Abstract
Adult numbers and sizes of mosquitoes were monitored for 2 yr in neighboring habitats on the western coast of Raiatea (Society Archipelago) in anticipation of testing new vector control technologies. Aedes polynesiensis Marks females comprised the overwhelming majority (≈99%) of the three species of mosquitoes captured in Biogent Sentinel traps placed at fixed sites on three small satellite islands (motus) of the western lagoon and on the shoreline of Raiatea. Aedes polynesiensis males, Aedes aegypti (L.), and Culex quinquefasciatus Say rarely were collected. Numbers of Ae. polynesiensis females per collection differed among trapping dates and locations, with the majority of females captured on two motus, Horea and Toamaro. Shoreline and Horea females had significantly longer mean wing lengths than females from Tiano and Toamaro. Thus, wing lengths were influenced more by local developmental conditions than overall numbers of adults. Significantly more females were captured during the wet season than the dry season. Nonetheless, at least on the two highly productive motus, dry-season females had larger wing lengths than their wet season counterparts. Local weather patterns predicted about half the variation in mosquito numbers. Differences in vector abundance observed when comparing neighboring motus are likely because of differences in human activity and mosquito suppression.
Keywords: Aedes polynesiensis population dynamic, filariasis vector, trap sampling, seasonality, adult size variation
Aedes polynesiensis Marks (Diptera: Culicidae) is the primary vector of Wuchereria bancrofti (Cobbold) (Spirurida: Onchocercidae), the causative agent of human lymphatic filariasis (Rosen 1955, Laigret et al. 1965), and a secondary vector of dengue in French Polynesia (Rosen et al. 1954, Maguire et al. 1971). Host seeking mostly is diurnal and outdoors, with peaks of activity at dawn and dusk (Russell 2004). Aedes polynesiensis uses a variety of natural and artificial containers for development, complicating vector control (Lardeux et al. 2002). Like many container-breeding mosquitoes, cohorts of Ae. polynesiensis respond to overcrowding and resource limitation during larval development through reduced survival, skewed sex ratios, and smaller size at emergence and delayed emergence, especially for females (Mercer 1999, Mercer et al. 2005).
Despite prolonged mass administration of prophylactic drugs (MDA) against W. bancrofti, lymphatic filariasis persists in French Polynesia (Esterre et al. 2001, Plichart et al. 2006). It has been hypothesized that the biology of Ae. polynesiensis contributes to the failure of MDA in these archipelagoes despite success elsewhere (Burkot et al. 2002, Lardeux et al. 2002, Pichon 2002). Aedes polynesiensis females are more efficient at transmission (which requires development of the parasite but is nonpropagative in the vector) as the number of ingested microfilariae decreases (as occurs during MDA) (Rosen 1955, Pichon et al. 1974, Failloux et al. 1995, Stolk et al. 2004). The coupling of MDA with effective vector control may be required to upset disease transmission (Burkot et al. 2002, Pichon 2002, Brelsfoard et al. 2008, Brelsfoard et al. 2009).
The small islands of the archipelago lend themselves to studies of vector biology on replicated sites. Raiatea is the largest of the Leeward Islands in French Polynesia and serves as the regional administrative and public health center for the western Society Archipelago. Like many “high islands” of the South Pacific, the lagoon of Raiatea contains several small satellite islands (motus) associated with the surrounding coral reef. Natural dispersal of Ae. polynesiensis is augmented across short and long distances by commercial trade; therefore, genetic differences are influenced by human transport in addition to mosquito flight distances, weather patterns, and natural barriers (Failloux et al. 1997). There is sufficient genetic exchange between Raiatea and its surrounding motus to maintain genetic homogeneity (Shiu et al. 1997).
In addition to Ae. polynesiensis, nine mosquito species are reported from the Society Archipelago (Belkin 1962, Huang 1977, Russell 2004, Brunes and Boussès 2009). Although Culex quinquefasciatus Say is common on numerous islands of French Polynesia and shares many developmental habitats with Ae. polynesiensis, the species is not an important vector of the subperiodic form of W. bancrofti found in French Polynesia (Iyengar 1965, Lardeux et al. 2002). The peridomestic species Aedes aegypti (L.), the primary vector of dengue in French Polynesia, is common in urban areas and villages of French Polynesia but less common in more rural settings where Ae. polynesiensis predominates. Culex annulirostris Skuse serves as a vector of encephalitis viruses elsewhere (Kay et al. 1984) and nocturnal periodic filariasis in New Guinea (Belkin 1962) but is not competent for the subperiodic form found in the eastern archipelagoes of the South Pacific (Lee et al. 1989). Most of the other mosquito species are rare endemics with limited distributions and unknown vector status. Although it is imperative to monitor unintended changes in nontarget species (including disease vectors) during control efforts, these species were not of primary interest in the current study.
We implemented a surveillance program for Ae. polynesiensis in Raiatea in anticipation of testing new vector-control technologies to augment MDA against filariasis in French Polynesia. We studied natural variation in mosquito numbers and sizes across time on four neighboring locations. The climate of Raiatea is subtropical oceanic with distinct hot, humid (December–February) and cooler, dry (March–November) seasons (Meyer 1996). Thus, we monitored adult numbers and size and local weather conditions of Raiatea during a 2-yr trapping study.
