Abstract
Background
High cholesterol is identified as a major risk factor for chronic non-communicable diseases, especially cardiovascular and cerebrovascular diseases. Monitoring trends of cholesterol levels and comparing trends across population groups are important to assess population distribution and risks related to cholesterol change over time. Cholesterol surveillance data are lacking, even in high-income countries.
Objectives
To describe the trends in cholesterol and triglyceride levels in different population groups and to estimate the risk of developing hypercholesterolemia and hypertriglyceridemia in Västerbotten County, Sweden during 1990–2010.
Designs and Methods
Since 1990, 133,082 individuals living in Västerbotten County, Northern Sweden, invited on their 30th, 40th, 50th and 60th birthdays, participated in the Västerbotten Intervention Program. Ten years after baseline data collection, 34,868 individuals were surveyed for a second time. In addition to a self-administered health questionnaire (that included information on socioeconomic status, demographics, self-reported health and lifestyle behaviours), blood cholesterol and triglyceride were examined.
Results
The level and prevalence of hypercholesterolemia decreased significantly from 1990 to 2007, but the trends began to increase during 2008–2010 in men, women, and in all educational groups. Men had significantly higher serum triglyceride levels than women and their cholesterol levels were similar to those of the women. This study shows that those with basic education and who live in rural inlands had consistently higher triglyceride level than those who live in the city and have higher educational attainments. People with basic education are also at higher risk of developing hypercholesterolemia and hypertriglyceridemia at 10-year follow-up; the risk is much higher among the older cohorts, particularly women. During 1990–2010, the proportion of participants who reported treatment with lipid-lowering agents increased from 1.1% to 9.6% among men and 0.5% to 5.3% among women. About 60% of those treated achieved treatment goals for cholesterol or triglycerides.
Conclusions
The increasing trend in cholesterol level in the Västerbotten population during 2008–2010 needs to be closely monitored. Addressing the unequal distribution of cholesterol, as well as other risk factors such as obesity, physical inactivity, high blood glucose, among those with basic education, and particularly among populations in rural areas are important to prevent higher burdens of chronic non-communicable diseases in this population.
Keywords: cholesterol, hypercholesterolemia, triglyceride, dyslipidaemia, trends, education, longitudinal studies, Sweden
In 2004, approximately 60% of global deaths were attributable to chronic non-communicable diseases, primarily cardiovascular diseases (CVD), cancers, and respiratory diseases. Cardiovascular diseases, including ischaemic heart disease and cerebrovascular disease, accounted for total mortality in 32% of men and 27% of women. The Global Burden of Disease project estimated that 22% of the 21.3 million global CVD deaths occurred in high-income countries, mainly in American and European countries. Of the 1.5 million CVD deaths in high income European countries, 41% were related to ischaemic heart disease and 25% to cerebrovascular disease (1).
Several large epidemiological studies show a reduction in CVD mortality over the last few decades (2–4). Results from the longitudinal Framingham Heart Study show a 59% decrease in coronary heart disease (CHD) death rates from 1950 to 1999 (2). The WHO MONICA study was conducted in 38 populations in 21 countries and reports annual percentage decreases in coronary event rates of 2.3% in men and 1.4% in women (5), but these rates differ significantly between and within the different study populations (3). In Australia, CHD mortality decreased by 74% in men and 81% in women over the period of 1968–2000, and the decrease was primarily attributable to reductions in population risk factors for primary and secondary prevention (4). In Sweden, the death rate from cardiovascular diseases among women aged 15–74 years decreased significantly from 128/100,000 in 1987 to 55/100,000 in 2010. The corresponding rates for men were 352/100,000 in 1987 and 126/100,000 in 2010. Yet, among Swedish adults, CVD was the leading cause of death for 41% of all deaths among women and 39% among men in 2010 (6).
Reduction of population serum cholesterol levels through primary and secondary prevention strategies, such as reduction of dietary fat, increasing use of lipid-lowering agents, better management of hypertension, and increasing use of antiplatelet and thrombolytic agents are believed to account for the reduction in CHD mortality (2, 4). In Australia, a decrease in population serum cholesterol levels, mainly due to changes in dietary saturated fat consumption, accounted for a reduction in CHD mortality of 22% among men and 20% among women over the period of 1968–2000 (4). The WHO MONICA study estimates 40% of men's and 15% of women's variability in coronary event rate trends are attributable to changes in the major CVD risk factors (ie, smoking, blood pressure, blood cholesterol, and body mass index) (5). Hypercholesterolemia (defined as blood cholesterol over 6.5 mmol/L) with raised low-density lipoprotein cholesterol (LDL-C), which is the main form of dyslipidaemia, are present in 20–80% of adults of European descent (7). The global population average of total cholesterol has changed by less than 0.1 mmol/L per decade between 1980 and 2008. A larger decrease was observed in Australasia, North America, and Western Europe (8). Decreased population serum cholesterol has also been reported in many countries that participate in the WHO MONICA project (9). In the Northern Sweden MONICA, the prevalence of hypercholesterolemia (cholesterol ≥ 7 mmol/L) in men aged 25–64 years decreased from 28.6% in 1986 to 10.5% in 2009; the corresponding decrease was from 26.2% to 8.4% in women. Mean cholesterol among men aged 25–64 years old decreased from 6.4 mmol/L in 1986 to 5.6 mmol/L in 2009; the corresponding numbers were 6.3 mmol/L and 5.4 mmol/L in women (10).
