Abstract
In contrast to viruses of plants and animals, viruses of fungi, mycoviruses, uniformly lack an extracellular phase to their replication cycle. The persistent, intracellular nature of the mycovirus life cycle presents technical challenges to experimental design. However, these properties, coupled with the relative simplicity and evolutionary position of the fungal host, also provide opportunities for examining fundamental aspects of virus–host interactions from a perspective that is quite different from that pertaining for most plant and animal virus infections. This chapter presents support for this view by describing recent advances in the understanding of antiviral defense responses against one group of mycoviruses for which many of the technical experimental challenges have been overcome, the hypoviruses responsible for hypovirulence of the chestnut blight fungus Cryphonectria parasitica. The findings reveal new insights into the induction and suppression of RNA silencing as an antiviral defense response and an unexpected role for RNA silencing in viral RNA recombination.
I. INTRODUCTION
Although widely distributed throughout the diverse taxonomic groups of the filamentous fungi, mycoviruses (viruses of fungi) share surprisingly similar lifestyles (recently reviewed by Nuss, 2010). With two reported exceptions (Dawe and Kuhn, 1983; Yu et al., 2010), all characterized mycoviruses have genomes composed of double-stranded (ds) or single-stranded (ss) RNA. In contrast to viruses of plants and animals, mycoviruses uniformly lack an extracellular phase to their replication cycle. Consequently, they are not infectious in the classical sense. Infections cannot be initiated by exposure of uninfected hyphae to cell extracts prepared from an infected fungal strain. Rather, mycoviruses are transmitted by intracellular mechanisms such as anastomosis (fusion of hyphae) or through asexual spores. Since the absence of exposure to the extracellular environment reduces the need for the formation of particles that protect viral genetic information, a significant number of mycoviruses, for example, members of the taxonomic families Narnaviridae and Hypoviridae, do not even encode capsid proteins (reviewed in Nuss, 2005).
As a result of an exclusively intracellular lifestyle and dependence on the host for transmission, mycovirus infections are persistent and generally absent of severe symptoms and cell death. The constraints of this virus–host relationship have contributed to the development of novel phenotypic characteristics of fundamental interest and potential practical value. These include the recent report that the ability of the endophytic fungus Curvularia protuberance to confer heat tolerance to the panic grass Dichanthelium lanuginosum requires the presence of the mycovirus Curvularia thermal tolerance virus (Marquez et al., 2007). Moreover, the virus-infected endophyte was able to confer heat tolerance to a crop plant, tomato. A growing number of mycoviruses have been reported to alter the ability of plant pathogenic fungi to cause disease (recently reviewed by Ghabrial and Suzuki, 2009; Pearson et al., 2009). These mycovirus infections generally result in reduced virulence, termed hypovirulence, and offer the potential for development of biological control strategies for a range of fungal diseases.
The persistent, intracellular nature of the mycovirus life cycle presents technical challenges to experimental design but also provides opportunities for examining fundamental aspects of virus–host interactions from a perspective that is quite different from that pertaining for most plant and animal virus infections. Recent advances in the understanding of antiviral defense responses against one group of mycoviruses for which many of the technical experimental challenges have been overcome, the hypoviruses responsible for hypovirulence of the chestnut blight fungus Cryphonectria parasitica, will be developed in this chapter as support for this view. The findings have revealed new insights into the induction and suppression of RNA silencing as an antiviral defense response and an unexpected role for RNA silencing in viral RNA recombination.
II. OVERCOMING TECHNICAL CHALLENGES TO MYCOVIRUS RESEARCH
Although initial interest in hypoviruses derived primarily from reports of virus-mediated control of chestnut blight in Europe and potential use in North America (reviewed by Anagnostakis, 1982; Dawe and Nuss, 2001; Heiniger and Rigling, 1994; Nuss, 1992), several key advancements in hypovirus and C. parasitica molecular biology and genomics subsequently led to the development of a robust experimental system that was able to overcome most of the technical challenges inherent in mycovirus research. Development of a hypovirus reverse genetics system, the principal advancement, depended on progress in three fronts: sequence determination of the prototypic hypovirus CHV-1/EP713 (Fig. 1; Shapira et al., 1991a), construction of a full-length infectious CHV-1/EP713 cDNA clone (Choi and Nuss, 1992), and development of a robust DNA transformation protocol for C. parasitica (Churchill et al., 1990). This eliminated two major technical hurdles: limitations in initiating fungal infections by an extracellular route and the inability to genetically manipulate mycovirus genetic information. Hypovirus infections were initiated by removing the cell wall from virus-free C. parasitica hyphae, introducing the viral cDNA by a transformation protocol (Choi and Nuss, 1992) or the viral coding strand RNA transcript synthesized from the cDNA copy by electroporation (Chen et al., 1994), followed by cell-wall regeneration (Fig. 2). The availability of the cDNA form of the viral RNA also afforded the means to generate mutant and chimeric hypoviruses (e.g., Chen et al., 2000; Craven et al., 1993), thereby allowing the field to progress from descriptive studies to hypothesis-driven experimental approaches. Infectious cDNA clones have now been constructed for three different hypovirus isolates that differ in symptom expression (Chen and Nuss, 1999; Choi and Nuss, 1992; Lin et al., 2007).
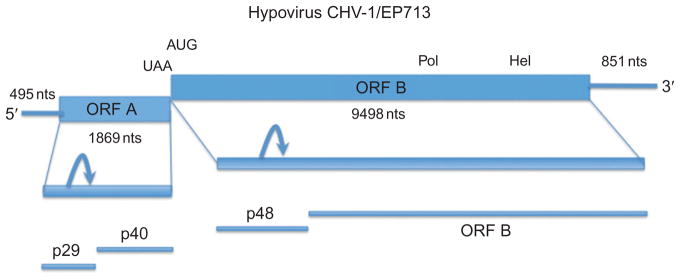
FIGURE 1.
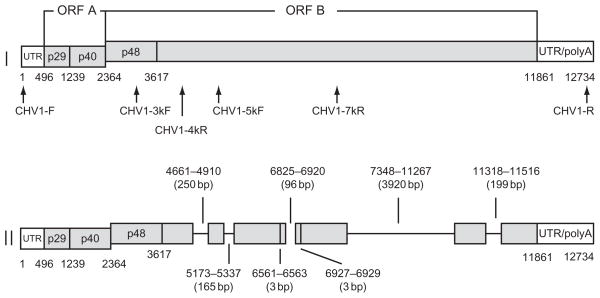
Genome organization and expression strategy for hypovirus CHV-1/EP713. The coding strand RNA (infectious; Chen et al., 1994) consists of 12,712 nucleotides excluding the poly(A) tail and contains two major coding domains, designated ORF A and ORF B (Shapira et al., 1991a). The junction between ORFs A and B consists of the pentanucleotide 5′-UAAUG-3′ in which the UAA portion serves as the termination codon for ORF A and the AUG serves as the ORF B initiation codon. Translation proceeds via a termination and reinitiation mechanism (Guo et al., 2009). ORF A encodes two poly-peptides, p29 and p40, that are released from a polyprotein p69 by an autocatalytic event (curved arrow) mediated by the papain-like protease activity of p29. ORF B encodes a very large polyprotein that contains polymerase (Pol) and helicase (Hel) coding domains and a p29-related N-terminal protein, p48, that is also autoproteolytically released from the larger polyprotein (curved arrow). Adapted with permission from Shapira et al. (1991a).
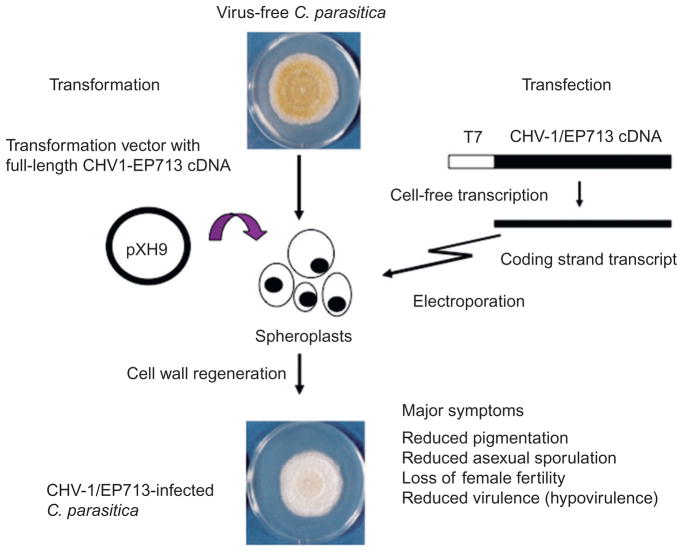
FIGURE 2.
