepulses 4 dB above bg (F(1,52) = 2.95, P = 0.092). Post hoc analyses for the 4 dB trial revealed no significant effects; however, the 8 dB and 16 dB PPI trials showed that rolipram only increased PPI in amphetamine-treated animals (veh-veh vs. veh-amph, P = 0.032 and P = 0.022; veh-amph vs. rol-amph, P = 0.037 and P = 0.038; veh-veh vs. rol-veh, P = NS; rol-veh vs. rol-amph, p = NS).
Fig. 4.
Rolipram (rol) ameliorates the impairment in PPI caused by amphetamine. Relative to vehicle, d-amphetamine (10 mg/kg; amph) disrupted PPI; however, pretreatment with rol (0.66 mg/kg) blocked this disruption; n=14 per group. ^ Main effect of amphetamine, P=0.05; post hoc * vs. veh-veh, P<0.05; @ vs. veh-amph, P<0.05.
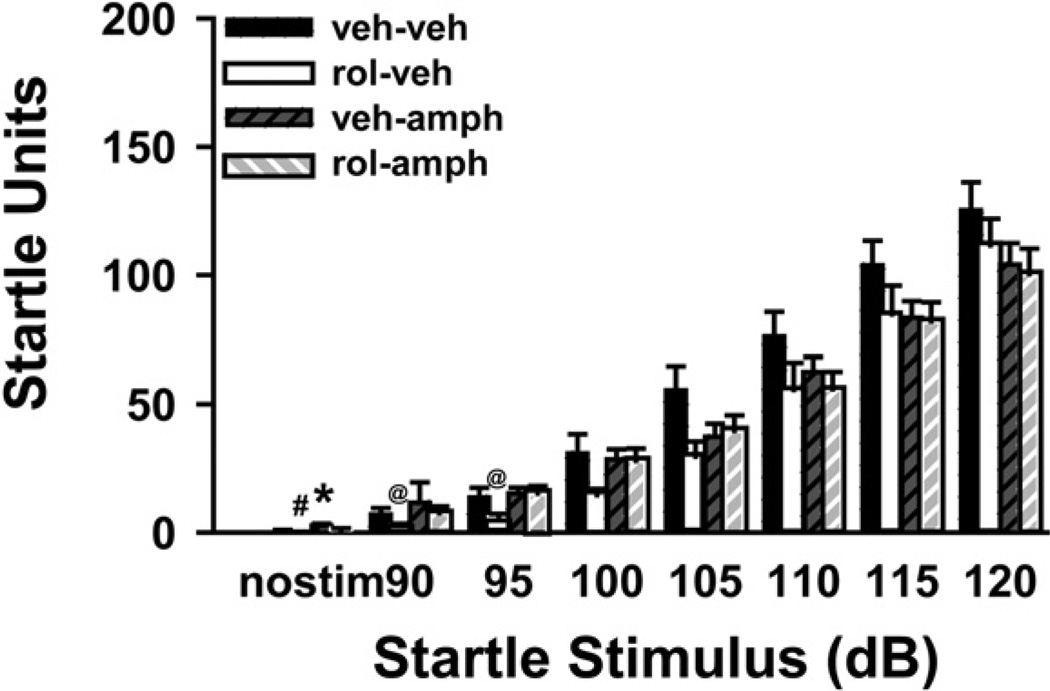
Rolipram does not reverse the effects of amphetamine on acoustic startle
Amphetamine causes a dose-related impairment in acoustic startle response in mice (Ralph-Williams et al., 2002) that is not reversed by antipsychotic medication. In general, treatment with either d-amphetamine (10 mg/kg), rolipram (0.66 mg/kg), or both reduced startle responses of C57BL/6J mice across dB intensities; however, this effect only reached the level of statistical significance in select trials (Fig. 5). In the “no stimulation” trial, there was a significant effect of treatment (H(3) = 25.98, P<0.001), with post hoc analyses showing that both groups of animals treated with amphetamine exhibited an increased response. There was also a significant effect of treatment in the 90 dB and 95 dB trials (H(3) = 10.26, P = 0.016 and H(3) = 10.01, P = 0.019, respectively), with post hoc analyses revealing rol-veh-treated mice showed significantly reduced responses only relative to veh-amph mice. For 100–120 dB trials, there was no significant effect of treatment. There was, however, a significant effect of treatment on habituation of the startle response (F(3,52) = 4.11, P = 0.011). Post hoc analyses showed that veh-amph-treated mice failed to demonstrate appreciable habitation (−8.53±8.4 units; veh-amph vs. veh-veh, rol-veh, and rolamph, P = 0.014–0.023). Rolipram pretreatment blocked amphetamine-disruption of habituation as rol-amph-treated mice showed levels of habituation (28.87±12.2 units) that did not significantly differ relative to veh-veh (38.09±12.7 units) or rol-veh-treated mice (40.99±11.3 units).