The Västerbotten Intervention Program (VIP) was developed in the mid 1980s in response to high myocardial infarction mortality rates in Västerbotten County in early 1980. In 1985, the mortality rate of myocardial infarction in Västerbotten County was 546/100,000 population/year in men and 324/100,000/year in women. These compare to rates of 136/100,000 population/year in men and 91/100,000 population/year in women living in Gotland County in southern Sweden (11). VIP started initially with a small-scale intervention program in Norsjö Municipality in Västerbotten County in 1985. This was expanded to the whole county during 1990–1992 (12). The VIP model includes population-based and individual prevention strategies. In the latter strategy, middle-aged individuals are invited to participate in health screening and individual counselling in primary health care centre. In addition to self-administered questionnaires, participants are offered fasting blood glucose and blood cholesterol examinations (13). The objectives of this study are to describe the trends in cholesterol and triglyceride levels in Västerbotten County, Sweden and to estimate the risk of developing hypercholesterolemia and hypertriglyceridemia in this population during 1990–2010.
Methods
Study participants
Since 1990, all individuals aged 30, 40, 50, and 60 years who live in Västerbotten County, Sweden are invited to participate in a health examination at their local health care centres. The response rate to this invitation has increased from 59% in 1995 to 69% in 2010. By 2010, 133,082 health examinations were conducted and recorded in a cross-sectional database. In the cross-sectional analysis, we excluded individuals aged 30 (n=9,136) as only few health centres continued to invite them after 1995. The panel data (n=34,868 individuals) consists of individuals who participated in follow-up examination ten years after their initial examination. We included individuals age 30 who participated during 1990–1995 in the panel data analysis. This allows individuals aged 30, 40 and 50 years to participate in the survey a second time when they turn 40, 50 or 60 years old. The panel data also consists of 1,042 individuals who had participated three times in VIP (in 1990, 2000, and 2010). We excluded the third observation from the panel analysis. Details about the study population have been reported elsewhere (13).
Measurements
Each participant completed a self-administered health questionnaire that included socioeconomic and demographic status, self-reported health, and lifestyle behaviours. Subjects were categorized into three groups based on their highest educational level: basic education (ie, those who completed only elementary and comprehensive compulsory school), medium education (ie, those who graduated from residential college for adult education or high school); and high education (ie, those who graduated from college). Based on their area of residence, participants were categorized into those who lived in (1) Umeå city, (2) Skellefteå town, (3) Lycksele town, (4) rural coastlands (communities of Nordmaling, Robertsfors, Vännas, and Vindeln), and (5) rural inlands (communities of Åsele, Bjurholm, Dorotea, Malå, Norsjö, Sorsele, Storuman, and Vilhelmina).
Staff at each health care centre performed blood pressure measurements and blood sampling. A venous blood sample was obtained after an overnight fast. Serum total cholesterol and triglycerides were analysed in each health care centre using Reflotron bench-top analysers (Boehringer Mannheim GmbH, Mannheim, Germany) (14). On Sept. 1, 2009, the VIP blood sampling protocol was updated and all blood samples were sent to the Department of Clinical Chemistry in the nearest hospital in Umeå, Lycksele and Skellefteå for analysis. To ensure comparability of results analysed through both methods, we conducted the calibration protocol on total cholesterol (n=1,197) and triglycerides (n=838) for VIP participants whose blood samples were analysed using both Reflotron and laboratory chemistry. Results from blood samples sent to the Departments of Clinical Chemistry were adjusted to Reflotron-comparable values using the following formula:
Reflotron-based serum triglyceride = 0.177 + (0.932 × serum triglyceride by laboratory chemistry)
Reflotron-based serum cholesterol = 0.170 + (0.939 × serum cholesterol by laboratory chemistry)
Participants with blood cholesterol levels ≥6.5 mmol/L and/or those who took lipid-lowering medication within 14 days were categorized as the hypercholesterolemia group. Serum triglyceride of ≥1.7 mmol/L was used to define hypertriglyceridemia.
Statistical analyses
We grouped observations from the last three years period separately, in order to ensure comparability of the six years interval (1990–1995, 1996–2001, and 2002–2007) used in other papers in the VIP series published earlier (15–18). Mean levels of total cholesterol and triglycerides were adjusted for age, and are presented graphically by sex, educational level and survey year. These time-trend data were also adjusted for age, educational level and sex when the data were presented graphically by geographical area. Prevalences of hypercholesterolemia and hypertriglyceridemia were adjusted for age and presented with 99% confidence intervals (CI). The 10-year risks of developing hypercholesterolemia or hypertriglyceridemia were calculated for each age cohort using the predicted probability following logistic regression analysis for each sex, controlling for age and educational level. Risk was calculated separately for cohorts recruited during 1990–1995 and 1996–2000. We compared the proportion of hypercholesterolemia and hypertriglyceridemia in the panel at baseline and 10-year follow-up. Finally, we calculated the proportion of men and women among those who reported taking lipid-lowering medication during the last 14 days with cholesterol ≥5 mmol/L and triglycerides ≥1.7 mmol/L, the recommended levels. Statistical analyses were conducted with Stata Version 11.2 (19). Time-trends were graphed with two-year moving averages using the graph function in Microsoft Excel. A p-value of 0.01 was used to signify statistical significance so as to take into consideration the large sample size.
Results
Of the 123,946 VIP participants aged 40, 50 and 60 years during 1990–2010, 121,972 (98.4%) individuals had complete information on cholesterol and educational levels and were included in the analyses. In total, 48.3% of the participants were men, 24.5% had basic education, and 48.4% had medium education. The proportion of participants aged 40, 50 and 60 years were 33.4%, 35.2%, and 31.4%, respectively. The panel analyses included 16,305 men and 18,563 women who had 10 –year follow-up data.
