Abstract
Sebaceous neoplasms are commonly considered in their relationship to the Muir-Torre Syndrome and the now well-documented loss of DNA mismatch repair proteins leading to microsatellite instability. However, sebaceous neoplasms showing microsatellite instability comprise only a subset of this group of tumors, and thus alternative tumorigenic mechanisms must exist. This paper explores the relationship of p53, a tumor suppressor implicated in other cutaneous malignancies, and sebaceous neoplasia. We examined 94 sebaceous tumors from 92 patients. Tumors with strong nuclear p53 staining were significantly associated with the diagnosis of sebaceous carcinoma compared to benign sebaceous lesions, most notably for periocular carcinomas. Importantly, nuclear mismatch repair protein expression was intact in all lesions showing p53 alterations, suggesting that p53 dysfunction may represent a divergent pathway in the molecular pathogenesis of these tumors.
Keywords: Sebaceous adenoma, sebaceoma, sebaceous carcinoma, p53, Muir-Torre Syndrome, DNA mismatch repair, microsatellite instability
INTRODUCTION
Numerous signaling pathways and genetic mutations have been implicated in tumor biology. Cutaneous sebaceous neoplasms comprise a group of lesions for which one particular pathway of tumorigenesis has been extensively studied, in part because of their association with the Muir-Torre syndrome (MTS). MTS refers to the association of cutaneous sebaceous neoplasia and internal visceral malignancies, most frequently colonic adenocarcinoma 1–3. Patients with Muir-Torre syndrome comprise a small subset of patients (less than 5%) with hereditary nonpolyposis colorectal cancer (HNPCC) syndrome, an autosomal dominant condition defined by mutation of one of several genes encoding DNA mismatch repair proteins. In MTS, most commonly MSH2 is affected and less frequently MLH1 or other members of this family such as MSH6; in HNPCC MLH1 and MSH2 are equally affected 4–6. The germline mutation, coupled with a second somatic mutation, results in defective repair of DNA base pair replication errors. These are most commonly encountered in short stretches of DNA characterized by repetitive mono- or dinucleotide repeats, termed microsatellites. Microsatellite instability predisposes patients to development of numerous tumors, including sebaceous neoplasms 7, 8.
Interestingly, several reports have proposed that specific characteristics of sebaceous tumor type, location, and histology may predict deficiency in mismatch repair enzymes, microsatellite instability, and the Muir-Torre syndrome 9–10. In keeping with the distribution of normal sebaceous glands, sebaceous tumors are most often found in the head and neck region. However, two groups have reported that mismatch repair deficiency is statistically more common in sebaceous tumors occurring outside of the head and neck region 11, 12. Furthermore, tumor type has been correlated to mismatch repair status, with nearly half of benign sebaceous tumors (adenomas and sebaceomas) displaying deficiencies in mismatch repair enzymes compared to only 15% of sebaceous carcinomas. More specifically, carcinomas in the head and neck region were shown to exhibit even less frequent mismatch repair deficiency 11. So, while mismatch repair deficiency within sebaceous lesions certainly plays a role in the pathogenesis of some of these tumors, it is clear that mechanisms beyond microsatellite instability must contribute to tumorigenesis in a subset of sebaceous lesions.
P53 is a well described tumor suppressor gene, whose loss is implicated in a myriad of cancers 13–15. Normally kept at low levels within the cell, p53 is activated by cell stressors such as oxidative stress and DNA damage to regulate the transcription of genes which protect the cell from further damage, including activation of DNA repair pathways and cell cycle arrest 13. The majority of p53 mutations occur in the DNA binding domain, resulting in impaired downstream regulation of cell cycle control and faulty or absent repair of DNA damage. UV radiation leads to the development of specific, “signature” p53 mutations and has been linked to various cutaneous cancers 13, 16. Although limited research exists currently on the relationship of p53 mutations and sebaceous neoplasms 17–20, p53 represents a potential factor in tumor initiation or progression. Immunohistochemical staining for nuclear p53 is often increased in tumors with p53 mutations. While nuclear overexpression is not specific for mutations in the P53 gene, it seems to correlate with dysregulation of the signaling pathway, thus representing a reasonable method to investigate integrity of the p53 pathway 21–22. The relationship of p53 dysregulation and microsatellite instability in sebaceous neoplasia has not been investigated.
The goal of this study was to determine the frequency of p53 impairment as assessed by strong nuclear p53 immunohistochemical staining within sebaceous neoplasms and to correlate these findings with DNA mismatch repair status, tumor site, and tumor histology.
