Abstract
Polyisoprenylation is a set of secondary modifications involving proteins whose aberrant activities are implicated in cancers and degenerative disorders. The last step of the pathway involves an ester-forming polyisoprenylated protein methyl transferase- and hydrolytic polyisoprenylated methylated protein methyl esterase (PMPMEase)-catalyzed reactions. Omega-3 and omega-6 polyunsaturated fatty acids (PUFAs) have been linked with antitumorigeneis and tumorigenesis, respectively. PUFAs are structurally similar to the polyisoprenyl groups and may interfere with polyisoprenylated protein metabolism. It was hypothesized that PUFAs may be more potent inhibitors of PMPMEase than their more polar oxidative metabolites, the prostaglandins. As such, the relative effects of PUFAs and prostaglandins on PMPMEase could explain the association between cyclooxygenase-2 (COX-2) expression in tumors, the chemopreventive effects of the non-steroidal anti-inflammatory (NSAIDs) COX-2 inhibitors and PUFAs. PUFAs such as arachidonic (AA), eicosapentaenoic (EPA) and docosahexaenoic (DHA) acids inhibited PMPMEase activity with Ki values of 0.12 to 3.7 μM. The most potent prostaglandin was 63-fold less potent than AA. The PUFAs were also more effective at inducing neuroblastoma cell death at physiologically equivalent concentrations. The lost PMPMEase activity in AA-treated degenerating cells was restored by incubating the lysates with COX-1 or COX-2. PUFAs may thus be physiological regulators of cell growth and could owe these effects to PMPMEase inhibition.
Keywords: isoprenylation, polyunsaturated fatty acids, prostaglandins, arachidonic acid, COX-2
1 Introduction
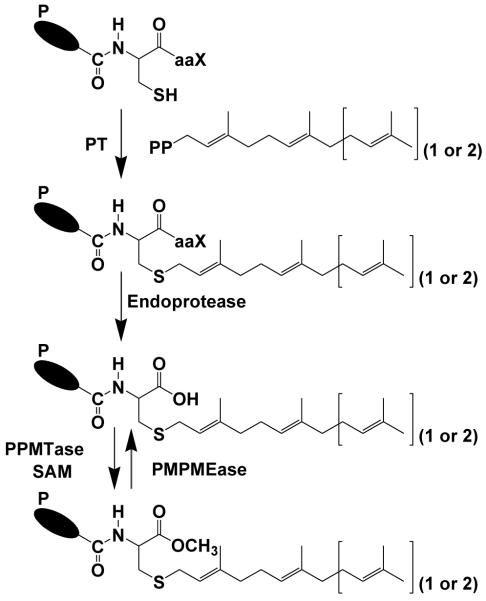
The polyisoprenylation pathway (Figure 1) involves a set of essential secondary modifications that culminate in the methylation of the carboxylate ion of the terminal polyisoprenylated cysteine in a reaction catalyzed by polyisoprenylated protein methyl transferase (PPMTase) (1, 2). The resulting esters are hydrolyzed by polyisoprenylated methylated protein methyl esterase (PMPMEase) (3-6). Both enzymes catalyze the reactions of the only reversible step of the pathway; their relative activities determining the balance between the two polyisoprenylated protein states. This could be of functional significance as it likely leads to conformational changes of the proteins. Although the structures and functions of the estimated 2% of eukaryotic proteins that undergo polyisoprenylation (7) are diverse, the Ras and Rab families of monomeric G-proteins are interesting because of their effects on cell viability (8). Mutations or gene amplification that cause their hyperactivities are implicated in about 30% of cancers (8). Enzymes of the polyisoprenylation pathway have thus long been regarded as viable targets for the development of anticancer agents (8-14).
Figure 1. The polyisoprenylation pathway.
PT, Polyisoprenyl transferase; PPMTase, polyisoprenylated protein methyltransferase; PMPMEase, polyisoprenylated methylated protein methyl esterase; SAM, S-adenosyl-L-methionine. aaX represents a tripeptide in which “a” is an aliphatic amino acid and “X” represents other amino acids. Together with the adjacent cysteine, they form the CaaX signal for polyisoprenylation in some proteins. P = polypeptide chain.
Reports linking long term use of non-steroidal anti-inflammatory drugs (NSAIDs) to reduced cancer risks have led to the discovery of increased cyclooxygenase-2 (COX-2) expression in cancer cell lines (15) and in tumors (16-18). COX-2 is one of the cyclooxygenases that initiate the synthesis of prostaglandins (PGs) from polyunsaturated fatty acids (PUFAs) such as arachidonic acid (AA) (19). Several other reports also validate elevated COX-2 expression in colorectal adenomas and adenocarcinomas (20). Although the general consensus appears to associate the tumor and cancer cell line expression of COX-2 to the generation of pro-inflammatory/tumorigenic prostaglandins (20, 22, 23), AA, an omega-6 PUFA, has recently been shown to induce the death of HL-60 human promyelocytic, Jurkat, Raji and rat lymphocyte cells at physiologically relevant concentrations (21). In most cell lines AA concentrations of 50-100 μM were found to be cytotoxic (21). The omega-3 polyunsaturated fatty acids (PUFAs) such as eicosapentaenoic (EPA) and docosahexaenoic (DHA) acids also inhibited Jurkat cell proliferation at concentrations of 25-100 μM (24). The consumption of foods rich in PUFAs has been widely associated with anticancer benefits and anticancer clinical trials have thus been conducted (25). Significantly lower tumor numbers and percentage of animals with colon adenocarcinomas were observed in experimental animals fed with diets rich in omega-3 PUFAs supplemented with celecoxib (26).
Pace-Asciak (27) reported the AA concentrations in human and rat plasma at 5.3 μg/mL (17.4 μM) and 23.8 μg/mL (78.3 μM), respectively. The concentration of free fatty acids (FFAs) has been reported at 323 nmol/g in mice brains with oleic acid (OA) and AA comprising 64.6 and 35.5 nmol/g, respectively (28). Others have reported the concentrations of FFAs in rat plasma at 206.6 μM of which 9.3 μM is AA (29). Total FFAs in rat blood vessels ranging from 467.9 to 642.4 μM have been reported (30). The AA concentrations were between 14.8 and 21.8 μM while those of the other FFAs ranged from 1.5 to 16.3 μM (30).
PUFAs have some common physicochemical properties with trans,trans-farnesyl and all trans-geranylgeranyl moieties by virtue of their hydrophobicity and multiple double bonds. This implies that some of the FFAs may assume structures that closely resemble those of the polyisoprenyl groups of polyisoprenylated proteins. Unlike the FFAs and the polyisoprenes, PGs are modified with polar groups such as carboxylic, hydroxyl and ketone groups that render them less hydrophobic than the FFAs and AA from which they are derived. From 10- to over 100-fold higher rates of AA metabolism into prostanoids has been reported in cancer patients (31). COX-2 is overexpressed in many tumors and cancer cell lines (15, 32-34). As such, the affinity and thus the inhibitory potency of prostanoids towards PMPMEase may be significantly diminished relative to the precursor PUFAs. The relative inhibition of PMPMEase by FFAs such as AA and PGs suggest that the elevated COX-2 levels in some cancers may spur the conversion of PUFAs such as AA into PGs, compromising the tissue’s ability to control PMPMEase activity and thus cell viability. The likelihood of this being the case is supported by our present study which shows an over 63- and 150-fold higher inhibitory potency for AA over prostaglandin A2 (PGA2) and prostaglandin E2 (PGE2) against PMPMEase, respectively.