Trends of population cholesterol and triglycerides in Västerbotten County
The age-adjusted cholesterol level had decreased from 5.90 mmol/L (99% CI 5.87–5.92) in 1990–1995 to 5.25 (5.23–5.27) in 2002–2007, and later increased to 5.31 (5.28–5.34) in 2008–2010 among men. The corresponding numbers were 5.82 (5.80–5.84), 5.24 (5.22–5.26), and 5.28 (5.25–5.31) in women (data not shown). These changes in cholesterol level had also been reflected in the reverting trend of hypercholesterolemia, which showed statistically significant increases from 14.7% to 18.3% in men and 9.9% to 12.5% in women between 2002–2007 and 2008–2010 (Table 1). Similar trends had also been observed in triglyceride level in men and women. It decreased from 1.65 mmol/L (1.62–1.67) to 1.55 (1.53–1.56) and later increased to 1.59 (1.57–1.62) in men. In women, it seemed that triglyceride level continued to decrease in the last 3 years period also. The levels of triglyceride in women changed from 1.38 (1.37–1.40) in 1990–1995 to 1.27 (1.26–1.28) in 2002–2007 and continued decreasing to 1.25 (1.24–1.27) in 2008–2010 (data not shown). These patterns were reflected in the prevalence of hypertriglyceridemia as shown in Table 1.
Table 1.
Prevalences of hypercholesterolemia (%) or hypertriglyceridemia (%) in the Västerbotten Intervention Programme during four periods from 1990–2010. Prevalences are adjusted for age. The numbers in brackets represent the 99% confidence intervals for the adjusted prevalence
| Prevalence (%) | Educational level | Total | ||||||
|---|---|---|---|---|---|---|---|---|
| Basic education | Medium education | High education | ||||||
| Men | Women | Men | Women | Men | Women | Men | Women | |
| Hypercholesterolemia | ||||||||
| 1990–1995 | 35.0 (33.3–36.8) | 37.7 (36.0–39.5) | 29.3 (27.8–30.9) | 21.3 (19.9–22.7) | 24.3 (22.1–26.6) | 15.7 (14.1–17.5) | 29.9(28.8–30.9) | 24.0(23.0–25.0) |
| 1996–2001 | 28.4 (26.7–30.1) | 27.6 (25.9–29.3) | 21.3 (20.1–22.5) | 15.5 (14.4–16.6) | 18.8 (17.1–20.6) | 12.5 (11.4–13.8) | 22.5 (21.7–23.4) | 17.0 (16.2–17.7) |
| 2002–2007 | 20.4 (18.7–22.1) | 18.6 (17.0–20.3) | 13.8 (13.0–14.7) | 9.1 (8.4–9.8) | 12.1 (11.0–13.4) | 7.0 (6.3–7.8) | 14.7 (14.1–15.4) | 9.9 (9.4–10.5) |
| 2008–2010 | 24.8 (21.9–27.9) | 20.0 (17.2–23.2) | 17.8 (16.5–19.2) | 12.8 (11.7–14.0) | 14.8 (13.1–16.6) | 8.7 (7.7–9.8) | 18.3 (17.3–19.4) | 12.5 (11.7–13.3) |
| Hypertriglyceridemia | ||||||||
| 1990–1995 | 37.1 (35.1–39.2) | 29.1 (27.4–30.9) | 34.4 (32.4–36.4) | 20.0 (18.5–21.5) | 29.4 (26.1–32.8) | 13.1 (11.4–15.0) | 34.9 (33.6–36.2) | 21.0 (20.1–22.0) |
| 1996–2001 | 30.1 (28.4–32.0) | 22.0 (20.5–23.6) | 27.9 (26.6–29.3) | 14.9 (13.9–16.0) | 24.0 (22.0–26.2) | 11.1 (10.0–12.4) | 27.8 (26.9–28.8) | 15.4 (14.7–16.1) |
| 2002–2007 | 33.2 (31.2–35.2) | 25.9 (24.0–27.8) | 31.8 (30.6–32.9) | 16.5 (15.5–17.5) | 26.4 (24.8–28.1) | 11.4 (10.5–12.4) | 30.7 (29.8–31.5) | 16.3 (15.6–17.0) |
| 2008–2010 | 35.7 (32.5–39.1) | 22.9 (19.9–26.2) | 33.0 (31.4–34.7) | 17.2 (15.9–18.7) | 27.6 (25.4–29.9) | 10.7 (9.6–12.0) | 31.9 (30.7–33.2) | 15.5 (14.6–16.4) |
Trends of population cholesterol and triglycerides in educational groups
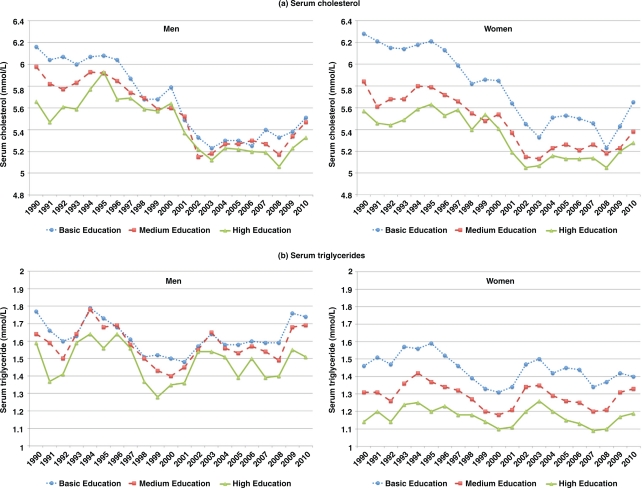
In general, men had significantly higher serum triglyceride levels than women. During 1990–2010, both men and women with basic education had consistently higher levels of age-adjusted cholesterol and triglycerides compared to those with medium or high educational levels. The differences in cholesterol and triglyceride levels across educational groups were more prominent among women than men. Differences in blood cholesterol levels among the educational groups were observed during the first 6 years (1990–1995) and became less prominent during 2002–2010, particularly among men. In both sexes and all educational groups, cholesterol levels decreased between 1990–2001, remained stable during 2002–2007, and showed an increasing trend from 2008–2010. In contrast, the triglyceride levels fluctuated during 1990–2010, and 2010 levels were similar to those in 1990 (Fig. 1).