Methods
Study Sites
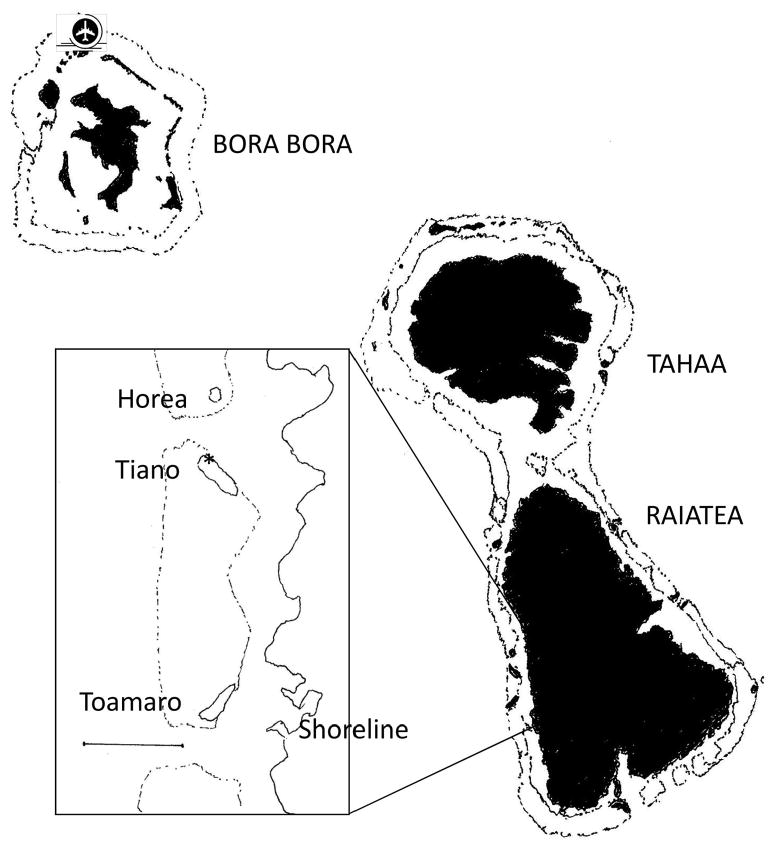
Three small neighboring motus and an adjacent shoreline location along the western coast of Raiatea (Tehurui and Vaiaau villages in the associated commune of Tumaraa) were the focus of this study (Fig. 1). Horea is the smallest of the motus surveyed (area = 1.41 ha; perimeter = 443 m; area/perimeter = 31.8, estimates using ArcView 9.2, ESRI Corp., Redlands, CA). This circular motu is held publicly, but hosted up to 20 temporary structures during the study and is semipermanently inhabited by area residents for fishing and holidays during which insecticides and wood smoke are used for mosquito control. The vegetation is typical of Polynesian islands with 60–80% canopy cover. An herbaceous layer is present for most of the motu. Mosquito developmental sites include artificial containers (e.g., appliances, buckets, cans, glass bottles, plastic and polystyrene containers, tires); coconuts; spathes; phytotelmata (tree holes, plant axils, and stumps); and burrows of the land crab Cardisoma carnifex Herbst (Decapoda: Gecarcinidae). Permanent blood sources include dogs, rats (although the relative paucity of rat-chewed coconuts suggests low densities), chickens, and other birds and humans.
Fig. 1.
Location of study sites (inset) relative to Raiatea and airport weather station, Society Archipelago, French Polynesia. Bar in inset represents 1 km.
Tiano is owned privately with three permanent structures. This elongate motu is the largest of the three included in the survey (area = 6.34 ha, perimeter = 1,169 m) and presents the greatest area relative to shoreline (area/perimeter = 54.2). The island is managed extensively against mosquitoes (source reduction and pesticides) with open (<20% cover) canopy centrally and denser (80–90%) natural canopy around the edge, particularly at the apices. The central portion of Tiano consists mostly of coconut palms (metal banded, to exclude rats). Putative mosquito developmental sites are limited to cryptic tree holes, crab burrows, and a temporary pool with stagnant freshwater adjacent to the dwellings. Blood sources include rats, humans, dogs, and birds.
Toamaro also is privately owned and has two structures used infrequently. The elongate motu is intermediate in size relative to the others (area = 4.06 ha, perimeter = 1,052 m, area/perimeter = 38.6), but has a more complete canopy of native vegetation (70–95%) and greater stratification with well-developed herbaceous and shrub layers. The island is managed occasionally with vegetation clearing, insecticide application, and burning during collection of coconuts to produce copra oil. Mosquito developmental sites are plentiful and include artificial containers, coconuts, phytotelmata, and numerous crab burrows. Blood sources include rats, birds, and occasionally dogs, cats, and humans. Closest linear distances from motu to shoreline for Horea, Tiano, and Toamaro were 700, 600, and 526 m, respectively, as estimated using Google Earth (Version 5.0, Google Inc., Mountain View, CA).
Adult Trapping
Trapping of adult mosquitoes was initiated on 17 August 2007. Biogent Sentinel (BiogentsAG, Regensburg, Germany) traps were hung from trees with openings at a height of 0.8 m at three fixed sites on each of the three motus; adjacent sites were spaced 50–175 m. Traps were placed at the apices and centers of the elongate motus (Tiano and Toamaro) and as far apart as possible on circular Horea; traps were placed amid habitat that was representative of vegetational coverage and putative larval developmental sites (including C. carnifex burrows) for each of the motus.
The traps were powered by rechargeable 12-V sealed gel-cell batteries. To ensure uniformity of capture, trap opening air velocities were confirmed to be >3 m/s by using a handheld anemometer (La Crosse Technology, Ltd., LA Crosse, WI) at the beginning and ending of each trapping period; spare batteries were available for replacement if velocities were too low. The orders of motu visitation and trap placements, as well as the trap numbers and batteries, were randomly assigned to reduce systematic error. Trapping was done during 20-min intervals; all trapping was completed during the morning peak of host-seeking activity reported for Ae. polynesiensis. Trapping was repeated at 14-d intervals (with the exception of two dates missed during October 2007 and January 2008) until 13 August 2009.
Adults also were captured in two (beginning 1 February 2008) and then three (22 May 2009) fixed shoreline sites along the western lagoon coast of Raiatea facing Toamaro. These representative sites include the roadside adjacent to the coast, under an open-sided garage, and within a seasonally flooded mudflat bordered with dense vegetation and a high density of crab burrows, respectively at 10-, 75-, and 170-m inland. Cover is sparse (10–40%) and consists of native, ornamental, and cultivated vegetation at the sites nearer the coast, whereas the native vegetation is dense (80–90%) at the inland site. Because of logistic constraints, the motus were visited sequentially in a randomly determined order, whereas those on the shoreline were visited first or last on any given trapping day.
Adult Size
Captured mosquitoes were identified, sex determined, and counted for each collection period. Wing lengths were measured for a subset of captured Ae. polynesiensis (N ≥ 10 per trap when available) by location and collection date by using a stereomicroscope fitted with a micrometer scale. Mean Ae. polynesiensis female numbers were compared among dates, locations, sites, trap numbers, and order of collection by using Wilcoxon rank sums and location, season, and their interaction by using the multiple regression model in JMP 9.0.1 software (SAS Institute, Cary, NC).