Illustration of the transformation (left) and transfection (right) protocols used to initiate infections with full-length hypovirus cDNAs. Both protocols require the generation of cell-wall-free spheroplasts from virus-free C. parasitica strains. For transformation, a plasmid, that contains a full-length hypovirus cDNA copy and an independent selectable marker gene, is introduced into C. parasitica spheroplasts by DNA-mediated transformation. Transformants that contain the chromosomally integrated plasmid and cDNA-derived cytoplasmically replicating hypovirus RNA are selected following cell-wall regeneration and growth in the presence of the appropriate antibiotic (Choi and Nuss, 1992). The hypovirus transfection system uses synthetic transcripts corresponding to the hypovirus coding strand RNA (12.7 kb in the case of hypovirus CHV-1/EP713) that are synthesized in a T7-polymerase-dependent cell-free transcription system. The synthetic transcripts are introduced into spheroplasts by electroporation and followed by cell-wall regeneration in the absence of any selection (Chen et al., 1994). Replicating hypoviruses are able to migrate through the cytoplasmic network of the regenerated hyphal colony. The CHV1-infected C. parasitica strains exhibit a number of virus-mediated symptoms that include reduced pigmentation, reduced asexual sporulation, loss of female fertility, and hypovirulence (reviewed in Dawe and Nuss, 2001). Adapted with permission from Nuss (2005).
Further refinements in C. parasitica genomics and transformation capabilities have added new dimensions to the hypovirus/C. parasitica experimental system. The C. parasitica genome sequence (43.9 Mbp) has been determined by the U.S. Department of Energy Joint Genome Institute Community Sequencing Program and is available to the research community at (http://genome.jgi-psf.org/Crypa2/Crypa2.home.html). The development of a C. parasitica strain that carries a mutation in the ku80 gene that encodes a key component of the nonhomologous-end-joining DNA repair pathway (Lan et al., 2008) has increased the efficiency for disrupting genes in the haploid C. parasitica genome from ~5% to ~85%. Since C. parasitica supports the replication of members of five virus families—Hypoviridae, Reoviridae, Narnaviridae, Partitiviridae, and Chrysoviridae (reviewed by Hillman and Suzuki, 2004)—these new capabilities provide a very rich resource for studies on fundamental aspects of virus–host interactions, including the RNA silencing antiviral defense response.
III. ANTIVIRAL DEFENSE MECHANISMS IN FUNGI
A. Vegetative incompatibility
Mycoviruses are able to readily spread through the hyphal network that comprises a fungal colony. The septa that compartmentalize the tube-shaped hyphae contain pores that allow free flow of virus particles or viral genetic information. However, transmission of mycoviruses between different strains of the same fungal species is often regulated by a genetic self/nonself recognition system termed heterokaryon or vegetative incompatibility (vic). Interactions between vegetative incompatible strains result in programmed cell death (PCD), preventing exchange of cellular contents (Leslie and Zeller, 1996). It has long been proposed that this genetic recognition system evolved as a defense mechanism to limit the transmission of viruses, transposable elements, and senescence plasmids (Caten, 1972). The results of extensive laboratory and field studies on the influence of the C. parasitica vic system on hypovirus transmission are generally consistent with this view.
The vic system in C. parasitica is controlled by at least six genetic loci with two alleles at each locus (Cortesi and Milgroom, 1998). While allelic differences at any of these vic loci result in PCD, significant variation has been observed in the influence of incompatibility at the different vic loci on virus transmission (Cortesi et al., 2001; Huber, 1996; Huber and Fulbright, 1994). For example, heteroallelism at the vic 4 locus causes PCD but does not restrict virus transmission (Cortesi et al., 2001). Moreover, the frequency of virus transmission can be significantly influenced by the allele that is present in the donor or the recipient (Cortesi et al., 2001). There is growing evidence to suggest that the vic locus-dependent variations in virus transmission, including allele-associated asymmetric transmission for a specific vic locus, is related to differences in the rate at which PCD occurs in either or both strains following an incompatible cell fusion event (Biella et al., 2002; Milgroom and Cortesi, 2004). Virus transmission is most restricted by a rapid PCD response and increases in frequency depending on the degree to which PCD is delayed. Biella et al. (2002) have also reported that the rate of PCD is influenced by virus infection raising the possibility that hypoviruses have evolved specific mechanisms to delay or modify the PCD pathway triggered by vic. Consistent with laboratory results, it is generally agreed that virus transmission and biological control appear to be more effective in C. parasitica populations that exhibit lower vic diversity (Anagnostakis, 1983; Milgroom and Cortesi, 2004). It is anticipated that the availability of the C. parasitica genome sequence will allow identification of the vic genes leading to a mechanistic understanding and potential modulation of incompatible reactions.
B. RNA silencing
While vic serves as an antiviral defense mechanism at the population level, RNA silencing provides a fungal antiviral defense response at the cellular level. This form of RNA interference is a key component of the innate immunity repertoire in plants and invertebrates (reviewed in Ding, 2010), with increasing evidence that it influences virus replication in animal cells (Parameswaran et al., 2010). The core elements of the cross-kingdom RNA silencing defense response consists of conserved ribonucleases: members of the Dicer-like and Argonaute-like protein families (Hammond, 2005). Dicer nucleases recognize viral ds and structured RNAs and use the associated RNase III-type activity to process these RNAs into small RNAs of 21–24 nts in length, termed virus-derived small (vs) RNAs. The vsRNAs are then incorporated into an effector complex termed the RNA-induced silencing complex (RISC) with the aid of an Argonaute family protein. One strand of the vsRNA is degraded and the remaining guide strand targets the effector complex to the cognate viral RNA, which is then cleaved by the Argonaute-associated RNAse H-like activity. As most clearly shown for C. elegans and for plants, the antiviral RNA silencing response is further amplified by host RNA-dependent RNA polymerases (RdRPs; reviewed in Ding, 2010).
The model fungus Neurospora crassa was one of the first organisms used to study RNA interference (reviewed by Li et al., 2010) and genetic screens with this organism resulted in the first identification of an RNAi pathway gene, the RdRP QDE-1 (Cogoni and Macino, 1999). In addition, N. crassa encodes two Dicer-like proteins, DCL-1 and DCL-2, two Argonaute-like proteins, QDE-2 and SMS-2, and a second RdRP, SAD-1. These core RNA silencing components were subsequently shown to contribute to two RNA silencing pathways: Quelling and meiotic silencing by unpaired DNA (MSUD). The Quelling pathway operates during the vegetative phase of growth and is dependent primarily on DCL-2, QDE-2, and QDE-1. However, DCL-1 can compensate for DCL-2 in the Quelling pathway following deletion of dcl-2, indicating a level of redundancy. MSUD operates only during meiosis to silence unpaired genes and is dependent only on DCL-1, SMS-2, and SAD-1. Although most of what is known about RNA silencing in fungi was derived from studies with N. crassa, the absence of a mycovirus experimental system for this fungus limited its use to examine whether RNA silencing also provides antiviral defense in fungi. Fortunately, C. parasitica is closely related phylogenetically to N. crassa (Dawe et al., 2003), so it was possible to take advantage of the C. parasitica/hypovirus experimental system to apply the information gained from the N. crassa studies to address this question.
Segers et al. (2007) cloned two C. parasitica Dicer-like genes based on the N. crassa Dicer gene sequences. Inspection of the recently released C. parasitica genome sequence generated by the JGI Community Sequencing Project confirmed that, like N. crassa, C. parasitica encodes two Dicer-like genes designated dcl1 and dcl2. Disruption of dcl1 and dcl2, independently or in combination, resulted in no obvious phenotypic changes. However, hypovirus CHV-1/EP713 infection of the dcl2 null mutant Δdcl2 or the double mutant Δdcl1/Δdcl2 resulted in a severely debilitated growth phenotype (Fig. 3), while infection of the Δdcl1 null mutant resulted in a phenotype indistinguishable from that of CHV-1/EP713-infected wild-type strain EP155. Infection of the Dicer mutant strains with the mycoreovirus MyRV1-Cp9B21 resulted in a similar set of results. MyRV1-Cp9B21-infected Δdcl2 and Δdcl1/Δdcl2 strains showed reduced growth and altered colony morphology relative to the MyRV1-Cp9B21-infected wild-type and Δdcl1 mutant strain, but not to the extent observed for the corresponding hypovirus-infected strains.