Fig. 5.
Rolipram (rol) neither augments nor reverses the effects of amphetamine on startle responses to higher intensity stimuli. d-Amphetamine (10 mg/kg; amph) generally reduced acoustic startle responses to higher intensity stimuli (105 dB and greater), but not to the level of statistical significance. As above, rol (0.66 mg/kg) reduced acoustic startle responses. There was neither a rescue nor an additive effect of rol plus amphetamine on startle responses to higher intensity stimuli; however, amphetamine appeared to block the ability of rol to reduce startle responses to lower intensity stimuli; n=14 mice per treatment group. Post hoc * vs. veh-veh, P<0.05; @ vs. veh-amph, P<0.05; # vs. veh-amph and rol-amph, P<0.05.
Rolipram induces catalepsy
The ability of a drug to cause catalepsy, is predictive of both its potential for antipsychotic efficacy as well as its liability to cause EPSs (Hoffman and Donovan, 1995). Rolipram was compared with haloperidol, a canonical antipsychotic that works through D2 dopamine receptor blockade (Table 1). Rolipram induced catalepsy at doses lower than those of haloperidol (χ2(2) = 7.85, P<0.05). The lowest dose of rolipram that caused catalepsy was 1.0 mg/kg, whereas the lowest dose for haloperidol was 5.0 mg/kg (Table 1); 0.66 mg/kg did not cause appreciable catalepsy.
Table 1.
Dose response for catalepsy induced by rolipram and haloperidol
| Dose (mg/kg, i.p) | Percent cataleptic |
|---|---|
| Rolipram | |
| 0.0 (Vehicle) | 0 (8) |
| 0.1 | 0 (10) |
| 0.66 | 0 (10) |
| 1.0 | 13 (8) |
| 5.0 | 46 (10) |
| 10.0 | 100 (8) |
| Haloperidol | |
| 0.0 (Vehicle) | 0 (8) |
| 0.1 | 0 (8) |
| 0.66 | 0 (8) |
| 1.0 | 0 (10) |
| 5.0 | 10 (10) |
| 10.0 | 80 (10) |
Both rolipram and haloperidol induced catalepsy 15 min after drug administration in a dose-dependent fashion. The size of each group is shown in parentheses.
DISCUSSION
By measuring PPI in C57BL6/J mice we have examined the potential antipsychotic activity of rolipram. Rolipram is a drug that increases cAMP levels by inhibiting PDE4, an enzyme that degrades cAMP. Our results show that rolipram increases baseline PPI in a dose-dependent manner and blocks the disruption of PPI caused by amphetamine. These effects of rolipram on PPI are elicited at marginally sub-cataleptic doses. These data support previous studies in endophenotypic mouse models of schizophrenia, in which rolipram rescued pharmacological disruption of sensory gating (Maxwell et al., 2004) and latent inhibition (Davis and Gould, 2005) as well as genetic disruption of PPI (Kelly et al., 2006).
The ability of a compound to increase baseline PPI in mice has been proposed as a valid pharmacologic screen for antipsychotic efficacy (Ouagazzal et al., 2001). C57BL/6J, BALB/cBYJ, MORO, 129/SvEv mice all exhibit significant increases in baseline PPI after administration of haloperidol, clozapine and risperidone. We found that rolipram increases baseline PPI in mice at doses (0.66–10 mg/kg) that may possibly be clinically meaningful. A preliminary open-label trial of rolipram in the treatment of schizophrenia, 0.64 mg/kg (45 mg/day) was effective in reducing Brief Psychiatric Rating Scale scores during the first week of treatment (Pietzcker et al., 1979). It should be noted, however, that it is problematic to compare effective doses for behavioral effects of rolipram between species due to differences in bioavailability (Krause and Kuhne, 1988). We can, however, compare doses used in the current experiments with others used in mice. The effective doses found in the current study (0.66–10 mg/kg) are similar to those shown to elicit anti-depressant (0.5 mg/kg/day; Zhang et al., 2002; Li et al., 2003) and anti-inflammatory effects in mice (6.25 mg/kg/day; Sommer et al., 1997), but are substantially higher than those shown to reverse memory deficits in mice (0.1 mg/kg; Zhang et al., 2000, 2004; Bourtchouladze et al., 2003). Further, the minimal dose of rolipram shown to increase PPI here (0.66 mg/kg) was similar that for haloperidol (1.0 mg/kg).