Weather Data
A stand-alone HOBO weather station (H21-SYS-A, Onset Computer Corporation, Pocasset, MA) was installed on 26 July 2007 in a clearing toward the northwestern apex of Tiano (16° 49′ 44.85″ S, 159° 29′ 21.4″ W); not only was this site central for the three motus, it offered the best security for the weather station because of the private status of Tiano. Probes for rainfall, barometric pressure, solar radiation, wind speed, wind gust speed, wind direction, air temperature, relative humidity, and subsurface soil temperature (−50 cm) collected data at 10-min intervals. An Onset HOBO U-Shuttle (U-DT-1, Onset Computer Corporation, Pocasset, MA) was used to download data during mosquito trapping on Tiano. Linear distances were estimated from the weather station to the center of Horea (0.8 km), Toamaro (2.2 km), and the Raiatea shoreline (2.8 km) (Google Earth 5.0).
Weather data were analyzed using HOBOware Pro Software Version 2.7.3 (Onset Computer Corporation, Pocasset, MA). Maxima, minima, means, and standard deviations were estimated for the 10-min logger intervals for each variable across the entire sampling period. In addition, the “Filter Series” of HOBO software was used to generate the maximum, minimum, and average (and total for rainfall) values for each measurement during biweekly intervals. Spearman rank correlations were run on all pair-wise means for weather data and for total mosquitoes collected concurrently. Because mosquito collections were not always concordant with the 14-d intervals upon which weather data were analyzed, mosquito collection periods were matched to the nearest preceding biweekly weather period.
Results
Adult Trapping
During 2 yr of collections, 13,865 adult mosquitoes in total were captured in BG-Sentinel traps on the three motus during the 51 collection periods (i.e., 153 samples). Three species were captured (Ae. polynesiensis, Ae. aegypti, and Cx. quinquefasciatus), with Ae. polynesiensis females making up 98.9% of the total (Table 1). By comparison, male Ae. polynesiensis were rare (125 individuals), representing 0.90% of trapped individuals. Aedes aegypti and Cx. quinquefasciatus were rarer still; males slightly outnumbered females for Ae. aegypti and both Cx. quinquefasciatus individuals captured were males. An Ae. aegypti female was captured for the first time on Tiano more than a year after initiation of collections (5 January 2009). Therefore, all three species were recovered from Tiano and Toamaro, whereas no Cx. quinquefasciatus was captured on Horea.
Table 1.
Total mosquitoes captured in BG-Sentinel traps on three Raiatea motus (17 August 2007–13 August 2009)
| Species and sex | Total | Mean (SE)a | Maxa | % total |
|---|---|---|---|---|
| Ap female | 13,713 | 29.682 (2.479) | 428 | 98.90 |
| Ap male | 125 | 0.271 (0.035) | 7 | 0.90 |
| Ae female | 11 | 0.024 (0.007) | 1 | 0.08 |
| Ae male | 14 | 0.030 (0.008) | 1 | 0.10 |
| Cx female | 0 | 0 | 0 | 0.00 |
| Cx male | 2 | 0.004 (0.003) | 1 | 0.01 |
| Total | 13,865 | 30.011 (1.396) | 431 | 100.00 |
Ap, Aedes polynesiensis; Ae, Aedes aegypti; Cx, Culex quinquefasciatus.
Per trap per collection.
An additional 569 adults were captured during 33 collection periods (i.e., 97 samples) at the Raiatea shoreline sites. All three species were captured, although the duration of collection period was shorter (Table 2). Aedes polynesiensis females again represented the majority of mosquitoes captured at the shoreline, although their proportion (88.8%) was lower relative to Ae. aegypti males (4.5%), Ae. polynesiensis males (3.8%), Ae. aegypti females (1.0%), and Cx. quinquefasciatus males (0.2%). As on the motus, no female Cx. quinquefasciatus was trapped during these morning collections.
Table 2.
Mosquitoes collected in BG-Sentinel traps on three Raiatea motus (17 August 2007–13 August 2009) and adjacent shoreline (1 February 2008–13 August 2009)
| Location | Ap females | Ap males | Ae females | Ae males | Cx males | Total |
|---|---|---|---|---|---|---|
| Toamaro | 54.6 (5.3)a | 0.418 (0.07)a | 0.039 (0.02)a | 0.046 (0.02)a | 0.007 (0.01)a | 55.08 (5.36)a |
| Horea | 31.6 (4.3)b | 0.288 (0.07)ab | 0.026 (0.01)a | 0.026 (0.01)a | 0 | 31.91 (4.35)b |
| Tiano | 3.49 (3.6)c | 0.111 (0.04)b | 0.007 (0.01)a | 0.020 (0.01)a | 0.007 (0.01)a | 3.63 (0.51)c |
| Shoreline | 5.30 (4.6)c | 0.227 (0.11)ab | 0.062 (0.04)a | 0.268 (0.13)a | 0.010 (0.01)a | 5.87 (0.89)c |
Numbers shown represent mean (SE) mosquitoes per trap per 20-min sampling period. Means followed by different letters within a column are significantly different by Tukey’s HSD on ranks.
Ap, Aedes polynesiensis; Ae, Aedes aegypti; Cx, Culex quinquefasciatus.
Numbers of Ae. polynesiensis females correlated significantly with Ae. polynesiensis males (Spearman’s rank correlation ρ = 0.432, N = 187, P < 0.0001) and Ae. aegypti females (ρ = 0.148, N = 187, P = 0.045) among the trap collections from the same date. All three species were captured in the same trap only once (17 July 2008 on the shoreline).