MATERIALS AND METHODS
Cases
This study utilized a large case series of sebaceous neoplasms previously described 11. The local institutional review board approved this study with a waiver of consent. Briefly, the tumors included 17 sebaceous hyperplasia serving as controls, 13 sebaceoma, 46 sebaceous adenoma, and 35 sebaceous carcinoma (both periocular (n= 10) and nonocular (n=22); site was unknown for three carcinomas). The criteria for diagnosis of each lesion were based on previously described criteria, with diagnoses reviewed and confirmed by three of the authors (A.J.L., S.S. and E.C.; AJL, American and EC, British board certified dermatopathologists). Sebaceous adenomas were defined as benign lesions with maintained organoid architecture, an increased basaloid cell population compared to sebaceous hyperplasia, but greater than 50% mature sebocyte differentiation 23. Sebaceomas, also benign lesions, had a loss of organoid architecture and more extensive (>50%) basaloid cell content compared to adenomas 24–26. Sebaceous carcinomas exhibited malignant features such as infiltrative architecture and/or cytologic atypia to a degree unacceptable in a benign lesion 27. Cystic change and keratoacanthoma-like architecture were assessed according to prior descriptions of these features 28–30. Patient demographics and tumor location were obtained from pathology reports.
Immunohistochemistry for p53 nuclear localization
Immunohistochemistry for p53 (clone DO7, dilution 1:100; DakoCytomation, Carpinteria, CA, USA) was performed on formalin-fixed, paraffin-embedded sections. Microscopically, p53 staining was assessed as either absent, some staining, or uniform nuclear staining. Some staining included patterns of weak nuclear and/or cytoplasmic staining in scattered cells. These patterns of staining generally do not correlate with mutated or dysfunctional p53 21, 31, 32. Staining was only considered positive if there was strong, uniform nuclear staining in the majority of the tumor cells, clearly distinct from surrounding, non-neoplastic tissue. This pattern of staining has been most closely linked to the identification of (predominately missense) p53 mutations 21, 31. The immunohistochemistry for mismatch repair enzymes MSH2, MLH1, and MSH6 was previously described and reported 11.
Statistical Analysis
For purposes of statistical analysis, patient demographics and tumor characteristics were summarized using tabulations for categorical variable and means and standard deviations for continuous variables. Relationships among categorical variables were assessed using the Chi-squared test for equal proportions or Fisher’s exact test as appropriate. Associations between age and categorical variables were determined using the unpaired Student’s t-test or one-way analysis of variance. All reported p-values were two sided and reported at a significance level of 5%. Analyses were performed with SAS for Windows (release 8.2; SAS Institute Inc., Cary, NC).
RESULTS
The analysis included 111 cases. These included seventeen cases of hyperplasia that were used as controls for the staining methods and were not a part of the primary analysis. There were 94 sebaceous neoplasms from 92 patients. Two patients had two tumors each. The diagnoses and immunohistochemical results were identical in both specimens from each of these two patients. The diagnoses for the 94 specimens comprised: 46 adenomas (49%), 13 sebaceomas (14%), and 35 carcinomas (37%).
Patient Characteristics and IHC
Seventeen cases of sebaceous hyperplasia from the head and neck served as controls. For fifteen of these cases, p53 staining was completely absent, while there was minimal, weak staining for two cases (scattered nuclei).
As previously reported 11, demographic information of age and sex was known in 80 of the 92 patients with sebaceous lesions. 47 (59%) were male and 33 (41%) were female. Patient age ranged from 34 to 94 years with a mean age of 70 years (SD, 14.7 years). Additional patient information, such as history of non-sebaceous skin cancers or visceral malignancy, was not available.
For the 94 specimens, tumor site was unavailable for 15 tumors (16%): 11 adenomas, 1 sebaceoma, and 3 carcinomas. Of the remaining 79 tumors, 82% were located in the head and neck region. Fourteen tumors (18%) occurred outside the head and neck area. The group of benign sebaceous neoplasms (adenomas and sebaceomas) was composed of 39 tumors in the head and neck region and 8 tumors located elsewhere. Of the sebaceous carcinomas, 26 tumors were located in the head and neck region and 6 were located elsewhere. Of head and neck sebaceous carcinomas, 10 were periocular and 16 were extraocular. As with previous reports 22, 33, 34, there was a significant association between periocular tumors and diagnosis (p=0.0223), with 10 of 14 (71%) periocular tumors diagnosed as carcinomas.
Of the 94 specimens, nine tumors demonstrated uniform, strong staining with p53 (9.6%). These tumors, considered to have a dysfunctional p53 pathway (and possibly mutated p53), comprised eight carcinomas (4 periocular) and one sebaceoma. Of these p53 positive tumors, tumor site was unknown in two carcinoma specimens; the remaining seven tumors were located in the head and neck region.
P53 staining was not statistically related to patient age or gender.
Association of p53 staining and tumor location
In the subset of tumors from the head and neck (n=65), seven specimens stained uniformly for p53 (11%; n= 7/65). No tumor from a non-head and neck region was positive for p53 staining (0%; n= 0/14) (Figure 1). However, when assessed for statistical significance, statistical significance was not achieved (p=0.2446), likely due to the small numbers of p53 positive specimens. However, there was a statistically significant percentage of tumors from the periocular region compared to the extraocular region (p=0.0201). Out of 14 periocular tumors (10 of which were carcinomas), 4 tumors (29%), all carcinomas, stained uniformly for p53. In contrast, only 3 of 61 extraocular tumors (5%) stained uniformly for p53. This result is likely due to the prevalence of carcinomas in the periocular region.