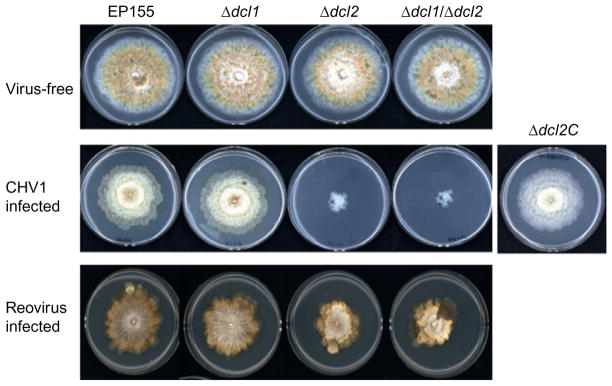
FIGURE 3.
Effect of mycovirus infection on C. parasitica Dicer gene deletion mutants. (Top row) Deletion of C. parasitica Dicer genes dcl1, dcl2, or both dcl1 and dcl2 resulted in no observable phenotypic changes in the absence of mycovirus infection. (Middle row) Hypovirus CHV-1/EP713-infected Dicer mutant strain Δdcl1 and a Δdcl2-2 C mutant, for which the Δdcl2 mutation was complemented with a wild-type dcl2 gene, exhibited symptoms identical to CHV-1/EP713-infected C. parasitica wild-type strain EP155. In sharp contrast, hypovirus infection of the Δdcl2 and Δdcl1/Δdcl2 double Dicer mutant strains resulted in a severe debilitation. (Bottom row) Infection of the Δdcl2 and double Dicer mutant strains with mycoreovirus MyRV1-Cp9B21 also resulted in a reduced growth phenotype relative to mycoreovirus-infected wild-type C. parasitica strain EP155, but not to the extent observed for hypovirus-infected strains carrying the dcl2 mutation (from Segers et al., 2007).
The accumulation of vsRNAs is a distinguishing characteristic of RNA silencing-based antiviral defense. Consistent with the increased susceptibility of the Δdcl2 mutant to hypovirus infection, Zhang et al. (2008) subsequently showed that hypovirus-derived vsRNAs of ~21–22 nt in length, and corresponding to both positive and negative strand RNA, accumulate in wild-type and the Δdcl1 mutant strain, but not in the Δdcl2 mutant. The combined results clearly demonstrate that the antiviral defense response in C. parasitica requires only a single Dicer, the Quelling homologue DCL2, with no indication of redundancy by dcl1 and dcl2.
A total of four Argonaute-like protein genes designated agl1–agl4, were identified through inspection of the C. parasitica draft genome sequence (Sun et al., 2009). The predicted proteins encoded by all four C. parasitica Argonaute-like genes contain the conserved PAZ and PIWI domains (reviewed in Song and Joshua-Tor, 2006) and consist of the same relative number of amino acids as the corresponding N. crassa orthologues (Catalanotto et al., 2000; Lee et al., 2003). In the paralogous grouping system proposed by Hammond et al. (2008b), AGL4 clustered with group M Argonaute-like proteins related to N. crassa SMS-2 involved in MSUD, while AGL1, AGL2, and AGL3 all clustered with group Q, related to N. crassa QDE-2 involved in Quelling (Sun et al., 2009).
As indicated in the top panel of Fig. 4, strains carrying a disruption mutation of the individual agl genes were indistinguishable from the parental strain EP155. Moreover, mutant strains Δagl1, Δagl3, and Δagl4 all responded to CHV-1/EP713 infection with phenotypic changes that were indistinguishable from that exhibited by CHV-1/EP713-infected wild-type strain EP155. However, as was observed following CHV-1/EP713 infection of the Δdcl2 mutant strain (Segers et al., 2007), a very severe debilitation phenotype was observed for the CHV-1/EP713-infected Δagl2 mutant strain (Fig. 4, middle panel). Complementation of the Δagl2 mutant with the intact agl2 coding domain restored the wild-type response to hypovirus infection (Fig. 4, lower panel).
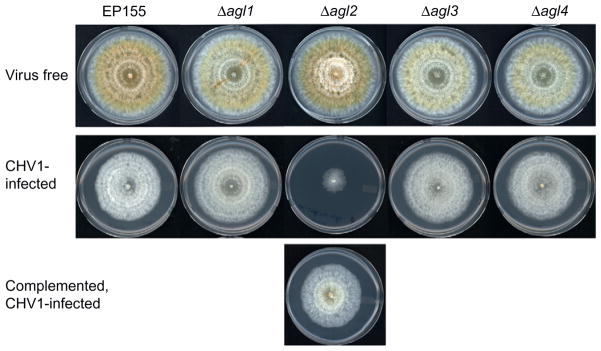
FIGURE 4.
Effect of hypovirus CHV-1/EP713 infection on C. parasitica Argonaute gene disruption mutant strains. As observed for the C. parasitica Dicer gene mutants, no phenotypic changes were observed for the four individual Argonaute gene disruption mutant strains in the absence of virus infection. Similarly, the response of the Argonaute mutant strains to hypovirus infection was indistinguishable from the response by wild-type strain EP155 with the exception of Δagl2, which responded to hypovirus infection with the severe debilitation phenotype observed for the Dicer dcl2 mutant strains (Fig. 3). Complementation of the Δagl2 mutant with the intact agl2 coding domain restored the wild-type response to hypovirus infection (from Sun et al., 2009).
The very similar severe levels of virus-induced symptoms observed for the C. parasitica Δdcl2 and Δagl2 mutants confirm a central role for RNA silencing as an antiviral defense response and indicate that this response is mediated by a single Dicer and a single Argonaute, as opposed to multiple Dicers and Argonautes observed in plants. Hammond et al. (2008a) detected vsRNAs derived from Aspergillus virus 341 indicating that this virus is a target of the Aspergillus RNA silencing machinery which consists of the single Dicer and single Argonaute that are encoded by this fungus. However, the 341 virus did not cause increased symptoms when moved into the Dicer or Argonaute mutant strains. As shown for higher eukaryotes (reviewed in Almeida and Allshire, 2005), RNA silencing also operates in fungi to target transposable elements (Murata et al., 2007; Nolan et al., 2005). The RNA silencing pathway was also shown to be induced in response to hairpin RNA in both C. parasitica (Sun et al., 2009) and N. crassa (Choudhary et al., 2007). Thus, it is likely that RNA silencing originated as a defense response against viruses and other invading nucleic acids in early eukaryotes and serves this role broadly within the Kingdom Fungi.
The absence of phenotypic change associated with disruption of RNA silencing in uninfected C. parasitica contrasts significantly with the effect of disruption of RNA silencing pathways in higher eukaryotes. A central role of RNA silencing in plants and animals is the production of micro-RNAs (miRNAs) from genome-encoded stem–loop RNA precursors and other endogenous small RNAs that direct regulation of developmental and metabolic pathways (Carrington and Ambrose, 2003; Mallory and Vaucheret, 2006). Thus, disruption of RNA silencing in these organisms results in developmental and metabolic defects. Although miRNA-like RNAs have recently been detected in fungi (Lee et al., 2010), evidence for a physiological role for the RNAs has not yet been forthcoming. Moreover, similar to the observations with C. parasitica, disruption of the RNA silencing pathways in a number of fungi, including N. crassa (Lee et al., 2010) and A. nidulans (Hammond et al., 2008a), does not result in obvious phenotypic change. One exception is the basal fungus Mucor circinelloides, where disruption of dcl2 results in reduced asexual sporulation (de Haro et al., 2009) and disruption of dcl1 results in defects in vegetative growth and hyphal morphology (Nicolas et al., 2007). Recent deep sequencing studies for M. circinelloides revealed a population of Dicer2 generated endogenous small RNAs that map to exons and regulate the expression of many protein-encoding genes (Nicolas et al., 2010). However, no miRNA-like RNAs were detected. Surprisingly, some fungi, for example, Ustilago maydis, appear to have completely lost the genes for the RNA silencing machinery (Nakayashiki and Nguyen, 2008). However, these fungi do not seem to suffer unusually high incidence of virus-induced symptoms, suggesting the possibility of additional as yet unidentified mechanisms used by filamentous fungi to modulate virus infection. In this regard, the genomes of some fungi contain quite high numbers of genes encoding RNA silencing proteins, for example, Coprinis cinereus contains genes for three Dicers, eight Argonautes, and seven RdRPs, while Stagonospora nodorum encodes four Dicers, six Argonautes, and four RdRPs (Nakayashiki and Nguyen, 2008), raising the possibility of redundant, perhaps hierarchical RNA silencing innate immunity pathways in some fungi.