In addition to improving baseline PPI, rolipram ameliorates amphetamine-induced disruption of PPI and habituation of the acoustic startle response. Although we cannot strictly rule out the possibility that the ability of rolipram to rescue amphetamine-induced disruptions in PPI simply reflects the ability of rolipram to increase baseline PPI, we believe several facts argue against this scenario. First, in this experiment (which employed two injections) rolipram only increased PPI in amphetamine-treated not vehicle-treated mice. Further, the difference in % PPI between veh-amph- and rol-amph-treated mice (8 dB above bg, 18%; 16 dB above bg, 16%) is twice that between veh-veh- and rol-veh-treated mice (8 dB above bg, 7%; 16 dB above bg, 8%). Finally, rolipram did not significantly affect baseline habituation of the startle response. It is also worth noting, that the ability of a drug to increase baseline PPI does not necessarily correspond to an ability to increase amphetamine-disrupted PPI (e.g. Brody et al., 2003; Ong et al., 2005). Taken together, we believe these data suggest that the ability of rolipram to rescue amphetamine-induced disruptions does not simply reflect a shifted baseline.
Amphetamine is a widely used pharmacologic model that attempts to mimic the disruption in PPI seen in patients with schizophrenia. The ability of amphetamine to disrupt PPI requires D2 receptors (Ralph et al., 1999; Ralph-Williams et al., 2002; but see Ralph-Williams et al., 2003) that inhibit cAMP production (Neves et al., 2002). Interestingly, the dose of amphetamine used here (10 mg/kg) increases cAMP levels in striatum but decreases cAMP levels in cortex of C57BL/6J mice (Kelly et al., 2006). It is likely that it is this reduction in cortical cAMP signaling that primarily mediates amphetamine-disruption of PPI because decreasing cAMP signaling in the brain of C57BL/6J mice is sufficient to induce PPI deficits (Kelly et al., 2006). The ability of rolipram to rescue amphetamine-induced deficits in PPI further suggests that it is the decreases in cortical cAMP—and not the increases in striatal cAMP—that mediate the effect of amphetamine on PPI. We believe this is further support for the hypothesis that PPI in C57BL/6J mice is not only regulated by a complex neural circuitry, but also a molecular signature that exhibits regional specificity (Kelly et al., 2006).
The ability of a compound to induce catalepsy is a measure of striatal dopamine receptor blockade (Wadenberg et al., 2000). This is predictive of both antipsychotic efficacy as well as a compound’s propensity to induce EPSs. Our results show that rolipram induces catalepsy with a potency similar to that of haloperidol, suggesting an antipsychotic-like effect. Although the lowest effective dose of rolipram to increase PPI (0.66 mg/kg) did not induce catalepsy, a dose only slightly higher did (1.0 mg/kg). Despite this ability to induce catalepsy, it should be noted that rolipram reduces vacuous chewing movements due to chronic haloperidol treatment (Sasaki et al., 1995).
The results presented here indicate that rolipram, a prototypical PDE4 inhibitor, may act as an effective antipsychotic. It will be of interest to future studies to determine if rolipram is capable of reversing amphetamine-induced hyperlocomotion or glutamatergic disruption of PPI, given that rolipram rescues glutamatergic disruption in tasks related to memory (Zhang et al., 2000) and latent inhibition (Davis and Gould, 2005). Although rolipram itself may be limited in its clinical utility by emetic and sedative side effects, there are newer compounds in development that appear to have reduced side effects, possibly due to a greater PDE4-subtype selectivity (Houslay et al., 2005). Our findings, combined with previous indications of improved learning and memory, mood, and, potentially, tardive dyskinesia, indicate that PDE4 inhibition represents a new frontier in antipsychotic development. Inhibition of intracellular second messenger cascades may provide the path to new and more effective treatments for patients with severe symptoms.
Acknowledgments
This work was supported by NIMH P50-MH064045 (S.J.K., S.J.S., J.T. and T.A.), NIMH K08-MH067091 (S.J.K.) and NIMH T32 MH019112 (M.P.K.) as well as a grant from the Tourettes Syndrome Association (M.P.K.). The authors would like to thank Dr. Mark Geyer for his help with experimental design.