Fig. 1.
Levels of (a) serum cholesterol (mmol/L) and (b) serum triglycerides (mmol/L) among adults in the Västerbotten Intervention Programme, 1990–2010. The levels are adjusted for age.
The age-adjusted prevalence of hypercholesterolemia decreased significantly in men and women, as well as all educational groups from 1990–1995 to 2002–2007. During 2008–2010, prevalences increased among men and women and all educational groups. Statistically significant increases were observed among men and women with medium education, and the prevalence of hypercholesterolemia in men increased from 13.8% in 2002–2007 to 17.8% in 2008–2010. Prevalences increased from 9.1% to 12.8% in women during corresponding periods. The age-adjusted prevalence of hypertriglyceridemia showed a decreasing trend from 1990 to 2001. After 2001, prevalences increased in men, women, and all educational groups. The prevalences of hypertriglyceridemia in men and women during 2008–2010 were similar to those during 1990–1995, except for women with basic education who had a significantly lower prevalence of 22.9% in 2008–2010, compared to a prevalence of 29.1% in 1990–1995 (Table 1).
Population trends in cholesterol and triglycerides in different geographical areas
In the earlier period, participants who lived in the town of Lycksele, rural coastlands, and rural inland areas showed a higher cholesterol level compared to those who lived in the city of Umeå or town of Skellefteå. The cholesterol level decreased over time in all geographical areas until 2003, except for those who lived in Lycksele where the decreasing trend rebounded earlier, in 2000. The cholesterol level among inhabitants of Lycksele increased from 5.29 mmol/L in 2001 to 5.66 mmol/L in 2010 (Fig. 2). The prevalence of hypercholesterolemia among the inhabitants of Lycksele decreased from 44% in 1993 to 12.4% in 2000; thereafter it increased steadily to the level of 24% in 2010. During 2002–2007, the prevalence of hypercholesterolemia in the other four regions was more than 5% lower than in Lycksele. Since 2007, it has increased in these four regions and the prevalence in the rural inlands reached the level of that in Lycksele in 2010 (data not shown). For those who live in the rural inlands, cholesterol levels reached the nadir in 2002 and increased to 5.5 mmol/L in 2010. Although the cholesterol levels were stable during 2002–2007 in the other three areas, there was an increasing trend during 2008–2010. In 2010, Umeå inhabitants had the lowest cholesterol levels (5.34 mmol/L) and prevalence of hypercholesterolemia (16%) as compared to VIP participants in other geographical areas. The serum triglyceride trends were more consistent: individuals who lived in the city of Umeå or town of Skellefteå had lower triglyceride levels during the whole observation period than individuals who lived in other areas. Inhabitants in the rural inlands also had higher triglyceride level throughout the observation period (Fig. 2).
Fig. 2.
Levels of (a) serum cholesterol (mmol/L) and (b) serum triglycerides (mmol/L) in the Västerbotten Intervention Programme, 1990–2010, across geographical areas. The levels are adjusted for age, educational levels and sex. The graphs represent two-year moving averages.
Ten-year risk of developing hypercholesterolemia or hypertriglyceridemia
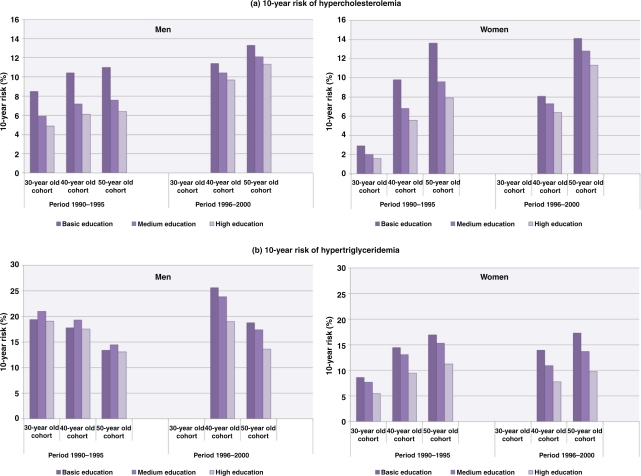
The panel data show that the ten-year risk of developing hypercholesterolemia was significantly higher among 40- and 50-year old men with medium or high education who were recruited during 1996–2000, compared to those recruited earlier. The same was true for 50-year old women with medium or high education. Men and women in all age cohorts who had basic education were at significantly higher risk of developing hypercholesterolemia than those with high education. This was true only among those who participated in the early period (1990–1995). The differences in risk across educational groups diminished in the later period. Younger women had significantly lower risk for hypercholesterolemia as compared to younger men. The risk differences between younger (30-year old) and older women (40- and 50-year olds) were larger than the different age-cohorts among men. These observations were similar across all educational groups. The 10-year risk of developing hypertriglyceridemia was higher among the older women (40- and 50-year olds) than their younger counterparts. Among 50-year old men, the risk of developing hypertriglyceridemia was lower than that of the 30-year old men. There was no difference in risk between men with basic and high education or between women with basic and medium education among those recruited in 1990–1995 (Fig. 3).
Fig. 3.
Ten-year risk of developing (a) hypercholesterolemia and (b) hypertriglyceridemia among participants in the Västerbotten Intervention Programme. Risk is presented as a function of age at the start of the 10-year period and education, adjusted for baseline levels of cholesterol and triglycerides.