2 Materials and methods
2.1 Materials
Saturated and polyunsaturated fatty acids and prostaglandins A2 (PGA2) and E2 (PGE2) were purchased either from Sigma-Aldrich (St. Louis, MO) or from VWR (West Chester, PA). COX-1 and COX-2 and all the other prostaglandins (PGs) were obtained from Cayman Chemical company (Ann Arbor, MI). N-(4-nitrobenzoyl)-S-trans, trans-farnesyl-L-cysteine methyl ester (RD-PNB) substrate was synthesized in our laboratory as previously described (35). This is similar to other polyisoprenylated small molecule substrates that have been used to characterize a polyisoprenylated protein-metabolizing esterase from rod outer segments (36).
2.2 Enzyme assays
PMPMEase used for the assays was the same as that previously described (5, 35). The substrate (RD-PNB), PGs and FFAs were dissolved in dimethylsulfoxide (DMSO). The enzyme assays and analysis were conducted as previously described (5, 35) except with a 15 min pre-incubation of the assay mixture with the FFAs or PGs before the addition of substrate. RD-PNB (1 mM) was incubated at 37°C with the enzyme in the presence of the FFAs or PGs in 100 mM Tris-HCl, pH 7.4 in a total incubation volume of 100 μL. Reactions were stopped by adding 200 μL of methanol and placing them on ice for at least 5 min before centrifugation at 5000×g for 5 min. The supernatant was analyzed by RP-HPLC. The product was separated from the substrate on a Hamilton PRP-1 RP-HPLC column (5 μm particles, 4.1 mm ID × 50 mm) with UV detection at 260 nm. The mobile phase consisted of a linear gradient of acetonitrile in 0.1% ethanolamine, from 30% at the start of the separation to 95% in 1 min. This was then maintained for a further 2 min before a 0.5 min re-equilibration at 30% acetonitrile for the next sample.
2.3 Effects of FFAs and PGs on cell viability
Oncogenic proteins such as Ras and Rho require polyisoprenylation to function (37). Since overexpression or mutations that render them constitutively active alters their activity in favor of excessive cell proliferation, their post-translational metabolism has been targeted for the development of anticancer drugs (8, 37). It was thus of interest to know whether the inhibition of PMPMEase by FFAs may be linked to the reported effects of FFAs on cell viability and whether metabolism of the PUFAs by COX-2 in tumors may lead to PGs’ lower PMPMEase-inhibitory potency and ability to control cell viability. Human neuroblastoma SH-SY5Y cells were initially cultured in a 1:1 mixture of Dulbecco’s modified Eagle’s Medium (DMEM) and F12 Medium (Invitrogen, Carlsbad, CA), supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum (Invitrogen, Carlsbad, CA), 100 U/ml penicillin and 100 μg/ml streptomycin (Invitrogen, Carlsbad, CA) in 75 cm2 vented culture flasks. Since PMPMEase is highly ubiquitous in mammalian tissues, the choice of cell type was based mainly on morphological characteristics. Treatment-induced changes in neuroblastoma cells are more easily discernable due to changes in the morphological processes. The cultures were incubated at 37°C in 5% CO2/95% humidified air. When cells had reached 80-90% confluence, they were trypsinized and seeded onto 96-well plates at a density of 105 or 24-well plates at the density of 5 × 105 and incubated at 37°C in 5% CO2/95% humidified air.
2.3.1 Cell viability assays
CellTiter-Blue Cell Viability Assay kit (CTB) with fluorescence readout was used to measure resazurin reduction as a marker of metabolic activity following treatment with FFAs. FFAs and PGs were dissolved in acetone (solvent) which is considerably less cytotoxic than DMSO (38). The final concentration of the acetone in each well was 1%. The human neuroblastoma SH-SY5Y cells in 96-well plates were exposed to varying concentrations of FFAs and PGs in serum-free DMEM/F12 for 24, 48 and 72 h. Resazurin (Promega, Madison, WI) was used to measure the cell viability according to the vendor instructions. Identical amounts of the FFAs were used to supplement the samples at 24 h for the 48 h exposure and 24 and 48 h for the 72 h exposure. In another experiment, the cells in 96-well plates were exposed to varying concentrations of FFAs in serum-containing DMEM/F12 (10% FBS) for 72 h. Resazurin (20 μL) was added to each well and the contents gently mixed and incubated in the dark for 2 h at room temperature before measurement of the fluorescence with excitation at 560 nm and emission at 590 nm using FLx 800 Microplate Flourescence Reader (Bio-Tek Instruments, Inc., Winooski, VT). Cell viability was expressed as the percentage of the fluorescence in the treated cells relative to that of the controls.
2.4 PMPMEase activity in FFA-treated human neuroblastoma SH-SY5Y cells
Human neuroblastoma SH-SY5Y cells were seeded in 24-well plates as described above. The cells were either treated with AA (0 - 200 μM), EPA (0 - 200 μM), or arachidic acid (200 μM) and incubated for 24 h. Some of the medium (320 μL) in each well was removed and Triton-X 100 (20 μL, 1%) was then added to the remaining 180 μL of medium. These were thoroughly mixed and aliquots of the resulting lysate were assayed for PMPMEase activity using RD-PNB as the substrate. Since cell death would be accompanied by the degradation of macromolecules in the cells, the low PMPMEase activity may be interpreted to imply the degradation of PMPMEase protein rather than its inhibition by FFAs. To test this, human neuroblastoma SH-SY5Y cells were treated with AA (0-50 μM) for 24 h. The AA concentrations were chosen such that if totally converted to PGs, the total PGs concentrations will be less than those expected affect PMPMEase activity. These were then lysed and aliquots pre-incubated at 37 °C with either COX-1 (23.5 U) or COX-2 (9 U) for 1 h. RD-PNB substrate was then added followed by further incubation at 37 °C for 3 h. The reactions were stopped, processed and analyzed for PMPMEase activity as described in the “enzyme assay” section. In order to assess the effect of non-specific cytotoxic agents on the effect of cell degeneration on PMPMEase activity cells were also treated with DMSO. The degenerating cells were lysed and aliquots analyzed for PMPMEase activity.
2.5 Statistical analysis
All results were expressed as the means ± S.E.M. The data were analyzed using one-way ANOVA. Statistical differences between control and treated groups were determined by Dunnett’s post-test comparisons. P-values of less than 0.05 were considered statistically significant. The concentrations that inhibited 50% of the activity (IC50) were calculated from a nonlinear regression curve using Graphpad Prism version 4.0 for Windows (San Diego, CA). The dose-response curves were obtained by plotting the percentage inhibition against the log of the concentrations.