Abbreviations
- ANOVA
analysis of variance
- bg
background
- EPS
extrapyramidal side effects
- PDE
phosphodiesterase
- PPI
prepulse inhibition of acoustic startle
- SNK
Student-Newman-Keuls
REFERENCES
- Adell A, Artigas F. The somatodendritic release of dopamine in the ventral tegmental area and its regulation by afferent transmitter systems. Neurosci Biobehav Rev. 2004;28:415–431. doi: 10.1016/j.neubiorev.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Baillie GS, Sood A, McPhee I, Gall I, Perry SJ, Lefkowitz RJ, Houslay MD. beta-Arrestin-mediated PDE4 cAMP phosphodiesterase recruitment regulates beta-adrenoceptor switching from Gs to Gi. Proc Natl Acad Sci U S A. 2003;100:940–945. doi: 10.1073/pnas.262787199. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Barad M, Bourtchouladze R, Winder DG, Golan H, Kandel E. Rolipram, a type iv-specific phosphodiesterase inhibitor, facilitates the establishment of long-lasting long-term potentiation and improves memory. Proc Natl Acad Sci U S A. 1998;95:15020–15025. doi: 10.1073/pnas.95.25.15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavo JA, Brunton LL. Cyclic nucleotide research: still expanding after half a century. Nat Rev Mol Cell Biol. 2002;3:710–718. doi: 10.1038/nrm911. [DOI] [PubMed] [Google Scholar]
- Bonbon D, Breulet M, Gerard-Vandenhove M, Guiot-Goffioul F, Plomteux G, Sastre-y-Hernandez M, Schratzer M, Troisfontaines B, van Frenckeill R, Wachtel H. Is phosphodiesterase inhibition a new mechanism of antidepressant action? A double blind double-dummy study between rolipram and desipramine in hospitalized major and/or endogenous depressives. Eur Arch Psychiatry Neurol Sci. 1988;238:2–6. doi: 10.1007/BF00381071. [DOI] [PubMed] [Google Scholar]
- Bourtchouladze R, Lidge R, Catapano R, Stanley J, Gossweiler S, Romashko D, Scott R, Tully T. A mouse model of Rubinstein-Taybi syndrome: defective long-term memory is ameliorated by inhibitors of phosphodiesterase 4. Proc Natl Acad Sci U S A. 2003;100:10518–10522. doi: 10.1073/pnas.1834280100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braff D, Stone C, Callaway E, Geyer M, Glick I, Bali L. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978;15:339–343. doi: 10.1111/j.1469-8986.1978.tb01390.x. [DOI] [PubMed] [Google Scholar]
- Brody SA, Geyer MA, Large CH. Lamotrigine prevents ketamine but not amphetamine-induced deficits in prepulse inhibition in mice. Psychopharmacology. 2003;169:240–246. doi: 10.1007/s00213-003-1421-2. [DOI] [PubMed] [Google Scholar]
- Censits DM, Ragland JD, Gur RC, Gur RE. Neuropsychological evidence supporting a neurodevelopmental model of schizophrenia: A longitudinal study. Schizophr Res. 1997;24:289–298. doi: 10.1016/s0920-9964(96)00091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. Rolipram attenuates MK-801-induced deficits in latent inhibition. Behav Neurosci. 2005;119:595–602. doi: 10.1037/0735-7044.119.2.595. [DOI] [PubMed] [Google Scholar]
- Dodge-Kafka KL, Soughayer J, Pare GC, Carlisle Michel JJ, Langeberg LK, Kapiloff MS, Scott JD. The protein kinase A anchoring protein mAKAP coordinates two integrated cAMP effector pathways. Nature. 2005;437:574–578. doi: 10.1038/nature03966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulawa SC, Geyer MA. Effects of strain and serotonergic agents on prepulse inhibition and habituation in mice. Neuropharmacology. 2000;39:2170–2179. doi: 10.1016/s0028-3908(00)00030-7. [DOI] [PubMed] [Google Scholar]
- Duncan E, Szilagyi S, Schwartz M, Kunzova A, Negi S, Efferen T, Peselow E, Chakravorty S, Stephanides M, Harmon J, Bugarski-Kirola D, Gonzenbach S, Rotrosen J. Prepulse inhibition of acoustic startle in subjects with schizophrenia treated with olanzapine or haloperidol. Psychiatry Res. 2003a;120:1–12. doi: 10.1016/s0165-1781(03)00161-6. [DOI] [PubMed] [Google Scholar]