Because Ae. polynesiensis females comprised ≈99% of adults captured overall, subsequent comparisons will focus on this species, although statistical outcomes for total mosquitoes were identical. Numbers of Ae. polynesiensis captured per trap per collection period were not normally distributed (Shapiro–Wilk’s W = 0.559, P < 0.0001). Mean numbers of females differed by date of collection (Wilcoxon rank sum χ2 = 119.7, df = 50, P < 0.0001). Relative peaks of total adult collections occurred between March and June 2008 and December 2008 and April 2009 (Fig. 2), but trap catches were not significantly correlated for similar dates between years (Pearson’s correlation coefficient r = −0.0053, P > 0.05). The strength of the correlation was improved little by shifting time frames by 1 or 2 wk to account for any time lag between rainfall and adult emergence (data not shown). Among the four trapping locations, mosquito numbers were significantly correlated for corresponding collection dates between years only for Tiano (Pearson’s r = 0.431, N = 25, P = 0.031).
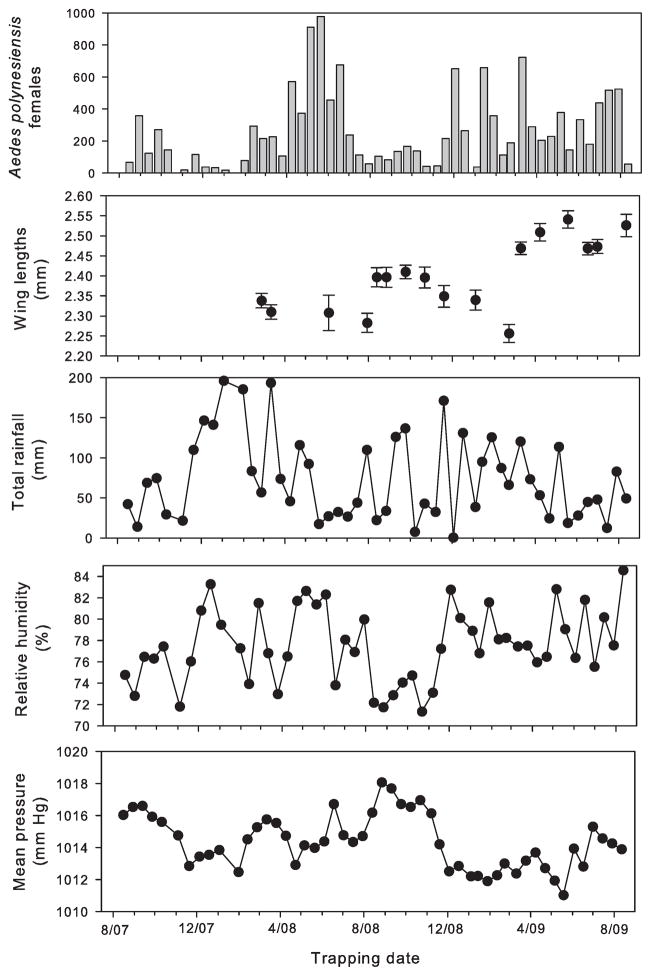
Fig. 2.
(A) Total Ae. polynesiensis females collected in BG-Sentinel traps on three Raiatea motus, (B) Ae. polynesiensis mean (SE) female wing lengths captured per date, (C) Total rainfall, (D) Mean relative humidity, and (E) Mean barometric pressure for each sample period.
Average numbers of Ae. polynesiensis females captured per collection date (across locations and traps) were highly variable but equivalent (Student’s t = −0.088, P = 0.93) during the first (mean ± SD = 267.92 ± 273.78) and second (260.0 ± 201.0) years (Fig. 2). Trap catches on a given date (across both years) were significantly correlated for Toamaro × Horea (ρ = 0.356, N = 51, P = 0.010); Toamaro × Tiano (ρ = 0.508, N = 51, P = 0.0001); and Tiano × shoreline (ρ = 0.549, N = 33, P = 0.001).
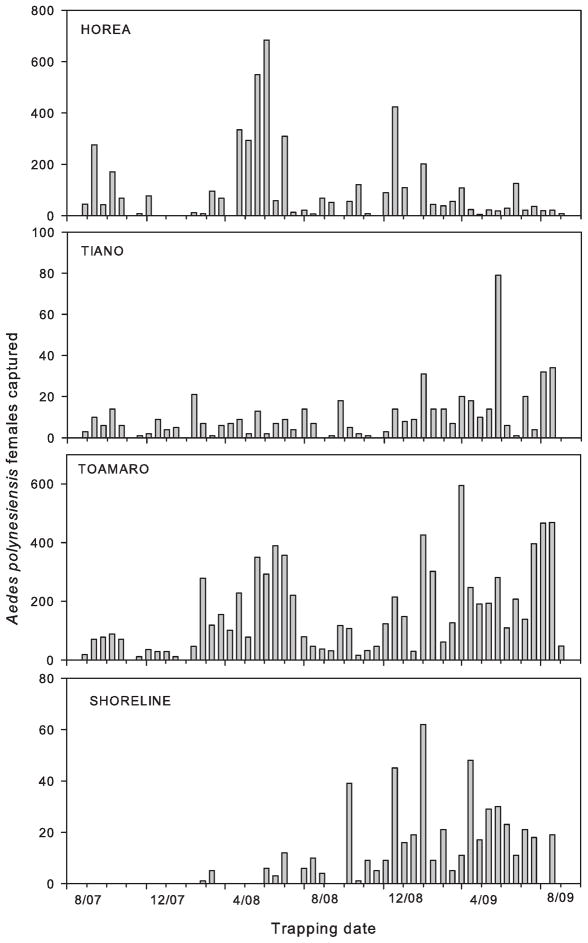
Collections also differed by location (χ2 = 168.1, df = 3, P < 0.0001) with significantly more Ae. polynesiensis females and males captured on Toamaro than on Tiano or the shoreline (Table 2). Mean numbers of Ae. polynesiensis captured on Horea were high in magnitude, similar to Toamaro, but with sufficient variability to be not significantly different from numbers on Tiano (Fig. 3). Although initiated later, trapping on the Raiatea shoreline yielded similar numbers between Tiano and the shoreline (Fig. 3). Within a location, site order (χ2 = 0.330, df = 3, P < 0.85) and specific Biogent trap used (χ2 = 1.88, df = 2, P < 0.39) did not influence mean numbers of female Ae. polynesiensis captured. However, numbers of female Ae. polynesiensis captured among trapping sites at a location were not uniform (χ2 = 8.27, df = 2, P < 0.016). In addition, fewer females were captured at the fourth collection location than the three visited earlier the same day (χ2 = 26.3, df = 3, P < 0.0001).