3 Results
3.1 FFAs inhibition of PMPMEase is dependent on chain-length, degree and type of unsaturation
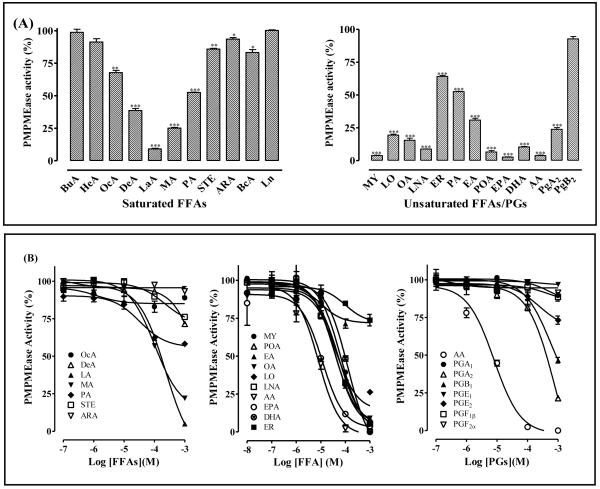
When the RD-PNB substrate was incubated with PMPMEase in the presence of 1 mM FFAs, the enzymatic activity was inhibited in a manner that depended on the lengths of the fatty acids and their degree and type of unsaturation. Of the saturated FFAs, the inhibitory potency decreased progressively from the 12-carbon lauric acid (LA) with either increasing or decreasing chain length (Figure 2A). The inhibition was increased by the degree of unsaturation and decreased by the length of the carbon chain (Figure 2A). Comparisons between palmitic (PA), Elaidic (EA) and palmitoleic (POA) acids reveal that the inhibition increased in the order: saturated<trans-unsaturated<cis-unsaturated.
Figure 2. (A) Effect of FFAs on PMPMEase activity.
Purified PMPMEase (5 μg) was incubated with RD-PNB in the presence of 1 mM saturated FFAs or unsaturated FFAs and prostaglandins as indicated. The reactions were then analyzed by HPLC. The residual enzymatic activities are expressed as the mean relative to the controls (± SEM, N=3). *P<0.05, **P<0.01 and ***P<0.001 versus untreated controls compared by ANOVA, followed by the Dunnett’s post-test. (B) Inhibition of PMPMEase by FFAs and PGs. Purified PMPMEase (5 μg) was incubated with RD-PNB in the presence of varying concentrations of the indicated FFAs or PGs for 1 h. The reactions were stopped with methanol and analyzed for the residual PMPMEase activity as described in the methods section. The results are expressed as the means (± SEM, N=3) relative to the controls.
A concentration-dependent analysis of the FFAs validated this inhibitory pattern. IC50 values ranging from 6.6 μM for AA to 232 μM for LA with the corresponding Ki values of 0.12 and 6.5 μM, respectively (Figure 2B and Table 1). The increased inhibitory effects due to increased FFAs unsaturation was counteracted by increasing carbon chain length as typified by the results for AA, EPA and DHA.
Table 1.
Analysis data for PMPMEase inhibition by FFAs and PGs
| FFAs/PGs | IC50 [μM] | Ki [μM] | |
|---|---|---|---|
| Octanoic acid | OcA | >1000 | >28 |
| Decanoic acid | DeA | >1000 | >28 |
| Lauric acid | LA | 230 | 6.5 |
| Myristic acid | MA | 100 | 2.8 |
| Palmitic acid | PA | >1000 | >28 |
| Stearic acid | STE | >1000 | >28 |
| Arachidic acid | ARA | >1000 | >28 |
| Myristoleic acid | MY | 63 | 1.8 |
| Palmitoleic acid | PA | 37 | 1.0 |
| Elaidic acid | EA | >1000 | >28 |
| Oleic acid | OA | 53 | 1.5 |
| Linoleic acid | LNA | 64 | 1.8 |
| Linolenic acid | LO | 22 | 0.62 |
| Arachidonic acid | AA | 6.6 | 0.12 |
| Eicosapentaenoic acid | EPA | 12 | 0.34 |
| Docosahexaenoic acid | DHA | 130 | 3.7 |
| Erucic acid | ER | >1000 | >28 |
| Prostaglandin A1 | PGA1 | >1000 | >28 |
| Prostaglandin A2 | PGA2 | 420 | 12 |
| Prostaglandin B1 | PGB1 | 580 | 16 |
| Prostaglandin E1 | PGE1 | >1000 | >28 |
| Prostaglandin E2 | PGE2 | >1000 | >28 |
| Prostaglandin F1β | PGF1β | >1000 | >28 |
| Prostaglandin F2α | PGF2α | >1000 | >28 |
3.2 PUFAs but not PGs are potent inhibitors of PMPMEase
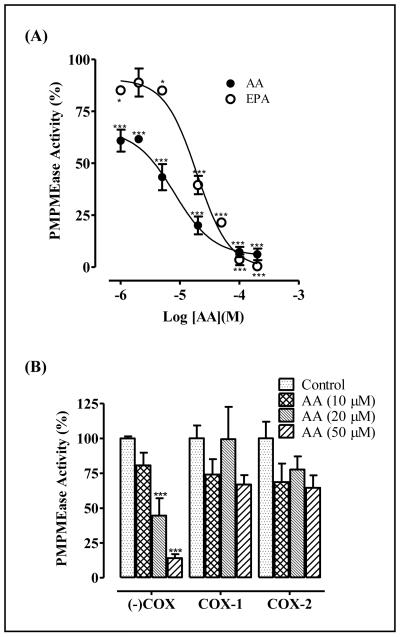
In order to test the effect of PUFAs conversion to PGs on PMPMEase inhibition, PMPMEase was incubated with substrate in the absence or presence of AA or selected prostaglandins. As shown in Figure 2B, the PGs were much less inhibitory against PMPMEase than AA. The most potent PG, PGA2 inhibited PMPMEase with an IC50 of 420 μM compared to 6.6 μM for AA (Table 1). This reflects an over 63-fold decrease in potency. All the other PGs were either not inhibitory or only marginally effective against PMPMEase even at 1 mM concentrations (Figure 2B, Table 1). As shown in Figure 3, the formation of PMPMEase enzymatic product over time was significantly inhibited by both AA (omega-6 PUFA) and EPA (omega-3 PUFA).
Figure 3. Inhibition of PMPMEase activity by FFAs.
PMPMEase (5 μg) was incubated with RD-PNB substrate (1 mM) in the presence of AA (•), EPA (▲) or control (○) at 37 °C for the indicated times. The reactions were stopped with methanol, processed and the product (P) was separated from the residual substrate (S) by HPLC as described in the methods. The results are expressed as the means (± SEM, N=3) relative to the controls. *P<0.05, **P<0.01 and ***P<0.001 versus untreated controls compared by paired t-test.