- Duncan EJ, Szilagyi S, Efferen TR, Schwartz MP, Parwani A, Chakravorty S, Madonick SH, Kunzova A, Harmon JW, Angrist B, Gonzenbach S, Rotrosen JP. Effect of treatment status on prepulse inhibition of acoustic startle in schizophrenia. Psychopharmacology (Berl) 2003b;167:63–71. doi: 10.1007/s00213-002-1372-z. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology. 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Gold JM, Goldberg RW, McNary SW, Dixon LB, Lehman AF. Cognitive correlates of job tenure among patients with severe mental illness. Am J Psychiatry. 2002;159:1395–1402. doi: 10.1176/appi.ajp.159.8.1395. [DOI] [PubMed] [Google Scholar]
- Gonul AS, Suer C, Coburn K, Ozesmi C, Oguz A, Yilmaz A. Effects of olanzapine on auditory p300 in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:173–177. doi: 10.1016/s0278-5846(02)00349-4. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Bizily SP, Tokarczyk J, Kelly MP, Siegel SJ, Kanes SJ, Abel T. Sensorimotor gating deficits in transgenic mice expressing a constitutively active form of g(s)alpha. Neuropsychopharmacology. 2004;29:494–501. doi: 10.1038/sj.npp.1300309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greba Q, Gifkins A, Kokkinidis L. Inhibition of amygdaloid dopamine D2 receptors impairs emotional learning measured with fear-potentiated startle. Brain Res. 2001;899:218–226. doi: 10.1016/s0006-8993(01)02243-0. [DOI] [PubMed] [Google Scholar]
- Gur RC, Ragland JD, Moberg PJ, Bilker WB, Kohler C, Siegel SJ, Gur RE. Computerized neurocognitive scanning: II. The profile of schizophrenia. Neuropsychopharmacology. 2001;25:777–788. doi: 10.1016/S0893-133X(01)00279-2. [DOI] [PubMed] [Google Scholar]
- Gur RE, Kohler C, Ragland JD, Siegel SJ, Bilker WB, Loughead J, Phend N, Gur RC. Neurocognitive performance and clinical changes in olanzapine-treated patients with schizophrenia. Neuropsychopharmacology. 2003;28:2029–2036. doi: 10.1038/sj.npp.1300275. [DOI] [PubMed] [Google Scholar]
- Hill SK, Ragland JD, Gur RC, Gur RE. Neuropsychological profiles delineate distinct profiles of schizophrenia, an interaction between memory and executive function, and uneven distribution of clinical subtypes. J Clin Exp Neuropsychol. 2002;24:765–780. doi: 10.1076/jcen.24.6.765.8402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman DC, Donovan H. Catalepsy as a rodent model for detecting antipsychotic drugs with extrapyramidal side effect liability. Psychopharmacology (Berl) 1995;120:128–133. doi: 10.1007/BF02246184. [DOI] [PubMed] [Google Scholar]
- Houslay MD, Adams DR. PDE4 cAMP phosphodiesterases: modular enzymes that orchestrate signalling cross-talk, desensitization and compartmentalization. Biochem J. 2003;370:1–18. doi: 10.1042/BJ20021698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houslay MD, Schafer P, Zhang KY. Keynote review: phosphodiesterase-4 as a therapeutic target. Drug Discov Today. 2005;10:1503–1519. doi: 10.1016/S1359-6446(05)03622-6. [DOI] [PubMed] [Google Scholar]
- Kanes S, Dains K, Cipp L, Gatley J, Hitzemann B, Rasmussen E, Sanderson S, Silverman M, Hitzemann R. Mapping the genes for haloperidol-induced catalepsy. J Pharmacol Exp Ther. 1996;277:1016–1025. [PubMed] [Google Scholar]
- Kanes SJ, Hitzemann BA, Hitzemann RJ. On the relationship between d2 receptor density and neuroleptic-induced catalepsy among eight inbred strains of mice. J Pharmacol Exp Ther. 1993;267:538–547. [PubMed] [Google Scholar]