Fig. 3.
Total Ae. polynesiensis females captured per date on three Raiatea motus and adjacent shoreline.
Adult Size
Aedes polynesiensis female wing lengths were not normally distributed (N = 695, W = 0.994, P = 0.004). Female Ae. polynesiensis from the shoreline (mean [SE] = 2.466 [0.014] mm) and Horea (2.428 [0.010] mm) had wing lengths that were equivalent (by Steel–Dwass method, q = 2.569) but significantly larger than their counterparts from Tiano (2.345 [0.008] mm) and Toamaro (2.374 [0.011] mm), which were equivalent. Mean female Ae. polynesiensis wing lengths also differed by date of collection for Horea (Wilcoxon’s χ2 = 66.416, df = 13, P < 0.0001) and Toamaro χ2 = 125.33, df = 14, P < 0.0001) but not Tiano (χ2 = 4.403, df = 8, P = 0.819 or the shoreline (χ2 = 5.962, df = 6, P = 0.432). The correlation between numbers of Ae. polynesiensis females captured and their mean wing lengths was not significant (ρ = −0.454, N = 17, P = 0.109, P = 0.675).
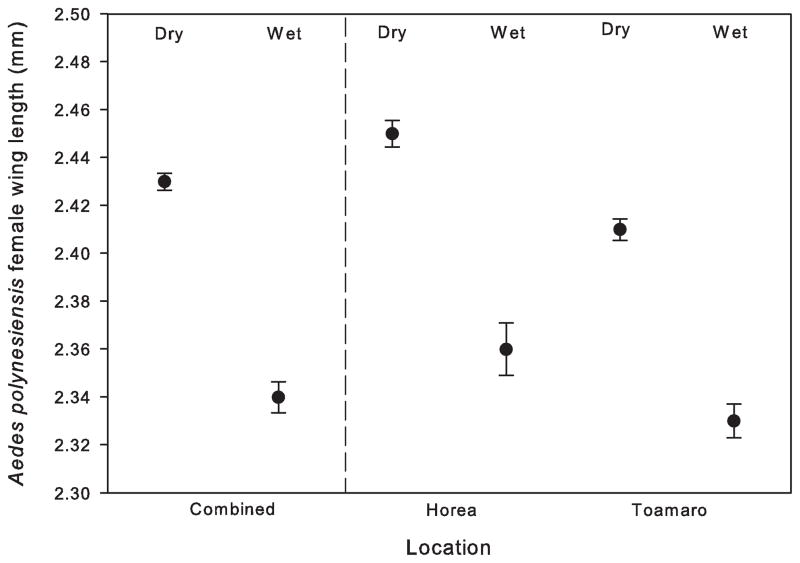
Sizes of Ae. polynesiensis females emerging from the densest populations were analyzed further for seasonal trends. Wing lengths of females collected on Horea and Toamaro during the wet (December–March) and dry (April–November) seasons were not normally distributed (N = 629, W = 0.991, P = 0.0006). There was a significant within the model effect (F = 15.04; P = 0.0001). Overall, mean female wing lengths were significantly longer in the dry season (2.43 [0.011] mm) than in the rainy season (2.35 [0.014] mm) (LS Means Student’s t = −5.56, p = 0.0001). There were no significant differences between Horea and Toamaro during the wet or the dry seasons (LS Means Tukey HSD Q = 2.58, p = 0.054 and p = 0.66, respectively, Fig. 4).
Fig. 4.
Ae. polynesiensis mean (SE) female wing lengths collected on Horea and Toamaro during the dry (April–November) and the wet (December–March) seasons (combined and individually).
Weather Data
Typical of the tropical oceanic climate of French Polynesia, weather conditions on Raiatea were variable during the period of study. However, some conditions fluctuated more than others. The weather station on Tiano recorded nine weather variables at 10-min intervals (N = 92,839 records); unitless coefficients of variation (SD/mean) allow for comparisons among variables (Table 3). Rainfall showed the greatest variability (CV = 8.72) with frequent high rainfall (>14 mm during 10-min intervals). During the sampling period, 3.62 m of rainfall was recorded by the weather station. Solar radiation also was variable; however, the instrument recorded during both day and night. The mean maximum solar radiation (which excludes nighttime measurements included in Table 3) was 1,174.8 W/m2. Wind speed (and gust speed) at 3 m above the ground also were variable, with likely consequences upon mosquito movement nearer the ground. The prevailing wind direction (125.66ø) corresponds to ESE subtropical trade winds. Although wind direction shifted to all quarters of the compass, it delivered air masses relatively consistent in air temperature and barometric pressure.
Table 3.
Weather conditions on Tiano motu July 2007–August 2009 from data collected at 10-min intervals
| Weather variable | Mean | SD | CV | Max | Min. |
|---|---|---|---|---|---|
| Rain (mm) | 0.039 | 0.340 | 8.72 | 14.6 | 0 |
| Air temperature (°C) | 27.517 | 2.349 | 0.085 | 48.007 | 20.246 |
| RH (%) | 77.297 | 8.772 | 0.113 | 95.8 | 45.9 |
| Pressure (mbar) | 1,014.61 | 2.211 | 0.002 | 1,021.55 | 1,006.85 |
| Solar radiation (W/m2) | 167.22 | 286.22 | 1.71 | 1,276.9 | 0.6 |
| Wind speed (m/s) | 0.851 | 0.965 | 1.13 | 9.09 | 0 |
| Gust speed (m/s) | 2.357 | 2.110 | 0.895 | 15.59 | 0 |
| Wind direction (ø) | 125.66 | 88.542 | 0.705 | 355.2 | 0 |
| Soil temperature (°C) | 26.575 | 2.179 | 0.082 | 34.255 | 20.555 |
SD, standard deviation; CV, coefficient of variation; RH, relative humidity.