IV. REGULATION OF THE FUNGAL ANTIVIRAL RNA SILENCING RESPONSE
A. Induction of the RNA silencing pathway in response to mycovirus infection
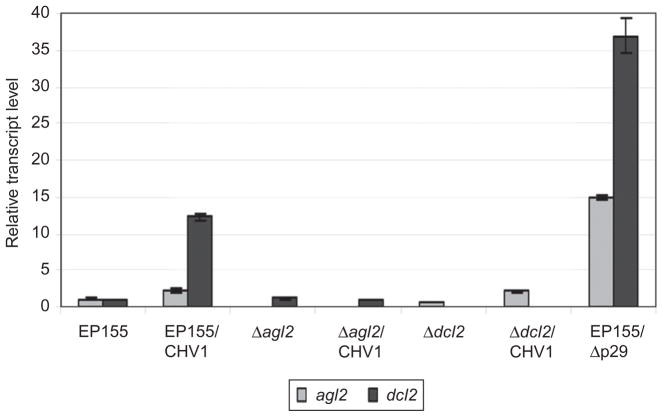
The requirement of a single Dicer gene, dcl2, and a single Argonaute gene, agl2, for RNA silencing antiviral defense in C. parasitica has allowed an examination of the activation of this response in the absence of potential contributions from multiple Dicers and Argonautes, as occurs in plants, and potentially in animal cells (Ding and Voinnet, 2007). Transcript levels for C. parasitica dcl1 and dcl2 were found to increase ~1.5- and ~15-fold, respectively, following either hypovirus or mycoreovirus infection. (Fig. 5; Zhang et al., 2008). In contrast, little to no increase in transcript levels was observed for the Argonaute genes following mycovirus infection (Qihong Sun, Gil H. Choi and Donald L. Nuss, unpublished observation). This included a modest twofold increase in the transcripts for agl2 that is required for antiviral defense (Fig. 5). However, the ~15-fold increase in dcl2 transcript accumulation in response to virus infection does not occur if the agl2 gene is disrupted (Fig. 5; Sun et al., 2009), while the approximately twofold increase in agl2 transcript accumulation still occurs in the virus-infected Δdcl2 disruption mutant. Thus, AGL2 appears to play an important regulatory role in the induction of dcl2 expression in response to virus infection.
FIGURE 5.
Accumulation of dcl2 (black columns) and agl2 (gray columns) transcripts in response to hypovirus infection. The relative level of dcl2 and agl2 transcripts were measured by semiquantitative RT-PCR for wild-type C. parasitica strain EP155, strain EP155 infected with hypovirus CHV-1/EP713 (EP155/CHV1), virus-free and CHV-1/EP713-infected mutant strain Δagl2 (Δagl2 and Δagl2/CHV1, respectively), virus-free and CHV-1/EP713-infected mutant strain Δdcl2 (Δdcl2 and Δdcl2/CHV1, respectively), and strain EP155 infected with a CHV-1/EP713 mutant virus that lacks the p29 suppressor of RNA silencing (EP155/Δp29) (from Sun et al., 2009).
B. Mycovirus-mediated suppression of the RNA silencing antiviral response
To combat RNA silencing-based antiviral defense, viruses of plants, insects, and mammals encode proteins, designated viral suppressors of RNA silencing (VSR), that employ a variety of mechanisms to suppress RNA silencing pathways (recently reviewed by Wu et al., 2010). While detailed characterization of mycovirus-encoded VSRs is currently limited to the hypovirus-encoded protein p29, these studies have uncovered a novel suppression strategy that targets the transcriptional induction of the RNA silencing pathway in response to virus infection.
A possible role for p29 as a VSR was suggested by striking similarities between p29 and one of the first VSRs to be identified, the plant potyvirus-encoded protein HC-Pro. Following the publication of the hypovirus CHV-1/EP713 nucleotide sequence, Koonin et al. (1991) presented evidence for a common ancestry between hypoviruses and members of the large plant potyvirus family and also noted that p29 and HC-Pro both exhibit papain-like protease activity and shared similarities in sequences surrounding the catalytic cysteine and histidine residues as well as the spacing of these essential residues relative to the respective cleavage sites. Suzuki et al. (2003) subsequently showed that the p29 protein could act in trans to enhance viral RNA accumulation. Similarities between p29 and HC-Pro extended to reports that both proteins enhanced the accumulation of homologous and heterologous viruses when expressed in trans (Pruss et al., 1996; Sun et al., 2006; Suzuki et al., 2003). Prompted by these reports, Segers et al. (2006) showed that p29 suppressed hairpin RNA-mediated gene silencing in C. parasitica and virus vector-based and agroinfiltration-induced RNA silencing in the plant Nicotiana benthamiana.
Consistent with a role for p29 in suppressing the fungal RNA-silencing-based antiviral defense response, deletion of p29 in the context of the hypovirus CHV-1/EP713 infectious cDNA clone, virus Δp29, was shown to result in a ~80% reduction in viral RNA accumulation (Suzuki and Nuss, 2002). Infection of the C. parasitica Δdcl2 mutant strain with the Δp29 virus resulted in an increase in viral RNA accumulation to a level approaching that of parental CHV-1/EP713 hypovirus in wild-type C. parasitica strain EP155 (Segers et al., 2007). This result indicated that p29 directly or indirectly counteracts DCL2 function.
Insight into one possible mechanism by which p29 suppresses the antiviral defense response came from the unexpected observation that dcl2 transcript levels increase by greater than 30 fold following infection by Δp29 virus compared to the 10- to 15-fold increase observed following wild-type CHV1/EP713 infection (Zhang et al., 2008; Fig. 5). Similarly, agl2 transcript accumulation increase to a significantly higher level in response to Δp29 than to CHV-1/EP713 (~14-fold vs. ~2-fold; Sun et al., 2009; Fig. 5). Notably, the response to Δp29 was specific for the Dicer and Argonaute genes that are required for antiviral defense. Transcript levels for dcl1 and agl1 increase less than twofold and transcripts for agl3 and agl4 were undetectable in Δp29-infected mycelia. Moreover, Sun et al., created C. parasitica transformed strains containing an integrated promoter/reporter construct consisting of a 1.5 kbp portion of the agl2 promoter fused to the green fluorescent protein coding region to demonstrate that the increase in agl2 transcript accumulation in response to Δp29 infection is indeed promoter dependent. A similar result has been obtained for dcl2 promoter/reporter constructs (Gil H. Choi and Donald L. Nuss, unpublished observations). These combined results suggest a novel mechanism in which a VSR represses the transcriptional induction of an RNA silencing pathway in response to virus infection.
V. RNA SILENCING CONTRIBUTES TO MYCOVIRUS RNA RECOMBINATION
One of the most unexpected observations to come from study of the RNA silencing antiviral defense response in C. parasitica was that RNA silencing contributes to hypovirus viral RNA recombination. Viral RNA recombination is one of the major components of viral evolution and a driving force behind the emergence of new viruses (reviewed by Nagy and Simon, 1997). Early molecular characterizations of hypovirus RNAs revealed the accumulation of significant levels of defective interfering (DI) RNAs (Shapira et al., 1991b; Tartaglia et al., 1986). DI RNAs are generated from the parental viral RNA genome as a result of recombination deletion events and then further selected for the presence of cis elements that promote their replication by the replication machinery provided by the parental helper virus (Roux et al., 1991). The presence of DI RNAs often results in the suppression of parental RNA accumulation, leading to attenuation of symptoms (Simon et al., 2004) and persistent virus infections (Huang and Baltimore, 1970). Efforts to use recombinant hypoviruses to express foreign genes also encountered limitations due to rapid recombination-mediated deletion of the nonviral nucleotide sequences (Suzuki et al., 2000). In this regard, genome instability represents an important obstacle to the use of recombinant RNA viruses as gene expression vectors or therapeutic agents (Lee et al., 2002; Paar et al., 2007; van den Born et al., 2007). Thus, the discovery of a role for RNA silencing in promoting hypovirus RNA recombination has potentially broad implications.