- Kapur S, Mamo D. Half a century of antipsychotics and still a central role for dopamine d2 receptors. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1081–1090. doi: 10.1016/j.pnpbp.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Kapur S, Seeman P. Does fast dissociation from the dopamine d(2) receptor explain the action of atypical antipsychotics? A new hypothesis. Am J Psychiatry. 2001;158:360–369. doi: 10.1176/appi.ajp.158.3.360. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Seidman LJ, Christensen BK, Hamer RM, Sharma T, Sitskoorn MM, Lewine RR, Yurgelun-Todd DA, Gur RC, Tohen M, Tollefson GD, Sanger TM, Lieberman JA. Comparative effect of atypical and conventional antipsychotic drugs on neurocognition in first-episode psychosis: A randomized, double-blind trial of olanzapine versus low doses of haloperidol. Am J Psychiatry. 2004;161:985–995. doi: 10.1176/appi.ajp.161.6.985. [DOI] [PubMed] [Google Scholar]
- Kehne JH, Boulis NM, Davis M. Effects of the phosphodiesterase inhibitor rolipram on the acoustic startle response in rats. Psychopharmacology (Berl) 1991;105:27–36. doi: 10.1007/BF02316860. [DOI] [PubMed] [Google Scholar]
- Kelly MP, Isiegas C, Cheung YF, Tokarczyk J, Yang X, Esposito MF, Rapoport DA, Fabian SA, Siegel SJ, Wand G, Houslay M, Kanes SJ, Abel T. Constitutive activation of Gαs causes deficits in sensorimotor gating due to PKA-dependent decreases in cAMP. Neuropsychopharmacology. 2006 doi: 10.1038/sj.npp.1301099. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause W, Kuhne G. Pharmacokinetics of rolipram in the rhesus and cynomolgus monkeys, the rat and the rabbit. Studies on species differences. Xenobiotica. 1988;18:561–571. doi: 10.3109/00498258809041693. [DOI] [PubMed] [Google Scholar]
- Li X, Witkin JM, Need AB, Skolnick P. Enhancement of antidepressant potency by a potentiator of AMPA receptors. Cell Mol Neurobiol. 2003;23:419–430. doi: 10.1023/A:1023648923447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery N, Giovanni L, Mozley LH, Arnold SE, Bilker WB, Gur RE, Moberg PJ. Relationship between clock-drawing and neuropsychological and functional status in elderly institutionalized patients with schizophrenia. Am J Geriatr Psychiatry. 2003;11:621–628. doi: 10.1176/appi.ajgp.11.6.621. [DOI] [PubMed] [Google Scholar]
- Lugnier C. Cyclic nucleotide phosphodiesterase (PDE) superfamily: A new target for the development of specific therapeutic agents. Pharmacol Ther. 2006;109:366–398. doi: 10.1016/j.pharmthera.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Maxwell CR, Kanes SJ, Abel T, Siegel SJ. Phosphodiesterase inhibitors: A novel mechanism for receptor-independent antipsychotic medications. Neuroscience. 2004;129:101–107. doi: 10.1016/j.neuroscience.2004.07.038. [DOI] [PubMed] [Google Scholar]
- Meltzer HY. Beyond control of acute exacerbation: Enhancing affective and cognitive outcomes. CNS Spectr. 2003;8:16–18. 22. doi: 10.1017/s1092852900008142. [DOI] [PubMed] [Google Scholar]
- Millar JK, Pickard BS, Mackie S, James R, Christie S, Buchanan SR, Malloy MP, Chubb JE, Huston E, Baillie GS, Thomson PA, Hill EV, Brandon NJ, Rain JC, Camargo LM, Whiting PJ, Houslay MD, Blackwood DH, Muir WJ, Porteous DJ. DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science. 2005;310:1187–1191. doi: 10.1126/science.1112915. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Duncan GE, Marx CE, Lieberman JA. Treatments for schizophrenia: A critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry. 2005;10:79–104. doi: 10.1038/sj.mp.4001556. [DOI] [PubMed] [Google Scholar]
- Mongillo M, McSorley T, Evellin S, Sood A, Lissandron V, Terrin A, Huston E, Hannawacker A, Lohse MJ, Pozzan T, Houslay MD, Zaccolo M. Fluorescence resonance energy transfer-based analysis of cAMP dynamics in live neonatal rat cardiac myocytes reveals distinct functions of compartmentalized phosphodiesterases. Circ Res. 2004;95:67–75. doi: 10.1161/01.RES.0000134629.84732.11. [DOI] [PubMed] [Google Scholar]