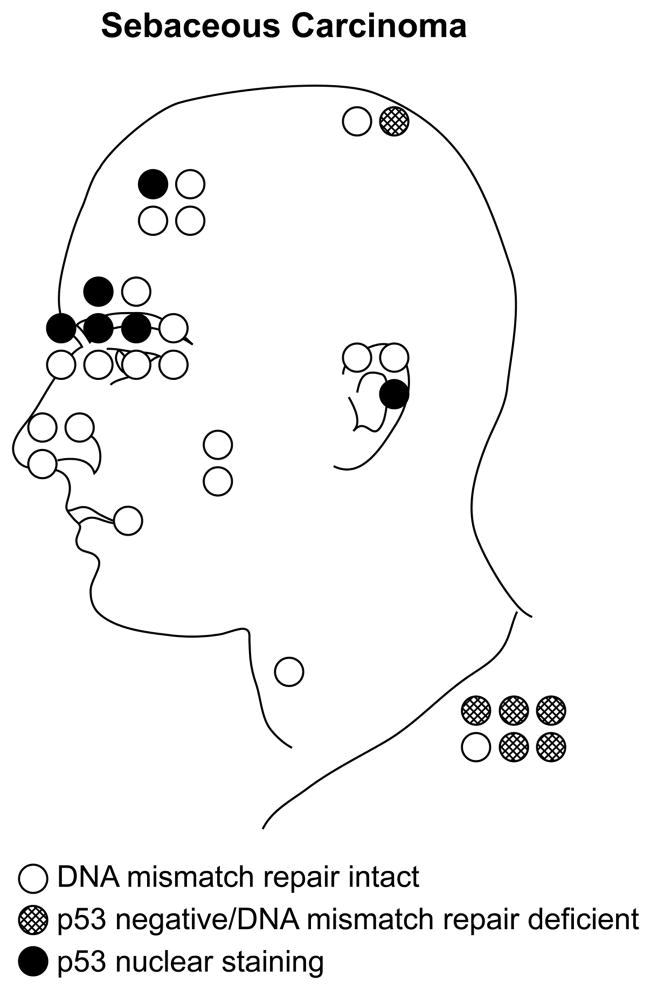
Figure 1.
Of 32 sebaceous carcinomas for which tumor site was known, 26 were located on the head and neck, while 6 were located elsewhere on the body. All carcinomas demonstrating p53 mutations for which tumor site was known were located in the head and neck region with a preponderance of p53 mutations found in periocular tumors (solid dark circles). In contrast, carcinomas showing mismatch repair protein deficiency (hatched circles) tended to be in non-head and neck locations. In addition to the tumors illustrated here, only three other tumors showed p53 mutations: a sebaceoma from the cheek, and two additional carcinomas for which tumor site was not known.
Association of p53 staining and diagnosis
Uniform, strong p53 staining was significantly associated with diagnosis. Benign sebaceous lesions (adenomas and sebaceomas) showed low rates of p53 staining (1.7%; n= 1/59) while carcinomas were more likely to stain uniformly for p53 (23%; n= 8/35; p= 0.0008 by Chi-squared test). The statistical significance carried over to individual diagnosis of adenoma, sebaceoma, and carcinoma as well, as determined by Fisher’s exact test (p=0.0014). No adenomas demonstrated p53 staining (0%; n=0/46) and only one sebaceoma showed uniform p53 staining (7.7%; n=1/13), but eight of 35 carcinomas showed uniform p53 staining (23%) (Table 1 and Figures 2 & 3).
Table 1.
P53 staining correlates with tumor type and location in sebaceous neoplasms.
| Diagnosis | Total number of diagnoses | Cases with p53 staining | Cases with MMRD | Cases with p53 staining and MMRD |
|---|---|---|---|---|
| Adenoma | 45 | 0 (0%) | 22 (48.9%) | 0 |
| Sebaceoma | 13 | 1 (7.7%) | 4 (30.8%) | 0 |
| Carcinoma | 35 | 8 (23%) | 5 (14.2%) | 0 |
Figure 2.