Note: The 30-year cohort during 1996–2000 is not presented as only few individuals were invited to the study since 1995. The 10-year risk was estimated using the predicted probability after logistic regression analysis for each sex, controlling for age and educational level.
The 40- and 50-year female cohorts showed statistically significant higher proportions of hypercholesterolemia and hypertriglyceridemia across all educational groups at 10-year follow-up compared to the proportions in the baseline survey. In contrast, 30-year women with medium or high education and 50-year men with basic or medium education had a significantly lower proportion of hypercholesterolemia at 10-year follow-up. Though not statistically significant, the proportion of hypercholesterolemia was lower among 30- and 40-year men at 10-year follow-up. This was observed in most educational groups. In men, no statistically significant difference was observed in the proportion of hypertriglyceridemia between baseline and 10-year follow-up except for a higher proportion of hypertriglyceridemia among 30- and 40-year men with medium education at 10-year follow-up (Table 2).
Table 2.
Proportions of men and women with hypercholesterolemia and hypertriglyceridemia during the baseline and 10-year follow-up periods in the Västerbotten Intervention Programme
| Groups* | Men | Women | ||
|---|---|---|---|---|
| Baseline | 10-year follow-up | Baseline | 10-year follow-up | |
| Hypercholesterolemia (%) | ||||
| 30-year-old panel | ||||
| Basic education | 13.2 (9.5–18.2) | 10.9 (7.5–15.7) | 4.4 (2.4–7.9) | 4.1 (2.1–7.6) |
| Medium education | 8.8 (7.6–10.1) | 9.4 (8.2–10.8) | 5.1 (4.2–6.2) | 3.1 (2.4–4.0) |
| High education | 8.4 (6.0–11.6) | 7.8 (5.5–11.1) | 4.6 (3.3–6.3) | 1.5 (0.8–2.6) |
| 40-year-old panel | ||||
| Basic education | 22.4 (20.0–25.0) | 21.8 (19.4–24.4) | 10.3 (8.6–12.3) | 13.6 (11.6–15.8) |
| Medium education | 19.1 (17.9–20.3) | 17.1 (16.0–18.3) | 9.3 (8.4–10.2) | 12.1 (11.2–13.2) |
| High education | 15.6 (13.8–17.5) | 14.5 (12.8–16.4) | 6.5 (5.7–7.6) | 9.4 (8.4–10.6) |
| 50-year-old panel | ||||
| Basic education | 31.4 (29.5–33.3) | 26.6 (24.8–28.4) | 26.4 (24.7–28.2) | 30.3 (28.5–32.1) |
| Medium education | 28.3 (26.7–29.9) | 25.0 (23.5–26.5) | 20.8 (19.5–22.2) | 28.1 (26.6–29.6) |
| High education | 23.6 (21.4–26.0) | 21.8 (19.7–24.1) | 18.0 (16.4–19.7) | 24.6 (22.8–26.5) |
| Hypertriglyceridemia (%) | ||||
| 30-year-old panel | ||||
| Basic education | 23.8 (18.0–30.8) | 28.6 (23.1–35.0) | 16.6 (12.0–22.4) | 19.7 (15.0–25.5) |
| Medium education | 23.4 (21.2–25.7) | 29.8 (27.8–31.8) | 11.2 (9.7–12.8) | 10.5 (9.1–11.9) |
| High education | 16.9 (11.9–23.3) | 22.3 (18.3–26.9) | 7.7 (5.8–10.1) | 6.4 (4.8–8.4) |
| 40-year-old panel | ||||
| Basic education | 34.2 (31.0–37.5) | 34.6 (31.7–37.6) | 13.8 (11.7–16.2) | 21.5 (19.1–24.1) |
| Medium education | 28.1 (26.6–29.7) | 32.7 (31.3–34.2) | 11.6 (10.6–12.7) | 16.8 (15.7–18.0) |
| High education | 24.1 (21.4–27.1) | 26.1 (23.9–28.4) | 7.9 (6.8–9.1) | 12.0 (10.8–13.3) |
| 50-year-old panel | ||||
| Basic education | 34.2 (32.0–36.4) | 30.5 (28.7–32.4) | 20.6 (19.0–22.4) | 26.9 (25.2–28.7) |
| Medium education | 32.5 (30.7–34.4) | 29.5 (28.0–31.1) | 17.4 (16.1–18.9) | 25.2 (23.8–26.7) |
| High education | 26.4 (23.7–29.4) | 27.3 (25.0–29.7) | 12.6 (11.1–14.3) | 17.8 (16.2–19.5) |
*Age of panel is age at baseline survey
Trends in self-reported treatment with lipid-lowering agents
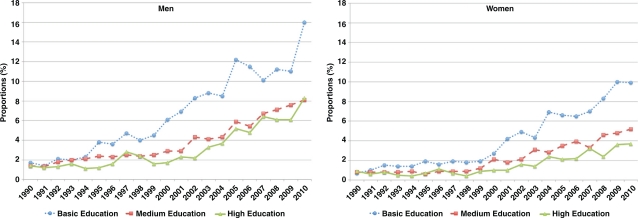
During 1990–2010, the proportion of VIP participants who reported treatment with lipid-lowering agents increased from 1.1% to 9.6% among men and from 0.5% to 5.3% among women. In both sexes, the increase was consistently higher among respondents with basic education than those with medium or high education, and the gaps became more prominent during the last 10 years (Fig. 4). Although the proportion on treatment increased over time, only half of those in each sex and educational group who were categorized as hypercholesterolemia reported treatment. In a more detailed analysis limited to those individuals with high cholesterol level (≥ 6.5 mmol/L), we observed that 50% of men with basic education reported taking lipid-lowering treatment within the two weeks preceding the survey. This proportion was higher than the 35% of men with medium education and 43% of men with high education. The corresponding numbers were 33%, 26%, and 23% among women with basic, medium, and high education, respectively. The treated proportion was lower when the analysed data included those with high serum triglycerides. Less than 15% of those with hypertriglyceridemia reported treatment during the two weeks preceding the survey, and the proportion was higher among men and women with basic education (data not shown).