3.3 FFAs inhibit the viability of human neuroblastoma SH-SY5Y cells
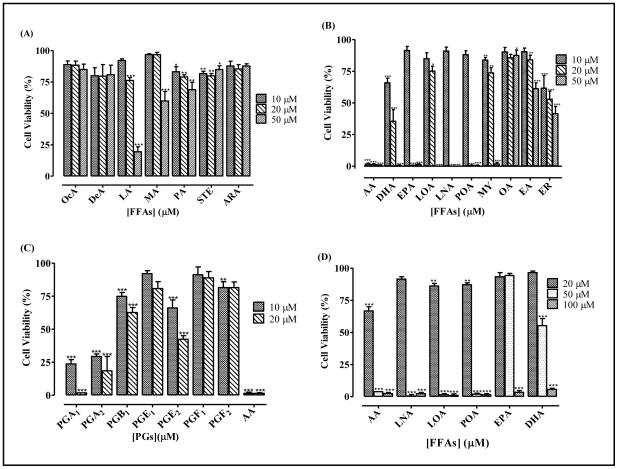
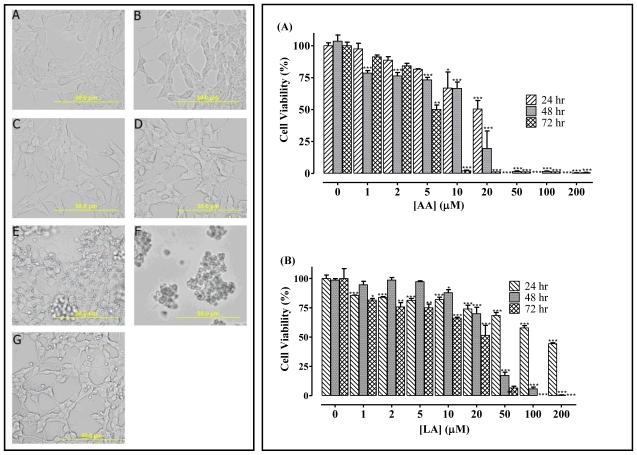
When human neuroblastoma cells were treated with varying concentrations of FFAs, the FFAs that were the most potent at inhibiting PMPMEase activity were also the most effective at inducing cell degeneration (Figure 4). Of the saturated FFAs, LA and myristic acid (MA) were the most effective at inducing the degeneration of human neuroblastoma cells. Similar to the pattern for PMPMEase inhibition, the PGs were significantly less effective at inducing cell degeneration than their biosynthetic precursor FFAs. As shown in Figure 4C, 10 μM AA induced the degeneration of 99 ± 0.1% of the cells. However, PGE2 and PGF2 only caused the degeneration of 34 ± 4.0% and 19 ± 5.6%, respectively. The cytotoxic potencies of the FFAs were reduced in the presence of 10% FBS, although they effectively inhibited cell viability at relatively higher concentrations compared to those in the serum free media. In most cases, cell degeneration was significant at concentrations of 20 μM and above (Figure 4D). Treatment of human neuroblastoma cells with AA concentrations that overlap the physiological levels and the saturated lauric acid resulted in cell degeneration (Figure 5). Also, images of neuroblastoma cells captured with an Olympus DP70 Camera after a 24 h exposure to ARA (200 μM) was compared to that of AA (1 - 50 μM). ARA is a saturated C20 analog of AA which does not inhibit PMPMEase. In like manner, it had no effect on cell viability even at 200 μM concentrations (Figure 5).
Figure 4. Relative induction of cell degeneration by FFAs and PGs.
Human neuroblastoma cells were cultured and seeded in 96-well plates as described in the methods. The cell viabilities were measured by fluorescence using the resazurin reduction assays after 72 h of treatment with the indicated (A) saturated FFAs, (B) PUFAs and (C) PGs. Cells were also treated with selected PUFAs in the presence of 10% FBS (D). The results are expressed as the means (± SEM, N=4) relative to the controls. *P<0.05, **P<0.01 and ***P<0.001 versus untreated control cells compared by ANOVA, followed by the Dunnett’s post-test.
Figure 5.
left panel -AA acid induces the degeneration of human neuroblastoma SH-SY5Y cells. Human neuroblastoma cells were cultured and seeded in 24-well plates as described in the methods. They were then treated with varying concentrations of AA. Images of the cells were captured after a 24 h exposure to AA with an Olympus DP70 Camera (left panel). These show (A) untreated, (B) acetone and (C) to (F) were treated with 1, 10, 20, 50 μM AA, respectively and (G) 200 μM ARA. Right panel -AA and LA induce the degeneration of human neuroblastoma SH-SY5Y cells. Human neuroblastoma cells were cultured and seeded in 96-well plates as described in the methods. At 24, 48 and 72 h after treatment with varying concentrations of (A) AA or (B) LA as indicated, cell viability was measured by fluorescence using the resazurin reduction assay. The results are expressed as the means (± SEM, N=4) relative to the controls. *P<0.05, **P<0.01 and ***P<0.001 versus untreated control cells compared by ANOVA, followed by the Dunnett’s post-test.
3.4 Inhibition of Neuroblastoma Cell PMPMEase by FFAs correlates with cytotoxicity
Although FFAs inhibited purified PMPMEase, it was not clear whether the observed effects on cell growth were directly related to PMPMEase inhibition. As such, cells were treated with varying concentrations of AA or EPA for 24 h and the lysate analyzed for PMPMEase activity. As shown in Figure 6A, the residual PMPMEase activity in the AA-treated cell lysate follows the same concentration-dependent profile as the AA effect on cell viability (Figure 5). The IC50 values of 7.3 μM and 19.3 μM were obtained for AA and EPA effect on cell PMPMEase activity respectively. ARA, a C20 saturated analog of AA was used as a control. ARA at 200 μM did not have any significant effect on cell PMPMEase activity and viability. The IC50 value of 7.3 μM was obtained for the AA effect on cell PMPMEase activity. This further suggests that the cell degeneration caused by AA and the other FFAs most likely results from PMPMEase inhibition. To test whether this loss of PMPMEase activity may be due to generalized protein degradation due to the cell death, lysate from degenerating cells following treatment with AA was pre-incubated with either COX-1 or COX-2 prior to the assay for PMPMEase activity. COX-1 and COX-2 was added to the reaction mixture to metabolize any residual AA into products unlikely to inhibit PMPMEase. As shown in Figure 6B, treatment with the COX enzymes resulted in the recovery of most of the enzyme activity. Analysis for PMPMEase activity revealed activity levels that were comparable to the controls, implying that the lost enzyme activity was due to the presence of AA in the treated cells. This is an indication that the absence of PMPMEase activity in the AA-treated cells is not due to PMPMEase degradation but to its inhibition by AA. Furthermore, DMSO-induced degenerated cells lost only 49 ± 3% of the enzyme activity.
Figure 6.
(A): PMPMEase activity in degenerating AA-treated human neuroblastoma SH-SY5Y cells. Human neuroblastoma cells were cultured and seeded in 24-well plates as described in the methods. They were then treated with the indicated concentrations of AA or EPA. After 24 h, the cells were lysed as described in the methods and the PMPMEase activity determined using RD-PNB as the substrate. (B): AA-treated, degenerating human neuroblastoma cells were lysed and aliquots were pre-incubated with either COX-1 (23.5 units) or COX-2 (9 units) for 1 h. The RD-PNB substrate was added and the mixture further incubated for 3 h to determine the PMPMEase activity. The results are relative to the controls and are the means (± SEM, N=4). ***P<0.001 versus untreated control cells compared by ANOVA, followed by the Dunnett’s post-test.