Trap catches during biweekly collections correlated poorly with weather variables measured during the previous fortnight (Fig. 2). Only mean maximum barometric pressure was consistent in being significantly correlated with mean numbers of Ae. polynesiensis females captured on Toamaro (ρ = −0.327, P = 0.026), Tiano (ρ = −0.342, P = 0.020), and the shoreline (ρ = 0.467, P = 0.011), but not Horea (ρ = 0.009, P = 0.953). Maximum solar radiation (ρ = −0.398, P = 0.006) and minimum air temperature (ρ = 0.328, P = 0.026) were significantly correlated with Toamaro trap catches, whereas maximum rain (ρ = 0.414, P = 0.026) and minimum soil temperatures (ρ = 0.373, P = 0.046) on Tiano were significantly correlated with shoreline Ae. polynesiensis female numbers.
The weather station failed to record data during two periods of the study: 6 June-4 July 2008 and 20 November 2008–1 February 2009. Therefore, air temperature, relative humidity, wind gust speed, and wind direction measurements generously were provided by Meteo France from data collected at the Bora Bora Airport (Fig. 1), 51 km NW of Tiano (Google Earth 5.0). Daily values from the Bora Bora Airport and Tiano correlated significantly for 68 overlapping days when both data sets were available (analysis not shown); therefore, the proxy data were used to fill gaps in weather measurements from Tiano.
For each of nine weather variables, mean, maximum and minimum values from 10-min measurements were calculated across 14-d intervals that corresponded with the frequency of mosquito sampling periods. These data were used in multiple regression analyses to explain the amount of variation in mosquito capture rates during the sampling period. The multiple regression model with all weather variables explained 66.5% of the variation in numbers of TOTAL MOSQUITOES captured for all locations, but represented a model that was not significant (F24, 21 = 1.741, P = 0.102). Only MAX PRESSURE (maximum atmospheric pressure) and MAX RH (maximum relative humidity) during the corresponding fortnightly period were significant factors in the model. Therefore, a forward stepwise regression was performed and MAX SOLAR, MEAN SOLAR, MAX AIR (temperature), MAX RH and MIN RH produced a significant model (R2 = 0.430; F5,40 = 6.04; P = 0.0003). Because Toamaro motu is the least managed of the motus, mosquito numbers may be determined more strongly by weather conditions. The forward stepwise regression analysis with MAX PRES, MIN PRES, MAX SOLAR, MEAN SOLAR, MAX WIND, MAX AIR, MAX RH, and MIN RH produced a significant model (F8,37 = 5.03, P=0.00003) that explained 52.1% of the variation in TOTAL MOSQUITOES captured on Toamaro.
Discussion
Despite the proximity of the four locations, there were dramatic differences in vector numbers sampled, including differences among the motu Ae. polynesiensis populations. Typically, motus have a coral base and are adjacent to channels cut by streams descending from steep valleys of the main island’s volcanic core (or its remnant on “low islands”). The three motus were selected because they are similar distances from the shoreline and share a portion of the lagoon. As a result, they have similar substrates and are exposed to similar ocean effects, weather patterns, and natural colonization rates. Contrarily, they represent the range of human use for motus, allowing for testing of control strategies against discrete mosquito populations subject to different human interventions.
All three motus host C. carnifex populations; the numerous, cryptic burrows of these land crabs represent important sources of Ae. polynesiensis mosquitoes that currently escape vector control efforts (Lardeux et al. 2002). Likewise, the cryptic nature of treeholes and other phytotelmata in native and ornamental vegetation make these developmental sites difficult to eliminate. Otherwise, developmental sites among the three motus are influenced heavily by the degree of human intervention. Although coconut palms are present on all three motus, their decaying fruits do not provide equivalent developmental opportunities for the three mosquito populations. On Tiano, coconut trees are banded to deny treetop access to rats while the fruits are on the trees and fallen coconuts are regularly collected and discarded. Trees are not banded on the other two motus, but fallen coconuts are collected more frequently on Horea than on Toamaro. None of the motus was occupied permanently; however, humans were encountered more frequently on Horea than on Toamaro. During the survey, the number of temporary dwellings on Horea increased and people were present on 14 survey dates (compared with one date on Toamaro). Although people and their domestic animals represent additional blood sources for mosquitoes and their refuse provides artificial containers for immatures, increased human activities (burning, clearing of vegetation, insecticide, and repellant application) during the second year likely had a greater impact on Horea than on Toamaro.
Toamaro is the least managed of the three motus. Other people besides researchers were encountered only once, and there was a single burning record for Toamaro during the 2 yr of the study. Aedes polynesiensis biting rates typically reach hundreds per hour everywhere on the motu except in presence of a steady breeze on the exposed windward shore (D.R.M., unpublished data). Interestingly, weather data better explained mosquito abundance on Toamaro than on the other motus or the shoreline. The Raiatea shoreline does not have an isolated mosquito population and was not intended for trial of new technology. Thus, it was not included in the preliminary sampling scheme. Similar to the motus, the three shoreline sample sites are <2 m above sea level. But these sample sites are adjacent to forested volcanic ridges, intervening riparian valleys, the road circling the island, and permanently occupied dwellings; therefore, the shoreline is much more heterogeneous and influenced by surrounding contiguous habitats. The shoreline was ultimately included in the sampling scheme for comparison purposes, particularly to identify any disparities in mosquito numbers relative to the motu populations. It is interesting that Tiano, the most managed of the motus, had mosquito numbers similar to the shoreline.
There was high variability in BG-Sentinel trap catches with only weak seasonal trends in mosquito numbers consistent between the 2 yr of collection. In addition, there was little consistency by date in size or numbers between years, with only Tiano’s numbers correlated between years. However, there were overwhelming numbers of female Ae. polynesiensis captured at all locations and during both years of the study. Mosquitoes were collected during the morning peak of host seeking to maximize capture of Ae. polynesiensis females.