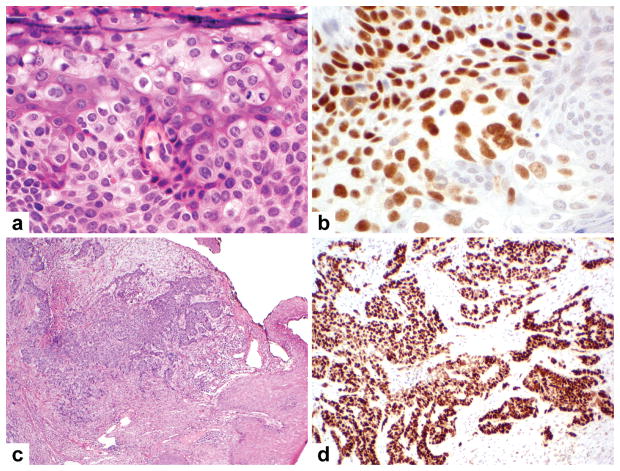
Sebaceous carcinomas, particularly those from the periocular region, were the most frequent sebaceous neoplasia to show p53 nuclear staining. A sebaceous carcinoma from the eyelid (A) demonstrates strong nuclear p53 immunoreactivity (B). Like many periocular sebaceous carcinomas, this one showed extensive pagetoid spread and mitotic figures. A sebaceous carcinoma from the ear (C), characterized by infiltrative growth pattern, focal necrosis, and poor differentiation, also demonstrates strong nuclear p53 staining (D).
Figure 3.
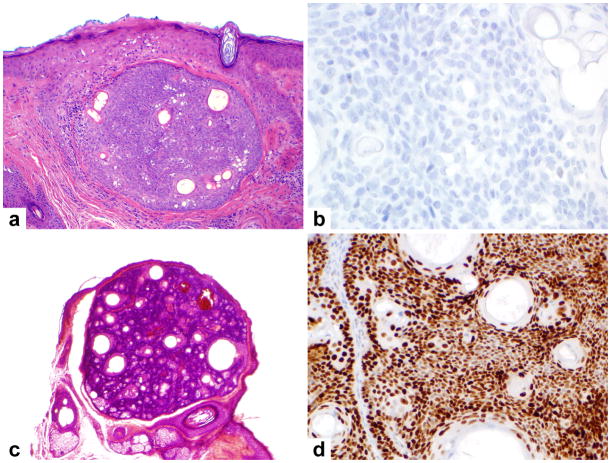
Most benign sebaceous neoplasms, such as this well-circumscribed sebaceoma (A), were not immunoreactive for p53 (B). A single sebaceoma from the cheek with lobular, benign architecture (C) showed strong p53 immunoreactivity (D).
Association of p53 staining and mismatch repair enzyme status
P53 staining results was significantly associated with mismatch repair enzyme status (p=0.0432). Uniform nuclear p53 staining (n=9) occurred only in specimens with intact mismatch repair protein expression, as assessed by immunohistochemistry (Table 1).
DISCUSSION
It is well established that a subset of sebaceous lesions harbor microsatellite instability and mutations in proteins encoding DNA mismatch repair enzymes 8, 11, 12, 35, 36. However, the presence of mismatch repair deficiency has been shown to occur less frequently in carcinomas than in benign sebaceous lesions and is seen more commonly in tumors occurring outside of the head and neck region 11, 12. Therefore, there are clearly other mechanisms contributing to the development of sebaceous lesions, particularly carcinomas.
Moreover, the head, neck and periorbital regions are exposed to frequent UV radiation, potentially making this an attractive factor in tumorigenesis of sebaceous neoplasia. Mutation of p53 and signaling alterations secondary to UV radiation 13, 37, the presence of p53 mutations in other non-melanoma skin cancers 13, 16, and previous reports noting p53 staining in sebaceous carcinomas are well documented 17–20. Dysfunction or lack of function of the p53 pathway appears to represent a potential mediator of an alternative mechanism of malignant sebaceous tumorigenesis (distinct from the microsatellite instability pathway). Proponents of field cancerization theory have demonstrated that numerous p53 clonal mutations occur as a result of chronic sun damage within the epidermis and can be present in “normal” appearing skin, thus putting patients at risk for the developing multiple skin cancers. The development of non-melanoma skin cancers are suggested to occur when p53 mutation involve the stem cell compartment of the epidermal proliferative unit 38, 39.
Interestingly, however, while a recent study by Kiyosaki and colleagues found a high percentage of p53 mutations in a small group of eyelid sebaceous carcinomas, the mutations were not the typical tandem mutations induced by UV damage 20. This finding raises the possibility that p53 dysregulation as a mechanism of sebaceous tumorigenesis may occur independently of UV damage. Indeed, periocular sebaceous carcinomas, which demonstrated the highest incidence of p53 nuclear staining in the present study, can often present on the conjunctival aspect of the eyelid, which is largely protected from solar damage.
The present study examined the relationship between tumor type and site with p53 dysregulation in sebaceous neoplasms as assessed by p53 immunohistochemistry. Intense nuclear p53 signal was significantly associated with tumor location (p=0.0201), occurring more frequently in the periocular region, and was significantly correlated with tumor type (p=0.0008), occurring almost exclusively in sebaceous carcinomas. Importantly, specimens demonstrating p53 staining showed intact DNA mismatch repair enzyme status, suggesting that p53 signaling alterations may represent an independent and distinct mechanism in the development of sebaceous carcinomas.