A. Contribution of RNA silencing to the production of hypovirus DI RNAs
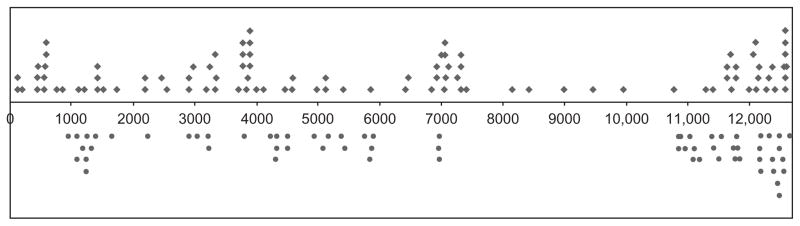
The cloning and sequence analysis of hypovirus-derived vsRNAs revealed a nonrandom distribution along the hypovirus CHV-1/EP713 genome with a paucity of vsRNAs corresponding to the region spanning map coordinates 7500–11,000 (Fig. 6; Zhang et al., 2008). Subsequent deep sequencing analysis of the hypovirus vsRNAs confirmed this general distribution pattern (Xuemin Zhang and Donald L. Nuss, unpublished). Zhang et al. (2008) proposed that the pattern may be due to the reduction in 12.7 kb full-length viral RNA accumulation caused by competition with DI RNAs resulting in less substrate for vsRNA biogenesis for the regions that are absent in the DI RNAs. Subsequent characterization of the major 8 kb DI RNA species revealed a total of seven deletions with a major 3920 nt deletion in the DI RNA corresponding directly to the region of low vsRNA abundance (Fig. 7; Zhang and Nuss, 2008). Thus, DI RNA accumulation clearly contributes to the uneven distribution of the vsRNAs along the hypovirus genome.
FIGURE 6.
Origins and polarities of Dicer dcl2 generated small RNAs (18–24 nts) derived from hypovirus CHV-1/EP713. The map positions along the 12.7 kb CHV-1/EP713 genome RNA from which the vsRNAs originated are indicated below the line representing the viral RNA. Virus-derived RNAs originating from the positive RNA strand are indicated above the line while vsRNAs originating from the negative RNA strand are indicated under the line (from Zhang et al., 2008). With permission, Copyright American Society for Microbiology. Doi: 10.1128/JVI.02324-07.
FIGURE 7.
Diagram of the structure of the major 8098-nt long CHV-1/EP713-derived DI RNA (II) relative to the full-length parental viral RNA (I). The positions of seven deletions found in the 8098 nt DI RNA are indicated as lines, whereas the regions retained in the DI RNA are indicated by gray bars. The positions of primer pairs used to amplify cDNA fragments for full-length viral and DI RNAs for sequence analysis are indicated below the full-length CHV-1/EP713 RNA diagram. The map coordinates and size of each deletion is indicated above or below the DI RNA diagram (from Zhang and Nuss, 2008).
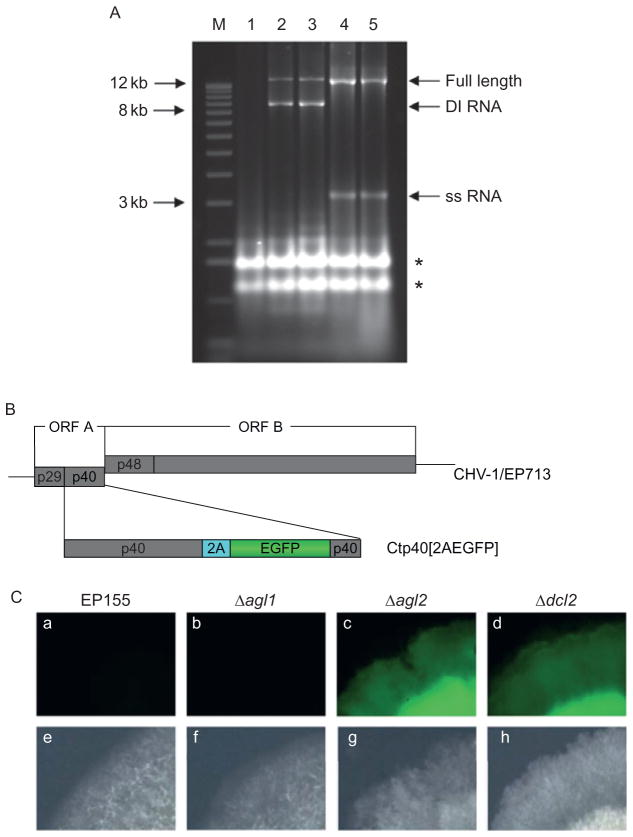
An unexpected role for RNA silencing in the generation of DI RNAs was uncovered following transfection of C. parasitica wild-type and Dicer mutant strains with infectious CHV-1/EP173 transcripts. The production of DI RNAs proceeded in a similar manner in the wild-type and Δdcl1 strains resulting in a predominance of DI RNAs relative to full-length viral RNA (Fig. 8A), while DI RNAs failed to form in the Δdcl2 mutant strain even after prolonged infection and subculturing of the infected mycelia. Additionally, the DI RNAs present in the wild-type strain EP155 were lost when CHV-1/EP713 RNA was transferred to the Δdcl2 strain by anastomosis (fusion of the hyphae) and then reappeared following several subcultures after anastomosis-mediated transfer of viral RNA back from the Δdcl2 strain to wild-type strain EP155, or to the Δdcl2 strain that had been complemented with the wild-type dcl2 gene. Very similar results were obtained when the set of experiments were performed with the Δagl1 and Δagl2 mutant strains (Fig. 8A; Sun et al., 2009). The combined results clearly establish that the Dicer and Argonaute genes, dcl2 and agl2, that are induced in response to virus infection and required for the antiviral defense response, also significantly contribute to hypovirus RNA recombination and DI RNA production.
FIGURE 8.
(A) Agarose gel (1%) analysis of total RNA isolated from virus-free wild-type C. parasitica strain EP155 (lane 1); hypovirus CHV-1/EP713 infected strain EP155 (lane 2); CHV-1/EP713 infected Argonaute mutant strain Δagl1 (lane 3); CHV-1/EP713 infected Argonaute mutant strain Δagl2 (lane 4); and CHV-1/EP713 infected Dicer mutant strain Δdcl2 (lane 5). The lane marked “M” contains 1-kb DNA size markers. The migration positions of replicative dsRNAs corresponding to full-length CHV-1/EP713 and related DI RNAs, and full-length CHV-1/EP713 single-stranded (ss) RNA are indicated by arrows at the left. The asterisks indicate the migration positions of C. parasitica ribosomal RNAs. (B) Diagram of recombinant hypovirus Ctp40[2AEGFP] that contains insertions of the enhanced green fluorescence protein (EGFP) gene and the Foot and Mouth Virus 2A protease gene at the 3′-end of the p40 coding region of hypovirus CHV-1/EP713. (C) Fluorescence micrographs of Argonaute and Dicer mutant strains infected by transfection with transcripts derived from recombinant hypovirus Ctp40[2AEGFP]. The colonies shown in the figure were photographed after two transfers, by which time fluorescence had been lost in the Ctp40[2AEGFP] infected EP155 wild-type (a) and Δagl1 mutant (b) strains. Fluorescence was retained in the Ctp40[2AEGFP] infected Δagl2 and Δdcl2 mutant strains (c and d, respectively) even after extended subculturing. Corresponding light micrographs are shown in (e–h) (from Sun et al., 2009).
Zhang and Nuss (2008) postulated that the RNA silencing pathway contributes to viral RNA recombination by providing 5′- and 3′-fragments of the viral RNA. In terms of the classical template switching model for RNA recombination (reviewed in Pathak and Nagy, 2009), cleavage of the viral RNA would promote disengagement of the viral RNA-dependent-RNA polymerase with the nascent RNA strand when it encounters the 5′-end of a cleaved template RNA 3′-fragment and provide 5′-fragments as substrate for template switching and strand completion. In this regard, Jaag et al. (2011) recently reported a role for the endoribonuclease RNase MRP in Saccharomyces cerevisiae and its homologue in N. benthamiana in promoting recombination of Tomato bushy stunt virus RNA. Consistent with the cleavage of hypovirus RNA by the RNA silencing pathway, the accumulation of hypovirus ss RNA is very significantly increased in both the Δdcl2 and Δagl2 mutant strains (Fig. 8A). The increased accumulation of hypovirus coding strand RNA and viral-encoded protein (Zhang and Nuss, unpublished) in the RNA silencing mutants is likely to be a major contributor to the severe debilitation phenotype observed in the CHV-1/EP713-infected Δdcl2 and Δagl2 mutant strains.
One could imagine that both the C. parasitica Dicer DCL2 and Argonaute AGL2 could contribute to fragmentation of viral RNA by dicing of structured RNA regions or vsRNA-guided slicing, respectively. However, the fact that dcl2 expression is not activated in response to virus infection in the absence of AGL2 (Sun et al., 2009) complicates a determination of the relative contribution of the two nucleases to promotion of hypovirus RNA recombination. Irrespective of the precise contributions of the RNA silencing ribonucleases to DI RNA production, the apparent absence of DI RNAs in the RNA silencing mutant strains strongly suggests that errors by the viral replicase alone are insufficient to explain the extent of DI RNA formation in hypovirus-infected C. parasitica. The loss and reemergence of hypovirus DI RNAs following anastomosis-mediated transfer to the Δdcl2 strain and back to the wild-type C. parasitica strain indicates a much more dynamic nature of DI RNA formation and decay than generally held.