- Mori T, Baba J, Ichimaru Y, Suzuki T. Effects of rolipram, a selective inhibitor of phosphodiesterase 4, on hyperlocomotion induced by several abused drugs in mice. Jpn J Pharmacol. 2000;83:113–118. doi: 10.1254/jjp.83.113. [DOI] [PubMed] [Google Scholar]
- Neves SR, Ram PT, Iyengar R. G protein pathways. Science. 2002;296:1636–1639. doi: 10.1126/science.1071550. [DOI] [PubMed] [Google Scholar]
- Ong JC, Brody SA, Large CH, Geyer MA. An investigation of the efficacy of mood stabilizers in rodent models of prepulse inhibition. J Pharmacol Exp Ther. 2005;315:1163–1171. doi: 10.1124/jpet.105.090845. [DOI] [PubMed] [Google Scholar]
- Ouagazzal AM, Jenck F, Moreau JL. Drug-induced potentiation of prepulse inhibition of acoustic startle reflex in mice: A model for detecting antipsychotic activity? Psychopharmacology (Berl) 2001;156:273–283. doi: 10.1007/s002130100763. [DOI] [PubMed] [Google Scholar]
- Paylor R, Crawley JN. Inbred strain differences in prepulse inhibition of the mouse startle response. Psychopharmacology. 1997;132:169–180. doi: 10.1007/s002130050333. [DOI] [PubMed] [Google Scholar]
- Percudani M, Barbui C, Tansella M. Effect of second-generation antipsychotics on employment and productivity in individuals with schizophrenia: An economic perspective. Pharmacoeconomics. 2004;22:701–718. doi: 10.2165/00019053-200422110-00002. [DOI] [PubMed] [Google Scholar]
- Perry SJ, Baillie GS, Kohout TA, McPhee I, Magiera MM, Ang KL, Miller WE, McLean AJ, Conti M, Houslay MD, Lefkowitz RJ. Targeting of cyclic AMP degradation to beta 2-adrenergic receptors by beta-arrestins. Science. 2002;298:834–836. doi: 10.1126/science.1074683. [DOI] [PubMed] [Google Scholar]
- Pietzcker A, Muller-Oerlinghausen B, Kehr W. Antipsychotic activity of rolipram in schizophrenia patients. A pilot study. Naunyn Schmiedebergs Arch Pharmacol. 1979;308 Suppl 1 Deutsche Pharmakologische Gesellschaft Fall Meeting Abstract 175. [Google Scholar]
- Ploeger GE, Spruijt BM, Cools AR. Effects of haloperidol on the acquisition of a spatial learning task. Physiol Behav. 1992;52:979–983. doi: 10.1016/0031-9384(92)90380-k. [DOI] [PubMed] [Google Scholar]
- Ploeger GE, Spruijt BM, Cools AR. Spatial localization in the Morris water maze in rats: acquisition is affected by intra-accumbens injections of the dopaminergic antagonist haloperidol. Behav Neurosci. 1994;108:927–934. doi: 10.1037//0735-7044.108.5.927. [DOI] [PubMed] [Google Scholar]
- Quednow BB, Kuhn KU, Beckmann K, Westheide J, Maier W, Wagner M. Attenuation of the prepulse inhibition of the acoustic startle response within and between sessions. Biol Psychology. 2006;71:256–263. doi: 10.1016/j.biopsycho.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Ralph RJ, Varty GB, Kelly MA, Wang YM, Caron MG, Rubinstein M, Grandy DK, Low MJ, Geyer MA. The dopamine d2, but not d3 or d4, receptor subtype is essential for the disruption of prepulse inhibition produced by amphetamine in mice. J Neurosci. 1999;19:4627–4633. doi: 10.1523/JNEUROSCI.19-11-04627.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph-Williams RJ, Lehmann-Masten V, Geyer MA. Dopamine d1 rather than d2 receptor agonists disrupt prepulse inhibition of startle in mice. Neuropsychopharmacology. 2003;28:108–118. doi: 10.1038/sj.npp.1300017. [DOI] [PubMed] [Google Scholar]
- Ralph-Williams RJ, Lehmann-Masten V, Otero-Corchon V, Low MJ, Geyer MA. Differential effects of direct and indirect dopamine agonists on prepulse inhibition: A study in d1 and d2 receptor knock-out mice. J Neurosci. 2002;22:9604–9611. doi: 10.1523/JNEUROSCI.22-21-09604.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randt CT, Judge ME, Bonnet KA, Quartermain D. Brain cyclic AMP and memory in mice. Pharmacol Biochem Behav. 1982;17:677–680. doi: 10.1016/0091-3057(82)90344-6. [DOI] [PubMed] [Google Scholar]