Previous reports have suggested alterations in p53 signaling as contributing to the pathogenesis of sebaceous lesions and reported p53 staining within these tumors 17–19. Gonzalez-Fernandez et al. examined a set of periocular sebaceous carcinomas, reporting p53 overexpression in 5 of 5 invasive sebaceous carcinomas. Two cases which were negative for p53 were diagnosed as in situ carcinomas without an invasive component 17. The high frequency of p53 positivity in their study can be accounted for by the selection of only a small number of periocular carcinomas examined. It does, however, correspond to our findings that this particular subset of sebaceous lesions are most likely to show p53 overexpression. McBride and colleagues examined a spectrum of sebaceous lesions, noting strong p53 overexpression in sebaceous carcinomas. However, this study did not comment on how many of the twelve carcinomas examined showed p53 staining, nor did it comment on whether tumor location impacted p53 staining 18. Moreover, benign sebaceous lesions examined in this study were also reported to have some degree of p53 staining, albeit in a fainter and topographically restricted pattern. Cabral and colleagues examined 27 benign and malignant sebaceous lesions. They found statistically significant increased percentages of p53 positive cells in carcinomas compared to adenomas and a trend for the intensity of p53 staining to be greater in carcinomas compared to benign lesions 19. Of the eight sebaceous carcinomas examined, only one was periocular. McBride’s and Cabral’s study differed from the present one in that p53 staining was assessed on a continuum, without a strict, categorical definition for positive staining. In the present study, focal or weak p53 staining was observed in 41% (n=38) of the specimens (data not shown); however, a strict criteria of uniform nuclear staining in the majority of cells was used to designate “positive staining”, as this pattern has been shown to correlate more closely with clonal p53 mutations and disruption of the p53 signaling network 21, 31.
Our study confirms previous reports that p53 is overexpressed in a subset of sebaceous tumors. This study expands the current body of knowledge by establishing a firm link between p53 dysregulation and the diagnosis of sebaceous carcinoma. Nearly one quarter of examined carcinomas showed p53 staining, while all adenomas were negative and only one sebaceoma was positive. Because the number of specimens examined in this study is larger than in previous similar studies, this study likely approximates the true incidence of p53 mutation and the frequency with which it is seen within subsets of sebaceous neoplasia. Our study also demonstrates a strong association between p53 dysregulation and periocular tumor location, however, this may be related to the overall increased prevalence of carcinomas in the periocular region. For seven p53-staining sebaceous tumors for which the tumor site was known, all were located in the head and neck region, and four were periocular. Interestingly, 4 out of 10 periocular carcinomas had nuclear p53 accumulation, compared to 4 out of 25 non-ocular carcinomas, indicating a propensity for p53 dysregulation in the periocular region. Moreover, while intense p53 nuclear accumulation was more common in the periocular carcinomas, it was also seen in non-ocular carcinomas, suggesting that p53 dysregulation may be a marker of “invasive” or “malignant” tumorigenesis. This idea is substantiated by results from a previous study noting p53 mutations in invasive periocular sebaceous carcinomas but not in-situ lesions 17. We lack an credible explanation for the single sebaceoma case with intense nuclear p53 reactivity. However, it is interesting to speculate that in this singular sebaceoma, perhaps a second mutation, downstream or in parallel of p53 signaling counteracted some of the “malignant” effects of the loss of p53 but still allowed proliferation of a benign neoplasm, or that p53 mutation by itself is insufficient to effect a malignant phenotype.
Finally, this study also compared p53 staining with expression of DNA mismatch repair proteins in sebaceous lesions, which correlates strongly with microsatellite instability and drives the phenotype in the Muir-Torre syndrome. Notably, in all sebaceous lesions with p53 overexpression, mismatch repair proteins were intact, confirming microsatellite stability. These data suggest that divergent signaling mechanisms can contribute to sebaceous neoplasia. While microsatellite instability may be one pathway whereby sebaceous tumors arise or progress, p53 signaling alteration may be another. Microsatellite instability and its concomitant downstream effectors may be particularly important for the development of benign sebaceous lesions 11, while the present data suggest that mutations in p53 may be more important in the development of malignancy and/or invasiveness. Importantly, this finding generally supports the widely held notion benign sebaceous neoplasms are not pre-malignant lesions; if this were the case, one would expect to see more of an overlap between p53 staining and loss of mismatch repair protein expression, rather than independent molecular signatures.
Interestingly, a recent study specifically examined periocular sebaceous carcinomas in patients with concurrent visceral malignancy (a Muir-Torre syndrome phenotype) and found that the fragile histadine triad gene (FHIT) was disrupted in a subset of cases with confirmed microsatellite stability 35. The authors suggested that mutations in FHIT provided another mechanism for sebaceous carcinoma formation. Similar in some ways to p53, Fhit has been linked to apoptosis, with FHIT mutations resulting in defective programmed cell death 40. Of note, another recent study documented a gain of function mutation in p53 in a sebaceous carcinoma with concomitant mutations in fragile sites 41, thus perhaps linking FHIT and p53 as similar or even synergistic in their mechanism of tumorigenesis. Additional literature regarding the relationship of Fhit and p53 is lacking, and links between the two tumor suppressors as well as their individual contributions to the development of sebaceous tumors require more investigation.