4 Discussion
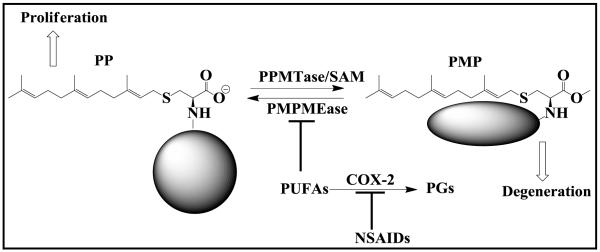
Consumption of foods rich in PUFAs has been widely reported to reduce the risks of cancers (39, 40). The apoptotic effects of omega-3 and omega-6 PUFAs on various tumor cell lines have been widely reported (23, 41-44). Reduced levels of prostatic PUFAs have also been reported in locally advanced prostate cancer (45). The mechanisms of these effects which suggest a beneficial role for PUFAs are not well understood. COX-2 expression in various tumors and cancer cell lines have led to the belief that its PGs products are tumorigenic (20). This theory has been supported by the lower cancer risks associated with prolonged use of NSAIDS (26). The finding of the current study that some FFAs are potent inhibitors of PMPMEase while all the PGs are over 63-fold less inhibitory than AA could explain the role of COX-2 in cancers as this implies that the conversion of PUFAs to PGs likely alters the metabolism and functions of polyisoprenylated proteins, albeit indirectly. PMPMEase, which has a high affinity for S-trans, trans-farnesylated and S-all trans-geranylgeranylated cysteinyl ester substrates (35, 46) is one of two enzymes that catalyze reactions in the last and only reversible step of the polyisoprenylation pathway. PMPMEase and the methyl ester-forming PPMTase may switch the conformations of polyisoprenylated proteins between functional and non-functional states (47). While acting as endogenous modulators of PMPMEase activity, FFAs may thus indirectly control polyisoprenylated protein effects on cell viability and the associated conditions (Figure 7).
Figure 7.
Possible interrelationship between PUFAs metabolism and cell viability: PUFAs inhibition of PMPMEase probably contributes to a natural equilibrium between methylated (PMP) and unmethylated (PP) polyisoprenylated proteins. COX-2 expression in tumors results in the conversion of PUFAs to PGs, the loss of the PMPMEase inhibitory potency and a shift in the equilibrium in favor of PP and cell proliferation. This may explain why long term use of NSAIDs and consumption of PUFAs-rich foods may owe their anticancer benefits to the increased tissue levels of PUFAs and the resulting PMPMEase inhibition.
The correlation between the relative inhibition of PMPMEase and the induction of cell degeneration by FFAs and PGs suggest that PMPMEase inhibition is intricate to the effects of the FFAs on cell viability. This is in agreement with previous reports showing that AA and other FFAs induce cell degeneration at concentrations that overlap physiological levels (21, 48). The 6.6 μM IC50 for AA against PMPMEase overlaps the 9.3 to 78.8 μM concentrations of unesterified AA in mammalian plasma (27, 29, 30, 49). Cerebral free AA has been measured at 35.5 μg/g of tissue (28). Although a substantial proportion of the AA and other FFAs may be protein-bound and the suppression of cell viability was indeed diminished in the presence of serum, the combined effect of all the inhibitory FFAs is likely to play a significant role in modulating PMPMEase activity and cell proliferation. Unlike AA and the other FFAs that inhibit PMPMEase and cell viability, the concentrations of the PGs that either only marginally inhibited or had no effect on PMPMEase are well above the 1024, 378 and 84 pg/mL (approximately 3, 1 and 0.02 nM) for PGA, PGE and PGF in human plasma, respectively (31). This implies that the PGs are unlikely to have any PMPMEase-dependent physiological effects.
The PGs differ from the FFAs and the polyisoprenyl moiety of polyisoprenylated proteins by virtue of the hydrophilic hydroxyl, ketone and carboxylic acid groups that likely diminish their ability to bind to the PMPMEase active site. Crystallographic studies of human carboxylesterase 1 (hCE1), the human isoform of PMPMEase, reveal an active site lined with hydrophobic amino acid residues (50). The cis-polyunsaturation of the FFAs hydrocarbon chain renders them structurally similar to the polyisoprenyl tails of the substrates. Molecular modeling studies of polyisoprenylated substrates reveal curved polyisoprenyl tails for trans,trans-farnesyl and all trans-geranylgeranyl substrates in their lowest energy conformations (35). Docking to hCE1/human PMPMEase reveal that polyisoprenylated substrates in these conformations fit the contours of the hydrophobic active site (46). Moreover, synthetic polyisoprenylated sulfonyl fluorides which are specific inhibitors of PMPMEase have been shown to induce cell degeneration (51). It appears that a combination of type and degree of unsaturation and the length of each FFA determine the relative inhibitory potency of the FFA on both PMPMEase and cell viability. While the moderate length saturated FFAs such as the C10 to C16 may be easily accommodated at the enzyme active site, the longer chain saturated, structurally linear FFAs may not be accessible while the very short chain BuA, HeA and OcA lack sufficient interactions required for high affinity binding. The fact that the FFAs which are not substrates for PGs synthesis inhibited PMPMEase as well as induce cell degeneration is an indication that PMPMEase inhibition is likely to be involved in the degeneration process. This may imply that the elevated levels of COX-2 in various cancers (15, 32-34) may convert the more effective PMPMEase-inhibiting AA, EPA and other PUFAs into ineffective prostanoids with significantly diminished abilities to inhibit PMPMEase and control cell proliferation. This is supported by the correlation between the AA effects on cell viability and the PMPMEase activity in the lysate from AA-treated cell as well as the fact that those FFAs that did not inhibit purified PMPMEase also had no observable effects on the cells. This is further substantiated by the recovery of PMPMEase activity in lysates from AA-induced degenerating cells. The PMPMEase inhibition by AA and other FFAs such as EPA and DHA may be the mechanism for the apoptotic effects that have been observed for these FFAs by various researchers (21, 52). The notion that PUFAs may be depleted in cell proliferation is better supported by PGE levels in the plasmas of cancer patients that were reported to be 10- to over 100-fold higher than in normal individuals (31). Lower PUFAs levels in cancer have been reported (45, 53).
PMPMEase is the last member of the polyisoprenylation pathway enzymes whose role as putative drug target has not been evaluated. It (under the pseudonym human carboxylesterase 1) is a member of the 11-gene signature whose expression in primary tumors is a strong indicator of rapid recurrence and poor therapeutic outcomes and death (54). Farnesylation inhibitors, which were developed as modulators of mutated constitutively active oncogenic Ras, have reached advanced phases of clinical trials (11, 55, 56). Likewise, PPMTase inhibitors have been developed as putative anticancer agents (57). The demonstration here that AA and other FFAs are potential endogenous modulators of cell viability through their interactions with PMPMEase is an indication that PMPMEase may be a pivotal enzyme of the polyisoprenylation pathway and therefore a valuable target for anticancer drug development.
That PMPMEase inhibition results in cell degeneration is important not only for their antitumor aspect but also for our understanding of diseases on the other extreme of the cell viability spectrum. Abnormally high tissue levels of PMPMEase-inhibitory FFAs may potentially be deleterious to tissues with low PMPMEase activities. Indeed, while about 30% of cancers are associated with abnormally high polyisoprenylated monomeric G-protein activities, diminished polyisoprenylated enzyme function have serious physiological consequences. PPMTase knockout is lethal in mice (58). Loss of function mutations of key proteins of the polyisoprenylation pathway enzymes result in extensive cell degeneration. For example, mutated defective Rab escort protein (REB-1) prevents the geranylgeranylation of Rab resulting in choroideremia (59, 60). Similarly, injection of S-adenosyl-L-methionine, a co-enzyme for PPMTase causes transient Parkinson’s disease (PD) -like effects (61) that are blocked by polyisoprenylated cysteine analogs (62). The reported neuronal degeneration (63) and Parkinsonism (64, 65) caused by exposure to organophosphorus compounds could be due to their potent inhibition of PMPMEase (3, 66). It appears therefore that care must be exercised when supplementing diets with cocktails of FFAs that inhibit PMPMEase as excessive PMPMEase inhibition may extend beyond merely controlling cell viability to the degeneration of vulnerable cells such as neurons.