Although they differ in size and human activities, Horea and Toamaro were similar in producing high mosquito numbers. Peaks in mosquito numbers overlapped between Horea and Toamaro during the first year (although their magnitudes differed). This likely reflects similarities in developmental sites, bloodmeal sources, and vegetational cover. However, mean female Ae. polynesiensis were larger overall from Horea than Toamaro and temporal trapping patterns differed for these two motus during the second year of collection (Fig. 3). Horea’s mosquito population did not reach equally high numbers March–August 2009. By comparison, numbers of mosquitoes trapped on Tiano were low (<35 in three traps) for all but one of the 51 dates. Extensive chemical control and source reduction on Tiano have reduced but not eliminated mosquito production on this motu; such sustained effort is impractical for vector control over larger temporal and spatial scales in French Polynesia. Equivalent wing lengths for Tiano mosquitoes with those from Toamaro (the most productive motu) suggest that conditions within developmental sites were similar even though fewer sites were available. Presumably, the low numbers of adults captured on Tiano are consistent with little immigration of mosquitoes from neighboring motus or the main island. The shoreline sites on Raiatea yielded relatively few mosquitoes but marginally greater species homogeneity than the motus.
Although there were no significant differences among collections as a function of the trap used, trap catches on the motus were not spatially uniform. On average, significantly more Ae. polynesiensis females were captured at the most central motu site. Exposure in the flight paths and distances from putative developmental sites presumably differed across the terrain. Whatever the cause, these data indicate heterogeneity in adult numbers, even across these small isolated habitats.
Trap collections depend upon the presence and activity of mosquitoes and may not reflect absolute numbers, host seeking, or putative disease transmission (Southwood and Henderson 2000). Because no carbon dioxide or artificial attractants were used, male Ae. polynesiensis (which intercept host-seeking females, unpublished data) were not adequately sampled by the protocol described here. If not because of a trapping bias, the paucity of male Ae. polynesiensis on these Raiatea motus would deserve additional attention. Departures from the expected 1:1 sex ratio for Ae. polynesiensis because of competition tend to favor males rather than reduce their production (Mercer 1999, Mercer et al. 2005). Trap catches also reflected very low numbers of Cx. quinquefasciatus. Individuals of this nocturnal species, which is the principal vector of periodic W. bancrofti in most of the world, were not captured on Horea (despite the presence of developmental sites) and were captured infrequently on Toamaro and the shoreline. Low trap catches of Cx. quinquefasciatus and of all mosquitoes at the fourth location sampled per collection day likely reflect non-optimal collection times and trapping methods. Nonetheless, it will be important to monitor other vector species as Ae. polynesiensis populations are targeted. Vector control should not augment vector status of other species nor negatively impact beneficial or “neutral” species in the target area.
One promising control strategy involves the mass release of incompatible male Ae. polynesiensis hosting a strain of maternally inherited Wolbachia pipientis Hertig & Wolbach (Alphaproteobacteria: Rickettsiales). Wolbachia are obligate endosymbionts that manipulate the reproduction of their hosts. In mosquitoes, Wolbachia cause cytoplasmic incompatibility (CI) when uninfected females mate with infected males (unidirectional CI) or infected females are inseminated by males carrying an incompatible strain (bidirectional CI). An incompatible strain of Ae. polynesiensis was developed through interspecific hybridization; mass release of infected males eliminated populations of wild-type Ae. polynesiensis in cage studies (Brelsfoard et al. 2008). Irradiation techniques have been developed specifically to target females infected with this strain that might escape mechanical sorting (Brelsfoard et al. 2009).
Because size of female mosquitoes tends to be related to fitness, vector control must consider both numbers and sizes of females in the target population. A control strategy that produces fewer but more robust females may fail at lowering disease transmission rates. There was high natural variability in Ae. polynesiensis female size throughout the study period. The mean sizes of female Ae. polynesiensis collected from Horea and Toamaro populations during the dry season were significantly greater than those collected during the rainy season, suggesting females are more robust during the dry season on these productive motus. However, shoreline females were, on average, larger than females captured on the motus, implying that the large populations on the motus develop under intense intraspecific competition. Reducing the numbers of adult mosquitoes is likely to ameliorate larval crowding, resulting in higher survival rates and larger, more fecund adults. A control strategy timed to coincide with the sparsest target population may encounter females larger than average.
A large number of Ae. polynesiensis was removed from the population of Toamaro in December 2008 for a separate study (data not shown). Although the removal of adults was timed to avoid biweekly collections, we were curious to measure potential impacts upon scheduled Biogent Sentinel trapping. Despite the extra collections, large numbers of Ae. polynesiensis were captured on Toamaro during December 2008-March 2009 (with no apparent effect on wing length); by contrast, there was no peak in numbers of mosquitoes captured on Horea between January and March 2009. At least during the short term, mosquito populations on motus appear to be resilient. Control strategies must be sustained, perhaps corresponding to the development of several natural cohorts. In addition, in view of high natural variability (between collections, between seasons, and among habitats), it is imperative to monitor Ae. polynesiensis female numbers for a sustained period after release of incompatible males.
In view of the variation in mosquito population patterns among the motus, it is inappropriate to generalize our results to additional motus influenced by different natural and anthropogenic effects. Nonetheless, we successfully monitored temporal and spatial patterns of mosquito numbers and investigated the effects of weather upon mosquito abundance for motus that would serve as targets for development of vector control technologies.
Acknowledgments
We thank Jerome Petit, the Richard B. Gump Biological Research Station, and Meteo France for providing weather data. This research was sponsored by grants from the National Institutes of Health (NIH) (AI)-067434 and the Bill and Melinda Gates Foundation BMGF no. 44190. This is publication 11-08-039 of the University of Kentucky Agricultural Experiment Station.