A limitation of this study is that immunohistochemistry was used as a marker for p53 mutation. The tumors were not sequenced for the presence of specific p53 genetic mutations. Previous reports have correlated the presence of compact, uniform nuclear staining for p53 with clonal mutations in the gene sequence 21–22. However, while nuclear accumulation of the p53 protein product, as assessed by immunohistochemistry, correlates with the presence of p53 mutations, immunohistochemistry may not detect all p53 isoforms, null or frame shift mutations, nor will it pick up mutations in the gene that alter the signaling pathways but do not cause nuclear accumulation 15, 42. In this regard, our study may underestimate sebaceous lesions with p53 mutations. Likewise, overestimation of p53 mutations may also occur because immunohistochemistry for p53 may actually detect mutations in proteins upstream or downstream of p53 which lead to nuclear accumulation of the protein 15, 22. Even in this case, the nuclear accumulation of p53 suggests a dysregulation of the signaling pathways involving p53 if not a mutation in the gene itself.
In conclusion, this study strongly suggests that p53 overexpression occurs in a set of primarily malignant sebaceous tumors that are microsatellite stable, particularly carcinomas in the periocular region, and provides a framework for future investigation of the role of p53 mutations in the pathogenesis of sebaceous tumors. Although DNA mismatch repair proteins and microsatellite instability receive much focused attention in sebaceous lesions because of linkage to the Muir-Torre syndrome, mutations in these genes account for only a subset of these tumors. In addition to establishing a clear relationship of tumor type and site to p53 overexpression, this study also provides evidence that p53 mutations are seen independent of microsatellite instability. Determination of specific p53 mutations within sebaceous tumors, particularly periocular sebaceous carcinomas, along with affected downstream targets and effectors may allow us to better dissect out the molecular mechanisms contributing to the development of these lesions.
Acknowledgments
This work is supported by the U.S. National Institutes of Health (1R01CA118916, SL & AJL) and the M. D. Anderson Physician Scientist Grant (AJL). Ms. Kim Vu is thanked for expert aid in figure preparation.
References
- 1.Cohen PR, Kohn SR, Davis DA, et al. Muir-Torre syndrome. Dermatol Clin. 1995;13(1):79–89. [PubMed] [Google Scholar]
- 2.Lachiewicz AM, Wilkinson TM, Groben P, et al. Muir-Torre syndrome. Am J Clin Dermatol. 2007;8(5):315–319. doi: 10.2165/00128071-200708050-00008. [DOI] [PubMed] [Google Scholar]
- 3.Pettey AA, Walsh JS. Muir-Torre syndrome: a case report and review of the literature. Cutis. 2005;75(3):149–155. [PubMed] [Google Scholar]
- 4.Mangold E, Pagenstecher C, Leister M, et al. A genotype-phenotype correlation in HNPCC: strong predominance of msh2 mutations in 41 patients with Muir-Torre syndrome. J Med Genet. 2004;41(7):567–572. doi: 10.1136/jmg.2003.012997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ponti G, Losi L, Pedroni M, et al. Value of MLH1 and MSH2 mutations in the appearance of Muir-Torre syndrome phenotype in HNPCC patients presenting sebaceous gland tumors or keratoacanthomas. J Invest Dermatol. 2006;126(10):2302–2307. doi: 10.1038/sj.jid.5700475. [DOI] [PubMed] [Google Scholar]
- 6.Chhibber V, Dresser K, Mahalingam M. MSH-6: extending the reliability of immunohistochemistry as a screening tool in Muir-Torre syndrome. Mod Pathol. 2008;21(2):159–164. doi: 10.1038/modpathol.3800997. [DOI] [PubMed] [Google Scholar]
- 7.Abdel-Rahman WM, Peltomaki P. Lynch syndrome and related familial colorectal cancers. Crit Rev Oncog. 2008;14(1):1–22. doi: 10.1615/critrevoncog.v14.i1.10. discussion 23–31. [DOI] [PubMed] [Google Scholar]