5 Conclusion
The inhibition of PMPMEase by PUFAs appears to suggest that FFAs such AA, EPA and DHA may be physiological regulators of PMPMEase activity. The loss of PMPMEase inhibitory potency when PUFAs are converted to PGs is also an indication that elevated COX-2 levels in tissues would be synonymous with lower PUFAs concentrations and higher PMPMEase activities. The combined results of the FFAs on PMPMEase and cell degeneration suggest that the elevated COX-2 levels in some tumors likely result in the destruction of cancer-preventing FFAs such as EPA, DHA and AA and not necessarily the formation of tumorigenic PGs as has been proposed (23).
Practical application.
Some PUFAs have been widely reported to have anticancer benefits. However, the molecular mechanisms for these effects are not well understood. The findings in the current manuscript appear to suggest that inhibition of PMPMEase may underlie their effects. They also imply that the expression of COX-2 in various tumors may serve to convert the PUFAs into significantly less inhibitory prostaglandins. From these findings, AA and the other PUFAs, rather than being substrates for the synthesis of tumorigenic agents may actually contribute in suppressing cell proliferation. This being congruent with the lower cancer risks associated with long term use of anti-inflammatory agents, the practical implications will likely include the nutritional and/or therapeutic management of cancer with the goal of maintaining suitable levels of the fatty acids in tissues.
Acknowledgements
This work was supported by NIH/NIGMS/SCORE Grant number GM 08111-35 and by the Pharmaceutical Research Center NIH/NCRR Grant number G12 RR0 3020. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
List of abbreviations used
- ARA
arachidic acid
- BcA
behenic acid
- BuA
butanoic acid
- COX-1
cycloxygenase-1
- COX-2
cycloxygenase-2
- DeA
decanoic acid
- EA
elaidic acid
- ER
erucic acid
- FFAs
free fatty acids
- hCE1
human carboxylesterase 1
- HeA
hexanoic acid
- LA
lauric acid
- Ln
lignoceric acid
- LNA
linolenic acid
- LO
linoleic acid
- MA
myristic acid
- MY
myristoleic acid
- NSAIDs
non-steroidal anti-inflammatory drugs
- OA
oleic acid
- OcA
octanoic acid
- PA
palmitic acid
- PD
Parkinson’s disease
- PGA2
prostaglandin A2
- PGE2
prostaglandin E2
- PGs
prostaglandins
- POA
palmitoleic acid
- PMPMEase
polyisoprenylated methylated protein methylesterase
- PPMTase
polyisoprenylated protein methyl transferase or isoprenyl carboxylmethyl transferase (icmt)
- RD-PNB
N-(4-nitrobenzoyl)-S-trans, trans-farnesyl-L-cysteine methyl ester
- STE
Stearic acid
Footnotes
7 Conflict of interests
The authors have declared no conflict of interest.
8 References
- 1.Ma YT, Gilbert BA, Rando RR. Farnesylcysteine analogs to probe role of prenylated protein methyltransferase. Methods Enzymol. 1995;250:226–234. doi: 10.1016/0076-6879(95)50075-8. [DOI] [PubMed] [Google Scholar]
- 2.Stephenson RC, Clarke S. Identification of a C-terminal protein carboxyl methyltransferase in rat liver membranes utilizing a synthetic farnesyl cysteine-containing peptide substrate. J Biol Chem. 1990;265:16248–16254. [PubMed] [Google Scholar]
- 3.Lamango NS. Liver prenylated methylated protein methyl esterase is an organophosphate-sensitive enzyme. J Biochem Mol Toxicol. 2005;19:347–357. doi: 10.1002/jbt.20100. [DOI] [PubMed] [Google Scholar]
- 4.Perez-Sala D, Tan EW, Canada FJ, Rando RR. Methylation and demethylation reactions of guanine nucleotide-binding proteins of retinal rod outer segments. Proc Natl Acad Sci U S A. 1991;88:3043–3046. doi: 10.1073/pnas.88.8.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oboh OT, Lamango NS. Liver prenylated methylated protein methyl esterase is the same enzyme as Sus scrofa carboxylesterase. J Biochem Mol Toxicol. 2008;22:51–62. doi: 10.1002/jbt.20214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duverna R, Ablordeppey SY, Lamango NS. Biochemical and Docking Analysis of Substrate Interactions with Polyisoprenylated Methylated Protein Methyl Esterase. Current cancer drug targets. 2010 doi: 10.2174/156800910791859443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nalivaeva NN, Turner AJ. Post-translational modifications of proteins: acetylcholinesterase as a model system. Proteomics. 2001;1:735–747. doi: 10.1002/1615-9861(200106)1:6<735::AID-PROT735>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 8.Konstantinopoulos PA, Karamouzis MV, Papavassiliou AG. Post-translational modifications and regulation of the RAS superfamily of GTPases as anticancer targets. Nat Rev Drug Discov. 2007;6:541–555. doi: 10.1038/nrd2221. [DOI] [PubMed] [Google Scholar]
- 9.Bredel M, Pollack IF, Freund JM, Hamilton AD, Sebti SM. Inhibition of Ras and related G-proteins as a therapeutic strategy for blocking malignant glioma growth. Neurosurgery. 1998;43:124–131. doi: 10.1097/00006123-199807000-00081. discussion 131-122. [DOI] [PubMed] [Google Scholar]
- 10.Ghobrial IM, Adjei AA. Inhibitors of the ras oncogene as therapeutic targets. Hematol Oncol Clin North Am. 2002;16:1065–1088. doi: 10.1016/s0889-8588(02)00050-3. [DOI] [PubMed] [Google Scholar]
- 11.Gotlib J. Farnesyltransferase inhibitor therapy in acute myelogenous leukemia. Curr Hematol Rep. 2005;4:77–84. [PubMed] [Google Scholar]
- 12.Winter-Vann AM, Casey PJ. Post-prenylation-processing enzymes as new targets in oncogenesis. Nat Rev Cancer. 2005;5:405–412. doi: 10.1038/nrc1612. [DOI] [PubMed] [Google Scholar]
- 13.Kloog Y, Cox AD. Prenyl-binding domains: potential targets for Ras inhibitors and anti-cancer drugs. Semin Cancer Biol. 2004;14:253–261. doi: 10.1016/j.semcancer.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Ohkanda J, Knowles DB, Blaskovich MA, Sebti SM, Hamilton AD. Inhibitors of protein farnesyltransferase as novel anticancer agents. Curr Top Med Chem. 2002;2:303–323. doi: 10.2174/1568026023394281. [DOI] [PubMed] [Google Scholar]
- 15.Hong SH, Avis I, Vos MD, Martinez A, Treston AM, Mulshine JL. Relationship of arachidonic acid metabolizing enzyme expression in epithelial cancer cell lines to the growth effect of selective biochemical inhibitors. Cancer Res. 1999;59:2223–2228. [PubMed] [Google Scholar]