References Cited
- Belkin JN. The mosquitoes of the South Pacific (Diptera: Culicidae) Vol. 2. University of California Press; Berkeley and Los Angeles, CA: 1962. p. 608.p. 412. [Google Scholar]
- Brelsfoard CL, Sechan Y, Dobson SL. Inter-specific hybridization yields strategy for South Pacific filariasis vector elimination. PLoS Negl Trop Dis. 2008;2:e129. doi: 10.1371/journal.pntd.0000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brelsfoard CL, StClair W, Dobson SL. Integration of irradiation with cytoplasmic incompatibility to facilitate a lymphatic filariasis vector elimination approach. Parasit Vectors. 2009;2:38–45. doi: 10.1186/1756-3305-2-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunes J, Boussès P. Description of Culex (Culex) sechani n. sp. from Tahiti (Society Islands) (Diptera, Culicidae) Bull Soc Entomol France. 2009;11:21–28. [Google Scholar]
- Burkot TR, Taleo G, Toeaso V, Ichimori K. Progress towards, and challenges for, the elimination of filariasis from Pacific-island communities. Ann Trop Med Parasitol. 2002;96(Suppl 2):61–69. doi: 10.1179/000349802125002419. [DOI] [PubMed] [Google Scholar]
- Esterre P, Plichart C, Sechan Y, Nguyen NL. The impact of 34 years of massive DEC chemotherapy on Wuchereria bancrofti infection and transmission: the Maupiti cohort. Trop Med Int Health. 2001;6:190–195. doi: 10.1046/j.1365-3156.2001.00682.x. [DOI] [PubMed] [Google Scholar]
- Failloux AB, Raymond M, Ung A, Glaziou P, Martin PMV, Pasteur N. Variation in the vector competence of Aedes polynesiensis for Wuchereria bancrofti. Parasitology. 1995;111:19–29. doi: 10.1017/s0031182000064568. [DOI] [PubMed] [Google Scholar]
- Failloux AB, Raymond M, Ung A, Chevillon C, Pasteur N. Genetic differentiation associated with commercial traffic in the Polynesian mosquito, Aedes polynesiensis Marks 1951. Biol J Linn Soc. 1997;60:107–118. [Google Scholar]
- Huang YM. The mosquitoes of Polynesia with a pictorial key to some species associated with filariasis and/or dengue fever. Mosq Syst. 1977;9:289–322. [Google Scholar]
- Iyengar MOT. South Pacific Commission Technical Paper No 148. 1965. Epidemiology of filariasis in the South Pacific; p. 183. [Google Scholar]
- Kay BH, I, Fanning D, Carley JG. The vector competence of Australian Culex annulirostris with Murray Valley Encephalitis and Kunjin viruses. Aust J Exp Biol Med Sci. 1984;62:641–650. doi: 10.1038/icb.1984.61. [DOI] [PubMed] [Google Scholar]
- Laigret J, Kessel JF, Malarde L, Bambridge B, Adams H. La lutte contre la filarioise lymphatique apériodic en Polynésie Française. Bull Soc Pathol Exot. 1965;5:895–916. [PubMed] [Google Scholar]
- Lardeux F, Rivière F, Séchan Y, Loncke S. Control of the Aedes vectors of the dengue viruses and Wuchereria bancrofti: the French Polynesian experience. Ann Trop Med Parasitol. 2002;96(Suppl 2):105–116. doi: 10.1179/000349802125002455. [DOI] [PubMed] [Google Scholar]
- Lee DJ, Hicks MM, Debenham ML, Griffiths M, Marks EN, Bryan JH, Russell RC. The Culicidae of the Australasian Region. Vol. 7. Australian Government Publishing Service; Canberra, Australia: 1989. [Google Scholar]
- Maguire T, MacNamara FN, Miles JAR, Spears GFS. Mosquito-borne infections in Fiji. II. Arthropod-borne virus infections. J Hyg. 1971;69:287–296. doi: 10.1017/s0022172400021513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer DR. Effects of larval density on the size of Aedes polynesiensis adults (Diptera: Culicidae) J Med Entomol. 1999;36:702–708. [PubMed] [Google Scholar]
- Mercer DR, Wettach GR, Smith JL. Effects of larval density and predation by Toxorhynchites amboinensis (Diptera: Culicidae) upon Aedes polynesiensis production. J Am Mosq Control Assoc. 2005;21:425–431. doi: 10.2987/8756-971X(2006)21[425:EOLDAP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Meyer JY. Status of Miconia calvescens (Melastomataceae), a dominant invasive tree in the Society Islands (French Polynesia) Pac Sci. 1996;50:66–76. [Google Scholar]
- Pichon G. Limitation and facilitation in the vectors and other aspects of the dynamics of filarial transmission: the need for vector control against Anopheles transmitted filariasis. Ann Trop Med Parasitol. 2002;96(Suppl 2):S143–S152. doi: 10.1179/000349802125002509. [DOI] [PubMed] [Google Scholar]
- Pichon G, Perrault G, Laigret J. Rendement parasitaire chez les vecteurs de filarioses. Bull World Health Org. 1974;51:517–524. [PMC free article] [PubMed] [Google Scholar]
- Plichart C, Sechan Y, Davies N, Legrand A-M. PCR and dissection as tools to monitor filarial infection of Aedes polynesiensis mosquitoes in French Polynesia. Filaria J. 2006;5 doi: 10.1186/1475–2883-5–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen L, Rozeboom LE, Sweet BH, Sabin AB. The transmission of dengue by Aedes polynesiensis Marks. Am J Trop Med Hyg. 1954;3:878–882. doi: 10.4269/ajtmh.1954.3.878. [DOI] [PubMed] [Google Scholar]
- Rosen L. Observations on the epidemiology of human filariasis in French Oceana. Am J Hyg. 1955;61:219–248. [Google Scholar]
- Russell RC. The relative attractiveness of carbon dioxide and octenol in CDC- and EVS-type light traps for sampling the mosquitoes Aedes aegypti (L.), Aedes polynesiensis Marks, and Culex quinquefasciatus Say in Moorea, French Polynesia. J Vector Ecol. 2004;29:309–314. [PubMed] [Google Scholar]
- Shiu S, Mercer DR, Martin PMV, Rodhain F, Raymond M, Failloux AB. Aedes polynesiensis in the Society Islands: environmental correlates of isoenzyme differentiation. Med Vet Entomol. 1997;11:349–354. doi: 10.1111/j.1365-2915.1997.tb00421.x. [DOI] [PubMed] [Google Scholar]
- Southwood TRE, Henderson PA. Ecological methods. 3. Blackwell Ltd; Oxford, United Kingdom: 2000. [Google Scholar]
- Stolk WA, Van Oortmarssen GJ, Subramanian S, Das PK, Borsdoom GJJM, Habbema JDF, DeVlas SJ. Assessing density dependence in the transmission of lymphatic filariasis: uptake and development of Wuchereria bancrofti microfilariae in the vector mosquitoes. Med Vet Entomol. 2004;18:57–60. doi: 10.1111/j.0269-283x.2004.0470.x. [DOI] [PubMed] [Google Scholar]