- 8.Kruse R, Ruzicka T. DNA mismatch repair and the significance of a sebaceous skin tumor for visceral cancer prevention. Trends Mol Med. 2004;10(3):136–141. doi: 10.1016/j.molmed.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Shalin SC, Lyle S, Calonje E, et al. Sebaceous neoplasia and the Muir-Torre syndrome: important connections with clinical implications. Histopathology. 56(1):133–147. doi: 10.1111/j.1365-2559.2009.03454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orta L, Klimstra DS, Qin J, et al. Towards Identification of Hereditary DNA Mismatch Repair Deficiency: Sebaceous Neoplasm Warrants Routine Immunohistochemical Screening Regardless of Patient’s Age or Other Clinical Characteristics. Am J Surg Pathol. 2009 doi: 10.1097/PAS.0b013e318199edca. [DOI] [PubMed] [Google Scholar]
- 11.Singh RS, Grayson W, Redston M, et al. Site and tumor type predicts DNA mismatch repair status in cutaneous sebaceous neoplasia. Am J Surg Pathol. 2008;32(6):936–942. doi: 10.1097/pas.0b013e31815b0cc2. [DOI] [PubMed] [Google Scholar]
- 12.Cesinaro AM, Ubiali A, Sighinolfi P, et al. Mismatch repair proteins expression and microsatellite instability in skin lesions with sebaceous differentiation: a study in different clinical subgroups with and without extracutaneous cancer. Am J Dermatopathol. 2007;29(4):351–358. doi: 10.1097/DAD.0b013e318057713c. [DOI] [PubMed] [Google Scholar]
- 13.Benjamin CL, Ananthaswamy HN. p53 and the pathogenesis of skin cancer. Toxicol Appl Pharmacol. 2007;224(3):241–248. doi: 10.1016/j.taap.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kruse JP, Gu W. Modes of p53 regulation. Cell. 2009;137(4):609–622. doi: 10.1016/j.cell.2009.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall PA, McCluggage WG. Assessing p53 in clinical contexts: unlearned lessons and new perspectives. J Pathol. 2006;208(1):1–6. doi: 10.1002/path.1913. [DOI] [PubMed] [Google Scholar]
- 16.Bolshakov S, Walker CM, Strom SS, et al. p53 mutations in human aggressive and nonaggressive basal and squamous cell carcinomas. Clin Cancer Res. 2003;9(1):228–234. [PubMed] [Google Scholar]
- 17.Gonzalez-Fernandez F, Kaltreider SA, Patnaik BD, et al. Sebaceous carcinoma. Tumor progression through mutational inactivation of p53. Ophthalmology. 1998;105(3):497–506. doi: 10.1016/S0161-6420(98)93034-2. [DOI] [PubMed] [Google Scholar]
- 18.McBride SR, Leonard N, Reynolds NJ. Loss of p21(WAF1) compartmentalisation in sebaceous carcinoma compared with sebaceous hyperplasia and sebaceous adenoma. J Clin Pathol. 2002;55(10):763–766. doi: 10.1136/jcp.55.10.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cabral ES, Auerbach A, Killian JK, et al. Distinction of benign sebaceous proliferations from sebaceous carcinomas by immunohistochemistry. Am J Dermatopathol. 2006;28(6):465–471. doi: 10.1097/01.dad.0000245200.65600.a4. [DOI] [PubMed] [Google Scholar]
- 20.Kiyosaki K, Nakada C, Hijiya N, et al. Analysis of p53 mutations and the expression of p53 and p21WAF1/CIP1 protein in 15 cases of sebaceous carcinoma of the eyelid. Invest Ophthalmol Vis Sci. 2010;51(1):7–11. doi: 10.1167/iovs.09-4127. [DOI] [PubMed] [Google Scholar]
- 21.Ren ZP, Ponten F, Nister M, et al. Two distinct p53 immunohistochemical patterns in human squamous-cell skin cancer, precursors and normal epidermis. Int J Cancer. 1996;69(3):174–179. doi: 10.1002/(SICI)1097-0215(19960621)69:3<174::AID-IJC4>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 22.Dasgupta T, Wilson LD, Yu JB. A retrospective review of 1349 cases of sebaceous carcinoma. Cancer. 2009;115(1):158–165. doi: 10.1002/cncr.23952. [DOI] [PubMed] [Google Scholar]
- 23.Rulon DB, Helwig EB. Cutaneous sebaceous neoplasms. Cancer. 1974;33(1):82–102. doi: 10.1002/1097-0142(197401)33:1<82::aid-cncr2820330115>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 24.Troy JL, Ackerman AB. Sebaceoma. A distinctive benign neoplasm of adnexal epithelium differentiating toward sebaceous cells. Am J Dermatopathol. 1984;6(1):7–13. [PubMed] [Google Scholar]
- 25.Nielsen TA, Maia-Cohen S, Hessel AB, et al. Sebaceous neoplasm with reticulated and cribriform features: a rare variant of sebaceoma. J Cutan Pathol. 1998;25(4):233–235. doi: 10.1111/j.1600-0560.1998.tb01725.x. [DOI] [PubMed] [Google Scholar]