Fig. 4.
Proportions of individuals who reported taking lipid-lowering treatment in the Västerbotten Intervention Programme, 1990–2010. Proportions are adjusted for age.
Control of population blood lipids with treatment
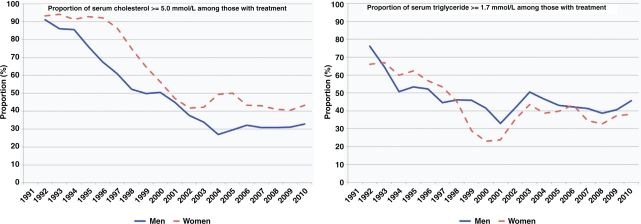
We analysed the proportion of respondents with serum cholesterol and triglyceride levels above the treatment goals of cholesterol <5.0 mmol/L and triglycerides <1.7 mmol/L who reported lipid-lowering treatment in the prior two weeks. Despite the large number of study participants, only 311 men and 173 women reported treatment in 2010. The proportion of ‘uncontrolled’ cholesterol dropped significantly from approximately 90% in 1990 to 30–40% in 2010. This was higher among women throughout the observation period. The proportion of ‘uncontrolled’ triglycerides decreased from approximately 70% in 1990 to 40% in 2010. Although the proportion was higher in women during the earlier period, the level have been higher among men since 1998 (Fig. 5).
Fig. 5.
Proportions of men and women reporting use of lipid-lowering treatment who did not reach treatment goals of serum cholesterol<5.0 mmol/L or serum triglycerides <1.7 mmol/L in the Västerbotten Intervention Program, 1990–2010. Proportions are adjusted for age. The graphs show two-year moving averages of the proportions.
Discussion
Unsustainable improvement – reversal of population cholesterol levels
Blood cholesterol levels among the northern Sweden population decreased steadily after 1990, reached a plateau during 2000–2007, and thereafter have increased among men and women as well as all educational groups. The observation period was short during 2008–2010, but the increasing trend in population cholesterol levels seen in this study area should be addressed seriously. This is especially important because the 2010 levels resemble patterns seen ten years ago. The northern Sweden MONICA study also reported a decline in cholesterol levels among their 25–64 years old population between 1986 and 2009. Women aged 25–64 years old consistently had cholesterol levels 1–5% higher than men during the survey years of 1986, 1990, 1994, 1999, 2004, and 2009 (10). Unlike the MONICA study, our study found that men had cholesterol levels 1–2% higher than women during 1990–2001. After that time, the levels have had a difference of less than 1%.
Our hypothesis was that the increase in population cholesterol levels during 2008–2010 was not uniform across different geographical regions in the study area. This was not proven. Instead, increases were observed consistently in all geographical areas, from the rural inlands to large cities. The population in the rural inlands has consistently shown higher serum triglycerides than those who live in other areas. This might be explained by the higher prevalence of overweight, obesity, and physical inactivity observed in rural inlands (15, 18). High serum triglycerides are associated with obesity, sedentary lifestyle and alcohol consumption (20).
This study also found a steady decrease in the prevalence of hypercholesterolemia until 2002–2007 followed by an increase during 2008–2010. We observed statistical significant increases in the prevalence between 2002–2007 and 2008–2010 only among respondents with medium education. Even though the 99% CI between 2002–2007 and 2008–2010 overlaps for men and women with basic and high education, the overlap are very marginal (except for women with basic education), which might disappear if we use 95% CI instead. Therefore being conservative, we believe that these results from this large population survey, which show an increase in the prevalence of hypercholesterolemia during the last three years, are robust.
Gaps were found in the prevalence of hypercholesterolemia among men and women. Individuals with basic education have a significantly higher prevalence of hypercholesterolemia than those with medium or high education. The higher prevalence of overweight and obesity (15), and physical inactivity (18) among individuals with basic education in this study area might explain the unfavourable lipid patterns in this population subgroup. These two factors are independent risk factor for hypertriglyceridemia (20). In women, endogenous hormones and exogenous reproductive hormones influence triglyceride levels. Higher triglyceride levels are observed among menopausal women. It is unclear whether the change in triglycerides is attributable to the menopausal hormonal transition or is an effect of ageing or lifestyle changes among postmenopausal women.
Risk of developing dyslipidaemia
The 10-year risks of developing hypercholesterolemia and hypertriglyceridemia were higher among men recruited in 1996–2000 than those recruited in the earlier period 1990–1995. These patterns were less prominent in women. Because the 10-year risk of hypercholesterolemia for those recruited in 1996–2000 was measured during follow-up in 2006–2010, one might hypothesize that the higher risk in the later cohort is associated with the changes in dietary patterns during this period. The low-carbohydrate high-fat diet, a more extreme form of carbohydrate-restricted diet, was introduced into Sweden in 2005. Since that time, it has received much interest from the general public and the debates have been intensive at the national level. The appropriateness of the diet for diabetic patients or for weight loss has been questioned (21). A systematic review conducted by the Swedish Council on Health Technology Assessment expert group concluded that scientific evidence for the effects of low-carbohydrate high-fat (LCHF) diets, at least in diabetic patients, is insufficient due to a lack of studies with adequate study quality. The experts stated that the more extreme form of a carbohydrate-restricted diet must be used with caution, and individuals eating a low-carbohydrate high-fat diet ought to observe whether the new diet incurs negative consequences on risk factors in general and LDL-cholesterol in particular. At the same time, the expert group suggested that both traditional low-fat diets and moderate carbohydrate-restricted diets with an increase in unsaturated fatty acids, could function well for diabetic patients (22). Dietary saturated fat is proven to increase LDL-cholesterol, yet combination diets rich in polyunsaturated fat might modify this effect (23).