B. Contribution of RNA silencing to hypovirus recombinant vector RNA instability
Suzuki et al. (2000) reported the development of replication-competent recombinant hypovirus expression vectors that contained foreign, nonviral sequences. The utility of hypoviruses as expression vectors was significantly limited by the rapid recombination and deletion of the foreign sequences, usually within two subcultures after transfection. The observation that hypovirus DI RNA formation was restricted in the RNA silencing mutant fungal strains prompted the testing of hypovirus expression vectors in the Δdcl2 and Δagl2 mutants. Initial studies were performed with hypovirus vector Ctp40[2AEGFP], which contains the enhanced green fluorescent protein (EGFP) coding domain proceeded by the foot and mouth virus 2A protease coding domain inserted into the p40 coding region just upstream of the ORF A–ORF B junction (Fig. 8B). As indicated in Fig. 8C, wild-type C. parasitica and the Δdcl1 and Δagl1 mutant strains transfected with Ctp40[2AEGFP] coding strand RNA produced RNAs deleted of the EGFP sequence and weak fluorescence by the first subculturing, followed by a rapid complete loss of fluorescence signal. The Ctp40[2AEGFP] transfected Δdcl2 (Zhang et al., 2008), and Δagl2 strains, in contrast, produced strong fluorescence and maintained the intact Ctp40[2AEGFP] vector RNA after extensive subculturing. Thus, the C. parasitica RNA silencing antiviral defense pathway contributes to both hypovirus DI RNA production and hypovirus vector RNA instability. The finding that foreign nucleotide sequences are stably retained in recombinant hypovirus vectors when the RNA silencing pathway is defeated raises the potential for the development of robust hypovirus-based protein expression systems. Unfortunately, the severe growth defect caused by hypovirus infection of the Δdcl2 and Δagl2 mutant strains presents a serious complication. However, the recent identification of hypovirus isolates that do accumulate high levels of viral RNA in the RNA silencing mutants without causing the severe growth defect observed for CHV-1/EP713 infection offers some possible resolution to this complication (Zhang and Nuss, unpublished).
VI. CONCLUDING REMARKS
Filamentous fungi have featured prominently as experimental systems for advancing biochemistry, genetics, and molecular and cellular biology (reviewed in Borkovich et al., 2004; Davis and Perkins, 2002). Recent examples include the use of N. crassa in foundation studies on RNA silencing and the identification of the first RNAi pathway gene (reviewed in Li et al., 2010). The potential utility of fungi as experimental systems is further enhanced when one layers on the rich diversity of biological interactions, replication strategies, host responses and phenotypic changes associated with mycovirus infections. The advances made with the hypovirus/C. parasitica experimental system clearly demonstrate that, like viruses of animals and plants, mycoviruses have significant utility for elucidating host functions and manipulating host phenotype (reviewed in detail in Dawe and Nuss, 2001; Nuss, 2005).
The demonstration that RNA silencing serves as a robust antiviral defense response in C. parasitica and limited evidence for physiologically functional miRNAs in fungi are consistent with the view that RNA silencing arose as an ancestral surveillance system to protect against invading nucleic acids, including viruses. Although activation of the RNA silencing antiviral defense response reduces hypovirus symptoms and viral ssRNA accumulation, it also results in the production of a new population of potentially bioactive vs RNAs. Given the persistent and widespread nature of mycovirus infections, the potential influence of mycovirus-derived small RNA populations on long-term fungal gene expression and evolution deserves further investigation. The apparent absence of RNA silencing component genes in some filamentous fungi and the over abundance of component genes in other fungi also raises the possibility of multiple or hierarchical RNA silencing antiviral pathways or fungal antiviral defense responses that are independent of RNA silencing.
While p29 is the only mycovirus-encoded viral suppressor of RNA silencing so far characterized, the novel interference with the transcriptional induction of the RNA silencing antiviral defense response by this VSR is intriguing. The striking similarities between p29 and the potyvirus-encoded VSR HC-Pro raise the question of whether a similar, but as yet undetected, mechanism may occur in plants. In this regard, the surprising observation that RNA silencing significantly contributes to hypovirus DI RNA production and recombinant viral RNA instability also begs the question of whether this observation will extend to a broader role of RNA silencing in recombination of plant and animal RNA viruses.
While of considerable fundamental interest, fungal RNA silencing antiviral pathways also need to be considered in any current or future use of mycoviruses for practical applications or therapeutic manipulation of the fungal host, for example, reducing fungal virulence or mycotoxin production. The ability to significantly increase the stability of foreign gene sequences in recombinant hypovirus vectors by inactivating the RNA silencing pathway certainly removes one major limitation to the use of such vectors for protein production. The influence of the RNA silencing pathway on the severity of symptoms resulting from hypovirus infection has implications for the use of mycoviruses for biological control. It may be possible to manipulate the RNA silencing pathway, for example, by the incorporation or removal of virus-encoded suppressors of RNA silencing, so that mycovirus infection still reduces the virulence of plant pathogenic fungi without negatively affecting ecological fitness and ability of hypovirulent strains to spread and persist in the ecosystem following introduction. An additional intriguing possibility follows from the observation by Biella et al. (2002) that hypovirus infection influences the frequency of vic-associated PCD. Could there be a link between the interactions of hypoviruses with the two principal antiviral defense strategies of vic and RNA silencing?
References
- Almeida R, Allshire RC. RNA silencing and genome regulation. Trends Cell Biol. 2005;15:251–258. doi: 10.1016/j.tcb.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Anagnostakis SL. Biological control of chestnut blight. Science. 1982;215:466–471. doi: 10.1126/science.215.4532.466. [DOI] [PubMed] [Google Scholar]
- Anagnostakis SL. Conversion to curative morphology in Endothia parasitica and its restriction by vegetative compatibility. Mycologia. 1983;75:777–780. [Google Scholar]
- Biella S, Smith ML, Aist JR, Cortesi P, Milgroom MG. Programmed cell death correlates with virus transmission in a filamentous fungus. Proc R Soc Lond Ser B. 2002;269:2269–2276. doi: 10.1098/rspb.2002.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkovich KA, Alex LA, Yarden O, Freitag M, Turner GE, Read ND, Seiler S, Bell-Pedersen D, Paietta J, Plesofsky N, Plamann M, Goodrich-Tanrikulu M, et al. Lessons from the genome sequence of Neurospora crassa: Tracing the path from genome blueprint to muticellular organism. Microbiol Mol Biol Rev. 2004;68:1–108. doi: 10.1128/MMBR.68.1.1-108.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington JC, Ambrose V. Role of microRNAs in plant and animal development. Science. 2003;301:336–338. doi: 10.1126/science.1085242. [DOI] [PubMed] [Google Scholar]
- Catalanotto C, Azzalin G, Macino G, Cogoni C. Gene silencing in worms and fungi. Nature. 2000;404:245. doi: 10.1038/35005169. [DOI] [PubMed] [Google Scholar]
- Caten CE. Vegetative incompatibility and cytoplasmic infection in fungi. J Gen Microbiol. 1972;72:221–229. doi: 10.1099/00221287-72-2-221. [DOI] [PubMed] [Google Scholar]
- Chen B, Nuss DL. Infectious cDNA clone of hypovirus CHV1-Euro7: A comparative virology approach to investigate virus-mediated hypovirulence of the chestnut blight fungus Cryphonectria parasitica. J Virol. 1999;73:985–992. doi: 10.1128/jvi.73.2.985-992.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Choi GH, Nuss DL. Attenuation of fungal virulence by synthetic infectious hypovirus transcripts. Science. 1994;264:1762–1764. doi: 10.1126/science.8209256. [DOI] [PubMed] [Google Scholar]