- 26.Misago N, Mihara I, Ansai S, et al. Sebaceoma and related neoplasms with sebaceous differentiation: a clinicopathologic study of 30 cases. Am J Dermatopathol. 2002;24(4):294–304. doi: 10.1097/00000372-200208000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Nelson BR, Hamlet KR, Gillard M, et al. Sebaceous carcinoma. J Am Acad Dermatol. 1995;33(1):1–15. doi: 10.1016/0190-9622(95)90001-2. quiz 16–18. [DOI] [PubMed] [Google Scholar]
- 28.Rutten A, Burgdorf W, Hugel H, et al. Cystic sebaceous tumors as marker lesions for the Muir-Torre syndrome: a histopathologic and molecular genetic study. Am J Dermatopathol. 1999;21(5):405–413. doi: 10.1097/00000372-199910000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Abbott JJ, Hernandez-Rios P, Amirkhan RH, et al. Cystic sebaceous neoplasms in Muir-Torre syndrome. Arch Pathol Lab Med. 2003;127(5):614–617. doi: 10.5858/2003-127-0614-CSNIMS. [DOI] [PubMed] [Google Scholar]
- 30.Dinneen AM, Mehregan DR. Sebaceous epithelioma: a review of twenty-one cases. J Am Acad Dermatol. 1996;34(1):47–50. doi: 10.1016/s0190-9622(96)90833-6. [DOI] [PubMed] [Google Scholar]
- 31.Seta T, Imazeki F, Yokosuka O, et al. Expression of p53 and p21WAF1/CIP1 proteins in gastric and esophageal cancers: comparison with mutations of the p53 gene. Dig Dis Sci. 1998;43(2):279–289. doi: 10.1023/a:1018889818855. [DOI] [PubMed] [Google Scholar]
- 32.Kazakov DV, Grossmann P, Spagnolo DV, et al. Expression of p53 and TP53 mutational analysis in malignant neoplasms arising in preexisting spiradenoma, cylindroma, and spiradenocylindroma, sporadic or associated with Brooke-Spiegler syndrome. Am J Dermatopathol. 2010;32(3):215–221. doi: 10.1097/DAD.0b013e3181b9678c. [DOI] [PubMed] [Google Scholar]
- 33.Buitrago W, Joseph AK. Sebaceous carcinoma: the great masquerader: emgerging concepts in diagnosis and treatment. Dermatol Ther. 2008;21(6):459–466. doi: 10.1111/j.1529-8019.2008.00247.x. [DOI] [PubMed] [Google Scholar]
- 34.Rao NA, Hidayat AA, McLean IW, et al. Sebaceous carcinomas of the ocular adnexa: A clinicopathologic study of 104 cases, with five-year follow-up data. Hum Pathol. 1982;13(2):113–122. doi: 10.1016/s0046-8177(82)80115-9. [DOI] [PubMed] [Google Scholar]
- 35.Goldberg M, Rummelt C, Foja S, et al. Different genetic pathways in the development of periocular sebaceous gland carcinomas in presumptive Muir-Torre syndrome patients. Hum Mutat. 2006;27(2):155–162. doi: 10.1002/humu.20281. [DOI] [PubMed] [Google Scholar]
- 36.Jones B, Oh C, Mangold E, et al. Muir-Torre syndrome: Diagnostic and screening guidelines. Australas J Dermatol. 2006;47(4):266–269. doi: 10.1111/j.1440-0960.2006.00292.x. [DOI] [PubMed] [Google Scholar]
- 37.Pfeifer GP, Besaratinia A. Mutational spectra of human cancer. Hum Genet. 2009;125(5–6):493–506. doi: 10.1007/s00439-009-0657-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Backvall H, Stromberg S, Gustafsson A, et al. Mutation spectra of epidermal p53 clones adjacent to basal cell carcinoma and squamous cell carcinoma. Exp Dermatol. 2004;13(10):643–650. doi: 10.1111/j.0906-6705.2004.00211.x. [DOI] [PubMed] [Google Scholar]
- 39.Backvall H, Wolf O, Hermelin H, et al. The density of epidermal p53 clones is higher adjacent to squamous cell carcinoma in comparison with basal cell carcinoma. Br J Dermatol. 2004;150(2):259–266. doi: 10.1111/j.1365-2133.2004.05683.x. [DOI] [PubMed] [Google Scholar]
- 40.Zanesi N, Croce CM. Fragile histidine triad gene and skin cancer. Eur J Dermatol. 2001;11(5):401–404. [PubMed] [Google Scholar]
- 41.Becker K, Goldberg M, Helmbold P, et al. Deletions of BRCA1/2 and p53 R248W gain-of-function mutation suggest impaired homologous recombination repair in fragile histidine triad-negative sebaceous gland carcinomas. Br J Dermatol. 2008;159(6):1282–1289. doi: 10.1111/j.1365-2133.2008.08783.x. [DOI] [PubMed] [Google Scholar]
- 42.Ireland AP, Clark GW, DeMeester TR. Barrett’s esophagus. The significance of p53 in clinical practice. Ann Surg. 1997;225(1):17–30. doi: 10.1097/00000658-199701000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]