- Rosengarten H, Quartermain D. Effect of prenatal administration of haloperidol, risperidone, quetiapine and olanzapine on spatial learning and retention in adult rats. Pharmacol Biochem Behav. 2002;72:575–579. doi: 10.1016/s0091-3057(02)00727-x. [DOI] [PubMed] [Google Scholar]
- Sachs G, Steger-Wuchse D, Kryspin-Exner I, Gur RC, Katschnig H. Facial recognition deficits and cognition in schizophrenia. Schizophr Res. 2004;68:27–35. doi: 10.1016/S0920-9964(03)00131-2. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Hashimoto K, Inada T, Fului S, Iyo I. Suppression of oro-facial movements by rolipram, a camp phosphodiesterase inhibitor, in rats chronically treated with haloperidol. Eur J Pharmacol. 1995;282:71–76. doi: 10.1016/0014-2999(95)00278-s. [DOI] [PubMed] [Google Scholar]
- Scuvee-Moreau J, Giesbers I, Dresse A. Effect of rolipram, a phosphodiesterase inhibitor and potential antidepressant, on the firing rate of central monoaminergic neurons in the rat. Arch Int Pharmacodyn Ther. 1987;288:43–49. [PubMed] [Google Scholar]
- Seeman P. Atypical antipsychotics: Mechanism of action. Can J Psychiatry. 2002;47:27–38. [PubMed] [Google Scholar]
- Serretti A, De Ronchi D, Lorenzi C, Berardi D. New antipsychotics and schizophrenia: A review on efficacy and side effects. Curr Med Chem. 2004;1:343–358. doi: 10.2174/0929867043456043. [DOI] [PubMed] [Google Scholar]
- Setlow B, McGaugh JL. Sulpiride infused into the nucleus accumbens posttraining impairs memory of spatial water maze training. Behav Neurosci. 1998;112:603–610. doi: 10.1037//0735-7044.112.3.603. [DOI] [PubMed] [Google Scholar]
- Sommer N, Martin R, McFarland HF, Quigley L, Cannella B, Raine CS, Scott DE, Loschmann PA, Racke MK. Therapeutic potential of phosphodiesterase type 4 inhibition in chronic autoimmune demyelinating disease. J Neuroimmunol. 1997;79:54–61. doi: 10.1016/s0165-5728(97)00111-2. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi T, Jayathilake K, Meltzer HY. The effect of melperone, an atypical antipsychotic drug, on cognitive function in schizophrenia. Schizophr Res. 2003;59:7–16. doi: 10.1016/s0920-9964(01)00329-2. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Bakshi V, Waikar M, Taaid N, Geyer MA. Seroquel, clozapine and chlorpromazine restore sensorimotor gating in ketamine-treated rats. Psychopharmacology (Berl) 1998;140:75–80. doi: 10.1007/s002130050741. [DOI] [PubMed] [Google Scholar]
- Wadenberg ML, Kapur S, Soliman A, Jones C, Vaccarino F. Dopamine d2 receptor occupancy predicts catalepsy and the suppression of conditioned avoidance response behavior in rats. Psychopharmacology (Berl) 2000;150:422–429. doi: 10.1007/s002130000466. [DOI] [PubMed] [Google Scholar]
- Zhang H, Huang Y, Jin SLC, Frith SA, Suvarna N, Conti M, O’Donnell JM. Antidepressant-like profile and reduced sensitivity to rolipram in mice deficient in the PDE4D phosphodiesterase enzyme. Neuropsychopharmacology. 2002;27:587. doi: 10.1016/S0893-133X(02)00344-5. [DOI] [PubMed] [Google Scholar]
- Zhang HT, Crissman AM, Dorairaj NR, Chandler LJ, O’Donnell JM. Inhibition of cyclic AMP phosphodiesterase (PDE4) reverses memory deficits associated with NMDA receptor antagonism. Neuropsychopharmacology. 2000;23:198–204. doi: 10.1016/S0893-133X(00)00108-1. [DOI] [PubMed] [Google Scholar]
- Zhang HT, Zhao Y, Huang Y, Dorairaj NR, Chandler LJ, O’Donnell JM. Inhibition of the phosphodiesterase 4 (PDE4) enzyme reverses memory deficits produced by infusion of the MEK inhibitor U0126 into the CA1 subregion of the rat hippocampus. Neuropsychopharmacology. 2004;29:1432–1439. doi: 10.1038/sj.npp.1300440. [DOI] [PubMed] [Google Scholar]