- Chen B, Geletka LM, Nuss DL. Using chimeric hypoviruses to fine-tune the interaction between a pathogenic fungus and its plant host. J Virol. 2000;74:7562–7567. doi: 10.1128/jvi.74.16.7562-7567.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi GH, Nuss DL. Hypovirulence of chestnut blight fungus conferred by an infectious cDNA. Science. 1992;257:800–803. doi: 10.1126/science.1496400. [DOI] [PubMed] [Google Scholar]
- Choudhary S, Lee HC, Maiti M, He Q, Cheng P, Liu Q, Lui Y. A double-stranded RNA response program important for RNA interference efficiency. Mol Cell Biol. 2007;27:3995–4005. doi: 10.1128/MCB.00186-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill ACL, Ciufetti LM, Hansen DR, Van Etten NK. Transformation of the fungal pathogen Cryphonectria parasitica with a variety of heterologous plasmids. Curr Genet. 1990;17:25–31. [Google Scholar]
- Cogoni C, Macino G. Gene silencing in Neurospora crassa requires a protein homologous to RNA-dependent RNA polymerase. Nature. 1999;399:166–169. doi: 10.1038/20215. [DOI] [PubMed] [Google Scholar]
- Cortesi P, Milgroom MG. Genetics of vegetative incompatibility in Cryphonectria parasitica. Appl Environ Microbiol. 1998;64:2988–2994. doi: 10.1128/aem.64.8.2988-2994.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortesi P, McCulloch CE, Song H, Lin H, Milgroom MG. Genetic control of horizontal virus transmission in the chestnut blight fungus, Cryphonectria parasitica. Genetics. 2001;159:107–118. doi: 10.1093/genetics/159.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven MG, Pawlyk DM, Choi GH, Nuss DL. Papain-like protease p29 as a symptom determinant encoded by a hypovirulence-associated virus of the chestnut blight fungus. J Virol. 1993;67:6513–6521. doi: 10.1128/jvi.67.11.6513-6521.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RH, Perkins DD. Timeline: Neurospora: A model of model microbes. Nat Rev Genet. 2002;3:397–403. doi: 10.1038/nrg797. [DOI] [PubMed] [Google Scholar]
- Dawe VH, Kuhn CW. Isolation and characterization of a double-stranded DNA mycovirus infecting the aquatic fungus Rhizidomyces. Virology. 1983;130:21–28. doi: 10.1016/0042-6822(83)90114-9. [DOI] [PubMed] [Google Scholar]
- Dawe AL, Nuss DL. Hypoviruses and chestnut blight: Exploiting viruses to understand and modulate fungal pathogenesis. Annu Rev Genet. 2001;35:1–29. doi: 10.1146/annurev.genet.35.102401.085929. [DOI] [PubMed] [Google Scholar]
- Dawe AL, McMains VC, Panglao M, Kasahara S, Chen B, Nuss DL. An ordered collection of expressed sequences from Cryphonectria parasitica and evidence of genomic microsynteny with Neurospora crassa and Magnaporthe grisea. Microbiology. 2003;149:2373–2384. doi: 10.1099/mic.0.26371-0. [DOI] [PubMed] [Google Scholar]
- De Haro JP, Calo S, Cervantes M, Nicolas FE, Torres-Martinez S, Ruiz-Vazquez RM. A single dicer gene is required for efficient gene silencing associated with two classes of small antisense RNAs in Mucor circinelloides. Eukaryot Cell. 2009;8:1486–1497. doi: 10.1128/EC.00191-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding SW. RNA-based antiviral immunity. Nat Rev Immunol. 2010;10:632–644. doi: 10.1038/nri2824. [DOI] [PubMed] [Google Scholar]
- Ding SW, Voinnet O. Antiviral immunity directed by small RNAs. Cell. 2007;130:413–426. doi: 10.1016/j.cell.2007.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghabrial SA, Suzuki N. Viruses of plant pathogenic fungi. Annu Rev Phytopathol. 2009;47:353–384. doi: 10.1146/annurev-phyto-080508-081932. [DOI] [PubMed] [Google Scholar]
- Guo LH, Sun L, Chiba S, Araki H, Suzuki N. Coupled termination/reinitiation for translation of the downstream open reading frame B of the prototypic hypovirus CHV1-EP713. Nucleic Acids Res. 2009;37:3645–3659. doi: 10.1093/nar/gkp224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond SM. Dicing and slicing: The core machinery of the RNA interference pathway. FEBS Lett. 2005;579:5822–5829. doi: 10.1016/j.febslet.2005.08.079. [DOI] [PubMed] [Google Scholar]
- Hammond TM, Andrewski MD, Roossinck MJ, Keller NP. Aspergillus mycoviruses are targets and suppressors of RNA silencing. Eukaryot Cell. 2008a;7:350–357. doi: 10.1128/EC.00356-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond TM, Bok JW, Andrewski MD, Reyes-Dominquez Y, Scazzocchio C, Keller NP. RNA silencing gene truncation in the filamentous fungus Aspergillus nidulans. Eukaryot Cell. 2008b;7:339–349. doi: 10.1128/EC.00355-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiniger U, Rigling D. Biological control of chestnut blight in Europe. Annu Rev Phytopathol. 1994;32:581–599. [Google Scholar]
- Hillman BI, Suzuki N. Viruses of the chestnut blight fungus. Adv Virus Res. 2004;63:423–473. doi: 10.1016/S0065-3527(04)63007-7. [DOI] [PubMed] [Google Scholar]
- Huang AS, Baltimore D. Defective viral particles and viral disease processes. J Mol Biol. 1970;47:275–291. doi: 10.1038/226325a0. [DOI] [PubMed] [Google Scholar]
- Huber DH. PhD thesis. Michigan State University; East Lansing, MI: 1996. Genetic analysis of vegetative incompatibility polymorphisms and horizontal transmission in the chestnut blight fungus Cryphonectria parasitica. [Google Scholar]
- Huber DH, Fulbright DW. Preliminary investigations on the effect of individual genes upon the transmission of dsRNA in Cryphonectria parasitica. In: Double ML, MacDonald WL, editors. Proceedings of the International Chestnut Conference. West Virginia University Press; Morgantown, WV: 1994. pp. 15–19. [Google Scholar]
- Jaag HM, Lu Q, Schmitt ME, Nagy PD. Role of RNase MRP in viral RNA degradation and RNA recombination. J Virol. 2011;85:243–253. doi: 10.1128/JVI.01749-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonin EV, Choi GH, Nuss DL, Shapira R, Carrington JC. Evidence for a common ancestry of a chestnut blight hypovirulence-associated double-stranded RNA and a group of positive-stranded RNA plant viruses. Proc Natl Acad Sci USA. 1991;88:10647–10651. doi: 10.1073/pnas.88.23.10647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan X, Yao Z, Zhou Y, Shang J, Lin H, Nuss DL, Chen B. Deletion of the cpku80 gene in the chestnut blight fungus, Cryphonectria parasitica, enhances gene disruption efficiency. Curr Genet. 2008;53:59–66. doi: 10.1007/s00294-007-0162-x. [DOI] [PubMed] [Google Scholar]
- Lee SG, Kim DY, Hyun BH, Bae YS. Novel design architecture for genetic stability of recombinant poliovirus: The manipulation of G/C contents and their distribution patterns increases the genetic stability of inserts in a poliovirus-based RPS-Vax vector system. J Virol. 2002;76:1649–1662. doi: 10.1128/JVI.76.4.1649-1662.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DW, Pratt RJ, McLaughlin M, Aramayo R. An argonaute-like protein is required for meiotic silencing. Genetics. 2003;164:821–828. doi: 10.1093/genetics/164.2.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HC, Li L, Gu W, Xue Z, Crosthwaite SK, Pertsemlidis A, Lewis Z, Freitag M, Selker EU, Mello C, Liu Y. Diverse pathways generate microRNA-like RNAs and dicer-independent small interfering RNAs in fungi. Mol Cell. 2010;38:1–12. doi: 10.1016/j.molcel.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie JF, Zeller KA. Heterokaryon incompatibility in fungi: More than just another way to die. J Genet. 1996;75:415–424. [Google Scholar]
- Li L, Chang SS, Liu Y. RNA interference pathways in filamentous fungi. Cell Mol Life Sci. 2010;67:3849–3863. doi: 10.1007/s00018-010-0471-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Lan X, Liao H, Parsley TB, Nuss DL, Chen B. Genome sequence, full-length infectious cDNA clone, and mapping of viral double-stranded RNA accumulation determinant of hypovirus CHV1-EP721. J Virol. 2007;81:1813–1820. doi: 10.1128/JVI.01625-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallory AC, Vaucheret H. Functions of microRNAs and related small RNAs in plants. Nat Genet. 2006;38(Suppl):S31–S36. doi: 10.1038/ng1791. [DOI] [PubMed] [Google Scholar]
- Marquez LM, Redman RS, Rodriguez RJ, Roossinck MJ. A virus in a fungus in a plant: Three-way symbiosis required for thermal tolerance. Science. 2007;315:513–515. doi: 10.1126/science.1136237. [DOI] [PubMed] [Google Scholar]
- Milgroom MG, Cortesi P. Biological control of chestnut blight with hypovirulence: A critical analysis. Annu Rev Phytopathol. 2004;42:311–338. doi: 10.1146/annurev.phyto.42.040803.140325. [DOI] [PubMed] [Google Scholar]