- 16.Kim YB, Kim GE, Cho NH, Pyo HR, Shim SJ, Chang SK, Park HC, Suh CO, Park TK, Kim BS. Overexpression of cyclooxygenase-2 is associated with a poor prognosis in patients with squamous cell carcinoma of the uterine cervix treated with radiation and concurrent chemotherapy. Cancer. 2002;95:531–539. doi: 10.1002/cncr.10684. [DOI] [PubMed] [Google Scholar]
- 17.Half E, Tang XM, Gwyn K, Sahin A, Wathen K, Sinicrope FA. Cyclooxygenase-2 expression in human breast cancers and adjacent ductal carcinoma in situ. Cancer Res. 2002;62:1676–1681. [PubMed] [Google Scholar]
- 18.Liao Z, Milas L. COX-2 and its inhibition as a molecular target in the prevention and treatment of lung cancer. Expert Rev Anticancer Ther. 2004;4:543–560. doi: 10.1586/14737140.4.4.543. [DOI] [PubMed] [Google Scholar]
- 19.Harizi H, Corcuff JB, Gualde N. Arachidonic-acid-derived eicosanoids: roles in biology and immunopathology. Trends Mol Med. 2008;14:461–469. doi: 10.1016/j.molmed.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Wang D, DuBois RN. Pro-inflammatory prostaglandins and progression of colorectal cancer. Cancer Lett. 2008;267:197–203. doi: 10.1016/j.canlet.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pompeia C, Lima T, Curi R. Arachidonic acid cytotoxicity: can arachidonic acid be a physiological mediator of cell death? Cell Biochem Funct. 2003;21:97–104. doi: 10.1002/cbf.1012. [DOI] [PubMed] [Google Scholar]
- 22.Hughes-Fulford M, Chen Y, Tjandrawinata RR. Fatty acid regulates gene expression and growth of human prostate cancer PC-3 cells. Carcinogenesis. 2001;22:701–707. doi: 10.1093/carcin/22.5.701. [DOI] [PubMed] [Google Scholar]
- 23.Hughes-Fulford M, Tjandrawinata RR, Li CF, Sayyah S. Arachidonic acid, an omega-6 fatty acid, induces cytoplasmic phospholipase A2 in prostate carcinoma cells. Carcinogenesis. 2005;26:1520–1526. doi: 10.1093/carcin/bgi112. [DOI] [PubMed] [Google Scholar]
- 24.Verlengia R, Gorjao R, Kanunfre CC, Bordin S, Martins De Lima T, Martins EF, Curi R. Comparative effects of eicosapentaenoic acid and docosahexaenoic acid on proliferation, cytokine production, and pleiotropic gene expression in Jurkat cells. J Nutr Biochem. 2004;15:657–665. doi: 10.1016/j.jnutbio.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 25.Berquin IM, Edwards IJ, Chen YQ. Multi-targeted therapy of cancer by omega-3 fatty acids. Cancer Lett. 2008;269:363–377. doi: 10.1016/j.canlet.2008.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reddy BS, Patlolla JM, Simi B, Wang SH, Rao CV. Prevention of colon cancer by low doses of celecoxib, a cyclooxygenase inhibitor, administered in diet rich in omega-3 polyunsaturated fatty acids. Cancer Res. 2005;65:8022–8027. doi: 10.1158/0008-5472.CAN-05-0212. [DOI] [PubMed] [Google Scholar]
- 27.Pace-Asciak CR. One-step rapid extractive methylation of plasma nonesterified fatty acids for gas chromatographic analysis. J Lipid Res. 1989;30:451–454. [PubMed] [Google Scholar]
- 28.Yasuda H, Kishiro K, Izumi N, Nakanishi M. Biphasic liberation of arachidonic and stearic acids during cerebral ischemia. J Neurochem. 1985;45:168–172. doi: 10.1111/j.1471-4159.1985.tb05489.x. [DOI] [PubMed] [Google Scholar]
- 29.Zhou L, Vessby B, Nilsson A. Quantitative role of plasma free fatty acids in the supply of arachidonic acid to extrahepatic tissues in rats. J Nutr. 2002;132:2626–2631. doi: 10.1093/jn/132.9.2626. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez de Turco EB, Belayev L, Liu Y, Busto R, Parkins N, Bazan NG, Ginsberg MD. Systemic fatty acid responses to transient focal cerebral ischemia: influence of neuroprotectant therapy with human albumin. J Neurochem. 2002;83:515–524. doi: 10.1046/j.1471-4159.2002.01121.x. [DOI] [PubMed] [Google Scholar]
- 31.Jaffe BM, Behrman HR, Parker CW. Radioimmunoassay measurement of prostaglandins E, A, and F in human plasma. J Clin Invest. 1973;52:398–405. doi: 10.1172/JCI107196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mobius C, Stein HJ, Spiess C, Becker I, Feith M, Theisen J, Gais P, Jutting U, Siewert JR. COX2 expression, angiogenesis, proliferation and survival in Barrett’s cancer. Eur J Surg Oncol. 2005;31:755–759. doi: 10.1016/j.ejso.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 33.Steffensen KD, Waldstrom M, Jeppesen U, Jakobsen E, Brandslund I, Jakobsen A. The prognostic importance of cyclooxygenase 2 and HER2 expression in epithelial ovarian cancer. Int J Gynecol Cancer. 2007;17:798–807. doi: 10.1111/j.1525-1438.2006.00855.x. [DOI] [PubMed] [Google Scholar]
- 34.Hashimoto N, Inayama M, Fujishima M, Shiozaki H. Clinicopathologic significance of expression of cyclooxygenase-2 in human esophageal squamous cell carcinoma. Hepatogastroenterology. 2007;54:758–760. [PubMed] [Google Scholar]
- 35.Lamango NS, Duverna R, Zhang W, Ablordeppey SY. Porcine Liver Carboxylesterase Requires Polyisoprenylation for High Affinity Binding to Cysteinyl Substrates. TOEIJ. 2009;2:12–27. doi: 10.2174/1874940200902010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tan EW, Rando RR. Identification of an isoprenylated cysteine methyl ester hydrolase activity in bovine rod outer segment membranes. Biochemistry. 1992;31:5572–5578. doi: 10.1021/bi00139a021. [DOI] [PubMed] [Google Scholar]
- 37.Wiemer AJ, Hohl RJ, Wiemer DF. The intermediate enzymes of isoprenoid metabolism as anticancer targets. Anticancer Agents Med Chem. 2009;9:526–542. doi: 10.2174/187152009788451860. [DOI] [PubMed] [Google Scholar]
- 38.Forman S, Kas J, Fini F, Steinberg M, Ruml T. The effect of different solvents on the ATP/ADP content and growth properties of HeLa cells. J Biochem Mol Toxicol. 1999;13:11–15. doi: 10.1002/(sici)1099-0461(1999)13:1<11::aid-jbt2>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 39.Moyad MA. An introduction to dietary/supplemental omega-3 fatty acids for general health and prevention: part I. Urol Oncol. 2005;23:28–35. doi: 10.1016/j.urolonc.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 40.Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (Maywood) 2008;233:674–688. doi: 10.3181/0711-MR-311. [DOI] [PubMed] [Google Scholar]
- 41.Pompeia C, Freitas JJ, Kim JS, Zyngier SB, Curi R. Arachidonic acid cytotoxicity in leukocytes: implications of oxidative stress and eicosanoid synthesis. Biol Cell. 2002;94:251–265. doi: 10.1016/s0248-4900(02)01200-5. [DOI] [PubMed] [Google Scholar]