Our study shows that the women in the 50-year old cohort had a significantly higher risk of developing hypercholesterolemia in the next 10 years than the 30-year old cohort. Although complex biological mechanisms between menopause and cholesterol are yet to be fully elucidated, different studies report a higher level of total cholesterol among postmenopausal women compared to premenopausal women (24–26). In the cross-sectional analysis of the Third French MONICA study, Agrinier et al. (2009) report higher age-adjusted total cholesterol and LDL-cholesterol in postmenopausal women. The association remained after adjustment for lipid-lowering therapy, diabetes, hormone replacement therapy, and blood pressure lowering therapy (26). Hak et al. (2004) assessed the contribution of genetic variation to cholesterol levels among postmenopausal women, and concluded that APOE genotype modifies the extent of cholesterol increase after menopause. Women with APOE3E3 have higher risk of hypercholesterolemia than women with APOE2E3 (27).
Treatment for dyslipidaemia: coverage and control
The increased use of lipid-lowering agents, despite a decreasing prevalence of hypercholesterolemia and hypertriglyceridemia, may reflect better coverage of the treatment among those in need. The proportion receiving treatment is about half of the prevalent hypercholesterolemia. However, the need of pharmacological treatment depends largely on whether the individuals also exhibit other cardiovascular risk markers and on the effectiveness of non-pharmacological interventions. The National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adults Treatment Panel III) recommended secondary prevention with lipid-lowering therapy based on different risk categories. Those with coronary heart disease risk should be provided with therapy if LDL-cholesterol is ≥3.4 mmol/L (130 mg/dL); those with no or a single risk factor are given drug therapy only if the LDL-cholesterol is ≥4.9 mmol/L (190 mg/dL) (28). Although the proportion of people taking lipid-lowering treatment has increased over time, around 40% of treated men and women have not reached treatment goals. Providing cholesterol-lowering agents as well as lifestyle modification for individuals with hypercholesterolemia is essential in preventing mortality from ischemic heart disease. The total cholesterol level is an independent risk factor for ischemic heart disease at all blood pressure levels. A total cholesterol reduction of 1 mmol/L reduces ischemic heart disease by half among the population aged 40–49 years, a third among those aged 50–69 years, and a sixth among those aged 70–89 years (29).
Strengths and limitations
This repeated cross-sectional and panel dataset is unique as it is integrated within the routine primary health care system and the same questionnaires and protocols have been used with only minor revisions over the last 20 years. This provides high quality, comparable datasets across time and allows trend measurements that are not feasible in many settings. This dataset can provide reliable trends in cholesterol levels. In contrast to the Northern Sweden MONICA study, which is conducted on a random sample of population in Norrbotten and Västerbotten counties every five year, our data is collected continuously and represents total population 40, 50 and 60 years old in Västerbotten county who are invited to the health examination.
While the protocol for cholesterol analysis changed in the last few years, we have ensured the comparability of the new and older data through a calibration study as reported in the methods section. As the regression coefficient is very close to 1 (0.932 for triglyceride and 0.939 for cholesterol), the intercept of the regression formula (about 0.17 for triglyceride or cholesterol) shows the difference between results from Reflotron and lab-chemistry, which is very small. These confirm that results from both measurements are very similar to each other. The increase in cholesterol levels in the last three years cannot be fully explained by this study. These needs further exploration with inclusion of dietary intake assessment and other lifestyle behaviour that is not within the scope of this paper.
Conclusion
Despite the decreasing trends of population cholesterol levels since 1990 among the adult Västerbotten population, the reversal in trends since 2008 needs to be monitored and seriously addressed. The consistent gaps across population levels reflect the influence of socio-demographic determinants in risk factor patterns. The geographical differences in cholesterol levels need further exploration by assessing the contribution of other risk factors or socio-demographic determinants. Promoting healthy lifestyles is important in reducing population levels of cholesterol. In turn, this can prevent fatalities from coronary heart disease and stroke.
Acknowledgement
This research was supported by the Umeä Centre for Global Health Research with support from FAS, the Swedish Council for Working Life and Social Research (grant no. 2006–1512).