- Murata T, Kadotani N, Yamaguchi M, Tosa M, Mayama S, Nakayashiki H. siRNA-dependent and-independent post-transcriptional cosuppression of the LTR-retrotransposon MAGGY in the phytopathogenic fungus Magnaporthe oryzae. Nucleic Acids Res. 2007;35:5987–5994. doi: 10.1093/nar/gkm646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy PD, Simon AE. New insights into the mechanisms of RNA recombination. Virology. 1997;235:1–9. doi: 10.1006/viro.1997.8681. [DOI] [PubMed] [Google Scholar]
- Nakayashiki H, Nguyen QB. RNA interference: Roles in fungal biology. Curr Opin Microbiol. 2008;11:494–502. doi: 10.1016/j.mib.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Nicolas FE, de Haro JP, Torres-Martinez S, Ruiz-Vazquez RM. Mutants defective in a Mucor circinelloides dicer-like gene are not compromised in siRNA silencing but display developmental defects. Fungal Genet Biol. 2007;44:504–516. doi: 10.1016/j.fgb.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Nicolas FE, Moxon S, de Haro JP, Calo S, Grigoriev IV, Torres-Martinez S, Moulton V, Ruiz-Vqzquez RM, Dalmay T. Endogenous short RNAs generated by Dicer-2 and RNA-dependent RNA polymerase 1 regulates mRNAs in the basal fungus Mucor circinelloides. Nucleic Acids Res. 2010;38:5535–5541. doi: 10.1093/nar/gkq301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan T, Braccini L, Azzalin G, De Toni A, Macino G, Cogoni C. The post-transcriptional gene silencing machinery functions independently of DNA methylation to repress a LINE1-like retrotransposon in Neurospora crassa. Nucleic Acids Res. 2005;33:1564–1573. doi: 10.1093/nar/gki300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuss DL. Biological control of chestnut blight: An example of virus-mediated attenuation of fungal pathogenesis. Microbiol Rev. 1992;56:561–576. doi: 10.1128/mr.56.4.561-576.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuss DL. Hypovirulence: Mycoviruses at the fungal-plant interface. Nat Rev Microbiol. 2005;3:632–642. doi: 10.1038/nrmicro1206. [DOI] [PubMed] [Google Scholar]
- Nuss DL. Mycoviruses. In: Borkovich KA, Ebbole DJ, editors. Cellular and Molecular Biology of Filamentous Fungi. ASM Press; Washington, DC: 2010. pp. 145–152. [Google Scholar]
- Paar M, Klein D, Saimons B, Gunzburg WH, Renner M, Portsmouth D. Effects of viral strain, transgene position, and target cell type on replication kinetics, genome stability, and transgene expression of replication-competent murine leukemia virus-based vectors. J Virol. 2007;81:6973–6983. doi: 10.1128/JVI.02470-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parameswaran P, Sklan E, Wilkins C, Burgon T, Samuel MA, Lu R, Ansel KM, Heissmeyer V, Einav S, Jackson W, Doukas T, Paranjape S, et al. Six RNA viruses and forty-one hosts: Viral small RNAs and modulation of small RNA repertories in vertebrate and invertebrate systems. PLoS Pathog. 2010;6:e1000764. doi: 10.1371/journal.ppat.1000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak KB, Nagy PD. Defective interfering RNAs: Foes of viruses and friends of virologist. Viruses. 2009;1:895–919. doi: 10.3390/v1030895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson MN, Beever RE, Boine B, Arthur K. Mycoviruses of filamentous fungi and their relevance to plant pathology. Mol Plant Pathol. 2009;10:115–128. doi: 10.1111/j.1364-3703.2008.00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruss G, Ge X, Shi X, Carrington JC, Vance VB. Plant viral synergism: The potyviral genome encodes a broad-range pathogenicity enhancer that transactivates replication of heterologous viruses. Plant Cell. 1996;9:859–868. doi: 10.1105/tpc.9.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux L, Simon AE, Holland JJ. Effects of defective interfering viruses on virus replication and pathogenesis. Adv Virus Res. 1991;40:181–211. doi: 10.1016/S0065-3527(08)60279-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segers GC, van Wezel R, Zhang X, Hong Y, Nuss DL. Hypovirus papain-like protease p29 suppresses RNA silencing in the natural fungal host and in a heterologous plant system. Eukaryot Cell. 2006;5:896–904. doi: 10.1128/EC.00373-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segers GC, Zhang X, Deng F, Sun Q, Nuss DL. Evidence that RNA silencing functions as an antiviral defense mechanism in fungi. Proc Natl Acad Sci USA. 2007;104:12902–12906. doi: 10.1073/pnas.0702500104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira R, Choi GH, Nuss DL. Virus-like genetic organization and expression strategy for double-stranded RNA genetic element associated with biological control of chestnut blight. EMBO J. 1991a;10:731–739. doi: 10.1002/j.1460-2075.1991.tb08004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira R, Choi GH, Hillman BI, Nuss DL. The contribution of defective RNAs to the complexity of virus-encoded double-stranded RNA populations present in hypovirulent strains of the chestnut blight fungus Cryphonectria parasitica. EMBO J. 1991b;10:741–746. doi: 10.1002/j.1460-2075.1991.tb08005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon AE, Roossinck MJ, Havelda Z. Plant virus satellite and defective interfering RNAs: New paradigms for a new century. Ann Rev Phytopathol. 2004;42:415–437. doi: 10.1146/annurev.phyto.42.040803.140402. [DOI] [PubMed] [Google Scholar]
- Song JJ, Joshua-Tor L. Argonaute and RNA-getting into the groove. Curr Opin Struct Biol. 2006;16:5–11. doi: 10.1016/j.sbi.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Sun L, Nuss DL, Suzuki N. Synergism between a mycoreovirus and a hypovirus mediated by the papain-like protease p29 of the prototypic hypovirus CHV1-EP713. J Gen Virol. 2006;87:3703–3714. doi: 10.1099/vir.0.82213-0. [DOI] [PubMed] [Google Scholar]
- Sun Q, Choi GH, Nuss DL. A single Argonaute gene is required for induction of RNA silencing antiviral defense and promotes viral RNA recombination. Proc Natl Acad Sci USA. 2009;106:17927–17932. doi: 10.1073/pnas.0907552106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Nuss DL. The contribution of p40 to hypovirus-mediated modulation of fungal host phenotype and viral RNA accumulation. J Virol. 2002;144:260–267. doi: 10.1128/JVI.76.15.7747-7759.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Geletka LM, Nuss DL. Essential and dispensable virus-encoded replication elements revealed by efforts to develop hypoviruses as gene expression vectors. J Virol. 2000;74:7568–7577. doi: 10.1128/jvi.74.16.7568-7577.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Maruyama K, Moriyama M, Nuss DL. Hypovirus papain-like protease p29 functions in trans to enhance viral double-stranded RNA accumulation and virus transmission. J Virol. 2003;77:11697–11707. doi: 10.1128/JVI.77.21.11697-11707.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia J, Paul CP, Fulbright DW, Nuss DL. Structural properties of double-stranded RNAs associated with biological control of chestnut blight fungus. Proc Natl Acad Sci USA. 1986;83:9109–9113. doi: 10.1073/pnas.83.23.9109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Born E, Posthuma CC, Knoops K, Snijder EJ. An infectious recombinant equine arteritis virus expressing green fluorescent protein from its replicase gene. J Gen Virol. 2007;88:1196–1205. doi: 10.1099/vir.0.82590-0. [DOI] [PubMed] [Google Scholar]
- Wu Q, Wang X, Ding SW. Viral suppressors of RNA-based viral immunity: Host targets. Cell Host Microbe. 2010;8:12–15. doi: 10.1016/j.chom.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Li B, Fu Y, Jiang D, Ghabrial SA, Li G, Peng Y, Xie J, Cheng J, Huang J, Yi X. A geminivirus-related DNA mycovirus that confers hypovirulence to a plant pathogenic fungus. Proc Natl Acad Sci USA. 2010;107:8387–8392. doi: 10.1073/pnas.0913535107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Nuss DL. A host dicer is required for defective viral RNA production and recombinant virus vector RNA instability for a positive sense RNA virus. Proc Natl Acad Sci USA. 2008;105:16749–16754. doi: 10.1073/pnas.0807225105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Segers G, Sun Q, Deng F, Nuss DL. Characterization of hypovirus-derived small RNAs generated in the chestnut blight fungus by an inducible DCL-2 dependent pathway. J Virol. 2008;82:2613–2619. doi: 10.1128/JVI.02324-07. [DOI] [PMC free article] [PubMed] [Google Scholar]