- 42.Dommels YE, Haring MM, Keestra NG, Alink GM, van Bladeren PJ, van Ommen B. The role of cyclooxygenase in n-6 and n-3 polyunsaturated fatty acid mediated effects on cell proliferation, PGE(2) synthesis and cytotoxicity in human colorectal carcinoma cell lines. Carcinogenesis. 2003;24:385–392. doi: 10.1093/carcin/24.3.385. [DOI] [PubMed] [Google Scholar]
- 43.Soyland E, Nenseter MS, Braathen L, Drevon CA. Very long chain n-3 and n-6 polyunsaturated fatty acids inhibit proliferation of human T-lymphocytes in vitro. European journal of clinical investigation. 1993;23:112–121. doi: 10.1111/j.1365-2362.1993.tb00750.x. [DOI] [PubMed] [Google Scholar]
- 44.Chan TA, Morin PJ, Vogelstein B, Kinzler KW. Mechanisms underlying nonsteroidal antiinflammatory drug-mediated apoptosis. Proc Natl Acad Sci U S A. 1998;95:681–686. doi: 10.1073/pnas.95.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Freeman VL, Meydani M, Hur K, Flanigan RC. Inverse association between prostatic polyunsaturated fatty acid and risk of locally advanced prostate carcinoma. Cancer. 2004;101:2744–2754. doi: 10.1002/cncr.20676. [DOI] [PubMed] [Google Scholar]
- 46.Duverna R, Ablordeppey SY, Lamango NS. Biochemical and docking analysis of substrate interactions with polyisoprenylated methylated protein methyl esterase. CCDT. doi: 10.2174/156800910791859443. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Philips MR, Pillinger MH, Staud R, Volker C, Rosenfeld MG, Weissmann G, Stock JB. Carboxyl methylation of Ras-related proteins during signal transduction in neutrophils. Science. 1993;259:977–980. doi: 10.1126/science.8438158. [DOI] [PubMed] [Google Scholar]
- 48.Kinsella JE, Black JM. Effects of polyunsaturated fatty acids on the efficacy of antineoplastic agents toward L5178Y lymphoma cells. Biochem Pharmacol. 1993;45:1881–1887. doi: 10.1016/0006-2952(93)90447-5. [DOI] [PubMed] [Google Scholar]
- 49.Zhou L, Xu N, Nilsson A. Tissue uptake and interconversion of plasma unesterified 14C linoleic acid in the guinea pig. Biochim Biophys Acta. 1997;1349:197–210. doi: 10.1016/s0005-2760(97)00131-8. [DOI] [PubMed] [Google Scholar]
- 50.Bencharit S, Edwards CC, Morton CL, Howard-Williams EL, Kuhn P, Potter PM, Redinbo MR. Multisite promiscuity in the processing of endogenous substrates by human carboxylesterase 1. J Mol Biol. 2006;363:201–214. doi: 10.1016/j.jmb.2006.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aguilar B, Amissah F, Duverna R, Lamango NS. Polyisoprenylation potentiates the inhibition of polyisoprenylated methylated protein methyl esterase and the cell degenerative effects of sulfonyl fluorides. Current cancer drug targets. 2011;11:752–762. doi: 10.2174/156800911796191015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martins de Lima T, Cury-Boaventura MF, Giannocco G, Nunes MT, Curi R. Comparative toxicity of fatty acids on a macrophage cell line (J774) Clin Sci (Lond) 2006;111:307–317. doi: 10.1042/CS20060064. [DOI] [PubMed] [Google Scholar]
- 53.Faas FH, Dang AQ, White J, Schaefer RF, Johnson DE. Decreased prostatic arachidonic acid in human prostatic carcinoma. BJU international. 2003;92:551–554. doi: 10.1046/j.1464-410x.2003.04387.x. [DOI] [PubMed] [Google Scholar]
- 54.Glinsky GV, Berezovska O, Glinskii AB. Microarray analysis identifies a death-from-cancer signature predicting therapy failure in patients with multiple types of cancer. J Clin Invest. 2005;115:1503–1521. doi: 10.1172/JCI23412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Doll RJ, Kirschmeier P, Bishop WR. Farnesyltransferase inhibitors as anticancer agents: critical crossroads. Curr Opin Drug Discov Devel. 2004;7:478–486. [PubMed] [Google Scholar]
- 56.Sebti SM. Protein farnesylation: implications for normal physiology, malignant transformation, and cancer therapy. Cancer Cell. 2005;7:297–300. doi: 10.1016/j.ccr.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 57.Marom M, Haklai R, Ben-Baruch G, Marciano D, Egozi Y, Kloog Y. Selective inhibition of Ras-dependent cell growth by farnesylthiosalisylic acid. J Biol Chem. 1995;270:22263–22270. doi: 10.1074/jbc.270.38.22263. [DOI] [PubMed] [Google Scholar]
- 58.Bergo MO, Lieu HD, Gavino BJ, Ambroziak P, Otto JC, Casey PJ, Walker QM, Young SG. On the physiological importance of endoproteolysis of CAAX proteins: heart-specific RCE1 knockout mice develop a lethal cardiomyopathy. J Biol Chem. 2004;279:4729–4736. doi: 10.1074/jbc.M310081200. [DOI] [PubMed] [Google Scholar]
- 59.Tolmachova T, Anders R, Abrink M, Bugeon L, Dallman MJ, Futter CE, Ramalho JS, Tonagel F, Tanimoto N, Seeliger MW, Huxley C, Seabra MC. Independent degeneration of photoreceptors and retinal pigment epithelium in conditional knockout mouse models of choroideremia. J Clin Invest. 2006;116:386–394. doi: 10.1172/JCI26617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perez-Sala D. Protein isoprenylation in biology and disease: general overview and perspectives from studies with genetically engineered animals. Front Biosci. 2007;12:4456–4472. doi: 10.2741/2401. [DOI] [PubMed] [Google Scholar]
- 61.Lamango NS, Nesby RA, Charlton CG. Quantification of S-adenosylmethionine-induced tremors: a possible tremor model for Parkinson’s disease. Pharmacol Biochem Behav. 2000;65:523–529. doi: 10.1016/s0091-3057(99)00220-8. [DOI] [PubMed] [Google Scholar]
- 62.Lamango NS, Charlton CG. Farnesyl-L-cysteine analogs block SAM-induced Parkinson’s disease-like symptoms in rats. Pharmacol Biochem Behav. 2000;66:841–849. doi: 10.1016/s0091-3057(00)00274-4. [DOI] [PubMed] [Google Scholar]
- 63.Glynn P. Axonal degeneration and neuropathy target esterase. Arh Hig Rada Toksikol. 2007;58:355–358. doi: 10.2478/v10004-007-0029-z. [DOI] [PubMed] [Google Scholar]
- 64.Bhatt MH, Elias MA, Mankodi AK. Acute and reversible parkinsonism due to organophosphate pesticide intoxication: five cases. Neurology. 1999;52:1467–1471. doi: 10.1212/wnl.52.7.1467. [DOI] [PubMed] [Google Scholar]
- 65.Arima H, Sobue K, So M, Morishima T, Ando H, Katsuya H. Transient and reversible parkinsonism after acute organophosphate poisoning. J Toxicol Clin Toxicol. 2003;41:67–70. doi: 10.1081/clt-120018273. [DOI] [PubMed] [Google Scholar]
- 66.Lamango NS, Koikov L, Duverna R, Abonyo BO, Hubbard JB. Non-cholinergic organophosphorus toxicity: possible mechanism involving the protein prenylation pathway. In: Webster LR, editor. Neurotoxicity Syndromes. Nova Science Publishers, Inc.; New York: 2007. pp. 37–68. [Google Scholar]