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
References
- 1.World Health Organization. The Global Burden of Disease: 2004 Update. Geneva: World Health Organization; 2008. [Google Scholar]
- 2.Fox CS, Evans JC, Larson MG, Kannel WB, Levy D. Temporal trends in coronary heart disease mortality and sudden cardiac death from 1950 to 1999: the Framingham Heart Study. Circulation. 2004;110:522–7. doi: 10.1161/01.CIR.0000136993.34344.41. [DOI] [PubMed] [Google Scholar]
- 3.Truelsen T, Mahonen M, Tolonen H, Asplund K, Bonita R, Vanuzzo D. Trends in stroke and coronary heart disease in the WHO MONICA Project. Stroke. 2003;34:1346–52. doi: 10.1161/01.STR.0000069724.36173.4D. [DOI] [PubMed] [Google Scholar]
- 4.Taylor R, Dobson A, Mirzaei M. Contribution of changes in risk factors to the decline of coronary heart disease mortality in Australia over three decades. Eur J Cardiovasc Prev Rehabil. 2006;13:760–8. doi: 10.1097/01.hjr.0000220581.42387.d4. [DOI] [PubMed] [Google Scholar]
- 5.Kuulasmaa K, Tunstall-Pedoe H, Dobson A, Fortmann S, Sans S, Tolonen H, et al. Estimation of contribution of changes in classic risk factors to trends in coronary-event rates across the WHO MONICA Project populations. Lancet. 2000;355:675–87. doi: 10.1016/s0140-6736(99)11180-2. [DOI] [PubMed] [Google Scholar]
- 6.Sweden National Board of Health and Welfare. Statistics – health and medical care: causes of death 2010. Stockholm: Sweden National Board of Health and Welfare; 2011. [Google Scholar]
- 7.Durrington P. Dyslipidaemia. Lancet. 2003;362:717–31. doi: 10.1016/S0140-6736(03)14234-1. [DOI] [PubMed] [Google Scholar]
- 8.Farzadfar F, Finucane MM, Danaei G, Pelizzari PM, Cowan MJ, Paciorek CJ, et al. National, regional, and global trends in serum total cholesterol since 1980: systematic analysis of health examination surveys and epidemiological studies with 321 country-years and 3.0 million participants. Lancet. 2011;3(77):578–86. doi: 10.1016/S0140-6736(10)62038-7. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. MONICA Monograph and Multimedia Sourcebook. Geneva: World Health Organization; 2005. [Google Scholar]
- 10.Eriksson M, Holmgren L, Janlert U, Jansson JH, Lundblad D, Stegmayr B, et al. Large improvements in major cardiovascular risk factors in the population of northern Sweden: the MONICA study 1986–2009. J Intern Med. 2011;269:219–31. doi: 10.1111/j.1365-2796.2010.02312.x. [DOI] [PubMed] [Google Scholar]
- 11.Statistics Sweden. Causes of death 1985. Stockholm: National Central Bureau of Statistics; 1987. [Google Scholar]
- 12.Weinehall L, Hellsten G, Boman K, Hallmans G. Prevention of cardiovascular disease in Sweden: the Norsjo community intervention programme –motives, methods and intervention components. Scand J Public Health Suppl. 2001;56:13–20. [PubMed] [Google Scholar]
- 13.Norberg M, Wall S, Boman K, Weinehall L. The Västerbotten Intervention Programme: background, design and implications. 2010;3 doi: 10.3402/gha.v3i0.4643. Global Health Action. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Schenck H, Treichl L, Tilling B, Olsson AG. Laboratory and field evaluation of three desktop instruments for assay of cholesterol and triglyceride. Clin Chem. 1987;33:1230–2. [PubMed] [Google Scholar]
- 15.Norberg M, Lindvall K, Stenlund H, Lindahl B. The obesity epidemic slows among the middle-aged population in Sweden while the socioeconomic gap widens. Global Health Action. 2010;3 doi: 10.3402/gha.v3i0.5149. 5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norberg M, Lundqvist G, Nilsson M, Gilljam H, Weinehall L. Changing patterns of tobacco use in a middle-aged population: the role of snus, gender, age, and education. Global Health Action. 2011;4 doi: 10.3402/gha.v4i0.5613. 5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindahl B, Stenlund H, Norberg M. Increasing glucose concentrations and prevalence of diabetes mellitus in northern Sweden, 1990–2007. Global Health Action. 2010;3 doi: 10.3402/gha.v3i0.5222. 5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ng N, Soderman K, Norberg M, Ohman A. Increasing physical activity, but persisting social gaps among middle-aged people: trends in Northern Sweden from 1990 to 2007. Global Health Action. 2011;4 doi: 10.3402/gha.v4i0.6347. 6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.StataCorp LP. Stata 11.2. TX, USA: 2009. [Google Scholar]
- 20.Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, et al. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123:2292–333. doi: 10.1161/CIR.0b013e3182160726. [DOI] [PubMed] [Google Scholar]
- 21.Mann J, Nye ER. Fad diets in Sweden, of all places. Lancet. 2009;374:767–9. doi: 10.1016/S0140-6736(09)61575-0. [DOI] [PubMed] [Google Scholar]
- 22.SBU. Mat vid diabetes: en systematisk litteraturöversikt (in Swedish) Stockholm: SBU the Swedish Council on Health Technology Assessment; 2010. [Google Scholar]
- 23.Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Saturated fat, carbohydrate, and cardiovascular disease. Am J Clin Nutr. 2010;91:502–9. doi: 10.3945/ajcn.2008.26285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pramparo P, Schargrodsky H, Boissonnet C, Champagne B, Silva S, Acevedo M, et al. Cardiovascular risk factors for heart disease and stroke in women by age and time since menopause, in seven Latin American cities: the CARMELA study. CVD Prevent Control. 2008;3:181–9. [Google Scholar]
- 25.de Aloysio D, Gambacciani M, Meschia M, Pansini F, Bacchi Modena A, Bolis PF, et al. The effect of menopause on blood lipid and lipoprotein levels. The Icarus Study Group. Atherosclerosis. 1999;147:147–53. doi: 10.1016/s0021-9150(99)00315-9. [DOI] [PubMed] [Google Scholar]
- 26.Agrinier N, Cournot M, Dallongeville J, Arveiler D, Ducimetiere P, Ruidavets JB, et al. Menopause and modifiable coronary heart disease risk factors: a population based study. Maturitas. 2010;65:237–43. doi: 10.1016/j.maturitas.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 27.Hak AE, Witteman JC, Hugens W, Keyzer JJ, Pop VJ, Uitterlinden AG, et al. The increase in cholesterol with menopause is associated with the apolipoprotein E genotype. A population-based longitudinal study. Atherosclerosis. 2004;175:169–76. doi: 10.1016/j.atherosclerosis.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 28.National Cholesterol Education Program (NCEP) Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 29.Lewington S, Whitlock G, Clarke R, Sherliker P, Emberson J, Halsey J, et al. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet. 2007;370:1829–39. doi: 10.1016/S0140-6736(07)61778-4. [DOI] [PubMed] [Google Scholar]